Central Adiponectin Signaling – A Metabolic Regulator in Support of Brain Plasticity
Abstract
Brain plasticity and metabolism are tightly connected by a constant influx of peripheral glucose to the central nervous system in order to meet the high metabolic demands imposed by neuronal activity. Metabolic disturbances highly affect neuronal plasticity, which underlies the prevalent comorbidity between metabolic disorders, cognitive impairment, and mood dysfunction. Effective pro-cognitive and neuropsychiatric interventions, therefore, should consider the metabolic aspect of brain plasticity to achieve high effectiveness. The adipocyte-secreted hormone, adiponectin, is a metabolic regulator that crosses the blood-brain barrier and modulates neuronal activity in several brain regions, where it exerts neurotrophic and neuroprotective properties. Moreover, adiponectin has been shown to improve neuronal metabolism in different animal models, including obesity, diabetes, and Alzheimer’s disease. Here, we aim at linking the adiponectin’s neurotrophic and neuroprotective properties with its main role as a metabolic regulator and to summarize the possible mechanisms of action on improving brain plasticity via its role in regulating the intracellular energetic activity. Such properties suggest adiponectin signaling as a potential target to counteract the central metabolic disturbances and impaired neuronal plasticity underlying many neuropsychiatric disorders.
Impaired brain plasticity is a hallmark of cognitive- and mood-related disorders, such as Alzheimer’s disease (AD) [1] and depression [2], which is emphasized by the high comorbidity among such conditions [3]. Brain function is tightly connected with the peripheral metabolism given the brain’s need for a continuous influx of peripheral glucose to support its high metabolic demands [4], such that metabolic dysfunctions eventually result in disruption of brain activity and impaired neuronal plasticity [4]. Not surprisingly, metabolic disorders are commonly associated with cognitive and mood impairments [5, 6], whereas AD itself is currently characterized by some as type 3 diabetes [7]. Given that metabolic disorders are an ever-growing problem [8, 9], it is expected that the incidence of cognitive- and mood-related problems will increase in the coming years. Such entangled nature of metabolism and brain plasticity suggests that effective treatments must tackle both ends: metabolism and plasticity, at the same time [5].
Adiponectin is a cytokine with remarkable pleiotropic properties. It has been shown to be anti-inflammatory, anti-atherosclerotic, anticarcinogenic, neuroprotective, pro-cognitive, and antidepressant [10, 11]. Being a cytokine with receptors expressed across several physiological systems [10], including the brain [12], adiponectin functions as a major metabolic regulator. Its relevance for proper brain functioning has been demonstrated by marked neurophysiological and behavioral impairments displayed by adiponectin deficient mice [13], resembling an AD-like pathology [14]. On the other hand, increasing central adiponectin signaling has been remarkably effective in counteracting central metabolic dysfunctions and increasing brain plasticity [15– 17].
Although genetic factors have an impact on metabolic functions, lifestyle factors also play a critical role [18]. In this regard, chronic psychological stress is a significant risk factor for developing metabolic dysfunctions [4]. Increasing energy availability is an elementary allostatic response during any stress reaction [4, 19]. However, it poses a challenge to the individual’s metabolic system if such stress is prolonged over days, weeks, or longer [4, 19]. Likewise, chronic stress is linked to central metabolic dysfunction that is followed by impaired neuroplasticity [20, 21]. Although chronic stress-associated neuronal atrophy is usually associated with downregulation of neurotrophic support [22, 23], the mechanisms by which stress specifically reduces neurotrophic support are largely unknown. There is a resurging interest in the metabolic changes induced by stress and how they could underlie stress-induced neuronal impairments [5, 24]. As remembered by Picard and colleagues, glucocorticoids were originally termed after their influence over glucose metabolism [24].
In this review, we aim to link the adiponectin’s neurotrophic and neuroprotective properties with its main role as a metabolic regulator, suggesting the improvements in brain plasticity observed by adiponectin treatment result from the regulation of the intracellular energetic activity. We first review the adiponectin signaling as a regulator of the peripheral and central metabolism and its relevance for brain plasticity, followed by a discussion on the negative impact of chronic psychological stress on neurodegeneration and the central metabolism, whereas cognitive and mood impairments are mentioned as representative behavioral changes associated with plasticity deficits. Finally, adiponectin’s neurotrophic and neuroprotective properties are reviewed and considered along with the restored intracellular metabolic functions.
CENTRAL ADIPONECTIN SIGNALING REGULATES CELLULAR METABOLISM AND SUPPORTS NEURONAL PLASTICITY
Adiponectin regulates peripheral metabolism
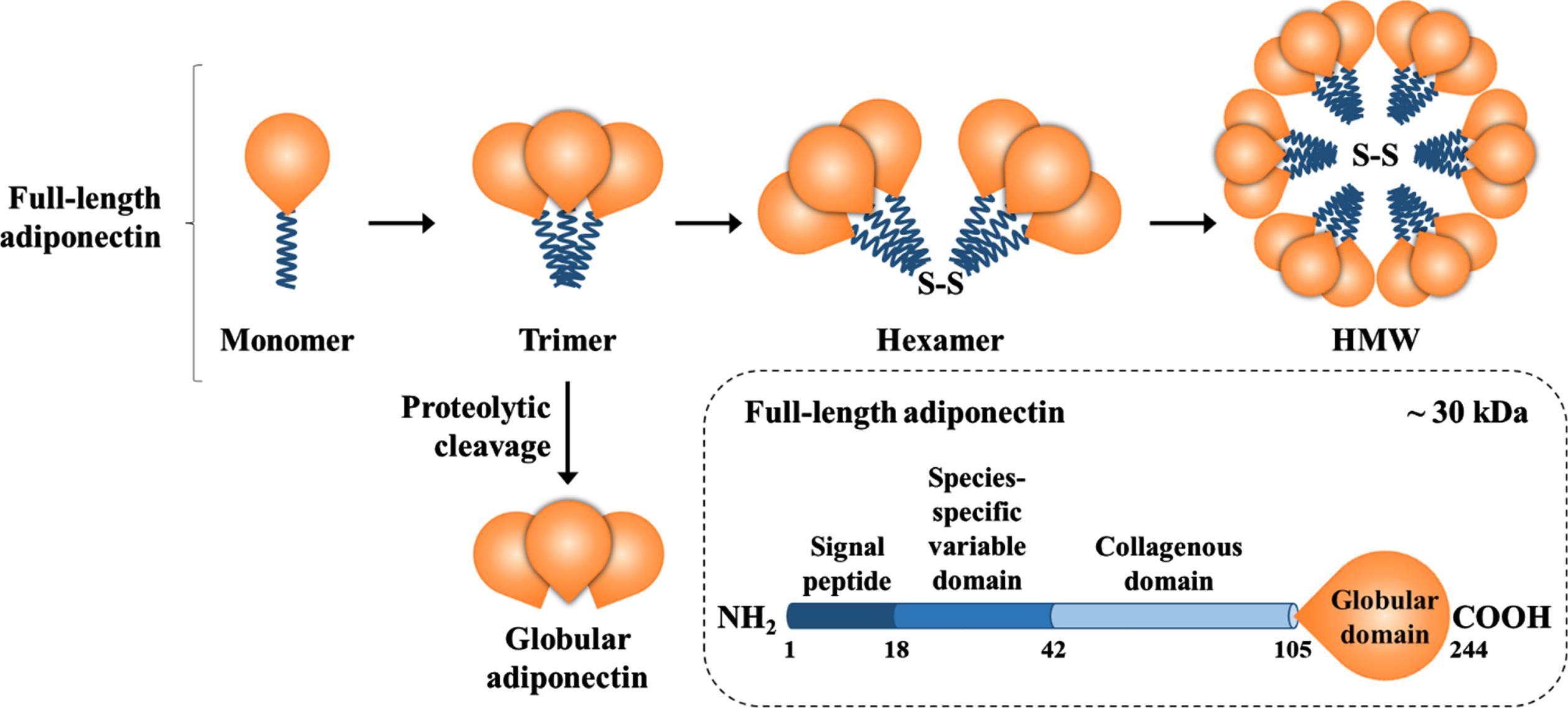
Adiponectin synthesis is controlled by a multitude of transcriptional factors, including the peroxisome proliferator-activated receptor (PPAR) gamma, and undergoes several post-transcriptional and post-translational steps involving different chaperones before its secretion [25]. Adiponectin synthesis and secretion are inversely proportional to the visceral adipose depot [26] and the adipocyte size [27]. Although the mechanisms for such negative modulation are largely unknown, the tumor necrosis factor (TNF)-α has been shown to negatively modulate circulating adiponectin levels as treating mice with recombinant TNF-α reduces circulating adiponectin levels [28] and pharmacologically blocking TNF-α in subjects with metabolic syndrome increases it [29]. Structurally, the 244-aminoacid protein consists of a globular domain, a collagen-like domain, a species-specific domain, and a signal peptide (e.g. full-length form) [10, 11]. The full-length adiponectin undergoes oligomerization and circulates as trimer, hexamer, or high-molecular-weight multimers, whereas trimers can be cleaved to globular adiponectin (Fig. 1) [11, 30].
Fig.1
Adiponectin composition and isoforms. Full-length adiponectin is composed of its three main domains plus a globular domain, which can be cleaved and circulate as globular adiponectin. Full-length adiponectin undergoes oligomerization and circulates as a trimer, hexamer, or high-molecular-weight multimer, whereas trimers and hexamers can cross the blood-brain barrier.
Adiponectin exerts its physiological functions mainly through the activation of two specific receptors, namely the AdipoR1 and AdipoR2 [31]. Whereas AdipoR2 has a moderate affinity for both adiponectin isoforms, AdipoR1 has a higher affinity for globular adiponectin in comparison with the full-length isoform [31]. Both receptors interact with the adaptor protein containing a pleckstrin homology domain, a phosphotyrosine domain, and a leucine zipper motif (APPL1) as their key mediator coupling receptor activation with downstream signaling pathways [32]. As an endosome-associated adaptor protein, APPL1 can modulate signaling specificity and crosstalk by anchoring effectors of different signaling pathways [33, 34]. APPL1 promotes the crosstalk between adiponectin receptors and its downstream signaling targets, including the activation of the PPARα [32, 35], the AMP-activated protein kinase (AMPK) [35], and other proteins associated with the insulin signaling pathway, such as phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) [36] (Fig. 2A).
Fig.2
Adiponectin signaling pathway regulates intracellular metabolism and neuronal plasticity in (A) physiological- and (B) chronic stress-associated conditions. (A) Activation of the adiponectin receptors, which are present at synapses, orchestrates signaling pathways that are essential for proper bioenergetic balance and plasticity. AdipoR1/2 phosphorylates the APPL1 and activates the AMPK and PPARα pathways, whereas APPL1 itself forms a complex with NMDA receptors and couples neuronal activity with the activation of the insulin signaling pathway (PI3K/Akt). PI3K/Akt induces mTORC1 activity, protein synthesis, and cellular growth by suppressing TSC1/2. Upon synaptic activation, increased AMPK signaling is necessary to upregulate substrate oxidation, mitochondrial respiration and biogenesis, maintaining the intracellular energetic reserves necessary for LTP maintenance. PPARα, on the other hand, directly upregulated CREB transcription. (B) Chronic stress downregulates PPARα and AMPK expression and phosphorylation, as well as downregulates the expression of upstream modulators of the PI3K/Akt pathway, such as vascular endothelial growth factor and insulin. Currently, a direct connection between APPL1 and stress-associated deficits remains unknown.
