Development of neuro-Behcet’s disease in a patient with operable HER2-positive breast cancer during neoadjuvant chemotherapy: A case report
Abstract
BACKGROUND:
Neuro-Behcet’s disease (NBD) is a variant of Behcet’s disease (BD). To our knowledge, there have been no previous reports on concurrent NBD in breast cancer patients undergoing chemotherapy.
CASE PRESENTATION:
Our patient had a history of BD and was asymptomatic. She was diagnosed with human epidermal growth factor receptor 2-positive breast cancer by core needle biopsy and was administered neoadjuvant chemotherapy. After four courses, in addition to the aggravation of the existing adverse events, headache, fever, dysarthria, and muscle weakness in the upper left and lower extremities appeared. On admission, she was diagnosed with acute NBD, and steroid therapy was initiated. After her symptoms improved gradually, she was discharged. Then, she underwent mastectomy and axillary lymph node dissection for breast cancer. Trastuzumab and pertuzumab plus tamoxifen were administered postoperatively. Two years postoperatively, no recurrence of breast cancer and NBD was noted.
CONCLUSION:
When chemotherapy is administered to breast cancer patients with a history of BD, it is necessary to select chemotherapy with as few adverse events as possible and to continue with treatment while paying attention to the risk of NBD.
1.Introduction
Bechet’s disease (BD) is characterized by systemic inflammation, as well as recurrent oral and genital ulcers, inflammatory skin lesions, and uveitis. The various systemic symptoms, including arthritis, gastrointestinal, neurologic, and vascular manifestations, as well as life-threatening complications, may occur due to the severe inflammation of the internal organs [1]. Neuro-Bechet’s disease (NBD) is one of the most serious pathological conditions; notably, 5–10% of the patients with BD present with neurological manifestations such as encephalitis and meningitis [2,3]. The diagnosis of NBD is made by correlating the clinical symptoms, magnetic resonance imagining (MRI) findings, and the results of the cerebrospinal fluid (CSF) analysis. Because the treatment of NBD depends on the severity of organ damage and symptoms, timely diagnosis of NBD is crucial [4].
The association between antineoplastic drugs and autoimmune diseases remains unclear. It has been reported that concomitant use of immune checkpoint inhibitors can exacerbate autoimmune disease [4]. On the other hand, another study reported that the risk of autoimmune disease reactivation induced by anti-human epidermal receptor-2 (HER2) monoclonal antibody is low [5].
Neoadjuvant or adjuvant chemotherapy and molecular targeted therapy are commonly used as the current standard of care for operable HER2-positive breast cancer. We report a patient with HER2-positive breast cancer who developed NBD during neoadjuvant chemotherapy and was difficult to diagnose and treat.
2.Case presentation
The patient was a 40-year-old woman. She became aware of a left breast mass in the last 8 months and consulted a breast surgeon. Mammography showed a dense, oval, finely serrated marginal mass with microcalcification in the left M-O segment (Fig. 1a). Ultra-sonography showed a 2.4 cm lobe-shaped tumor on the left C area (Fig. 1b), and swollen axillary lymph nodes measuring 13 mm and 11 mm were found in the left axilla (Fig. 1c). She underwent a core needle biopsy, which indicated the presence of invasive ductal carcinoma. Immunohistochemistry results indicated that she was estrogen receptor (ER)-positive, progesterone receptor (PgR)-positive, and human epidermal growth factor receptor 2 (HER2)-positive. Positron emission tomography-computed tomography (PET-CT) indicated left axillary lymph node metastasis (Fig. 1d). She was diagnosed with left breast cancer (C, T2N1M0, stage IIB). Neoadjuvant chemotherapy (NAC) with TCbHP (docetaxel + carboplatin + trastuzumab + pertuzumab) commenced. After one course, grade 2 stomatitis, general fatigue, and decreased appetite were observed. Moreover, following four courses, there was an aggravation of adverse events, notably headache, fever, recurrent oral aphthae, facial erythema, dysarthria, disturbance of consciousness, and left hemiplegia. Blood biochemistry revealed very high levels of C-reactive protein, but there were no other significant abnormalities (Table 1). She was thus hospitalized urgently for detailed examination and treatment. Fluid-attenuated inversion recovery MRI showed high intensity in the basal ganglia and marked compression of both ventricles (Fig. 2a). Although the cerebrospinal fluid (CSF) pressure and the CSF sugar/blood sugar ratio showed no abnormalities, mononuclear cell-dominant cell increase and elevated levels of protein and interleukin-6 were observed in the CSF (Table 2).
