Ageing, Cognitive Decline, and Effects of Physical Exercise: Complexities, and Considerations from Animal Models
Abstract
In our ageing global population, the cognitive decline associated with dementia and neurodegenerative diseases represents a major healthcare problem. To date, there are no effective treatments for age-related cognitive impairment, thus preventative strategies are urgently required. Physical exercise is gaining traction as a non-pharmacological approach to promote brain health. Adult hippocampal neurogenesis (AHN), a unique form of brain plasticity which is necessary for certain cognitive functions declines with age and is enhanced in response to exercise. Accumulating evidence from research in rodents suggests that physical exercise has beneficial effects on cognition through its proneurogenic capabilities. Given ethical and technical limitations in human studies, preclinical research in rodents is crucial for a better understanding of such exercise-induced brain and behavioural changes.
In this review, exercise paradigms used in preclinical research are compared. We provide an overview of the effects of different exercise paradigms on age-related cognitive decline from middle-age until older-age. We discuss the relationship between the age-related decrease in AHN and the potential impact of exercise on mitigating this decline. We highlight the emerging literature on the impact of exercise on gut microbiota during ageing and consider the role of the gut-brain axis as a future possible strategy to optimize exercise-enhanced cognitive function. Finally, we propose a guideline for designing optimal exercise protocols in rodent studies, which would inform clinical research and contribute to developing preventative strategies for age-related cognitive decline.
INTRODUCTION
Due to the ageing global population and our increased longevity, the incidence of dementia and neurodegenerative diseases, such as Alzheimer’s disease (AD) has increased in recent decades. Cognitive decline is a core symptom, which currently presents significant health, economic and societal challenges. According to the World Health Organization, the number of people aged 60 years and older is predicted to double by 2050 [1]. Moreover, the incidence of dementia is set to triple by 2050 [2] (Fig. 1). There are currently no treatments to effectively reverse the cognitive impairment associated with age, and there is so far only limited success in disease-modifying therapies for AD and other neurodegenerative diseases that share cognitive decline as a main symptom. Thus, preventative strategies, including non-pharmacological strategies, which can promote brain health and curb the increase in age-related cognitive impairment are urgently required.
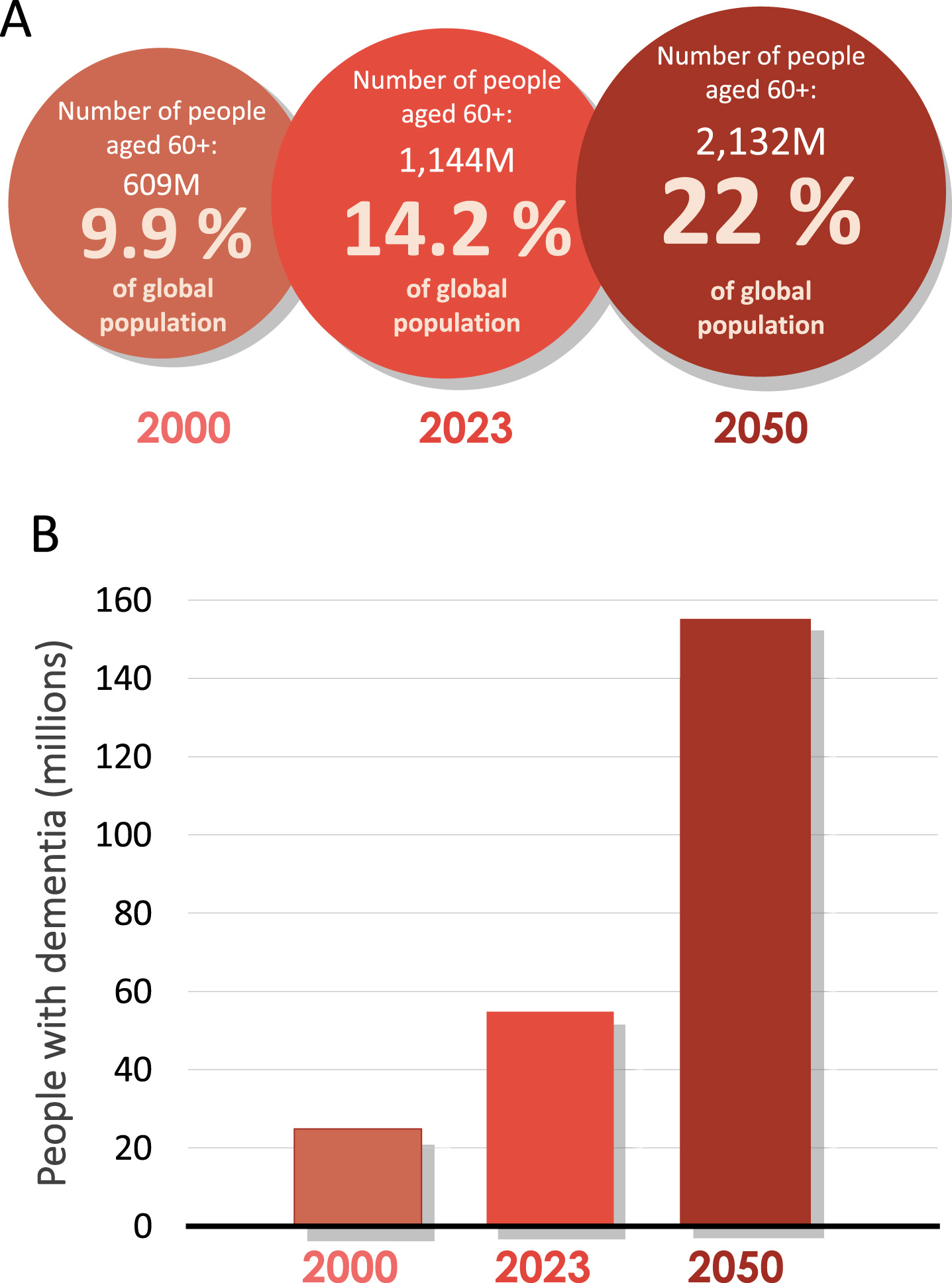
Fig. 1
An ageing population is leading to an increase in the prevalence of dementia worldwide. A) Number of people and percentage of global population aged 60 and over in 2000, 2023, and predicated for 2050. Data source: World Health Organization (2023). B) Approximate and predicted numbers of people living with dementia worldwide (2).
Emerging evidence from studies in rodents [3–5] and humans [6–8] have highlighted the beneficial effects of physical exercise training in ageing. The neurobiology underlying the beneficial effects of physical exercise remains, however, poorly understood. Extensive research has now shown that the hippocampus, a key brain region regulating cognitive and mood functions [9–11] is particularly sensitive to environmental regulators, including exercise. The hippocampus is a component of the limbic system located in the medial temporal lobe that retains considerable levels of plasticity until old age. For instance, new neurons are produced in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) throughout life, in a process called adult hippocampal neurogenesis (AHN) [12]. This unique form of brain plasticity is maintained in the adult brain, yet it declines with increasing age and its demise has been implicated in mechanisms underlying AD and dementia [13, 14]. Conversely, increasing physical activity in rodents is a robust enhancer of AHN [15, 16] and can attenuate deficits in AHN in the aged brain [4, 5]. In terms of the functional relevance of AHN, rodent studies have shown that AHN is primarily involved in cognitive tasks which require spatial and contextual memory [17, 18] including pattern separation [19, 20], which is the ability to distinguish between similar but not identical memories and contexts. It has also been implicated in the regulation of antidepressant action [21–23] and forgetting [24–26].
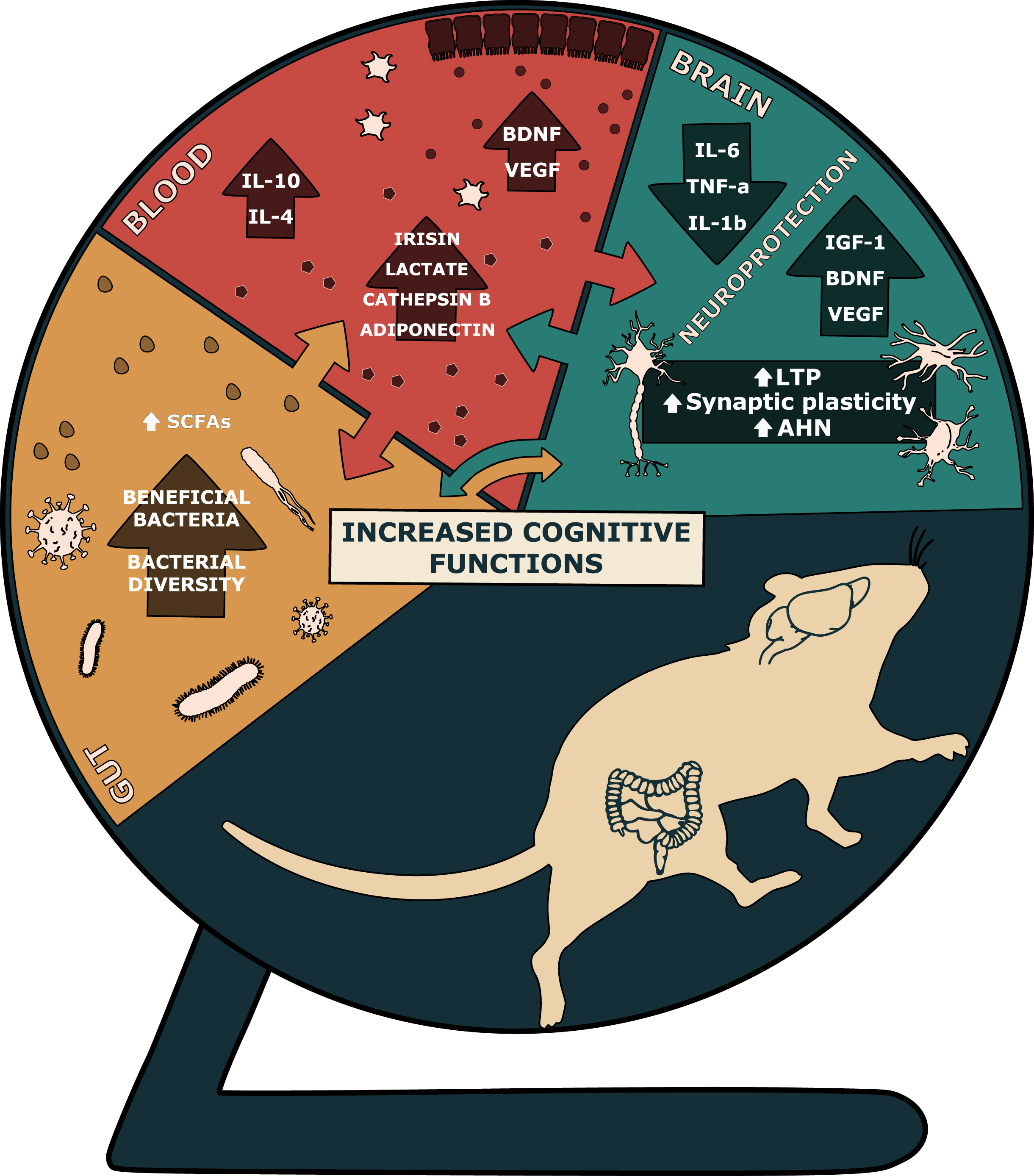
Exercise-driven cognitive enhancement is also mediated by other processes in the brain besides AHN, such as the increase in long-term potentiation (LTP) and synaptic plasticity [27], or the reduction in neuroinflammation [28] (Fig. 2). Multiple exercise-related systemic factors modulate AHN and cognitive functions. Amongst them are myokines (irisin and cathepsin B) [29], metabolites (lactate) [29], hormones (adiponectin) [30], anti-inflammatory cytokines such as interleukin 10 and 4 (IL-10 and IL-4) [31], and neurotrophic factors such as brain derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) [32] (Fig. 2). Recent studies indicate that gut microbiota are a susceptibility factor for the decline in AHN and associated cognitive function in AD [33]. Importantly, exercise-driven changes in the gut-microbiota composition [34] have been shown to play important roles in the modulation of AHN [35] and related behaviours [36] (Fig. 2). Thus, AHN may be a key element in maintaining hippocampal integrity and function, which may potentially be harnessed and enhanced by exercise in order to prevent cognitive decline and associated symptoms of dementia during ageing. Investigating mechanisms such as the role of the gut-brain-axis in the exercise-induced modulation of hippocampal cellular and behavioural changes will provide important information for preventative strategies to delay the onset of cognitive decline. In this respect, preclinical research is crucial for uncovering exercise-induced peripheral, brain and behavioural changes, which would not be ethically or technically feasible in humans. Of the many factors to be considered when investigating such changes in rodent studies, the choice of the exercise protocol requires particular attention, given that the type, intensity and duration of physical activity can induce diverse effects.
Fig. 2
Central and systemic effects of physical exercise. Abbreviations: AHN: adult hippocampal neurogenesis; BDNF: brain derived neurotrophic factor; IGF-1: insulin-like growth factor 1; IL-4: interleukin 4; IL-6: interleukin 6; IL-10: interleukin 10; IL-1β: interleukin 1β; LTP: long-term potentiation; SCFAs: short-chain fatty acids; TNF-α: tumor necrosis factor α; VEGF: vascular endothelial growth factor.
In this review, we compare and contrast different exercise protocols used in healthy middle and old aged rodents for exercise-induced changes in age-related cognitive decline. We consider the role of AHN as a key cellular mediator in these changes, which may be harnessed for therapeutic gain. We further consider the emerging literature on changes in the gut microbiota during ageing and in response to exercise with a view to providing insight into possible strategies to optimize gut health for cognitive gain. Finally, based on the evidence garnered to date, we propose optimal exercise regimens for rodent research, which would inform clinical research to help develop preventative strategies for cognitive decline during ageing.
EXERCISE REGIMENS USED IN PRECLINICAL RESEARCH
The majority of physical exercise protocols used in preclinical research are either voluntary or involuntary (forced) exercise. The most common voluntary exercise intervention is wheel running, where rodents have free access to running wheels in their home cage environment. Commonly used wheel types include saucer-like wheels, which are usually made of plastic, with a slightly tilted, nearly horizontal orientation, and vertical, steel running wheels. It has been reported that mice are more prone to run in horizontal-like plastic wheels compared to vertical steel wheels which are generally heavier and as such may require some extra effort to be set in motion by a mouse [37]. The number of wheel revolutions are automatically recorded by sensors and software connected to the wheel. Therefore, the voluntary running wheel activity is typically measured as number of wheel revolutions over a period of 24 hours and expressed as distance ran per 24 hours (km/day or m/day) [38]. In the majority of studies employing running wheels, rodents have unlimited access to the wheels [39], but some studies report limiting wheel access to rodents to only a few hours per day [40] or for a few days per week [41]. Controls are commonly housed without or with a locked wheel. The latter is used to control for cage enrichment effects, but it should be noted that the presence of a locked wheel can affect certain aspects of AHN [42]. In one study using limited wheel access, one month of daily running for 4 h and 8 h on alternative days had a greater stimulatory effect on AHN compared to running for 24 hours on alternative days or compared to unrestricted voluntary wheel running [43]. Thus, while restrictions in wheel access helps to control and limit the total amount of wheel running, there are conflicting reports in the literature on the impact of limited access wheel running on AHN due to the variety of running durations and frequency of access.
Rodents with unlimited access to running wheels predominantly run during the dark phase with the majority of running occurring at the beginning of the dark phase and tapering off over the course of the night [44]. Rodents commonly increase their daily running distance during the first couple of weeks, termed the acquisition phase, before reaching a steady level of daily running, termed the maintenance phase [45]. Moreover, it has been reported that the daily voluntary running distance is higher when rodents are socially isolated compared to group-housed conditions [46]. A study examining sex differences in voluntary wheel running has shown that female rats voluntarily run on wheels daily for higher distances compared to males [44]. Similarly, more recent studies have reported higher numbers of daily wheel revolutions in female compared to male rats [47, 48], indicative of higher voluntary running activity in female compared to male rodents. Interestingly, ovariectomized 3-month-old female Sprague-Dawley rats run shorter daily distances on wheels compared to ovariectomized rats treated with estrogen replacement, suggesting a potential role of the sex hormone estrogen in running distance [49, 50]. In addition, aged rodents are reported to run less, as evident by their distance run on wheels in 24 hours compared to young adults [51, 52]. Despite the different levels of exercise across sex and age, the main benefits of voluntary wheel running lies in its capacity to enrich the environment for rodents, allowing them to naturally and actively engage in an innate ability to run. Indeed, in experimental conditions where the cage environment is larger and enriched, the opportunity to exercise forms an essential component of the beneficial effects of an enriched environment on various brain measures, including AHN [53]. Interestingly, voluntary wheel running contributes to stress reduction and resilience in aged mice, possibly through an exercise-induced increase in cell proliferation [54, 55].
In contrast to voluntary regimens, involuntary exercise protocols require rodents to run for a predetermined duration, intensity and frequency in specialized, often closed, treadmills or on motorized running wheels [56–59]. Other types of involuntary exercise protocols include forced swimming exercise [60] and resistance training [60, 61], although they are less commonly used, as they may involve confounding aspects like stress and fatigue. Treadmill exercise regimes are classified as low-, moderate- or high-intensity, depending on frequency, duration and speed. Although there are no fixed protocols defining treadmill intensities, low-intensity treadmill exercise typically reaches a maxim speed of 11 m/min, while moderate and high intensities reach a maxim speed of 14 and 22 m/min, respectively [15]. To help rodents become familiar with treadmill exercise and to facilitate proficiency in running, they are conditioned to the treadmill protocol by gradually increasing the intensity (speed and duration) during each running session. A useful example is the protocol used by [62] in 5- and 24-month-old Sprague-Dawley rats, which consisted of 6 weeks of exercise (7 days/week). Training sessions lasted for 30 minutes, with the first 5 minutes at an intensity of 2 m/min, followed by another 5 minutes at 5 m/min and finally by 20 minutes at the highest intensity of 8 m/min.
