Overexpression of lncRNA ANRIL promoted the proliferation and migration of prostate cancer cells via regulating let-7a/TGF-β1/ Smad signaling pathway
Abstract
Long non-coding RNAs (lncRNAs) were playing critical roles in tumorigenesis. However, in prostate cancer, the roles and mechanisms of lncRNAs especially ANRIL were largely unknown. We investigated the effects of ANRIL on the proliferation and migration of prostate cancer cells using CCK-8 assay and Transwell migration assay. Real-time PCR and western blotting assays were used to analyze the levels of ANRIL, let-7a, TGF-
1.Introduction
Prostate cancer is one of the most frequent malignant cancers in the reproductive and urinary system, and the second most common cancer in the world [1, 2]. Many studies suggested that long non-coding RNAs (lncRNAs) were playing critical roles in tumorigenesis. However, in prostate cancer, the roles and mechanisms of lncRNAs especially ANRIL were largely unknown.
ANRIL was significantly up-regulated in cervical cancer tumors and cell lines, and high ANRIL expression was associated with advanced the International Federation of Gynecology and Obstetrics (FIGO) stage, lymph node metastasis and poor overall survival than those with low ANRIL expression, and functional study showed that ANRIL could promote the proliferation, migration and invasion of cervical cancer via PI3K/Akt pathways [3]. And Zhang et al. further found that ANRIL knockdown inhibited the proliferation and invasion, and promoted apoptosis of cervical cancer cells by sponging miR-186 [4]. In pancreatic cancer, ANRIL was also overexpressed, and its overexpression promoted epithelial-mesenchymal transition by activating the ATM-E2F1 signaling pathway both in vitro and in vivo study [5]. In osteosarcoma, ANRIL was also upregulated, and HIF-1
In the present study, we found that ANRIL was overexpressed in prostate cancer, and knockdown of ANRIL using siRNAs inhibited the proliferation and migration of prostate cancer cells. We further found that knockdown of ANRIL significantly inhibited the TGF-
2.Materials and methods
2.1Patients
Fresh tissues of prostate cancer and adjacent normal tissues from 8 patients were collected by the Department of Urology, Yunnan Tumor Hospital and the Third Affiliated Hospital of Kunming Medical University (Kunming, China). Primary tumor tissues and the adjacent normal tissues from the same patients were separated and then immediately stored at
2.2Cell culture
The human prostate cancer cell lines including LNCap, PC3 and DU145 were (American Type Culture Collection, Manassas, VA, USA) cultured in RPMI-1640 medium (Invitrogen corporation, USA) with 10% fetal bovine serum (Hyclone, USA), penicillin (100 U/ml) and streptomycin (100 mg/ml). The cells were maintained at 37
2.3Cell transfection
The cells were seeded in six-well plates and transfected with lncRNA ANRIL siRNAs or negative control siRNA or let-7a inhibitor using lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) following the manufacture’s protocol. The lncRNA ANRIL siRNA-1 sequence was GGUCAUCUCAUUGCUCUAU; the lncRNA ANRIL siRNA-2 sequence was GCCCAAUUAUGCUGUGGUA. In addition, commercial anti-let-7a oligonucleotide (inhibitor) was purchased from GenePharma (Shanghai, China). And the lncRNA ANRIL siRNAs and negative control siRNA were also synthesized by GenePharma (Shanghai, China).
2.4Cell proliferation assay
Cellular proliferation was measured by Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) based on manufacturer’s instructions. Twenty-four hours after transfection, cells were seeded into 96-well plates at a density of 8
2.5Cell migration assay
Transwell assay was performed to detect the migration ability of transfected prostate cancer cells. The cells were digested and seeded in the upper chamber at a density of 3
2.6Total RNA extraction and Real-time PCR assay
Total RNA was isolated from cancer cells using the RNeasy Mini Kit as described by the manufacturer (Qiagen, Hilden, Germany) and used for Real-time PCR assay.
Real-time PCR was performed to detect the relative expression levels of lncRNA ANRIL. The PCR reactions were performed in a total volume of 20
Hairpin-itTM let-7a qRT-PCR Primer Set (Gene- Pharma) was used for the measurement of the relative quantity of let-7a. The mRNA expression of let-7a was normalized to the endogenous expression of U6.