Fig. 1.
a. Mammogram. A dense, oval, finely serrated marginal mass with microcalcifications can be seen in the left M-O segment. b. Echocardiogram. A 1.5 × 1.6 × 2.4 cm lobulated mass shadow with unclear borders can be seen in the left C segment. c. Ultrasonographic scan. Left axillary swollen axillary lymph nodes of 13 mm and 11 mm in size can be seen. d. Positron emission tomography-computed tomography scan Hyper-uptake consistent with the mass can be seen in the left breast and left axillary lymph node (left breast: SUVmax, 15.2, left axillary lymph node: SUVmax, 9.5). No obvious distant metastases can be seen. SUVmax, maximum standardized uptake value.
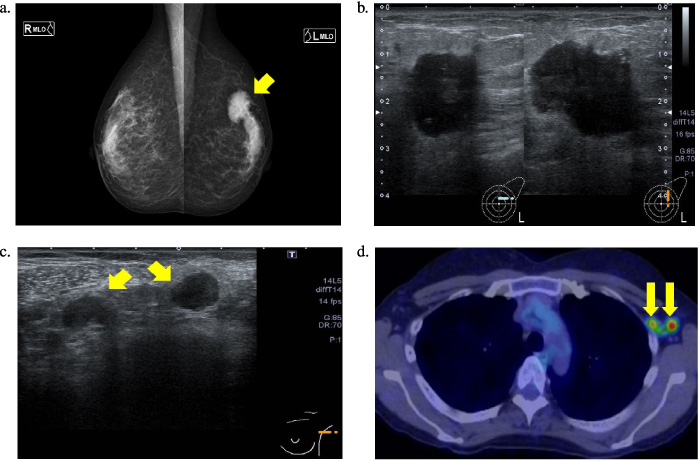
Fig. 2.
a. Head FLAIR-MRI scan obtained at admission. High-density areas in both basal ganglia and marked compression of both ventricles can be seen. FLAIR-MRI, Fluid-attenuated inversion recovery-magnetic resonance imaging b. Occipital FLAIR-MRI obtained 2 weeks after the start of treatment. A reduction in the high-density areas of the bilateral basal ganglia and an improvement in the compression of the bilateral ventricles can be seen. FLAIR-MRI, Fluid-attenuated inversion recovery-magnetic resonance imaging. c. Occipital FLAIR-MRI obtained 3 months after the start of treatment. Further reduction of the high-density areas of the bilateral basal ganglia and further improvement of the compression of the bilateral ventricles can be seen. FLAIR-MRI, Fluid-attenuated inversion recovery-magnetic resonance imaging.
