Contributions of Adult-Generated Granule Cells to Hippocampal Pathology in Temporal Lobe Epilepsy: A Neuronal Bestiary
Abstract
Hippocampal neurogenesis continues throughout life in mammals – including humans. During the development of temporal lobe epilepsy, newly-generated hippocampal granule cells integrate abnormally into the brain. Abnormalities include ectopic localization of newborn cells, de novo formation of abnormal basal dendrites, and disruptions of the apical dendritic tree. Changes in granule cell position and dendritic structure fundamentally alter the types of inputs these cells are able to receive, as well as the relative proportions of remaining inputs. Dendritic abnormalities also create new pathways for recurrent excitation in the hippocampus. These abnormalities are hypothesized to contribute to the development of epilepsy, and may underlie cognitive disorders associated with the disease as well. To test this hypothesis, investigators have used pharmacological and genetic strategies in animal models to alter neurogenesis rates, or ablate the newborn cells outright. While findings are mixed and many unanswered questions remain, numerous studies now demonstrate that ablating newborn granule cells can have disease modifying effects in epilepsy. Taken together, findings provide a strong rationale for continued work to elucidate the role of newborn granule cells in epilepsy: both to understand basic mechanisms underlying the disease, and as a potential novel therapy for epilepsy.
BODY
Hippocampal granule cell structure
The hippocampal dentate gyrus contains three primary subregions: the dentate molecular layer, the granule cell body layer and the dentate hilus. Hippocampal dentate granule cell axons arborize in the hilus, their cell bodies are restricted to the tightly-packed cell body layer, and their dendrites occupy the molecular layer. The molecular layer is further subdivided into inner, middle and outer lamina. Axons from excitatory mossy cells, interneurons and neurons from the contralateral hippocampus [1] target the inner molecular layer, while inputs from medial and lateral entorhinal cortex target the middle and outer molecular layers, respectively (Fig. 1). Within the granule cell body layer, cell positioning tends to follow an age gradient, with the oldest cells located proximal to the molecular layer, while cells generated later in development take up positions closer to the hilus. This gradient follows from an “outside-in” pattern of proliferation and migration. Specifically, during development, granule cells are produced from precursors located in what will become the hilar region, and migrate outward to form the cell body layer [2]. Later-generated granule cells take up positions beneath previously generated neurons, forming the age gradient. Adult-generated granule cells are produced from a residual population of progenitor cells located in the subgranular zone, a thin layer of cells sandwiched between the granule cell body layer and the dentate hilus. Migration of adult-generated neurons follows the developmental pattern, with the new cells typically migrating only tens of microns from the subgranular zone to occupy the innermost region of the granule cell body layer [3, 4]. The precise structural organization of the dentate – with afferents restricted to specific layers and granule cell dendrites developing to meet these inputs – has profound functional consequences for dysmorphic granule cells.
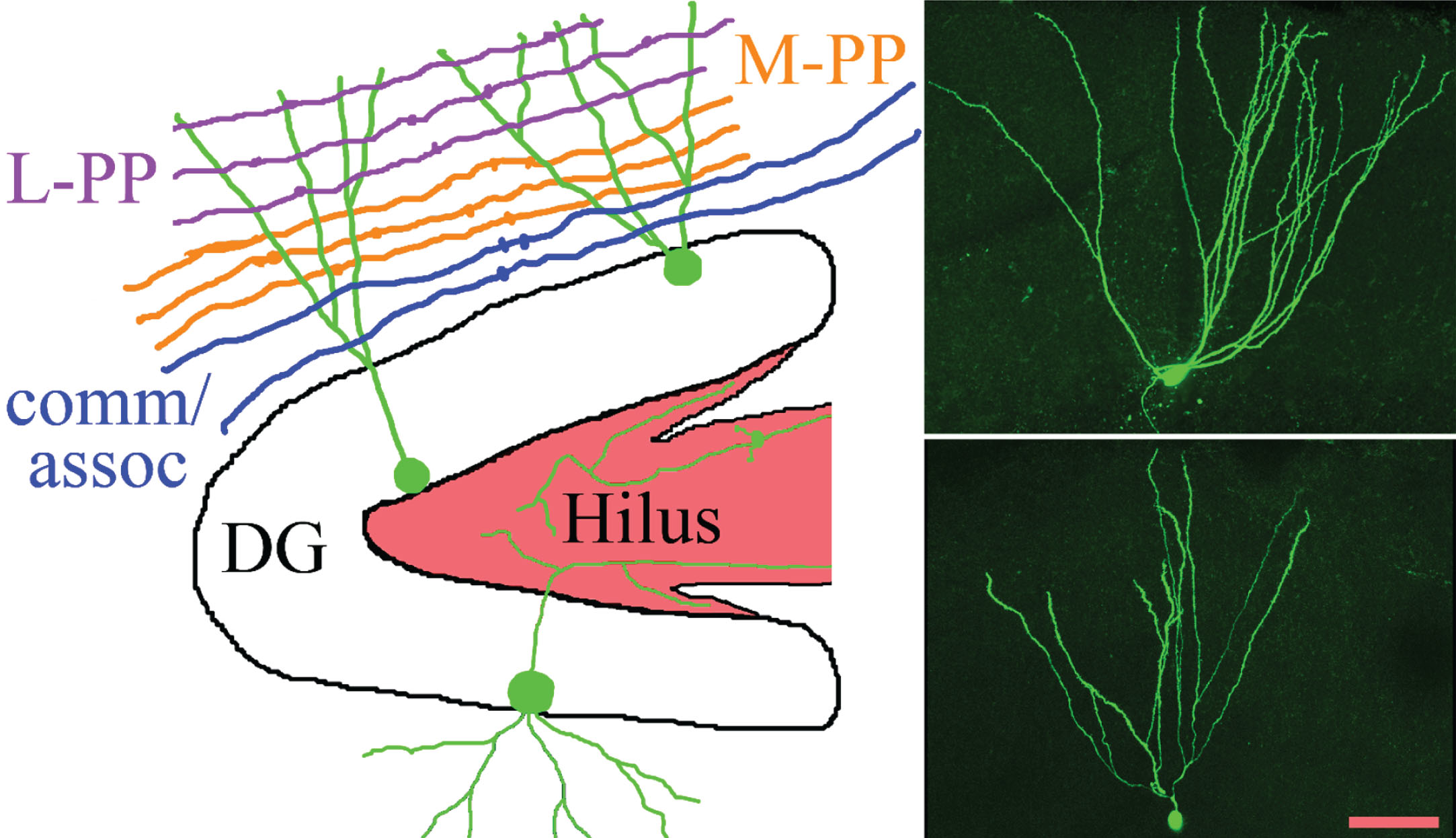
Fig.1
The schematic shows the gross organization of afferent inputs to the molecular layer of the dentate gyrus. Inputs from lateral perforant path (L-PP), medial perforant path (M-PP) and from associational/commissural fibers (comm/assoc) target the outer, middle and inner molecular layer, respectively. Hippocampal granule cells (green) possess cell bodies located in the dentate granule cell body layer (DG) and project dendrites into the molecular layer. Granule cell mossy fiber axons project into the hilus. Micrographs show two biocytin-filled granule cells, revealing the characteristic fanlike spread of the dendritic trees. Scale bar = 50μm.
Here, common somatic and dendritic abnormalities exhibited by granule cells in the epileptic brain will be reviewed. The functional implications of these abnormalities will also be discussed. Granule cell axonal abnormalities (i.e. mossy fiber sprouting) have been reviewed extensively elsewhere [5–7], so they will not be covered in-depth.
Ectopic granule cells
Ectopic granule cells are a common finding in temporal lobe epilepsy, and manifest in two discrete locations: the dentate hilus and the dentate molecular layer. The mechanisms regulating the appearance of abnormal cells in these two regions appear to be distinct. This is inferred from the observation that aberrant migration into the dentate hilus appears to affect primarily newly-generated granule cells, while migration in the opposite direction – into the dentate molecular layer – can affect new and old granule cells.
Hilar ectopic granule cells have been consistently observed in animal models of temporal epilepsy [8] and in patients with the disease [9]. Cell birthdating studies indicate that the vast majority of hilar ectopic cells are newly-generated. Cells born after an epileptogenic insult appear in the dentate hilus, while cells born before the insult do not [10]. Recently, Singh and colleagues [11] used a clonal analysis approach to explore the role of individual granule cell progenitors in producing hilar ectopic granule cells. Using the Brainbow transgene to express distinct fluorochromes in individual granule cell progenitors, they were able to “color code” clonal clusters; the grouping of daughter cells produced by an individual progenitor. This study revealed that progenitor cells that produced hilar ectopic daughter cells tended to generate only ectopic cells, while progenitors that produced cells correctly localized to the granule cell body layer continued to produce only positionally-normal cells (Fig. 2). These findings suggest cell-intrinsic or regionally localized abnormalities drive hilar ectopic cell migration. An abnormal progenitor cell – producing primarily ectopic daughter cells – could account for the observed findings. Alternatively, the progenitor cell may be functionally normal, but it could be localized to a region in which migratory cues are disrupted, with this local disruption driving ectopic cell generation.