Table 1
Characteristics of patients with prostate cancer in the present study
| NO. | Age | pT status | pN status | Gleason score |
| 1 | 45 | T2 | N0 | 6 |
| 2 | 54 | T2 | N0 | 7 |
| 3 | 61 | T3 | N0 | 9 |
| 4 | 39 | T4 | N0 | 8 |
| 5 | 48 | T3 | N0 | 8 |
| 6 | 55 | T2 | N1 | 9 |
| 7 | 64 | T4 | N1 | 7 |
| 8 | 54 | T2 | N1 | 9 |
2.7Western blotting assay
Cells from each group were detached with trypsin, centrifuged, and washed 2 times with pre-chilled PBS. Cell lysis buffer was subsequently added and incubated on ice for protein extraction. Protein concentration was determined using the BCA Protein Assay Kit (Beyotime Biotechnology, China). Equal amounts of proteins were separated via 12% SDS-PAGE and then then transferred to a PVDF membrane (Millipore Corporation, Billerica, MA, USA). The membrane was soaked in 10% skimmed milk (in PBS, PH 7.2, containing 0.1% Tween-20) for 2 h and incubated with an appropriate amount of primary antibody at 4
2.8Statistical analyses
All quantitative data are presented as mean
3.Results
3.1lncRNA ANRIL is overexpressed in prostate cancer, and knockdown of lncRNA ANRIL inhibits the proliferation and migration of prostate cancer cells
We detected the expression of lncRNA ANRIL in 8 prostate cancer tissues and corresponding nornal tissues using Real-time PCR, and the results showed that lncRNA ANRIL was significantly overexpressed (Fig. 1A). Using siRNAs of lncRNA ANRIL, we significantly reduced the expression of ANRIL in three cell lines of prostate cancer including LNCap, PC3 and DU145 (Fig. 1B). Using Transwell assay, we found that knockdown of ANRIL significantly inhibited the migration of prostate cancer cells LNCap, PC3 and DU145 (Fig. 1C and D). Using CCK-8 assay, we further found that knockdown of ANRIL significantly suppressed the proliferation of prostate cancer cells LNCap, PC3 and DU145 (Fig. 1E).
Figure 1.
lncRNA ANRIL is overexpressed in prostate cancer, and knockdown of lncRNA ANRIL inhibits the proliferation and migration of prostate cancer cells. A. lncRNA ANRIL was overexpressed in prostate cancer detected by Real-time PCR assay (