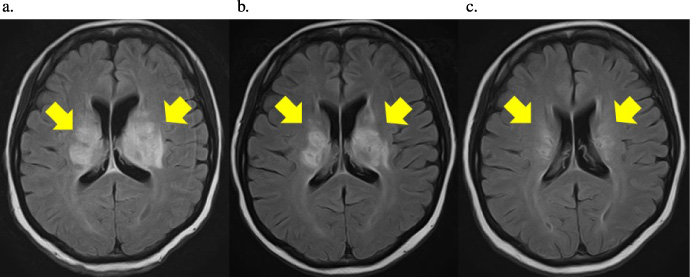
Table 1
Blood biochemistry findings
| Examination | Value |
| Total protein | 6.8 g/dL |
| Albumin | 3.3 g/dL |
| Total bilirubin | 0.5 mg/dL |
| Aspartate aminotransferase | 22 U/L |
| Alanine aminotransferase | 6 U/L |
| Alkaline phosphatase | 65 U/L |
| Lactate dehydrogenase | 345 U/L |
| γ-Glutamyl transferase | 21 U/L |
| Creatinine | 1.03 mg/dL |
| Urea nitrogen | 37 mg/dL |
| Glucose | 86 mg/dL |
| Sodium | 142 mEq/L |
| Potassium | 5.2 mEq/L |
| Chloride | 100 mEq/L |
| Calcium | 9.6 mg/dL |
| Leukocytes | 8.5 × 103∕μL |
| Neutrophils | 79.30% |
| Lymphocytes | 14.70% |
| Monocytes | 5.40% |
| Eosinophils | 0.20% |
| Basophils | 0.40% |
| Red blood cell | 282 × 104∕μL |
| Hemoglobin | 9.0 g/dL |
| Hematocrit | 26.80% |
| Platelets | 26.4 × 104∕μL |
| Active partial thromboplastin time | 26.6 sec |
| Prothrombin time-international normalized ratio | 0.95 |
| Fibrinogen | 576 mg/dL |
| D-dimer | 1.7 μg/mL |
| C-reactive protein | 10.2 mg/dL |
| Immunoglobulin G | 1115 mg/dL |
| Immunoglobulin A | 467 mg/dL |
| Immunoglobulin M | 160 mg/dL |
| Complement component 3 | 150 mg/dL |
| Complement component 4 | 41 mg/dL |
| Anti-doublestranded DNA antibody | <10 IU/mL |
| Anti-Smith antibody | <1.0 U/mL |
| Anti-ribonucleoprotein antibody | 2.0 U/mL |
| Anti-Sjogren’s syndrome A antibody | 2.4 U/mL |
| Anti-cardiolipin antibody | <8 U/mL |
| Anti-nuclear antibody | <40 U/mL |
| Mumps | Negative |
| Herpes simplex virus | Negative |
| Varicella-zoster virus | Negative |
| Cytomegalovirus | Negative |
| Japanese encephalitis virus | Negative |
| Epstein-Barr virus | Negative |
| Aspergillus antigen | Negative |
| β-D glucan | 7.9 pg/mL |
Table 2
Cerebrospinal fluid analysis
| Examination | Value |
| Cerebrospinal fluid (CSF) pressure | 156 cmH2O |
| Leukocyte count | 44.3/mm3 |
| Protein | 55 mg/dL |
| Glucose level | 55 mg/dL |
| Ratio of CSF glucose level to serum glucose | 0.53 |
| Appearance | Crystal clear |
| Predominant cell type | Mononuclear leukocytes |
| Malignant cell | Negative |
| Interleukin-6 | 137 pg/ml |
2.1.Past medical history
She was diagnosed with BD at 32 years of age and has been taking colchicine 1.0 mg/day since then with good control.
She has also been using prophylactic medication for asthma since childhood.
2.2.Imaging tests
Mammography: A dense, oval, finely serrated marginal mass with microcalcification was found in the left M-O segment (Fig. 1a).
Ultra-sonography: A 1.5 × 1.6 × 2.4-cm lobe-shaped tumor shadow with an unclear border was observed on the left C area (Fig. 1b). Swollen axillary lymph nodes measuring 13 mm and 11 mm were found in the left axilla (Fig. 1c).
PET-CT: Hyper-uptake, consistent with the mass in the left breast and left axillary lymph node (left breast: maximum standardized uptake value (SUV)max, 15.2, left axillary lymph node: SUVmax, 9.5). No obvious distant metastases were observed (Fig. 1d).
Histopathological examination: Immunostaining revealed ER-positive (Allred score = 5 + 2), PgR-positive (Allred score = 4 + 3), and HER2-positive (with fluorescent in situ hybridization amplification) status, and Ki-67 index = 50%, indicating invasive ductal carcinoma.
2.3.Neurological findings at admission
Glasgow Coma Scale: E3V1M6
Manual Muscle Test upper extremity: right 4/left 2; lower extremity: right 4/left 2.
2.4.Laboratory findings at admission
Blood biochemistry: C-reactive protein, 10.2 mg/dL (Table 1).