Adaptation to aerobic training occurs over the longer term and notably also with age [63]. Accordingly, a protocol has been devised such that a maximum oxygen consumption (max VO2) of 60-70% is maintained. It consisted of a low-moderate exercise intensity of 60 min/day at 12 m/min with a 5° treadmill slope when rats were 8 months of age, which was then reduced and reached a minimal intensity of 30 min/day at 4–6 m/min with 0° slope at 18 months of age. It demonstrates that exercise protocols should be adapted to the rodents age in order to maintain a constant intensity [63]. Running speed has been identified as a crucial factor for maximal oxygen consumption [64]. While it is possible to regulate speed and thus oxygen consumption using treadmill protocols, it has been shown that voluntary exercise resulted in small or null changes in maximum oxygen consumption after 8 weeks of exercise [65]. In this regard and coupled with the fact that it is possible to minimise inter-individual variability in exercise intensity using treadmills, which is often not possible using unlimited voluntary wheel running protocols [65], treadmill exercise is advantageous for controlling experimental variables. Short bouts of exercise are also employed in treadmill protocols through the use of intermittent training, as it is proposed to mimic the way rodents would spontaneously run [44]. It has been shown that when the distance run by rats was identical, using either a voluntary wheel, or involuntarily and intermittently on a motorised wheel, those on the voluntary wheels ran at a higher speed, for less time and were less anxious [58]. Since then, intermittent exercise protocols using different running intensities have been developed. For example, a mild-intensity, intermittent exercise protocol consisted of 4–6 minutes bouts at 10 m/min repeated twice with 1 minute of rest (4 days/week for 4 weeks) [66]; a moderate-intensity intermittent training (MIIT) protocol consisted of 10 min at 10 m/min, 4 minutes at 20 m/min and 3 minutes at 12 m/min repeated for 45 minutes (5 days/week for 8 weeks) [67]; and a high-intensity intermittent treadmill training (HIIT) protocol consisted of very short bouts of exercise lasting only for 10–30 s at the extremely high intensity of 60 m/min interspaced with 2.5 min of recovery (5 times/week for 4 weeks) [68]. Regardless of the intensity, all intermittent training protocols showed beneficial effects on brain and cognition [66–68].
One aspect to consider in treadmill studies is that rodents are forced to run while restrained in small running lanes, which may cause stress or anxiety. Furthermore, many of the treadmill protocols use electrical or mechanical stimuli to motivate the animal to continue to run [66, 69–71], which induces a stress response. It has been shown that the levels of corticosterone are higher in mice undergoing treadmill exercise with shock stimulations compared to sedentary mice [72]. Nonetheless, voluntary wheel running is also associated with an increase in corticosterone concentrations compared to sedentary controls, but these higher levels normalize after 4 weeks of running [73]. Strategies to avoid the use of electrical stimuli and reduce potential stress during treadmill running include tactile incentives to run, such as the presence of a metal beaded curtain at the end of the running lane [66], and food motivation in which rats receive a food reward at the end of each training session [63]. Some voluntary wheel running protocols also used food to increase the motivation to run. For example, conditioned running wheel training is used in aged rodents through the programmed delivery of food pellets based on the number of wheel rotations [5]. While food reward may be a strategy to overcome a potential low motivation to run, it may have central effects. For example, food reward stimulates the dopaminergic system which plays a role in the proliferation of neural precursor cells [74].
Swimming exercise has been developed for use in rodent research based on the innate ability of rodents to swim. It has been reported that rodents can adapt to swimming exercise similarly to humans based on measures of lactate threshold [75]. A variety of swimming protocols have been used in preclinical research. For example, a mild intensity (50–65% of rat’s aerobic capacity) forced swimming exercise protocol for 6 weeks has been devised. This protocol included a week of adaptation during which the swimming duration was set at 10 minutes on the first day and was then increased daily for 6 days, to a total of 60 minutes [76]. Interestingly, rats were trained in groups (5–8 per group) because the swimming intensity was increased by the social interaction amongst rats. While this study did not use any resistance, swimming with resistance has been employed in young, middle- and older-aged rats [60, 77]. The duration of swimming exercise was 20 minutes daily with a resistance attached to the tail equal to 3% of their body weight for a total of 4 weeks [77], or for a longer duration and intensity, that lasted daily for 1 hour with a resistance equal to 5% of their body weight for a total of 12 weeks [60]. A high-intensity interval training (HIIT) protocol in swimming exercise, (14 bouts of exercise/session, 20 sec/bout + 10 sec ITI) with rats carrying a weight (9% of body weight increased by 1% of body weight each week) attached to their tail has also been recently developed [78]. It is difficult however to determine the exact intensity in swimming exercise compared to treadmill exercise paradigms.
Most of the reported exercise paradigms in rodents, including treadmill and voluntary wheel running as well as swimming, are commonly, though not always accurately referred to as ‘aerobic’ exercise. However, high intensity treadmill training, treadmill HIIT and swimming HIIT have been reported as anaerobic exercise paradigms [79–82]. The transition from aerobic to anaerobic training depends on experimental species, biological sex and age, and importantly, should be evaluated by specific physiological parameters, such as blood lactate concentration [79]. Due to the limited number of studies reporting such type of measurements and thus the lack of clarity on whether the exercise paradigms employed in experimental studies are aerobic or anaerobic, we will refrain from defining exercise paradigms described in this review as aerobic or anaerobic.
Exploring the mechanisms that underlie the impacts of exercise on hippocampal functions will yield crucial insights for developing preventive approaches for the onset of cognitive decline. Furthermore, an understanding of such mechanisms will yield new strategies for the development of exercise mimetics [83–85]. Such mimetics will potentially enable beneficial interventions for both healthy individuals and those enduring hippocampal-mediated disease, especially those that are not capable of physical exercise. Given the variability in training protocols currently used in preclinical research, the following sections will review the effects of different types of exercise paradigms on cognitive functions and brain plasticity. This will aid in refining exercise type, intensity and duration for studies of age-related cognitive decline, taking into consideration the specific animal model and experimental design.
EXERCISE-INDUCED CHANGES IN AGE-RELATED COGNITIVE DECLINE
Cognitive impairment is emerging as a significant health challenge due to our ageing population. Evidence to suggest that preventative strategies using non-pharmacological approaches like exercise may have beneficial effects has prompted a surge of exercise research, aimed at finding optimal protocols for studies in aged and more recently in middle-aged rodents. Middle age is a transitioning period of the lifespan, associated with cognitive and emotional changes that may become more pronounced in older age (Box 1: “How old are middle aged humans and rodents?”) [86–88]. There is limited research on the effects of physical exercise on cognition during middle age, although it could represent a critical time window when therapeutic strategies may help to prevent cognitive decline [3, 89–91]. Furthermore, there is a shortage of comparative research examining the effects of various exercise paradigms on cognitive functions. Nevertheless, what is known about the effects of physical exercise interventions on cognitive functions in middle- and older- aged rodents will be reviewed in the following sections.
EXERCISE AND AGE-RELATED CHANGES IN SPATIAL LEARNING AND MEMORY
Age-related changes in spatial learning and memory
Ageing disrupts the capacity for spatial learning and memory [41, 96]. Changes in these abilities are classically assessed in rodents using behavioural tasks such as the Morris Water Maze (MWM). For example, 21-month-old Sprague-Dawley rats showed impairments in both spatial learning and memory compared to 3-month-old rats [41]. Similarly, older-aged C57/BL mice (19 months) showed impaired spatial learning and memory compared to younger mice [96]. On the other hand, while older-aged Wistar rats (18 months) exhibited impaired spatial memory compared with younger animals (3 months), their spatial learning abilities were similar to the younger animals [97]. Individual differences in water maze performance also emerge with increasing age in rats, such that it is possible to distinguish age-impaired and age-unimpaired animals when large groups of animals are used [98, 99]. Notably, these cognitive differences correlate with changes in AHN [98, 99] suggesting that different levels of resilience to cognitive aging among individuals may be associated with rates of AHN. The age-related decline in spatial learning and memory begins in middle age. For example, both middle- and older-aged (12 and 18 months) C57BL/6 mice showed reduced learning abilities during the training days and reduced performance in the probe trial of the MWM when compared to 3-month-old mice [87]. Moreover, spatial learning ability declines further with advancing age. For example, 18-month-old Sprague-Dawley rats showed an increased efficiency path and lower latency to find the platform in the last training day of the MWM compared to 28-month-old rats [69]. These age-related behavioural changes highlight the importance of identifying interventions such as optimal exercise regimens that may delay or attenuate the effect of advancing age on spatial learning and memory. Indeed, physical exercise has been demonstrated to improve spatial learning memory in the MWM in older-aged rodents, as described in detail in the following section.
Effects of physical exercise on spatial learning and memory in older aged rodents
Physical exercise has been reported to counteract age-related impairments in spatial learning and memory in the MWM in older-aged rats [69, 100] and mice [87, 96] (summarized in Table 1). However, discrepancies have also been reported. Eight weeks of high-intensity treadmill exercise did not improve spatial learning in 18-month-old male Wistar rats [97]. Likewise, 12 weeks of voluntary wheel running or forced swim exercise (with resistance) did not ameliorate spatial learning in 20-month-old female Wistar rats [60]. The reasons underlying this lack of a beneficial effect of exercise in these studies are not clear. However, it is unlikely to be related to exercise duration since a shorter exercise intervention (7 weeks of treadmill running) increased spatial learning in 23-month-old Brown-Norway/F344 rats compared to sedentary controls [101]. It is also unlikely that the rat strain is responsible for differences in behavioural outcomes as previous studies using the same strain of male and female rat (Wistar) have showed that similar exercise paradigms increased spatial learning ability [66, 93].
Table 1
Effects of physical activity on spatial learning and memory, spatial working and reference memory, recognition memory and pattern separation in middle and older aged rodents. Symbols: ↑: increased; ↓: decreased; **: studies involving middle-aged rodents (10–15 months of age). Abbreviations: A: Albino; FS: forced swimming; FTR: forced treadmill running; ITI: inter-trial interval; mo: months old; MWM: Morris water maze; NOR: novel object recognition; OIR: object identity recognition; ORT: object recognition test; RAM: radial arm maze; ref. mem.: reference memory; SD: Sprague-Dawley; SLR: spontaneous location recognition; VO2: oxygen consumption; VWR: voluntary wheel running; w: week; W: Wistar; work. mem.: working memory
| Voluntary exercise | ||||
| Species, age, sex | Exercise intervention | Behaviour | Exercise effects | Reference |
| Rat (SD), 21 mo, male | VWR (8 w, 3days/week) | MWM | ↑ learning ↑ memory | [41] |
| Rat (W), 20 mo, female | VWR (VE) (12 w) FS (12 w, 5days/w, 1 h/day, resistance) | MWM | No effects on learning ↑ memory | [60] |
| Mouse (BALB-cByJ), 19 mo, male | VWR (6 w) | MWM | ↑ swimming speed ↑ memory and reversal memory | [39] |
| **Mouse (C57BL/6), 4, 9, 14 mo, male and female | VWR (unlimited access to wheels from 3 mo) | Y-maze for ref. mem. | ↑ spatial reference memory in 9 mo | [109] |
| **Rat (SD), 12 mo, male | VWR (40 w) | SLR | ↑ spatial pattern separation | [118] |
| Mouse (Swiss), 6 and 20 mo, female | Enriched environment since weaning (including free access to running wheels) | OIR | No effects on object recognition memory | [112] |
| Rat (F344), 18 mo, male | Food conditioned exercise (18 w, average 4 km/w) | MWM | ↑ swimming speed ↑ learning ↑ short and long memory | [5] |
| Involuntary exercise | ||||
| Species, age, sex | Exercise intervention | Behaviour | Exercise effects | Reference |
| **Rat (W), 3 and 12 mo, male | FS (4 w, 20 min/day with resistance) | ORT | ↑ object recognition memory (short and long term) | [77] |
| Rat (W), 2 and 20 mo, male | FS (6 w, 3 days/w, 14 bouts/day of 20 s + 10 s ITI with resistance) | NOR | ↑ object recognition memory in 20 mo | [78] |
| Rat (Brown Norway / F344), 23 mo, male | FTR (7 w, 5 days/w, 15 min/day at 8 m/min, electric shock) | MWM | ↑ learning | [101] |
| Rat (W), 24 mo, female | FTR (4 w, 4 days/w, 3 min at 2 m/min + two bouts of 4 - 6 min at 10 m/min with 1 min resting interval, tactile incentive) | MWM | ↑ learning ↑ memory | [66] |
| Rat (W), 6 18 26 mo, male | FTR (6 months, 3 days/week, 40 min/day at 6-18 m/min, VO2 max 60%) | MWM ORT | ↑ learning in 26 mo ↑ working memory ↑ object recognition memory in 26 mo | [93] |
| **Rat (SD), 14 to 18 mo (middle aged only sed) or 14 to 28 mo (old ex and sed)), male | FTR (14 moths, 5 days/w, 20 min/day at 20 m/min) | MWM | ↑ learning | [69] |
| Rat (W), 3 and 18 mo, male | FTR (8 w, at 30 m/min) | MWM | No effects on learning ↑ memory | [97] |
| Mouse (C57/BL), 19 mo, male | FTR (6 w, 5 days/w, 15–60 min/day at 16–24 rotations/min) | MWM NOR | ↑ learning ↑ memory ↑ object recognition memory | [96] |
| **Mouse (C57BL/6), 3, 9/12, >18 mo, male | FTR (6 w, 5 days/w, 60 min/day at 10, 8 or 6 m/min for the young, middle aged and older age respectively) | MWM ORT | ↑ learning in 18 mo ↑ memory in 9/12 mo ↑ object recognition memory in 9/12 and 18 mo | [87] |
| Rat (SD), 5 and 24 mo | FTR (6 w, 7 days/w, 30 min/day at 8 m/min) | RAM for work. mem. | ↑ spatial working memory in 24 mo | [62] |
| Rat (W), 8 and 18 mo, male | FTR (24 or 64 w, 3 days/w, at a low-moderate intensity based on VO2 max test (60–70%) reaching a maximum of 60 min/day at 14 m/min and 10° slope) | RAM for work. and ref. mem. | ↑ spatial reference memory (↑ % of correct entries and ↓ total time in 8 and 18 mo, ↑ success variable in 8 mo) | [107] |
| Rats (A), 4–6 and 23–24 mo, male | FTR (6 w, 5 days/w, 20 min/day at 24 m/min from 3rd w) | Y-maze for work. mem. | ↑ spatial working memory in aged rats | [104] |
| Rat (W), 8 and 18 mo, male | FTR (24 or 64 w, 3 days/w, at a low-moderate intensity based on VO2 max test (60–70%) reaching a maximum of 60 min/day at 12 m/min and 5° slope) motivated by food reward | ORT | ↑ object recognition memory in 8 and 18 mo | [63] |
One factor that could be responsible for discrepancies in exercise-induced changes in learning and memory is the intensity of exercise. For example, the very high intensity of the treadmill exercise protocol (30 m/min) used in [97] did not induce any change in learning abilities compared to studies where learning abilities were ameliorated by similar treadmill exercise interventions but with lower intensities of either 8–10 m/min [66, 101] or 5–20 m/min [69, 93]. Other considerations to note when interpreting these findings is that there is a variety of approaches in analysing behaviour in the MWM. For example, [39] concluded that 6 weeks of voluntary exercise improved spatial learning in 19-month-old mice based on an increase in swimming speed. However, exercise did not reduce the latency of mice to reach the platform during the training days, which is also indicative of learning. Thus, this finding could be interpreted as an improvement in the general spatial navigation abilities (moving faster in the pool) rather than in spatial learning abilities (learning the platform location in the pool). Besides the beneficial effects of physical activity on spatial learning abilities during older age, studies have demonstrated that physical exercise, irrespective of being voluntary or involuntary, improved age-related spatial memory loss in rats [60, 66, 97, 100] and mice [39, 87, 96].
Very few studies have reported the effects of physical exercise on spatial learning and memory during middle age, despite this perhaps being a key intervention period to delay the age-related decline in cognition. Interestingly, six weeks of moderate-intensity treadmill running ameliorated a decline in spatial learning in older-aged mice (18 months), but not in middle-aged (12 months), compared to younger adult mice (3 months) [87]. In conclusion, while physical exercise, both voluntary and involuntary, can improve spatial learning and memory in aged rodents [87, 100], others have reported no effects of physical exercise on spatial learning [60, 97]. While the reasons underlying these discrepancies are unknown, the exercise type and intensity as well as the inclusion of resistance is likely to play a role.
EXERCISE AND AGE-RELATED CHANGES IN SPATIAL WORKING AND REFERENCE MEMORY
Age-related changes in spatial working and reference memory
Spatial working memory can be assessed by evaluating spontaneous alternation behaviour (SAB) in the Y-maze or T-maze [102] as well as in the radial arm maze (RAM) [103]. An age-related impairment in spatial working memory has been reported in 23 to 24-month-old male rats compared to 4 to 6-months-old rats when tested in the Y-maze task [104]. Similarly, analysis of SAB in a T-maze task showed that Sprague-Dawley rats (21 months) displayed lower spatial working memory compared to younger 7-month-old rats [105]. Similar data were obtained from testing older age (24 months) Sprague-Dawley rats compared to 5-month-old rats in the RAM task, further demonstrating the age-related reduction of spatial working memory [62]. This decline has been reported to start in late adulthood and middle age. For example, middle-aged (9 and 12 months) virgin female NMRI (Naval Medical Research Institute, outbred Swiss-type mice) mice showed reduced percentages of alternations in a Y-maze task compared to 3-month-old mice [86]. Similarly, middle-aged (9 months) male Wistar rats showed a higher error rate in the RAM task when compared to younger (3 months) rats [106]. Interestingly, however, 8-month-old rats showed a better spatial working memory in the RAM apparatus compared to 18-month-old rats [107], indicating that advancing age exacerbates the age-related decline in spatial working memory abilities.