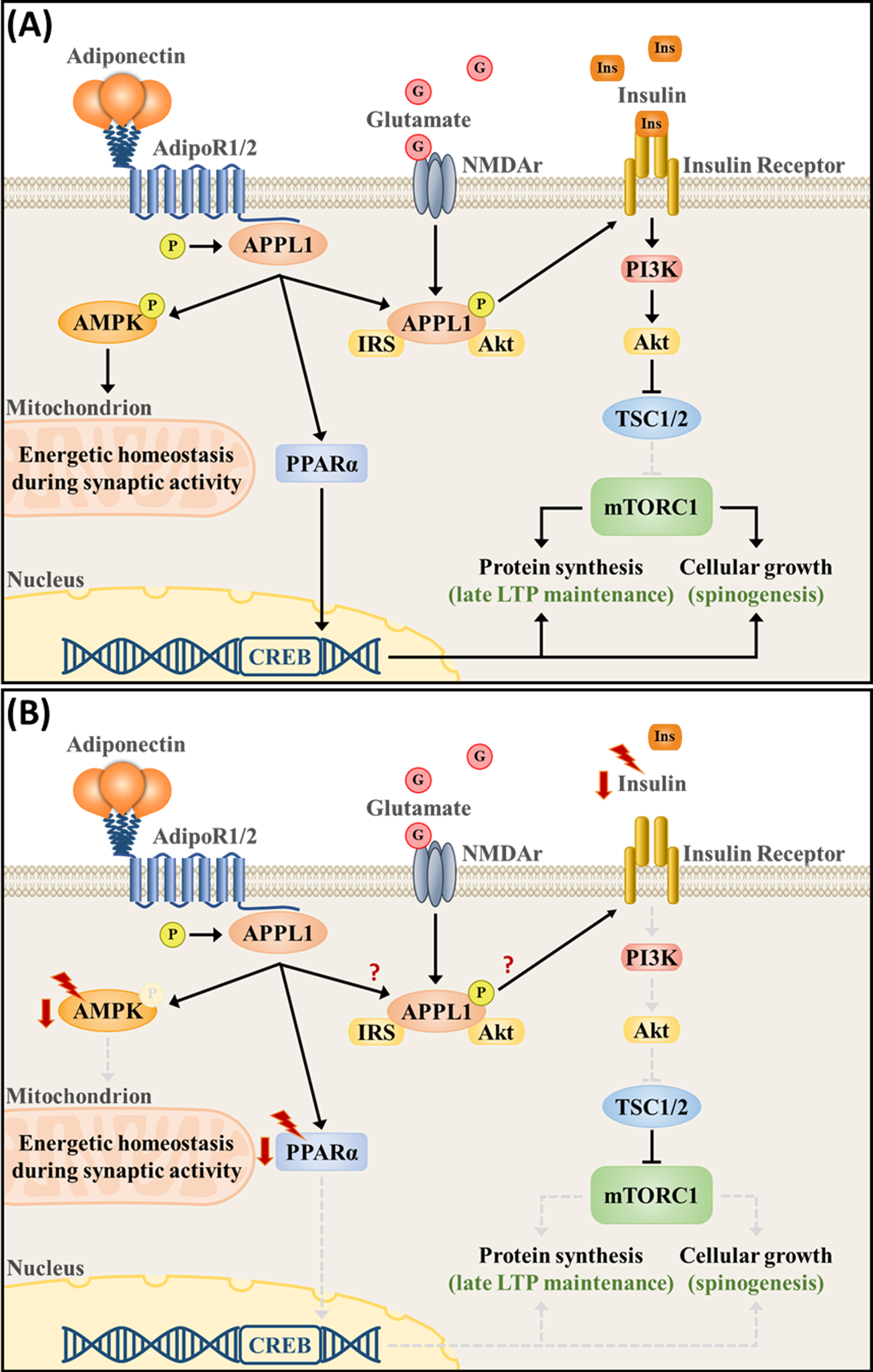
PPARα is a transcription factor that functions as a lipid sensor. PPARα responds to starvation by regulating the expression of several genes associated with fatty acid metabolism, including fatty acid uptake and oxidation [37]. In parallel, PPARα also downregulates several proinflammatory factors [37]. AMPK, on the other hand, functions as the cellular energy sensor. AMPK is activated upon circumstances that pose a challenge for adenosine triphosphate (ATP) production, such as hypoxia and glucose deprivation [38– 40]. Being the canonical catabolic pathway, AMPK senses an increase in adenosine monophosphate to ATP ratio, leading to the phosphorylation of proteins that regulate the catabolic process, such as increased gluconeogenesis and fatty acid oxidation, while suppressing anabolic mechanisms, such as glycolysis and protein synthesis, resulting in increased energy availability [38].
APPL1 also mediates the crosstalk between the adiponectin and insulin signaling pathways, as suggested by the insulin resistance and impaired insulin signaling observed in APPL1 knockout mice [36]. Specifically, APPL1 facilitates the interaction of insulin-receptor substrates 1 and 2 with the insulin receptor β, which is dependent on the APPL1 phosphorylation on the Ser401 site [36]. Interestingly, APPL1Ser401 phosphorylation is also promoted by adiponectin, which mediates the adiponectin’s insulin-sensitizing properties [36]. However, insulin is necessary for the downstream activation of the PI3K/Akt cascade [36]. Such pathway is part of the canonical anabolic pathway as it inhibits the tuberous sclerosis proteins 1 and 2 activity (which suppress the activity of the mammalian target of rapamycin complex 1 [mTORC1]) and activates the mTORC1, resulting in protein synthesis and cell growth [38, 41] (Fig. 2A).
On the one hand, adiponectin signals the initiation of catabolic reactions through activation of the AMPK pathway, interrupting protein synthesis and cell growth. On the other hand, it facilitates anabolic processes through the PI3K/Akt pathway, fostering protein synthesis and cell growth. It is, therefore, intriguing how adiponectin activates antagonistic processes simultaneously. One possible explanation relies on the organism’s nutritional state. In the absence of glucose and, hence, insulin (starvation), the adiponectin-induced activation of AMPK and PPARα pathways prevails, resulting in the AMPK activation of the tuberous sclerosis proteins 1 and 2 and consequent suppression of mTORC1 (catabolism) [38, 41]. On the other hand, in the presence of insulin and growth factor stimulation (nutrient-rich state), adiponectin facilitates the PI3K/Akt pathway activation, which interacts with the glycogen synthase kinase 3 beta to inhibit AMPK activity [42], interrupting catabolic processes and leading to protein synthesis and cell growth through activation of the mTORC1 (anabolism). Activation of such downstream signaling pathways evidences the adiponectin’s role as an insulin sensitizer to improve glucose metabolism and fatty acid oxidation, eventually counteracting hyperglycemia in metabolic disorders [43– 46].
The APPL1, PPARα, AMPK, and PI3K/Akt are also expressed in the neuronal tissues, where they have an important role in supporting neuronal metabolism and plasticity.
Adiponectin downstream signaling pathways regulate neuronal metabolism and plasticity
Brain activity relies on a disproportional amount of energy requirements compared with the rest of the body. Roughly 20% of the body’s metabolic resources are used by the central nervous system (CNS), although it accounts for only 2% of the total body weight [47]. Since the brain contains virtually no energetic reserves, central glucose levels are maintained through a constant influx of peripheral glucose through the blood-brain barrier, which is facilitated by the glucose transporter 1 [4]. Such high energetic requirements result mostly from synaptic activity (i.e., generation of action potentials as well as re-establishment and maintenance of membrane potential) [48, 49]. Therefore, excitatory glutamatergic transmission largely determines the brain’s energetic demands [48, 49]. Not surprisingly, neuronal mitochondria are mostly located at synapses where oxidative respiration is tightly connected with neuronal activation [49]. Given that energy requirements are directly proportional to synaptic activity, increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking due to synaptic potentiation naturally results in increased energy demands [49]. Therefore, tight regulation of intracellular metabolism is the basis for proper synaptic and structural plasticity.
PPARα is expressed in all hippocampal subfields [50] and has been implicated in glutamate metabolism and transmission [51]. It can promote glutamate transporter 1 endocytosis in astrocytes and downregulate the level of endogenous glutamate receptors antagonists, such as kynurenic acid [51]. In the hippocampus, it indirectly modulates the expression of synaptic-related genes (e.g., GluN2A and GluR1) via direct upregulation of cAMP response element-binding protein (CREB) transcription, which is necessary for adequate calcium influx and acquisition of long-term spatial memory [50] (Fig. 2A).
In the hypothalamus, the AMPK signaling pathway serves as a master metabolic sensor. The different hormones associated with fasting (e.g., ghrelin) or feeding (e.g., leptin) activate or inhibit hypothalamic AMPK, respectively, and determine the subsequent metabolic functions based on AMPK activity (e.g., decreased energy expenditure and increased food intake) [52]. At the cellular level, constitutive AMPK is not necessary for proper neuronal development, although in vitro overactivation of AMPK using its agonist, 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR), impairs axogenesis by inhibiting mTORC1 activity [53]. Nutrient sufficiency is required for the brain-derived neurotrophic factor (BDNF)-induced activation of mTORC1 [54]. Upon glucose starvation, increased AMPK activity directly blocks BDNF-induced activation of mTORC1 and subsequent protein synthesis [54]. Furthermore, AMPK is recruited upon synaptic activation to sustain intracellular ATP levels, upregulating mitochondrial respiration and glycolysis to meet the energy requirements upon cellular activation [55]. Energy-depleted cells fail to activate the mitogen-activated protein kinase pathways [55]. Blocking AMPK activity impairs long-term potentiation (LTP) and inhibits the protein-synthesis-dependent late phase of LTP in the hippocampal cornu Ammonis 1 (CA1) subregion, which leads to deficits in contextual fear memory learning [55]. Moreover, AMPK is also necessary for activity-induced immediate early-genes expression (Arc, Egr1, cFos) [55]. AMPK, therefore, signals cellular metabolic adjustments upon synaptic activation (Fig. 2A).
Insulin receptors are also present at the synapse level [56], where insulin-receptor substrates are translocated upon increased N-methyl-D-aspartate (NMDA) receptor activity [57]. The insulin signaling in dendritic spines is relevant for the maintenance of activity-dependent synaptic efficiency and experience-induced structural plasticity in terms of dendritic complexity [58]. The NMDA receptor forms a complex composed of 77 proteins, amidst which APPL1 can be found [59]. APPL1 directly interacts with the NMDA receptor complex and couple NMDA receptor activity with recruitment and activation of the PI3K/Akt pathway in postsynaptic densities [60] (Fig. 2A). Such APPL1-mediated interaction with the PI3K/Akt pathway is necessary for appropriate spine and synapse formation, such that knocking down APPL1 activity in cultured hippocampal neurons impairs dendritic spine formation [61]. In parallel, dendritic spine-located APPL1 senses synaptic activity and is translocated to the nucleus upon neuronal activation, where it facilitates transcription necessary for late-phase LTP maintenance. Blocking APPL1 activity impairs late LTP but not LTP induction nor early LTP [62].
