Mild Cognitive Impairment with a High Risk of Progression to Alzheimer’s Disease Dementia (MCI-HR-AD): Effect of Souvenaid® Treatment on Cognition and 18F-FDG PET Scans
Abstract
Background:
Previous studies have shown that Souvenaid (medical food) can have benefits on memory, cognition, and function in early Alzheimer’s disease (AD) and mild cognitive impairment (MCI).
Objective:
Demonstrate that Souvenaid could improve or maintain cognition and has an effect on neurodegeneration biomarkers.
Methods:
This cohort study was carried out from June 2015 through December 2016 in the Neurology Department, Infanta Cristina Hospital, Madrid, Spain. MCI-HR-AD were recruited using Petersen criteria, neuropsychology (NPS), and 18F-FDG PET scans to confirm the high risk of progression to dementia with one year of follow-up. Age, sex, vascular risk factors (VRF), and NPS values (Barcelona brief version) were analyzed. 18F-FDG PET scans were analyzed as a visual procedure. The study was approved by the Research Committee of ICH. Statistical analysis was made with SPSS 22.0 version.
Results:
Subjects included 43 MCI patients (58.5% women; mean age 69.78±7.89): 17 receiving Souvenaid® treatment (ST), 24 receiving no treatment (WT) and 2 who withdrew. No differences were seen in VRF, only hypercholesterolemia, and were less prevalent in the ST group (p = 0.002). The rate of progression to dementia was 48.8% (no differences between groups, p = 0.654). A second round of 18F-FDG PET scans showed a significance worsening of glucose metabolism in WT (p = 0.001) versus ST, in which it was low (p = 0.050). For NPS testing, there was a significant worsening in memory performance in the WT group (p = 0.011) and a stabilization in ST (p = 0.083), as well as in executive functions and attention (worsening in WT, p = 0.014). For the Subjective Changing Scale (SCS), caregivers indicated a stabilization/improvement in ST (p = 0.017).
Conclusion:
Souvenaid had a significant effect on several cognitive domains, and on SCS in patients with MCI-HR-AD. Its intervention had an impact on preservation on 18F-FDG PET scans.
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative condition which is highly prevalent in old age [1–4]. The World Health Organization (WHO) and Alzheimer’s Disease International (ADI) estimate that costs for care of older people will continue to increase and that the number of diagnosed dementia will reach 132 million patients [2, 3]. According to the Alzheimer’s Association, 13% of people over 65 suffer from this disease in developed countries, and this number is increasing in developing countries. AD has a significant socio-economic impact, which will lead to increased economic burden in healthcare systems all over the world [1–4].
AD has an insidious onset with episodic memory loss being one of the earliest reported symptoms. Progress toward effective therapies has been hampered because by the time cognitive symptoms emerge, significant pathological change has already taken place.
Aging is considered the principal risk factor for sporadic AD. Other potential risk factors include depression in midlife, low education level, obesity, hypertension in the midlife, dyslipidemia, metabolic syndrome, and diabetes [5–8].
The initial asymptomatic phase (preclinical AD) continues into a prodromal phase with mild, but noticeable, cognitive impairment but functional autonomy [9, 10], and eventual progression to dementia. This gradual progression creates a window of opportunity for pharmacological and non-pharmacological interventions in early disease stages.
Prevention trials have involved multimodal, non-pharmacological approaches including dietary intervention [11–14]. Diet is an important modifiable risk factor for dementia [15].
The LipiDiDiet group is a research consortium, which has studied the preclinical and clinical impact of nutrition in AD. This research contributed to the development of the medical food Souvenaid (Nutricia, Zoetermeer, the Netherlands). The results of the clinical trial in prodromal AD reported stabilization of cognition and function (Clinical Dementia Rating Scale Sum of Boxes, CDR-SB) and amelioration of hippocampal atrophy (magnetic resonance imaging, MRI) over a two-year period [16].