Spatial reference memory, another form of spatial memory that can be tested in the Y-maze or RAM has also been reported to be impaired in older age. Eighteen-month-old rats showed a lower percentage of correct entries in the RAM task compared to eight-month-old rats indicative of an age-related decline in spatial reference memory [107]. Moreover, it was demonstrated in senescence accelerated mouse prone 8 (SAMP8) mice that the onset of the impaired reference memory in the Y-maze occurs in late adulthood, given that 6-month-old mice as well as 9-month-old mice showed a lower preference for the novel arm compared to younger mice [108]. Taken together, the literature suggests that impairments in spatial working and reference memory occur in middle-age and are exacerbated in older ages in both rats and mice.
Effects of physical exercise on spatial working and reference memory in older aged rodents
Only a few studies have investigated the effects of physical exercise on working and reference memory in older aged rodents (summarized in Table 1). Voluntary wheel running initiated at 3 months of age and continuing throughout the experiment increased spatial reference memory in the Y-maze at 9 months of age [109]. In agreement, lifelong, moderate-intensity treadmill training improved spatial reference memory in the RAM task in both 8-month-old and 18-month-old male Wistar rats, although, the exercise intervention had a more robust effect in 8-month-old rats compared with the older aged rats [107]. On the other hand, the same study reported that lifelong moderate-intensity treadmill training had no effects on spatial working memory in the RAM at either age [107]. However, six weeks of low- or high-intensity treadmill training has been reported to improve spatial working memory in older aged rats compared to age-matched sedentary rats, suggesting that the effects of treadmill running on spatial working memory may be dependent on the intensity or the duration of the treadmill training. Specifically, six weeks of low intensity (30 min/day at 8 m/min, 7 days/week) treadmill training significantly improved age-related decline in spatial working memory in the RAM in 24-month-old Sprague Dawley rats [62]. Similarly, six weeks of high intensity treadmill exercise (20 min/day at 24 m/min, 5 days/week) enhanced spatial working memory in the Y-maze task in 24-month-old male albino rats when compared to age-matched sedentary controls [104].
Overall, there is a dearth of studies investigating the effects of physical exercise on spatial reference and working memory in older aged rodents, with considerable variation in the results, and hence it is rather difficult to make firm conclusions. Nevertheless, the published studies suggest that long term voluntary and moderate intensity treadmill exercise can improve reference memory in both late adulthood and in older age, with greater effects apparent in late adulthood. Moreover, low and high, but not moderate, intensity can improve spatial working memory in older aged rodents. Future studies are required to determine the optimal exercise intervention for ameliorating age-related spatial working and reference memory impairments in rodents during the susceptible times of middle and older age.
EXERCISE AND AGE-RELATED CHANGES IN RECOGNITION MEMORY
Age-related changes in recognition memory
Recognition memory in rodents is commonly measured using the novel object recognition (NOR) test [110]. Studies have reported that ageing impairs object recognition memory in rats (Wistar, 20 months vs 2 months) [78] and mice (C57/BL, 19 months vs 2 months) [96], but conflicting results have also been reported. For example, it has been reported that neither older-aged (18 months) nor middle-aged (12 months) male C57BL/6 mice exhibited impaired object recognition memory compared to young (3 months) mice [87]. Recognition memory declines with advancing age as evident from impaired object recognition in 18-month-old female Sprague-Dawley rats compared to 5-month-old rats [111]. Moreover, older rats (28 months) preferred the familiar over the novel object, indicative of a stronger impairment in recognition memory at 28 compared to 18 months of age [111]. Indeed, the onset of recognition memory decline might occur at middle age based on a study showing that middle-aged (12 months) male Wistar rats had reduced preference for the novel object compared to 3-month-old rats [77]. This further supports the importance of identifying interventions, like optimal exercise regimens for middle-aged rodents, that may delay the effects of advancing age on recognition memory.
Effects of physical exercise on recognition memory in older aged rodents
Physical exercise has been found to ameliorate age-associated impairments in recognition memory in both middle- and old-aged rodents [77, 78, 96] (summarized in Table 1). Indeed, six weeks of treadmill training improved object recognition memory in 19-month-old C57/BL mice compared to age-matched sedentary controls [96]. Similarly, a longer duration of six months of treadmill exercise improved object recognition memory in 32-month-old male Wistar rats compared to age-matched sedentary counterparts [93]. Moderate-intensity treadmill exercise, initiated at 2 months of age reduced the age-related impairments in object recognition memory in 18-month-old Wistar rats compared to sedentary age-matched rats [63]. In addition, six weeks of treadmill exercise initiated during middle age (9 to 12 months) as well as older age (18 months) increased object recognition memory in male C57BL/6 mice [87]. Other forms of exercise have also improved recognition memory in aged rodents. For example, six weeks of forced swimming exercise (with resistance) reduced the age-related decline in object recognition memory in 20-month-old Wistar rats when compared to age-matched sedentary rats [78]. Similarly, four weeks of forced swimming exercise (with resistance) in middle-aged (12 months) male Wistar rats ameliorated object recognition memory compared to the sedentary rats when tested 1.5 and 24 hours after the acquisition phase, indicative of improved short- and long-term object recognition memory [77].
While most of the studies examining the effect of physical exercise on recognition memory have used either treadmill or forced swimming exercise, one study has examined the effects of voluntary wheel running as part of an enriched environment compared to standard cage environment on recognition memory in young and aged Swiss mice [112]. The environment included not just free access to running wheels but also access to ropes, tunnels and toys since weaning. When tested for novel object recognition, young adult (5 months) as well as aged (20 months) mice, were able to distinguish familiar from novel objects, regardless of the environment [112]. Taken together, forced treadmill and swimming exercise have been shown to ameliorate recognition memory in both middle- and older-aged rodents, but more investigation is needed to reveal the potential effects of voluntary wheel running on recognition memory during middle and older age.
EXERCISE AND AGE-RELATED CHANGES IN PATTERN SEPARATION
Age-related changes in pattern separation
Studies conducted in humans, rats and mice have generally reported that ageing impairs pattern separation ability [111, 113, 114]. Multiple behavioural tasks have been developed to assess pattern separation in rodents including the delayed non-matching to position (DNMP) test, touchscreen-based tasks (location discrimination (LD); visual discrimination (VD); paired associates learning (PAL)), and the spontaneous location recognition (SLR) test. It has been reported that unlike 5-month-old female Sprague-Dawley rats, neither 18-month-old nor 28-month-old female Sprague-Dawley rats could discriminate between objects in a novel location in the SLR task, suggesting a reduced spatial pattern separation ability in older age [111]. An age-related reduction in visual pattern separation has also been shown; sixteen month-old male C57BL/6J mice exhibited decreased visual pattern separation ability compared to 3-month-old mice when tested in the touchscreen-based LD task [115]. Similar age-related impairments in pattern separation were reported in the VD and PAL tasks. Older-aged (17 and 21 months) but not middle-aged (12 months) male C57BL/6J mice exhibited a decrease in visual pattern separation in the touchscreen-based VD task compared to 3-month-old mice [115]. In agreement, 16- and 21-month-old male C57BL/6J mice showed impaired performance in visual pattern separation in an operant touchscreen task compared to 3-6 months-old mice [116]. Similar results were obtained from rats in the operant touchscreen PAL task, whereby 24-month-old Fischer 344×Brown Norway F1 hybrid rats showed reduced visual pattern separation ability compared to 4-month-old rats [117]. However, given that this task is based on visual stimuli, results may be associated with an impaired visual ability in aged animals [115]. In this regard, a modification of the touchscreen-based location discrimination protocol was applied in a study examining behaviours in older-aged (22 months) C57/BL6 mice, as they exhibited difficulties in habituating to the touch screen [114]. While there is consensus that ageing decreases pattern separation abilities in older-aged rodents, further investigation is needed to determine pattern separation ability during middle age.
Effects of physical exercise on pattern separation in older-aged rodents
A limited number of studies have investigated physical exercise intervention as a strategy to improve pattern separation in healthy, older aged rodents (summarized in Table 1). This is perhaps not surprising given that pattern separation behavioural tasks were only developed more recently compared to tests assessing spatial learning and memory, spatial working and reference memory and recognition memory. Nevertheless, the evidence suggests that physical exercise improves pattern separation in older-aged rodents. Forty weeks of voluntary running initiated during adulthood has been reported to improve pattern separation ability in the SLR task (small configuration) in 12-month-old Sprague-Dawley rats compared to the age-matched sedentary counterparts [118]. To the best of our knowledge, there are no reports on the effects of treadmill training or other types of forced exercise on pattern separation in older-aged rodents. Furthermore, there is no reported evidence on the effects of physical activity on pattern separation during middle age, even though this could represent a susceptible time frame for intervention. Therefore, future investigation is needed to determine whether physical exercise during middle age can prevent or significantly delay the age-related impairments in pattern separation, and whether alternative exercise protocols can induce comparable improvements as induced by voluntary exercise. Interestingly, physical exercise also increases forgetting to facilitate the encoding of new overlapping memories via the clearance of old memories in aged rodents. Detailed analysis of this topic is beyond the scope of this review, but further information can be found in Box 2 (Box 2: “The role of exercise-mediated forgetting in the encoding of new overlapping memories”).

EXERCISE-INDUCED CHANGES IN AGE-RELATED DECLINE IN ADULT HIPPOCAMPAL NEUROGENESIS
New neurons are generated in the adult brain in the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus, through a process called adult hippocampal neurogenesis (AHN) (Box 3: “Adult hippocampal neurogenesis”). The rate of AHN varies throughout life, but typically declines with age (Fig. 3). During the transitioning period of middle age (Box 1: “How old are middle aged humans and rodents?”), rates of AHN drop and reach low rates during senescence [13]. The age-related reduction in AHN has been hypothesised to be one of the cellular mechanisms mediating age-related cognitive decline. Indeed, AHN has been shown to be necessary for certain cognitive functions such as spatial learning and memory [18], and pattern separation [19, 40], and its levels correlate with individual differences in cognitive performance of older-aged rats [98, 99, 123].
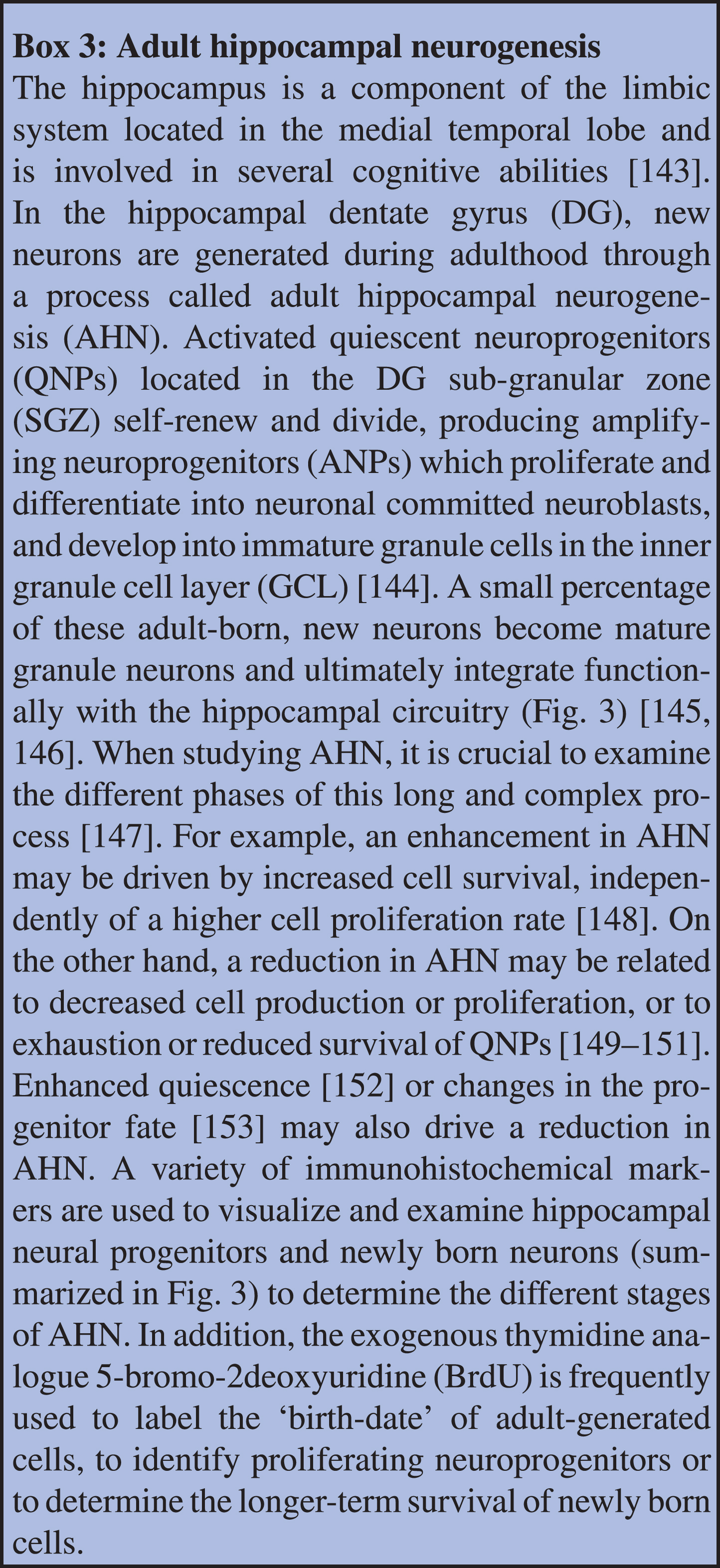
Fig. 3
Stages of adult hippocampal neurogenesis in the dentate gyrus of the hippocampus in adulthood (top panel) and older age (bottom panel). Abbreviations: DCX: doublecortin; DG: dentate gyrus; GFAP: glial fibrillary acidic protein; PSA-NCAM: polysialic-acid neural cell adhesion molecule; SGZ: sub-granular zone; SOX 2: SRY-related HMG box.
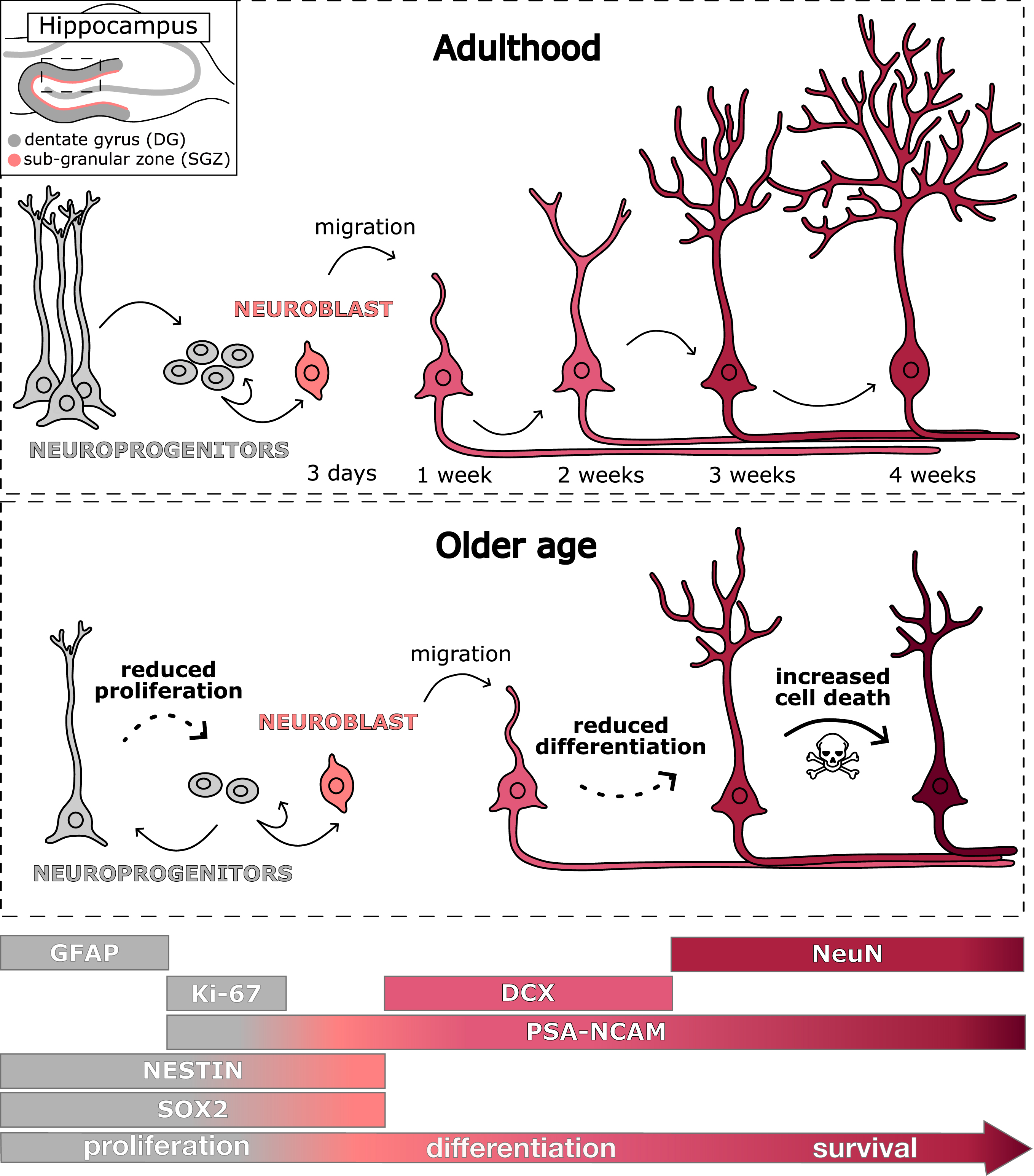
Exercise has been reported to increase AHN in adolescent [124], adult [15, 16] as well as older-aged rodents [4, 5], and has been proposed as a strategy to ameliorate the age-related reduction in AHN. Nevertheless, few studies have focused on the effect of exercise on AHN during middle age, despite this representing a particularly susceptible time for interventions aimed at delaying the onset of age-related cognitive decline. In addition, there is a dearth of studies comparing the effects of different exercise paradigms on the cellular stages of AHN. Although studies in rodents report a significant effect of physical exercise on neurogenesis and hippocampal cognitive measures, heterogeneous findings have been reported regarding the presence and functional role of AHN in humans [125–128]. Studies have been openly debated [129–135], focusing on methodological aspects and issues of post mortem tissue integrity and patient information [136–139]. Moreover, due to technical and ethical limitations, the functional role of AHN in the modulation of cognitive abilities in humans has not been directly demonstrated to date. Nevertheless, human studies have reported that aerobic exercise (walking for a duration of 40 minutes) correlated with increased hippocampal volume (a proxy for enhanced AHN) and improved memory [11, 140]. Furthermore, an exercise-related increase in hippocampal volume has also been associated with increased serum concentrations of BDNF, which stimulates AHN, at least in rodents [11]. Arguably, the number of new neurons that may be generated in the adult or senescent brain in humans is low [13, 126, 134]. However, given the homogeneity between neuronal properties of the rodent and human brain, adult-born neurons are likely to exert a significant influence on hippocampal neuronal network integrity [141], which is important for certain cognitive functions [142].
