Synchronous primary breast angiosarcoma with invasive ductal carcinoma
Abstract
Primary angiosarcoma (PAS) of the breast is an extremely uncommon variant of breast malignancies. Highly aggressiveness and dismal prognosis characterize this endothelial neoplasm. We report here an unusual case of PAS of the breast occurring in a 46-year-old woman associated with concurrent bilateral invasive ductal carcinoma and ovarian metastases.
1.Introduction
Angiosarcomas (AS) are rare soft tissue malignant tumour of vascular origin. AS of the breast is uncommon, accounting less than 0.1% of all breast malignant tumors and 8 to 10% of mammary sarcoma [1]. The first case of breast angiosarcoma has been reported by Borman in 1907 [2]. Two types of breast AS are individualized: Primary AS which affects often young women [35–42 years] with no known risk factors and secondary AS occurring especially in older women following radiotherapy for breast cancer [3]. AS have a poor prognosis owing to its high rate of local recurrences and its propensity to metastasize hematogenously [4]. Here, we report, to the best of our knowledge, the first case of both primary breast angiosarcoma and bilateral ductal invasive carcinoma with ovarian metastases.
2.Case presentation
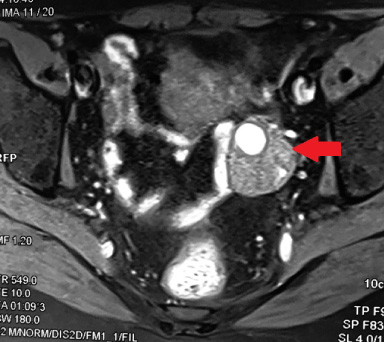
In November 2020, a 46-year-old nulliparous woman, consulted for a right breast mass. She had no previous exposure to radioactive or chemical substances. Mammography showed a large irregular lesion with hydric tonality measuring 9 × 7 × 5 cm in lower quadrant and in the retroareolar region of the right breast with micro calcifications. Brest ultrasound (US) revealed large microlobulated hypoechoic lesion in the right breast, associated with multiple single cysts. These findings were classified BI-RADS5. Besides, the examination of the left breast affirmed the presence of a 2 cm irregular solid lesion classified also BIRADS 5. Our patient underwent US-guided core biopsy of suspicious bilateral lesions. Pathological study confirmed the diagnosis of high grade angiosarcoma (CD31+/CD34+) in the right breast associated with an invasive ductal carcinoma in the left breast. Initial body computed tomography was normal. A right mastectomy and a left lumpectomy with axillary dissection were performed. The pathological study of the right mastectomy specimen showed the existence of 2 tumors; one lobulated with a spongy and hemorrhagic appearance measuring 8 × 8 × 5 cm corresponding to angiosarcoma and a second indurate and whitish mass measuring 3 × 2 × 2.5 cm related to an invasive ductal carcinoma. The margins were free of tumor and immunohistochemistry showed a luminal A sub-type (positive hormone receptor with negative HER2). The left tumor measured 2.5 cm. It was a triple negative molecular subtype (negative hormone receptor with negative HER2) and there was no nodal involvement. Thus, our patient had a primary angiosarcoma of the right breast with a concurrent bilateral invasive ducal carcinoma. One-week postoperatively, the patient presented abdominal pain. Computed tomography showed a mixed solid and cystic hypervascular structure in the left ovary measuring 52 × 37 mm without lung, liver, and bones metastases. The left ovarian infiltration was classified as AdnexMR score 5 on MRI (Fig. 1).
Fig. 1.
Abdominal MRI: Left ovarian infiltration classified as AdnexMR score 5.
With the findings of oligometastatic disease which could have been due to either angiosarcoma or breast carcinoma, surgery for solitary metastasis was indicated to decide on systemic treatment as it depends on the pathological study of metastasis. Furthermore, ovarian biopsy was not possible. After the decision of the local multidisciplinary consultation meeting the decision was to perform ovarian surgery for diagnostic and therapeutic purposes. The patient underwent laparoscopic bilateral oophorectomy to ensure castration. Histology revealed a high grade metastatic angiosarcoma involving left ovarian parenchyma with positive estrogen receptor. The patient died before starting chemotherapy based on doxorubicin and cyclophosphamide because of disease progression with lung, liver and bone metastases. In fact, she was reluctant to chemotherapy.