2.5.Plain MRI of the head
Fluid-attenuated inversion recovery MRI showed high intensity in the basal ganglia and marked compression of both ventricles (Fig. 2a).
2.6.Cerebrospinal fluid analysis
The leukocyte count in the cerebrospinal fluid was 44.3/mm3, and mononuclear cell-dominant cell increase and elevated levels of protein and interleukin-6 were observed (Table 2).
2.7.Course after admission
From the time of admission, steroid pulse therapy (SoluMedrol® at 1000 mg/day administered as a drip for three days) was started. The stomatitis improved quickly after the start of the treatment. Next, the steroid was tapered off gradually, and the patient was able to communicate and walk. Two weeks after the start of the treatment, the brain MRI showed a reduction in the high-density areas of the bilateral basal ganglia and a reduction in the compression of the bilateral ventricles (Fig. 2b). A head MRI performed 3 months after the start of the treatment showed a further reduction in the high-density areas in the bilateral basal ganglia (Fig. 2c). Prednisolone 10 mg/day was administered perioperatively. One month after discharge, she underwent a left partial mastectomy and axillary lymph node dissection. A pathological examination of the resected specimens revealed the presence of residual invasive ductal carcinoma, but none of the resected axillary lymph nodes showed the presence of metastasis (n = 0∕8). The therapeutic effect of preoperative chemotherapy was Grade 2b. Postoperative therapy with trastuzumab, pertuzumab, and tamoxifen was continued for 8 months. At the 2-year postoperative follow-up, there was no recurrence of breast cancer or NBD.
3.Discussion
Although the cause of BD is still unknown, it involves repeated acute inflammatory attacks, with mainly recurrent oral aphthae, vulvar ulcers, uveitis, and skin symptoms. Depending on the characteristics of the affected organ, specific types such as intestinal, vascular, and neurological types may develop. In particular, NBD is one of the most serious pathological conditions; notably, 5–10% of the patients with BD present with neurological manifestations such as encephalitis and meningitis [2]. Early diagnosis and treatment are important, as the prognosis is poor in the absence of appropriate treatment [6]. The characteristic imaging findings of acute NBD include a relatively large brain stem and the presence of basal ganglia lesions. Steroids are generally the treatment of first choice [7]. After a comprehensive search in the PubMed database, to the best of our knowledge, we did not find any papers reporting a causal relationship between breast cancer or chemotherapy and NBD. In our patient’s case, in addition to her previous medical history of BD, the physical and mental stress, which then triggered the onset of NBD, may have been due to the side effects of the chemotherapy, such as stomatitis, general fatigue, and decreased appetite. Because NBD symptoms are difficult to distinguish from the adverse events caused by chemotherapy, a delay in the diagnosis may cause life-threatening or serious sequelae.
The differential diagnosis, in this case, includes paraneoplastic syndrome and specific symptoms attributable to the adverse events of chemotherapy. The diagnostic criteria for paraneoplastic neurological disorders include (1) a diagnosis of cancer within 4 years from the onset of neurological symptoms; (2) the presence of neurological symptoms; (3) the absence of other neurological disorders; and (4) at least one of the following findings: CSF analysis showing inflammation with negative cytology, a brain MRI showing a lesion in the temporal lobe, or the detection of epileptic activity in the temporal lobes using an electroencephalogram [8–10]. Currently, breast cancer-related neurologic paraneoplastic syndromes include sensory and motor-type neuropathies, paraneoplastic cerebellar degeneration, opsoclonus–myoclonus syndrome, stiff person syndrome, encephalomyelitis (including limbic encephalopathy), and paraneoplastic retinopathy [11]. In the present case, although no antineural antibodies in the serum and CSF were measured, the presence of neurological symptoms and a diagnosis of cancer within 4 years from the onset of neurological manifestations were consistent with the diagnostic criteria for paraneoplastic neurological disorders. However, the possibility of paraneoplastic neurological disorders is unlikely to be considered due to the presence of a history of BD, the absence of a lesion in the temporal lobe on MRI, and the onset of symptoms after the beginning of chemotherapy. Nonetheless, if a breast cancer patient without a history of BD presents with neurological symptoms unexplained by other neurological diseases, it may be preferable to have her tested for anti-neural antibodies.