The active component of Souvenaid (Fortasyn Connect®) is a multinutrient combination, containing docosahexaenoic acid (DHA); eicosapentaenoic acid (EPA); uridine monophosphate; choline; vitamins B12, B6, C, E, and folic acid; phospholipids; and selenium [17]. These nutrients were selected based on their biological properties, involved in metabolic pathways (Kennedy and PEMP pathways), and specifically combined to enhance efficacy in phospholipid turn over and improvement of synaptic formation. In animal models, including transgenic AD mice, dietary intervention with this multinutrient combination has been shown to enhance phospholipid synthesis, to maintain white and gray matter integrity, to reduce the impact of amyloid-induced neurodegeneration and loss of functional connectivity, to increase numbers of hippocampal cholinergic synapses, and to improve cholinergic neurotransmission and hippocampus-dependent cognitive performance [17–26].
In two previous randomized clinical trials, Souvenaid improved memory performance in patients with mild AD, over 3 and 6 months, respectively [28, 29]. Furthermore, increased neurophysiological measures of synaptic activity, and enhanced functional connectivity in the brain [30] were reported in the longer study. Another study in patients with more advanced AD, who were on stable AD medication, showed no significant add-on effect of the multinutritional intervention [31].
Across four clinical trials, Souvenaid was well tolerated with a positive safety profile, alone and in combination with cholinesterase inhibitors and memantine. The results of the clinical trials of Souvenaid are consistent in their report of benefits in cognition and memory with better outcomes in earlier intervention [16]. The size effect of this nutritional intervention has been published recently [32]. Small to moderate effect sizes have been observed on primary outcome memory function in patients with mild AD in two separate randomized controlled trials [28, 29]. These effect sizes are clinically detectable and similar to those seen in cholinesterase inhibitors.
No effects were observed on the co-primary outcome Alzheimer’s Disease Assessment Scale—Cognitive Subscale (ADAS-cog), due to the lack of sensitivity of this outcome in very early AD patients.
To further investigate the presumed effect of this multinutrient combination on synaptic function, a Dutch double-blind randomized controlled parallel-group single-center study exploring the Effect of this specific Nutritional Intervention on cerebral Glucose Metabolism in early Alzheimer’s disease has been examined and designated as NL-ENIGMA (Dutch Trial Register NTR4718, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC54718) [33].
In this present study, the mode of action of the specific multinutrient combination is further explored using 18F-fluoro-deoxyglucose (18F-FDG-PET) and neuropsychological tests in clinical practice.
The results of one trial using EEG suggest that Souvenaid preserves the organization of brain networks in patients with mild AD within 24 weeks, hypothetically counteracting the progressive network disruption over time in AD. This result strengthens the hypothesis that Souvenaid affects synaptic integrity and function [34].
In this work, we want to replicate the results obtained by Souvenaid in this trial preserving the organization of brain networks using FDG-PET, a technique that is a direct index for synapse function and density because the uptake of 18F-FDG is driven by synaptic terminals generating ATP for synthesis, release, and recycling of neurotransmitters, the maintenance of the normal resting potential, and the recovery from action potentials [35, 36]. We examine patients with MCI with high rate of progression to AD dementia.
METHODS
43 patients from the Neurology Department of Infanta Cristina, Parla, Madrid, Spain Hospital, diagnosed with MCI according to Petersen criteria [37] were included. For diagnosis of MCI, impairment in one or more cognitive domains has to be present, based on clinical interpretation of performances on a neuropsychological test battery (Barcelona brief version battery) [38], whereas independency of functional abilities is preserved.
18F-FDG scans (w300 MBq, 90–110 min post injection) was considered positive when abnormal binding was seen in at least one cortical ROI (i.e., lateral temporal, frontal, posterior cingulate, precuneus, and parietal). Scans were divided into normal, mild (temporal low glucose metabolism (LGM) uni- or bilateral), medium (temporo-parietal LGM unilateral), and high (temporo-parietal LGM bilateral).