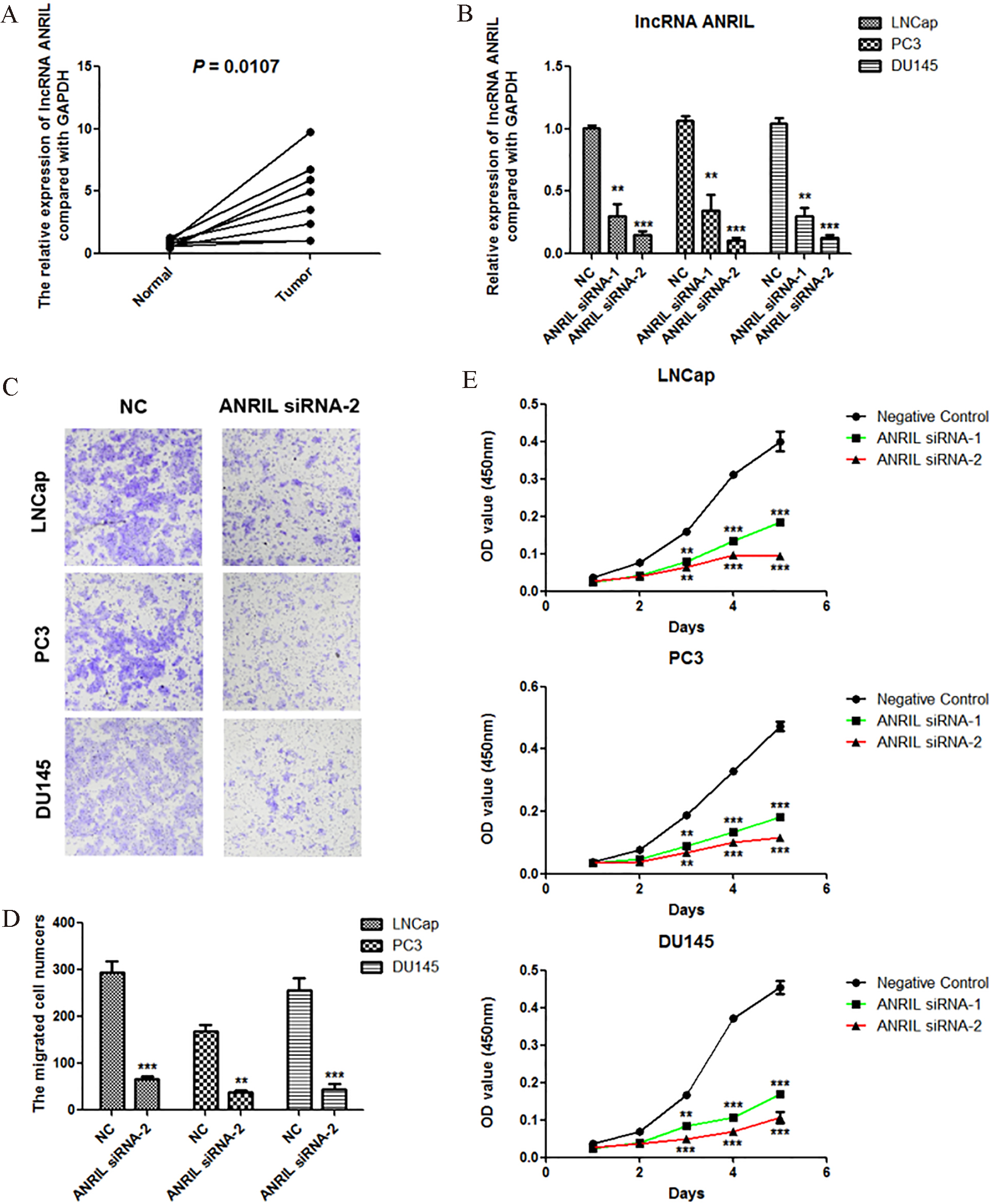
Figure 2.
Knockdown of lncRNA ANRIL inactivates the TGF-
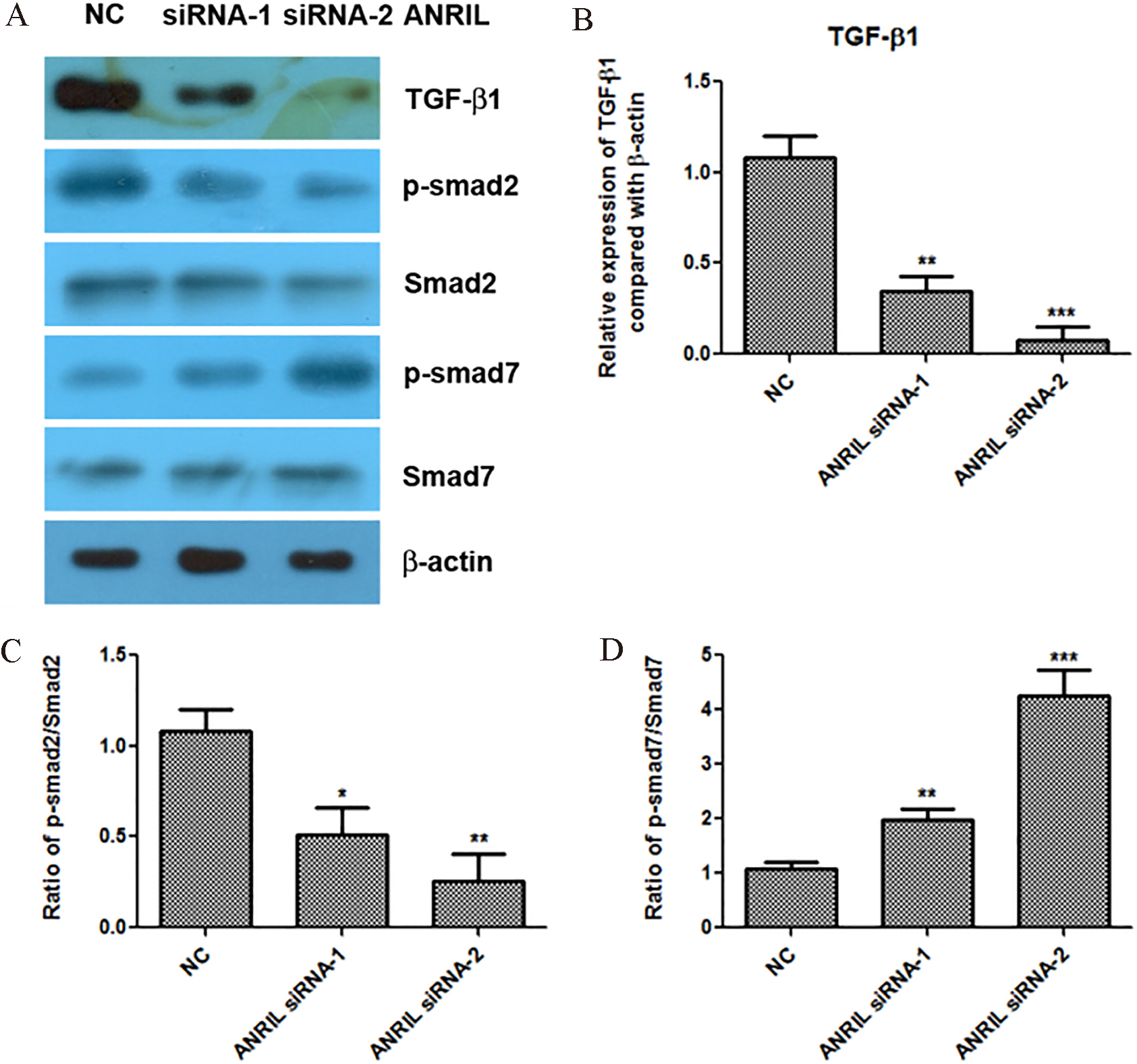
Figure 3.
Knockdown of lncRNA ANRIL increases the expression of let-7a, and let-7a inhibitor reactivates the TGF-
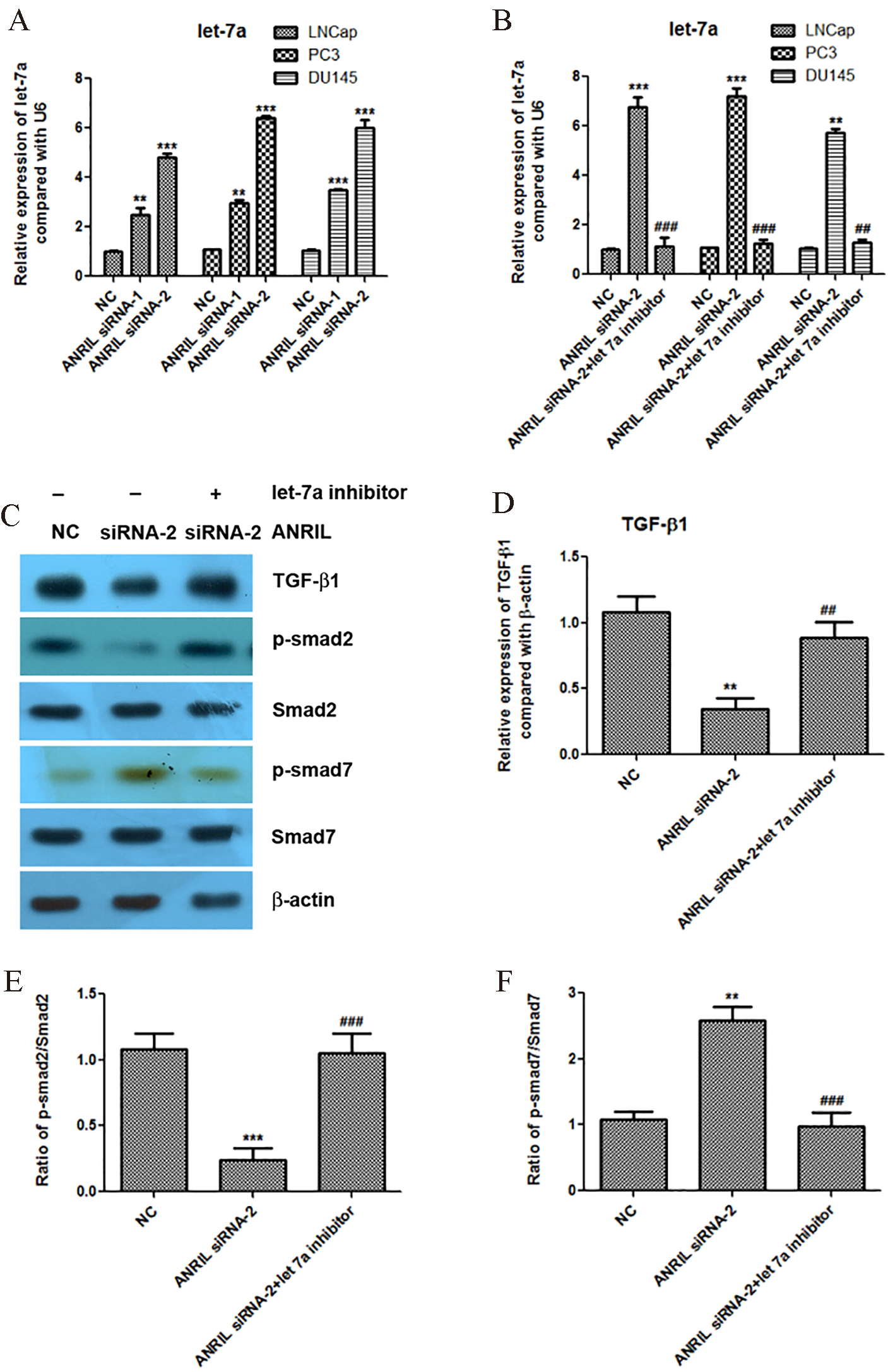
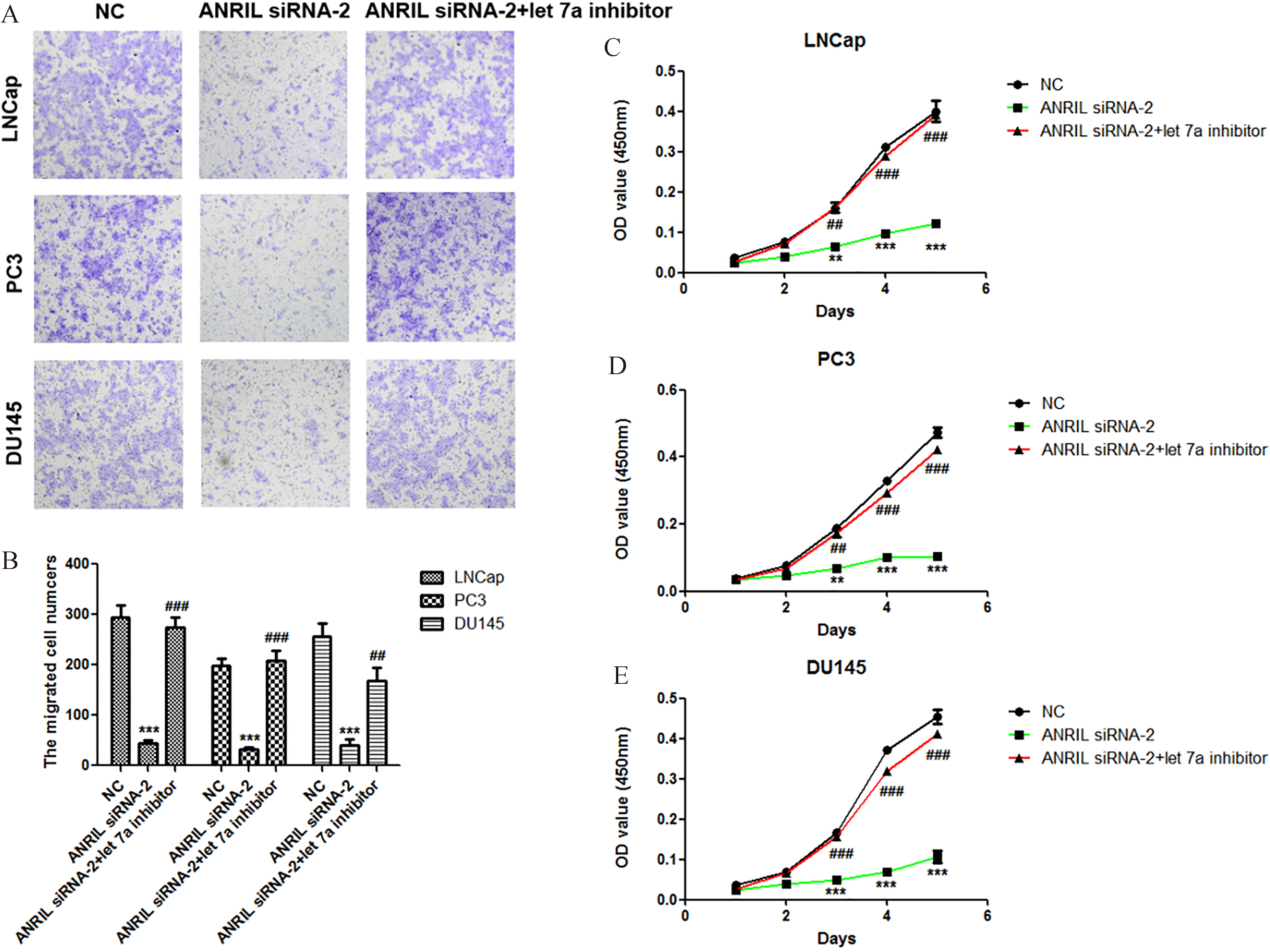
Figure 4.
Knockdown of let-7a recovers the abilities of prostate cancer cells proliferation and migration at the condition of ANRIL silencing. A. Effect of let-7a inhibitor at condition of ANRIL silencing on the migration of LNCap, PC3 and DU145 cells. The migration of cells was measured by Transwell migration assay, and statistical analysis was done using Student’s test. E. Effect of let-7a inhibitor at condition of ANRIL silencing on the proliferation of LNCap, PC3 and DU145 cells. The viability of cells was measured by Cell Counting Kit-8, and statistical analysis was done using Student’s test.