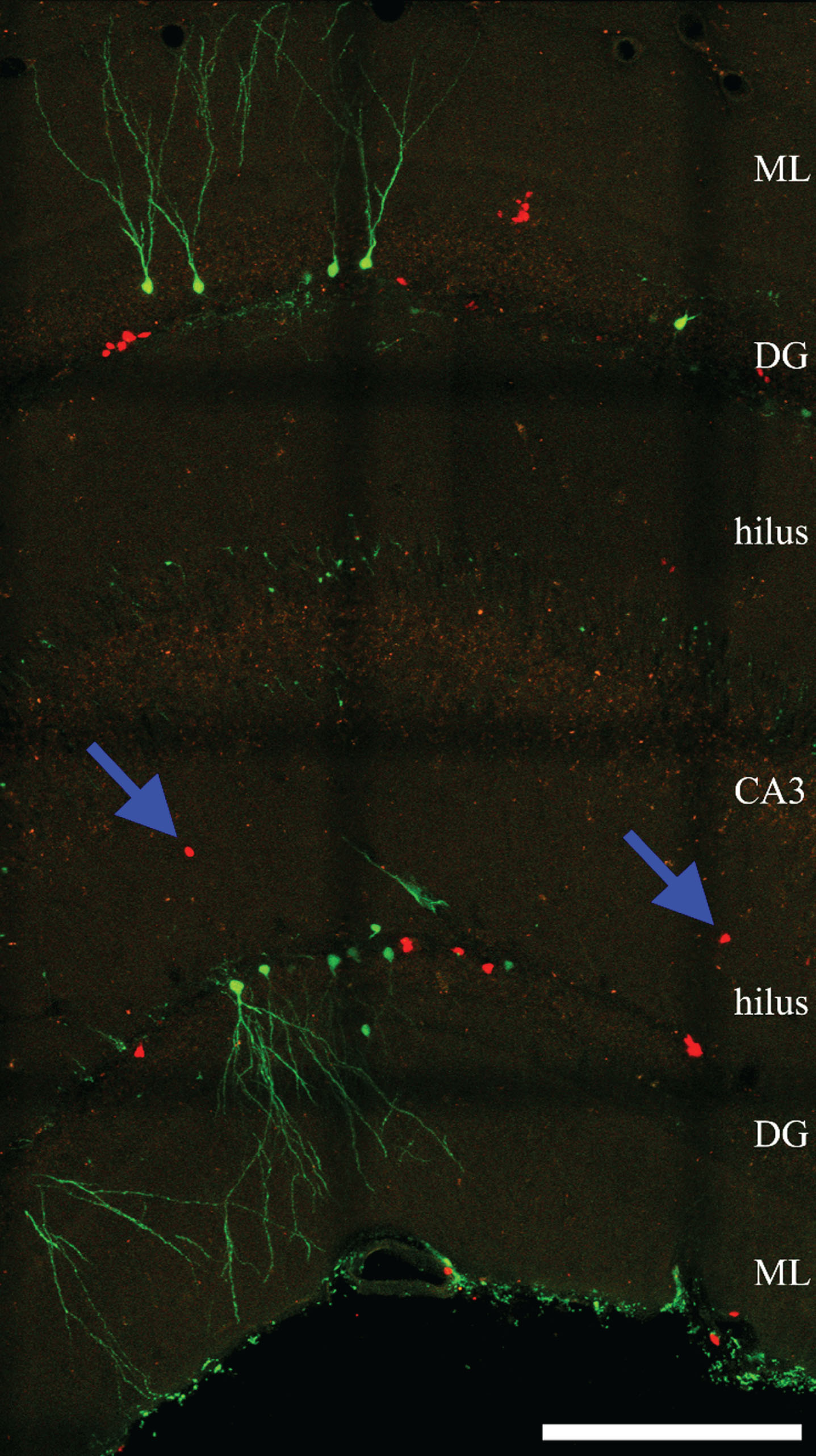
Fig.2
Confocal maximum projection of YFP- and RFP-expressing newly-generated granule cells from an epileptic Gli1-CreERT2, Gt(ROSA)26Sortm1 (CAG - Brainbow2.1) Cle/J “brainbow” reporter mouse (Singh et al., 2016). Blue arrows denote ectopic RFP-expressing cells located in the dentate hilus. ML, molecular layer. DG, dentate granule cell layer. CA3, CA3 pyramidal cell layer. Scale bar = 200μm.
Granule cell dispersion also leads to malpositioning of granule cells in epilepsy, but the phenomena is anatomically and mechanistically distinct from hilar ectopic granule cells. Granule cell dispersion occurs when the normally compact granule cell body layer spreads, and granule cell somas migrate into the molecular layer. In contrast to hilar ectopic granule cells, dispersion into the molecular layer can affect both newly-generated and mature granule cells. Neurogenesis is not required for the phenomena [12, 13], and dispersion can lead to repositioning of the majority of granule cells in the dentate gyrus (Fig. 3). Granule cell dispersion is most prominent in the rodent intrahippocampal kainic acid model of epilepsy [14], but occurs to a lesser extent in the systemic kainic acid model [15], and is also evident in tissue resected from patients with epilepsy [16]. In the kainic acid model, dispersion appears to be driven in part by somatic hypertrophy. Granule cell somas more than double in size, which, presumably, forces the cell body layer to spread [17]. More significantly, however, down-regulation of reelin in the molecular layer has been shown to be a driving force. Reelin is a secreted glycoprotein that plays an important role in regulating neuronal migration during development. Following epileptogenic brain injury, reelin expression decreases [18, 19]. Moreover, blocking reelin function in naïve mice can induce granule cell dispersion, and providing exogenous reelin following epileptogenic injury can prevent dispersion [20]. Recent work suggests that reelin acts by controlling the directionality of granule cell migration [21]. Although direct observation of cell migration in vivo is challenging, in vitro studies have revealed at least one unusual mechanism by which migration of adult neurons can occur. Specifically, time-lapse imaging of granule cells in slice culture revealed that granule cell somas can migrate upwards through an apical dendrite, repositioning the soma into the molecular layer (Fig. 4) [22, 23]. This process of somatic translocation has not been directly observed in vivo, but the presence of characteristic dendritic abnormalities caused by the process suggest it occurs in epileptic animals.
Fig.3
Confocal images showing granule cell dispersion in the intrahippocampal kainic acid model of epilepsy. Images show YFP-expressing granule cells (green) in the dentate gyrus of a Thy1-YFP transgenic mouse. The Thy1 promoter leads to YFP expression in a subset of CNS neurons, including granule cells. Granule cells are co-labeled with the granule cell marker Prox1. The bottom row shows the dentate gyrus in the hemisphere that was injected with kainic acid, while the top row shows the dentate in the non-injected hemisphere. Pronounced granule cell dispersion and somatic hypertrophy is evident in the injected hemisphere, affecting essentially all granule cells. Scale bar = 50μm.