Souvenaid (specific multinutrient combination Fortasyn Connect®) intervention was offered to all patients. Those who refused were included in control group. Some patients in the prodromal phase of the disease were given cholinesterase inhibitors as a primary treatment. These patients were then given a combination of treatments (Souvenaid and cholinesterase inhibitors/memantine).
Procedures
This longitudinal study, with a 1-year follow-up, was carried out from June 2015 to December 2016 in the Neurology Department of Infanta Cristina Hospital, Madrid, Spain.
Baseline demographic information, including age, sex, education, family history of AD, date of diagnosis MCI or dementia, and rate of progression to dementia, was recorded.
All patients had a neurological history, physical examination, neurological examination, neuropsychological test exploration, CT scans, blood samples (including total protein levels), and 18F-FDG-PET imaging. The imaging was conducted in the Department of Nuclear Medicine at the University Getafe Hospital.
Neuropsychological tests and 18F-FDG-PET imaging were conducted at the inclusion visit, approximately 8 months later, and checked again at the 1-year follow-up (dependent on the delay in clinical visit). On the last clinical visit, we included a neurological examination and the Subjective Changing Scale (SCS) completed by the caregiver.
PET assessment and analyses
18F-FDG-PET scan was performed on a Siemens PET-CT scanner. Patients were in a fasting state for at least 6 h prior to scanning. An intravenous line was placed in an upper extremity 15 min prior to administration of approximately 185 MBq (5mCi) of 18F-FDG, dissolved in 5 mL of saline. After waiting 30 min at rest and without visual or auditory stimuli, the PET scan was initiated with a low-dose CT scan for attenuation correction of PET data and subsequent acquisition of PET, 1 bed of 10 min duration. The analysis was visual.
Cognitive assessment
Cognitive measures were made according to the Barcelona battery (brief version) which included orientation, memory, language, executive functions, gnosis, and praxis domains. This battery was administered once at baseline and then repeated 8 months later. The results were divided in accordance with the Barcelona brief version rules, with the following cut-off: deficient (p < 5), low performance (p5–p20), medium performance (p25–p75), and high performance (p > 80). Patients included in high performance group were excluded from the study.
Statistical analyses
A descriptive analysis was performed. Comparisons between the groups [Souvenaid treatment (ST) versus no treatment (WT)] were conducted using chi-square and/or Fisher’s exact tests for categorical variables, and Student t-test and Mann-Whitney test for continuous variables. McNemar’s test and Test of Marginal Homogeneity were used to determine differences during follow up.
In all analyses, differences of p < 0.05 were considered statistically significant. Analyses were performed using IBM SPSS Statistics for Windows, version 22.0.
Several potential covariates and possible intervention effect moderators were defined: Mini-Mental State Examination at screening, diagnosis of dementia, relevant medical events, relevant medication, coexisting diseases, all the demographic and other baseline variables, and product compliance.
Ethical and legal considerations
The study 224/2017 was approved by the Research Committee of ICH in 2015.
RESULTS
Between June 2015 to December 2016, 43 participants were included to the study: 17 receiving Souvenaid® treatment (ST), 24 without treatment (WT), and 2 who withdrew. The main reasons for dropout were negative to intake. Two patients in the WT group refused 18F-FDG PET scans. Two patients refused neuropsychological (NPS) examination (one in each group). Mean age was 69.78 (±7.89 SD) years, and 58.5% of participants were women. Further baseline characteristics of study participants are shown in (Table 1).
Table 1
Baseline characteristics
| Baseline characteristics | ST (n = 17) | WT (n = 24) | p |
| Age (y) Mean (±SD) | 72.18 (±6.34) | 68.08 (±8.54) | 0.102 |
| Sex n (%) | 9 (52.9%) men | 8 (33.3%) men | 0.209 |
| 8 (47.1%) women | 16 (66.7%) women | ||
| Hypercholesterolemia n (%) | 2 (14.3%) | 15 (68.2%) | 0.002 |
| High blood pressure n (%) | 9 (52.9%) | 10 (41.7%) | 0.476 |
| Diabetes mellitus n (%) | 2 (11.8%) | 4 (16.7%) | 0.512 |
| Total proteins Mean (±SD) | 7.023 (±0.54) | 6.96 (±0.47) | 0.722 |
ST, Souvenaid treatment; WT, without treatment; SD, standard deviation; n, number.