3.2Knockdown of lncRNA ANRIL inactivates the TGF-β
TGF-
3.3Knockdown of lncRNA ANRIL increases the expression of let-7a, and let-7a inhibitor reactivates the TGF-β
We further found that knockdown of lncRNA ANRIL significantly increased the expression of let-7a in the prostate cancer cells LNCap, PC3 and DU145 (Fig. 3A). In order to confirm that let-7a is involved in the regulation of TGF-
3.4Knockdown of let-7a recovers the abilities of prostate cancer cells proliferation and migration at the condition of ANRIL silencing
In order to confirm that let-7a is involved in the regulation of prostate cancer cell proliferation and migration by lncRNA ANRIL, we reduced the expression of let-7a at the condition of ANRIL silencing, and then analyzed the activities of cell proliferation and migration. Importantly, we found that knockdown of let-7a at the condition of ANRIL silencing significantly promoted the migration and proliferation of prostate cancer cells LNCap, PC3 and DU145 compared with ANRIL silencing group (Fig. 4A–E). These results indicated that knockdown of lncRNA ANRIL inhibited the prostate cancer cell proliferation and migration through upregulation of let-7a.
4.Discussion
The present study found that lncRNA ANRIL was overexpressed in prostate cancer, and knockdown of ANRIL inhibited the proliferation and migration of prostate cancer cells. We further found that knockdown of lncRNA ANRIL inactivated the TGF-
Although Sun et al. reported that downregulation of ANRIL repressed tumorigenicity and enhanced cisplatin-induced cytotoxicity via regulating let-7a in nasopharyngeal carcinoma [13]. However, the relationships among ANRIL, let-7a and TGF-
Taken together, our findings revealed that overexpression of lncRNA ANRIL promoted the proliferation and migration of prostate cancer cells via regulating let-7a/TGF-
Acknowledgments
This study was supported by the Yunnan Provincial Research Foundation for Basic Research, China (No. 2014FB068).