Whether adiponectin can be expressed in the nervous tissue is still debatable. However, low-molecular-weight adiponectin, both the globular and full-length isoforms, can cross the blood-brain barrier [63, 64]. Moreover, adiponectin receptors are found in synapses [13] and are widely expressed throughout the CNS, including the ventral tegmental area [65], dorsal raphe nucleus [66], medial prefrontal cortex (mPFC), hippocampus, and amygdala [12]. Given the relevance of the adiponectin’s downstream targets for the modulation of central metabolism and maintenance of neuronal activity, adiponectin is likely to be one of the key peripheral factors modulating neuronal metabolism and plasticity [30, 67]. Such rationale has been supported by the impairments observed in mice with chronic adiponectin deficiency [13, 14, 17].
Adiponectin deficiency leads to impaired neuronal metabolism and plasticity
Adiponectin deficiency greatly impairs intracellular metabolism in the CNS. Chronic adiponectin deficiency in aged adiponectin knockout mice leads to hippocampal insulin resistance, which is associated with reduced phosphorylation levels of AMPK, Akt, and PI3K [13, 14, 17], despite normal insulin plasma levels and normal hippocampal insulin receptor expression [14]. Moreover, Akt phosphorylation is impaired in response to intra-hippocampal insulin administration in adiponectin knockout mice [14]. Remarkably, such impairment is reversed by adiponectin in cultured insulin-resistant neuronal cells, since adiponectin pretreatment improves Akt phosphorylation in response to insulin challenge [14].
Disrupted CNS metabolism in adiponectin deficient mice is associated with impaired functional and structural plasticity. Adiponectin knockout mice have reduced presynaptic and postsynaptic protein expression, including synaptophysin (presynaptic), synaptosomal-associated protein 23, synapse-associated protein 102, and postsynaptic density protein 95 (PSD-95) (postsynaptic) [14, 17]. These postsynaptic proteins are associated with NMDA receptors and glutamatergic transmission. In agreement with that, such animals also present reduced levels of the glutamate receptor subunits GluA1, GluN1, and GluN2A (but not GluN2B) along with reduced phosphorylated CREB [13]. Such impairments in glutamatergic transmission are accompanied by reduced synaptic plasticity, dendritic complexity, spine density, and neurogenesis in the hippocampal region [13, 17, 68]. Not surprisingly, chronic adiponectin deficiency leads to impaired spatial learning and memory, contextual and cued fear conditioning, and increased anxiety-like behavior [13, 14, 17]. Moreover, adiponectin knockout mice do not benefit from the physical exercise-induced increase in hippocampal neurogenesis and antidepressant effects [69].
Lifestyle factors greatly interfere with metabolism, circulating adiponectin levels, cognition, and mood. Imbalance in the input/output energy levels due to excessive energy intake and low physical activity levels can lead to increased adiposity, obesity, and diabetes, all associated with reduced circulating adiponectin levels [70– 72], cognitive impairments [74– 76], and reduced structural and functional plasticity [74– 76]. Likewise, chronic psychological stress, which will be thoroughly discussed next, is linked to reduced adiponectin signaling and impaired peripheral and central metabolism.
CHRONIC STRESS IMPAIRS ADIPONECTIN SIGNALING AND DISRUPTS NEURONAL PLASTICITY
Chronic stress impairs peripheral and central metabolism
Stress is an adaptive response to external and internal stimuli, which aims to adjust the physiological functions to cope with the encountered challenges [79]. In this regard, the stress response can arise from metabolic disturbances, such as those induced by dietary and exercise habits (i.e. metabolic stress) [80], or environmental factors, which are perceived as threatening the subject’s well-being (i.e. psychological stress) [81].
In psychological stress, the central survival circuitry detects innate and learned threats and activates the hypothalamus-pituitary-adrenal axis, leading to the release of catecholamines (autonomic response) and glucocorticoids (e.g. cortisol, endocrine response) [82]. Glucocorticoids are key modulators of glucose metabolism [4, 19]. They increase plasma glucose levels and promote gluconeogenesis and glycogen storage in the liver, increase lipolysis and visceral fat, reduce glucose uptake in the skeletal muscles, and antagonize insulin production [4, 19]. Such metabolic adjustments induced by the survival response are an important adaptive response to acute stimuli perceived as threatening. Nonetheless, chronic psychological stress eventually results in hyperglycemia and insulin resistance [4, 19]. Not surprisingly, it is a risk factor for type 2 diabetes and impacts the disease’s prognosis [83]. Here, we focus on the metabolic disturbances associated with chronic psychological stress, henceforth simply referred to as stress.
Psychological stress can be experimentally induced in animals through exposure to naturalistic stressors, such as food, water, or sleep deprivation, or social stressors, such as social isolation and social defeat by exposure to a stronger male aggressor (referred to [84] for in-depth review). Interestingly, it has also been suggested that stress can potentially downregulate adiponectin expression. Dexamethasone, a glucocorticoid receptor (GR) agonist, reduces circulating adiponectin levels and leads to metabolic disorders in rodents [85, 86]. In humans, reduced plasma adiponectin levels were observed in firefighters diagnosed with post-traumatic stress disorder [87]. Moreover, glucocorticoids were shown to reduce adiponectin transendothelial movement, suggesting that stress can reduce adiponectin availability to targeted tissues even if serum concentrations are unchanged [88].
The CNS’s reliance on glucose as its main energetic source renders it vulnerable to such metabolic disturbances. In rodents, chronic stress induces hippocampal hyperglycemia [21], increases hippocampal glycogen synthesis [89], and reduces hippocampal and mPFC mitochondrial respiratory chain activity [90, 91]. Such stress-induced disturbances in the central metabolism were accompanied by increased depression-like [90, 91] and impaired spatial memory [21, 89]. Likewise, intra-hippocampal glucose infusion impairs spatial memory [21]. On the other hand, lowering blood glucose levels pharmacologically rescues spatial memory performance in hyperglycemic stressed mice [21].
The stress-induced impairments on neuronal metabolism and plasticity are largely mediated by decreased PPARα, AMPK, and PI3K/Akt expression and activity.
Chronic stress impairs the adiponectin’s downstream signaling pathways and disrupts neuronal plasticity
In rodents, three isoforms of PPAR are co-expressed in the brain circuits responsible for regulating stress responses, playing a role in several neuropsychiatric disorders by modulating anti-inflammatory and metabolic processes [92]. PPAR-α is one of the three isoforms of the nuclear PPAR family [93], whose physiological functions are associated with cell differentiation, metabolism, and energy homeostasis [94]. Upon binding to endogenous and synthetic ligands, PPARα is transferred to the nucleus to dimerize with the retinoic acid x receptor and bind to the peroxisome proliferative response element to regulate the transcription of the target gene [95]. It is abundantly expressed in the hippocampus, amygdala, prefrontal cortex, thalamic nucleus, vomeronasal nucleus, and ventral tegmental region [96], where it modulates the production of BDNF [97], a critical neurotrophic factor governing neuronal plasticity. Of note, hippocampal PPARα expression is downregulated by chronic stress (Fig. 2B, which leads to increased expression of depression-like behaviors [98].
Chronic stress has also been linked with reduced AMPK activity and expression in several experimental models (Fig. 2B). In vitro, corticosterone significantly reduces AMPK phosphorylation in a time-dependent fashion in both astrocytes (from 2-h to 72-h incubation time) [99] and PC12 cells (24-h incubation time) [100]. In vivo, different chronic stress protocols were shown to reduce AMPK phosphorylation in the mPFC [99], hippocampus [101– 103] and cortex [104]. Noteworthy, the hippocampus and mPFC are two regions that have been classically involved in mood and cognitive performance. Congruently, chronic stress-induced reduction in AMPK signaling was associated with increased depression- and anxiety-like behavior [99, 103] and impaired spatial memory [101– 103].
There are different mechanisms through which decreased AMPK activity can be linked with chronic stress outcomes. First, as previously discussed, AMPK functions as a metabolic switch that transiently activates catabolic pathways to adjust the increased intracellular metabolic demands upon neuronal activation, a process that is essential for further activation of the mitogen-activated protein kinase pathways, protein synthesis, and synaptic plasticity [55]. Second, stress-induced reduction in the central expression of GR results in glucocorticoid resistance and hypercortisolemia [82], which is associated with impaired plasticity and neuronal atrophy [105, 106]. Remarkably, pharmacological activation of AMPK in cultured astrocytes protects against the dexamethasone-induced reduction in GR, whereas its pharmacological or genetic inhibition results in reduced GR expression [99]. Finally, AMPK also upregulates BDNF signaling. One-hour incubation of cultured primary hippocampal neurons with AICAR significantly increases AMPK phosphorylation and BDNF levels in a dose-dependent manner [101]. Likewise, both acute (30 min) [107] and sub-chronic (7 days) [101] systemic administration of AICAR increases hippocampal BDNF levels, which can last for up to 14 days after the last sub-chronic dose administration [101].
Chronic stress can impair the PI3K/Akt signaling pathway by downregulating the expression of upstream modulators, such as the vascular endothelial growth factor [108] and insulin [109] (Fig. 2B). Chronic corticosterone treatment dysregulates the vascular endothelial growth factor expression, resulting in reduced activation of the PI3K/Akt/mTOR signaling pathway [110]. Moreover, chronic stress disrupts the insulin-mediated activation of this pathway, resulting in cognitive impairment in healthy [109], AD [111], and diabetic [112] rodents. The PI3K/Akt pathway is the canonical anabolic pathway, further targeting proteins like the glycogen synthase kinase 3 beta [113], mTOR [114], and forkhead box protein O (FOXO) [115], all involved in protein synthesis and cellular growth. Disrupting these pathways result in an overall shift in the molecular and cellular mechanisms mediating synaptic and structural plasticity.
Finally, the impact of chronic stress on APPL1 expression and phosphorylation, to our knowledge, remains to be explored. However, considering the previously reported relevance of APPL1 in linking glutamatergic activity with the activation of the insulin signaling pathway [60, 61, 116, 117], it is reasonable to speculate that disturbed APPL1 activity likely mediates the deleterious effect of chronic stress on neuronal metabolism and plasticity. Although the specific involvement of APPL1 with mood and cognition is still lacking, APPL1 was found differentially expressed in postmortem brain samples of AD subjects [118]. Specifically, APPL1 was observed to aggregate in granules around hippocampal pyramidal cells, mostly in the CA1 and subiculum areas, a feature present only in AD patients but not in normally aged controls [118]. However, at the current state of the literature, it is not possible to draw a direct connection between APPL1 and stress-associated deficits nor is it possible to indicate it as a therapeutic target.
Targeting the adiponectin’s downstream signaling pathways results in therapeutic response
The hippocampal PPARα has been previously established as a novel antidepressant target [119] given its upregulating properties over the BDNF/CREB signaling pathway [97], which is necessary for the antidepressant effects. Specifically, genetic overexpression of hippocampal PPARα produces antidepressant effects by promoting CREB-mediated BDNF biosynthesis [98]. On the other hand, PPARα-associated antidepressant effects were also linked with the activation of the mesolimbic dopaminergic system [120]. Fenofibrate enriched-diet, a PPARα agonist, can prevent and alleviate chronic stress-induced depression-like behaviors by increasing activity of the ventral tegmental area [120].
As previously addressed, AMPK upregulates BDNF signaling, which is a key mediator of antidepressant treatments like ketamine [121] and physical exercise [122]. In agreement with that, both acute ketamine administration [107] and chronic physical exercise [101, 102] increased hippocampal AMPK activity, whereas pre-treatment of animals with Compound C, an AMPK inhibitor, reduced the ketamine rapid-antidepressant effects [107]. On the other hand, AMPK was also shown to be a key mediator of the physical exercise-associated increase in neuroplasticity and cognition. In stressed mice, six injections with Compound C once every 4 days during 21 days of treadmill running significantly reduced the exercise improved spatial memory and blocked the exercise-associated increase in BDNF, synaptophysin, and PSD-95 mRNA expression [101]. Such blunted effect on synaptic protein expression and neurotrophic support abolished exercise-induced adult neurogenesis, dendritic complexity, and spine density [101, 102].