There were no differences in vascular risk factors (VRF), and hypercholesterolemia was less prevalent in the ST group (p = 0.002) (Fig. 2).
The age of the participants at the beginning of the study was the same between groups (Fig. 1) and total protein was also the same in both groups (Fig. 3).
Fig.1
Age at onset.
Fig.2
Cardiovascular risk factors. HTA, hypertension; DM, diabetes mellitus.

Fig.3
Total proteins in both groups.
In neuropsychological testing, each cognitive domain was evaluated. At baseline, a lower performance was observed in the ST group (p < 5 in 75% versus 34.8%, p = 0.033). There was a significant worsening in memory performance in the WT group (p = 0.011) from baseline, whereas stabilization was observed in the ST group (p = 0.083). Similarly for executive function and attention, the WT group experienced a worsening (p = 0.014) from baseline, while stabilization was seen in the in ST group.
Around 26% of the ST patients (n = 5) were receiving other treatment at the beginning (cholinesterase inhibitors, because of abnormal 18F-FDG PET scans or worse NPS performance). There were no differences between groups, comparing ST (monotherapy) with WT.
The rate of progression to dementia was higher in the bi-therapy group (ST monotherapy p < 0.008, WT p < 0.009), compared with monotherapy.
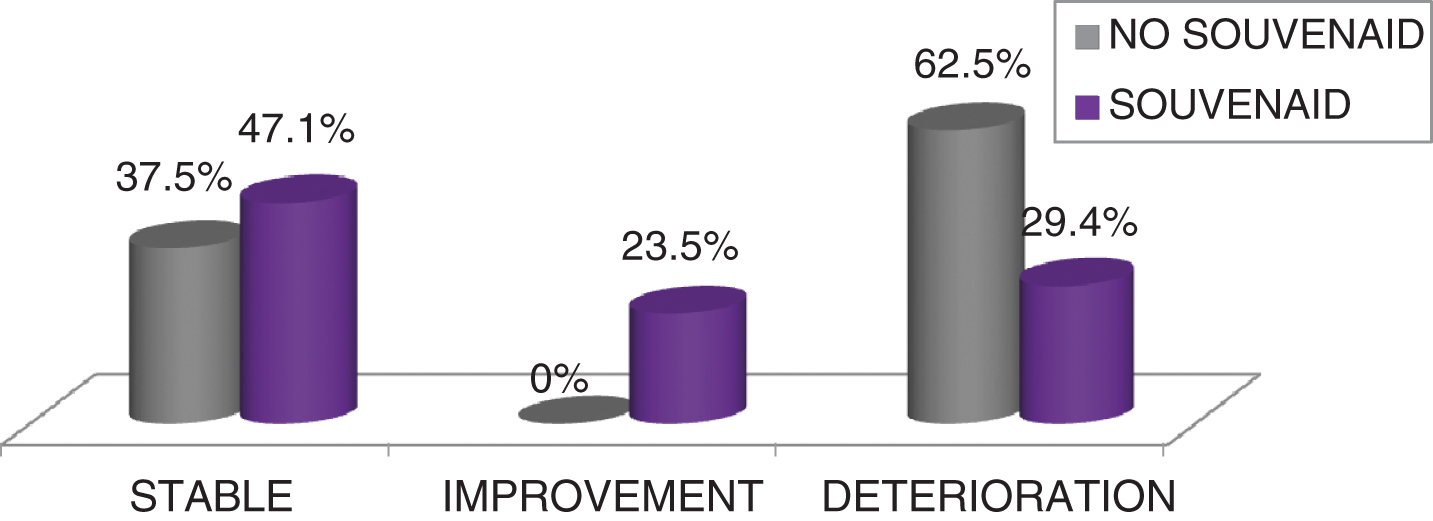
In the SCS, caregivers reported a stabilization or improvement in ST versus WT group (p = 0.017).