Conflict of interest
None.
References
[1] | E.M. Peyromaure, K. Mao, Y. Sun, S. Xia, N. Jiang, S. Zhang, G. Wang, Z. Liu and B. Debre, A comparative study of prostate cancer detection and management in China and in France, Can J Urol 16: ((2009) ), 4472–4477. |
[2] | A. Jemal, F. Bray, M.M. Center, J. Ferlay, E. Ward and D. Forman, Global cancer statistics, CA Cancer J Clin 61: ((2011) ), 69–90. |
[3] | D. Zhang, G. Sun, H. Zhang, J. Tian and Y. Li, Long non-coding RNA ANRIL indicates a poor prognosis of cervical cancer and promotes carcinogenesis via PI3K/Akt pathways, Biomed Pharmacother 85: ((2017) ), 511–516. |
[4] | J.J. Zhang, D.D. Wang, C.X. Du and Y. Wang, Long Noncoding RNA ANRIL Promotes Cervical Cancer Development By Acting as a Sponge of miR-186, Oncol Res ((2017) ). |
[5] | S. Chen, J.Q. Zhang, J.Z. Chen, H.X. Chen, F.N. Qiu, M.L. Yan, Y.L. Chen, C.H. Peng, Y.F. Tian and Y.D. Wang, The over expression of long non-coding RNA ANRIL promotes epithelial-mesenchymal transition by activating the ATM-E2F1 signaling pathway in pancreatic cancer: An in vivo and in vitro study, Int J Biol Macromol 102: ((2017) ), 718–728. |
[6] | X. Wei, C. Wang, C. Ma, W. Sun, H. Li and Z. Cai, Long noncoding RNA ANRIL is activated by hypoxia-inducible factor-1alpha and promotes osteosarcoma cell invasion and suppresses cell apoptosis upon hypoxia, Cancer Cell Int 16: ((2016) ), 73. |
[7] | M. Taheri, F. Pouresmaeili, M.D. Omrani, M. Habibi, S. Sarrafzadeh, R. Noroozi, A. Rakhshan, A. Sayad and S. Ghafouri-Fard, Association of ANRIL gene polymorphisms with prostate cancer and benign prostatic hyperplasia in an Iranian population, Biomark Med 11: ((2017) ), 413–422. |
[8] | K.L. Yap, S. Li, A.M. Munoz-Cabello, S. Raguz, L. Zeng, S. Mujtaba, J. Gil, M.J. Walsh and M.M. Zhou, Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a, Mol Cell 38: ((2010) ), 662–674. |
[9] | Y. Jin, Z. Cui, X. Li, X. Jin and J. Peng, Upregulation of long non-coding RNA PlncRNA-1 promotes proliferation and induces epithelial-mesenchymal transition in prostate cancer, Oncotarget 8: ((2017) ), 26090–26099. |
[10] | L. Ratz, M. Laible, L.A. Kacprzyk, S.M. Wittig-Blaich, Y. Tolstov, S. Duensing, P. Altevogt, S.M. Klauck and H. Sultmann, TMPRSS2:ERG gene fusion variants induce TGF-beta signaling and epithelial to mesenchymal transition in human prostate cancer cells, Oncotarget 8: ((2017) ), 25115–25130. |
[11] | A. Hamidi, J. Song, N. Thakur, S. Itoh, A. Marcusson, A. Bergh, C.H. Heldin and M. Landstrom, TGF-beta promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85alpha, Sci Signal 10: ((2017) ). |
[12] | J.J. Zhao, S. Hao, L.L. Wang, C.Y. Hu, S. Zhang, L.J. Guo, G. Zhang, B. Gao, Y. Jiang, W.G. Tian and D.L. Luo, Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-beta/Smad signaling pathway, Oncotarget 7: ((2016) ), 57903–57918. |
[13] | M. Sun, H. Song, S. Wang, C. Zhang, L. Zheng, F. Chen, D. Shi, Y. Chen, C. Yang, Z. Xiang, Q. Liu, C. Wei and B. Xiong, Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer, J Hematol Oncol 10: ((2017) ), 79. |




