Exercise in a Pill: The Latest on Exercise-Mimetics
Abstract
There is increasing evidence that an active lifestyle benefits both body and brain. However, not everyone may be able to exercise due to disease, injury or aging-related frailty. Identification of cellular targets activated by physical activity may lead to the development of new compounds that can, to some extent, mimic systemic and central effects of exercise. This review will focus on factors relevant to energy metabolism in muscle, such as the 5’ adenosine monophosphate-activated protein kinase (AMPK) - sirtuin (SIRT1) - Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) pathway, and the molecules affecting it. In particular, putative exercise-mimetics such as AICAR, metformin, and GW501516 will be discussed. Moreover, plant-derived polyphenols such as resveratrol and (-)epicatechin, with exercise-like effects on the body and brain will be evaluated.
INTRODUCTION
“Methinks that the moment my legs begin to move, my thoughts begin to flow”
- David Henry Thoreau -
‘Thoreau’s Journal’ August 19, 1851
Thoreau was among those intellectuals who saw physical activity as a fundamental component of keeping their mind active and the spring of inspiration flowing. Exercise benefits young and old organisms, including increased skeletal mass, improvement in the cardiovascular system and metabolic regulation, as well as in brain functions associated with cognition, memory and mood. Indeed, benefits of an active lifestyle for the brain are being supported by an increasing amount of scientific evidence. In particular, exercise promotes adult hippocampal neurogenesis and neuronal plasticity [1, 2], and is associated with increased memory performance and cognition, and is considered to counter cognitive decline caused by aging and by neurodegenerative diseases [3–5].
The vast beneficial consequences of exercise might not be within reach of debilitated, diseased and elderly patients. The development of compounds capable of activating cellular targets of exercise may be a new therapeutic approach. Indeed, recent research indicates that factors secreted by skeletal muscle during exercise may exert beneficial effects on brain function [6–9]. This review will focus on the identified targets relevant to energy metabolism in muscle, such as the 5’ adenosine monophosphate-activated protein kinase (AMPK) - sirtuin (SIRT1) - Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) pathway, and the molecules affecting it.
Skeletal muscle is the most abundant tissue in the human body and the most highly activated organ in response to physical activity. Aerobic exercise affects skeletal muscle by inducing a substantial switch in composition from fast-twitching, glycolytic type IIb fibers to the more oxidative, slow-twitching type I fibers [10]. Endurance training results in an increase in mitochondrial biogenesis and activity [11], vascularization, oxygen consumption [12] and an overall improvement of aerobic capacity [13]. Furthermore, the resulting activation of signaling pathways relevant to energy metabolism, such as the AMPK-SIRT1-PGC-1α pathway in muscle may contribute to the benefits of exercise for brain function. In this context, compounds such as AICAR, metformin, and GW501516 will be discussed. Moreover, the capacity of dietary supplements, such as resveratrol and epicatechin, to mimic effects of exercise on body and brain will be evaluated (Fig. 1).
AMPK-SIRT1-PGC-1α PATHWAY, A POSSIBLE TARGET?
Repeated muscle contractions affect a plethora of cellular allosteric factors, such as ATP/AMP concentration, Ca++ availability, and NAD+ levels, which in turn regulate enzymes whose activity is at the core of metabolic regulating pathways. Sirtuin 1 (SIRT1) is sensitive to NAD+ levels, and, via its deacetylase ability, is known to regulate up to 40 different downstream proteins [14], including AMPK and PGC-1α which are essential for muscle energy metabolism and mitochondrial biogenesis.
AMPK
AMPK is a master regulator of cellular metabolism. It is a heterotrimeric Ser/Thr kinase formed by three distinct subunits: the α subunit is responsible for the catalytic activity, while β and γ are regulatory subunits. AMPK is responsive to the cellular ratio of ATP:AMP, which is greatly reduced by ATP consumption during muscle contraction. Increasing AMP concentration leads to the formation of an AMP-AMPK complex on the γ subunit, which activates the catalytic α subunit of AMPK [15]. Moreover, once AMP binds AMPK, the complex has increased affinity with AMPK-kinase Liver Kinase B1 (LKB1), which in turn phosphorylates Thr172, massively increasing AMPK catalytic activity [16]. AMP-AMPK complex prevents dephosphorylation of Thr172, maintaining the kinase in the active form [15].
In skeletal muscle, AMPK activation induces a switch of cellular metabolism from anabolic to catabolic, blocking energy-consuming processes and promoting ATP synthesizing processes from fatty acid oxidation [17], glycosylation and glucose uptake [18]. Such modifications are rapidly induced by direct phosphorylation of metabolic enzymes, while a slower, long-lasting effect is obtained by regulating transcription. AMPK is therefore a transcriptional regulator, because it directly phosphorylates, among others, PGC-1α [19], whose activity modulates mitochondrial biogenesis.
SIRT1
Deacetylase SIRT belongs to a family of 7 proteins called sirtuins, so named for being the mammalian homologs of the yeast silent information regulator (SIR2) protein. Studies showed that SIRT1 is crucial for downstream activation of AMPK by promoting the deacetylation of AMPK-kinase (LKB1), which in turn activates AMPK [20]. Interestingly, SIRT1 acts as a metabolic sensor via changes in intracellular redox state. Increasing cellular levels of NAD+ activates SIRT1 and promotes PGC-1α activity, playing an important role in role in mitochondrial biogenesis [21]. Indeed, SIRT1 and PGC-1α form a complex. In this form, NAD+ driven deacetylation by SIRT1 activates PGC-1α promoting its specific activity as a transcriptional factor on mitochondrial respiratory- and fatty acid metabolism-related genes [22]. Interestingly, SIRT1, in conditions of overexpression or knock-out, can also act as a PGC-1α inhibitor, reducing mitochondrial activity [23], suggesting there is an important role for SIRT1 in exercise-induced mitochondrial biogenesis.
PGC-1α
Endurance exercise activates PGC-1α by stimulating P38 MAPK in skeletal muscle, and activated PGC-1α enhances mitochondrial biogenesis. PGC-1α is a crucial co-activator of a huge variety of downstream transcriptional factors involved in fatty acid oxidation and mitochondrial biogenesis, ultimately boosting cellular respiratory rate. Impaired endurance, abnormal fiber composition and flawed mitochondrial-related gene regulation are observed in muscle specific PGC-1α knock-out models [24, 25]. These results support the idea that PGC-1α has an important role in angiogenesis, mitochondrial biogenesis and muscle fiber type transition during exercise.
PPARδ
PPARδ is a nuclear hormone receptor which acts as a transcriptional regulator of more than 100 genes and in doing so plays a crucial role in a variety of biological processes, from energy regulation to development and differentiation [26]. The role of PPARδ in skeletal muscle has been widely studied and is known to affect mitochondrial biogenesis, lipid metabolism and oxidative processes, slow/fast twitch fiber regulation, weight reduction, impairment of liver gluconeogenesis, and regulation of inflammatory processes [27, 28].
EXERCISE-MIMETICS
Exercise is an effective tool to counteract a wide variety of metabolic problems, age-related loss of function and physiological issues. Overall, physical activity is a cornerstone of a healthy lifestyle. Unfortunately, exercise often is difficult to implement as an intervention for patients with conditions requiring better weight management and improved glucose metabolism. An active lifestyle has also been shown to enhance mood and cognition, and may delay the onset of neurodegenerative diseases. The beneficial effects of exercise on the body and brain are likely not replaced by one single pill. The complex underlying molecular mechanisms and modes of action provide multiple opportunities for pharmacological strategies for the various diseases. To better understand the molecular mechanisms, numerous studies focus on the systemic metabolic networks of transcriptional activators and interconnected enzymes involving AMPK, SIRT1, and PGC-1α. However, the possibility of minimal or even detrimental effects on brain function of candidate compounds targeting these peripheral pathways must also be considered. Indeed, differences between effects of exercise-mimetics on central versus peripheral systems could be another factor limiting the viability of a pharmacological alternative to exercise (Fig. 2, Table 1).
AICAR
The compound 5-Aminoimidazole-4-carboxamide ribonucleotide (also known as AICAR or AICA-ribotide) is the analog of AMP and intermediate metabolite of the purine synthesis pathway. AICAR is an endogenous substance, and is the active agent of the drug 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside. AICA-riboside is phosphorylated by the cellular adenosine kinase and, as AMP, activates AMPK by binding the AMPK γ subunit and promoting phosphorylation of Thr172 [29].
Effects of AICAR on peripheral organs
AICAR affects many organs and regulates a plethora of metabolic processes, in part by replicating the effects of exercise in both in vivo and in vitro studies. For instance, AICAR can mimic exercise by increasing glucose transporter type-4 (GLUT-4), hexokinase activity, resting glycogen content and muscle mitochondria numbers [30, 31]. AICAR has been also reported to increase angiogenesis and vascularization by inducing VEGFa expression in a fashion similar to exercise [32], although not necessarily acting through AMPK activation, rather via independent, alternative, and still unclear mechanisms [33].
Similar to exercise, AICAR also promotes liver fat consumption and inhibits lipogenesis as well as reduces circulating triglyceride levels and blood pressure [34, 35]. Furthermore, this compound can reduce hyperglycemia and improve glucose tolerance in obese diabetic mice in an insulin-independent way: reducing rate of gluconeogenesis in the liver, while increasing GLUT-4 mediated glucose uptake [36]. AICAR is also known for its direct AMPK-dependent effects on inflammatory process, resulting in a reduction of cytokine levels and inflammation. For instance, in vitro incubation of human aortic smooth muscle cells with AICAR reduced vascular inflammation and pro-inflammatory cytokine levels in a dose-dependent manner [37]. In vivo, treatment of mice with AICAR lowered cytokine levels in the lungs and reduced inflammation of the airways [38].