Age-related changes in AHN
Changes in precursor and expansion stage, differentiation and early maturation, postmitotic maturation and late survival stages of hippocampal neurogenesis have been observed during ageing in rodents (summarized in Table 2) [12]. Recent studies have shed light on the mechanisms underlying these changes. Ageing alters the functions of the quiescent neuroprogenitor pool, such as their metabolic activity and chromatin accessibility dynamics [154, 155]. A disruption of the balance between neural stem cell quiescence and activity is thought to be a leading cause of the age-related decrease in AHN [154]. Additionally, slower proliferation of the activated neuroprogenitors coupled with a higher return to quiescence further contributes to the observed age-related reduction in AHN [156].
Table 2
Age-related changes in different stages of AHN in the hippocampus. Symbols: ↑: increased; ↓: decreased; **: studies involving middle-aged rodents (10–15 months of age). Abbreviations: A: Albino; BrdU: 5-bromo-2deoxyuridine; DCX: doublecortin; DG: dentate gyrus; F: Fischer; GCL: granule cell layer; GFP: green fluorescent protein; MA: middle-aged; mo: months-old; O: older-aged; PCNA: proliferating cell nuclear assay; PSA-NCAM: polysialic-acid neural cell adhesion molecule; SD: Sprague Dawley; SGZ: sub-granular zone; Y: young
| Species, age, sex | Precursor and expansion | Differentiation and early maturation | Postmitotic maturation and late survival | Reference |
| Rat (SD), 5 and 24 mo | / | / | ↓ BrdU+ cells in DG (6 week old cells) | [62] |
| Rat (F 344), 6 and 21 mo, female | ↓ BrdU+ cells in GCL (1 day old cells) | ↓ PSA-NCAM+ neurons in GCL | ↓ BrdU+ cells in GCL (6 week old cells) | [157] |
| Rat (SD), 5 (Y), 18 (MA) and 28 (O) mo, female | / | ↓ DCX+ neurons in GCL in MA and O compared to Y | / | [111] |
| Rat (A), 4–6 and 23–24 mo, male | ↓ PCNA+ cells in DG | / | / | [104] |
| **Rat (F 344), 4 (Y), 12 (MA), 24 (O) mo | ↓ Ki67+ cells and BrdU+ cells in SGZ in MA and O compared to Y | ↓ DCX+ cells and their dendritic growth in DG in MA and O compared to Y | No effects on % of BrdU+/DCX+ on BrdU+ (6 hour and 12 day old) cells in SGZ | [159] |
| Mouse (C57/BL6), 2 and 17 mo, male | ↓ Ki67+ cells in DG | ↓ DCX+ neurons in DG ↓ DCX+ neurons with tertiary dendrites in DG | ↓ BrdU+ cells in DG (6 week old cells) | [113] |
| Mouse (C57BL/6), 3 and 19 mo male | / | / | No changes in number of BrdU+ cells in DG but–% of newly-born astrocytes (4 week old cells) | [4] |
| **Mouse (transgenic Nestin-GFP), 2 and 12 mo | ↓ GFP+ (nestin) cells in DG ↓ Ki67 + /GFP+ neurons in DG | ↓ DCX+ cells in DG | / | [156] |
Precursor and expansion: Advanced age has been shown to reduce the proliferation rate of neuroprogenitor cells (NPCs) in the hippocampal DG of both rats and mice. The first report to quantify age-related changes in AHN came from a study in which the authors used BrdU labelling with immunohistochemistry analysis to show that a decrease in proliferation of NPCs in the SGZ occurred in 21-month old Fischer 344 rats compared to 6-month old rats [157]. Similarly, 17-month-old male C57BL/6 mice were shown to have a lower number of Ki67+ cells in the DG when compared to 2-month-old male mice [113], and lower numbers of proliferating Ki67+ NPCs and immature neurons have been reported in middle-aged (12 months) compared to 2-month-old mice [156]. In agreement, fewer proliferating Ki67+ or BrdU+ NPCs have also been found in middle-aged (12 months) compared to 4-month-old Fischer 344 rats, suggesting that during middle age, changes in the plasticity of the NPCs arise that may relate to their age-related decline [158].
Differentiation and early maturation: Age-related reductions in the numbers of immature and differentiating neurons have also been reported. Older-aged (21 months) female Fischer 344 rats have fewer differentiating neurons in the GCL compared to 6-month-old rats [157]. The number of immature neurons (DCX+ cells) was also found to be reduced in the GCL of 18- and 28-month old compared to 5-month-old Sprague-Dawley rats [111]. Similarly, 17-month-old C57/BL6 male mice were found to have a lower number of DCX+ neurons and a lower number of DCX+ neurons with a complex dendritic arborisation compared to 2-month-old mice [113]. The age related decline in neuronal differentiation has also been found to occur at middle age as demonstrated by the reduced number and differentiation of DCX+ cells in the SGZ of the DG at 12 months compared to 4 months of age in Fischer 344 rats [159]. Reduced dendritic maturation has been shown in newly-born granule cells of middle-aged (12 months) transgenic nestin-GFP mice compared to 2-month-old counterparts [156]. In addition, chronic in vivo imaging of genetically targeted radial glial-like NPCs in the DG of mice has shown that the number of these cells was significantly reduced in middle-aged (12-months) compared to 2-month-old mice. Moreover, middle-aged mice had a higher number of proliferating NPCs returning to quiescence and a higher number of targeted NPCs undergoing cell death compared to young mice [156].
Postmitotic maturation and late survival: The number of surviving cells labelled with BrdU (4-week-old cells) in the GCL of the hippocampus was significantly reduced in middle- and older-aged (12 and 27 months) female Fischer 344 rats compared to 6-month-old counterparts [157]. However, the rate of the survival of these 4-week old cells was not significantly different between 12- and 27- month old animals [157], suggesting that the age-related reduction in cell survival already occurs during middle age. A reduction in surviving BrdU+ cells (6-week-old) was reported in 24-month-old compared to 5-month-old Sprague-Dawley rats [62] and in 21-month-old compared to 6-month-old Fischer 344 rats [157]. Older aged (17 months) C57/BL6 mice had fewer surviving cells labelled with BrdU (6-week-old cells) in the DG compared to 2-month-old mice [113]. Lower abundance or reduced survival of cells labelled only with proliferation markers may also indicate a reduction in the astrocytic pool, given that stem cells of the DG also convert into astrocytes [153, 160]. Analysis of hippocampal astrocytic production in older-aged rodents is beyond this review, but it may bring novel insights to the field given that astrocytes are involved in memory consolidation and cognitive functions [161, 162] and are also indirectly involved in adult neurogenesis [163].
In conclusion, evidence suggests that each stage of AHN is impacted by ageing, with middle age representing a crucial window of time during which a reduction in cell proliferation and survival of newly born neurons arises, and could potentially, be modified at that age.
Effects of physical exercise on AHN in older-aged rodents
Precursor and expansion: Preclinical studies have demonstrated that voluntary exercise counteracts age-related decline in hippocampal cell proliferation (summarized in Table 3). Eleven days of voluntary wheel running has been shown to increase the number of newly born cells (BrdU+) in the DG of 17-month-old C57BL/6J mice compared to aged-matched sedentary controls [54]. In contrast, others have reported that six weeks of voluntary wheel running did not increase the number of BrdU+ and Ki67+ cells in the DG of 17-month-old C57/BL6 mice compared to sedentary controls [113]. Treadmill exercise has been reported to ameliorate the age-related decline in hippocampal cell proliferation in the DG of older aged rodents (summarized in Table 3). Six weeks of high-intensity treadmill running increased the number of proliferating (PCNA+) cells in the DG of 24-month-old Albino rats compared to age-matched sedentary controls [104]. Food conditioned running, which consists of wheel running activity motivated by food reward, has also been reported to increase the number of newly born cells (BrdU+ cells) in the hippocampus of 18-month-old F344 rats compared to the age-matched sedentary controls [5].
Table 3
Effects of exercise on different stages of AHN in the hippocampus in middle- and old-aged rodents. Symbols: ↑: increased; ↓: decreased; **: studies involving middle-aged rodents (10-15 months of age). Abbreviations: A: Albino; BrdU: 5-bromo-2deoxyuridine; DCX: doublecortin; DG: dentate gyrus; FTR: forced treadmill running; GCL: granule cell layer; GFAP: glial fibrillary acidic protein; MAP2: microtubule associated protein 2; mo: months old; NG2: neuron-glial antigen 2; O: older-aged; PCNA: proliferating cell nuclear assay; SD: Sprague Dawley; SGZ: sub granular zone; VWR: voluntary wheel running; Y: young
| Voluntary exercise | |||||
| Species, age, sex | Exercise intervention | Precursor and expansion phase | Differentiation and early maturation | Postmitotic maturation and late survival | Reference |
| Rat (F344), 18 mo, male | Food conditioned exercise (18 weeks, average 4 km/week) | ↑BrdU+cells in DG (injected 16 weeks after the experiment onset) | No effects on % of (BrdU+/DCX+, BrdU+/NeuN+, BrdU+/NG2+, BrdU+/GFAP+) but ↑ % of total BrdU+ neurons in hippocampus | / | [5] |
| Mouse (BALB-cByJ), 19 mo, male | VWR (∼6 weeks) | / | / | ↑ BrdU+ cells in GCL (injected for 10 days at the beginning of the experiment) | [39] |
| Mouse (C57/BL6), 2 and 17 mo, male | VWR (6 weeks, unlimited access) | No effects on BrdU+ cells and Ki67+ cells in DG in O | ↑ DCX+ and DCX+ with tertiary dendrites in DG in O | / | [113] |
| Mouse (C57BL/6), 3 and 19 mo, male | VWR (45 days) | / | / | ↑ BrdU+ cells in DG in Y and O ↑BrdU+/NeuN+ neurons in DG in Y and O (injected in the 1st week) | [4] |
| Mouse (C57BL/6), 3 and 22 mo, male | VWR (75 days) | / | / | ↑ BrdU+ and BrdU+/NeuN+ neurons in DG in Y No effects in O | [114] |
| Mouse (C57BL/6J), 2 and 17 mo, female | VWR (11 days) | ↑ BrdU+ cells in DG (injected 10 times/day for 11 days) in O | / | / | [54] |
| **Mouse (C57BL/6J), 15 mo, female | VWR (6 months) | / | Exercise tented to increase the maturation of DCX+ cells | ↑ BrdU+ and BrdU+/NeuN+ neurons in DG | [3] |
| Involuntary exercise | |||||
| Species, age, sex | Exercise intervention | Precursor and expansion phase | Differentiation and early maturation | Postmitotic maturation and late survival | Reference |
| **Mouse (C57BL/6J), 8 and 12 mo, male | FTR (1 week of habituation, 5 or 8 weeks of exercise, 60 min/day at 11 m/min) | ↑ BrdU+ and BrdU+/DCX+ cells in DG (injected during the last week of exercise) | ↑ DCX+ cells and their neurite growth | ↑BrdU+/MAP2+ | [164] |
| Involuntary exercise | |||||
| Species, age, sex | Exercise intervention | Precursor and expansion phase | Differentiation and early maturation | Postmitotic maturation and late survival | Reference |
| Rat (SD), 5 and 24 mo | FTR (6 weeks of exercise, 7 days/week, 30 min/day at 8 m/min) | / | / | ↑BrdU+ cells in DG (6 week old cells) | [62] |
| Rats (A), 4–6 and 23–24 mo, male | FTR (6 weeks of exercise, 5 days/week, 20 min/day at 24 m/min from 3rd week) | ↑ PCNA+ cells in DG of O | / | / | [104] |
Differentiation and early maturation: Exercise-driven promotion of the maturation of newly born neurons in the hippocampus has been reported (summarized in Table 3): six weeks of voluntary exercise modestly increased the number of DCX+ cells, while it significantly increased the number of DCX+ neurons with a more mature morphology (presence of tertiary dendrites) in 17-month-old C57/BL6 male mice compared to sedentary mice [113].
Postmitotic maturation and late survival: Physical activity has been shown to enhance the survival of newly born cells and neurons in the hippocampus of older-aged rodents (summarized in Table 3). Forty-five days of voluntary wheel running increased the number of surviving newly born cells (BrdU+) and neurons (BrdU+/NeuN+) in the DG of 19-month-old C57BL/6 mice compared to the sedentary controls [4]. Similarly, six weeks of voluntary wheel running increased the number of surviving newly born cells in the GCL of the hippocampus in 19-month-old BALB-cByj mice [39]. In contrast, three months of voluntary wheel running did not increase the number of surviving newly born cells or neurons in the DG of 22-month-old C57/BL6 male mice compared to the sedentary animals [114]. However, the number of mice involved in the above-mentioned study was low (n = 4), which could perhaps explain the absence of statistical significance. Treadmill intervention has also been reported to increase the survival of hippocampal newly born cells. Six weeks of low-intensity treadmill exercise increased the number of 6-weeks-old BrdU+ cells in the hippocampus of 24-month-old male Sprague-Dawley rats compared to sedentary animals [62].
Considering that middle age is increasingly recognised as a pivotal time when age-related decline in proliferation and survival occurs, it is important to also investigate the impacts of physical activity on AHN not just in older age, but also in middle age. Currently, relatively few studies have highlighted the effects of physical activity on AHN during this time of the lifespan (summarized in Table 3). Voluntary running initiated in adulthood and lasting for 6 months was shown to increase the survival of newly born hippocampal cells and neurons in the DG in middle-aged (15 months) C57BL/6J female mice compared to sedentary animals [3], while improving memory function and BDNF levels. Intriguingly, a recent study reported that voluntary running throughout middle age in male C57BL/6J mice contributes to maintaining the connections of adult born neurons in the hippocampus with brain regions crucial for different cognitive functions such as the perirhinal cortex, subiculum and entorhinal cortex [141]. Six weeks of moderate intensity treadmill exercise increased the number of proliferating cells as well as immature neurons and promoted their neurite growth in the DG of 12-month-old male C57BL/6J mice compared to the sedentary age-matched control mice [164]. The same study reported that nine weeks of moderate intensity treadmill exercise also increased the survival of newly born neurons in the hippocampus of middle-aged (12-month-old) mice compared to the sedentary animals [164]. Taken together, results suggest that voluntary as well as forced treadmill exercise during middle and old age stimulate the proliferation, maturation and survival stages of AHN.
GUT MICROBIOTA AS A MEDIATOR OF AGE-RELATED COGNITIVE DECLINE
The gut microbiota, a complex community of microorganisms residing in the gastrointestinal tract has emerged as a critical mediator of age-related and AD-related decreases in cognitive abilities and AHN [33, 35, 36]. Ageing has been associated with a decline in gastrointestinal function and a change in gut microbiota composition [165–167], and AHN is known to be vulnerable to gut-microbiota mediated changes [168, 169]. Indeed, studies have shown that germ-free (GF) Swiss Webster mice display increased AHN [170], while alterations in the gut microbiota composition with antibiotic cocktails negatively impacts AHN in rats and mice [171–173]. Dysbiosis, which is an imbalance of the gut microbiota composition, can lead to increased systemic inflammation and the release of pro-inflammatory cytokines into the bloodstream, which can, in turn, affect AHN [174]. Variations in the gut microbiota composition impacts the brain through various pathways, including the vagus nerve (which can influence AHN [175, 176]) as well as the secretion of hormones [177], neurotransmitters [178] and microbial metabolites like short-chain fatty acid [179] into the systemic circulation. Thus, understanding the intricate relationship between the gut microbiota and age-related reductions in AHN holds promising therapeutic potential for mitigating cognitive decline in ageing populations. In the following paragraphs, we consolidate current knowledge regarding alterations in the gut microbiota composition that occur with ageing, with a particular emphasis on the middle age stage of life. Furthermore, we provide a comprehensive overview of the latest insights into how various forms of physical activity impact the gut microbiota composition in rodents across middle-aged and older-aged populations.
Age-related changes in gut microbiota
Ageing is associated with several changes in physiological function including inflammation, sarcopenia and alterations in the composition of the gut microbiome. Several contributing factors to these age-related alterations in gut microbiota include reduced dietary fibre intake, prolonged residence in care facilities, sedentary behaviour, the use of non-steroidal anti-inflammatory drugs, and antibiotic usage [180]. Middle age in particular is a critical period during which individuals are more sensitive to the impact of environmental and lifestyle factors, and is associated with weight gain, a decline in physical activity, and increased risk of cardiovascular disease and diabetes [181]. These lifestyle changes, concomitant with the emergence of cardio-metabolic diseases, have an impact on the gut microbiota.
