Isokinetic testing of muscle strength of older individuals with sarcopenia or frailty: A systematic review
Abstract
BACKGROUND
: Sarcopenia is a component of frailty, which is a common geriatric syndrome for which the quantification of muscle strength is important.
OBJECTIVE:
Describe studies that have used isokinetic testing for detecting sarcopenia and determine whether there is an isokinetic strength level that can best detect sarcopenia.
METHODS
: A systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Our literature search involved the following key words: (isokinet*) AND ((sarcopenia) OR (frailty) OR (muscle weakness). Sarcopenia reference values for isokinetic knee flexion and extension at 60
RESULTS:
A total of 19 studies that were relevant and lacked major methodological flaws were included. Most measured peak torque of the knee extensors and/or flexors. The measurements were found to be valid and responsive. The net moment weighted averages for knee extension torques were 83
CONCLUSIONS:
The isokinetic strength values reported herein can be used to identify sarcopenia in older men and women.
1.Introduction
Numerous studies have described muscle strength as decreasing with age. Such decreases are a highlight of geriatric syndromes such as sarcopenia and frailty. As both sarcopenia and frailty typically lead to mobility limitations and a loss of overall self-sufficiency in the later stages; the identification of muscle weakness is important for both mentioned conditions. The identification of sarcopenia and frailty is typically evaluated by handgrip strength in clinical practice [1]. Handgrip strength has practical advantages, as it is easy to measure and interpret relative to available reference values [1, 2]. On the other hand, handgrip strength has disadvantages; it does not focus on muscles underlying mobility limitations [3] or untoward events such as falls [4, 5]. Although research studies on sarcopenia sometimes include isokinetic strength measurements, there is still a lack of consensus as to the what level of isokinetic strength best differentiates individuals who are versus are not sarcopenic. The purposes of this systematic review, therefore were to summarize studies in which isokinetic measures have been used for detecting sarcopenia and to determine an isokinetic strength cut-point for its designation.
2.Methods
The present systematic review is reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [6]. The following electronic bibliographic databases were searched: Web of Science, Scopus, and PubMed. Potentially relevant articles were identified on February 10, 2019. We used the same search string in all the databases (Table 1). After deleting duplicates, article titles and abstracts were examined to determine whether the articles identified by the searches addressed the isokinetic testing of muscle strength and focused on patients/participants aged
Table 1
Search results in electronic databases
| Database | Key | Number |
|---|---|---|
| Web of Science | TOPIC: (isokinet*) AND TOPIC: (sarcopenia) OR TOPIC: (frailty) OR TOPIC: (muscle weakness) | 580 |
| Scopus | (TITLE-ABS-KEY (isokinet*)) AND ((TITLE-ABS-KEY(sarcopenia) OR TITLE-ABS-KEY (frailty) OR TITLE-ABS-KEY (muscle AND weakness))) | 766 |
| PubMed | Search (((isokinet*) AND ((sarcopenia) OR (frailty) OR (muscle weakness))) | 575 |
We included all the articles from cross-sectional studies, validation studies, and randomized controlled trials in which isokinetic dynamometry was used for diagnosing participants aged
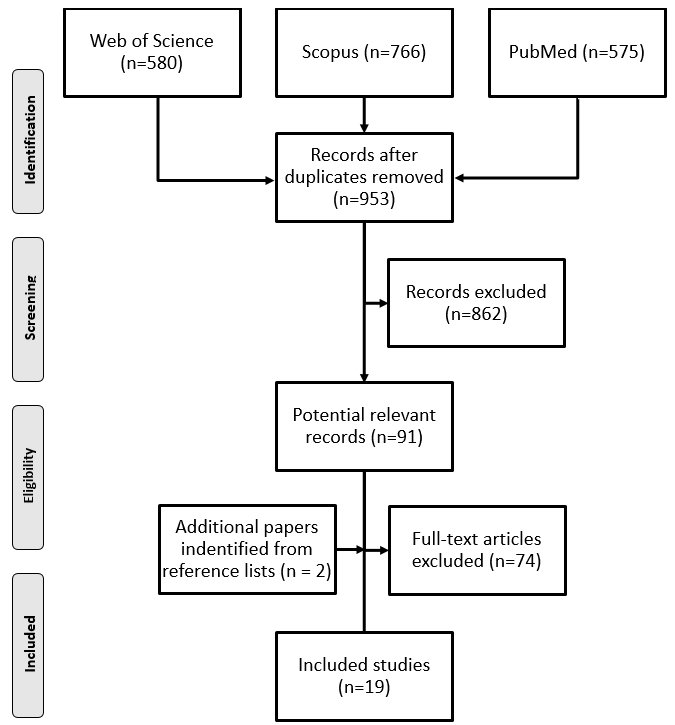
Figure 1.