Effects of AICAR on the brain
The most interesting feature of exercise-mimetics is their ability to replicate, to some extent, the beneficial effect of exercise on brain functions. Daily AICAR (500 mg/kg) treatment in young mice for one week improved spatial memory and increased neurogenesis one month later [6]. Even in 2-year-old mice, longer treatment (14 days) produced memory and motor coordination benefits [39]. These effects are likely to be indirect consequences of peripheral AICAR administration [9], given the extremely low permeability through the blood brain barrier [40] the lack of spatial memory improvement by AICAR in muscle specific AMPKα2 mutant mice [39], and the adverse effect of direct intracranial infusion on synaptic plasticity and spatial memory in rats [41]. It should also be noted that in young mice, longer (14 days) treatment failed to improve spatial memory and neurogenesis, raising the possibility of negative consequences of chronic administration [39].
Divergence between effects of short and long administration correlates neural plasticity with a study in skeletal muscle, where 14 days of AICAR failed to increase SIRT1 expression, contrary to 7 days of administration [42]. In a recent study, we made a side-by-side comparison between effects of short- and long-term AICAR administration and exercise regimens, on gastrocnemius muscle and brain in young C57Bl/6 male mice. Both interventions induced similar AMPK pathway activation in skeletal muscle after both short (3–7 days) and longer (14 days) administration. However, in the brain only the short-term treatment was beneficial. Specifically, in the dentate gyrus of the hippocampus, brain-derived neurotrophin (BDNF) levels and neuronal proliferation were elevated by short-term treatment, while no increase was reported after longer administration [43]. Moreover, gene expression profiles in brain areas crucial for learning and memory processes exhibited divergent regulation between longer AICAR administration and exercise treatment [43] (Fig. 2).
A possible reason for lack of beneficial effects in the brain after 14 days of AICAR administration could be elevated inflammatory cytokine IL-1b and divergent expression of nitric oxide (NO) synthetic enzymes, suggesting an up-regulation of inflammation and oxidative stress [43]. Given the aforementioned possibility that AICAR indirectly affects the brain, and the evidence of in vitro modulation of inflammatory responses and cytokine levels in rat microglia through both AMPK-dependent and -independent mechanisms [44], an indirect pro-inflammatory effect of long term AICAR administration on the brain seems possible. Moreover, AICAR is known to increase skeletal muscle release of IL-6 into the bloodstream, which can cross the blood brain barrier and is an active pro-inflammatory cytokine [31].
To some extent, these findings show that AICAR can improve the parameters that are enhanced by exercise, however these have a short half-life [45] and off-target effects may occur because of various distributions of AMPK receptor subtypes in different tissues [46]. Such observations are corroborated by a recent study by Jang et al., which showed an increase in BDNF levels, an increase neuronal proliferation and a decrease in AMPK activation in the brain - but not in the periphery - upon 7 days of AICAR treatment in young but not in old mice. Moreover, the activation of AMPK, measured after acute AICAR administration was more prominent than after subchronic AICAR treatment, thus supporting the possibility that brain tissues could be particularly sensitive to extended activation or over-activation of AMPK [47]. Therefore, the precise dosage and duration of administration must be carefully adjusted to the aim of treatments, in order to elicit effects that mimic those of exercise, and to minimize side effects.
Metformin
Metformin is a compound that belongs to the biguanidine class, commonly used in clinical treatment as an anti-diabetic drug. Despite numerous studies, the mechanisms of action of metformin are still unclear. Metformin’s primary target within cells is the mitochondrial respiratory chain. In fact, metformin has a transient inhibitory effect on Complex I, possibly via accumulation of positively charged metformin molecules within the mitochondria. Complex I inhibition in turn reduces the concentration of ATP in favor of AMP. As seen in the previous section, this decline of ATP/AMP ratio activates AMPK. Likewise, both AICAR and metformin activate AMPK and have similar roles in hepatic glucose production even though the mode of AMPK activation is different [48]. Moreover, metformin acts as a metabolic inhibitor and disrupts the downstream cAMP–PKA pathway [49].
Peripheral organ effects of metformin
Exercise and metformin are prescribed treatments for type 2 diabetes [50–52]. Exercise and metformin reduce circulating glucose levels by stimulating GLUT-4 membrane translocation and expression, both in adipose tissue and skeletal muscle which increases their glucose uptake. However, in terms of hepatic gluconeogenesis [53], they have contrasting roles. Indeed, exercise increases hepatic gluconeogenesis while metformin greatly reduces gluconeogenesis, thus reducing endogenous glucose synthesis up to 30% [54].
Chronic metformin administration causes significant weight loss both in rodents and humans [55, 56], similar to exercise. This weight loss is at least in part due to the anorexigenic effect of metformin, as reported in animal models of diabetes [51] and in human patients [57]. The underlying mechanism is still unclear. It has been proposed that metformin reduces hypothalamic Neuropeptide Y (NPY) gene expression, partly via regulation by AMPK, as shown after direct intracranial injection (200μg metformin) in fasting rats [58]. NPY is known to induce an increase in food intake while decreasing physical activity [59]. Moreover, blood levels of leptin have been shown to correlate with the anorexigenic effect of metformin, raising the possibility that metformin administration also induces hypothalamic sensitivity to leptin by promoting leptin receptor expression [60]. The hypothalamus is a crucial brain region for energy balance and regulation [61] and by acting upon it, metformin could reduce food intake both in diabetic [57] and non-diabetic [62] conditions.
Metformin can reduce blood pressure in diabetic patients [63]. In addition, metformin improves multiple cardiovascular risk factors by reducing body mass index, total cholesterol, HDL and VHDL levels, along with blood glucose levels, pulse rate and blood pressure in obese subjects [64].
In vitro studies report an inhibitory effect of metformin on TNFα-mediated inflammatory processes in vascular smooth muscle cells [65]. In addition, in rat cultures, metformin inhibited expression of inflammatory chemokines and cytokines such as ICAM-1, CCS-2, TGFβ 1 and NF-kB [66]. In vivo, a Spanish study reports obese diabetic patients treated with metformin showed reduced levels of C-reactive protein (hsCRP), TNFα and TLR 2/4 [67]. Moreover, a meta-analysis over five different studies reports that serum levels of pro-inflammatory IL-6 cytokine might be reduced by metformin, although not consistently throughout the analyzed studies [68]. It seems metformin may modulate inflammatory and immunological responses similar to exercise. However, more studies are necessary to evaluate the anti-inflammatory role of metformin.
Effects of metformin on the brain
Interestingly, some indications that beneficial effects of exercise on the brain might be inducible by metformin treatment are starting to appear. Treatment with metformin for 38 days in female mice increased hippocampal neurogenesis and improved spatial memory performance [69]. In mouse models of ischemia, metformin increases angiogenesis and neurogenesis via AMPK activation, and regulates macrophages and microglia in the recovering brain [70]. Such findings were confirmed by another study using mouse stroke models, which showed increased hippocampal neurogenesis in an AMPK-dependent fashion [71]. Interestingly, nitric oxide synthase (NOS) pathways have been implicated as well, similar to the beneficial effects promoted by AICAR in mice [43, 71]. Metformin was also reported to act as a neuroprotective drug in neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. For instance, metformin-induced AMPK activation was beneficial in a mouse model of Alzheimer’s disease, resulting in a protective effect on memory retention, albeit only in females [72]. Furthermore, in a Parkinson’s disease mouse model treatment for 21 days with oral metformin (500 mg/kg) increased levels of BDNF in substantia nigra pars compacta, reduced oxidative stress markers, and improved performance in motor skills [73].
Long-term metformin treatment may eventually induce cognitive impairments. Indeed, a study of over 125 diabetic patients showed that those who took metformin had more cognitive decline [74]. This is of interest, since diabetic patients are more at risk for Alzheimer’s disease. Another study of over 14,000 patients also linked long term metformin treatment to increased risk of Alzheimer’s disease [75]. It has been proposed that the mechanisms underlying negative effects might be related to interference of metformin with absorption of circulating levels of vitamin B12, important for memory function [76]. However, supplementation of vitamin B12 was not effective in counteracting cognitive decline in symptomatic, non-anemic older people with moderate vitamin B12 deficiency [77].
GW501516
The compound GW501516 is a selective agonist of Peroxisome Proliferator-Activator Receptor δ (PPARδ). Interestingly, GW501516 was developed for its possible beneficial effects on metabolic disorders and cardiovascular diseases, but the discovery of its carcinogenic properties in pre-clinical studies on animals led to GW501516’s abandonment [78]. Nonetheless, in the last decade, several studies have linked this drug to exercise-mimetic properties [79–81]. Like AICAR, the drug has now been listed as an illegal substance by the World Anti-Doping Agency [27].