Although this patient had a history of oral aphthae due to BD, she was asymptomatic, as the disease was controlled when the NAC commenced. Mild stomatitis, malaise, and loss of appetite were noted after completing one course of preoperative chemotherapy; however, the treatment was continued as these symptoms are known to be common adverse events of chemotherapy. However, we also observed adverse symptoms unlikely to be caused by ordinary chemotherapy, such as dysarthria, disturbance of consciousness, and hemiplegia. Hence, the decision to conduct a brain MRI was made based on the possibility of NBD due to the patient’s medical history of BD, along with the appearance of non-specific neurological disorders as symptoms of a chemotherapy-induced adverse event. Although she was not evaluated by contrast-enhanced MRI due to a history of asthma, a T2-weighted MRI of the head showed high intensity in a wide area around the bilateral basal ganglia and a marked compression of the bilateral ventricles. In addition, she had recurrent oral aphthae and facial erythema, diarrhea, and central nervous system symptoms, meeting the diagnostic criteria of an incomplete form of BD. In addition, she presented with an acute-onset headache, fever, left hemiparesis, and hypercytosis of the CSF, which was in accordance with the guidelines for acute NBD [7]. For the treatment of acute NBD, the systemic administration of large doses of steroids (initial dose of prednisone at 1 g/day) is recommended during the onset and exacerbation of neurological symptoms [12]. In addition, steroid pulse therapy is required for severe meningoencephalitis. It is particularly important to start treatment as early as possible after the onset of the disease because delaying treatment increases the possibility of refractory sequelae [13]. In our patient’s case, prednisone pulse therapy at 1000 mg/day was continued for 3 days from the time of admission, followed by maintenance therapy with intravenous prednisone at 120 mg/day for 1 week, with the prednisone being tapered gradually to 30 mg/day. Although it is difficult to specify the exact appearance of symptoms in this case, treatment was started within 3 days from the onset of speech difficulty and left upper and lower leg muscle weakness. Approximately 2 weeks after the start of the treatment, although mild paralysis of the upper left and lower extremities persisted, a significant improvement was observed on the head MRI, and the patient was discharged from the hospital in good condition on the 21st hospital day. After discharge, rehabilitation was continued, and approximately 1 month after the start of NBD treatment, the patient’s physical strength returned to the baseline level, and no other symptoms were observed at the latest follow-up. The diagnosis was difficult in this case because the patient was undergoing NAC for breast cancer; however, the NBD diagnosis at a relatively early stage and the early initiation of treatment contributed to its success.
There is insufficient scientific evidence to confirm that physical or psychological stress induces NBD. However, psychological, environmental, and physiological stressors are known to adversely affect body homeostasis [14]. Specifically, they exacerbate autoimmune diseases, many of which are mediated by stressful actions on the immune system [15,16]. It has been reported that stress can induce proinflammatory or type 1–type 2 cytokine-mediated immune responses, leading to an inability to modulate innate and adaptive immune responses. Interactions between physical and psychological factors and low quality of life are associated with BD progression [17]. Islam et al. investigated the effects of stress and environmental factors on the induction of BD using a herpes simplex virus (HSV-1) infected mouse model. It was demonstrated that environmental factors, immune dysfunction, and HSV-1 infection might be triggers of BD. Therefore, it is suggested that physical or psychological stress induced by chemotherapy may have triggered NBD in this case.