The PRISMA flowchart of the review selection process.
2.1Calculation of weighted averages
To approximate the values of muscle strength, we calculated weighted averages when possible. We used either the baseline values in the RCTs or the values for sarcopenic/frail individuals in cross-sectional studies. The weighted average was calculated by Eq. (1).
(1)
where
(2)
where
(3)
The standard error was defined by Eq. (4).
(4)
The 95% confidence interval (CI) for the weighted average was computed as the lower limit and upper limit by Eq. (5).
(5)
The standard deviation (SD) was calculated by Eq. (6).
(6)
Then, we compared the weighted averages with the reference values for healthy older adults by the independent
3.Results
Out of the 1,921 articles identified by the database search, we included a total of 17. An additional two articles were identified by hand searches. Ultimately, 19 articles that met the inclusion criteria were included in the review (Fig. 1).
There were 12 cross-sectional studies [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18], four randomized controlled trials [19, 20, 21, 22], one prospective study [23], one multicenter clinical trial [24], and one validation study [25] that were included. Five studies focused on frail people, 10 focused on people with sarcopenia and the other studies focused on people with muscle functioning problems that were defined differently. The randomized controlled trials examined the influence of several physical activity interventions on different muscle-related measures. One validation study tried to establish the validity of a novel portable trunk-muscle torque measuring instrument [25]. The cross-sectional studies tried to explore different relationships among muscle function or muscle mass and several independent variables, such as plasma levels of interleukin, the extent of apoptosis activation, and bone mass density. The main topic of one study was estimating the reference values of isokinetic measures in females with frailty [18] (Table 2).
The most frequently measured isokinetic strength was that of knee extension, followed by knee flexion. Both were measured with participants in a sitting position. The most commonly reported angular velocity was 60
The peak torque (Nm) in knee extension as well as in flexion varied considerably among the studies. In extension, the lowest and highest values in males net moment were 57
4.Discussion
Isokinetic dynamometry is a commonly used procedure for measuring muscle strength in athletes of many sports. Nevertheless, its potential is not fully realized in older people with muscle problems. Especially in people with sarcopenia, isokinetic dynamometry can be informative. This method is useful mainly in measuring lower extremity strength, which is essential for locomotion and is one of the most important factors of self-sufficiency in the elderly population. This study clearly documented that sex-specific isokinetic strength of the knee flexors and extensors at 60
Table 2
Summary of studies evaluating isokinetic strength in the sarcopenia population
| Study | Study type | Participants | Aims |
|---|---|---|---|
| Binder [19] | Randomized controlled trial | American older adults ( | Evaluate the changes in FFM and fat mass in response to progressive resistance exercise training |
| Binder [20] | Randomized controlled trial | American older adults ( | Determine the effects of intensive exercise training on measures of physical frailty |
| Brown [7] | Cross-sectional | American community-dwelling sedentary males and females ( | Examine the relationships between multiple physical factors believed to be associated with frailty, including isometric and dynamic strength, range of motion, sensation, coordination, balance, and reaction time, and the physical performance test. |
| Cramer [21] | Multicenter randomized controlled trial | American, Belgian, Italian, Mexican, Polish | |