Peripheral effects of GW501516
GW501516 has been show to affect expression of endurance-related genes and metabolic pathways. Interestingly, GW501516 has a synergistic effect with exercise. Indeed, combined with a running regimen it improved endurance performance by 70% [79]. More recently, a metabolomic study comparing 3 weeks of exercise alone and in combination with GW501516 confirmed the synergistic effect of the compound, with an endurance increase of 70%. Moreover, in sedentary conditions, the compound elicited an endurance increase of nearly 50%. These new data highlight the possibility that exercise and GW501516 exert beneficial effects through two different mechanisms. Although both treatments increased mitochondrial fatty acid oxidation and fat metabolism in skeletal muscle, exercise acts by promoting catabolism, glycolysis and gluconeogenesis, while GW501516 regulates branched chain amino acid and ketone body pathways [80]. It is also worth mentioning that another PPARδ activator, GW0742, recently administered for 4 weeks in combination with AICAR (5 mg/kg and 500 mg/kg, respectively) to 7-week-old male Balb/c mice, increased running both in training and sedentary cohorts (138–179% and 355% increase), along with a shift to fat as main energy source [82].
Numerous in vitro and in vivo studies report a strong connection between GW501516, skeletal muscle fat metabolism, insulin sensitivity and circulating glucose levels, showing that this compound could potentially be beneficial in treating obesity and diabetes [83–85]. For instance, through activation of fatty acid metabolism, this drug reduced weight gain rate by almost 50% in high fat diet-induced obese mice, without an anorexigenic effect and therefore did not reduce food intake. This study reported a reduction of fat mass and a significant increase in oxygen consumption after GW501516 administration, reduced liver lipid accumulation, and significantly affected circulating insulin levels, ameliorating diet-induced insulin sensitivity [86]. Moreover, in a transgenic mouse model of obesity, circulating glucose levels are reduced by this compound [86].
As seen for the previous compounds and similar to exercise, GW501516 can also affect the inflammatory response. In fact, in vitro studies showed that GW501516 can prevent IL-6 release by interfering with NF-kB activity in both human [87] and murine [88] skeletal muscle cells. Furthermore, daily oral administration of 3 mg/kg reduced lipid-induced endoplasmatic reticulum stress in high-fat diet mice as well as in myotube cultures via crosstalk between PPARδ and AMPK pathway activation, thereby reducing levels of inflammatory markers like NF-kB, TNFα and IL-6 [89]. However, activation of PPARδ is not always beneficial. In a murine asthma model, the compound did not inhibit airway inflammation [90].
Effects of GW501516 on the brain
Administration of GW501516 has also been shown to affect the central nervous system. Interestingly, when aggregating rat brain cells were treated in vitro with IFNγ and LPS to induce inflammation, the compound induced both anti- and pro-inflammatory properties, reducing TNFα and inducible NOS (iNOS) levels, but also upregulating pro-inflammatory cytokines such as IL-6 [91]. Increased neuronal levels of IL-6 have been linked to in vivo excitotoxic damage, both in damaged and proximal gray matter, as well as in astrocytes [92], and are associated with neurodegeneration and microglial activation [93].
GW501516 treatment may also produce positive effects on the brain. Indeed, in vitro and in vivo studies reported that direct infusion might have a neuroprotective effect against neuronal damage, reducing both ischemia-induced brain damage in rats and MPTP-induced dopaminergic neuronal death in Parkinson’s disease mouse models [94]. Moreover, in young female C57Bl/6 mice spatial memory and dentate gyrus neurogenesis are enhanced by GW501516 treatment [6]. Similar to AICAR, GW501516 hardly crosses the blood brain barrier [94]. Therefore, cognitive improvements are possibly induced by indirect exercise-mimetic effects on skeletal muscle [6, 9, 79].
DIETARY SUPPLEMENTS
Nutrition is increasingly recognized as a crucial player in proper brain development and function. Providing the very fundamental materials for brain structure and connections, dietary components can affect cognition, neuronal activity and synaptic plasticity on a cellular level. In particular, plant-derived polyphenols, may positively affect brain function and induce metabolic effects similar to those induced by exercise (Table 2).
Resveratrol
The stilbene-structured compound named resveratrol is a polyphenol naturally occurring in plants [95]. Resveratrol sources are abundant in the human diet, including peanuts, blue- and blackberries. Ultimately its main dietary source in the Mediterranean diet is wine. In red wine there is resveratrol in seeds, skin and woods of the grapes [96].
Resveratrol is a highly lipophilic molecule, but despite its easy adsorption, it is known for its scarce bioavailability. However, the molecular properties of resveratrol allow it to both cross the cellular membrane and interact with membrane receptors. Therefore, resveratrol could activate pathways at the extracellular, cytoplasmatic or nuclear level [97].
Resveratrol affects a plethora of functions and mechanisms throughout the body, such as inflammation, oxidative stress, and mitochondrial respiratory chain function. Interestingly, in vitro administration of resveratrol has been reported to affect SIRT1, either directly [98] or indirectly by activating AMPK, as seen in mouse myotubes after 8 hours of exposure to 50μM resveratrol [21]. This effect on the SIRT1-AMPK pathway, along with resveratrol-induced increased availability of cAMP, which in turns also activates PGC-1α highlights the potential of resveratrol as a metabolism-regulating, exercise-mimetic molecule.
Effects of resveratrol on peripheral organs
The effect of resveratrol on lifespan extension was reported initially in yeast and subsequently in worms [99] and fish [100]. Resveratrol is considered to regulate energy metabolism in a SIRT1-dependent fashion [98]. Lifespan and health conditions are also improved by resveratrol in obese rodents, but no clear result of increased lifespan of mammals on a regular diet has been reported [101].
The role of resveratrol in mitochondrial and glucose metabolism has been widely studied in rodents. In particular, oral resveratrol administration increases oxygen consumption, aerobic capacity and insulin sensitivity in a high-fat diet regimen [102–104]. Resveratrol also has well-known antioxidant capabilities, both in vitro and, to a minor extent, in vivo [106]. Such a protective effect contributes to resveratrol’s capability of improving cardiovascular functions. In vitro studies on human umbilical cord cells showed that by increasing the activity and expression of the endothelial NOS (eNOS) enzyme through the SIRT1/AMPK pathway, resveratrol increases bioavailability of NO, therefore promoting endothelial vasculature [107, 108]. Similar effects on eNOS have been reported in vivo [109]. Specifically, six month old male ApoE-KO mice treated with 100 mg/kg resveratrol for 7 days, showed a marked elevation of a NOS cofactor tetrahydrobiopterin and reversed the uncoupling of eNOS.
When tested in parallel with an exercise regimen, resveratrol attenuated exercise-induced oxidative stress damage in young and old rodents. In 3 and 27 month old C57Bl/6J mice, after 10 days of resveratrol-supplemented diet, the compound vastly reduced oxidative stress damage to muscle fibers [110]. Resveratrol exerts its effect both in combination with and in absence of exercise regimens. A resveratrol-enriched diet (146 mg/kg/day), via activation of the SIRT1/AMPK pathway, increased running endurance in rats trained on treadmills for 12 weeks [111]. Resveratrol failed, however, to improve endurance in rats in the absence of exercise training. Similarly, a resveratrol-enriched diet paired with regular exercise improved endurance in aged mice [112]. Interestingly, young C57Bl/6J and KKay male mice fed with a 400 mg/kg resveratrol-enriched diet showed almost twice the running endurance of their controls, both in the standard and high-fat diet paradigm [102]. The actual efficacy of resveratrol alone still requires further studies, as there is contradictory evidence. In aged humans, 8 weeks of 250 mg/day resveratrol intake alone and in combination with high-intensity exercise training showed that resveratrol alone fails to improve muscle resistance and endurance in healthy aged men [113]. It has indeed been postulated that resveratrol might be more apt as a performance enhancer in the presence of exercise, rather than as a substitute for exercise entirely [111].
Effects of resveratrol on the brain
Resveratrol, possibly via its direct effect on the SIRT1-AMPK pathway, shows beneficial effects on neuronal activity and brain functions. Old male C57Bl/6 mice, which received 150 mg resveratrol/kg food up to 12 months before testing, improved their spatial memory performance on a Y-maze test and displayed more stress-free behavior in the open-field test. Also, resveratrol was reported to improve vascular density in the hippocampus [114]. Resveratrol has additionally been shown to promote developmental neurogenesis via SIRT1- and AMPK-dependent mechanisms in vitro [115]. In vivo the compound increased adult hippocampal neurogenesis and neuronal survival in conditions of hippocampal neuro-inflammation in a mouse model of chronic fatigue [116]. In prenatally stressed rats, increase in new neuron number and hippocampal BDNF expression was reported postnatally in the offspring of rats exposed to restraint stress and resveratrol during gestation [117]. See, however, a recent paper that shows that the compound inhibits adult hippocampal neurogenesis in wildtype C57Bl/6 under standard conditions [118].
Resveratrol shows promising neuroprotective capabilities. For instance, up to 10 g/kg intravenous resveratrol solution administered before and after induction of brain ischemia in rats reduced brain infarct area and edema, and benefited brain mitochondrial function [119]. In addition, in a variety of neurodegenerative disorders such as Parkinson’s [120], Alzheimer’s [121], Huntington’s [122] disease and Amyotrophic Lateral Sclerosis [123], resveratrol had direct neuroprotective ability, both in vitro and in vivo (for a dedicated review, see Tellone et al., 2015 [124]).
Although resveratrol intake reflects exercise-like effects in improving mitochondrial function and insulin sensitivity in obesity, and has neuro-protective functions in neurodegenerative models, it is still necessary to elucidate the mechanisms of action and to determine the adequate intake dosages.
Epicatechin
(-)Epicatechin is a monomeric flavanol commonly found in fruits: primarily apples, berries, grapes, and cocoa. Belonging to the broader class of flavonoids, (-)epicatechin is a polyphenolic molecule, linking two aromatic carbon rings with a carbon bridge [125], (Fig. 1).