Directly targeting the AMPK activity, on the other hand, elicits therapeutic effects. In cultured primary hippocampal neurons, AICAR increases dendritic branching and length, which is protective against the corticosterone-induced reduction in dendritic complexity [102]. Moreover, AICAR treatment reduces hippocampal oxidative stress and prevents chronic stress-induced depression, anxiety, and cognitive impairments [103]. Similarly, 14 days of metformin administration, an antidiabetic drug relying on AMPK activation, improved chronic corticosterone-induced depression-like behavior, which was associated with increased expression of GR in the mPFC [99]. Likewise, sub-chronic treatment with AICAR (7 days) improves the cognitive performance of non-stressed mice [101].
PI3K/Akt downstream targets are key mediators of rapid-acting antidepressant drugs, such as the mTORC1-dependent antidepressant effects induced by ketamine [123]. Moreover, FOXO3a, another target of the PI3K/Akt pathway, plays an essential role in depression development and treatment [124]. Studies have shown that mice with FOXO3a gene deletion exhibit depression-like behavior [115], whereas antidepressant drugs, such as venlafaxine [125], exert antidepressant effects by modulating FOXO3a.
In summary, stress-induced downregulation of adiponectin expression and consequent metabolic dysregulation leads to impairments in neuronal plasticity that are largely mediated by a reduction in the activity of the adiponectin downstream signaling pathways. Moreover, directly targeting such pathways significantly restores neuronal metabolism and plasticity, suggesting the increase in adiponectin signaling as a prominent strategy for restoring neuronal metabolism and improving brain plasticity (Fig. 2).
INCREASING ADIPONECTIN SIGNALING AS A THERAPEUTIC STRATEGY FOR IMPROVING CENTRAL METABOLISM AND NEURONAL PLASTICITY IMPAIRMENTS
Adiponectin is the most abundant plasma protein [126], rendering its use as a direct treatment unsuitable. Nonetheless, two different compounds, AdipoRon and Osmotin, have been showing promising therapeutic properties by targeting the adiponectin signaling system. AdipoRon is a synthetic, orally active small molecule that agonizes both adiponectin receptors [127], whereas Osmotin is a plant-based protein homologous with adiponectin [128]. Both compounds have been experimentally tested to counteract neurological impairments associated with central metabolic dysfunctions, including those associated with AD, diabetes, obesity, and others.
Alzheimer’s disease has been increasingly understood as a metabolic disorder, with some classifying it as type 3 diabetes [7, 129]. [17] On the other hand, exogenous administration of adiponectin in several animal models of AD resulted in marked therapeutic effects. The treatment of acute hippocampal slices with adiponectin [130] increased synaptic plasticity in a transgenic animal model of AD, whereas 48 h of in vitro AdipoRon incubation rescued the amyloid β-induced impairments in dendritic complexity and neural stem cells’ proliferation [16]. AdipoRon administered centrally (intracerebroventricular infusion) [16] or orally [15] improved hippocampal insulin sensitivity and neuroinflammation [15], increased dendritic complexity, spine density [15], and cell proliferation [16] in transgenic animal models of AD. Such effects were accompanied by reduced anxiety-like behavior and improved spatial memory [15, 16]. Moreover, in a recent study investigating the therapeutic effects of long-term Osmotin treatment, the authors observed increased hippocampal synaptic protein expression, increased dendritic complexity and spine density, and increased synaptic plasticity in three different models of AD [17].
Interestingly, the impairments in neuroplasticity, cognitive performance, and anxiety-like behavior presented by aged adiponectin knockout mice (mentioned in the previous sections) are associated with increased amyloid β and tau phosphorylation in the hippocampus and frontal cortex [14]. Moreover, adiponectin deficiency further worsens the anxiety and spatial memory impairments in transgenic AD animal models [15]. Therefore, the neurophysiological and behavioral alterations induced by chronic adiponectin deficiency are associated with features resembling an AD-like pathology.
Although targeting the central adiponectin signaling system to counteract AD neuropathology has been promising in animals, the clinical observations regarding adiponectin and cognitive function are rather contradictory [131, 132]. High circulating adiponectin levels are associated with mild cognitive impairment and AD [131, 133], which was also associated with smaller hippocampal volume and increased Aβ deposition in women [134]. Moreover, in a long-term prospective study adiponectin was the only independent factor positively associated with increased risk for developing all-cause dementia and AD during the 13-year follow-up [132]. The mechanisms involved with such adiponectin paradox, which extend beyond AD [135, 136], are still unknown. Adiponectin resistance resulting from reduced expression of adiponectin receptors and/or the downstream adiponectin signaling targets has been suggested as one of the possible mechanisms [136]. Indeed, postmortem samples from patients with AD showed lower expression levels of AdipoR1 in the cortex and hippocampus compared with aged healthy subjects [17].
Obesity and diabetes were the first metabolic disorders to be associated with lower circulating adiponectin levels [44, 137] and to hint at the adiponectin’s role as a metabolic regulator [44, 126]. Streptozotocin-induced diabetes reduces the hippocampal levels of adiponectin [138] whereas high-fat diet-induced obesity reduces the adiponectin receptors’ expression in the hippocampus [139]. Not surprisingly, this is associated with impaired hippocampal functional and structural plasticity, including reduced expression of PSD-95, LTP, dendritic complexity, spine density, and neurogenesis [77, 78, 138]. Physical exercise is a potent treatment for metabolic disorders. Our group has demonstrated that adiponectin is a key mediator of the voluntary wheel running neurogenic and antidepressant effects, both in physiological conditions [69] as well as in diabetic mice [138]. In humans, high-intensity exercise appears to have the strongest effect over peripheral adiponectin expression [140, 141]. High-intensity aerobic exercise increased the high-molecular-weight to total adiponectin ratio in obese men [140], whereas in obese young female adolescents high-intensity interval training increased plasma adiponectin levels twice as much when compared to moderate intensity [141]. In young lean subjects, on the other hand, acute high-intensity running increased serum adiponectin levels by 10.5%, although reducing its levels in the cerebrospinal fluid by 21.3%. The decrease could be due to an exercise-induced decrease in the blood-brain barrier permeability [142]. Even though, serum and CSF adiponectin levels were positively correlated with the subject’s cognitive performance after exercise [142]. Moreover, we have recently demonstrated that 14 days of AdipoRon treatment restored the cognitive and anxiogenic profile in streptozotocin-induced diabetes to levels equivalent to voluntary wheel running, which was associated with improved hippocampal synaptic plasticity and improved dendritic complexity, spine density, and neurogenesis [77].
The mechanisms mediating the therapeutic effects of adiponectin in AD, diabetes, and obesity are associated with the activation of the AdipoR1/AMPK pathway. Knocking down AdipoR1, but not AdipoR2, blocked the AdipoRon-associated increase in structural plasticity in the in vitro amyloid β-induced model of AD [16]. Moreover, blocking the AMPK activity in vivo before AdipoRon treatment abrogated its effects on spatial memory and neurogenesis in a transgenic animal model of AD [16]. A similar observation was found for Osmotin treatment, which increased hippocampal and cortical expression of AdipoR1 and the activity of its downstream protein targets, including AMPK, Akt, and PI3K in different models of AD [17]. Similarly, When the AdipoR1/AMPK pathway was blocked, Osmotin improvements in terms of neuronal metabolism, plasticity, and cognition were abrogated [17].
As addressed previously, neuronal activation is the main factor driving the high metabolic demands of the central nervous system [48, 49]. It transiently depletes the intracellular energy levels mostly due to the increased activity of ATP-driven ionic pumps intended to reestablish the membrane potential [48, 49]. In agreement with that, sustained neuronal activation can lead to depletion of intracellular energy levels excitotoxic damage [143, 144]. Interestingly, adiponectin counteracted such metabolic stress and was neuroprotective in the kainic acid- [145– 147], and glutamate-induced excitotoxicity [148], ethanol-induced apoptosis [149], and neuronal injury induced by oxygen-glucose deprivation [150]. In the kainic acid-induced excitotoxicity, Osmotin was able to increase neuronal viability and restore hippocampal synaptic plasticity [151]. Furthermore, in an animal model of vascular dementia, AdipoRon treatment increased spatial memory and rescued neuronal survival and axonal integrity in the hippocampal CA1 regions, cortex, and striatum [152].
The AMPK signaling pathway is likely to be involved in such neuroprotective properties [153]. Shortly after glutamate-induced cellular excitation, increased AMPK activity upregulates the membrane translocation of the glucose transporter 3, resulting in increased glucose influx and hyperpolarization of the mitochondria [39]. Such AMPK-dependent restoration of intracellular glucose availability is essential for a rapid recovery of the intracellular ATP levels, which increases neuronal survival and reduces excitotoxic damage [40]. Moreover, we observed that the neurogenic and pro-cognitive effects of AdipoRon treatment in diabetic mice are mediated by increased mitochondrial function [77], suggesting the neuroprotective effects of adiponectin signaling can be mediated by increased mitochondrial activity.
The role of increased central adiponectin signaling in physiological conditions, however, is less clear. In vivo electrophysiological recording of the hippocampal dentate gyrus displayed an increase in LTP formation and prevented long-term depression induction upon 10 minutes of i.c.v adiponectin infusion [154]. Interestingly, adiponectin infusion per se increased the field excitatory postsynaptic potential amplitude [154]. However, local infusion of AdipoRon reduced intrinsic neuronal excitability in the hippocampal dentate gyrus subregion [155] and downregulated spontaneous neuronal activation in the ventral tegmental area [65]. Moreover, 2 h incubation of hippocampal slices with adiponectin impaired LTP in the hippocampal CA1 region [156]. On the other hand, 10 min of AdipoRon application through bath perfusion tended to increase short-term synaptic plasticity in the hippocampal dentate gyrus [77]. Therefore, although adiponectin relevance for synaptic activity has been suggested, it is still not clear what determines the direction of its effect. One possible explanation relies on the dual role of its downstream targets, such as the AMPK signaling pathway, which could also underly the previously mentioned adiponectin paradox
Although AMPK is essential for proper synaptic function and plasticity [39, 40, 55], its overactivity can result in opposite effects. Metformin, a first-line anti-diabetic drug and strong activator of AMPK, blocks the high-frequency stimulation-induced activation of the mTOR pathway and impairs LTP maintenance in the hippocampus [157]. Moreover, 0.1 and 0.3 mM of AICAR incubation leads to increased dendritic length and branching in cultured hippocampal slices, whereas 1 mM leads to a reduction of such parameters [102]. Noteworthy, abnormal AMPK phosphorylation has been found in neuronal substrates of several neurological disorders, including Alzheimer’s and Parkinson’s disease [158]. As previously discussed, AMPK is an inhibitor of the mTOR cascade [38], whose activity is essential for protein synthesis [41] and consolidation of plasticity changes [159]. Nonetheless, if the adiponectin-associated reduction in neuronal excitability and synaptic plasticity under physiological conditions are mediated by AMPK overexpression and if such mechanism is involved in the adiponectin paradox requires further investigation.