| Spanish, Swiss, English older people ( | Evaluate the effects of 2 high-quality oral nutritional supplements differing in amount and type of key nutrients | ||
| Cramer [8] | Cross-sectional | American, Belgian, Italian, Mexican, Polish | |
| Spanish, Swiss, English older people ( | Quantify systematic variability and intraindividual variability among three consecutive repetitions of concentric isokinetic knee extension and knee flexion muscle actions to determine velocity-related differences in peak torque and mean power in healthy elderly older adults versus sarcopenic and malnourished older adults | ||
| de Oliveira [9] | Cross-sectional | Brazilian older females ( | Examine the associations between FFM, knee extensor strength and sarcopenia with aerobic fitness indexes in elderly females |
| Gadelha [23] | Prospective | Brazilian older females ( | Assess the association between different stages of sarcopenia and the incidence of falls over 18 months |
| Jenkins [24] | Multicenter clinical trial | American, Belgian, Italian, Mexican, Polish | |
| Spanish, Swiss, English older people ( | Determine the test – retest reliability and minimum detectable change scores for 7 common clinical measurements of muscle strength and physical function | ||
| Kim [11] | Cross-sectional | Korean older people ( | Clarify the relationship between muscle mass and physical performance in older adults with weak muscle strength |
| Kim [10] | Cross-sectional | Korean older people ( | Investigate the relationship between body composition parameters and functional limitation in older adults |
| Lima [12] | Cross-sectional | Brazilian older females ( | Examine the association between FFM & muscle strength with bone mineral density sites and to compare the bone mineral density values between sarcopenic and nonsarcopenic older females |
| Lustosa [13] | Cross-sectional | Brazilian older females ( | Compare the performance of the knee extensors test (by isokinetic dynamometer) and plasma levels of interleukin-6 & soluble receptors of tumor necrosis factor alpha between sarcopenic & nonsarcopenic community-dwelling elderly females |
| Marzetti [14] | Cross-sectional | American community-dwelling older adults ( | Explore the relationship between the extent of apoptosis activation in the skeletal muscle & measures of muscle mass & physical performance in older persons |
| Merriwether[15] | Cross-sectional | American community-dwelling older adults ( | Classify individuals using two common sarcopenic indices: appendicular lean mass.height |
|
Table 2, continued | |||
|---|---|---|---|
| Study | Study type | Participants | Aims |
| Parra-Rodríguez [16] | Cross-sectional | Mexican older people ( | Cross-culturally adapt and validate the Spanish-language version of the sarcopenia questionare |
| Sasaki [25] | Validation study | Study 1: Healthy Japanese students ( | Establish the validity of a novel portable trunk muscle torque measuring instrument |
| Tsekoura [22] | Randomized controlled trial | Greek; older people ( | Investigate the effects of a three-month group-based versus home-based exercise program on muscular, functional/physical performance & quality of life |
| Tuttle [17] | Cross-sectional | American older adults ( | Determine impairments that contribute to early-onset physical frailty in individuals with diabesity & peripheral neuropathy |
| Van Roie [18] | Cross-sectional | Belgian institutionalized females ( | Examine the relationships between muscle strength, speed of movement, muscle mass, & functional performance & determine optimal threshold values below which physical frailty occurs |
FFM
Table 3
Summary of the methods of measuring isokinetic strength and determining sarcopenia in the selected studies and their key findings
| Study | Isokinetic dynamometry | Sarcopenia or frailty diagnostics | Key finding |
|---|---|---|---|