There were no differences in PET-FDG scans at baseline (p = 0.321). (See Table 2).
Table 2
18F-FDG PET scans results (number and percentage)
| Normal | Mild impairment | Moderate impairment | Severe impairment | p | |
| ST Group n(%) | n (%) | n (%) | n (%) | n (%) | 0.050 |
| First 18F-FDG PET scans | 6 (35.29%) | 8 (47.06%) | 2 (11.76%) | 1 (5.88%) | |
| Second 18F-FDG PET scans | 3 (17.65%) | 8 (47.06%) | 5 (29.41%) | 1 (5.88%) | |
| WT Group | 0.001 | ||||
| First 18F-FDG PET scans | 14 (63.64%) | 8 (36.36%) | 0 (0.00%) | 0 (0.00%) | |
| Second 18F-FDG PET scans | 8 (36.36%) | 7 (31.82%) | 7 (31.82%) | 0 (0.00%) |
ST, Souvenaid treatment; WT, without treatment. p, McNemar’s test for ST group and test of marginal homogeneity for WT group.
At follow-up, 18F-FDG PET scans showed a significant worsening of glucose metabolism in WT (p = 0.001) versus ST, in which it was low (p = 0.050) (See Table 3).
Table 3
Main results. Comparison between groups
| WT | ST | |
| PET | Significant worsening | Mild worsening |
| p = 0.001 | p = 0.050 | |
| Memory | Worsening | Stabilization |
| p = 0.011 | p = 0.053 | |
| Praxis | Stabilization | Stabilization |
| p = 0.157 | p = 1 | |
| Orientation | Stabilization | Stabilization |
| p = 0.082 | p = 0.999 | |
| Executive functions | Worsening | Stabilization |
| p = 0.012 | p = 0.135 | |
| Attention | Worsening | Stabilization |
| p = 0.014 | p = 0.157 | |
| Gnosis | Stabilization | Stabilization |
| p = 0.151 | p = 1 |
There were 3 patients with amyloid PET scans (one in ST and 2 in WT). In the ST patient, the SCS showed a mild improvement against the other two with a mild worsening.
There were no significant differences in the rate of progression to dementia between groups. The rate of progression to dementia was 48.8% (p = 0.654).
In the SCS, caregivers reported a stabilization or improving in ST versus WT (p = 0.017) (see Fig. 4).
Fig.4
Subjective Changing Scale (SCS): differences between groups.
DISCUSSION
This study aims to explore the effect of Souvenaid on cerebral glucose metabolism in mild to very mild patients with high risk of progression to AD, using neuropsychological variables, and 18F-FDG scans over 1 year of follow up and in real life clinical practice. A positive effect of the nutritional intervention was observed, compared with controls, in cognitive and imaging parameters but not in progression to dementia. These results are in line with previous trials of Souvenaid in early AD [16].
This is the first Souvenaid study to include functional neuroimaging. 18F-FDG-PET is a well-established method to study synapse function [26–28].
The LipiDiDiet group, a research consortium, has studied preclinical and clinical impacts of nutrition in AD. This research contributed to the development of the medical food Souvenaid (Nutricia; Zoetermeer, the Netherlands). A clinical trial on the use of Souvenaid in prodromal AD reported that it stabilized cognition and function (CDR-SB) and ameliorated hippocampal atrophy (MRI) over a two-year period [16].
The active component of Souvenaid includes, in addition to other nutrients (e.g., B vitamins, vitamins C and E) [17], three food constituents which, when given together, promote synaptogenesis [19, 20]. These are choline, an omega-3 fatty acid [docosahexaenoic acid (DHA) or eicosopentaenoic acid (EPA)]; and uridine monophosphate (UMP). The omega-3 fatty acid and choline are true nutrients: their consumption in foods raises their levels in the blood and brain [20, 21]. In contrast, the uridine in the blood of adult humans is more like a hormone than a nutrient, in that it is derived not from dietary sources but from synthesis in and secretion from the liver [20, 21]. This is because most of the uridine in foods is present in a form (e.g., as RNA) which is not bioavailable in adult humans [22]. Infants do obtain dietary uridine from bioavailable sources, because much of the uridine in mothers’ milk or most infant formulas is present as UMP, the same bioavailable form as in Souvenaid [23].