Chemotherapy and anti-HER2 therapy (trastuzumab and pertuzumab) are the current standard therapeutic strategies for operable HER2-positive breast cancer because they improve the prognosis significantly [18,19]. Therefore, in the case of HER2-positive breast cancer, there was concern that not using the standard treatments based only on a history of BD may increase the disadvantages for patients. In this case, the treatment with TCbHP commenced as NAC; however, the target course could not be completed due to the onset of NBD. A pathological examination of the surgical specimens revealed the presence of residual invasive ductal carcinoma; however, there was no metastasis to the axillary lymph nodes. Therefore, NAC was effective. The KATHERINE trial reported that the prognosis is further improved by the administration of trastuzumab emtansine (T-DM1) rather than trastuzumab + pertuzumab as postoperative adjuvant therapy to patients with non-pathologic complete response who are administered NAC for HER2-positive breast cancer [20]. Although the association between antineoplastic drugs and autoimmune diseases remains unclear, it has been reported that concomitant use of immune checkpoint inhibitors can exacerbate autoimmune disease cite4. On the other hand, a previous study reported that the risk of autoimmune disease reactivation induced by anti-human epidermal receptor-2 monoclonal antibodies, including T-DM1, is low (15.8%) [5]. However, it should be noted that the study did not include patients with a history of BD. In this case, we chose anti-HER2 therapy with trastuzumab and pertuzumab as the adjuvant therapy and tamoxifen as hormonal therapy, given the possibility of a recurrence of BD due to physical or psychological stress induced by the adverse events caused by T-DM1. With the addition of immune checkpoint inhibitors to the treatment of breast cancer, their use in the future for breast cancer patients with such autoimmune diseases may require caution. Furthermore, because the safety of chemotherapy for patients after NBD treatment has not yet been clarified, it is important to select a treatment strategy that aims to not only suppress breast cancer recurrence, but also to prevent the onset of NBD.
4.Conclusion
The relationship between breast cancer, chemotherapy, and NBD remains unclear. When chemotherapy is administered to breast cancer patients with a history of BD, it is necessary to select chemotherapy with as few adverse events as possible and continue with treatment while paying attention to the risk of NBD. If the onset of NBD is suspected, chemotherapy should be discontinued immediately, and treatment for NBD must commence after confirming the diagnosis.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and informed consent
The study was approved by the Ethics Committee of the Saitama Medical Center.
Consent for publication
Approval for the publication of this case report has been obtained from the patient.
Availability of data and materials
All the data generated or analyzed during this study are included in this published article.
Author contributions
Conception: TN and HS.
Preparation of the manuscript: TN, HS, AM, and YK.
Literature search: TN and HS carried out the
Clinical management of the patient: TN and HS.
Surgical treatment: HS.
Revision for important intellectual content: HS, TK, TO, and TK.
All authors read and approved the final manuscript.
References
[1] | Kim D, , Nakamura K, , Kaneko F, , Alpsoy E, , Bang D, Mucocutaneous manifestations of Behçet’s disease: Pathogenesis and management from perspectives of vasculitis, Front Med (Lausanne), 9: : 987393, (2022) . doi:10.3389/fmed.2022.987393. |
[2] | Akman-Demir G, , Serdaroglu P, , Tasçi B, Clinical patterns of neurological involvement in Behçet’s disease: Evaluation of 200 patients, Brain, 122: : 2171–2182, (1999) . doi:10.1093/brain/122.11.2171. |
[3] | Farah S, , Al-Shubaili A, , Montaser A, , Hussein JM, , Malaviya AN, , Mukhtar M , Behçet’s syndrome: A report of 41 patients with emphasis on neurological manifestations, J Neurol Neurosurg Psychiatry, 64: : 382–384, (1998) . doi:10.1136/jnnp.64.3.382. |