| Binder [20] | Concentric knee extension & flexion; PT @ 60 | Frailty: 1) modified PPT score between 18–32 2) peak aerobic power between 10–18 ml.kg | Responsiveness: No SGNF |
| Binder [19] | Concentric knee extension & flexion; PT @ 60 | Frailty: 1) modified PPT score between 18–32 2) peak aerobic power between 10–18 ml.kg | Responsiveness: Exercise intervention had a SGNF larger effect on the study group than on the control group in terms of maximum voluntary knee extensor & flexor torque. |
| Brown [7] | Concentric knee extension & flexion; PT @ 60 | Frailty: modified PPT score of not frail (32–36 points), mildly frail (25–31 points), or moderately frail (17–24 points). | Validity: SGNF correlation between the PPT & knee extension 60 |
| Cramer [21] | Dominant extremity concentric knee extension & flexion; PT & mean power @ 60 | Sarcopenia: EWGSOP algorithm | Responsiveness: In individuals with mild-moderate sarcopenia, but not severe sarcopenia, consumption of the high-protein oral nutritional supplements SGNF |
|
Table 3, continued | |||
|---|---|---|---|
| Study | Isokinetic dynamometry | Sarcopenia or frailty diagnostics | Key finding |
| Cramer [8] | Dominant extremity concentric knee extension & flexion; PT & mean power (W) @ 60 & 180 | Sarcopenia: EWGSOP algorithm | Validity: 1) repetition with the highest PT value may be the best indicator of maximal strength, while the average may indicate strength maintenance in sarcopenic elderly individuals 2) intraindividual variability among repetitions reflected functional decrements in sarcopenic elderly individuals compared with healthy elderly individuals 3) decreases in PT from 60 |
| de Oliveira [9] | Dominant extremity concentric knee extension; peak torque & PT relative to body weight @ 60 | Sarcopenia: AFFMI below 5.45 kg/m | Validity: 1) both absolute & relative PT were SGNF lower in the sarcopenic group than in the control group 2) quadriceps strength was SGNF related to aerobic capacity indices in older females. |
| Gadelha [23] | Dominant extremity concentric knee extension; PT @ 60 | Sarcopenia: EWGSOP algorithm | Validity: 1) PT in the sarcopenic groups SGNF |
| Jenkins [24] | Dominant extremity concentric knee extension & flexion; PT @ 60 | Sarcopenia: EWGSOP algorithm | Reliability: For isokinetic testing in sarcopenic or malnourished older adults, to perform familiarization trial measurements is necessary. |
| Kim [11] | Right leg concentric knee extension; PT @ 60 | Muscle weakness: using cutoff values equal to the 25th percentile PT in males & females separately | Validity: Muscle mass was not SGNF associated with physical performance in weak older adults. Measures of muscle strength may be of greater clinical importance in weak older adults than muscle mass alone. |
| Kim [10] | Bilateral concentric knee extension; PT @ 60 | Functional limitation: SPPB | Validity: Obesity measured by BMI, the percentage of fat mass & the abdominal fat area is not SGNF associated with functional limitations in older adults. |
| Lima [12] | Dominant extremity concentric knee extension; PT & relative knee extensor PT @ 60 | Sarcopenia: AFFMI below 5.45 kg/m | Validity: SGNF correlation between muscle strength & bone mineral density, but the correlation was weaker when corrected for body weight. |
| Lustosa [13] | Bilateral concentric knee extension; PT, mean power & work normalized by body @ 60 & 180 | Sarcopenia: EWGSOP algorithm | Validity: Sarcopenic elderly females showed low levels of performance in the lower limbs, predisposing them to greater vulnerability in functional activities that require agility & postural stability. |
| Marzetti [14] | Dominant extremity concentric knee extension; PT @ 60 | High-functioning | Validity: SGNF correlation between PT & apoptotic signaling. |
| Merriwether [15] | Concentric knee extension, knee flexion, ankle dorsiflexion & ankle plantarflexion; PT @ 60 | Sarcopenia: 1) AFFMI below 5.45 kg/m | Validity: 1) together, nonsarcopenic group SGNF |