These three compounds are essential precursors in the biosynthesis of the phosphatide molecules that comprise the bulk of synaptic membranes (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol). The key biochemical steps in the conversions of DHA or EPA, choline, or uridine to the phosphatides are all catalyzed by enzymes which have low affinities for their substrates [20]. Thus, administering each substrate increases the saturation of its metabolizing enzyme and the rate at which new product is formed, ultimately raising brain phosphatide levels [19].
Administering the mixture of phosphatide precursors also increases brain levels of the major proteins in synaptic membranes, possibly via uridine’s activation of P2Y receptors [24]. Hence both of the key constituents (phospholipids and proteins) of functionally-complete membranes are formed when animals or humans receive the three precursors. This, in turn, enhances the production of dendritic spines [25], the immediate cytologic precursor of new synapses, thereby partly correcting the deficiency in the spines that is characteristic of AD and other dementias [26], and enabling enhanced synaptogenesis.
In animal models, including transgenic AD mice, administration of the three precursors, alone or as a constituent of Souvenaid, has also been shown to maintain the integrity of white and gray matter, reduce the loss of functional connectivity, increase cholinergic hippocampal synapses and cholinergic neurotransmission, and facilitate hippocampus-dependent cognitive performance [17–26].
A previous clinical study in patients with mild AD using EEG as a biomarker demonstrated an effect of the multinutrient combination on functional connectivity and brain network organization, suggesting that its mode of action includes alteration of synapse function [39].
As with all real-world studies, this has some limitations. The first one is the brief neuropsychological test battery used for the study, and the other is the visual assessment of 18F-FDG-PET scans.
Secondly, in the 5 patients with bi-therapy (ST and cholinesterase inhibitors), compared with the other (monotherapy or WT), there were no differences in the values, only in the rate of progression to dementia. Obviously, these patients were more impaired (although MCI criteria), therefore it was right to begin treatment sooner, with both therapies (ST and cholinesterase inhibitors). Interestingly, these patients progressed to dementia more quickly.
Another thing to consider is the option of to treat or not. We offered the treatment to everybody. All those who refused (we offered all the information about benefits and inconvenience) to take the treatment were included in the without treatment group. Another thing to consider is that the patients who withdrew from taking treatment did so for one reason, they did not want to drink a fluid. This is something to consider in the methodology of the study. They obviously stopped the follow up.
Overall, the results of this study support the hypothesis that Souvenaid, a multinutrient combination, can benefit patients with MCI and those who are at risk of progressing to AD. The NL-ENIGMA study will provide more information about this [33].
Souvenaid, as a medical food, had a significant effect on several cognitive domains in patients with AD in dementia stage and also in MCI. In our study, caregivers report benefits in the SCS in patients with MCI-HR-AD. Furthermore, its intervention had an impact on preservation as seen in 18F-FDG PET scans and in many cognitive domains (memory, attention, and executive functions).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
Some of the authors of this study are affiliated with Nutrica as indicated in their respective addresses. Nutrica funded portions of this study.