Despite its relatively low bioavailability, epicatechin has been extensively studied and shown to be biologically active on a different number of metabolic and structural processes.
Effects of (-)epicatechin on peripheral systems
Similar to exercise, (-)epicatechin has a remarkable effect on regulation of cardiovascular processes and oxidative stress management. In vitro, (-)epicatechin improved NO production by affecting the eNOS pathway in human coronary artery endothelial cells [126]. Similarly in vivo, 10 days of pretreatment with 1 mg/kg/day (-)epicatechin in Sprague-Dawley rats conferred cardiovascular protection against epithelial damage induced by ischemia, via reduction of tissue levels of oxidative stress [127]. Also, vasodilation and blood pressure are regulated by oral (-)epicatechin consumption in human subjects in a dose-dependent manner. This effect has been linked to NO levels as inhibition of NO synthase reduces effects of (-)epicatechin on the vasculature [128].
Endurance is affected by (-)epicatechin as well. (-)Epicatechin alone can elicit an improvement, albeit this is magnified upon combination with exercise. Treatment with 1 mg/kg (-)epicatechin for two weeks improved treadmill performance and time to fatigue in mice, as well as increased mitochondrial protein expression, both by (-)epicatechin alone and in combination with exercise [129]. Moreover, (-)epicatechin combined with treadmill exercise induced an increase in vascularization and mitochondrial biogenesis in mouse muscle, improving exercise-induced fatigue tolerance [130].
In humans, (-)epicatechin benefits peripheral systems as well. A randomized double blind study in elderly sedentary human subjects showed a direct effect on the AMPK-SIRT1-PGC-1α pathway in skeletal muscles: after three months of daily intake of 20 g (-)epicatechin-rich cocoa, protein levels of LKB1 and activated AMPK and PGC-1α increased. In addition, VO2 and exercise performance improved in the (-)epicatechin group [131]. Moreover, diabetic patients with affected skeletal muscle structure and performance, showed improvement in muscle growth and differentiation markers compatible with myofiber regeneration, after chronic consumption of (-)epicatechin-rich cocoa (approximately 100 mg/day for 3 months) [132].
(-)Epicatechin consumption in elderly sarcopenia patients (25 mg for 7 days) improved the ratio of plasma follistatin/myostatin levels, as well as increased hand strength [133]. In addition, vasodilation and blood pressure were shown to be affected by oral consumption in human subjects in a dose-dependent manner. This effect was linked to NO levels as inhibition of NO synthase reduces (-)epicatechin’s vascular effects [134].
Effects of (-)epicatechin on the brain
(-)Epicatechin is a lipophilic compound that crosses the blood brain barrier and has been shown to elicit a direct effect on brain function and neuronal activity. Indeed, studies in both animal models and humans reported a promising link between (-)epicatechin consumption and cognition. Daily oral administration of up to 30 mg/kg epicatechin to female C57Bl/6J mice improved spatial memory, increased dentate granule cell spine density, enhanced hippocampal vascularization and expression of genes important for synaptic plasticity [135]. Comparable observations were made in mouse models of aging [136–138]. Furthermore, three months of (-)epicatechin consumption in C57Bl/6J male mice reduced anxiety in the open field and elevated plus maze tests, possibly via hippocampal and cortical monoaminergic (monoamine oxidase) and neurotrophic (BDNF) systems [139].
The dentate gyrus of the hippocampus is crucial for cognition and its functional decline is strongly linked to age-related memory loss [140]. After 3 months of daily administration of a cocoa drink rich in flavanols, including (-)epicatechin, elderly subjects were tested for cognitive, dentate gyrus-dependent tasks and showed reversal of memory decline comparable to a shift to a three decades younger group [141], highlighting the potential of (-)epicatechin to counteract age-related cognitive decline. Even more so, epicatechin has shown promising neuroprotective effects in preventing the onset of neurodegenerative diseases [142], in mouse models of Parkinson’s [143] and Alzheimer’s disease [144].
CONCLUSIONS
An active lifestyle, despite the promising of compounds currently under study, remains the preferred choice for improving body and brain function. Indeed, the mechanisms of action of exercise-mimetics still require further investigation, and the possibility of a treatment capable of replacing exercise in its entirety is remote. In order to achieve an artificial exercise regimen, potential adverse effects of prolonged treatment with exercise-mimetics have to be overcome. Nonetheless, a possible use of this class of compounds could be envisioned in parallel with light training paradigms, as “crutch-drugs” helping to achieve a more complete exercise-induced benefit, both on brain and on peripheral functions. This is especially poignant for conditions, such as morbid obesity or neurodegenerative diseases, which may preclude exercise.
ACKNOWLEDGMENTS
This work was supported by the National Institute on Aging Intramural Research Program. We thank Susan Lubejko for editorial assistance and Thomas Wynn for illustration.
REFERENCES
[1] | Kobilo T , Liu QR , Gandhi K , Mughal M , Shaham Y , van Praag H . Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learning & Memory. (2011) ;18: (9):605–9. |
[2] | Christie BR , Eadie BD , Kannangara TS , Robillard JM , Shin J , Titterness AK . Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Medicine. (2008) ;10: (2):47–58. |
[3] | Hayes SM , Alosco ML , Forman DE . The Effects of Aerobic Exercise on Cognitive and Neural Decline in Aging and Cardiovascular Disease. Current Geriatrics Reports. (2014) ;3: (4):282–90. |
[4] | Svensson M , Lexell J , Deierborg T . Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabilitation and Neural Repair. (2015) ;29: (6):577–89. |
[5] | Voss MW , Vivar C , Kramer AF , van Praag H . Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences. (2013) ;17: (10):525–44. |
[6] | Kobilo T , Yuan C , van Praag H . Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learning & Memory. (2011) ;18: (2):103–7. |
[7] | Wrann CD , White JP , Salogiannnis J , Laznik-Bogoslavski D , Wu J , Ma D , et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metabolism. (2013) ;18: (5):649–59. |
[8] | Agudelo LZ , Femenia T , Orhan F , Porsmyr-Palmertz M , Goiny M , Martinez-Redondo V , et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. (2014) ;159: (1):33–45. |
[9] | Moon HY , Becke A , Berron D , Becker B , Sah N , Benoni G , et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metabolism. (2016) ;24: :332–40. |
[10] | Colliander EB , Dudley GA , Tesch PA . Skeletal muscle fiber type composition and performance during repeated bouts of maximal, concentric contractions. Eur J Appl Physiol Occup Physiol. (1988) ;58: (1-2):81–6. |
[11] | Adhihetty PJ , Irrcher I , Joseph AM , Ljubicic V , Hood DA . Plasticity of skeletal muscle mitochondria in response to contractile activity. Experimental Physiology. (2003) ;88: (1):99–107. |
[12] | Abruzzo PM , Esposito F , Marchionni C , di Tullio S , Belia S , Fulle S , et al. Moderate exercise training induces ROS-related adaptations to skeletal muscles. International Journal of Sports Medicine. (2013) ;34: (8):676–87. |
[13] | Fluck M . Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. The Journal of Experimental Biology. (2006) ;209: (Pt 12):2239–48. |
[14] | Nogueiras R , Habegger KM , Chaudhary N , Finan B , Banks AS , Dietrich MO , et al. Sirtuin 1 and sirtuin Physiological modulators of metabolism. Physiological Reviews. (2012) ;92: (3):1479–514. |
[15] | Gowans GJ , Hawley SA , Ross FA , Hardie DG . AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metabolism. (2013) ;18: (4):556–66. |
[16] | Hawley JA , Holloszy JO . Exercise: It’s the real thing! Nutrition Reviews (2009) ;67: (3):172–8. |
[17] | Vavvas D , Apazidis A , Saha AK , Gamble J , Patel A , Kemp BE , et al. Contraction-induced changes in acetyl-CoA carboxylase and 5’-AMP-activated kinase in skeletal muscle. J Biol Chem. (1997) ;272: (20):13255–61. |
[18] | Kurth-Kraczek EJ , Hirshman MF , Goodyear LJ , Winder WW . 5’ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. (1999) ;48: (8):1667–71. |
[19] | Jager S , Handschin C , St-Pierre J , Spiegelman BM . AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America. (2007) ;104: (29):12017–22. |