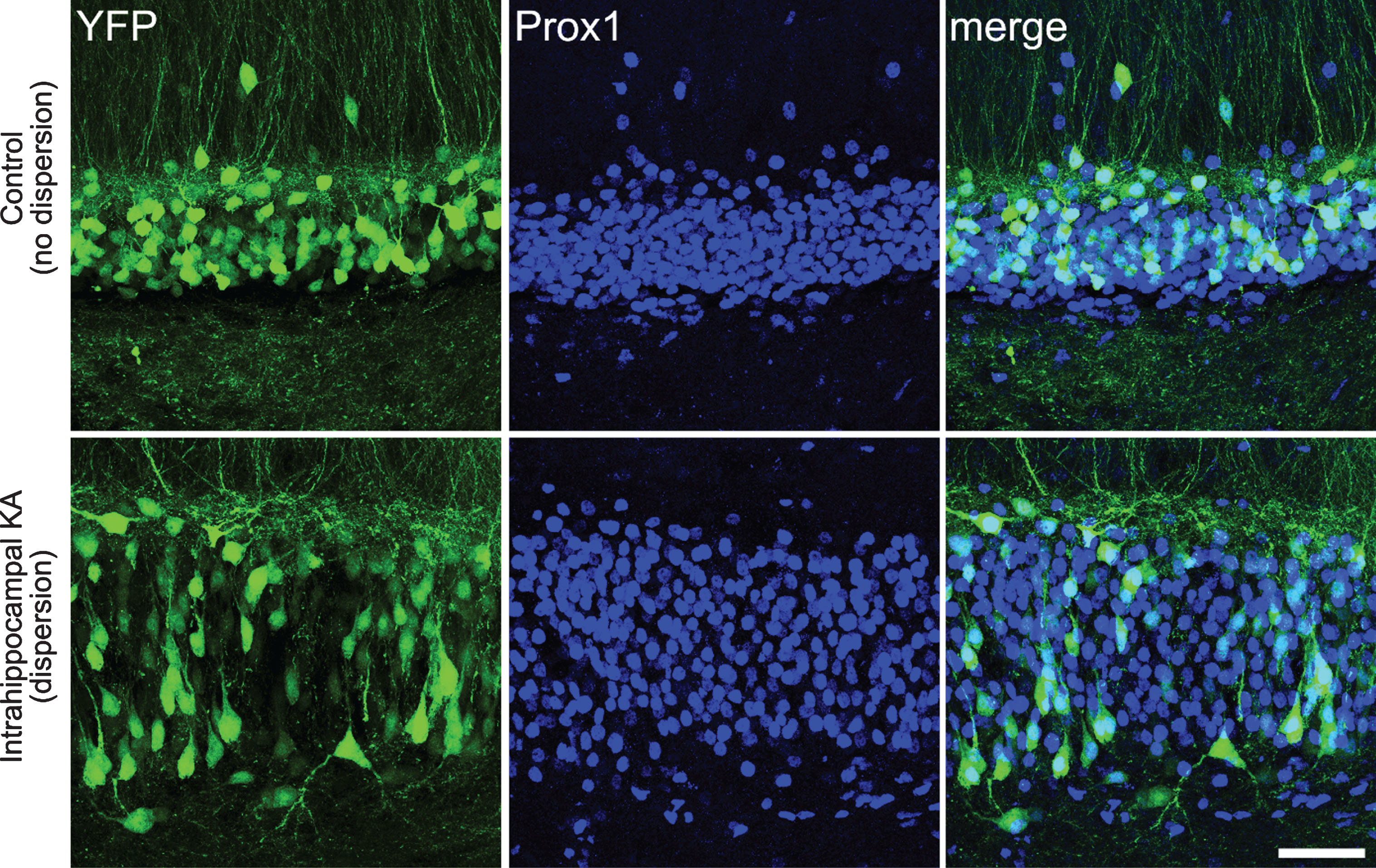
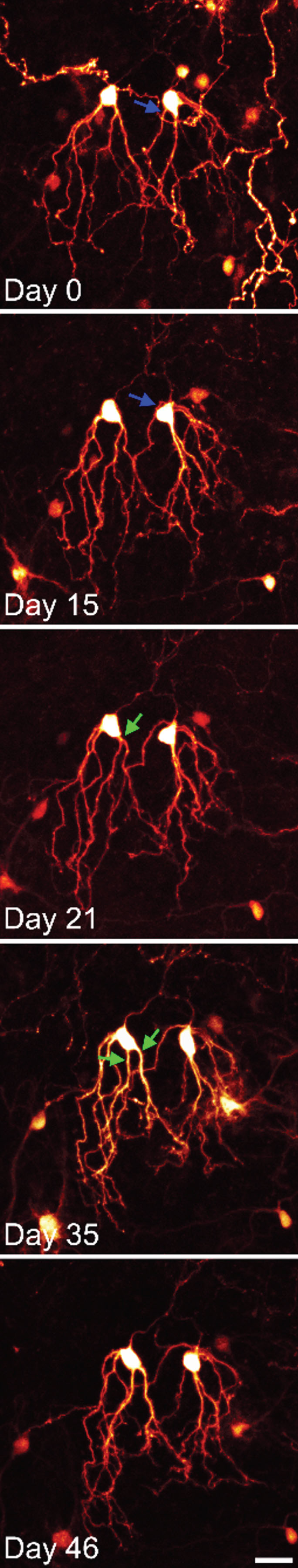
Fig.4
Time series confocal images of YFP-expressing granule cells in an organotypic hippocampal slice culture. The two granule cells shown were imaged over a period of 6 weeks. Both cells show modest somatic translocation, with the soma moving into the apical dendrites. In both cases, this distorts the apical dendritic tree. The blue arrow shows an apical dendrite, which is shifted to the basal pole of the cell. The green arrows show the soma absorbing a dendritic branch point, converting a single primary dendrite into two primary dendrites. Scale bar = 20μm.
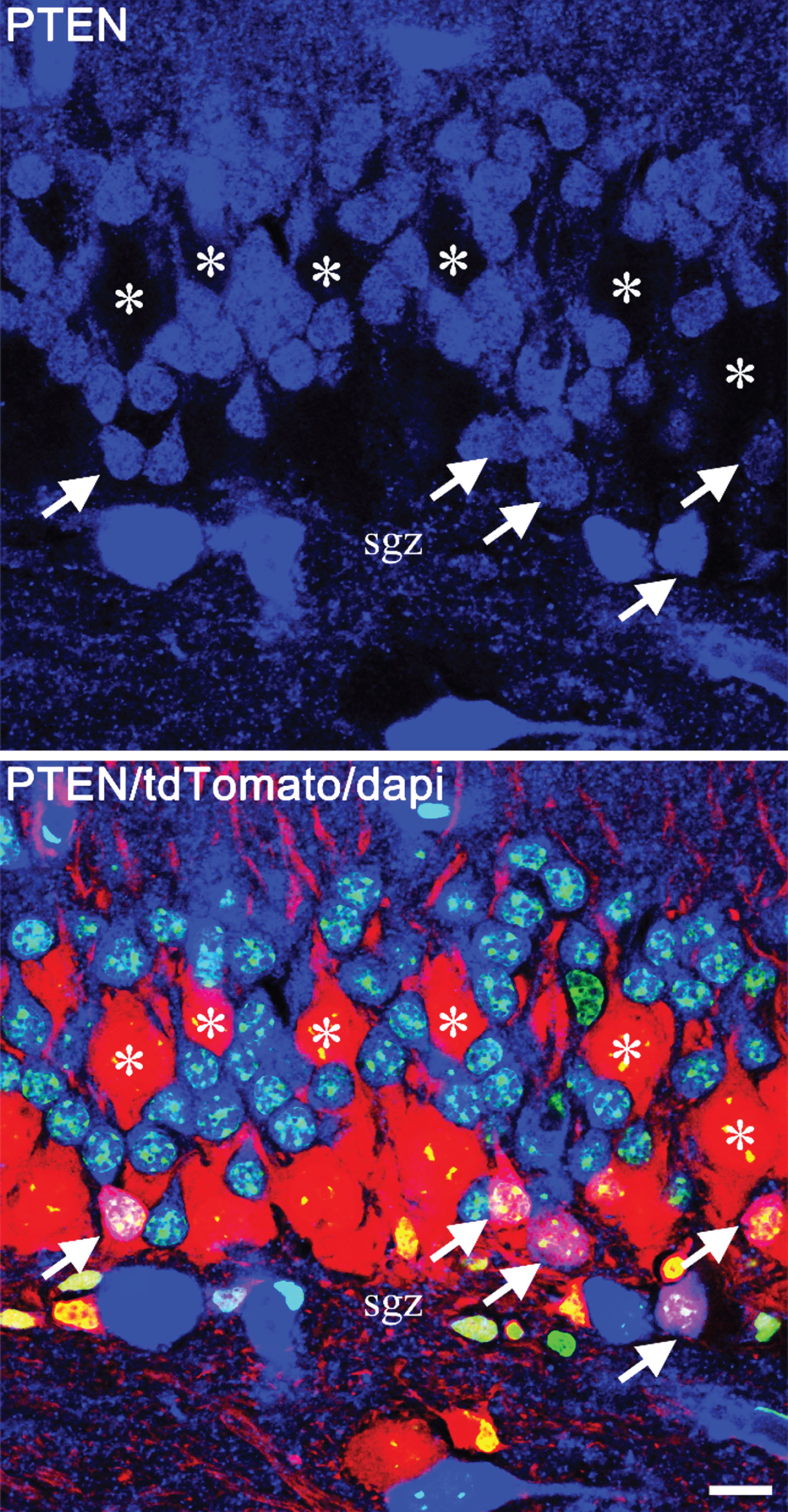
Granule cell dispersion can also impact newly-generated granule cells. This is clearly demonstrated in PTEN knockout models of epilepsy (Fig. 5). PTEN is a phosphatase that inhibits activation of the mTOR pathway. mTOR signaling regulates neuronal migration, growth and plasticity. Loss of PTEN from granule cell progenitors produces daughter cells which migrate deeper into the granule cell layer, taking positions closer to the molecular layer border [24]. Interestingly, granule cell somatic hypertrophy also occurs following PTEN deletion [25, 26], and the mTOR pathway is robustly activated throughout the granule cell layer following kainic acid treatment [27]. Moreover, treatment of animals with the mTOR antagonist rapamycin after intrahippocampal kainic acid injection mitigates granule cell dispersion [28] directly linking the mTOR pathway to the phenomena.
Fig.5
Confocal images showing mosaic deletion of PTEN from newborn hippocampal granule cells in a Gli1-CreERT2, PTENfl/fl, tdTomato reporter mouse. PTEN is shown in blue, tdTomato in red and dapi in green. The top panel shows PTEN alone, and the lower panel shows the merged image. PTEN-expressing newborn cells (arrows) are located in the inner third of the granule cell body layer, close to the subgranular zone (sgz). The much larger PTEN KO granule cells (asterisks), on the other hand, are distributed through the cell body layer, with some migrating close to the molecular layer border. Scale bar = 10μm.