| Parra-Rodríguez [16] | Bilateral concentric knee extension; PT & power @ 60 | Sarcopenia: EWGSOP algorithm | Validity: SGNF correlation between PT & power for knee extension & the SARC-F total score & measures related to sarcopenia. |
|
Table 3, continued | |||
|---|---|---|---|
| Study | Isokinetic dynamometry | Sarcopenia or frailty diagnostics | Key finding |
| Sasaki [25] | |||
| Trunk flexion & extension; PT in 10 | – | Validity: The cutoff points for flexion & extension torque were 2.0 Nm/kg & 3.0 Nm/kg, respectively, in males, & the cutoff points for flexion & extension torque were 0.8 Nm/kg & 1.7 Nm/kg, respectively, in females. | |
| Tsekoura [22] | Concentric knee extension & flexion; PT relative to body weight @ 90 | Sarcopenia: EWGSOP algorithm | Responsiveness: Group-based compared to home-based exercise yielded SGNF |
| Tuttle [17] | Right leg concentric knee extension & ankle plantarflexion; PT @ 60 | Frailty: using the 9-item, modified PPT | Validity: Individuals with diabesity & peripheral neuropathy are particularly likely to be classified as frail. Early identification & interventions aimed at improving lower-extremity function may be important to mitigate the early onset of functional decline. |
| Van Roie [18] | Knee extension; PT @ 60 | Frailty: modified PPT – not frail (32–36), mildly frail (25–31), & moderatelyfrail (17–24) points | Validity: 1) normalized PT SGNF differed between frailty groups & not frail group 2) SGNF correlation between normalized PT & PPT score ( |
PT
Table 4
Reported values of knee extension and flexion peak torque in sarcopenic and non-sarcopenic populations
| Study | Group type and time of testing | Knee extension 60 | Knee flexion 60 | ||||
| Males | Together | Females | Males | Together | Females | ||
| Harbo [26] | Healthy reference values 60–70 years | 166 |
| 131 | 91 |
| 49 |
| Binder [20] | Frailty home exercise |
| 80 |
|
| 57 |
|
| Frailty home exercise post 12w |
| 81 |
|
| 60 |
| |
| Frailty pre guided exercise |
| 89 |
|
| 61 |
| |
| Frailty post guided 12w exercise |
| 98 |
|
| 66 |
| |
| Binder [19] | Frailty home exercise | 89 |
| 70 | 55 |
| 50 |
| Frailty home exercise post 12w | 75 |
| 68 | 55 |
| 50 | |
| Frailty home exercise post 24w | 74 |
| 73 | 58 |
| 51 | |
| Frailty home exercise post 36w | 90 |
| 72 | 58 |
| 50 | |
| Frailty pre guided exercise | 96 |
| 66 | 63 |
| 46 | |
| Frailty post guided 12w exercise | 101 |
| 70 | 69 |
| 50 | |
| Frailty post guided 24w exercise | 112 |
| 79 | 77 |
| 52 | |
| Frailty post guided 36w exercise | 115 |
| 75 | 78 |
| 56 | |
| Cramer [21] | Severe sarcopenia control |
| 50 [31, 64] |
|
|
|
|
| Sarcopenia control |
| 62 [48, 88] |
|
|
|
| |
| Sarcopenia normal gait control |
| 61 [46, 82] |
|
|
|
| |
| Sarcopenia normal grip control |
| 71 [54, 98] |
|
|
|
| |
| Severe sarcopenia experimental |
| 48 [31, 62] |
|
|
|
| |
| Sarcopenia experimental |
| 64 [45, 81] |
|
|
|
| |
| Sarcopenia normal gait experimental |
| 60 [41, 75] |
|
|
|
| |
| Sarcopenia normal grip experimental |
| 76 [59, 111] |
|
|
|
| |
| Cramer [8] | Sarcopenia and malnourished | 78 (70,86) |
| 49 (46,52) | 31 (28,34) |
| 18 (16,20) |
| Healthy elderly | 85 (78,92) |
| 42 (38,46) | 45 (41,49) |
| 26 (24,28) | |
| de | Sarcopenia |
|
| 70 |
|
|
|
| Oliveira [9] | Non sarcopenia |
|
| 100 |
|
|
|
| Gadelha [23] | Several sarcopenia |
|
| 63 |
|
|
|
| Sarcopenia |
|
| 87 |
|
|
| |
| Presarcopenia |
|
| 108 |
|
|
| |
| Non sarcopenia |
|
| 90 |
|
|
| |
| Jenkins [24] | Sarcopenia familiarized 1 | 83 |
| 51 | 42 |
| 27 |
| Sarcopenia familiarized 2 | 89 |
| 53 | 46 |
| 29 | |
| Sarcopenia familiarized 3 after 12w | 93 |
| 57 | 50 |
| 32 | |
| Sarcopenia familiarized 4 after 24w | 94 |
| 57 | 52 |
| 32 | |
| Kim [11] | Muscle weakness SPPB | 57 |
| 38 |
|
|
|
| Normal muscle SPPB | 83 |
| 54 |
|
|
| |
| Lima [12] | Sarcopenia |