[20] | Lan F , Cacicedo JM , Ruderman N , Ido Y . SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. The Journal of Biological Chemistry. (2008) ;283: (41):27628–35. |
[21] | Canto C , Gerhart-Hines Z , Feige JN , Lagouge M , Noriega L , Milne JC , et al. AMPK regulates energy expenditure by modulating NAD+metabolism and SIRT1 activity. Nature. (2009) ;458: (7241):1056–60. |
[22] | Gerhart-Hines Z , Rodgers JT , Bare O , Lerin C , Kim SH , Mostoslavsky R , et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. (2007) ;26: (7):1913–23. |
[23] | Nemoto S , Fergusson MM , Finkel T . SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1alpha. The Journal of Biological Chemistry. (2005) ;280: (16):16456–60. |
[24] | Lin J , Wu H , Tarr PT , Zhang CY , Wu Z , Boss O . Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. (2002) ;418: (6899):797–801. |
[25] | Handschin C , Chin S , Li P , Liu F , Maratos-Flier E , Lebrasseur NK , et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. The Journal of Biological Chemistry. (2007) ;282: (41):30014–21. |
[26] | Matsakas A , Narkar VA . Endurance exercise mimetics in skeletal muscle. Curr Sports Med Rep. (2010) ;9: (4):227–32. |
[27] | Pokrywka A , Cholbinski P , Kaliszewski P , Kowalczyk K , Konczak D , Zembron-Lacny A . Metabolic modulators of the exercise response: Doping control analysis of an agonist of the peroxisome proliferator-activated receptor δ (GW16) and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). J Physiol Pharmacol. (2014) ;65: (4):469–76. |
[28] | Wang YX , Zhang CL , Yu RT , Cho HK , Nelson MC , Bayuga-Ocampo CR , et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. (2004) ;2: (10):e294. |
[29] | Corton JM , Gillespie JG , Hawley SA , Hardie DG . 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells?. Eur J Biochem. (1995) ;229: (2):558–65. |
[30] | Sanchez J , Nozhenko Y , Palou A , Rodriguez AM . Free fatty acid effects on myokine production in combination with exercise mimetics. Molecular Nutrition & Food Research. (2013) ;57: (8):1456–67. |
[31] | Lauritzen HP , Brandauer J , Schjerling P , Koh HJ , Treebak JT , Hirshman MF , et al. Contraction and AICAR stimulate IL-6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes. (2013) ;62: (9):3081–92. |
[32] | Ouchi N , Shibata R , Walsh K . AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circulation Research. (2005) ;96: (8):838–46. |
[33] | Zwetsloot KA , Westerkamp LM , Holmes BF , Gavin TP . AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. The Journal of Physiology. (2008) ;586: (24):6021–35. |
[34] | Yang Z , Wang X , He Y , Qi L , Yu L , Xue B , et al. The full capacity of AICAR to reduce obesity-induced inflammation and insulin resistance requires myeloid SIRT1. PLoS One. (2012) ;7: (11):e49935. |
[35] | Buhl ES , Jessen N , Pold R , Ledet T , Flyvbjerg A , Pedersen SB , et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features. Diabetes. (2002) ;51: (7):2199–206. |
[36] | Viollet B , Lantier L , Devin-Leclerc J , Hebrard S , Amouyal C , Mounier R , et al. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Frontiers in Bioscience. (2009) ;14: :3380–400. |
[37] | He G , Zhang YW , Lee JH , Zeng SX , Wang YV , Luo Z , et al. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Molecular and Cellular Biology. (2014) ;34: (2):148–57. |
[38] | Kim TB , Kim SY , Moon KA , Park CS , Jang MK , Yun ES , et al. Five-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates poly (I:C)-induced airway inflammation in a murine model of asthma. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology. (2007) ;37: (11):1709–19. |
[39] | Kobilo T , Guerrieri D , Zhang Y , Collica SC , Becker KG , van Praag H . AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learning & Memory. (2014) ;21: (2):119–26. |
[40] | Marangos PJ , Loftus T , Wiesner J , Lowe T , Rossi E , Browne CE , et al. Adenosinergic modulation of homocysteine-induced seizures in mice. Epilepsia. (1990) ;31: (3):239–46. |
[41] | Dash PK , Orsi SA , Moore AN . Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. The Journal of Neuroscience. (2006) ;26: (31):8048–56. |
[42] | Suwa M , Nakano H , Radak Z , Kumagai S . Short-term adenosine monophosphate-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism: Clinical and Experimental. (2011) ;60: (3):394–403. |
[43] | Guerrieri D , van Praag H . Exercise-mimetic AICAR transiently benefits brain function. Oncotarget. (2015) ;6: (21):18293–313. |
[44] | Labuzek K , Liber S , Gabryel B , Okopien B . AICAR (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside) increases the production of toxic molecules and affects the profile of cytokines release in LPS-stimulated rat primary microglial cultures. Neurotoxicology. (2010) ;31: (1):134–46. |
[45] | Karagounis LG , Hawley JA . The 5’ adenosine monophosphate-activated protein kinase: Regulating the ebb and flow of cellular energetics. The International Journal of Biochemistry & Cell Biology. (2009) ;41: (12):2360–3. |
[46] | Wu J , Puppala D , Feng X , Monetti M , Lapworth AL , Geoghegan KF . Chemoproteomic analysis of intertissue and interspecies isoform diversity of AMP-activated protein kinase (AMPK). The Journal of Biological Chemistry. (2013) ;288: (50):35904–12. |
[47] | Jang S , Kim H , Jeong J , Lee SK , Kim EW , Park M , et al. Blunted response of hippocampal AMPK associated with reduced neurogenesis in older versus younger mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry. (2016) ;71: :57–65. |
[48] | Hardie DG . Metformin-acting through cyclic AMP as well as AMP? Cell Metabolism. (2013) ;17: (3):313–4. |
[49] | Pernicova I , Korbonits M . Metformin–mode of action and clinical implications for diabetes and cancer. Nature Reviews Endocrinology. (2014) ;10: (3):143–56. |
[50] | Cheng JT , Huang CC , Liu IM , Tzeng TF , Chang CJ . Novel Mechanism for Plasma Glucose – Lowering Action of Metformin in Streptozotocin-Induced Diabetic Rats. Diabetes. (2006) ;55: (3):819–25. |
[51] | Matsui Y , Hirasawa Y , Sugiura T , Toyoshi T , Kyuki K , Ito M . Metformin reduces body weight gain and improves glucose intolerance in high-fat diet-fed C57BL6J mice. Biol Pharm Bull. (2010) ;33: (6):963–70. |
[52] | Salpeter SR , Buckley NS , Kahn JA , Salpeter EE . Meta-analysis: Metformin treatment in persons at risk for diabetes mellitus. The American Journal of Medicine. (2008) ;121: (2):149–57 e2. |
[53] | Bergman BC , Horning MA , Casazza GA , Wolfel EE , Butterfield GE , Brooks GA . Endurance training increases gluconeogenesis during rest and exercise in men. American Journal of Physiology Endocrinology and Metabolism. (2000) ;278: (2):E244–51. |
[54] | Hundal RS , Krssak M , Dufour S , Laurent D , Lebon V , Chandramouli V , et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. (2000) ;49: (12):2063–9. |
[55] | Lim SS , Norman RJ , Clifton PM , Noakes M . The effect of comprehensive lifestyle intervention or metformin on obesity in young women. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD. (2011) ;21: (4):261–8. |
[56] | Seifarth C , Schehler B , Schneider HJ . Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Experimental and Clinical Endocrinology & Diabetes: Official Journal, German Society of Endocrinology [and] German Diabetes Association. (2013) ;121: (1):27–31. |
[57] | Paolisso G , Amato L , Eccellente R , Gambardella A , Tagliamonte MR , Varricchio G , et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest. (1998) ;28: (6):441–6. |
[58] | Duan Y , Zhang R , Zhang M , Sun L , Dong S , Wang G , Zhang J , Zhao Z . Metformin inhibits food intake and neuropeptide Y gene expression in the hypothalamus. Neural Regeneration Research. (2013) ;8: (25):2379–88. |
[59] | Mercer RE , Chee MJ , Colmers WF . The role of NPY in hypothalamic mediated food intake. Frontiers in Neuroendocrinology. (2011) ;32: (4):398–415. |
[60] | Aubert G , Mansuy V , Voirol MJ , Pellerin L , Pralong FP . The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression. Metabolism: Clinical and Experimental. (2011) ;60: (3):327–34. |
[61] | Tritos NA , Elmquist JK , Mastaitis JW , Flier JS , Maratos-Flier E . Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. (1998) ;139: (11):4634–41. |
[62] | Smith DL Jr , Elam CF Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, et al. Metformin supplementation and life span in Fischer-344 rats. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. (2010) ;65: (5):468–74. |
[63] | Giugliano D , De Rosa N , Di Maro G , Marfella R , Acampora R , Buoninconti R , et al. Metformin Improves Glucose, Lipid Metabolism, and Reduces Blood Pressure in Hypertensive, Obese Women. Diabetes Care. (1993) ;16: (10):1387–90. |