Displacement of granule cells to the hilus vs. the dentate molecular layer will result in very different functional consequences for the dentate network. Because of the layer-specific patterning of afferent inputs, ectopic granule cells with their dendrites confined to the hilus can no longer receive normal association/commissural inputs, or direct inputs from entorhinal cortex (although polysynaptic input from perforant path is still evident) [29]. In contrast, granule cells displaced to the molecular layer can still receive input from entorhinal cortex, but may receive reduced or no associational/commissural input – depending on whether or not they retain processes in the inner molecular layer. Several groups have now examined hilar ectopic granule cell physiology, revealing a range of abnormalities, including a tendency for burst firing and altered excitation/inhibition ratios [30–33].
On the other hand, molecular layer or “semilunar” granule cells, which are normally present in small numbers, possess large dendritic trees with increased lateral extent and frequent axon collaterals in the inner molecular layer. These cells also exhibit distinct physiological properties, including lower input resistance and less spike frequency adaption [34]. Moreover, epileptogenic brain injury increases excitability among these neurons [35] and promotes the migration of additional granule cells to the molecular layer. Whether or not semilunar granule cells in healthy animals are distinct from granule cells that take up positions in the molecular layer after injury is unclear; but the latter do share some features, including greater lateral spread of their dendrites [36].
Granule cells with aberrant basal dendrites
In rodents, hippocampal granule cells typically exhibit only apical dendrites. Basal dendrites, projecting into the hilus, are absent. During the development of epilepsy in rodents, both granule cells that were immature at the time of the insult and those born after the insult develop basal dendrites (Fig. 6). This can lead to a dramatic shift in the percentage of granule cells with basal dendrites: from >1% in controls to ≈20% in epileptic animals [10]. The incidence of basal dendrites can vary considerably, both among models [37–42] and among animals generated using the same model [43]. Interestingly, hilar-projecting granule cell basal dendrites are common in healthy non-human primates and humans [44–46]. Hilar-projecting basal dendrites are also common in tissue resected from patients with epilepsy. The unavailability of healthy control tissue, however, limits conclusions about whether or not the incidence of basal dendrites increases during human epilepsy [47, 48].
Fig.6
Basal dendrites create a new pathway for recurrent excitatory contacts in the epileptic dentate gyrus. Neuronal reconstruction shows a biocytin-filled PTEN KO cell (green) and a biocytin-filled PTEN-expressing (purple) cell from a PTEN KO (Gli1-CreERT2, PTENfl/fl) mouse. The KO cell exhibits a large basal dendrite. The arrow denotes a point where the axon of the wildtype cell occupies the same focal plane in the z-axis as the PTEN KO cell basal dendrite, suggesting that a synaptic contact could be formed. Scale bar = 50μm.
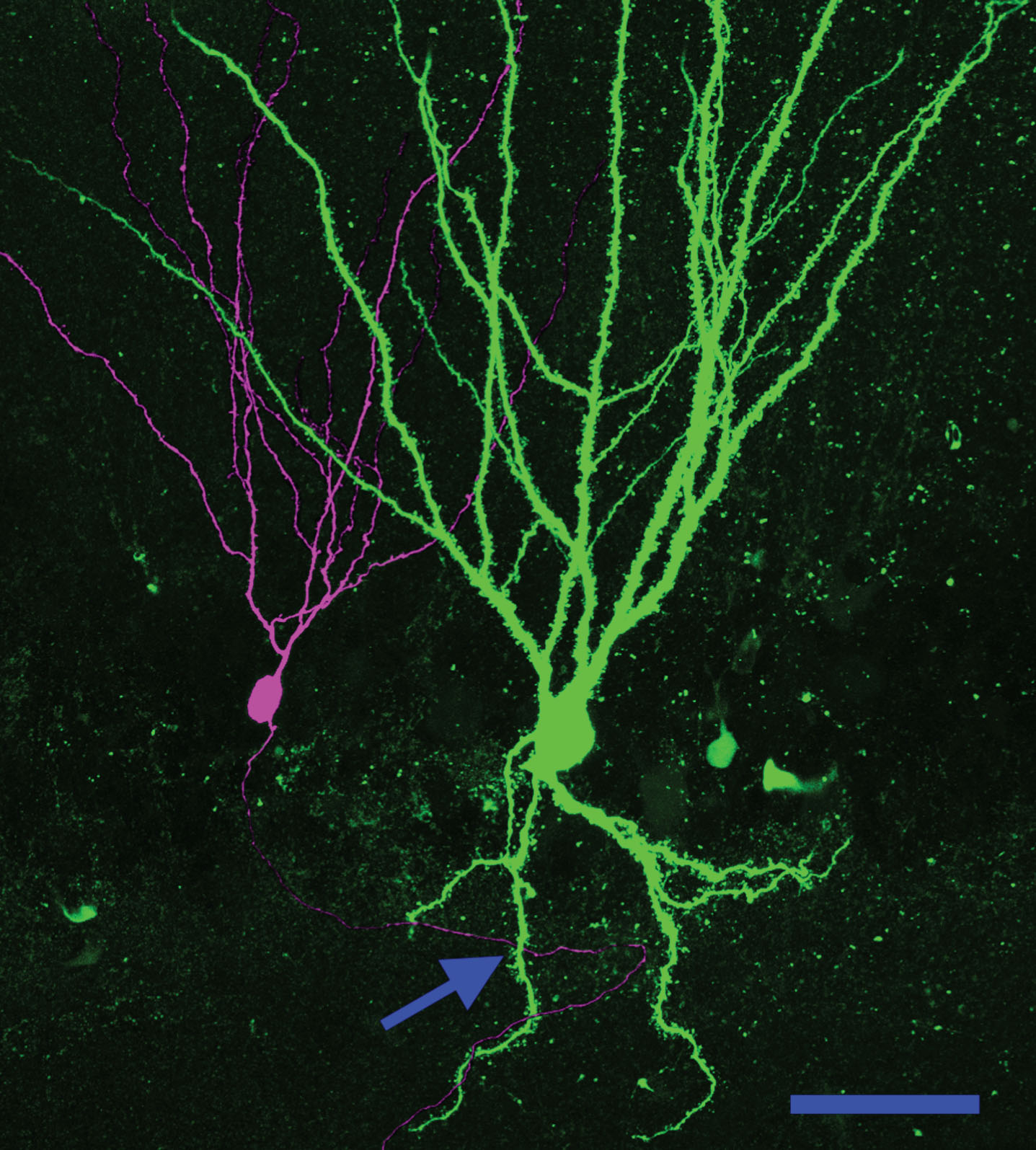
Granule cell basal dendrites are innervated by the mossy fiber axons of other granule cells, creating recurrent excitatory circuits [49–52]. The de novo creation of recurrent circuits is hypothesized to promote epileptogenesis by increasing hippocampal excitability. Physiological evidence of recurrent circuitry has been found in numerous epilepsy models by recording field potential activity from the granule cell layer while stimulating the perforant path in acute hippocampal slices [53]. In tissue from normal animals, each stimulus produces only a single population spike: evidence of the tight control of granule cell firing characteristic of the normal brain. In tissue from epileptic animals, by contrast, a single stimulus can induce multiple population spikes. These secondary spikes are hypothesized to be mediated by recurrent circuitry, allowing activity to re-invade the dentate. Consistent with this interpretation, targeted deletion of PTEN from a subset of granule cells leads to the development of basal dendrites on >90% of the knockout cells, and unusually robust secondary spikes following perforant path stimulation (Fig. 7) [54]. Basal dendrites are a promising candidate for mediating this recurrent activity, although mossy fiber sprouting could also play a role, as could impaired inhibition [53].
Fig.7
Responses to lateral perforant path (LPP) stimulation of increasing amplitude (60, 80, 200 and 400μA) from a control mouse and a PTEN KO mouse. In slices from the control mouse (A) the field excitatory post-synaptic potential (fEPSP) increased in amplitude with greater stimulation current and was followed by the appearance of a single population spike (negative going event) once threshold was reached. The slice from the PTEN KO mouse (B) also showed increasing fEPSP slope with increasing current, however, multiple population spikes were evoked. C: Hypothesized mechanism for the generation of multiple population spikes. Perforant path stimulation evokes an fEPSP in granule cell dendrites (1) leading to a population spike (2) which creates a secondary fEPSP in a granule cell basal dendrite (3). This recurrent activation provokes a secondary population spike (4). Portions of this image are reprinted from LaSarge et al. [54].