|
| 75 |
|
|
|
| Non sarcopenia |
|
| 98 |
|
|
| |
| Lustosa [13] | Sarcopenia |
|
| 49 |
|
|
|
| Non sarcopenia |
|
| 54 |
|
|
| |
| Marzetti [14] | Low-functioning |
| 87 |
|
|
|
|
| High-functioning |
| 118 |
|
|
|
| |
| Merriwether | Sarcopenia by ALM.ht | 90 | 70 | 61 | 60 | 49 | 44 |
| [15] | Non sarcopenia by ALM.ht | 100 | 85 | 79 | 66 | 55 | 52 |
| Sarcopenia by SMI | 95 | 78 | 70 | 63 | 53 | 48 | |
| Non sarcopenia by SMI | 114 | 72 | 72 | 81 | 48 | 47 | |
| Parra-Rodríguez [16] | Sarcopenia |
| 57 |
|
|
|
|
| Tuttle [17] | Several frailty |
| 84 |
|
|
|
|
| Moderate frailty |
| 114 |
|
|
|
| |
| Mild to no frailty |
| 132 |
|
|
|
| |
| Weighted average | Muscle weakness (e.g. sarcopenia, frailty) | 82.8 |
| 59.7 | 47.1 |
| 36.4 |
Note: values in Nm are expressed as the mean
Table 5
Reported values of knee extension and flexion peak torque muscle strength in sarcopenic and non-sarcopenic populations
| Note: values in Nm are expressed as the mean | |||||||
|---|---|---|---|---|---|---|---|
| Study | Group type and time of testing | Knee extension 180 | Knee flexion 180 | ||||
| Males | Together | Females | Males | Together | Females | ||
| Cramer [8] | Sarcopenia and malnourished | 45 (39,51) |
| 29 (26,32) | 37 (31,43) |
| 22 (19,25) |
| Healthy elderly | 57 (52,62) |
| 25 (23,27) | 33 (30,36) |
| 20 (18,22) | |
| Jenkins [24] | Sarcopenia familiarized 1 | 50 |
| 30 | 17 |
| 18 |
| Sarcopenia familiarized 2 | 53 |
| 32 | 20 |
| 19 | |
| Sarcopenia familiarized 3 after 12 w | 56 |
| 34 | 20 |
| 20 | |
| Sarcopenia familiarized 4 after 24 w | 57 |
| 34 | 17 |
| 18 | |
| Lustosa [13] | Sarcopenia |
|
| 31 |
|
|
|
| Non sarcopenia |
|
| 26 |
|
|
| |
| Tsekoura [22] | Sarcopenia group exercise baseline | R: 34 | R: 27 | ||||
| Sarcopenia home exercise baseline | R: 38 | R: 28 | |||||
| Sarcopenia control group | R: 36 | R: 25 | |||||
| Sarcopenia group exercise 12 w | R: 40 | R: 34 | |||||
| Sarcopenia home exercise 12 w | R: 40 | R: 30 | |||||
| Sarcopenia control group 12 w | R: 33 | R: 24 | |||||
| Sarcopenia group exercise 24 w | R: 39 | R: 33 | |||||
| Sarcopenia home exercise 24 w | R: 37 | R: 29 | |||||
| Sarcopenia control group 24 w | R: 31 | R: 26 | |||||
Normative values for the healthy elderly population have been presented for isometric and isokinetic knee flexion and extension and for other muscle groups [26, 27, 28], where the strength of the knee extensors and flexors decreases with age by 2% or more per year [29]. Therefore, it is difficult to determine cutoff points and reference values for multifactorial syndromes such as sarcopenia. However, our reported sarcopenia values (Table 4) are similar to the isokinetic knee extension cutoff points (94.5 Nm in males and 62.3 Nm in females), which have been reported for predicting slow gait speeds [30]. This finding suggests that these cutoff points are useful in forecasting sarcopenia because decreases in muscle strength start to lead to a slower gait speed when there is a clear sign of sarcopenia progression. One of the main limitations of our reported reference values is the absence of isokinetic strength values relative to participants’ body mass; 2 studies [18, 25] successfully found sarcopenia cutoff values (Table 3) for females in knee extension (1.46 Nm/kg
Selecting muscle groups or muscle chains to test is an important issue in the diagnosis of sarcopenia. We found that most studies focused on the knee extensors and flexors as relevant muscle groups. This selection seems to be justified by knowledge from the rehabilitation field; the knee extensors are the first group of muscles to lose strength with any form of hypokinesis, and knee flexors decrease in strength when knee stability is disrupted. Only one study reported trunk flexion and extension strength [25], two studies reported ankle dorsiflexion and ankle plantarflexion strength [7, 15] and no studies reported