One notable effect of ageing on the gut microbiota is a decrease in microbial diversity, characterized by a reduction in the abundance and variety of bacterial species. This decline in diversity is often referred to as dysbiosis, as it can result in an imbalance between beneficial and potentially harmful microbes. Several investigations have revealed that alterations in the gut microbiota composition due to ageing in humans are characterized by a reduction in species diversity, heightened inter-individual variations, elevated levels of Proteobacteria, and diminished populations of beneficial bacteria like Bifidobacterium. These shifts in microbial composition have been linked to an increased vulnerability to pathogen infections and disruptions in the integrity of the gut mucosal barrier [183, 184]. In centenarians, a decrease in short chain fatty-acid (such as butyrate) producing bacteria and an increase in Proteobacteria leads to an increased production of pro-inflammatory cytokines (IL-6 and IL-8) [185], which may underlie the increased low-grade inflammation observed in ageing known as ‘inflammaging’ [186].
In rodents, few studies have investigated the effect of ageing on the gut microbiota. It has been shown that the Firmicutes/Bacteroidetes ratio is altered in 17-month old mice [187], similar to that reported in older aged humans [182], which is indicative of age-related gut-dysbiosis [188]. One study showed that middle age is a vulnerable period for gut microbiota compositional and metabolite changes, coupled with neuroinflammation in 10-month-old mice [189]. In terms of the relevance of the gut microbiome in healthy ageing, a recent study showed that transferring the gut microbiota via faecal microbial transplant (FMT) from young mice (3–4 months) into old mice (19–20 months) attenuated age-associated impairments in cognitive behaviours as well as changes in the hippocampal metabolome and transcriptome, but not hippocampal neurogenesis of aged recipient mice [190]. Interestingly, in a reverse experiment in which gut microbiota was transplanted from older aged mice (24 months) into young (3 months) recipient mice via FMT, a decrease in spatial learning and memory and a differential expression of proteins involved in synaptic plasticity and neurotransmission was observed [191]. A similar study was performed in rats, in which the authors transferred gut microbiota from older aged rats (20–24 months) into young recipients (3 months old rats) and observed a decrease in working memory concurrent with a decrease in BDNF in the prefrontal cortex and hippocampus. The authors compared the gut microbiota composition between the 3-month-old and 20–24 month old rats and showed a decrease in diversity induced by ageing as well as an increase in the Firmicutes/Bacteroidetes ratio [192] suggesting a gut dysbiosis. Further research is needed to reveal the role of the aged gut microbiome in AHN and the related cognitive ability of pattern separation. Moreover, very few studies have investigated the gut microbiome composition in middle age, although it could represent a critical time window when therapeutic strategies may help to prevent cognitive decline. Nevertheless, studies to date show that age-related changes in gut microbiome lead to behavioural deficits, and thus suggest that identifying ways of modifying the composition of gut microbiota through approaches such as exercise may offer promise to maintain cognitive ability.
Effects of physical exercise on gut microbiota in older-aged rodents
A growing body of evidence points to the fact that exercise can change gut microbial composition [34] and related metabolite profile, and that those changes confer benefits on health and/or age-related disease in humans [193]. In aged animals, the effect of life-long exercise in mice (from 5 weeks old to 26 months old) revealed that voluntary wheel running reduced the relative abundance of the anaerobic bacteria Lachnospiraceae and Ruminococcaceae compared with sedentary counterparts. Interestingly, the authors compared this microbiome signature of aged running mice with the microbiome of a rat strain of high-capacity running and showed that the microbiome signature was similar between rodents with inherited or acquired aerobic training performance [194]. Moreover, long term endurance training (on a treadmill) between 4 to 11 months old in a mouse model of ageing (PolgAmut/mut mice) attenuated the decrease in bacterial diversity observed in sedentary older-aged mice [195].
In middle age (12 month) mice, high intensity interval training (on treadmill) intervention could dynamically alter gut microbiota profiles [196]. When compared to their sedentary counterparts, 11-month-old rats exposed to voluntary wheel running for 6 weeks, displayed an increase in the relative abundance of Bifidobacteria, and a concomitant increase in intestinal Akt, which is known to regulate peripheral glucose uptake and insulin sensitivity. The authors also showed increased performance in working memory in the exercising group [197]. It is important to note, however, that a consensus still needs to be reached about the beneficial effect of increasing or decreasing the bacterial diversity on health, regardless of age [198–200]. In addition, most of the cited studies did not analyse cognitive functions of middle- and older-aged rodents, although this could provide important insights on whether the exercise-driven changes in the gut microbiome may mediate a delay in the onset of age-related cognitive decline.
BEST PRACTICE IN PLANNING PRECLINICAL EXPERIMENTS WITH PHYSICAL EXERCISE
Select the animal: strain, age and biological sex
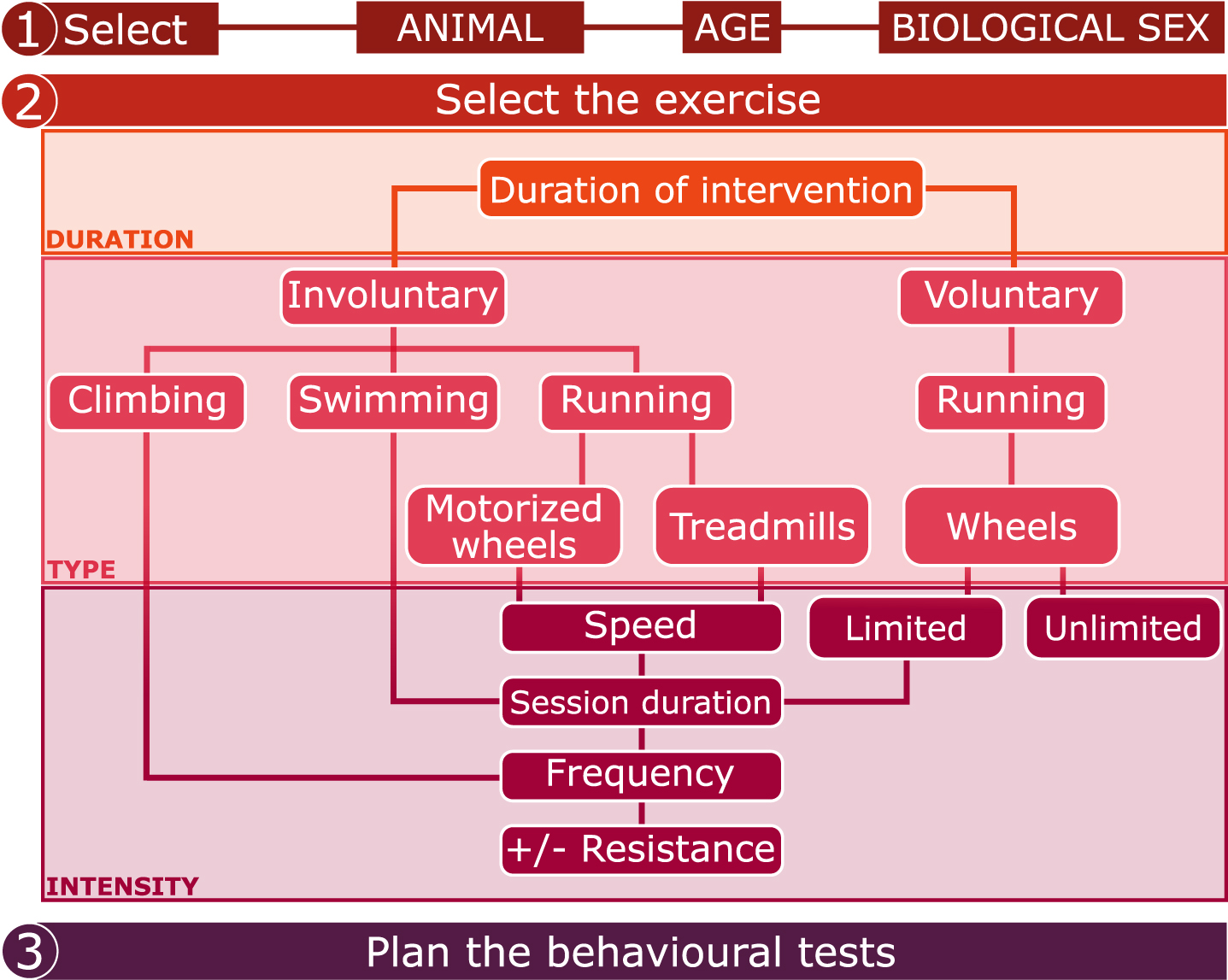
The first step in planning an animal study is selecting the animal (or animal model), its age and biological sex (Fig. 4), which is dependent on the scientific question. It is important to note that there are strain-dependent differences in response to exercise training in mice. For example, when tested for endurance performance after four weeks of treadmill training, FVB/NJ mice were reported to run for longer distances compared with C57BL/6J and Balb/c mice, indicative of a greater response to training [201]. In addition, mouse models with strong motivation for engaging in voluntary wheel running have been developed [202]. Interestingly, strain-specific differences in the AHN response to physical exercise have been reported. For example, analysis of 9 different mouse strains (C57BL/6, BALB/c, A/J, C3 H/HeL, DBA/1, DBA/2, I29/SvJ, and FVB) revealed that DBA/2 mice showed the lowest levels of basal neurogenesis and that forced treadmill exercise increased AHN in all the strains except from DBA/2 [203]. In addition, voluntary running activity has differential effects on neurotrophic factor levels (involved in the stimulation of AHN) in the hippocampus of Sprague-Dawley, Brown Norway, Dark Agouti and PVG rats [204]. Given that strain-derived discrepancies have been shown in a variety of cognitive tasks in rats, the behavioural phenotype intended to be examined should be taken into account when choosing rodent strain. For example, comparison between Sprague-Dawley (SD), Wistar (W) and Long-Evans (LE) rats revealed that SD rats are poor learners in the radial-arm-maze task, indicative of a lower spatial working and reference memory [205]. Thus, using SD rats, might introduce floor effects in the assessment of spatial working and reference memory. Moreover, Wistar rats showed lower visual discrimination learning ability and long-term memory (tested in a touchscreen operant chamber) compared to Long Evans, suggesting that pigmented rats (such as Long Evans) may have higher cognitive abilities compared to albino (such as Wistar) rats [206]. Another plausible explanation for the results obtained in the above-mentioned study is that Long Evans rats have greater visual acuity compared to albino rats [207]. This factor is crucial given that many cognitive tests are based on visual stimuli. Evidence suggests that visual abilities are strain-dependent in mice also [208].
Fig. 4
Checklist for planning preclinical experiments involving physical exercise.
A further choice in the experimental planning is the age of the rodent. Older-aged rodents are reported to voluntarily run less compared to young adults, as evident by distance run on wheels in 24 hours [51, 52]. This difference may be associated with physiological changes related to age, which impact on the adaptation of the rodents’ body to the specific exercise regimen. Indeed, a study reported that a low-moderate intensity (maximum oxygen consumption of 60–70%) was obtained with 60 min/day at 12 m/min with 5° treadmill slope at 8 months of age, but a different combination of duration, speed and slope (30 min/day at 4–6 m/min with 0° slope) was necessary at 18 months of age in order to maintain the same intensity [63]. Thus, the exercise intervention should be adapted to age.
Another crucial factor for experimental planning of exercise studies is biological sex.
Female rodents voluntarily run more compared to age-matched males [47, 48] and this may be dependent on estrogen concentrations [49, 50]. Thus, it is important to consider the sex-dependent variability in exercise levels when planning mixed sex studies. Studies in humans report that exercise induces greater cognitive benefits in females than in males [209], but induces an increase in hippocampal volume in males but not in females [210]. These reports suggest that there may be contradictory mechanisms underlying exercise-induced cognitive changes, which are dependent on biological sex, and support the consideration of biological sex in exercise studies. In rodents, physical exercise also induces differential effects on cognitive functions in males versus females. For example, treadmill exercise improved pattern separation abilities in male, but not female, middle-aged (11–14 months-old) Sprague-Dawley rats in a model of middle cerebral artery occlusion [71]. However, in the above-mentioned study the intensity of the treadmill intervention was established for males, and then applied to females, suggesting that the treadmill exercise protocol should perhaps be optimised for both male and female rodents. Given that the response to physical activity in males and females may differ due to sex-specific physiology, more comparative studies or studies involving mixed-sex population are needed to investigate sex-derived differences in exercise-induced effects on cognition and hippocampal plasticity and thus to determine optimal exercise regimens for female rodents. A knowledge of the sex-dependent response to exercise in preclinical research will ultimately help to inform the effects of exercise in men and women, and in the development of personalized therapeutic interventions for hippocampal-mediated disease.
Select the exercise regimen
Exercise regimens of different duration, type and intensity are used in preclinical research (summarized in Fig. 4). The exercise regimen should be adapted to the animal, age and sex. Regarding the duration of the exercise intervention, involuntary treadmill training lasting just 7 or 10 days has been shown to improve cognitive functions [71, 211, 212], and similar results were obtained with involuntary treadmill running lasting for several weeks or months [62, 213, 214] in male rodents. These results suggest that both short and long treadmill training interventions can induce beneficial effects on cognition. Beneficial effects of voluntary exercise on cognition have been observed after at least 4 weeks in male and female rodents [103, 124], but very short interventions (i.e. days) of voluntary exercise have rarely been shown to improve cognitive functions. To understand how the different durations of exercise affects hippocampal-related behaviours, it is important to investigate the underlying changes in AHN (Box 4: “Possible factors that confound the effects of physical exercise on AHN”). While it is well known that exercise lasting for weeks or months enhances the levels of AHN in rodents, as described above, few studies have reported the effects of shorter training durations on AHN. An interesting study was designed to investigate the effects of short durations (1, 3, 5, 7, 14 and 28 days) of voluntary wheel running on the expression of hippocampal BDNF in 2-month-old male Sprague-Dawley rats [215]. BDNF was found to be significantly higher in the runners compared to the sedentary controls but only after 28 days of training [215]. Nevertheless, more recent data show that 10 days of voluntary running enhanced the cell proliferation in the DG of the hippocampus in female C57BL/6 mice compared to sedentary animals [54]. In addition, 3 days of voluntary wheel running induced long-term morphological maturation and enhanced synaptic plasticity in hippocampal new-born granule neurons (labelled with GFP-expressing retrovirus) in adult male Sprague-Dawley rats compared to sedentary animals [216]. Different durations (1, 3, 7, 14, 21, 28 days) of treadmill exercise enhanced the proliferation of hippocampal cells at all time points compared to sedentary conditions, despite inducing the highest effect between 3 and 21 days of exercise in 5-week-old male Sprague-Dawley rats [217]. Based on a review of the effects of one week and one month of voluntary running on hippocampal plasticity in young adult male mice [218], it has been suggested that one week of voluntary exercise is sufficient to increase the number of amplifying neuroprogenitors in the hippocampus and to modify the synaptic contacts of 1-week old immature granule cell. On the other hand, long-term exercise acts through diverse mechanisms, such as the release of grow factors (such as BDNF) or the modulation of new neuronal networks. In conclusion, the duration of the exercise intervention should be carefully evaluated depending on the readouts of interest, given that diverse durations of physical exercise exert different changes on hippocampal plasticity and consequently on hippocampal-dependent cognitive functions.
Given that the type and intensity of exercise can exert diverse effects on behaviours and AHN, the choice of exercise should carefully reflect these effects when considering experimental outcomes. For example, both voluntary and involuntary exercise have been found to improve spatial learning in the MWM in older-aged rodents. However, studies have also reported no effects of both voluntary and involuntary exercise on spatial learning in the MWM in male and female older-aged rats [60, 97]. Discrepancies in the exercise-induced changes in spatial learning may arise from the intensity of exercise. For instance, an extremely high intensity of a treadmill exercise protocol (30 m/min) [97] induced no change in learning abilities compared to that reported in studies where enhanced learning abilities through similar treadmill interventions were observed but at lower intensities [66, 69, 93, 101]. The absence of an exercise-related improvement of learning abilities in aged rats may also be linked to the inclusion of resistance in the exercise regimen, as demonstrated by studies where forced swim exercise with resistance showed no impact on spatial learning [60]. Because there is a dearth of studies investigating the effects of different types and intensities of physical exercise on spatial reference and working memory, recognition memory and pattern separation in older aged rodents, as well as a lack of direct comparisons amongst exercise protocols on the proliferation, maturation and survival of new neurons, it is difficult to draw firm conclusions about the optimal physical exercise protocol to induce the most robust effects on cognitive function and AHN (Box 4: “Possible factors that confound the effects of physical exercise on AHN). For example, studies in young adult male rodents reported that treadmill exercise at a lower intensity exerts a greater increase in the number of hippocampal newly born cell and neurons when compared to moderate or high intensity treadmill training [15, 217, 219]. Thus, further investigation is needed to determine whether low intensity treadmill training could induce the greatest effects on AHN in middle- and older-aged rodents when compared to moderate or high intensity training.
Plan the behavioural tests
The final step concerns the choice of behavioural test (Fig. 4). Careful planning of the behavioural tests is required when using involuntary exercise as well as voluntary running with limited access to wheels. To mitigate against any potential fatigue or acute effects of exercise on a behavioural outcome, a major consideration is the alignment of the behavioural tests in terms of time and duration relative to the exercise protocol, especially when a battery of behavioural tests is planned. Burghardt and colleagues combined treadmill running with multiple behavioural tests (elevated-plus-maze, open-field test, social interaction, conditioned freezing) and performed behavioural testing weekly, 72 hours following the last exercise session [52]. This resulted in a washout period to mitigate against any potential acute effects of each training session. This however may lead to different behavioural outcomes compared to results from studies where the cognitive tests are performed soon after the exercise. Another approach is the performance of the behavioural tests (Y-maze, open field test, elevated-plus maze, Morris Water Maze) immediately preceding the exercise session [224], but excessive fatigue or stress due to the multiple interactions of the rodents with the experimenters may result. Finally, the fact that forced exercise may induce stress to rodents should also be considered when planning anxiety-related or stress-related behavioural tasks as it may confound behavioural outcomes.