![Responses to lateral perforant path (LPP) stimulation of increasing amplitude (60, 80, 200 and 400μA) from a control mouse and a PTEN KO mouse. In slices from the control mouse (A) the field excitatory post-synaptic potential (fEPSP) increased in amplitude with greater stimulation current and was followed by the appearance of a single population spike (negative going event) once threshold was reached. The slice from the PTEN KO mouse (B) also showed increasing fEPSP slope with increasing current, however, multiple population spikes were evoked. C: Hypothesized mechanism for the generation of multiple population spikes. Perforant path stimulation evokes an fEPSP in granule cell dendrites (1) leading to a population spike (2) which creates a secondary fEPSP in a granule cell basal dendrite (3). This recurrent activation provokes a secondary population spike (4). Portions of this image are reprinted from LaSarge et al. [54].](https://content.iospress.com:443/media/bpl/2018/3-2/bpl-3-2-bpl170056/bpl-3-bpl170056-g007.jpg)
Granule cells with disorganized apical dendritic trees
Epileptogenic insults in animal models disrupt the apical dendritic structure of newly-generated granule cells. Cells that are mature at the time of the insult are resistant to this form of disruption [55]. Disruption can manifest as an overall disorganization of the dendritic tree, but a few recurring patterns are also evident. One such abnormality is a failure of dendritic self-avoidance. In normal animals, the dendrites and dendritic branches of a given granule cell will project away from each other, creating an even, fan-like spread in the molecular layer. Granule cells generated in the epileptic brain, by contrast, frequently develop a more columnar appearance, occupying overlapping space in the molecular layer (Fig. 8). Abnormalities of this nature have been described in the pilocarpine model of epilepsy [10, 55], the PTEN knockout model of epilepsy [56] and in tissue resected from patients with intractable temporal lobe epilepsy [57]. The functional significance of the normal, fanlike spread of granule cell dendrites has yet to be fully elucidated. This spreading may allow the cells to effectively sample afferent fibers entering the molecular layer via the perforant path. Recent computational modeling work supports the conclusion that elaborate granule cell dendritic trees are critical for maintaining sparse granule cell firing, a key trait for effective pattern separation [58]. Collapsed dendritic trees, therefore, may impair the ability of these cells to process information.
Fig.8
Images show granule cell reconstructions of PTEN expressing (control) and PTEN knockout (KO) cells from Gli1-CreERT2, PTENfl/fl mice. Cell morphology was revealed by biocytin filling. Cells are projected from above (left, cells A–D), looking down from the top of the dendritic tree towards the soma, and in profile (right, a–d). Note the more limited spread of the dendritic tree among KO cells, and frequent overlapping dendrites. Reconstructions are color-coded by depth. Scale bars = 100μm. Imaged reproduced from Santos et al. [56].
![Images show granule cell reconstructions of PTEN expressing (control) and PTEN knockout (KO) cells from Gli1-CreERT2, PTENfl/fl mice. Cell morphology was revealed by biocytin filling. Cells are projected from above (left, cells A–D), looking down from the top of the dendritic tree towards the soma, and in profile (right, a–d). Note the more limited spread of the dendritic tree among KO cells, and frequent overlapping dendrites. Reconstructions are color-coded by depth. Scale bars = 100μm. Imaged reproduced from Santos et al. [56].](https://content.iospress.com:443/media/bpl/2018/3-2/bpl-3-2-bpl170056/bpl-3-bpl170056-g008.jpg)
A second abnormality is the appearance of newborn granule cells with proximal dendrites that project obliquely into the molecular layer (Fig. 9). This pattern contrasts with the typical pattern, in which granule cell primary dendrites project radially from the soma, reaching the molecular layer by the shortest possible route. This abnormality has also been observed in the pilocarpine model [55] and in tissue from patients with epilepsy [57]. The predicted functional consequences, however, are distinct from the collapsed dendritic trees. Specifically, obliquely-projecting primary dendrites increase dendritic extent in the granule cell layer and inner molecular layer, which could increase the percentage of associational/commissural inputs received by the cells, and also increase the dendritic extent available for innervation by sprouted mossy fiber axons targeting these same regions.
Fig.9
Neurolucida reconstructions of Thy1-GFP-expressing newborn hippocampal granule cells from mice rendered epileptic using the pilocarpine status epilepticus model. Dendrites and somas are shown in black, axons in blue and basal dendrites in green. Red lines denote the hilar—granule cell body layer border, the granule cell body layer—molecular layer border, and the hippocampal fissure, from top to bottom, respectively. The primary dendrites of these granule cells project obliquely into the molecular layer, rather than directly – as is typical for this cell type. The abnormality gives the cells a “windswept” appearance. Scale bar = 100μm. Portions of this image are reprinted from Santos et al. [55].
![Neurolucida reconstructions of Thy1-GFP-expressing newborn hippocampal granule cells from mice rendered epileptic using the pilocarpine status epilepticus model. Dendrites and somas are shown in black, axons in blue and basal dendrites in green. Red lines denote the hilar—granule cell body layer border, the granule cell body layer—molecular layer border, and the hippocampal fissure, from top to bottom, respectively. The primary dendrites of these granule cells project obliquely into the molecular layer, rather than directly – as is typical for this cell type. The abnormality gives the cells a “windswept” appearance. Scale bar = 100μm. Portions of this image are reprinted from Santos et al. [55].](https://content.iospress.com:443/media/bpl/2018/3-2/bpl-3-2-bpl170056/bpl-3-bpl170056-g009.jpg)
Newborn granule cells as a therapeutic target for anti-epileptogenesis
Abnormal newborn granule cells are hypothesized to contribute to epileptogenesis. Physiological studies [59, 60] and in vivo manipulations of granule cell activity support an import role for the dentate in regulating seizures [61]. If the newborn granule hypothesis of epilepsy is correct, then eliminating these cells should be protective. Indeed, studies to manipulate neurogenesis in rodent models of epilepsy have been ongoing for more than a decade. The earliest studies used a variety of antimitotic agents to limit neurogenesis, beginning either just before or immediately after an epileptogenic brain injury in rodents. Two of three early studies produced promising results, demonstrating a significant reduction in seizure frequency [62, 63], while the third found no effect of treatment [64]. A notable caveat of all three of these initial studies was the use of pharmacological approaches, with the potential for off-target effects.
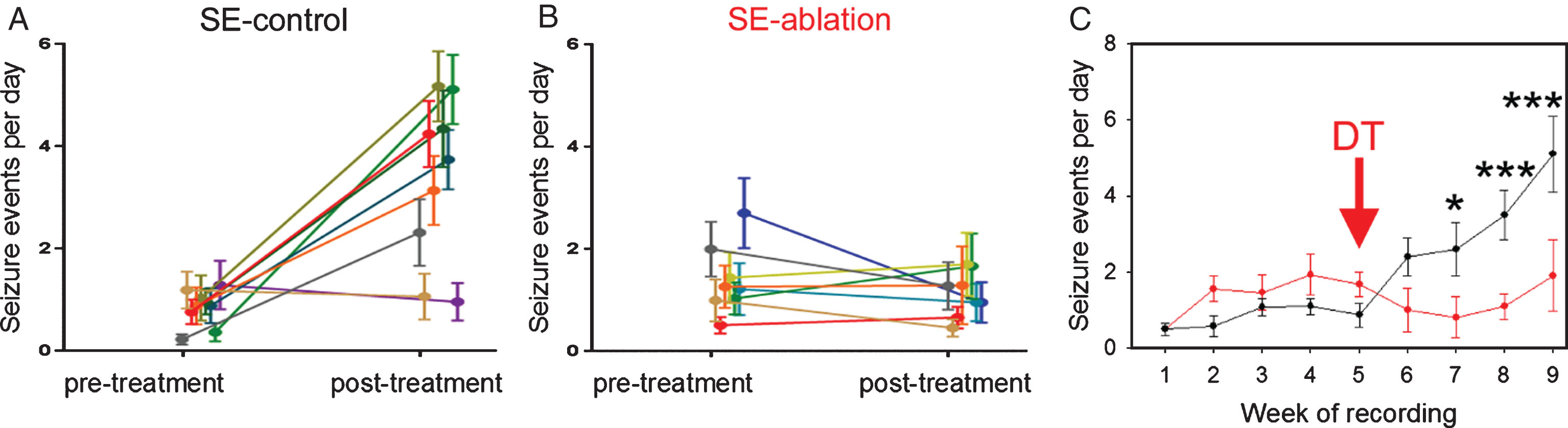
To increase selectivity and specificity, investigators have taken advantage of new, genetic strategies to target granule cell progenitors. Specifically, by using progenitor cell-specific gene promotor sequences, the “DNA scissors” cre-recombinase can be selectively expressed in newborn granule cells. Cre-recombinase recognizes specific genetic sequences termed “lox p” sites and splices out DNA sequences flanked by these sites. Crossing a cre-expressing line with mouse lines containing cell-killing genes preceded by lox p flanked stop codons can then be used to selectively ablate newborn cells. Using two independent strategies, investigators have demonstrated that ablating newborn granule cells just before, or following, an epileptogenic insult produced a roughly 50% decrease in seizure frequency when the animals were examined months later [65, 66]. Follow up work by Hosford and colleagues [67] demonstrated that ablation of newborn granule cells was still effective in animals already exhibiting spontaneous seizures (Fig. 10). This finding is of particular relevance for clinical translation. Specifically, since only a subset of patients suffering an epileptogenic injury go on to develop epilepsy, decisions to treat will be much easier if physicians are able to wait until patients are symptomatic.