hip abduction strength. Although trunk flexion and extension strength have been used to estimate sarcopenia cutoff points [25], we must mention that this test probably has lower validity than the other tests because the complex movements involved in this test are difficult and require more familiarization with the test and because trunk flexors and extensors are often shortened. Regarding ankle muscle groups, we recommend ankle dorsiflexion due to its relationship with physical performance and reported differences between healthy and sarcopenic populations [7, 15]; however, we do not recommend ankle plantarflexion, as its relations and differences have not been reported [7, 15]. The lack of evaluations of the hip abductors is quite surprising because of its relation to falls in the elderly population [31] and the relation between functional muscle activity and the strength ratios of the hip abductor muscles to the knee extensor and flexor muscles [32]. Therefore, we recommend testing of the knee extensors and flexors, which are the most frequently reported muscle groups, and suggest performing a verification study focused on the hip abductors for estimating strength in individuals with sarcopenia.
Seven studies considered multiple speeds of contraction in their approach, where most studies used 60
Another finding is that many previous studies reported isokinetic strength in groups of individuals of both sexes, which makes interpretation of the results impossible because the decreases in muscle strength are dramatically larger in males than in females; additionally, females are reported to have less muscle strength than males [27, 33, 34]. Therefore, we strongly recommend reporting isokinetic values by sex.
5.Conclusion
The isokinetic knee extension strength values at 60
Acknowledgments
This review was supported by the research grants of Charles University, Czech Republic (PRIMUS/19/HUM /012 and the project Q41).
Conflict of interest
The authors have no conflicts of interest to declare.
References
[1] | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. (2010) ; 39: (4): 412-423. |
[2] | Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) ; 15: (2): 95-101. |
[3] | Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom. (2015) ; 18: (4): 467-471. |
[4] | Lloyd RS, Oliver JL, Hughes MG, Williams CA. Reliability and validity of field-based measures of leg stiffness and reactive strength index in youths. J Sports Sci. (2009) ; 27: (14): 1565-1573. |
[5] | Buchner DM, Cress ME, De Lateur BJ, Esselman PC, Margherita AJ, Price R, et al. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. J Gerontol A Biol Sci Med Sci. (1997) ; 52: (4): M218-224. |
[6] | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) ; 151: (4): 264-269. |
[7] | Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. (2000) ; 55: (6): M350-355. |
[8] | Cramer JT, Jenkins NDM, Mustad VA, Weir JP. Isokinetic dynamometry in healthy versus sarcopenic and malnourished elderly: beyond simple measurements of muscle strength. J Appl Gerontol. (2017) ; 36: (6): 709-732. |
[9] | de Oliveira RJ, Bottaro M, Mota AM, et al. Association between sarcopenia-related phenotypes and aerobic capacity indexes of older women. J Sports Sci Med. (2009) ; 8: (3): 337-343. |
[10] | Kim JH, Choi SH, Lim S, et al. Sarcopenia and obesity: gender-different relationship with functional limitation in older persons. J Korean Med Sci. (2013) ; 28: (7): 1041-1047. |
[11] | Kim KE, Jang SS, Lim S, et al. Relationship between muscle mass and physical performance: Is it the same in older adults with weak muscle strength? Age Ageing. (2012) ; 41: (6): 799-803. |
[12] | Lima RM, Bezerra LMA, Rabelo HT, et al. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. J Clin Densitom. (2009) ; 12: (1): 35-41. |