3.Discussion
To the best of our knowledge, this is the fourth reported case in the literature with breast PAS and concurrent invasive breast carcinoma and the first case associated with ovarian metastases (Table 1). Microscopically, the important aspects that lead to the diagnosis of AS are presence of anastomizing vascular channels, infiltrative pattern of breast parenchyma, cellular atypia and mitoses with a possible existence of solid areas containing atypical cells with high proliferative rate, necrosis, or hemorrhage (“blood lakes”) [5]. On the immunohistochemistry, CD31, Factor VIII, CD34 and Fli1 positivity confirms the diagnosis of AS. AS are classified into low-, intermediate- and high grade. Grading of AS is based on features evocative of degree of vascular differentiation and overall growth patterns [6]. It has been demonstrated in several studies that a higher grade was associated with poorer prognosis [5]. In the English literature, we found only three reported cases of AS with concomitant breast carcinoma. We summarized those cases in the table 1 [6–8]. Metastases are identified in approximately 50% of AS. The Commonest sites of metastases from AS are liver, lung, or bones [9]. Ovarian metastases from AS are exceptional in contrast to other tumors such as colon cancer, stomach, lung, and pancreas cancer [10]. Cases of ovary metastases from AS were often found at postmortem in the context of disseminated AS.
Table 1
Breast angiosarcoma cases described in the literature
| Author | Age (year) | Gender | Diagnosis | Side | Treatment | Recurrence or metastases |
| Ryan [7] | 40 | Female | (1) Angiosarcoma | Left | Mastectomy | 5 months |
| (2) Carcinoma | Left axillary lymph node = carcinoma | |||||
| (after retrospective examination) | ||||||
| Britt [6] | 59 | Female | Lobular carcinoma + angiosarcoma | Left | Modified radical mastectomy | 12 months |
| Lung and cutaneous recurrence of angiosarcoma | ||||||
| Ni [8] | 33 | Female | (1) Invasive carcinoma | Right | Lumpectomy | 8 months |
| (2) Angiosarcoma | In the Surgical site Angiosarcoma (low and high grade) | |||||
| (after retrospective examination) | ||||||
| Our case | 46 | Female | (1) Angiosarcoma | Right | Right mastectomy | Lung, liver, bones and ovarian metastases |
| (2) Bilateral carcinoma | Left lumpectomy |
The treatment of breast AS differs significantly from that of a classic breast carcinoma. Surgery is the standard treatment for resectable tumors. A simple excision is not recommended because it enhances the risk of local recurrence at the operative site. Owing to the rarity of this entity, no randomized trial has compared breast conserving surgery with mastectomy, but based on guidelines for management of sarcomas, BCS (wide local excision with negative margins) of tumors may be adequate in the management of breast angiosarcoma. Mastectomy will be invariably required in large tumors, like in our case. Margin positivity is recognized as a poor prognostic factor. Therefore, obtaining a free margin is important in order to improve outcomes. Axillary dissection is not mandatory since most studies have shown axillary lymph node involvement in less than 10% [2]. Our patient underwent a simple mastectomy as treatment of breast angiosarcoma. The place of radiotherapy in the adjuvant setting persists controversial but it should be delivered in case of microscopically positive margins. In some reports, a benefit of adjuvant chemotherapy in disease free and overall survival has been documented. The most common chemotherapeutic drugs used were adriamycin, ifosfamide, cyclophosphamide, vincristine, and paclitaxel. With the findings of oligometastatic disease which could have been due to either angiosarcoma or breast carcinoma, surgery for solitary metastasis was indicated to decide on systemic treatment as it depends on the pathological study of metastasis. Furthermore, ovarian biopsy was not possible. After the decision of the local multidisciplinary consultation meeting the decision was to perform ovarian surgery for diagnostic and therapeutic purposes. In case of metastatic or locally advanced and unresectable angiosarcoma, doxorubicin-based chemotherapy remains the first-line standard treatment with a progression-free survival of 3.7–5.4 months. Although, it has been reported that weekly paclitaxel is well tolerated and efficient in this setting [11]. A benefit of a metronomic chemotherapy regimen associated with beta blockers (Propranolol) has been also documented in the management of several cases of relapsing, metastatic angiosarcoma [12].