CONCLUSIONS
Physical exercise has been identified as a non-pharmacological approach to prevent age-related neurodegeneration and dementia. Pre-clinical research is necessary to examine exercise-induced brain and behavioural effects, which would not be feasible in humans. A variety of exercise types are used in preclinical research, including voluntary and involuntary regimens. The most common voluntary exercise intervention is running on saucer-like horizontal wheels or vertical steel wheels while involuntary running regimens require rodents to run for a predetermined duration, intensity and frequency in specialized treadmills. Additionally, other involuntary exercise regimens developed for rodents are swimming and resistance exercise, although those are less commonly used. The use of involuntary treadmill running has the advantage of minimizing inter-individual variability in response to exercise, otherwise exhibited by rats of different sex or age. However, the use of electric shock to promote running may trigger a stress response and act as a possible confounder to the effects of exercise. Similarly, food motivation to run voluntarily on wheels represents another possible confounder due to activation of the dopaminergic system. Different types, intensities and duration of exercise exert various effects on cognitive abilities in older-aged rodents. Both voluntary and involuntary running improve spatial learning abilities in older-aged rodents, but not if applied at high intensity or in the presence of a resistance. Long term voluntary and moderate intensity treadmill exercise improve reference memory in older age, while low and high, but not moderate, intensity improves spatial working memory in older-aged rodents. In addition, forced treadmill and swimming exercise have been shown to ameliorate recognition memory in older-aged rodents, but the potential effect of voluntary wheel running is yet to be determined. Evidence suggests that voluntary running improves pattern separation in older-aged rodents, whereas no reports were found on the effects of treadmill training or other types of forced exercise on pattern separation in older-aged rodents. It should be noted that if the choice of exercise regimen is limited, animal strain, age and sex may influence behavioural outcomes. Voluntary as well as forced treadmill exercise during older age stimulate the proliferation, maturation and survival stages of AHN, an important central mechanism mediating the effects of exercise on cognitive function. Currently, the lack of comparisons between exercise protocols on AHN makes it difficult to draw firm conclusions about the optimal physical exercise regimen for AHN-mediated cognitive changes in older-aged rodents. The gut microbiota has been identified as a key mediator of exercise-driven changes in cognitive function in older-aged rodents, possibly through the modulation of AHN. Further research is needed to clarify the mechanisms underlying the exercise-induced changes in the aged gut microbiome and consequently an optimal exercise regimen to stimulate those changes. Recent research suggests that age-related cognitive decline arises during middle age, together with changes in AHN and the gut microbiota composition. Despite middle age representing a window for strategies aimed at delaying a potential decline in cognition, there is a dearth of studies investigating the effects of different exercise regimens during middle age. Future research investigating the impact of exercise during middle age may uncover novel mechanisms and predictors of cognitive decline, which are modifiable by exercise. Strategies to optimise exercise regimes in preclinical research will inform clinical approaches to prevent and delay age-related dementia, one of the most significant global concerns in current society.
ACKNOWLEDGMENTS
This work was supported by Science Foundation Ireland (SFI) under Grant Number SFI/FFP/6820. P.J.L. is supported by Alzheimer Nederland and the Zon-MW Memorabel program MODEM. J.D.M. and P.J.L. are supported by the Center for Urban Mental Health of the University of Amsterdam.
DECLARATION OF COMPETING INTEREST
None.
REFERENCES
[1] | United Nations, Department of Economic and Social Affairs PD (2020).World Population Ageing 2019 [Internet]. World Population Ageing. 2019. Available from:https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf |
[2] | Nichols E , Steinmetz JD , Vollset SE , Fukutaki K , Chalek J , Abd-Allah F , et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Heal. (2022) ;7: (2):e105–25. |
[3] | Marlatt MW , Potter MC , Lucassen PJ , van Praag H Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice (2013) ;72: (6):943–52. |
[4] | Van Praag H , Shubert T , Zhao C , Gage FH Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. (2005) ;25: (38):8680–5. |
[5] | Speisman RB Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. 2014. pp. 25-43. |
[6] | Duzel E , Van Praag H , Sendtner M Can physical exercise in old age improve memory and hippocampal function? Brain (2016) ;139: (3):662–73. |
[7] | Hayes SM , Alosco ML , Hayes JP , Cadden M , Peterson M , Allsup K , et al. Physical Activity Is Positively Associated with Episodic Memory in Aging. Aging (Albany NY). (2016) ;21: (10):780–90. |
[8] | Gaitán JM , Moon HY , Stremlau M , Dubal DB , Cook DB , Okonkwo OC , et al. Effects of Aerobic Exercise Training onSystemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front Endocrinol(Lausanne). (2021) ;12: , 1–18. |
[9] | Broadbent NJ , Gaskin S , Squire LR , Clark RE Object recognition memory and the rodent hippocampus. Learn Mem. (2010) ;17: (1):794–800. |
[10] | Cohen SJ , Stackman RW Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res [Internet] (2015) ;285: 105–17Available from:http://dx.doi.org/10.1016/j.bbr.2014.08.002. |
[11] | Erickson KI , Voss MW , Prakash RS , Basak C , Szabo A , Chaddock L , et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. (2011) ;108: (7):3017–22. |
[12] | Kozareva DA , Cryan JF , Nolan YM Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell. (2019) ;18: (5):2016–20. |
[13] | Moreno-Jiménez EP , Flor-García M , Terreros-Roncal J , Rábano A , Cafini F , Pallas-Bazarra N , et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients withAlzheimer’s disease. Nat Med [Internet]. (2019) ;25: (4)554–60Available from:http://dx.doi.org/10.1038/s41591-019-0375-9. |
[14] | Salta E , Lazarov O , Fitzsimons CP , Tanzi R , Lucassen PJ , Choi SH Adult hippocampal neurogenesis in Alzheimer’s disease: A roadmap to clinical relevance. Cell Stem Cell [Internet]. (2023) ;30: (2)120–36Available from:https://doi.org/10.1016/j.stem.2023.01.002. |
[15] | Lou jie S , Liu yan J , Chang H , Chen jie P Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. (2008) ;1210: 48–55. |
[16] | Rhodes JS , Jeffrey S , Girard I , Mitchell GS , Van Praag H , Garland T , et al. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. (2003) ;117: (5):1006–16. |
[17] | Shors TJ , Miesegaes G , Beylin A , Zhao M , Rydel T , Gould E Neurogenesis in the adult is involved in the formation of trace memories. Nature. (2001) ;414: (6866)938–938. |
[18] | Deng W , Saxe MD , Gallina IS , Gage FH Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. (2009) ;29: (43):13532–42. |
[19] | Clelland ACD , Choi M , Romberg C , Jr GDC , Fragniere A , Jessberger S , et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. (2009) ;325: (5937)210–3. |
[20] | Sahay A , Scobie KN , Hill AS , O’Carroll CM , Kheirbek MA , Burghardt NS , et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. October. (2011) ;472: (7344)466–70. |
[21] | Boldrini M , Hen R , Underwood MD , Rosoklija GB , Dwork AJ , Mann JJ , et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry [Internet]. (2012) ;72: (7)562–71Available from:http://dx.doi.org/10.1016/j.biopsych.2012.04.024. |
[22] | Dupret D , Revest JM , Koehl M , Ichas F , De Giorgi F , Costet P , et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. (2008) ;3: (4). |
[23] | Malberg JE , Eisch AJ , Nestler EJ , Duman RS Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. (2000) ;20: (24):9104–10. |
[24] | Akers KG , Martinez-Canabal A , Restivo L , Yiu AP , De Cristofaro A , Hsiang HL , et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science (80-). (2014) ;344: (6184)598–602. |
[25] | Kitamura T , Saitoh Y , Takashima N , Murayama A , Niibori Y , Ageta H , et al. Adult Neurogenesis Modulates the Hippocampus-Dependent Period of Associative Fear Memory. Cell [Internet]. (2009) ;139: (4)814–27Available from:http://dx.doi.org/10.1016/j.cell.2009.10.020. |
[26] | Frankland PW , Köhler S , Josselyn SA Hippocampal neurogenesis and forgetting. Trends Neurosci. (2013) ;36: (9):497–503. |
[27] | Vints WAJ , Levin O , Fujiyama H , Verbunt J , Masiulis N Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front Neuroendocrinol [Internet].93.. (2022) ;66: (March):100993. Available from:https://doi.org/10.1016/j.yfrne.2022.100993. |
[28] | Di Benedetto S , Müller L , Wenger E , Düzel S , Pawelec G Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev [Internet]. (2017) ;75: 114–28Available from:http://dx.doi.org/10.1016/j.neubiorev.2017.01.044. |
[29] | Nay K , Smiles WJ , Kaiser J , McAloon LM , Loh K , Galic S , et al. Molecular mechanisms underlying the beneficial effects of exercise on brain function and neurological disorders. Int J Mol Sci. (2021) ;22: (8). |
[30] | Yau SY , Li A , Xu A , So KF Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: An alternative anti-depressive treatment? Neural Regen Res (2015) ;10: (1):7–9. |
[31] | Gleeson M , Bishop NC , Stensel DJ , Lindley MR , Mastana SS , Nimmo MA The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol [Internet]. (2011) ;11: (9):607–10Available from:http://dx.doi.org/10.1038/nri3041. |
[32] | Walsh JJ , Tschakovsky ME Exercise and circulating bdnf: Mechanisms of release and implications for the design of exercise interventions. Appl Physiol Nutr Metab. (2018) ;43: (11):1095–104. |
[33] | Grabrucker S , Marizzoni M , Silajdžić E , Lopizzo N , Mombelli E , Nicolas S , et al. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain [Internet]. 2023; Available from:http://www.ncbi.nlm.nih.gov/pubmed/37849234 |
[34] | Mitchell CM , Davy BM , Hulver MW , Neilson AP , Bennett BJ , Davy KP Does Exercise Alter Gut Microbial Composition? A Systematic Review. Medicine and Science in Sports and Exercise. (2019) ;51: , 160–7. |
[35] | Sarubbo F , Cavallucci V , Pani G The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells. (2022) ;11: (3). |
[36] | Leung K , Thuret S Gut microbiota: A modulator of brain plasticity and cognitive function in ageing. Healthc. (2015) ;3: (4):898–916. |
[37] | Grégoire CA , Bonenfant D , Le Nguyen A , Aumont A , Fernandes KJL Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis. PLoS One. (2014) ;9: (1). |
[38] | Yau SY , Lau BWM , Zhang ED , Lee JCD , Li A , Lee TMC , et al. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience. (2012) ;222: , 289–301. |
[39] | Gibbons TE , Pence BD , Petr G , Ossyra JM , Mach HC , Bhattacharya TK , et al. Voluntary wheel running, but not a dietcontaining (-)-epigallocatechin-3-gallate and β-alanine, improves learning, memory and hippocampalneurogenesis in aged mice. Behav Brain Res [Internet]. (2014) ;272: 131–40Available from:http://dx.doi.org/10.1016/j.bbr.2014.05.049. |
[40] | Choi SH , Bylykbashi E , Chatila ZK , Lee SW , Pulli B , Clemenson GD , et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science (80-). (2018) ;361: (6406). |
[41] | Shen K , Liu X , Chen D , Chang J , Zhang Y , Kou X Voluntary wheel-running exercise attenuates brain aging of rats through activating miR-130a-mediated autophagy. Brain Res Bull [Internet]. (2020) ;172: (April 2020):203–11Available from:https://doi.org/10.1016/j.brainresbull.2021.04.027. |
[42] | Bednarczyk MR , Hacker LC , Fortin-Nunez S , Aumont A , Bergeron R , Fernandes KJL Distinct stages of adult hippocampal neurogenesis are regulated by running and the running environment. Hippocampus. (2011) ;21: (12):1334–47. |
[43] | Nguemeni C , McDonald MW , Jeffers MS , Livingston-Thomas J , Lagace D , Corbett D Short- and Long-term Exposure to Low and High Dose Running Produce Differential Effects on Hippocampal Neurogenesis. Neuroscience [Internet]. (2018) ;369: 202–11Available from:https://doi.org/10.1016/j.neuroscience.2017.11.026. |
[44] | Eikelboom R , Mills R A microanalysis of wheel running in male and female rats. Physiol Behav. (1988) ;43: (5):625–30. |
[45] | Greenwood BN , Fleshner M Voluntary Wheel Running: A Useful Rodent Model for Investigating the Mechanisms of Stress Robustness and Neural Circuits of Exercise Motivation Benjamin. Physiol Behav. (2016) ;176: (1):139–48. |
[46] | Pan-Vazquez A , Rye N , Ameri M , McSparron B , Smallwood G , Bickerdyke J , et al. Impact of voluntary exercise and housing conditions on hippocampal glucocorticoid receptor, miR-124 and anxiety. Mol Brain [Internet]. (2015) ;8: (1):1–13Available from:http://dx.doi.org/10.1186/s13041-015-0128-8. |
[47] | Jaehne EJ , Kent JN , Lam N , Schonfeld L , Spiers JG , Begni V , et al. Chronic running-wheel exercise from adolescence leads to increased anxiety and depression-like phenotypes in adulthood in rats: Effects on stress markers and interaction with BDNF Val66Met genotype. Dev Psychobiol. (2023) ;65: (1):1–13. |
[48] | Liang chu N Sex-Dependent Wheel Running Effects on High Fat Diet Preference, Metabolic Outcomes, and Performance on the Barnes Maze in Rats. 2020; |
[49] | Berchtold NC , Kesslak JP , Pike CJ , Adlard PA , Cotman CW Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. (2001) ;14: (12):1992–2002. |
[50] | Mathis V , Points L , Pope B , Lee CMJ , Mohamed M , Rhodes JS , et al.Estrogen-mediated individual differences in female rat voluntary running behavior. J Appl Physiol. 2024; |
[51] | Garvey SM , Russ DW , Skelding MB , Dugle JE , Edens NK Molecular and metabolomic effects of voluntary running wheel activity on skeletal muscle in late middle-aged rats. Physiol Re. (2015) ;3: (2):1–17. |
[52] | Burghardt PR , Fulk LJ , Hand GA , Wilson MA The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. (2004) ;1019: (1–2):84–96. |
[53] | Kobilo T , Liu QR , Gandhi K , Mughal M , Shaham Y , van Praag H Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. (2011) ;18: (9):605–9. |
[54] | Kannagara TS , Lucero MJ , Gil-Mohapel J , Drapala RJ , Simpson JM , Christie BR , et al. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging [Internet]. (2011) ;14: (4):535–62Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958625/pdf/nihms960157.pdf. |
[55] | Mul JD , Soto M , Cahill ME , Ryan RE , Takahashi H , So K , et al. Voluntary wheel running promotes resilience tochronic social defeat stress in mice: a role for nucleus accumbens Δ FosB. Neuropsychopharmacology[Internet]. (2018) ;(February)Available from:http://dx.doi.org/10.1038/s41386-018-0103-z. |
[56] | Lloyd BA , Hake HS , Ishiwata T , Farmer CE , Loetz EC , Fleshner M , et al. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav Brain Res. (2017) ;176: (1):139–48. |
[57] | Zhang L , Fan Y , Kong X , Hao W Neuroprotective effect of different physical exercises on cognition and behavior function by dopamine and 5-HT level in rats of vascular dementia. Behav Brain Res [Internet]. (2020) ;388: (April):112648Available from:https://doi.org/10.1016/j.bbr.2020.112648 |
[58] | Leasure JL , Jones M Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. (2008) ;156: (3):456–65. |
[59] | Herrera JJ , Fedynska S , Ghasem PR , Wieman T , Clark PJ , Gray N , et al. Independent of Exercise Controllability (2017) ;43: (9):1190–202. |
[60] | Belviranlı M , Okudan N Differential effects of voluntary and forced exercise trainings on spatial learning ability and hippocampal biomarkers in aged female rats. Neurosci Lett [Internet]. (2022) ;773: (February):136499Available from:https://doi.org/10.1016/j.neulet.2022.136499. |
[61] | Maryam K , Ali H Physiology & Behavior Aerobic and resistance exercises affect the BDNF / TrkB signaling pathway, and hippocampal neuron density of high-fat diet-induced obese elderly rats. Physiol Behav [Internet]. (2023) ;264: (November 2022):114140Available from:https://doi.org/10.1016/j.physbeh.2023.114140. |
[62] | Kim SE , Ko IG , Kim BK , Shin MS , Cho S , Kim CJ , et al. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol [Internet]. (2010) ;45: (5):357–65Available from:http://dx.doi.org/10.1016/j.exger.2010.02.005. |