[13] | Lustosa LP, Batista PP, Pereira DS, Pereira LSM, Scianni A, Ribeiro-Samora GA. Comparison between parameters of muscle performance and inflammatory biomarkers of non-sarcopenic and sarcopenic elderly women. Clin Interv Aging. (2017) ; 12: : 1183-1191. |
[14] | Marzetti E, Lees HA, Manini TM, et al. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: An exploratory study. PLoS ONE. (2012) ; 7: (2): e32829. |
[15] | Merriwether EN, Host HH, Sinacore DR. Sarcopenic indices in community-dwelling older adults. J Geriatr Phys Ther. (2012) ; 35: (3): 118-125. |
[16] | Parra-Rodríguez L, Szlejf C, García-González AI, Malmstrom TK, Cruz-Arenas E, Rosas-Carrasco O. Cross-cultural adaptation and validation of the Spanish-language version of the sarc-f to assess sarcopenia in Mexican community-dwelling older adults. J Am Med Dir Assoc. (2016) ; 17: (12): 1142-1146. |
[17] | Tuttle LJ, Bittel DC, Bittel AJ, Sinacore DR. Early-onset physical frailty in adults with diabesity and peripheral neuropathy. Can J Diabetes. (2018) ; 42: (5): 478-483. |
[18] | Van Roie E, Verschueren SM, Boonen S, et al. Force-velocity characteristics of the knee extensors: an indication of the risk for physical frailty in elderly women. Arch Phys Med Rehabil. (2011) ; 92: (11): 1827-1832. |
[19] | Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. (2002) ; 50: (12): 1921-198. |
[20] | Binder EF, Yarasheski KE, Steger-May K, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. (2005) ; 60: (11): 1425-1431. |
[21] | Cramer JT, Cruz-Jentoft AJ, Landi F, et al. Impacts of high-protein oral nutritional supplements among malnourished men and women with sarcopenia: a multicenter, randomized, double-blinded, controlled trial. J Am Med Dir Assoc. (2016) ; 17: (11): 1044-1055. |
[22] | Tsekoura M, Billis E, Tsepis E, et al. The effects of group and home-based exercise programs in elderly with sarcopenia: a randomized controlled trial. J Clin Med. (2018) ; 7: (12): pii: E480. |
[23] | Gadelha AB, Vainshelboim B, Ferreira AP, Neri SGR, Bottaro M, Lima RM. Stages of sarcopenia and the incidence of falls in older women: A prospective study. Arch Gerontol Geriatr. (2018) ; 79: : 151-157. |
[24] | Jenkins NDM, Cramer JT. Reliability and minimum detectable change for common clinical physical function tests in sarcopenic men and women. J Am Geriatr Soc. (2017) ; 65: (4): 839-846. |
[25] | Sasaki E, Sasaki S, Chiba D, et al. Age-related reduction of trunk muscle torque and prevalence of trunk sarcopenia in community-dwelling elderly: Validity of a portable trunk muscle torque measurement instrument and its application to a large sample cohort study. PLoS ONE. (2018) ; 13: (2): e0192687. |
[26] | Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. (2012) ; 112: (1): 267-275. |
[27] | Neder JA, Nery LE, Shinzato GT, Andrade MS, Peres C, Silva AC. Reference values for concentric knee isokinetic strength and power in nonathletic men and women from 20 to 80 years old. J Orthop Sports Phys Ther. (1999) ; 29: (2): 116-126. |
[28] | Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. (1997) ; 78: (1): 26-32. |
[29] | Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985). (2000) ; 88: (4): 1321-1326. |
[30] | Fragala MS, Alley DE, Shardell MD, Harris TB, McLean RR, Kiel DP, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. (2016) ; 64: (1): 144-150. |
[31] | Lloyd BD, Williamson DA, Singh NA, Hansen RD, Diamond TH, Finnegan TP, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. J Gerontol A Biol Sci Med Sci. (2009) ; 64: (5): 599-609. |
[32] | Stastny P, Tufano JJ, Lehnert M, Golas A, Zaatar A, Xaverova Z, et al. Hip abductors and thigh muscles strength ratios and their relation to electromyography amplitude during split squat and walking lunge exercises. Acta Gymnica. (2015) ; 45: (2): 51-59. |
[33] | Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. (2001) ; 56: (5): B209-217. |
[34] | Frontera WR, Bigard X. The benefits of strength training in the elderly. Sci Sports. (2002) ; 17: (3): 109-116. |