Our patient was proposed for chemotherapy based on doxorubicin and cyclophosphamide but unfortunately, she died before receiving any treatment.
4.Conclusions
Due to the rarity of this entity, clinician should be aware about the possibility of the coexistence of two distinct mammary malignancies especially when discovering vascular lesions in the breast.
Acknowledgements
We acknowledge patients who participated in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and the national research committee of Habib Bourguiba hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Patient consent for publication
Consent obtained directly from patient.
Conflict of interest
None declared.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Author contributions
W.B.K. idea, writing; M.L. data collection, writing; A.K. supervision, revision.
All the authors reviewed the manuscript.
References
[1] | Boufettal H, , Noun M, , Hermas S, , Samouh N, , Benayad S, , Karkouri M , Breast angiosarcoma: A case report, Ann Pathol, 33: (3): 217–220, (2013) . |
[2] | Lokanatha D, , Anand A, , Lakshmaiah KC, , Govind Babu K, , Jacob LA, , Suresh Babu MC , Primary breast angiosarcoma - a single institution experience from a tertiary cancer center in South India, Breast Dis, 37: (3): 133–138, (2018) . |
[3] | Gutkin PM, , Ganjoo KN, , Lohman M, , von Eyben R, , Charville GW, , Nazerali RS , Angiosarcoma of the breast: Management and outcomes, Am J Clin Oncol, 43: (11): 820–825, (2020) . |
[4] | Sasahara A, , Tanabe M, , Hayashi K, , Konishi T, , Oya M, , Sakiyama K , A case of primary breast angiosarcoma with multiple discontinuous small lesions, Surgical Case Reports, 5: (1): 157, (2019) . |
[5] | Yan M, , Gilmore H, , Bomeisl P, , Harbhajanka A, Clinicopathologic and immunohistochemical study of breast angiosarcoma, Ann Diagn Pathol, 54: : 151795, (2021) . |
[6] | Britt LD, , Lambert P, , Sharma R, , Ladaga LE, Angiosarcoma of the breast. Initial misdiagnosis is stillcommon, Arch Surg, 130: (2): 221–223, (1995) . |
[7] | Ryan JF, , Kealy WF, Concomitant angiosarcoma and carcinoma of the breast: A case report, Histopathology, 9: (8): 893–899, (1985) . |
[8] | Ni Y, , Xie X, , Bu H, , Zhang Z, , Wei B, , Yin L , Concurrent primary angiosarcoma and invasive ductal carcinoma in the same breast, J Clin Pathol, 66: (3): 263–264, (2013) . |
[9] | Wang J, , Fisher C, , Thway K, Angiosarcoma of the breast with solitary metastasis to the ovary during pregnancy: An uncommon pattern of metastatic disease, Case Rep Oncol Med, 2013: : 209610, (2013) . |
[10] | Souza FF, , Katkar A, , den Abbeele ADV, , Dipiro PJ, Breast angiosarcoma metastatic to the ovary, Case Rep Med, 2009: : 381015, (2009) .. |
[11] | Esposito E, , Avino F, , di Giacomo R, , Donzelli I, , Marone U, , Melucci MT , Angiosarcoma of the breast, the unknown—a review of the current literature, TranslCancer Res, 8: (Suppl 5): S510–S517, (2019) . |
[12] | Pramanik R, , Gogia A, , Malik PS, , Gogi R, Metastatic primary angiosarcoma of the breast: Can we tame it the metronomic way, Indian J Med Paediatr Oncol, 38: (2): 228–231, (2017) . |