[4] | Johnson DB, , Balko JM, , Compton ML, , Chalkias S, , Gorham J, , Xu Y , Fulminant myocarditis with combination immune checkpoint blockade, N Engl J Med, 375: : 1749–1755, (2016) . doi:10.1056/nejmoa1609214. |
[5] | Illarramendi JJ, , Salgado E, , Cruz SDL, , Esteban JI, , Arraras JI, , Asin G 1409 Autoimmune disease and immunosuppressive therapy in relapsed long-term breast cancer survivors treated with cyclin-dependent kinase inhibitors and human epidermal receptor-2 monoclonal antibodies. in: Regular and Young Investigator Award Abstracts. . BMJ Publishing Group Ltd; (2023) . |
[6] | Hirohata S, , Kikuchi H, , Sawada T, , Nagafuchi H, , Kuwana M, , Takeno M , Retrospective analysis of long-term outcome of chronic progressive neurological manifestations in Behcet’s disease, J Neurol Sci, 349: : 143–148, (2015) . doi:10.1016/j.jns.2015.01.005. |
[7] | Hirohata S, , Kikuchi H, , Sawada T, , Nagafuchi H, , Kuwana M, , Takeno M , Clinical characteristics of neuro-Behcet’s disease in Japan: A multicenter retrospective analysis, Mod Rheumatol, 22: : 405–413, (2012) . doi:10.3109/s10165-011-0533-5. |
[8] | Said S, , Cooper CJ, , Reyna E, , Alkhateeb H, , Diaz J, , Nahleh Z, Paraneoplastic limbic encephalitis, an uncommon presentation of a common cancer: Case report and discussion, Am J Case Rep, 14: : 391–394, (2013) . doi:10.12659/AJCR.889560. |
[9] | Graus F, , Delattre JY, , Antoine JC, , Dalmau J, , Giometto B, , Grisold W , Recommended diagnostic criteria for paraneoplastic neurological syndromes, J Neurol Neurosurg Psychiatry, 75: : 1135–1140, (2004) . doi:10.1136/jnnp.2003.034447. |
[10] | Gultekin SH, , Rosenfeld MR, , Voltz R, , Eichen J, , Posner JB, , Dalmau J, Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumour association in 50 patients, Brain, 123 (Pt7): : 1481–1494, (2000) . doi:10.1093/brain/123.7.1481. |
[11] | Fanous I, , Dillon P, Paraneoplastic neurological complications of breast cancer, Exp Hematol Oncol, 5: : 29, (2015) . doi:10.1186/s40164-016-0058-x. |
[12] | Kalra S, , Silman A, , Akman-Demir G, , Bohlega S, , Borhani-Haghighi A, , Constantinescu CS , Diagnosis and management of Neuro-Behçet’s disease: International consensus recommendations, J Neurol, 261: : 1662–1676, (2014) . doi:10.1007/s00415-013-7209-3. |
[13] | Hirohata S, , Kikuchi H, , Sawada T, , Nagafuchi H, , Kuwana M, , Takeno M , Analysis of various factors on the relapse of acute neurological attacks in Behçet’s disease, Mod Rheumatol, 24: : 961–965, (2014) . doi:10.3109/14397595.2014.891496. |
[14] | Liu YZ, , Wang YX, , Jiang CL, Inflammation: The common pathway of stress-related diseases, Front Hum Neurosci, 11: : 316, (2017) . doi:10.3389/fnhum.2017.00316. |
[15] | Dhabhar FS, Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology, Neuroimmunomodulation, 16: : 300–317, (2009) . doi:10.1159/000216188. |
[16] | Dhabhar FS, Effects of stress on immune function: The good, the bad, and the beautiful, Immunol Res, 58: : 193–210, (2014) . doi:10.1007/s12026-014-8517-0. |
[17] | Uğuz F, , Dursun R, , Kaya N, , Cilli AS, Quality of life in patients with Behçet’s disease: The impact of major depression, Gen Hosp Psychiatry, 29: : 21–24, (2007) . doi:10.1016/j.genhosppsych.2006.10.001. |
[18] | Gianni L, , Pienkowski T, , Im YH, , Roman L, , Tseng LM, , Liu MC , Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial, Lancet Oncol, 13: : 25–32, (2012) . doi:10.1016/S1470-2045(11)70336-9. |
[19] | von Minckwitz G, , Procter M, , de Azambuja E, , Zardavas D, , Benyunes M, , Viale G , APHINITY Steering Committee and Investigators, Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer, N Engl J Med, 377: : 122–131, (2017) . doi:10.1056/NEJMoa1703643. |
[20] | von Minckwitz G, , Huang CS, , Mano MS, , Loibl S, , Mamounas EP, , Untch M , KATHERINE Investigators, Trastuzumab emtansine for residual invasive HER2-positive breast cancer, N Engl J Med, 380: : 617–628, (2019) . doi:10.1056/NEJMoa1814017. |