REFERENCES
[1] | Santana I , Farinha F , Freitas S , Rodrigues V , Carvalho Å ((2015) ) [The epidemiology of dementia and Alzheimer disease in Portugal: Estimations of prevalence and treatment-costs] (in Portuguese). Med Port 28: , 182–188. |
[2] | Alzheimer’s Association ((2015) ) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11: , 332–384. |
[3] | Prince M , Albanese E , Prina M ((2014) ) World Alzheimer Report 2014. Dementia and risk reduction: An analysis of protective and modifiable factors. Alzheimer’s Disease International, London, UK. |
[4] | Chiang K , Koo EH ((2014) ) Emerging therapeutics for Alzheimer’s disease. Ann Rev Pharmacol Toxicol 54: , 381–405. |
[5] | Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ ((2012) ) National Institute on Aging—Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8: , 1–13. |
[6] | Castello MA , Soriano S ((2013) ) Rational heterodoxy: Cholesterol reformation of the amyloid doctrine. Ageing Res Rev 12: , 282–288. |
[7] | Castello MA , Soriano S ((2014) ) On the origin of Alzheimer’s disease. Trials and tribulations of the amyloid hypothesis. Ageing Res Rev 13: , 10–12. |
[8] | Drachman DA ((2014) ) The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimers Dement 10: , 372–380. |
[9] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[10] | Dubois B , Hampel H , Feldman HH , Scheltens P , Aisen P , Andrieu S , Bakardjian H , Benali H , Bertram L , Blennow K , Broich K , Cavedo E , Crutch S , Dartigues JF , Duyckaerts C , Epelbaum S , Frisoni GB , Gauthier S , Genthon R , Gouw AA , Habert MO , Holtzman DM , Kivipelto M , Lista S , Molinuevo JL , O’Bryant SE , Rabinovici GD , Rowe C , Salloway S , Schneider LS , Sperling R , Teichmann M , Carrillo MC , Cummings J , Jack CR Jr , Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA ((2016) ) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12: , 292–323. |
[11] | Zandi PP , Anthony JC , Khachaturian AS , Stone SV , Gustafson D , Tschanz JT , Norton MC , Welsh-Bohmer KA , Breitner JC ; Cache County Study Group ((2004) ) Reduced risk of Alzheimer disease in users of anti- oxidant vitamin supplements: The Cache County Study. Arch Neurol 61: , 82–88. |
[12] | Engelhart MJ , Geerlings MI , Ruitenberg A , van Swieten JC , Hofman A , Witteman JCM , Breteler MM ((2002) ) Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 287: , 3223–3229. |
[13] | Ngandu T , Lehtisalo J , Solomon A , Levälahti E , Ahtiluoto S , Antikainen R , Bäckman L , Hänninen T , Jula A , Laatikainen T , Lindström J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385: , 2255–2263. |
[14] | Drieu S , Guyonnet S , Coley N , Cantet C , Bonnefoy M , Bordes S , Bories L , Cufi MN , Dantoine T , Dartigues JF , Desclaux F , Gabelle A , Gasnier Y , Pesce A , Sudres K , Touchon J , Robert P , Rouaud O , Legrand P , Payoux P , Caubere JP , Weiner M , Carrié I , Ousset PJ , Vellas B ; MAPT Study Group ((2017) ) Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol 16: , 377–389. |
[15] | van Gelder BM , Tijhuis M , Kalmijn S , Kromhout D ((2007) ) Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am J Clin Nutr 85: , 1142–1147. |
[16] | Soininen H , Solomon A , Visser PJ , Hendrix SB , Blennow K , Kivipelto M , Hartmann T ; LipiDiDiet clinical study group ((2017) ) 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): A randomised, double-blind, controlled trial. Lancet Neurol 16: , 965–975. |
[17] | van Wijk N , Broersen LM , de Wilde MC , Hageman RJ , Groenendijk M , Sijben JW , Kamphuis PJ ((2014) ) Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J Alzheimers Dis 38: , 459–479. |
[18] | de Wilde MC , Hogyes E , Kiliaan AJ , Farkas T , Luiten PG , Farkas E ((2003) ) Dietary fatty acids alter blood pressure, behavior and brain membrane composition of hypertensive rats. Brain Res 988: , 9–19. |