[64] | Gokcel A , Gumurdulu Y , Karakose H , Melek Ertorer E , Tanaci N , BascilTutuncu N , et al. Evaluation of the safety and efficacy of sibutramine, orlistat and metformin in the treatment of obesity. Diabetes Obes Metab. (2002) ;4: (1):49–55. |
[65] | Kim SA , Choi HC . Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. (2012) ;425: (4):866–72. |
[66] | Gu J , Ye S , Wang S , Sun W , Hu Y . Metformin inhibits nuclear factor-κB activation and inflammatory cytokines expression induced by high glucose via adenosine monophosphate-activated protein kinase activation in rat glomerular mesangial cells in vitro. Chin Med J. (2014) ;127: (9):1755–60. |
[67] | Andrews M , Soto N , Arredondo M . Effect of metformin on the expression of tumor necrosis factor-alpha, Toll like receptors 2/4 and C reactive protein in obese type-2 diabetic patients. Revista Medica De Chile. (2012) ;140: (11):1377–82. |
[68] | Xu X , Du C , Zheng Q , Peng L , Sun Y . Effect of metformin on serum interleukin-6 levels in polycystic ovary syndrome a systematic review. BMC Womens Health. (2014) ;14: (93). |
[69] | Wang J , Gallagher D , DeVito LM , Cancino GI , Tsui D , He L , et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. (2012) ;11: (1):23–35. |
[70] | Jin Q , Cheng J , Liu Y , Wu J , Wang X , Wei S , et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain, Behavior, and Immunity. (2014) ;40: :131–42. |
[71] | Liu Y , Tang G , Zhang Z , Wang Y , Yang GY . Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neuroscience Letters. (2014) ;579: :46–51. |
[72] | DiTacchio KA , Heinemann SF , Dziewczapolski G . Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. Journal of Alzheimer’s Disease. (2015) ;44: (1):43–8. |
[73] | Patil SP , Jain PD , Ghumatkar PJ , Tambe R , Sathaye S . Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. (2014) ;277: :747–54. |
[74] | Moore EM , Mander AG , Ames D , Kotowicz MA , Carne RP , Brodaty H , et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. (2013) ;36: (10):2981–7. |
[75] | Imfeld P , Bodmer M , Jick SS , Meier CR . Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: A population-based case-control study. Journal of the American Geriatrics Society. (2012) ;60: (5):916–21. |
[76] | Moore E , Mander A , Ames D , Carne R , Sanders K , Watters D . Cognitive impairment and vitamin B A review. International Psychogeriatrics/IPA. (2012) ;24: (5):541–56. |
[77] | Dangour AD , Allen E , Clarke R , Elbourne D , Fletcher AE , Letley L , et al. Effects of vitamin B-12 supplementation on neurologic and cognitive function in older people: A randomized controlled trial. The American Journal of Clinical Nutrition. (2015) ;102: (3):639–47. |
[78] | Sahebkar A , Chew GT , Watts GF . New peroxisome proliferator-activated receptor agonists: Potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opinion on Pharmacotherapy. (2014) ;15: (4):493–503. |
[79] | Narkar VA , Downes M , Yu RT , Embler E , Wang YX , Banayo E , et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. (2008) ;134: (3):405–15. |
[80] | Chen W , Gao R , Xie X , Zheng Z , Li H , Li S , et al. A metabolomic study of the PPARdelta agonist GW16 for enhancing running endurance in Kunming mice. Scientific Reports. (2015) ;5: :9884. |
[81] | Zizola C , Kennel PJ , Akashi H , Ji R , Castillero E , George I , et al. Activation of PPARdelta signaling improves skeletal muscle oxidative metabolism and endurance function in an animal model of ischemic left ventricular dysfunction. Am J Physiol Heart Circ Physiol. (2015) ;308: (9):H1078–85. |
[82] | Manio MC , Inoue K , Fujitani M , Matsumura S , Fushiki T . Combined pharmacological activation of AMPK and PPARdelta potentiates the effects of exercise in trained mice. Physiological Reports. (2016) ;4: (5). |
[83] | Barroso E , Rodriguez-Rodriguez R , Chacon MR , Maymo-Masip E , Ferrer L , Salvado L , et al. PPARbeta/delta ameliorates fructose-induced insulin resistance in adipocytes by preventing Nrf2 activation. Biochimica Et Biophysica Acta. (2015) ;1852: (5):1049–58. |
[84] | Lee CH , Kang K , Mehl IR , Nofsinger R , Alaynick WA , Chong LW , et al. Peroxisome proliferator-activated receptor delta promotes very low-density lipoprotein-derived fatty acid catabolism in the macrophage. Proceedings of the National Academy of Sciences of the United States of America. (2006) ;103: (7):2434–9. |
[85] | Wang YX , Lee CH , Tiep S , Yu RT , Ham J , Kang H , et al. Peroxisome-proliferator-activated receptor activates fat metabolism to prevent obesity. Cell. (2003) ;113: (2):159–70. |
[86] | Tanaka T , Yamamoto J , Iwasaki S , Asaba H , Hamura H , Ikeda Y , et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. (2003) ;100: (26):15924–9. |
[87] | Coll T , Alvarez-Guardia D , Barroso E , Gomez-Foix AM , Palomer X , Laguna JC , et al. Activation of peroxisome proliferator-activated receptor-delta by GW16 prevents fatty acid-induced nuclear factor-kappaB activation and insulin resistance in skeletal muscle cells. Endocrinology. (2010) ;151: (4):1560–9. |
[88] | Remels AH , Langen RC , Gosker HR , Russell AP , Spaapen F , Voncken JW , et al. PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab. (2009) ;297: (1):E174–83. |
[89] | Salvado L , Barroso E , Gomez-Foix AM , Palomer X , Michalik L , Wahli W , et al. PPARbeta/delta prevents endoplasmic reticulum stress-associated inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. (2014) ;57: (10):2126–35. |
[90] | Trifilieff A , Bench A , Hanley M , Bayley D , Campbell E , Whittaker P . PPAR-alpha and -gamma but not -delta agonists inhibit airway inflammation in a murine model of asthma: In vitro evidence for an NF-kappaB-independent effect. British Journal of Pharmacology. (2003) ;139: (1):163–71. |
[91] | Defaux A , Zurich MG , Braissant O , Honegger P , Monnet-Tschudi F . Effects of the PPAR-beta agonist GW16 in an in vitro model of brain inflammation and antibody-induced demyelination. Journal of Neuroinflammation. (2009) ;6: :15. |
[92] | Acarin L , González B , Castellano B . Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immatur rat brain. European Journal of Neuroscience. (2000) ;12: :3505–20. |
[93] | üttler E , Tarabin V , Schwaninger M . Interleukin-6 (IL-6): A possible neuromodulator induced by neuronal activity. Neuroscientist. (2002) ;8: (3):268–75. |
[94] | Iwashita A , Muramatsu Y , Yamazaki T , Muramoto M , Kita Y , Yamazaki S , et al. Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo. The Journal of Pharmacology and Experimental Therapeutics. (2007) ;320: (3):1087–96. |
[95] | Rege SD , Geetha T , Griffin GD , Broderick TL , Babu JR . Neuroprotective effects of resveratrol in Alzheimer disease pathology. Frontiers in Aging Neuroscience. (2014) ;6: :218. |
[96] | Soleas GJ , Diamandis EP , Goldberg DM . Resveratrol: A molecule whose time has come? And gone? Clinical Biochemistry. (1997) ;30: (2):91–113. |
[97] | Gambini J , Ingles M , Olaso G , Lopez-Grueso R , Bonet-Costa V , Gimeno-Mallench L . Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Medicine and Cellular Longevity. (2015) :837042. |
[98] | Howitz KT , Bitterman KJ , Cohen HY , Lamming DW , Lavu S , Wood JG , et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. (2003) ;425: :191–6. |
[99] | Wood JG , Rogina B , Lavu S , Howitz K , Helfand SL , Tatar M . Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. (2004) ;430: (7000):686–9. |
[100] | Valenzano DR , Terzibasi E , Genade T , Cattaneo A , Domenici L , Cellerino A . Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Current Biology. (2006) ;16: (3):296–300. |
[101] | Pearson KJ , Baur JA , Lewis KN , Peshkin L , Price NL , Labinskyy N . Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metabolism. (2008) ;8: (2):157–68. |
[102] | Lagouge M , Argmann C , Gerhart-Hines Z , Meziane H , Lerin C , Daussin F . Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. (2006) ;127: (6):1109–22. |
[103] | Baur JA , Pearson KJ , Price NL , Jamieson HA , Lerin C , Kalra A . Resveratrol improves health and survival of mice on a high-calorie diet. Nature. (2006) ;444: (7117):337–42. |
[104] | Hong HJ , Kang W , Kim DG , Lee DH , Lee Y , Han CH . Effects of resveratrol on the insulin signaling pathway of obese mice. Journal of Veterinary Science. (2014) ;15: (2):179–85. |
[105] | Stojanovic S , Sprinz H , Brede O . Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Archives of Biochemistry and Biophysics. (2001) ;391: (1):79–89. |
[106] | Bradamante S , Barenghi L , Villa A . Cardiovascular protective effects of resveratrol. Cardiovascular Drug Reviews. (2004) ;22: (3):169–88. |