CONCLUSION
Neuronal plasticity underlies virtually all functions of the nervous system, being especially relevant for learning and adaptation. Likewise, neuroplasticity impairments are usually reflected in aberrant cognition and mood regulation. The CNS reliance on peripheral energetic resources renders it highly vulnerable to prolonged metabolic oscillations, such as those induced by chronic stress. Novel pro-cognitive and neuropsychiatric drugs are required to consider both aspects to achieve increased efficacy. Adiponectin stands out as a candidate, as experimental research has been showing it can counteract many of the metabolic features associated with impaired neuronal plasticity, resulting in improvements of cognition and psychiatric-like behaviors in several animal models. However, further research is needed. Adiponectin overactivation in physiological conditions can result in reduced neuronal plasticity and the adiponectin paradox observed in clinical settings warns on the necessity for a deeper understanding of adiponectin signaling before therapeutic strategies are advisable.
Declaration: Some sections of the text are part of the first author’s thesis [160] and were adequate to be included in this review.
ACKNOWLEDGMENTS AND FUNDING
The work was supported by funding awarded to SY YAU (Hong Kong Research Grant Council, General Research Fund15100018; 15104019 &15104620); Seed funding support from Mental Health Research Center, and Research Institute for Smart Ageing at Hong Kong Polytechnic University.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Soria Lopez JA , González HM , Léger GC . Alzheimer’s disease. In: Handbook of Clinical Neurology [Internet]. Elsevier B.V.; 2019. p. 231-55. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128047668000133 |
[2] | Duman RS , Aghajanian GK , Sanacora G , Krystal JH . Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nature Medicine [Internet]. (2016) ;22: (3):238–49. Available from: http://dx.doi.org/10.1038/nm.4050 |
[3] | Chi S , Wang C , Jiang T , Zhu X-C , Yu J-T , Tan L . The prevalence of depression in Alzheimer’s disease: a systematic review and meta-analysis. Current Alzheimer research [Internet]. (2015) ;12: (2):189–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25654505 |
[4] | van der Kooij MA . The impact of chronic stress on energy metabolism. Molecular and Cellular Neuroscience [Internet]. (2020) ;107: :103525. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1044743120301482 |
[5] | Kapogiannis D , Mattson MP . Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. The Lancet Neurology [Internet]. (2011) ;10: (2):187–98. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1474442210702775 |
[6] | Dunbar JA , Reddy P , Davis-Lameloise N , Philpot B , Laatikainen T , Kilkkinen A , et al. Depression: An Important Comorbidity With Metabolic Syndrome in a General Population. Diabetes Care [Internet]. (2008) ;31: (12):2368–73. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/dc08-0175 |
[7] | Norwitz NG , Mota AS , Norwitz SG , Clarke K . Multi-Loop Model of Alzheimer Disease: An Integrated Perspective on the Wnt/GSK3β, α-Synuclein, and Type 3 Diabetes Hypotheses. Frontiers in Aging Neuroscience [Internet]. 2019;11. Available from: https://www.frontiersin.org/article/10.3389/fnagi.2019.00184/full |
[8] | Susan van D , Beulens JWJ , Yvonne T . van der S, Grobbee DE, Nealb B. The global burden of diabetes and its complications: an emerging pandemic. European Journal of Cardiovascular Prevention & Rehabilitation [Internet]. (2010) ;17: (1_suppl):s3–8. Available from: https://academic.oup.com/eurjpc/article/17/1 suppl/s3-s8/5932331 |
[9] | Smith KB , Smith MS . Obesity Statistics. Primary Care: Clinics in Office Practice [Internet]. (2016) ;43: (1):121–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0095454315000986 |
[10] | Brochu-Gaudreau K , Rehfeldt C , Blouin R , Bordignon V , Murphy BD , Palin M-F . Adiponectin action from head to toe. Endocrine [Internet]. (2010) ;37: (1):11–32. Available from: http://link.springer.com/10.1007/s12020-009-9278-8 |
[11] | Wang Zv , Scherer PE . Adiponectin, the past two decades. Journal of Molecular Cell Biology [Internet]. (2016) Apr 1 [cited 2019 Nov 13];8: (2):93–100. Available from: https://academic.oup.com/jmcb/article-lookup/doi/10.1093/jmcb/mjw011 |
[12] | Liu J , Guo M , Zhang D , Cheng S-Y , Liu M , Ding J , et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proceedings of the National Academy of Sciences [Internet]. (2012) ;109: (30):12248–53. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1202835109 |
[13] | Bloemer J , Pinky PD , Smith WD , Bhattacharya D , Chauhan A , Govindarajulu M , et al. Adiponectin Knockout Mice Display Cognitive and Synaptic Deficits. Frontiers in Endocrinology [Internet]. (2019) ;10: (November):1–12. Available from: https://www.frontiersin.org/article/10.3389/fendo.2019.00819/full |
[14] | Ng RC-L , Cheng O-Y , Jian M , Kwan JS-C , Ho PW-L , Cheng KK-Y , et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Molecular Neurodegeneration [Internet]. (2016) ;11: (1):71. Available from: http://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-016-0136-x |
[15] | Ng RC , Jian M , Ma OK , Bunting M , Kwan JS , Zhou G-J , et al. Chronic oral administration of adipoRon reverses cognitive impairments and ameliorates neuropathology in an Alzheimer’s disease mouse model. Molecular Psychiatry [Internet]. 2020 Mar 4; Available from: http://dx.doi.org/10.1038/s41380-020-0701-0 |
[16] | Liu B , Liu J , Wang J gong , Liu C lan , Yan H jing . AdipoRon improves cognitive dysfunction of Alzheimer’s disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway. Experimental Neurology [Internet]. (2020) ;327: (February):113249. Available from: https://doi.org/10.1016/j.expneurol.2020.113249 |
[17] | Ali T , Rehman SU , Khan A , Badshah H , Abid N bin , Kim MW , et al. Adiponectin-mimetic novel nonapeptide rescues aberrant neuronal metabolic-associated memory deficits in Alzheimer’s disease. Molecular Neurodegeneration [Internet]. (2021) Dec 13;16: (1):23. Available from: https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-021-00445-4 |
[18] | Zmora N , Suez J , Elinav E . You are what you eat: diet, health and the gut microbiota. Nature Reviews Gastroenterology & Hepatology [Internet]. (2019) ;16: (1):35–56. Available from: http://www.nature.com/articles/s41575-018-0061-2 |
[19] | Kuo T , McQueen A , Chen T-C , Wang J-C . Regulation of Glucose Homeostasis by Glucocorticoids. In: Advances in Experimental Medicine and Biology [Internet]. Springer New York LLC; 2015. pp. 99-126. Available from: http://link.springer.com/10.1007/978-1-4939-2895-8_5 |
[20] | Woo H , Hong CJ , Jung S , Choe S , Yu S-W . Chronic restraint stress induces hippocampal memory deficits by impairing insulin signaling. Molecular Brain [Internet]. (2018) ;11: (1):37. Available from: https://molecularbrain.biomedcentral.com/articles/10.1186/s13041-018-0381-8 |
[21] | van der Kooij MA , Jene T , Treccani G , Miederer I , Hasch A , Voelxen N , et al. Chronic social stress-induced hyperglycemia in mice couples individual stress susceptibility to impaired spatial memory. Proceedings of the National Academy of Sciences [Internet]. (2018) ;115: (43):E10187–96. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1804412115 |
[22] | Duman RS , Monteggia LM . A Neurotrophic Model for Stress-Related Mood Disorders. Biological Psychiatry [Internet]. (2006) ;59: (12):1116–27. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006322306002319 |
[23] | Chattarji S , Tomar A , Suvrathan A , Ghosh S , Rahman MM . Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nature Neuroscience [Internet]. (2015) ;18: (10):1364–75. Available from: http://www.nature.com/doifinder/10.1038/nn.4115 |
[24] | Picard M , Juster R-P , McEwen BS . Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nature Reviews Endocrinology [Internet]. (2014) ;10: (5):303–10. Available from: http://www.nature.com/articles/nrendo.2014.22 |
[25] | Swarbrick MM , Havel PJ . Physiological, Pharmacological, and Nutritional Regulation of Circulating Adiponectin Concentrations in Humans. Metabolic Syndrome and Related Disorders [Internet]. (2008) ;6: (2):87–102. Available from: http://www.liebertpub.com/doi/10.1089/met.2007.0029 |
[26] | Park K-G , Park KS , Kim M-J , Kim H-S , Suh Y-S , Ahn JD , et al. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Research and Clinical Practice [Internet].. (2004) ;63: (2):135–42. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168822703002523 |
[27] | Bahceci M , Gokalp D , Bahceci S , Tuzcu A , Atmaca S , Arikan S . The correlation between adiposity and adiponectin, tumor necrosis factor α, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? Journal of Endocrinological Investigation [Internet]. (2007) ;30: (3):210–4. Available from: http://link.springer.com/10.1007/BF03347427 |
[28] | He Y , Lu L , Wei X , Jin D , Qian T , Yu A , et al. The multimerization and secretion of adiponectin are regulated by TNF-alpha. Endocrine [Internet]. (2016) ;51: (3):456–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26407855 |
[29] | Lo J , Bernstein LE , Canavan B , Torriani M , Jackson MB , Ahima RS , et al. Effects of TNF-alpha neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. American journal of physiology Endocrinology and metabolism [Internet]. (2007) ;293: (1):E102–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17374698 |
[30] | Formolo DA , Lee TH-Y , Yau S-Y . Increasing Adiponergic System Activity as a Potential Treatment for Depressive Disorders. Molecular Neurobiology [Internet]. 2019 May 28;Available from: http://link.springer.com/10.1007/s12035-019-01644-3 |
[31] | Yamauchi T , Kamon J , Ito Y , Tsuchida A , Yokomizo T , Kita S , et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature [Internet]. (2003) ;423: (6941):762–9. Available from: http://www.nature.com/articles/nature01705 |
[32] | Ruan H , Dong LQ . Adiponectin signaling and function in insulin target tissues. Journal of Molecular Cell Biology [Internet]. (2016) ;8: (2):101–9. Available from: https://academic.oup.com/jmcb/article-lookup/doi/10.1093/jmcb/mjw014 |
[33] | Pálfy M , Reményi A , Korcsmáros T . Endosomal crosstalk: meeting points for signaling pathways. Trends in Cell Biology [Internet]. (2012) ;22: (9):447–56. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0962892412001079 |
[34] | Diggins NL , Webb DJ . APPL1 is a multifunctional endosomal signaling adaptor protein. Biochemical Society Transactions [Internet]. (2017) ;45: (3):771–9. Available from: https://portlandpress.com/biochemsoctrans/article/45/3/771/66938/APPL1-is-a-multifunctional-endosomal-signaling |
[35] | Yamauchi T , Nio Y , Maki T , Kobayashi M , Takazawa T , Iwabu M , et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nature Medicine [Internet]. . (2007) ;13: (3):332–9. Available from: http://www.nature.com/articles/nm1557 |