[63] | Pietrelli A , Matković L , Vacotto M , Lopez-Costa JJ , Basso N , Brusco A Aerobic exercise upregulates theBDNF-Serotonin systems and improves the cognitive function in rats. Neurobiol Learn Mem. (2018) ;155: , 528–42. |
[64] | Høydal MA , Wisløff U , Kemi OJ , Ellingsen Ø Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. Eur J Prev Cardiol. (2007) ;14: (6):753–60. |
[65] | Lambert MI , Noakes TD Spontaneous running increases VO2max and running performance in rats. Am Phsiological Soc. 1990;. |
[66] | Aguiar AS , Castro AA , Moreira EL , Glaser V , Santos ARS , Tasca CI , et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev [Internet]. (2011) ;132: (11-12):560–7Available from:http://dx.doi.org/10.1016/j.mad.2011.09.005. |
[67] | Zhang J , Gao Q , Gao J , Lv L , Liu R , Wu Y , et al.brain sciences Moderate-Intensity Intermittent Training Alters the DNA Methylation Pattern of PDE4D Gene in Hippocampus to Improve the Ability of Spatial Learning and Memory in Aging Rats Reduced by D-Galactose. 2023; |
[68] | Okamoto M , Mizuuchi D , Omura K , Lee M , Oharazawa A , Yook JS , et al. High-intensity Intermittent Training Enhances Spatial Memory and Hippocampal Neurogenesis Associated with BDNF Signaling in Rats. Cereb Cortex. (2021) ;31: (9):4386–97. |
[69] | Chen L , Chao FL , Lu W , Zhang L , Huang CX , Yang S , et al. Long-Term Running Exercise Delays Age-Related Changes in White Matter in Rats. Front Aging Neurosci. (2020) ;12: , 1–15. |
[70] | Gomes S , Santos P , Simões R , Mortara RA , Scorza FA , Cavalheiro EA , et al. Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflammation. (2013) ;10: (61):2–7. |
[71] | Cohan CH , Youbi M , Saul I , Ruiz AA , Furones CC , Patel P , et al. Sex-Dependent Differences in Physical Exercise-Mediated Cognitive Recovery Following Middle Cerebral Artery Occlusion in Aged Rats. Front Aging Neurosci. (2019) ;11: , 1–10. |
[72] | Khataei T , Romig-Martin SA , Harding AMS , Radley JJ , Benson CJ Comparison of murine behavioural and physiological responses after forced exercise by electrical shock versus manual prodding. Exp Physiol. (2021) ;106: (4):812–9. |
[73] | Fediuc S , Campbell JE , Riddell MC Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol. (2006) ;100: (6):1867–75. |
[74] | Höglinger GU , Rizk P , Muriel MP , Duyckaerts C , Oertel WH , Caille I , et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. (2004) ;7: (7):726–35. |
[75] | Gobatto CA , De Mello MAR , Sibuya CY , De Azevedo JRM , Dos Santos LA , Kokubun E Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol - A Mol Integr Physiol. (2001) ;130: (1):21–7. |
[76] | Cheng M , Cong J , Wu Y , Xie J , Wang S , Zhao Y , et al. Chronic Swimming Exercise Ameliorates Low-Soybean-Oil Diet-Induced Spatial Memory Impairment by Enhancing BDNF-Mediated Synaptic Potentiation in Developing Spontaneously Hypertensive Rats. Neurochem Res [Internet]. (2018) ;43: (5):1047–57Available from:http://dx.doi.org/10.1007/s11064-018-2515-x. |
[77] | Cechella JL , Leite MR , Gai RM , Zeni G The impact of a diphenyl diselenide-supplemented diet and aerobic exercise on memory of middle-aged rats. Physiol Behav [Internet]. (2014) ;135: 125–9Available from:http://dx.doi.org/10.1016/j.physbeh.2014.06.006. |
[78] | Amirazodi F , Mehrabi A , Amirazodi M , Parsania S , Rajizadeh MA , Esmaeilpour K The Combination Effects of Resveratrol and Swimming HIIT Exercise on Novel Object Recognition and Open-field Tasks in Aged Rats. Exp Aging Res [Internet]. (2020) ;46: (4):336–58Available from:https://doi.org/10.1080/0361073X.2020.1754015. |
[79] | Teixeira PSA , Mendes SVD , Leal-Cardoso JH , Ceccatto VM Anaerobic threshold employed on exercise training prescription and performance assessment for laboratory rodents: A short review. Life Sci [Internet]. (2016) ;151: 1–6Available from:http://dx.doi.org/10.1016/j.lfs.2016.02.016. |
[80] | Contarteze RVL , Manchado FDB , Gobatto CA , De Mello MAR Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol - A Mol Integr Physiol. (2008) ;151: (3):415–22. |
[81] | Pengam M , Goanvec C , Moisan C , Simon B , Albacète G , Féray A , et al. Moderate intensity continuous versus high intensity interval training: Metabolic responses of slow and fast skeletal muscles in rat. PLoS One. (2023) ;18: (10 October):1–27. |
[82] | Constans A , Pin-Barre C , Molinari F , Temprado JJ , Brioche T , Pellegrino C , et al. High-intensity interval training is superior to moderate intensity training on aerobic capacity in rats: Impact on hippocampal plasticity markers. Behav Brain Res. (2021) ;398: (July 2020). |
[83] | Hawley JA , Joyner MJ , Green DJ Mimicking exercise: what matters most and where to next? J Physiol (2021) ;599: (3):791–802. |
[84] | Guerrieri D , Moon HY , van Praag H Exercise in a Pill: The Latest on Exercise-Mimetics. Brain Plast. (2017) ;2: (2):153–69. |
[85] | Gubert C , Hannan AJ Exercise mimetics: harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov. (2021) ;20: (11):862–79. |
[86] | Lamberty Y , Gower AJ Age-related changes in spontaneous behavior and learning in NMRI mice from middle to old age. Physiol Behav. (1992) ;51: (1):81–8. |
[87] | Tsai SF , Ku NW , Wang TF , Yang YH , Shih YH , Wu SY , et al. Long-Term Moderate Exercise Rescues Age-Related Decline in Hippocampal Neuronal Complexity and Memory. Gerontology. (2018) ;64: (6):551–61. |
[88] | Dohm-Hansen S , English JA , Lavelle A , Fitzsimons CP , Lucassen PJ , Nolan YM The ‘middle-aging’; brain. Trends Neurosci. (2024) Apr;47: (4):259–272. doi: 10.1016/j.tins.2024.02.00110/05/2024.Epub 2024 Mar 19. PMID: 38508906. |
[89] | Barha CK , Liu-Ambrose T Sex differences in exercise efficacy: Is midlife a critical window for promoting healthy cognitive aging? FASEB J (2020) ;34: (9):11329–36. |
[90] | Erickson K , Hillman C , Stillman C , Ballard R , Bloodgood B , Conroy D , et al. the Physical Activity Guidelines. Med Sci Sport Exerc. (2020) ;51: (6):1242–51. |
[91] | Bensalem J , Servant L , Alfos S , Gaudout D , Layé S Dietary Polyphenol Supplementation Prevents Alterations of Spatial Navigation in Middle-Aged Mice (2016) ;10: (February):1–16. |
[92] | Prenderville JA , Kennedy PJ , Dinan TG , Cryan JF Adding fuel to the fire: The impact of stress on the ageing brain. Trends Neurosci [Internet]. (2015) ;38: (1):13–25Available from:http://dx.doi.org/10.1016/j.tins.2014.11.001. |
[93] | Téglás T , Németh Z , Koller Á , Van der Zee EA , Luiten PGM , Nyakas C Effects of Long-Term ModerateIntensity Exercise on Cognitive Behaviors and Cholinergic Forebrain in the Aging Rat. Neuroscience. (2019) ;411: , 65–75. |
[94] | Erickson KI , Donofry SD , Sewell KR , Brown BM , Stillman CM Cognitive Aging and the Promise of Physical Activity. Annu Rev Clin Psychol. (2022) ;18: , 417–42. |
[95] | Castells-Sánchez A , Roig-Coll F , Dacosta-Aguayo R , Lamonja-Vicente N , Sawicka AK , Torán-Monserrat P , et al. Exercise and Fitness Neuroprotective Effects: Molecular, Brain Volume and Psychological Correlates and TheirMediating Role in Healthy Late-Middle-Aged Women and Men. Front Aging Neurosci. (2021) ;13: (March):1–11. |
[96] | Bao C , He C , Shu B , Meng T , Cai Q , Li B , et al. Aerobic exercise training decreases cognitive impairment caused by demyelination by regulating ROCK signaling pathway in aging mice. Brain Res Bull [Internet]. (2021) ;168: (June 2020):52–62Available from:. |
[97] | Chodari L , Derafshpour L , Jafari A , Ghasemi M , Mehranfard N Exercise may alleviate age-related spatial memoryimpairment by rescuing β -adrenergic receptor dysregulation via both G protein –dependent andβ -arrestin –dependent mechanisms in rat hippocampus. Brain Res [Internet]. (2023) ;1804: (November 2022):148250Available from:https://doi.org/10.1016/j.brainres.2023.148250. |
[98] | Montaron MF , Charrier V , Blin N , Garcia P , Abrous DN Responsiveness of dentate neurons generated throughout adult life is associated with resilience to cognitive aging. Aging Cell. (2020) ;19: (8):1–12. |
[99] | Drapeau E , Mayo W , Aurousseau C , Le Moal M , Piazza PV , Abrous DN Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. (2003) ;100: (SUPPL. 2):14385–90. |
[100] | Shen Y , Zhou M , Cai D , Filho DA , Fernandes G , Cai Y , et al. CCR5 closes the temporal window for memory linking. bioRxiv [Internet]. (2021) ;(March 2021):2021.10.07.463602.Available from:http://biorxiv.org/content/early/2021/10/09/2021.10.07.463602.abstract |
[101] | Albeck DS , Sano K , Prewitt GE , Dalton L Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. (2006) ;168: (2):345–8. |
[102] | Kraeuter katrin A , Guest PC The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice (2019) ;1916: :105–11. |
[103] | Anderson BJ , Rapp DN , Baek DH , McCloskey DP , Coburn-Litvak PS , Robinson JK Exercise influences spatial learning in the radial arm maze. Physiol Behav. (2000) ;70: (5):425–9. |
[104] | El-Domiaty HF , El-Roghy ES , Salem HR Combination of magnesium supplementation with treadmill exercise improves memory deficit in aged rats by enhancing hippocampal neurogenesis and plasticity: a functional and histological study. Appl Physiol Nutr Metab. (2022) ;47: (3):296–308. |
[105] | Lohninger S , Strasser A , Bubna-littitz H The effect of L -carnitine on T-maze learning ability in aged rats (2001) ;32: , 245–53. |
[106] | Peltonen I , Jalkanen AJ , Sinervä V , Puttonen KA , Männistö PT Different effects of scopolamine and inhibition of prolyl oligopeptidase on mnemonic and motility functions of young and 8- to 9-month-old rats in the radial-arm maze. Basic Clin Pharmacol Toxicol. (2010) ;106: (4):280–7. |
[107] | Pietrelli A , Lopez-Costa J , Goñi R , Brusco A , Basso N Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle-aged and old rats. Neuroscience [Internet]. (2012) ;202: :252–66Available from:http://dx.doi.org/10.1016/j.neuroscience.2011.11.054. |
[108] | Paçcesová A , Holubová M , Hrubá L , Strnadová V , Neprašová B , Pelantová H , et al. Age-related metabolic and neurodegenerative changes in SAMP8 mice. aging-us [Internet]. (2022) ;14: (18):7300–27Available from:www.aging-us.com. |
[109] | Morgan JA , Singhal G , Corrigan F , Jaehne EJ , Jawahar MC , Baune BT The effects of aerobic exercise on depression-like, anxiety-like, and cognition-like behaviours over the healthy adult lifespan of C57BL/6 mice. Behav Brain Res [Internet]. (2018) ;337: (September 2017):193–203Available from:http://dx.doi.org/10.1016/j.bbr.2017.09.022. |
[110] | Antunes M , Biala G The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn Process. (2012) ;13: (2):93–110. |
[111] | Canatelli-Mallat M , Chiavellini P , Lehmann M , Goya RG , Morel GR Age-related loss of recognition memory and its correlation with hippocampal and perirhinal cortex changes in female Sprague Dawley rats. Behav Brain Res. (2022) ;435: , 0–1. |
[112] | Diniz DG , Oliveira MA , Lima CM , Fôro CAR , Sosthenes MCK , Bento-Torres J , et al. Age, environment, object recognition and morphological diversity of GFAP-immunolabeled astrocytes. Behav Brain Funct. (2016) ;12: (1):1–19. |
[113] | Wu M V. , Luna VM , Hen R , Running rescues a fear-based contextual discrimination deficit in aged mice. Front Syst Neurosci. (2015) ;9: (AUGUST):1–10. |
[114] | Creer DJ , Romberg C , Saksida LM , Van Praag H , Bussey TJ Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. (2010) ;107: (5):2367–72. |
[115] | Buscher N , van Dorsselaer P , Steckler T , Talpos JC Evaluating aged mice in three touchscreen tests that differ in visual demands: Impaired cognitive function and impaired visual abilities. Behav Brain Res [Internet]. (2017) ;333: :142–9Available from:http://dx.doi.org/10.1016/j.bbr.2017.06.053. |
[116] | Houlton J , Zhou LYY , Barwick D , Gowing EK , Clarkson AN Stroke induces a BDNF-dependent improvement in cognitive flexibility in aged mice. Neural Plast. (2019) ;2019. |
[117] | Smith SM , Zequeira S , Ravi M , Johnson SA , Hampton AM , Ross AM , et al. Age-related impairments on the touchscreen paired associates learning (PAL) task in male rats. Neurobiol Aging [Internet]. (2022) ;109: :176–91Available from:https://doi.org/10.1016/j.neurobiolaging.2021.09.021. |
[118] | Crowley EK , Nolan YM , Sullivan AM Neuroprotective effects of voluntary running on cognitive dysfunction in anα-synuclein rat model of Parkinson’s disease. Neurobiol Aging [Internet]. (2018) ;65: :60–8Available from:https://doi.org/10.1016/j.neurobiolaging.2018.01.011. |
[119] | Epp JR , Botly LCP , Josselyn SA , Frankland PW Voluntary Exercise Increases Neurogenesis and Mediates Forgetting of Complex Paired Associates Memories. Neuroscience. (2021) ;475: , 1–9. |
[120] | Epp JR , Mera RS , Köhler S , Josselyn SA , Frankland PW Neurogenesis-mediated forgetting minimizes proactive interference. Nat Commun. (2016) ;7: , 5–12. |
[121] | Li C , Liu T , Li R , Zhou C Effects of exercise on proactive interference in memory: potential neuroplasticity and neurochemical mechanisms. Psychopharmacology (Berl). (2020) ;237: (7):1917–29. |
[122] | Scott GA , Terstege DJ , Roebuck AJ , Gorzo KA , Vu AP , Howland JG , et al. Adult neurogenesis mediates forgetting of multiple types of memory in the rat. Mol Brain [Internet]. (2021) ;14: (1):1–13Available from:https://doi.org/10.1186/s13041-021-00808-4. |
[123] | Drapeau E , Montaron MF , Aguerre S , Abrous DN Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. (2007) ;27: (22):6037–44. |
[124] | O’Leary JD , Hoban AE , Murphy A , O’Leary OF , Cryan JF , Nolan YM Differential effects of adolescent and adult-initiated exercise on cognition and hippocampal neurogenesis. Hippocampus. (2019) ;29: (4):352–65. |
[125] | Sorrells SF , Paredes MF , Cebrian-silla A , Sandoval K , Qi D , Kevin W , et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nat Publ Gr [Internet]. 555377-81. . (2018) ;555: (7696):377–81Available from:http://dx.doi.org/10.1038/nature25975. |
[126] | Boldrini M , Fulmore CA , Tartt AN , Dwork AJ , Mann JJ , Boldrini M , et al. Short Article Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. (2018) ;589–99. |
[127] | Lucassen PJ , Fitzsimons CP , Salta E , Maletic-Savatic M Adult neurogenesis, human after all (again): Classic, optimized, and future approaches. Behav Brain Res [Internet]. (2020) ;381: (November 2019):112458Available from:https://doi.org/10.1016/j.bbr.2019.112458. |
[128] | Moreno-Jiménez EP , Terreros-Roncal J , Flor-García M , Rábano A , Llorens-Martín M Evidences foradult hippocampal neurogenesis in humans. J Neurosci. (2021) ;41: (12):2541–53. |
[129] | Alvarez-Buylla A , Cebrian-silla A , Sorrells SF , Nascimento MA , Paredes MF , Garcia-verdugo JM , et al. Comment on “Impact of neurodegenerative diseases on human adult hippocampal neurogenesis”. Science (80-). (2022) ;555: (7696):377–81. |
[130] | Terreros-Roncal J , Moreno-Jiménez EP , Flor-García M , Rodríguez-Moreno CB , Trinchero MF , Márquez-Valadez B , et al. Response to Comment on “Impact of neurodegenerative diseases on human adulthippocampal neurogenesis”. Science. (2022) ;376: (6590):eabn7270. |
[131] | Tartt AN , Fulmore CA , Liu Y , Rosoklija GB , Dwork AJ , Arango V , et al. Considerations for Assessing the Extent of Hippocampal Neurogenesis in the Adult and Aging Human Brain. Cell Stem Cell. (2018) ;23: (6):782–3. |
[132] | Gallardo-Caballero M , Rodríguez-Moreno CB , Álvarez-Méndez L , Terreros-Roncal J , Flor-García M , Moreno-Jiménez EP , et al. Prolonged fixation and post-mortem delay impede the study of adult neurogenesis inmice. Commun Biol. (2023) ;6: (1):1–12. |
[133] | Lucassen PJ , Toni N , Kempermann G , Frisen J , Gage FH , Swaab DF Limits to human neurogenesis—really?Mol Psychiatry(2020) ;25: (10):2207–9. |
[134] | Kempermann G , Gage FH , Aigner L , Song H , Curtis MA , Thuret S , et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell. (2018) ;23: (1):25–30. |
[135] | Tosoni G , Ayyildiz D , Bryois J , Macnair W , Fitzsimons CP , Lucassen PJ , et al. Mapping human adult hippocampal neurogenesis with single-cell transcriptomics: Reconciling controversy or fueling the debate?. Neuron [Internet]. (2023) ;111: (11):1714–1731.e3.Available from:https://doi.org/10.1016/j.neuron.2023.03.010. |
[136] | Franjic D , Skarica M , Ma S , Arellano JI , Tebbenkamp ATN , Choi J , et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells.Neuron [Internet]. (2021) ;110: (3):452–469.e14.Available from:https://doi.org/10.1016/j.neuron.2021.10.036. |
[137] | Nano PR , Bhaduri A Mounting evidence suggests human adult neurogenesis is unlikely. Neuron [Internet]. (2022) ;110: (3):353–5Available from:https://doi.org/10.1016/j.neuron.2022.01.004. |