[107] | Wallerath T , Deckert G , Ternes T , Anderson H , Li H , Witte K , et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. (2002) ;106: (13):1652–8. |
[108] | Xia N , Forstermann U , Li H . Resveratrol and endothelial nitric oxide. Molecules. (2014) ;19: (10):16102–21. |
[109] | Xia N , Daiber A , Habermeier A , Closs EI , Thum T , Spanier G , et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. The Journal of Pharmacology and Experimental Therapeutics. (2010) ;335: (1):149–54. |
[110] | Ryan MJ , Jackson JR , Hao Y , Williamson CL , Dabkowski ER , Hollander JM , et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. (2010) ;65: (8):815–31. |
[111] | Dolinsky VW , Jones KE , Sidhu RS , Haykowsky M , Czubryt MP , Gordon T , et al. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. The Journal of Physiology. (2012) ;590: (11):2783–99. |
[112] | Murase T , Haramizu S , Ota N , Hase T . Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. (2009) ;10: (4):423–34. |
[113] | Olesen J , Gliemann L , Bienso R , Schmidt J , Hellsten Y , Pilegaard H . Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. The Journal of Physiology. (2014) ;592: (8):1873–86. |
[114] | Oomen CA , Farkas E , Roman V , van der Beek EM , Luiten PG , Meerlo P . Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Frontiers in Aging Neuroscience. (2009) ;1: :4. |
[115] | Dasgupta B , Milbrandt J . Resveratrol stimulates AMP kinase activity in neurons. Proceedings of the National Academy of Sciences of the United States of America. (2007) ;104: (17):7217–22. |
[116] | Moriya J , Chen R , Yamakawa J , Sasaki K , Ishigaki Y , Takahashi T . Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biological & Pharmaceutical Bulletin. (2011) ;34: (3):354–9. |
[117] | Madhyastha S , Sekhar S , Rao G . Resveratrol improves postnatal hippocampal neurogenesis and brain derived neurotrophic factor in prenatally stressed rats. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. (2013) ;31: (7):580–5. |
[118] | Park HR , Kong KH , Yu BP , Mattson MP , Lee J . Resveratrol inhibits the proliferation of neural progenitor cells and hippocampal neurogenesis. The Journal of Biological Chemistry. (2012) ;287: (51):42588–600. |
[119] | Yousuf S , Atif F , Ahmad M , Hoda N , Ishrat T , Khan B , et al. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Research. (2009) ;1250: :242–53. |
[120] | Wu Y , Li X , Zhu JX , Xie W , Le W , Fan Z , et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neuro-Signals. (2011) ;19: (3):163–74. |
[121] | Ma T , Tan MS , Yu JT , Tan L . Resveratrol as a therapeutic agent for Alzheimer’s disease. BioMed Research International. (2014) ;2014: :350516. |
[122] | Pasinetti GM , Wang J , Marambaud P , Ferruzzi M , Gregor P , Knable LA , et al. Neuroprotective and metabolic effects of resveratrol: Therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Experimental Neurology. (2011) ;232: (1):1–6. |
[123] | Song L , Chen L , Zhang X , Li J , Le W . Resveratrol ameliorates motor neuron degeneration and improves survival in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. BioMed Research International. (2014) ;2014: :483501. |
[124] | Tellone E , Galtieri A , Russo A , Giardina B , Ficarra S . Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxidative Medicine and Cellular Longevity. (2015) ;2015: :392169. |
[125] | de Pascual-Teresa S , Moreno DA , Garcia-Viguera C . Flavanols and anthocyanins in cardiovascular health: A review of current evidence. International Journal of Molecular Sciences. (2010) ;11: (4):1679–703. |
[126] | Ramirez-Sanchez I , Maya L , Ceballos G , Villarreal F . (-)-Epicatechin Activation of Endothelial Cell Endothelial Nitric Oxide Synthase, Nitric Oxide, and Related Signaling Pathways. Hypertension. (2010) ;55: (6):1398–405. |
[127] | Yamazaki KG , Romero-Perez D , Barraza-Hidalgo M , Cruz M , Rivas M , Cortez-Gomez B , et al. Short- and long-term effects of (-)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. (2008) ;295: (2):H761–7. |
[128] | Fraga CG , Oteiza PI . Dietary flavonoids: Role of (-)-epicatechin and related procyanidins in cell signaling. Free Radical Biology & Medicine. (2011) ;51: (4):813–23. |
[129] | Nogueira L , Ramirez-Sanchez I , Perkins GA , Murphy A , Taub PR , Ceballos G , et al. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. The Journal of Physiology. (2011) ;589: (Pt 18):4615–31. |
[130] | Lee I , Huttemann M , Kruger A , Bollig-Fischer A , Malek MH . (-)-Epicatechin combined with 8 weeks of treadmill exercise is associated with increased angiogenic and mitochondrial signaling in mice. Frontiers in Pharmacology. (2015) ;6: :43. |
[131] | Taub PR , Ramirez-Sanchez I , Patel M , Higginbotham E , Moreno-Ulloa A , Roman-Pintos LM , et al. Beneficial effects of dark chocolate on exercise capacity in sedentary subjects: Underlying mechanisms. A double Blind, Randomized, Placebo Controlled Trial. Food & Function. (2016) ;7: (9):3686–93. |
[132] | Taub PR , Ramirez-Sanchez I , Ciaraldi TP , Gonzalez-Basurto S , Coral-Vazquez R , Perkins G , et al. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and type 2 diabetes: Restorative effects of (-)-epicatechin-rich cocoa. Clinical Science. (2013) ;125: (8):383–9. |
[133] | Gutierrez-Salmean G , Ciaraldi TP , Nogueira L , Barboza J , Taub PR , Hogan MC , et al. Effects of (-)-epicatechin on molecular modulators of skeletal muscle growth and differentiation. The Journal of Nutritional Biochemistry. (2014) ;25: (1):91–4. |
[134] | Schroeter H , Heiss C , Balzer J , Kleinbongard P , Keen CL , Hollenberg NK , et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proceedings of the National Academy of Sciences of the United States of America. (2006) ;103: (4):1024–9. |
[135] | van Praag H , Lucero MJ , Yeo GW , Stecker K , Heivand N , Zhao C , et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. The Journal of Neuroscience. (2007) ;27: (22):5869–78. |
[136] | Rodrigues J , Assuncao M , Lukoyanov N , Cardoso A , Carvalho F , Andrade JP . Protective effects of a catechin-rich extract on the hippocampal formation and spatial memory in aging rats. Behavioural Brain Research. (2013) ;246: :94–102. |
[137] | Unno K , Yamamoto H , Maeda K , Takabayashi F , Yoshida H , Kikunaga N , et al. Protection of brain and pancreas from high-fat diet: Effects of catechin and caffeine. Physiology & Behavior. (2009) ;96: (2):262–9. |
[138] | Moreno-Ulloa A , Nogueira L , Rodriguez A , Barboza J , Hogan MC , Ceballos G , et al. Recovery of Indicators of Mitochondrial Biogenesis, Oxidative Stress, and Aging With (-)-Epicatechin in Senile Mice. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. (2015) ;70: (11):1370–8. |
[139] | Stringer TP , Guerrieri D , Vivar C , van Praag H . Plant-derived flavanol (-)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Translational Psychiatry. (2015) ;5: :e493. |
[140] | Yassa MA , Mattfeld AT , Stark SM , Stark CE . Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. (2011) ;108: (21):8873–8. |
[141] | Brickman AM , Khan UA , Provenzano FA , Yeung LK , Suzuki W , Schroeter H , et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nature Neuroscience. (2014) ;17: (12):1798–803. |
[142] | Subash S , Essa MM , Al-Adawi S , Memon MA , Manivasagam T , Akbar M . Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regeneration Research. (2014) ;9: (16):1557–66. |
[143] | Bitu Pinto N , da Silva Alexandre B , Neves KR , Silva AH , Leal LK , Viana GS . Neuroprotective Properties of the Standardized Extract from Camellia sinensis (Green Tea) and Its Main Bioactive Components, Epicatechin and Epigallocatechin Gallate, in the 6-OHDA Model of Parkinson’s Disease. Evidence-Based Complementary and Alternative Medicine: ECAM. (2015) ;2015: :161092. |
[144] | Cox CJ , Choudhry F , Peacey E , Perkinton MS , Richardson JC , Howlett DR , et al. Dietary (-)-epicatechin as a potent inhibitor of betagamma-secretase amyloid precursor protein processing. Neurobiology of Aging. (2015) ;36: (1):178–87. |
Figures and Tables
Fig.1
Overview of the cellular effects of exercise and exercise-mimetics. AMP-Kinase (AMPK) is activated by AICAR, Metformin and Resveratrol in skeletal muscle. Activated AMPK positively regulates signaling pathways involved in endurance capacity, fat metabolism, mitochondrial biogenesis. The compound GW501516 also has metabolic effects by selectively activating transcription factor PPAR-δ. Nitric oxide synthase (NOS) is stimulated by (-)Epicatechin and Resveratrol, affecting vasodilation and angiogenesis in the brain and periphery. (-)Epicatechin also increases BDNF expression through the TrkB-AKT-CREB pathway. These molecules exert partial exercise-like effects on brain and skeletal muscle.