[36] | Ryu J , Galan AK , Xin X , Dong F , Abdul-Ghani MA , Zhou L , et al. APPL1 Potentiates Insulin Sensitivity by Facilitating the Binding of IRS1/2 to the Insulin Receptor. Cell Reports [Internet]. (2014) ;7: (4):1227–38. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211124714002939 |
[37] | Contreras Av , Torres N , Tovar AR . PPAR-α as a Key Nutritional and Environmental Sensor for Metabolic Adaptation. Advances in Nutrition [Internet]. . (2013) ;4: (4):439–52. Available from: https://academic.oup.com/advances/article/4/4/439/4259631 |
[38] | Hardie DG . AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature Reviews Molecular Cell Biology [Internet]. (2007) ;8: (10):774–85. Available from: http://www.nature.com/articles/nrm2249 |
[39] | Weisova P , Concannon CG , Devocelle M , Prehn JHM , Ward MW . Regulation of Glucose Transporter 3 Surface Expression by the AMP-Activated Protein Kinase Mediates Tolerance to Glutamate Excitation in Neurons. Journal of Neuroscience [Internet]. (2009) ;29: (9):2997–3008. Available from: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.0354-09.2009 |
[40] | Connolly NMC , Dussmann H , Anilkumar U , Huber HJ , Prehn JHM . Single-Cell Imaging of Bioenergetic Responses to Neuronal Excitotoxicity and Oxygen and Glucose Deprivation. Journal of Neuroscience [Internet]. (2014) ;34: (31):10192–205. Available from: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.3127-13.2014 |
[41] | Zhu J , Thompson CB . Metabolic regulation of cell growth and proliferation. Nature Reviews Molecular Cell Biology [Internet]. (2019) ;20: (7):436–50. Available from: http://www.nature.com/articles/s41580-019-0123-5 |
[42] | Suzuki T , Bridges D , Nakada D , Skiniotis G , Morrison SJ , Lin JD , et al. Inhibition of AMPK Catabolic Action by GSK3. Molecular Cell [Internet]. (2013) ;50: (3):407–19. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1097276513002505 |
[43] | Yamauchi T , Kamon J , Minokoshi Y , Ito Y , Waki H , Uchida S , et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine [Internet]. (2002) ;8: (11):1288–95. Available from: http://www.nature.com/articles/nm788 |
[44] | Yamauchi T , Kamon J , Waki H , Terauchi Y , Kubota N , Hara K , et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine [Internet].. (2001) ;7: (8):941–6. Available from: http://www.nature.com/articles/nm0801_941 |
[45] | Berg AH , Combs TP , Du X , Brownlee M , Scherer PE . The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature Medicine [Internet].. (2001) ;7: (8):947–53. Available from: http://www.nature.com/articles/nm0801_947 |
[46] | Fruebis J , Tsao T-S , Javorschi S , Ebbets-Reed D , Erickson MRS , Yen FT , et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proceedings of the National Academy of Sciences [Internet]. (2001) Feb 13 [cited 2019 Nov 13];98: (4):2005–10. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.98.4.2005 |
[47] | Mink JW , Blumenschine RJ , Adams DB . Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology [Internet]. (1981) ;241: (3):R203–12. Available from: https://www.physiology.org/doi/10.1152/ajpregu.1981.241.3.R203 |
[48] | Mergenthaler P , Lindauer U , Dienel GA , Meisel A . Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in Neurosciences [Internet]. Oct. (2013) ;36: (10):587–97. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0166223613001306 |
[49] | Harris JJ , Jolivet R , Attwell D . Synaptic Energy Use and Supply. Neuron [Internet]. (2012) ;75: (5):762–77. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0896627312007568 |
[50] | Roy A , Jana M , Corbett GT , Ramaswamy S , Kordower JH , Gonzalez FJ , et al. Regulation of Cyclic AMP Response Element Binding and Hippocampal Plasticity-Related Genes by Peroxisome Proliferator-Activated Receptor α . Cell Reports [Internet]. (2013) ;4: (4):724–37. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211124713003902 |
[51] | Wójtowicz S , Strosznajder AK , Jeżyna M , Strosznajder JB . The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochemical Research [Internet]. (2020) ;45: (5):972–88. Available from: http://link.springer.com/10.1007/s11064-020-02993-5 |
[52] | Ramamurthy S , Ronnett G . AMP-Activated Protein Kinase (AMPK) and Energy-Sensing in the Brain. Experimental Neurobiology [Internet]. (2012) ;21: (2):52–60. Available from: http://www.en-journal.org/journal/view.html?doi=10.5607/en.2012.21.2.52 |
[53] | Williams T , Courchet J , Viollet B , Brenman JE , Polleux F . AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proceedings of the National Academy of Sciences [Internet]. (2011) ;108: (14):5849–54. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1013660108 |
[54] | Ishizuka Y , Kakiya N , Witters LA , Oshiro N , Shirao T , Nawa H , et al. AMP-activated protein kinase counteracts brain-derived neurotrophic factor-induced mammalian target of rapamycin complex 1 signaling in neurons. Journal of Neurochemistry [Internet]. (2013) ;127: (1):66–77. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jnc.12362 |
[55] | Marinangeli C , Didier S , Ahmed T , Caillerez R , Domise M , Laloux C , et al. AMP-Activated Protein Kinase Is Essential for the Maintenance of Energy Levels during Synaptic Activation. iScience [Internet]. (2018) ;9: :1–13. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2589004218301652 |
[56] | Abbott M-A , Wells DG , Fallon JR . The Insulin Receptor Tyrosine Kinase Substrate p58/53 and the Insulin Receptor Are Components of CNS Synapses. The Journal of Neuroscience [Internet]. (1999) ;19: (17):7300–8. Available from: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.19-17-07300.1999 |
[57] | Hori K , Yasuda H , Konno D , Maruoka H , Tsumoto T , Sobue K . NMDA receptor-dependent synaptic translocation of insulin receptor substrate p53 via protein kinase C signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience [Internet].. (2005) ;25: (10):2670–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15758177 |
[58] | Chiu SL , Chen CM , Cline HT . Insulin Receptor Signaling Regulates Synapse Number, Dendritic Plasticity, and Circuit Function In Vivo. Neuron.. (2008) ;58: (5):708–19. |
[59] | Husi H , Ward MA , Choudhary JS , Blackstock WP , Grant SGN . Proteomic analysis of NMDA receptor-adhesion protein signaling complexes [Internet]. 2000. Available from: http://neurosci.nature.com |
[60] | Wang Y-b , Wang J-j , Wang S-h , Liu S-S , Cao J-y , Li X-m , et al. Adaptor Protein APPL1 Couples Synaptic NMDA Receptor with Neuronal Prosurvival Phosphatidylinositol 3-Kinase/Akt Pathway. Journal of Neuroscience [Internet]. (2012) ;32: (35):11919–29. Available from: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.3852-11.2012 |
[61] | Majumdar D , Nebhan CA , Hu L , Anderson B , Webb DJ . An APPL1/Akt signaling complex regulates dendritic spine and synapse formation in hippocampal neurons. Molecular and Cellular Neuroscience [Internet]. . (2011) ;46: (3):633–44. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1044743111000078 |
[62] | Wu Y , Lv X , Wang H , Qian K , Ding J , Wang J , et al. Adaptor protein APPL1 links neuronal activity to chromatin remodeling in cultured hippocampal neurons. Luo Z-G, editor. Journal of Molecular Cell Biology [Internet]. . (2021) ;13: (5):335–46. Available from: https://academic.oup.com/jmcb/article/13/5/335/5940026 |
[63] | Kos K , Harte AL , da Silva NF , Tonchev A , Chaldakov G , James S , et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. Journal of Clinical Endocrinology and Metabolism [Internet]. (2007) [cited 2019 Aug 23];92: (3):1129–36. Available from: http://www. |
[64] | Kubota N , Yano W , Kubota T , Yamauchi T , Itoh S , Kumagai H , et al. Adiponectin Stimulates AMP-Activated Protein Kinase in the Hypothalamus and Increases Food Intake. Cell Metabolism [Internet]. (2007) ;6: (1):55–68. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1550413107001593 |
[65] | Sun F , Lei Y , You J , Li C , Sun L , Garza J , et al. Adiponectin modulates ventral tegmental area dopamine neuron activity and anxiety-related behavior through AdipoR1. Molecular Psychiatry [Internet].. (2018) ;24: (1):126–44. Available from: http://dx.doi.org/10.1038/s41380-018-0102-9 |
[66] | Li C , Meng F , Garza JC , Liu J , Lei Y , Kirov SA , et al. Modulation of depression-related behaviors by adiponectin AdipoR1 receptors in 5-HT neurons. Molecular Psychiatry [Internet]. 2020; Available from: http://dx.doi.org/10.1038/s41380-020-0649-0 |
[67] | Lee TH-Y , Formolo DA , Kong T , Lau SW-Y , Ho CS-L , Leung RYH , et al. Potential exerkines for physical exercise-elicited pro-cognitive effects: Insight from clinical and animal research. In: International Review of Neurobiology [Internet]. Academic Press Inc.; 2019. pp. 361-95. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0074774219300224 |
[68] | Zhang D , Wang X , Lu X-Y . Adiponectin Exerts Neurotrophic Effects on Dendritic Arborization, Spinogenesis, and Neurogenesis of the Dentate Gyrus of Male Mice. Endocrinology [Internet]. (2016) ;157: (7):2853–69. Available from: https://academic.oup.com/endo/article/157/7/2853/2422920 |
[69] | Yau SY , Li A , Hoo RLC , Ching YP , Christie BR , Lee TMC , et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proceedings of the National Academy of Sciences of the United States of America [Internet]. (2014) ;111: (44):15810–5. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1415219111 |
[70] | Nielsen MB , Çolak Y , Benn M , Nordestgaard BG . Low Plasma Adiponectin in Risk of Type 2 Diabetes: Observational Analysis and One- and Two-Sample Mendelian Randomization Analyses in 756,219 Individuals. Diabetes [Internet]. (2021) ;70: (11):2694–705. Available from: http://diabetes.diabetesjournals.org/lookup/doi/10.2337/db21-0131 |
[71] | Bellissimo MP , Hsu E , Hao L , Easley K , Martin GS , Ziegler TR , et al. Relationships between plasma apelin and adiponectin with normal weight obesity, body composition, and cardiorespiratory fitness in working adults. Journal of Clinical & Translational Endocrinology [Internet]. (2021) ;24: :100257. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214623721000090 |
[72] | Engin A . Adiponectin-Resistance in Obesity. In: Advances in Experimental Medicine and Biology [Internet]. Springer New York LLC; 2017. pp. 415-41.Available from: http://link.springer.com/10.1007/978-3-319-48382-5_18 |
[73] | Vuong E , Nothling J , Lombard C , Jewkes R , Peer N , Abrahams N , et al. Peripheral adiponectin levels in anxiety, mood, trauma- and stressor-related disorders: A systematic review and meta-analysis. Journal of Affective Disorders [Internet]. (2020) ;260: :372–409. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165032719306111 |
[74] | Li Z , Chen J , Feng Y , Zhong S , Tian S , Dai Z , et al. Differences in verbal and spatial working memory in patients with bipolar II and unipolar depression: an MSI study. BMC Psychiatry [Internet]. (2021) ;21: (1):568. Available from: https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-021-03595-3 |