Fig.10
Cell ablation treatment blocks epilepsy progression. Nestin-CreERT2, DTrfl/fl mice were generated to induce expression of the diphtheria toxin receptor among newborn granule cells. Epilepsy was induced using the pilocarpine status epilepticus model. Following one month of 24/7 video-EEG monitoring, animals were treated with saline (SE-control) or diphtheria toxin (SE-ablation). Pre-treatment and post-treatment seizure frequencies (A,B) are shown for SE-control mice (left, black) and SE-ablation mice (middle, red). Each line shows the means±SEM for one animal. (C) Average number of seizure events during each week of recording for SE-control (black) and SE-ablation (red) groups (DT was given during week 5, red arrow). Ablation treatment prevented the dramatic increase in seizure frequency evident in SE-control animals. *p < 0.05, ***p < 0.001.
Finally, recent work suggests newborn granule cells may promote epilepsy in a traumatic brain injury model. Specifically, Neuberger and colleagues [68] used a VEGFR2 antagonist to prevent the increase in neurogenesis that occurs following fluid percussion injury in rats [69]. Injured rats exhibited enhanced network excitability and reduced latency to develop seizures following kainic acid exposure. VEGFR2 antagonism prevented both changes.
To date, none of the anti-neurogenic approaches tested in animal models have led to a complete elimination of seizures, which may reflect inherent limitations with the current cell ablation strategies that still leave some abnormal granule cells behind. Consistent with this interpretation, use of a third genetic strategy to ablate newborn cells, which produced a much more modest reduction in newborn granule cell numbers, failed to reduce seizure frequency [70]. The need for near-complete ablation of abnormal cells is also in line with both computational studies, which indicate that aberrant connectivity among as little as 5% of the granule cell population is sufficient to destabilize the circuit [71]. The need for near-complete ablation is also consistent with the granule cell-specific PTEN knockout model of epilepsy, in which PTEN deletion from just 10% of granule cells caused spontaneous seizures [41].
Uncertainty remains as to exactly whether, how, and when newborn granule cells contribute to epileptogenesis. For example, Zhu and colleagues [72] used the proliferative cell toxin methylazoxymethanol acetate (MAM) to eliminate newborn granule cells from mice in the pilocarpine model of epilepsy. Despite effective reductions in ectopic cells, mossy fiber sprouting, and cells with basal dendrites, no impact on seizure frequency was observed. While it is possible that MAM had off-target effects that occluded any positive effect on seizures, the finding is not the only unexpected result: In 2015, Iyengar [73] and colleagues demonstrated that ablating newborn cells before a challenge with kainic acid increased the severity of the resulting seizures, suggesting that the presence of newborn granule cells before status epilepticus is protective. Cho and colleagues [65], on the other hand, observed no effect of ablation on status epilepticus induced by pilocarpine. In addition, in this work the investigators found that pre-ablation was effective at reducing spontaneous seizures, but pre- plus post-ablation was not. Since both cells born before and after the epileptogenic insult integrate abnormally, this latter finding was unexpected, as it produced a more effective reduction in abnormal cells. Differences among mouse strains, timepoints, seizure models or other technical issues could provide trivial explanations for these contradictory findings [74]. Unanticipated complexity in the role played by newborn cells may also be important.
Answering several key questions may clarify these findings. First, whether all newborn granule cells are pathological, or whether the newborn population contains a mix of pathological and beneficial cells needs to be determined. Anatomical studies suggest that some newborn neurons in epileptic animals may promote hyperexcitability, while others receive reduced input [83]. Physiological studies of newborn cells in epileptic animals support the possibility of a mixed population [33, 75–77]. Current techniques for ablating newborn cells act on the entire population. If the epileptic dentate contains both pathological and beneficial newborn cells, the net effect on seizures would vary depending on the relative ratio of the two. Secondly, the contribution of newborn cells to epilepsy may follow complex temporal dynamics. Cells born just before the insult may initially be beneficial (protection from status [78]) but then become pathological (developing abnormal features). Newborn cells could also play a transient role, promoting epileptogenesis over the first few months, but then becoming disengaged from the process. In this latter case, ablation may lose efficacy over time. A third key question is whether neurogenesis is important for all types of temporal lobe epilepsy. Epileptogenesis is associated with an extensive array of brain changes that are hypothesized to play a causal role, including cell loss, inflammation, gene expression changes, and changes in non-neuronal cells. Aberrant neurogenesis may be one of many variables contributing to epileptogenesis, and its role may vary from essential in some models, to irrelevant in others. Within the pilocarpine model, for example, neuropathology can vary widely, even among animals with similar seizure frequencies [43, 79]. The mechanisms underlying seizures in these animals may also differ. Determining which models require adult neurogenesis, and whether and how these models relate to human epilepsy is important. Finally, the function of normal granule cells in healthy brains remains an intense area of research. Why human adult-neurogenesis persists for this one cell type in one discrete brain region [80, 81] remains a mystery, as does the exact computational role played by these cells [82]. As our understanding of normal adult-neurogenesis advances, we will continue to gain new insights into pathological neurogenesis in epilepsy.
Conclusions
Here, we reviewed the key somatic and dendritic abnormalities exhibited by newly-generated hippocampal granule cells in models of temporal lobe epilepsy. Following the classic premise that “neuronal form predicts neuronal function”, abnormalities evident in the epileptic dentate gyrus will profoundly disrupt the normal physiology of the structure. Displacement of granule cells to ectopic locations radically alters the types of afferent inputs the cells can receive, while distortions of the dendritic tree likely impact the breadth of inputs received from upstream neurons and the relative proportions of inputs from different neuron types. Although the functional implications of these changes have yet to be fully elucidated, several studies using independent techniques have demonstrated that targeted ablation of newborn granule cells can significantly reduce seizure frequency. Treatments have been found to be effective when applied during the latent period – before the onset of spontaneous seizures – and after the onset of spontaneous seizures. Together, these findings implicate newly-generated granule cells as playing an important role in temporal lobe epilepsy and as a potential therapeutic target for the disease.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Neurological Disorders and Stroke (SCD, Awards R01NS065020 and R01NS062806). We would like to thank Keri Kaeding for useful comments on this manuscript, and Maria Ashton for editorial assistance. We also would like to thank the Cincinnati Children’s Hospital Medical Center Confocal Imaging Core for their assistance with confocal image acquisition and analysis, and the contribution of images by Brian Murphy, Shatrunjai Singh, Mary Dusing, Ray Pun and Victor Santos. The author declares no competing financial interests.