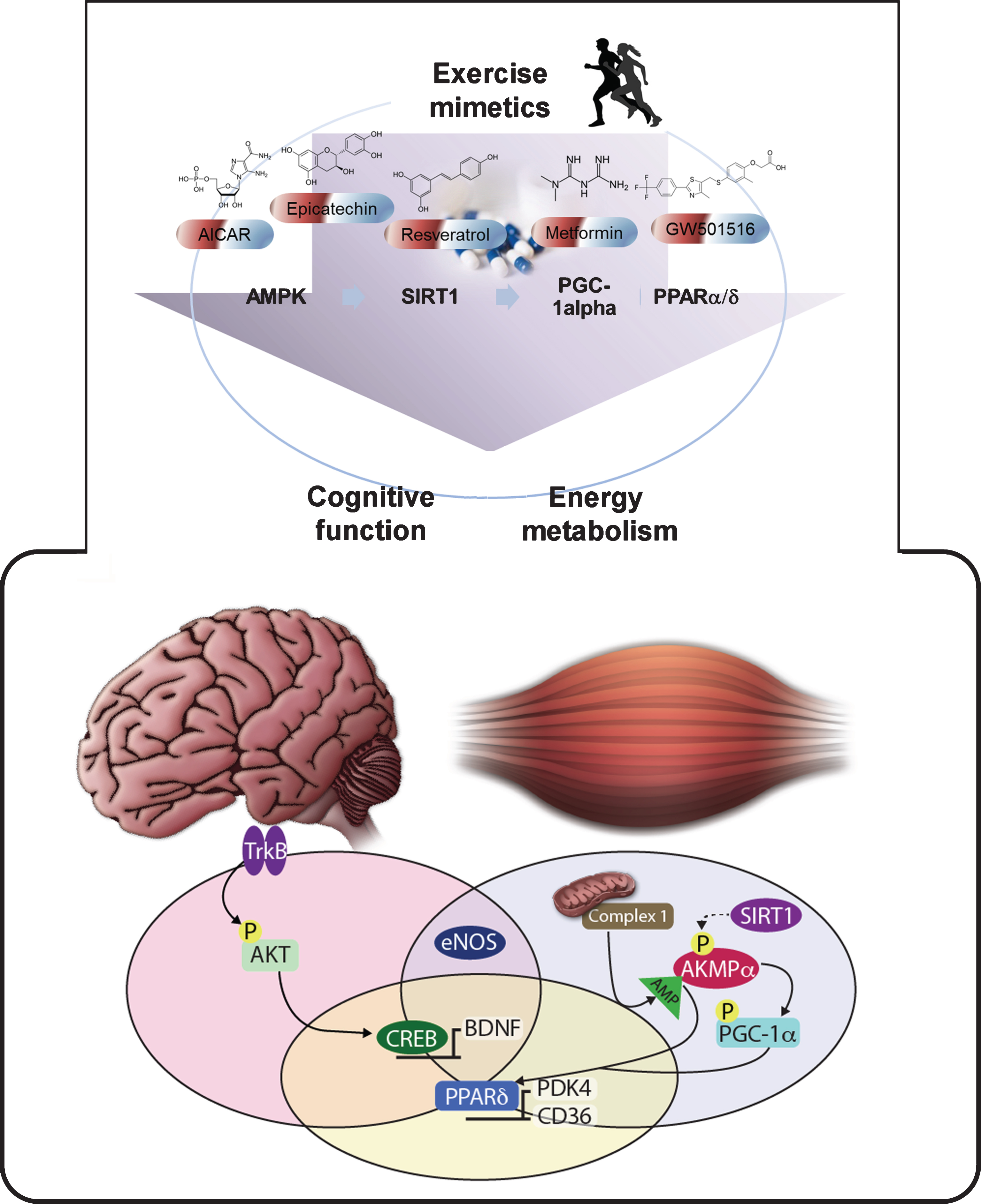
Fig.2
Comparison of effects of exercise and AICAR treatment on the Dentate Gyrus. A) Immunoblotting of AMPK activation in Dentate Gyrus (DG) of C57Bl/6J mice, after 7 days and 14 days of treatment in control (CTR), AICAR (ACR) and voluntary running (RUN) mice. Phosphorylated AMPK is increased by both interventions at 14 days. B) BDNF protein levels are transiently increased by AICAR: DG BDNF protein levels are elevated after 7 days in ACR and RUN groups, however after 14 days only in the RUN but not the ACR group. C) Microarray analysis of the Dentate Gyrus. Heat map of selected neuro-related GO Term gene classes: red represents up-regulated classes, green represents down-regulated classes. For every class the Z-ratio value is reported. Bold, underlined Z-ratio values represent classes with a Selector value above 2 or below – 2. Note the inversion in gene expression that occurs with 14 days of AICAR treatment as compared to the 7 day time-point. D) Cell genesis in C57Bl/6J mice is transiently increased by AICAR: DG BrdU-positive cell number increases in ACR and RUN groups at 7 days, but is elevated only in the RUN and not the ACR group at 14 days. The photomicrographs of BrdU-positive cells at 7 (left column) and 14 (right column) days are prepared from hippocampal sections derived from CONTROL (first row), AICAR (second row) and RUN (third row) mice. Scale bars, 250μm in overview images and 25μm in inserts. (*p < 0.05; compared to CTR; **p < 0.05 compared to CTR and ACR). Error bars denote S.E.M. Data and images from Guerrieri and van Praag, 2015 [43].
![Comparison of effects of exercise and AICAR treatment on the Dentate Gyrus. A) Immunoblotting of AMPK activation in Dentate Gyrus (DG) of C57Bl/6J mice, after 7 days and 14 days of treatment in control (CTR), AICAR (ACR) and voluntary running (RUN) mice. Phosphorylated AMPK is increased by both interventions at 14 days. B) BDNF protein levels are transiently increased by AICAR: DG BDNF protein levels are elevated after 7 days in ACR and RUN groups, however after 14 days only in the RUN but not the ACR group. C) Microarray analysis of the Dentate Gyrus. Heat map of selected neuro-related GO Term gene classes: red represents up-regulated classes, green represents down-regulated classes. For every class the Z-ratio value is reported. Bold, underlined Z-ratio values represent classes with a Selector value above 2 or below – 2. Note the inversion in gene expression that occurs with 14 days of AICAR treatment as compared to the 7 day time-point. D) Cell genesis in C57Bl/6J mice is transiently increased by AICAR: DG BrdU-positive cell number increases in ACR and RUN groups at 7 days, but is elevated only in the RUN and not the ACR group at 14 days. The photomicrographs of BrdU-positive cells at 7 (left column) and 14 (right column) days are prepared from hippocampal sections derived from CONTROL (first row), AICAR (second row) and RUN (third row) mice. Scale bars, 250μm in overview images and 25μm in inserts. (*p < 0.05; compared to CTR; **p < 0.05 compared to CTR and ACR). Error bars denote S.E.M. Data and images from Guerrieri and van Praag, 2015 [43].](https://content.iospress.com:443/media/bpl/2016-2017/2-2/bpl-2-2-bpl160043/bpl-2-bpl160043-g002.jpg)
Table 1
Compounds exerting exercise-like effects on body and brain. We provide an overview of the effects of AICAR, Metformin and GW501516 on central (gray shading) and peripheral systems.
| Agonist | Target | Dosage | Species | Function | Mechanism | References |
| AICAR | AMPK | 500 mg/kg/day, 4 weeks | Mice | Reduced epidermal fat mass | PPARdelta, Ucp3, Cpt 1b, Pdk4 | [79] |
| Enhanced endurance capacity | ||||||
| 300 mg/kg/day, 4 weeks | Mice | Angiogenesis | VEGF | [32] | ||
| 0.5 mg/kg/day, 7 weeks | Rats | Reduced hyperglycemia | GLUT4 | [35] | ||
| (subcutaneous injection) | Lower TG levels &Blood pressure | |||||
| 100 mg/kg/day, 3 days | Mice | Reduced airway inflammation | Lowering lung cytokines | [34] | ||
| 150 mg/kg/day, 5 weeks | Reduced macrophage inflammation | SIRT1 | ||||
| 500 mg/kg/day, 3 to 7 days | Mice | Improved spatial memory | Hippocampal neurogenesis, BDNF | [6, 43] | ||
| Metformin | Indirect | 300 mg/kg/day, 14 days | Mice | Improved Insulin sensitivity | AMPK, PGC-1alpha, GLUT4 | [52,54] |
| AMPK | Inhibition of gluconeogenesis | |||||
| 300 mg/kg/day, 7 days | Human | 6% weight loss | [56] | |||
| 2500 mg/kg/day, 6 months | Human | weight loss | [57] | |||
| 850 mg, twice a day for 3 months | Human | Lower Blood pressure | [64] | |||
| 850 mg/day, 6 months | Human | Reduced BMI, cholesterol, HDL, VHDL, glucose level | [55] | |||
| 200 mg/kg, 38 days | Mice | Improved spatial memory | Hippocampal neurogenesis | [69] | ||
| 50 mg/kg, 7 days | Mice | Regulation of macrophages and microglia | Angiogenesis &Neurogenesis | [71] | ||
| 200 mg/kg, 14 days | Mice | Hippocampal neurogenesis | AMPK | [72] | ||
| 500 mg/kg/day, 21 days | Mice | Improved motor skills in Alzheimer’s model | Reduced oxidative stress | [74] | ||
| Enhanced BDNF | ||||||
| GW501516 | PPARdelta | 5 mg/kg/day, 4 weeks | Mice | Changes in fiber composition (to slow-twitch fibers) | Transcriptional process | [79] |
| 10 mg/kg/day, 7 days | Mice | Reduced lipid accumulation | [85] | |||
| 1–10 mg/kg, 3 weeks | Mice | Inhibition of leukocyte-endothelial cell interaction | Lowering TNFalpha, iCAM, vCAM | [89] | ||
| and E-selectin | ||||||
| 5 mg/kg/day, 1 week | Mice | Improved spatial memory | Hippocampal neurogenesis | [6] | ||
| 2 mg/kg/day, 14 days | Mice | Brain vessel protection | MnSOD | [96] |
Table 2
Natural compounds with exercise-like effects on body and brain. The flavanols Resveratrol and (-)Epicatechin can partially mimic effects of exercise on body and brain (gray shading).
| Agonist | Target | Dosage | Species | Function | Mechanism | References |
| Resveratrol | AMPK, SIRT1 | 400 mg/kg/day, 15 weeks | Mice | Increased mitchondrial oxygen consumption | PGC1, SIRT1 | [103, 104] |
| Increased aerobic activity | ||||||
| Reduced blood glucose &Body weight | ||||||
| 150μg/food g, 30 days | Mice | Spatial memory improvement | Neurogenesis | [114] | ||
| 50 mg/kg, 7 days | Mice | Recovery from ischemia | Angiogenesis, Macrophage µglia regulation | [71] | ||
| (-)Epicatechin | AMPK, CREB | 1 mg/kg, 2∼8 weeks | Mice | Improvement of treadmill performance | Mitochondria | [130] |
| Vascularization | [138] | |||||
| 25 mg, 7 days | Human | Improvement of hand strength | Follistatin/myostatin | [132] | ||
| up to 30 mg, 6 weeks | Mice | Spatial memory improvement | Hippocampal vascularization, Neuronal spine density | [135] | ||
| Rats | [136] | |||||
| 200 mg/L of catechin solution | Rats | [137] | ||||
| 4 mg/day in water, 14 weeks | Mice | Reduced anxiety | Increased monoamine and BDNF levels | [139] | ||
| Cocoa flavanol drink for 3 months | Human | Reversal of memory decline | [142] |