[75] | Walsh JJ , Caldwell HG , Neudorf H , Ainslie PN , Little JP . Short-term ketone monoester supplementation improves cerebral blood flow and cognition in obesity: A randomized cross-over trial. The Journal of Physiology [Internet]. (2021) ;599: (21):4763–78. Available from: https://onlinelibrary.wiley.com/doi/10.1113/JP281988 |
[76] | Anita NZ , Zebarth J , Chan B , Wu C-Y , Syed T , Shahrul D , et al. Inflammatory markers in type 2 diabetes with vs. without cognitive impairment; A systematic review and meta-analysis. Brain, Behavior, and Immunity [Internet]. 2021 Nov; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0889159121006012 |
[77] | Lee TH , Ahadullah , Christie BR , Lin K , Siu PM , Zhang L , et al. Chronic AdipoRon Treatment Mimics the Effects of Physical Exercise on Restoring Hippocampal Neuroplasticity in Diabetic Mice. Molecular Neurobiology [Internet]. 2021; Available from: https://link.springer.com/10.1007/s12035-021-02441-7 |
[78] | Song J , Kang SM , Kim E , Kim C-H , Song H-T , Lee JE . Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study. Cell Death & Disease [Internet]. (2015) ;6: (8):e1844–e1844. Available from: http://www.nature.com/articles/cddis2015220 |
[79] | McEwen BS , Wingfield JC . The concept of allostasis in biology and biomedicine. Hormones and Behavior [Internet]. (2003) ;43: (1):2–15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0018506X02000247 |
[80] | Collao N , Farup J , de Lisio M . Role of Metabolic Stress and Exercise in Regulating Fibro/Adipogenic Progenitors. Frontiers in Cell and Developmental Biology [Internet]. 2020;8. Available from: https://www.frontiersin.org/article/10.3389/fcell.2020.00009/full |
[81] | Berjot S , Gillet N . Stress and Coping with Discrimination and Stigmatization. Frontiers in Psychology [Internet]. 2011;2(FEB). Available from: http://journal.frontiersin.org/article/10.3389/fpsyg.2011.00033/abstract |
[82] | Ness D , Calabrese P . Stress Effects on Multiple Memory SystemInteractions.Neural Plasticity [Internet]. 2016; 2016:1-20. Available from: http://www.hindawi.com/journals/np/2016/4932128/ |
[83] | Hackett RA , Steptoe A . Type 2 diabetes mellitus and psychological stress — a modifiable risk factor. Nature Reviews Endocrinology [Internet]. (2017) ;13: (9):547–60. Available from: http://www.nature.com/articles/nrendo.2017.64 |
[84] | Patchev VK , Patchev A v , Schering Pharma B . Experimental models of stress. Dialogues in Clinical Neuroscience [Internet]. (2006) ;8: (4):417–32. Available from: https://pubmed.ncbi.nlm.nih.gov/17290800/ |
[85] | Mohammed MA , Mahmoud MO , Awaad AS , Gamal GM , Abdelfatah D . Alpha lipoic acid protects against dexamethasone-induced metabolic abnormalities via APPL1 and PGC-1 α up regulation. Steroids [Internet]. (2019) ;144: :1–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0039128X19300066 |
[86] | Bönisch C , Irmler M , Brachthäuser L , Neff F , Bamberger MT , Marschall S , et al. Dexamethasone treatment alters insulin, leptin, and adiponectin levels in male mice as observed in DIO but does not lead to alterations of metabolic phenotypes in the offspring. Mammalian Genome [Internet]. (2016) ;27: (1-2):17–28. Available from: http://link.springer.com/10.1007/s00335-015-9616-5 |
[87] | Na K-S , Kim E-K , Park J-T . Decreased plasma adiponectin among male firefighters with symptoms of post-traumatic stress disorder. Journal of Affective Disorders [Internet]. (2017) ;221: :254–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165032716313970 |
[88] | Dang TQ , Yoon N , Chasiotis H , Dunford EC , Feng Q , He P , et al. Transendothelial movement of adiponectin is restricted by glucocorticoids. Journal of Endocrinology [Internet]. (2017) ;234: (2):101–14. Available from: https://joe.bioscientifica.com/view/journals/joe/234/2/JOE-16-0363.xml |
[89] | Zhao Y , Zhang Q , Shao X , Ouyang L , Wang X , Zhu K , et al. Decreased Glycogen Content Might Contribute to Chronic Stress-Induced Atrophy of Hippocampal Astrocyte volume and Depression-like Behavior in Rats. Scientific Reports [Internet]. (2017) ;7: (1):43192. Available from: http://www.nature.com/articles/srep43192 |
[90] | Tagliari B , Noschang CG , Ferreira AGK , Ferrari OA , Feksa LR , Wannmacher CMD , et al. Chronic variable stress impairs energy metabolism in prefrontal cortex and hippocampus of rats: prevention by chronic antioxidant treatment. Metabolic Brain Disease [Internet]. . (2010) ;25: (2):169–76. Available from: http://link.springer.com/10.1007/s11011-010-9194-x |
[91] | Detka J , Kurek A , Kucharczyk M , Głombik K , Basta-Kaim A , Kubera M , et al. Brain glucose metabolism in an animal model of depression. Neuroscience [Internet].. (2015) ;295: :198–208. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306452215002833 |
[92] | Nisbett KE , Pinna G . Emerging therapeutic role of PPARα in cognition and emotions. Vol. 9, Frontiers in Pharmacology. Frontiers Media S.A.; 2018. |
[93] | Tang W , Wang L , Wang Y , Zong Z , Gao Z , Liu X , et al. Peroxisome proliferator-activated receptor-α activation protects against endoplasmic reticulum stress-induced HepG2 cell apoptosis. Molecular and Cellular Biochemistry. (2014) ;385: (1-2). |
[94] | Cheng HS , Tan WR , Low ZS , Marvalim C , Lee JYH , Tan NS . Exploration and development of PPAR modulators in health and disease: An update of clinical evidence.Vol. 20, International Journal of Molecular Sciences. MDPI AG; 2019. |
[95] | Chawla A , Repa JJ , Evans RM , Mangelsdorf DJ . Nuclear Receptors and Lipid Physiology: Opening the X-Files. Science. (2001) ;294: (5548). |
[96] | Warden A , Truitt J , Merriman M , Ponomareva O , Jameson K , Ferguson LB , et al. Localization of PPAR isotypes in the adult mouse and human brain. Scientific Reports. (2016) ;6: (1). |
[97] | Castrén E , Rantamäki T . The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Developmental Neurobiology. (2010) ;70: (5). |
[98] | Song L , Wang H , Wang YJ , Wang JL , Zhu Q , Wu F , et al. Hippocampal PPARα is a novel therapeutic target for depression and mediates the antidepressant actions of fluoxetine in mice. British Journal of Pharmacology. . (2018) ;175: (14):2968–87. |
[99] | Yuan S-Y , Liu J , Zhou J , Lu W , Zhou H-Y , Long L-H , et al. AMPK Mediates Glucocorticoids Stress-Induced Downregulation of the Glucocorticoid Receptor in Cultured Rat Prefrontal Cortical Astrocytes. Bertrand L, editor. PLOS ONE [Internet]. (2016) ;11: (8):e0159513. Available from: https://dx.plos.org/10.1371/journal.pone.0159513 |
[100] | Ma R , Zhou G , Qu M , Yi J , Tang Y , Yang X , et al. Corticosterone induces neurotoxicity in PC12 cells via disrupting autophagy flux mediated by AMPK/mTOR signaling. CNS Neuroscience & Therapeutics [Internet]. (2020) ;26: (2):167–76. Available from: https://onlinelibrary.wiley.com/doi/10.1111/cns.13212 |
[101] | Kim D-M , Leem Y-H . Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience [Internet]. . (2016) ;324: :271–85. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306452216002426 |
[102] | Leem Y-H , Chang H . The ameliorating effect of exercise on long-term memory impairment and dendritic retraction via the mild activation of AMP-activated protein kinase in chronically stressed hippocampal CA1 neurons. Journal of Exercise Nutrition & Biochemistry [Internet]. . (2018) ;22: (3):35–41. Available from: http://e-pan.org/journal/view.php?doi=10.20463/jenb.2018.0022 |
[103] | Yu X , Hu Y , Huang W , Ye N , Yan Q , Ni W , et al. Role of AMPK/SIRT1-SIRT3 signaling pathway in affective disorders in unpredictable chronic mild stress mice. Neuropharmacology [Internet]. (2020) ;165: :107925. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0028390819304964 |
[104] | Zhu S , Wang J , Zhang Y , Li V , Kong J , He J , et al. Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Research [Internet]. (2014) ;1576: :81–90. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006899314007847 |
[105] | Woolley CS , Gould E , McEwen BS . Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Research [Internet]. (1990) ;531: (1-2):225–31. Available from: https://linkinghub.elsevier.com/retrieve/pii/000689939090778A |
[106] | Watanabe Y , Gould E , Cameron HA , Daniels DC , McEwen BS . Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus [Internet]. (1992) ;2: (4):431–5. Available from: https://onlinelibrary.wiley.com/doi/10.1002/hipo.450020410 |
[107] | Xu SX , Zhou ZQ , Li XM , Ji MH , Zhang GF , Yang JJ . The activation of adenosine monophosphate-activated protein kinase in rat hippocampus contributes to the rapid antidepressant effect of ketamine. Behavioural Brain Research. (2013) ;253: :305–9. |
[108] | Suzuma K , Naruse K , Suzuma I , Takahara N , Ueki K , Aiello LP , et al. Vascular Endothelial Growth Factor Induces Expression of Connective Tissue Growth Factor via KDR, Flt1, and Phosphatidylinositol 3-Kinase-Akt-dependent Pathways in Retinal Vascular Cells. Journal of Biological Chemistry. (2000) ;275: (52). |
[109] | Woo H , Hong CJ , Jung S , Choe S , Yu SW . Chronic restraint stress induces hippocampal memory deficits by impairing insulin signaling. Molecular Brain. (2018) ;11: (1). |
[110] | Howell KR , Kutiyanawalla A , Pillai A . Long-Term Continuous Corticosterone Treatment Decreases VEGF Receptor-2 Expression in Frontal Cortex. PLoS ONE. (2011) ;6: (5). |
[111] | Han B , Yu L , Geng Y , Shen L , Wang H , Wang Y , et al. Chronic Stress Aggravates Cognitive Impairment and Suppresses Insulin Associated Signaling Pathway in APP/PS1 Mice. Journal of Alzheimer’s Disease.. (2016) ;53: (4):1539–52. |
[112] | Bi T , Zhan L , Zhou W , Sui H . Effect of the ZiBuPiYin Recipe on Diabetes-Associated Cognitive Decline in Zucker Diabetic Fatty Rats After Chronic Psychological Stress. Frontiers in Psychiatry. 2020;11. |
[113] | Mai L , Jope RS , Li X . BDNF-mediated signal transduction is modulated by GSK3β and mood stabilizing agents. Journal of Neurochemistry. (2002) ;82: (1). |
[114] | Gomez O , Sanchez-Rodriguez A , Le M , Sanchez-Caro C , Molina-Holgado F , Molina-Holgado E . Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. British Journal of Pharmacology. (2011) ;163: (7). |
[115] | Polter A , Yang S , Zmijewska AA , van Groen T , Paik J-H , DePinho RA , et al. Forkhead Box, Class O Transcription Factors in Brain: Regulation and Behavioral Manifestation. Biological Psychiatry.(2009) ;65: (2). |
[116] | Wang J , Lu W , Chen L , Zhang P , Qian T , Cao W , et al. Serine 707 of APPL1 is Critical for the Synaptic NMDA Receptor-Mediated Akt Phosphorylation Signaling Pathway. Neuroscience Bulletin [Internet]. . (2016) ;32: (4):323–30. Available from: http://link.springer.com/10.1007/s12264-016-0042-9 |