[19] | Wurtman RJ , Ulus IH , Cansev M , Watkins CJ , Wang L , Marzloff G ((2006) ) Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res 1088: , 83–92. |
[20] | Wurtman RJ , Cansev M , Sakamoto T , Ulus IH ((2009) ) Use of phosphatide precursors to promote synaptogenesis. Annu Rev Nutr 29: , 59–87. |
[21] | Wurtman RJ ((2010) ) Nutritional modifiers of aging brain function: Increasing the formation of brain synapses by administering uridine and other phosphatide precursor. Nutr Rev 68(Suppl 2): , S88–101. |
[22] | Gasser T , Moyer JD , Handschumacher RE ((1981) ) Novel single-pass exchange of circulating uridine in rat liver. Science 213: , 777–778. |
[23] | Wurtman RJ ((2014) ) A nutrient combination that can affect synapse formation. Nutrients 6: , 1701–1710. |
[24] | Cansev M ((2016) ) Synaptogenesis: Modulation by availability of membrane phospholipid precursors. Neuromolecular Med 18: , 426–440. |
[25] | Sakamoto T , Cansev M , Wurtman RJ ((2007) ) Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res 1182: , 50–59. |
[26] | Catala I , Ferrer I , Galofre E , Fabregues I ((1988) ) Decreased numbers of dendritic spines on cortical pyramidal neurons in dementia. A quantitative Golgi study on biopsy samples. Hum Neurobiol 6: , 255–259. |
[27] | Wang L , Albrecht MA , Wurtman RJ ((2007) ) Dietary supplementation with uridine-5’-monophosphate (UMP), a membrane phosphatide precursor, increases acetylcholine level and release in striatum of aged rat. Brain Res 1133: , 42–48. |
[28] | Scheltens P , Kamphuis PJ , Verhey FRJ , Olde Rikkert MG , Wurtman RJ , Wilkinson D , Twisk JW , Kurz A ((2010) ) Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement 6: , 1–10.e1. |
[29] | Scheltens P , Twisk JW , Blesa R , E , von Arnim C , A , Harrison J , Swinkels S , Stam C , de Waal H , R , Wieggers R , Vellas B , Kamphuis P ((2012) ) Efficacy of Souvenaid in mild Alzheimer’s disease: Results from a randomized, controlled trial. J Alzheimers Dis 31: , 225–236. |
[30] | de Wilde MC , Penke B , van der Beek EM , Kuipers AA , Kamphuis PJ , Broersen LM ((2011) ) Neuroprotective effects of a specific multi-nutrient intervention against Abeta42-induced toxicity in rats. J Alzheimers Dis 27: , 327–339. |
[31] | Shah RC , Kamphuis PJ , Leurgans S , Swinkels SH , Sadowsky CH , Bongers A , Rappaport SA , Quinn JF , Wieggers RL , Scheltens P , Bennett DA ((2013) ) The S-Connect study: Results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther 5: , 59. |
[32] | Cummings J , Scheltens P , McKeith I , Blesa R , Harrison JE , Bertolucci PH , Rockwood K , Wilkinson D , Wijker W , Bennett DA , Shah RC ((2017) ) Effect size analyses of Souvenaid in patients with Alzheimer’s disease. J Alzheimers Dis 55: , 1131–1139. |
[33] | Scheltens N , Kuyper I , Boellaard R , Barkhof F, Teunissen CE , Broersen LM , Lansbergen MM , van der Flier WM , van Berckel BNM , Scheltens P ((2016) ) Design of the NL-ENIGMA study: Exploring the effect of Souvenaid on cerebral glucose metabolism in early Alzheimer’s disease. Alzheimers Dement (N Y) 2: , 233–240. |
[34] | de Waal H , Stam CJ , Lansbergen MM , Wieggers RL , Kamphuis PJ , Scheltens P , Maestú F , van Straaten EC ((2014) ) The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: A randomised controlled study. PLoS One 9: , e86558. |
[35] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[36] | deWilde MC , Kamphuis PJ , Sijben JW , Scheltens P ((2011) ) Utility of imaging for nutritional intervention studies in Alzheimer’s disease. Eur J Pharmacol 668: , S59–69. |
[37] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: , 183–194. |
[38] | |
[39] | Waal A , Stam C , Lansbergen M , Wieggers R , Kamphuis P , Scheltens P , Maestú F , van Straate E ((2014) ) Souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: A randomised controlled study. PLoS One 9: , e86558. |