REFERENCES
[1] | Leranth C , Hajszan T . Extrinsic afferent systems to the dentate gyrus. Progress in Brain Research. (2007) ;163: :63–84. doi:10.1016/s0079-6123(07)63004-0 |
[2] | Altman J , Bayer SA . Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. The Journal of Comparative Neurology. (1990) ;301: (3):365–81. doi:10.1002/cne.903010304 |
[3] | Mathews EA , Morgenstern NA , Piatti VC , Zhao C , Jessberger S , Schinder AF , et al. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. The Journal of Comparative Neurology. (2010) ;518: (22):4479–90. doi:10.1002/cne.22489 |
[4] | Kempermann G , Song H , Gage FH . Neurogenesis in the adult hippocampus. Cold Spring Harbor Perspectives in Biology. (2015) ;7: (9):a018812. doi:10.1101/cshperspect.a018812 |
[5] | Buckmaster PS . Mossy Fiber Sprouting in the Dentate Gyrus. In: th, Noebels JL , Avoli M , Rogawski MA , Olsen RW , Delgado-Escueta AV , editors. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda (MD): National Center for Biotechnology Information (US). Michael A Rogawski, Antonio V Delgado-Escueta, Jeffrey L Noebels, Massimo Avoli and Richard W Olsen.; (2012) . |
[6] | Houser CR , Zhang N , Peng Z , Huang CS , Cetina Y . Neuroanatomical clues to altered neuronal activity in epilepsy: From ultrastructure to signaling pathways of dentate granule cells. Epilepsia. (2012) ;53: (Suppl 1):67–77. doi:10.1111/j.1528-1167.2012.03477.x |
[7] | Buckmaster PS . Does mossy fiber sprouting give rise to the epileptic state? Advances in Experimental Medicine and Biology. (2014) ;813: :161–8. doi:10.1007/978-94-017-8914-1_13 |
[8] | Pierce JP , McCloskey DP , Scharfman HE . Morphometry of hilar ectopic granule cells in the rat. The Journal of Comparative Neurology. (2011) ;519: (6):1196–218. doi:10.1002/cne.22568 |
[9] | Parent JM , Elliott RC , Pleasure SJ , Barbaro NM , Lowenstein DH . Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Annals of Neurology. (2006) ;59: (1):81–91. doi:10.1002/ana.20699 |
[10] | Walter C , Murphy BL , Pun RY , Spieles-Engemann AL , Danzer SC . Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. The Journal of Neuroscience. (2007) ;27: (28)7541–52. doi:10.1523/jneurosci.0431-07.2007 |
[11] | Singh SP , LaSarge CL , An A , McAuliffe JJ , Danzer SC . Clonal analysis of newborn hippocampal dentate granule cell proliferation and development in temporal lobe epilepsy. eNeuro. (2015) ;2: (6).doi:10.1523/eneuro.0087-15.2015 |
[12] | Kralic JE , Ledergerber DA , Fritschy JM . Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. The European Journal of Neuroscience. (2005) ;22: (8):1916–27. doi:10.1111/j.1460-9568.2005.04386.x |
[13] | Nitta N , Heinrich C , Hirai H , Suzuki F . Granule cell dispersion develops without neurogenesis and does not fully depend on astroglial cell generation in a mouse model of temporal lobe epilepsy. Epilepsia. (2008) ;49: (10)1711–22. doi:10.1111/j.1528-1167.2008.01595.x |
[14] | Bouilleret V , Ridoux V , Depaulis A , Marescaux C , Nehlig A , Le Gal La Salle G . Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: Electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. (1999) ;89: (3):717–29. |
[15] | Jessberger S , Romer B , Babu H , Kempermann G . Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Experimental Neurology. (2005) ;196: (2):342–51. doi:10.1016/j.expneurol.2005.08.010 |
[16] | Houser CR . Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Research. (1990) ;535: (2):195–204. |
[17] | Suzuki F , Junier MP , Guilhem D , Sorensen JC , Onteniente B . Morphogenetic effect of kainate on adult hippocampal neurons associated with a prolonged expression of brain-derived neurotrophic factor. Neuroscience. (1995) ;64: (3):665–74. |
[18] | Haas CA , Dudeck O , Kirsch M , Huszka C , Kann G , Pollak S , et al. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. The Journal of Neuroscience. (2002) ;22: (14):5797–802. doi: 20026621 |
[19] | Gong C , Wang TW , Huang HS , Parent JM . Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. The Journal of Neuroscience. (2007) ;27: (8):1803–11. doi:10.1523/jneurosci.3111-06.2007 |
[20] | Haas CA , Frotscher M . Reelin deficiency causes granule cell dispersion in epilepsy. Experimental Brain Research. (2010) ;200: (2):141–9. doi:10.1007/s00221-009-1948-5 |
[21] | Wang S , Brunne B , Zhao S , Chai X , Li J , Lau J , et al. Trajectory analysis unveils reelin’s role in the directed migration of granule cells in the dentate gyrus. The Journal of Neuroscience. (2017) .doi:10.1523/jneurosci.0988-17.2017 |
[22] | Murphy BL , Danzer SC . Somatic translocation: A novel mechanism of granule cell dendritic dysmorphogenesis and dispersion. The Journal of Neuroscience. (2011) ;31: (8):2959–64. doi:10.1523/jneurosci.3381-10.2011 |
[23] | Chai X , Munzner G , Zhao S , Tinnes S , Kowalski J , Haussler U , et al. Epilepsy-induced motility of differentiated neurons. Cerebral Cortex (New York, NY:1991). (2014) ;24: (8):2130–40. doi:10.1093/cercor/bht067 |
[24] | Getz SA , DeSpenza T Jr , Li M , Luikart BW . Rapamycin prevents, but does not reverse, aberrant migration in pten knockout neurons. Neurobiology of Disease. (2016) ;93: :12–20. doi:10.1016/j.nbd.2016.03.010 |
[25] | Kwon CH , Luikart BW , Powell CM , Zhou J , Matheny SA , Zhang W , et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. (2006) ;50: (3):377–88. doi:10.1016/j.neuron.2006.03.023 |
[26] | Williams MR , DeSpenza T Jr , Li M , Gulledge AT , Luikart BW . Hyperactivity of newborn pten knock-out neurons results from increased excitatory synaptic drive. The Journal of Neuroscience. (2015) ;35: (3):943–59. doi:10.1523/jneurosci.3144-14.2015 |
[27] | Zeng LH , Rensing NR , Wong M . The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. The Journal of Neuroscience. (2009) ;29: (21):6964–72. doi:10.1523/jneurosci.0066-09.2009 |
[28] | Shima A , Nitta N , Suzuki F , Laharie AM , Nozaki K , Depaulis A . Activation of mtor signaling pathway is secondary to neuronal excitability in a mouse model of mesio-temporal lobe epilepsy. The European Journal of Neuroscience. (2015) ;41: (7):976–88. doi:10.1111/ejn.12835 |
[29] | Scharfman HE , Sollas AE , Berger RE , Goodman JH , Pierce JP . Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. (2003) ;121: (4):1017–29. |
[30] | Scharfman HE , Goodman JH , Sollas AL . Granule-like neurons at the hilar/Ca3 border after status epilepticus and their synchrony with area Ca3 pyramidal cells: Functional implications of seizure-induced neurogenesis. The Journal of Neuroscience. (2000) ;20: (16):6144–58. |
[31] | Zhan RZ , Nadler JV . Enhanced tonic gaba current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. Journal of Neurophysiology. (2009) ;102: (2):670–81. doi:10.1152/jn.00147.2009 |
[32] | Zhan RZ , Timofeeva O , Nadler JV . High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. Journal of Neurophysiology. (2010) ;104: (6):3293–04. doi:10.1152/jn.00663.2010 |
[33] | Althaus AL , Sagher O , Parent JM , Murphy GG . Intrinsic neurophysiological properties of hilar ectopic and normotopic dentate granule cells in human temporal lobe epilepsy and a rat model. Journal of Neurophysiology. (2015) ;113: (4)1184–94. doi:10.1152/jn.00835.2014 |
[34] | Williams PA , Larimer P , Gao Y , Strowbridge BW . Semilunar granule cells: Glutamatergic neurons in the rat dentate gyrus with axon collaterals in the inner molecular layer. The Journal of Neuroscience. (2007) ;27: (50):13756–61. doi:10.1523/jneurosci.4053-07.2007 |
[35] | Gupta A , Elgammal FS , Proddutur A , Shah S , Santhakumar V . Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain injury. The Journal of Neuroscience. (2012) ;32: (7):2523–37. doi:10.1523/jneurosci.4141-11.2012 |
[36] | Freiman TM , Eismann-Schweimler J , Frotscher M . Granule cell dispersion in temporal lobe epilepsy is associated with changes in dendritic orientation and spine distribution. Experimental Neurology. (2011) ;229: (2):332–8. doi:10.1016/j.expneurol.2011.02.017 |
[37] | Spigelman I , Yan XX , Obenaus A , Lee EY , Wasterlain CG , Ribak CE . Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. (1998) ;86: (1):109–20. |
[38] | Ribak CE , Tran PH , Spigelman I , Okazaki MM , Nadler JV . Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. The Journal of Comparative Neurology. (2000) ;428: (2):240–53. |
[39] | Diaz-Cintra S , Xue B , Spigelman I , Van K , Wong AM , Obenaus A , et al. Dentate granule cells form hilar basal dendrites in a rat model of hypoxia-ischemia. Brain Research. (2009) ;1285: :182–7. doi:10.1016/j.brainres.2009.06.034 |
[40] | Murphy BL , Hofacer RD , Faulkner CN , Loepke AW , Danzer SC . Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis. Epilepsia. (2012) ;53: (5):908–21. doi:10.1111/j.1528-1167.2012.03463.x |
[41] | Pun RY , Rolle IJ , Lasarge CL , Hosford BE , Rosen JM , Uhl JD , et al. Excessive activation of mtor in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. (2012) ;75: (6):1022–34. doi:10.1016/j.neuron.2012.08.002 |
[42] | Buckmaster PS , Dudek FE . In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. Journal of Neurophysiology. (1999) ;81: (2):712–21. doi:10.1152/jn.1999.81.2.712 |