[138] | Lipp HP , Bonfanti L Adult Neurogenesis in Mammals: Variations and Confusions. Brain Behav Evol. (2016) ;87: (3):205–21. |
[139] | Kuhn HG , Toda T , Gage FH Adult hippocampal neurogenesis: A coming-of-age story. J Neurosci. (2018) ;38: (49):10401–10. |
[140] | Chaddock L , Erickson KI , Prakash SR , Kim JS , Voss MW , VanPatter M , et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res [Internet]. (2010) ;23: (1):1–7Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf%0Ahttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC2688697/pdf/nihms-110443.pdf. |
[141] | Vivar C , Peterson B , Pinto A , Janke E , van Praag H Running throughout Middle-Age Keeps Old Adult-Born Neurons Wired. eNeuro. (2023) ;10: (5):1–15. |
[142] | Price RB , Duman R Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry [Internet]. (2020) ;25: (3):530–43Available from:http://dx.doi.org/10.1038/s41380-019-0615-x. |
[143] | Knierim JJ The hippocampus. Curr Biol. (2015) ;25: (23):R1116–21. |
[144] | O’Leary OF , Cryan JF A ventral view on antidepressant action: Roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol Sci [Internet]. (2014) ;35: (12):675–87Available fromhttp://dx.doi.org/10.1016/j.tips.2014.09.011. |
[145] | van Praag H , Schinder AF , Christie BR , Toni N , Palmer TD , Gage FH Functional neurogenesis in the adult hippocampus. Nature. (2002) ;415: , 1030–4. |
[146] | Vivar C , van Praag H Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits. (2013) ;7: , 1–13. |
[147] | Pawluski JL , Brummelte S , Barha CK , Crozier TM , Galea LAM Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol [Internet]. (2009) ;30: (3):343–57Available from:http://dx.doi.org/10.1016/j.yfrne.2009.03.007. |
[148] | Olson AK , Eadie BD , Ernst C , Christie BR Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. (2006) ;16: (3):250–60. |
[149] | bin Imtiaz MK , Jaeger BN , Bottes S , Machado RAC , Vidmar M , Moore DL , et al. Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity. Cell Stem Cell [Internet]. (2021) ;28: (5):967–977.e8.Available from:https://doi.org/10.1016/j.stem.2021.01.015. |
[150] | Dulken BW , Buckley MT , Negredo PN , Cayrol R , Leeman DS , George BM , et al. Single cell analysis reveals T cell infiltration in old neurogenic niches. (2020) ;571: (7764):205–10. |
[151] | Encinas JM , Sierra A Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res [Internet]. (2012) ;227: (2):433–9Available from:http://dx.doi.org/10.1016/j.bbr.2011.10.010. |
[152] | Degirmenci B , Durak H , Ellidokuz E , Tankurt E Return to quiescence of murine neural stem cells by degradation of a pro-activation protein. J Nucl Med. (2016) ;35: (9):1569. |
[153] | Encinas JM , Michurina TV , Peunova N , Park JH , Tordo J , Peterson DA , et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell [Internet]. (2011) ;8: (5):566–79Available from:http://dx.doi.org/10.1016/j.stem.2011.03.010. |
[154] | Urbán N , Blomfield IM , Guillemot F Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron. (2019) ;104: (5):834–48. |
[155] | Yeo RW , Zhou OY , Zhong BL , Sun ED , Navarro Negredo P , Nair S , et al. Chromatin accessibility dynamics of neurogenic niche cells reveal defects in neural stem cell adhesion and migration during aging. Nat Aging. (2023) ;3: (7):866–93. |
[156] | Wu Y , Bottes S , Fisch R , Zehnder C , Cole JD , Pilz GA , et al. Chronic in vivo imaging defines age-dependent alterations of neurogenesis in the mouse hippocampus. Nat Aging. (2023) ;. |
[157] | Kuhn HG , Dickinson-Anson H , Gage FH Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. (1996) ;16: (6):2027–33. |
[158] | Hattiangady B , Rao MS , Shetty AK Plasticity of hippocampal stem/progenitor cells to enhance neurogenesis in response to kainate-induced injury is lost by middle age. Aging Cell. (2008) ;7: (2):207–24. |
[159] | Rao MS , Hattiangady B , Shetty AK The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. (2006) ;5: (6):545–58. |
[160] | Bonzano S , Crisci I , Podlesny-Drabiniok A , Rolando C , Krezel W , Studer M , et al. Neuron-Astroglia Cell Fate Decision in the Adult Mouse Hippocampal Neurogenic Niche Is Cell-Intrinsically Controlled by COUP-TFI In Vivo. Cell Re. (2018) ;24: (2):329–41. |
[161] | Zorec R , Horvat A , Vardjan N , Verkhratsky A Memory formation shaped by astroglia. Front Integr Neurosci. (2015) ;9: , 1–7. |
[162] | Santello M , Toni N , Volterra A Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci [Internet]. (2019) ;22: (2):154–66Available from:http://dx.doi.org/10.1038/s41593-018-0325-8. |
[163] | Morrens J , van den Broeck W , Kempermann G Glial cells in adult neurogenesis. Glia. (2012) ;60: (2):159–74. |
[164] | Wu CW , Chang YT , Yu L , Chen HI , Jen CJ , Wu SY , et al. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. (2008) ;105: (5):1585–94. |
[165] | Scott KA , Ida M , Peterson VL , Prenderville JA , Moloney GM , Izumo T , et al. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav Immun [Internet]. (2017) ;65: :20–32Available from:http://dx.doi.org/10.1016/j.bbi.2017.02.004. |
[166] | An R , Wilms E , Masclee AAM , Smidt H , Zoetendal EG , Jonkers D Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut (2018) ;67: (12):2213–22. |
[167] | Cryan JF , Boehme M , Dinan TG Is the fountain of youth in the gut microbiome? J Physiol. (2019) ;597: (9):2323–4. |
[168] | Guzzetta KE , Cryan JF , O’Leary OF Microbiota-Gut-Brain Axis Regulation of Adult Hippocampal Neurogenesis. Brain Plast. (2022) ;8: (1):97–119. |
[169] | Dohm-Hansen S , Donoso F , Lucassen PJ , Clarke G , Nolan YM The gut microbiome and adult hippocampal neurogenesis: A new focal point for epilepsy?. Neurobiol Dis [Internet]. (2022) ;170: :105746Available from: https://doi.org/10.1016/j.nbd.2022.105746. |
[170] | Ogbonnaya ES , Clarke G , Shanahan F , Dinan TG , Cryan JF , O’Leary OF Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry [Internet]. (2015) ;78: (4):e7–9Available from:Available from:http://dx.doi.org/10.1016/j.biopsych.2014.12.023. |
[171] | Ruiz-González R , Lajud N , Tejeda-Martínez AR , Flores-Soto ME , Valdez-Alarcón JJ , Tellez LA , et al. Antibiotic-induced microbiota depletion in normally-reared adult rats mimics the neuroendocrine effects of earlylife stress. Brain Res. (2022) ;1793(June). |
[172] | Liu G , Yu Q , Tan B , Ke X , Zhang C , Li H , et al. Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome. Gut Microbes [Internet]. (2022) ;14: (1)Available from:https://doi.org/10.1080/19490976.2022.2104089. |
[173] | Möhle L , Mattei D , Heimesaat MM , Bereswill S , Fischer A , Alutis M , et al. Ly6Chi Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Re. (2016) ;15: (9):1945–56. |
[174] | Chesnokova V , Pechnick RN , Wawrowsky K Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun. (2016) ;58: , 1–8. |
[175] | O’Leary OF , Ogbonnaya ES , Felice D , Levone BR , Conroy L , Fitzgerald P ,, et al. The vagus nerve modulates BDNF expression and neurogenesis in the hippocampus. Eur Neuropsychopharmacol [Internet]. (2018) ;28: (2) 307–16Available from:http://dx.doi.org/10.1016/j.euroneuro.2017.12.004. |
[176] | Fülling C , Dinan TG , Cryan JF Gut Microbe to Brain Signaling: What Happens in Vagus. . . .. Neuron. (2019) ;101: (6):998–1002. |
[177] | de Weerth C Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev [Internet]. (2017) ;83: :458–71Available from:https://doi.org/10.1016/j.neubiorev.2017.09.016. |
[178] | Strandwitz P Neurotransmitter modulation by the gut microbiota. Brain Res [Internet]. (2018) ;1693: :128–33Available from: https://doi.org/10.1016/j.brainres.2018.03.015. |
[179] | Krautkramer KA , Fan J , Bäckhed F Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol [Internet]. (2021) :77–9419: (2)Available from: http://dx.doi.org/10.1038/s41579-020-0438-4. |
[180] | O’Toole PW , Jeffery IB Gut microbiota and aging. Science (80-). 350. (2015) ;350: (6265):1214–5. |
[181] | Zheng Y , Manson JE , Yuan C , Liang MH , Grodstein F , Stampfer MJ , et al. Associations ofweight gain from early to middle adulthood with major health outcomes later in life. JAMA - J Am Med Assoc. (2017) ;318: (3):255–69. |
[182] | Mariat D , Firmesse O , Levenez F , Guimarăes VD , Sokol H , Doré J , et al. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. (2009) ;9: , 1–6. |
[183] | Lahtinen SJ , Tammela L , Korpela J , Parhiala R , Ahokoski H , Mykkänen H , et al. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age (Omaha). (2009) ;31: (1):59–66. |
[184] | Claesson MJ , Jeffery IB , Conde S , Power SE , O’connor EM , Cusack S , et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. (2012) ;488: (7410):178–84. |
[185] | Biagi E , Nylund L , Candela M , Ostan R , Bucci L , Pini E , et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS One. (2010) ;5: (5). |
[186] | Franceschi C , Bonafè M , Valensin S , Olivieri F , De Luca M , Ottaviani E , et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. (2000) ;908: , 244–54. |
[187] | Fransen F , van Beek AA , Borghuis T , El Aidy S , Hugenholtz F , van der Gaast - de Jongh C , et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. (2017) ;8: , 1–12. |
[188] | Stojanov S , Berlec A , Štrukelj B The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. (2020) ;8: (11):1–16. |
[189] | Boehme M , van de Wouw M , Bastiaanssen TFS , Olavarría-Ramírez L , Lyons K , Fouhy F , et al. Mid-lifemicrobiota crises: middle age is associated with pervasive neuroimmune alterations that are reversed by targetingthe gut microbiome. Mol Psychiatry [Internet]. (2020) ;25: (10):2567–83Available from:http://dx.doi.org/10.1038/s41380-019-0425-1. |
[190] | Boehme M , Guzzetta KE , Bastiaanssen TFS , van de Wouw M , Moloney GM , Gual-Grau A , et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging. (2021) ;1: (8):666–76. |
[191] | D’Amato A , Di Cesare Mannelli L , Lucarini E , Man AL , Le Gall G , Branca JJV , et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- And neurotransmission-related proteins in young recipients. Microbiome. (2020) ;8: (1):1–19. |
[192] | Li Y , Ning L , Yin Y , Wang R , Zhang Z , Hao L , et al. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats (2020) ;12: (9):7801–17. |
[193] | Monda V , Villano I , Messina A , Valenzano A , Esposito T , Moscatelli F , et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. (2017) )2017. |
[194] | Anhê FF , Zlitni S , Barra NG , Foley KP , Nilsson MI , Nederveen JP , et al. Life-long exercise training and inherited aerobic endurance capacity produce converging gut microbiome signatures in rodents. Physiol Re. (2022) ;10: (5):1–10. |
[195] | Houghton D , Stewart CJ , Stamp C , Nelson A , Aj Ami J , Petrosino JF , et al. Impact of Age-Related Mitochondrial Dysfunction and Exercise on Intestinal Microbiota Composition. Journals Gerontol - Ser A Biol Sci Med Sci. (2018) ;73: (5):571–8. |
[196] | Wang G , Zhou H , Zhang L , Li R , Luo L , Yu Z , et al. Effects of high-intensity interval training on gut microbiota profiles in 12 months’ old ICR mice. J Physiol Biochem. (2020) ;76: (4):539–48. |
[197] | Bakonyi P , Kolonics A , Aczel D , Zhou L , Mozaffaritabar S , Molnár K , et al. Voluntary exercise does notincrease gastrointestinal motility but increases spatial memory, intestinal eNOS, Akt levels, and Bifidobacteriaabundance in the microbiome. Front Physiol. (2023) ;14: , 1–11. |
[198] | Clarke SF , Murphy EF , O’Sullivan O , Lucey AJ , Humphreys M , Hogan A , et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. (2014) ;63: (12):1913–20. |
[199] | Simpson CA , Diaz-Arteche C , Eliby D , Schwartz OS , Simmons JG , Cowan CSM The gut microbiota in anxiety and depression - A systematic review. Clin Psychol Rev [Internet]. (2021) ;83: :101943Available from:https://doi.org/10.1016/j.cpr.2020.101943. |
[200] | Gomaa EZ Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol [Internet]. (2020) ;113: (12):2019–40Available from:https://doi.org/10.1007/s10482-020-01474-7. |
[201] | Massett MP , Berk BC Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol - Regul Integr Comp Physiol. (2005) ;288: (4 57-4):1006–13. |
[202] | Rhodes JS , Hosack GR , Girard I , Kelly AE , Mitchell GS Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl). (2001) ;158: (2):120–31. |
[203] | Kim JW , Nam SM , Yoo DY , Jung HY , Kim IY , Hwang IK , et al. Comparison of Adult Hippocampal Neurogenesis and Susceptibility to Treadmill Exercise in Nine Mouse Strains. Neural Plast. (2017) )2017. |
[204] | Johnson RA , Mitchell GS Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin- Effects of rat strain. Brain Res. (2003) ;983: (1-2):108–14. |
[205] | Gök-Saraç Ç , Wesierska M , Jakubowska-Doğru E Comparison of spatial learning in the partially baited radial-arm maze task between commonly used rat strains: Wistar, Spargue-Dawley, Long-Evans, and outcrossed Wistar/Sprague-Dawley. Learn Behav. (2014) ;43: (1):83–94. |
[206] | Martis LS , Krog S , Tran TP , Bouzinova E , Christiansen SL , Møller A , et al. The effect of rat strain and stress exposure on performance in touchscreen tasks. Physiol Behav. (2018) )184: (November 2017):83–90. |
[207] | Prusky GT , Harker KT , Douglas RM , Whishaw IQ Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res. (2002) ;136: (2):339–48. |
[208] | Wong AA , Brown RE Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes, Brain Behav. (2006) ;5: (5):389–403. |
[209] | Barha CK , Davis JC , Falck RS , Nagamatsu LS , Liu-Ambrose T Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol. (2017) ;46: , 71–85. |
[210] | Barha CK , Best JR , Rosano C , Yaffe K , Catov JM , Liu-Ambrose T Sex-specific relationship between long-term maintenance of physical activity and cognition in the health ABC Study: Potential role of hippocampal and dorsolateral prefrontal cortex volume. Journals Gerontol - Ser A Biol Sci Med Sci. (2020) ;75: (4):764–70. |
[211] | Griffin ÉW , Bechara RG , Birch AM , Kelly ÁM Exercise enhances hippocampal-dependent learning in the rat:Evidence for a BDNF-related mechanism. Hippocampus. (2009) ;19: (10):973–80. |
[212] | Cardoso dos FS , Franç EF , Serra FT , Victorino AB , de Almeida AA , Fernandes J , et al. Aerobic exercise reduces hippocampal ERK and p38 activation and improves memory of middle-aged rats. Hippocampus. (2017) ;27: (8):899–905. |
[213] | Sun LN , Li XL , Wang F , Zhang J , Wang DD , Yuan L , et al. High-intensity treadmill running impairs cognitive behavior and hippocampal synaptic plasticity of rats via activation of inflammatory response. J Neurosci Res. (2017) ;95: (8):1611–20. |
[214] | Yu F , Xu B , Song C , Ji L , Zhang X Treadmill exercise slows cognitive deficits in aging rats by antioxidation and inhibition of amyloid production. Neuroreport. (2013) ;24: (6):342–7. |
[215] | Adlard PA , Perreau VM , Engesser-Cesar C , Cotman CW The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. (2004) ;363: (1):43–8. |
[216] | Lattanzi D , Savelli D , Pagliarini M , Cuppini R , Ambrogini P Short-Term, Voluntary Exercise Affects Morpho-Functional Maturation of Adult-Generated Neurons in Rat Hippocampus. Int J Mol Sci. (2022) )23: (12). |
[217] | Kim YP , Kim HB , Jang MH , Lim B V Kim YJ Kim H , et al. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. (2003) ;24: (2):114–7. |
[218] | Vivar C , Van Praag H Running changes the brain: The long and the short of itPhysiology. (2017) ;32: (6):410–24. |
[219] | Ra SM , Kim H , Jang MH , Shin MC , Lee TH , Lim BV , et al. Treadmill running and swimming increase cell proliferation in the hippocampal dentate gyrus of rats. Neurosci Lett. (2002) ;333: (2):123–6. |
[220] | Cameron HA , McKay RDG Restoring production of hippocampal neurons in old age. Nat Am Inc. (1999) )2: (10). |
[221] | Gould E , Cameron HA , Daniels DC , Woolley CS , McEwen BS Adrenal Hormones Suppress Cell Division in the Adult Rat Dentate Gyrus. J Neurosci. 1992;(September). |
[222] | Joëls M Corticosteroids and the brain. J Endocrinol. (2018) ;238: (3):R121–30. |
[223] | Ávila-Gámiz F , Pérez-Cano AM , Pérez-Berlanga JM , Mullor-Vigo RM , Zambrana-Infantes EN , Santín LJ , et al. Sequential treadmill exercise and cognitive training synergistically increase adult hippocampal neurogenesis in mice. Physiol Behav. 2023;266(April). |
[224] | Tai F , Wang C , Deng X , Li R , Guo Z , Quan H , et al. Treadmill exercise ameliorates chronic REM sleep deprivation-induced anxiety-like behavior and cognitive impairment in C57BL/6J mice. Brain Res Bull [Internet]. 2020;164:198-207. Available from:https://doi.org/10.1016/j.brainresbull.2020.08.025 |