[117] | Fernández-Monreal M , Sánchez-Castillo C , Esteban JA . APPL1 gates long-term potentiation via its plekstrin homology domain. Journal of Cell Science [Internet]. (2016) ;129: (14):2793–803. Available from: https://journals.biologists.com/jcs/article/doi/10.1242/jcs.183475/260163/APPL1-gates-long-term-potentiation-via-its |
[118] | Ogawa A , Yamazaki Y , Nakamori M , Takahashi T , Kurashige T , Hiji M ,et al.. Characterization and distribution of adaptor protein containing a PH domain, PTB domain and leucine zipper motif (APPL1) in Alzheimer’s disease hippocampus: an immunohistochemical study. Brain Research [Internet]. (2013) ;1494: :118–24. Available from: https://linkinghub.elsevier.com/retrieve/pii/S000689931201877X |
[119] | Chen C , Shen JH , Xu H , Chen P , Chen F , Guan YX ,et al.. Hippocampal PPARα is involved in the antidepressant-like effects of venlafaxine in mice. Brain Research Bulletin. (2019) ;153: :171–80. |
[120] | Scheggi S , Melis M , de Felice M , Aroni S , Muntoni AL , Pelliccia T ,et al.. PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors. Neuropharmacology. (2016) ;110: :251–9. |
[121] | Duman RS , Li N , Liu R-J , Duric V , Aghajanian G . Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology [Internet].. (2012) ;62: (1):35–41. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3195863/pdf/nihms327857.pdf |
[122] | Vaynman S , Ying Z , Gomez-Pinilla F . Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. (2004) ;20: (10):2580–90. |
[123] | Li N , Lee B , Liu RJ , Banasr M , Dwyer JM , Iwata M ,et al.. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. (2010) ;329: (5994):959–64. |
[124] | Wang H , Quirion R , Little PJ , Cheng Y , Feng Z-P , Sun H-S ,et al.. Forkhead box O transcription factors as possible mediators in the development of major depression. Neuropharmacology. (2015) ;99: . |
[125] | Wang H , Zhou X , Huang J , Mu N , Guo Z , Wen Q ,et al.. The role of Akt/FoxO3a in the protective effect of venlafaxine against corticosterone-induced cell death in PC12 cells. Psychopharmacology. (2013) ;228: (1). |
[126] | Kadowaki T , Yamauchi T . Adiponectin and Adiponectin Receptors. Endocrine Reviews [Internet].. (2005) ;26: (3):439–51. Available from: https://academic.oup.com/edrv/article/26/3/439/2355263 |
[127] | Okada-Iwabu M , Yamauchi T , Iwabu M , Honma T , Hamagami KI , Matsuda K ,et al.. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature [Internet]. (2013) ;503: (7477):493–9. Available from: http://dx.doi.org/10.1038/nature12656 |
[128] | Anil Kumar S , Hima Kumari P , Shravan Kumar G , Mohanalatha C , Kavi Kishor PB . Osmotin: A plant sentinel and a possible agonist of mammalian adiponectin. Frontiers in Plant Science. (2015) ;6: (MAR) |
[129] | de la Monte SM , Tong M . Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochemical Pharmacology [Internet]. (2014) ;88: (4):548–59. Available from: https://linkinghub.elsevier.com/retrieve/pii/S000629521300796X |
[130] | Wang M , Jo J , Song J . Adiponectin improves long-term potentiation in the 5XFAD mouse brain. Scientific Reports [Internet]. (2019) [cited 2019 Aug 23];9: (1). Available from: https://doi.org/10.1038/s41598-019-45509-0 |
[131] | Une K , Takei YA , Tomita N , Asamura T , Ohrui T , Furukawa K ,et al.. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. European Journal of Neurology [Internet]. (2011) ;18: (7):1006–9. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1468-1331.2010.03194.x |
[132] | van Himbergen TM , Beiser AS , Ai M , Seshadri S , Otokozawa S , Au R ,et al.. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Archives of neurology [Internet].. (2012) ;69: (5):594–600. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22213409 |
[133] | Khemka VK , Bagchi D , Bandyopadhyay K , Bir A , Chattopadhyay M , Biswas A ,et al.. Altered Serum Levels of Adipokines and Insulin in Probable Alzheimer’s Disease. Journal of Alzheimer’s Disease [Internet].. (2014) ;41: (2):525–33. Available from: https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD-140006 |
[134] | Wennberg AMV , Gustafson D , Hagen CE , Roberts RO , Knopman D , Jack C ,et al.. Serum Adiponectin Levels, Neuroimaging, and Cognition in the Mayo Clinic Study of Aging. Bowman G, editor. Journal of Alzheimer’s Disease [Internet]. (2016) ;53: (2):573–81. Available from: https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD-151201 |
[135] | Menzaghi C , Trischitta V . The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes.. (2018) ;67: (1):12–22. |
[136] | Sente T , van Berendoncks AM , Hoymans VY , Vrints CJ . Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. Journal of Cachexia, Sarcopenia and Muscle [Internet].. (2016) ;7: (3):261–74. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jcsm.12086 |
[137] | Arita Y , Kihara S , Ouchi N , Takahashi M , Maeda K , Miyagawa J ,et al.. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and biophysical research communications [Internet].. (1999) ;257: (1):79–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10092513 |
[138] | Yau S-Y , Lee TH-Y , Li A , Xu A , So K-F . Adiponectin Mediates Running-Restored Hippocampal Neurogenesis in Streptozotocin-Induced Type 1 Diabetes in Mice. Frontiers in Neuroscience [Internet]. (2018) ;12: (OCT):1–10. Available from: https://www.frontiersin.org/article/10.3389/fnins.2018.00679/full |
[139] | Song J , Kang SM , Kim E , Kim C-H , Song H-T , Lee JE . Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study. Cell Death & Disease [Internet]. (2015) ;6: (8):e1844–e1844. Available from: http://www.nature.com/articles/cddis2015220 |
[140] | Numao S , Katayama Y , Hayashi Y , Matsuo T , Tanaka K . Influence of acute aerobic exercise on adiponectin oligomer concentrations in middle-aged abdominally obese men. Metabolism: Clinical and Experimental. . (2011) ;60: (2):186–94. |
[141] | Racil G , ben Ounis O , Hammouda O , Kallel A , Zouhal H , Chamari K ,et al.. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. European journal of applied physiology [Internet]. (2013) ;113: (10):2531–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23824463 |
[142] | Schön M , Kovaničová Z , Košutzká Z , Nemec M , Tomková M , Jacková L ,et al.. Effects of running on adiponectin, insulin and cytokines in cerebrospinal fluid in healthy young individuals. Scientific Reports. (2019) ;9: (1). |
[143] | Armada-Moreira A , Gomes JI , Pina CC , Savchak OK , Gonçalves-Ribeiro J , Rei N ,et al.. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Frontiers in Cellular Neuroscience [Internet]. 2020;14. Available from: https://www.frontiersin.org/article/10.3389/fncel.2020.00090/full |
[144] | Dong X , Wang Y , Qin Z . Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacologica Sinica [Internet]. (2009) ;30: (4):379–87. Available from: http://www.nature.com/articles/aps200924 |
[145] | Jeon BT , Shin HJ , Kim J bin , Kim YK , Lee DH , Kim KH ,et al.. Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain Research Reviews [Internet]. (2009) ;61: (2):81–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S016501730900054X |
[146] | Shah SA , Lee HY , Bressan RA , Yun DJ , Kim MO . Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death and Disease. 2014; |
[147] | Qiu G , Wan R , Hu J , Mattson MP , Spangler E , Liu S ,et al.. Adiponectin protects rat hippocampal neurons against excitotoxicity. AGE [Internet]. (2011) ;33: (2):155–65. Available from: http://link.springer.com/10.1007/s11357-010-9173-5 |
[148] | Yue L , Zhao L , Liu H , Li X , Wang B , Guo H ,et al.. Adiponectin Protects against Glutamate-Induced Excitotoxicity via Activating SIRT1-Dependent PGC-1 α Expression in HT22 Hippocampal Neurons. Oxidative Medicine and Cellular Longevity [Internet]. . (2016) ;2016: :1–12. Available from: https://www.hindawi.com/journals/omcl/2016/2957354/ |
[149] | Naseer MI , Ullah I , Narasimhan ML , Lee HY , Bressan RA , Yoon GH ,et al.. Neuroprotective effect of osmotin against ethanol-induced apoptotic neurodegeneration in the developing rat brain. Cell Death & Disease [Internet]. (2014) ;5: (3):e1150–e1150. Available from: http://www.nature.com/articles/cddis201453 |
[150] | Wang B , Guo H , Li X , Yue L , Liu H , Zhao L ,et al.. Adiponectin Attenuates Oxygen-Glucose Deprivation-Induced Mitochondrial Oxidative Injury and Apoptosis in Hippocampal HT22 Cells via the JAK2/STAT3 Pathway. Cell Transplantation [Internet].. (2018) ;27: (12):1731–43. Available from: http://journals.sagepub.com/doi/10.1177/0963689718779364 |
[151] | Shah SA , Lee HY , Bressan RA , Yun DJ , Kim MO . Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death & Disease [Internet]. (2014) Jan 30 [cited 2019 Oct 25];5: (1):1026. Available from: http://www.nature.com/articles/cddis2013538 |
[152] | Miao W , Jiang L , Xu F , Lyu J , Jiang X , He M ,et al.. Adiponectin ameliorates hypoperfusive cognitive deficits by boosting a neuroprotective microglial response. Progress in Neurobiology [Internet]. (2021) ;205: :102125. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0301008221001398 |
[153] | Qiu G , Wan R , Hu J , Mattson MP , Spangler E , Liu S ,et al.. Adiponectin protects rat hippocampal neurons against excitotoxicity. AGE [Internet]. (2011) Jun 15;33: (2):155–65. Available from: http://link.springer.com/10.1007/s11357-010-9173-5 |
[154] | Pousti F , Ahmadi R , Mirahmadi F , Hosseinmardi N , Rohampour K . Adiponectin modulates synaptic plasticity in hippocampal dentate gyrus. Neuroscience Letters [Internet]. (2018) Jan [cited 2019 Jul 1];662: :227–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304394017308686 |
[155] | Zhang D , Wang X , Wang B , Garza JC , Fang X , Wang J ,et al.. Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Molecular Psychiatry [Internet]. (2017) ;22: (7):1044–55. Available from: http://www.nature.com/doifinder/10.1038/mp.2016.58 |
[156] | Weisz F , Piccinin S , Mango D , Ngomba RT , Mercuri NB , Nicoletti F ,et al.. The role of adiponectin receptors in the regulation of synaptic transmission in the hippocampus. Synapse [Internet]. (2017) ;71: (5):e21964. Available from: http://doi.wiley.com/10.1002/syn.21964 |
[157] | Potter WB , O’Riordan KJ , Barnett D , Osting SMK , Wagoner M , Burger C ,et al.. Metabolic Regulation of Neuronal Plasticity by the Energy Sensor AMPK. Manzoni OJ, editor. PLoS ONE [Internet].e. (2010) ;5: (2):8996. Available from: https://dx.plos.org/10.1371/journal.pone.0008996 |
[158] | Vingtdeux V , Davies P , Dickson DW , Marambaud P . AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathologica [Internet].. (2011) ;121: (3):337–49. Available from: http://link.springer.com/10.1007/s00401-010-0759-x |
[159] | Hoeffer CA , Klann E . mTOR signaling: At the crossroads of plasticity, memory and disease. Trends in Neurosciences [Internet]. (2010) ;33: (2):67–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0166223609001878 |
[160] | Formolo DA . Adiponectin as a rapid-acting antidepressant: an investigation of its mechanisms of action. 2021. |