[43] | Hester MS , Danzer SC . Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. The Journal of Neuroscience. (2013) ;33: (21):8926–36. doi:10.1523/jneurosci.5161-12.2013 |
[44] | Seress L , Mrzljak L . Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations. Brain Research. (1987) ;405: (1):169–74. |
[45] | Seress L . Morphological variability and developmental aspects of monkey and human granule cells: Differences between the rodent and primate dentate gyrus. Epilepsy Research Supplement. (1992) ;7: :3–28. |
[46] | Al-Hussain S , Al-Ali S . A Golgi study of cell types in the dentate gyrus of the adult human brain. Cellular and Molecular Neurobiology. (1995) ;15: (2):207–20. |
[47] | Franck JE , Pokorny J , Kunkel DD , Schwartzkroin PA . Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. (1995) ;36: (6):543–58. |
[48] | von Campe G , Spencer DD , de Lanerolle NC . Morphology of dentate granule cells in the human epileptogenic hippocampus. Hippocampus. (1997) ;7: (5)472–88. doi:10.1002/(SICI)1098-1063(1997)7:5<472::AID-HIPO4>3.0.CO;2-J |
[49] | Austin JE , Buckmaster PS . Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. The Journal of Comparative Neurology. (2004) ;476: (3):205–18. doi:10.1002/cne.20182 |
[50] | Thind KK , Ribak CE , Buckmaster PS . Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. The Journal of Comparative Neurology. (2008) ;509: (2):190–202. doi:10.1002/cne.21745 |
[51] | Sanchez RM , Ribak CE , Shapiro LA . Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy. Epilepsia. (2012) ;53: (Suppl 1):98–108. doi:10.1111/j.1528-1167.2012.03481.x |
[52] | Kelly T , Beck H . Functional properties of granule cells with hilar basal dendrites in the epileptic dentate gyrus. Epilepsia. (2017) ;58: (1):160–71. doi:10.1111/epi.13605 |
[53] | Sutula TP , Dudek FE . Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: An emergent property of a complex system. Progress in Brain Research. (2007) ;163: :541–63. doi:10.1016/s0079-6123(07)63029-5 |
[54] | LaSarge CL , Pun RY , Muntifering MB , Danzer SC . Disrupted hippocampal network physiology following pten deletion from newborn dentate granule cells. Neurobiology of Disease. (2016) ;96: :105–14. doi:10.1016/j.nbd.2016.09.004 |
[55] | Santos VR , de Castro OW , Pun RY , Hester MS , Murphy BL , Loepke AW , et al. Contributions of mature granule cells to structural plasticity in temporal lobe epilepsy. Neuroscience. (2011) ;197: 348–57. doi:10.1016/j.neuroscience.2011.09.034 |
[56] | Santos VR , Pun RYK , Arafa SR , LaSarge CL , Rowley S , Khademi S , et al. Pten deletion increases hippocampal granule cell excitability in male and female mice. Neurobiology of Disease. (2017) ;108: :339–51. doi:10.1016/j.nbd.2017.08.014 |
[57] | Scheibel ME SA . Hippocampal Pathology in Temporal Lobe Epilepsy: A Golgi Survey. In: MAB B , editor. Epilepsy: Its Phenomena in Man. New York: Raven Press; (1973) . pp. 311–37. |
[58] | Chavlis S , Petrantonakis PC , Poirazi P . Dendrites of dentate gyrus granule cells contribute to pattern separation by controlling sparsity. Hippocampus. (2017) ;27: (1):89–110. doi:10.1002/hipo.22675 |
[59] | Fujita S , Toyoda I , Thamattoor AK , Buckmaster PS . Preictal activity of subicular, Ca1, and dentate gyrus principal neurons in the dorsal hippocampus before spontaneous seizures in a rat model of temporal lobe epilepsy. The Journal of Neuroscience. (2014) ;34: (50):16671–87. doi:10.1523/jneurosci.0584-14.2014 |
[60] | Dengler CG , Yue C , Takano H , Coulter DA . Massively augmented hippocampal dentate granule cell activation accompanies epilepsy development. Scientific Reports. (2017) ;7: :42090. doi:10.1038/srep42090 |
[61] | Krook-Magnuson E , Armstrong C , Bui A , Lew S , Oijala M , Soltesz I . In vivo evaluation of the dentate gate theory in epilepsy. The Journal of Physiology. (2015) ;593: (10):2379–88. doi:10.1113/jp270056 |
[62] | Jung KH , Chu K , Kim M , Jeong SW , Song YM , Lee ST , et al. Continuous cytosine-B-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. The European Journal of Neuroscience. (2004) ;19: (12):3219–26. doi:10.1111/j.0953-816X.2004.03412.x |
[63] | Jung KH , Chu K , Lee ST , Kim J , Sinn DI , Kim JM , et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiology of Disease. (2006) ;23: (2):237–46. doi:10.1016/j.nbd.2006.02.016 |
[64] | Pekcec A , Fuest C , Muhlenhoff M , Gerardy-Schahn R , Potschka H . Targeting epileptogenesis-associated induction of neurogenesis by enzymatic depolysialylation of ncam counteracts spatial learning dysfunction but fails to impact epilepsy development. Journal of Neurochemistry. (2008) ;105: (2):389–400. doi:10.1111/j.1471-4159.2007.05172.x |
[65] | Cho KO , Lybrand ZR , Ito N , Brulet R , Tafacory F , Zhang L , et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nature Communications. (2015) ;6: :6606. doi:10.1038/ncomms7606 |
[66] | Hosford BE , Liska JP , Danzer SC . Ablation of newly generated hippocampal granule cells has disease-modifying effects in epilepsy. The Journal of Neuroscience. (2016) ;36: (43):11013–23. doi:10.1523/jneurosci.1371-16.2016 |
[67] | Hosford BE RS , Liska JP , Danzer SC . Ablation of peri-insult generated granule cells after epilepsy onset halts disease progression. Scientific Reports. (2017) ;7: (1)18015. doi:10.1038/s41598-017-18237-6 |
[68] | Neuberger EJ , Swietek B , Corrubia L , Prasanna A , Santhakumar V . Enhanced dentate neurogenesis after brain injury undermines long-term neurogenic potential and promotes seizure susceptibility. Stem Cell Reports. (2017) ;9: (3):972–84. doi:10.1016/j.stemcr.2017.07.015 |
[69] | Shapiro LA . Altered hippocampal neurogenesis during the first 7 days after a fluid percussion traumatic brain injury. Cell Transplantation. (2017) ;26: (7):1314–8. doi:10.1177/0963689717714099 |
[70] | Brulet R , Zhu J , Aktar M , Hsieh J , Cho KO . Mice with conditional neurod1 knockout display reduced aberrant hippocampal neurogenesis but no change in epileptic seizures. Experimental Neurology. (2017) ;293: :190–8. doi:10.1016/j.expneurol.2017.04.005 |
[71] | Morgan RJ , Soltesz I . Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proceedings of the National Academy of Sciences of the United States of America. (2008) ;105: (16):6179–84. doi:10.1073/pnas.0801372105 |
[72] | Zhu K , Yuan B , Hu M , Feng GF , Liu Y , Liu JX . Reduced abnormal integration of adult-generated granule cells does not attenuate spontaneous recurrent seizures in mice. Epilepsy Research. (2017) ;133: :58–66. doi:10.1016/j.eplepsyres.2017.04.004 |
[73] | Iyengar SS , LaFrancois JJ , Friedman D , Drew LJ , Denny CA , Burghardt NS , et al. Suppression of adult neurogenesis increases the acute effects of kainic acid. Experimental Neurology. (2015) ;264: :135–49. doi:10.1016/j.expneurol.2014.11.009 |
[74] | Danzer SC . Adult neurogenesis: Opening the gates of troy from the inside. Epilepsy Currents. (2015) ;15: (5):263–4. doi:10.5698/1535-7511-15.5.263 |
[75] | Jakubs K , Nanobashvili A , Bonde S , Ekdahl CT , Kokaia Z , Kokaia M , et al. Environment matters: Synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. (2006) ;52: (6):1047–59. doi:10.1016/j.neuron.2006.11.004 |
[76] | Wood JC , Jackson JS , Jakubs K , Chapman KZ , Ekdahl CT , Kokaia Z , et al. Functional integration of new hippocampal neurons following insults to the adult brain is determined by characteristics of pathological environment. Experimental Neurology. (2011) ;229: (2):484–93. doi:10.1016/j.expneurol.2011.03.019 |
[77] | Gao F , Song X , Zhu D , Wang X , Hao A , Nadler JV , et al. Dendritic morphology, synaptic transmission, and activity of mature granule cells born following pilocarpine-induced status epilepticus in the rat. Frontiers in Cellular Neuroscience. (2015) ;9: :384. doi:10.3389/fncel.2015.00384 |
[78] | Drew LJ , Kheirbek MA , Luna VM , Denny CA , Cloidt MA , Wu MV , et al. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus. (2016) ;26: (6):763–78. doi:10.1002/hipo.22557 |
[79] | Danzer SC , He X , Loepke AW , McNamara JO . Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy. Hippocampus. (2010) ;20: (1):113–24. doi:10.1002/hipo.20589 |
[80] | Eriksson PS , Perfilieva E , Bjork-Eriksson T , Alborn AM , Nordborg C , Peterson DA , et al. Neurogenesis in the adult human hippocampus. Nature Medicine. (1998) ;4: (11):1313–7. doi:10.1038/3305 |
[81] | Spalding KL , Bergmann O , Alkass K , Bernard S , Salehpour M , Huttner HB , et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. (2013) ;153: (6):1219–27. doi:10.1016/j.cell.2013.05.002 |
[82] | Christian KM , Song H , Ming GL . Functions and dysfunctions of adult hippocampal neurogenesis. Annual Review of Neuroscience. (2014) ;37: :243–62. doi:10.1146/annurev-neuro-071013-014134 |
[83] | Murphy BL , Pun RY , Yin H , Faulkner CR , Loepke AW , Danzer SC . Heterogeneous integration of adult-generated granule cells into the epileptic brain. J Neurosci. (2011) ;31: (1):105–17. doi:10.1523/JNEUROSCI.2728-10.2011 |



