Running Improves Pattern Separation during Novel Object Recognition
Abstract
Running increases adult neurogenesis and improves pattern separation in various memory tasks including context fear conditioning or touch-screen based spatial learning. However, it is unknown whether pattern separation is improved in spontaneous behavior, not emotionally biased by positive or negative reinforcement. Here we investigated the effect of voluntary running on pattern separation during novel object recognition in mice using relatively similar or substantially different objects.We show that running increases hippocampal neurogenesis but does not affect object recognition memory with 1.5 h delay after sample phase. By contrast, at 24 h delay, running significantly improves recognition memory for similar objects, whereas highly different objects can be distinguished by both, running and sedentary mice. These data show that physical exercise improves pattern separation, independent of negative or positive reinforcement. In sedentary mice there is a pronounced temporal gradient for remembering object details. In running mice, however, increased neurogenesis improves hippocampal coding and temporally preserves distinction of novel objects from familiar ones.
It is well known that the dentate gyrus is critically important for pattern separation within the hippocampal network [1, 2]. Furthermore, it was shown, that adult neurogenesis in the dentate supports pattern separation during hippocampus-dependent memory tasks. This notion is based on the observation that ablation of adult neurogenesis disrupts distinction of similar memories and disturbs differential population coding of similar memory items in the hippocampal CA3 network [3–5]. For example, animals with intact adult neurogenesis can distinguish similar context during context fear conditioning, closely spaced items on a spatial touch screen or neighboring arms in an 8-arm radial maze [6–8]. After ablation of adult neurogenesis, however, similar items cannot be distinguished anymore, whereas distinct items, like distinct context, can still be remembered by the animals. Furthermore, newly generated neurons are particularly important during the first 4 weeks after mitosis, as pattern separation in context fear conditioning is most sensitive to manipulations targeted to this young population of granule cells [9, 10]. Interestingly, this time period largely overlaps with a critical period for enhanced synaptic plasticity and synaptic integration of the newly generated young neurons into the hippocampal circuitry [11–13]. The enhanced plasticity contributes to neuronal pattern separation, as blocking synaptic plasticity in a cohort of newly generated young granule cells (<6 weeks post mitosis) by genetic deletion of NR2B receptors strongly reduced context discrimination after context fear conditioning [14].
Physical exercise was reported to increase hippocampal stem cell proliferation and adult neurogenesis [15]. As a consequence hippocampus-dependent learning and memory formation is improved by voluntary wheel running in mice [16, 17]. In particular, pattern separation in a touch screen task was shown to be more precise in running mice as compared to sedentary animals [18]. Remarkably, all the behavioral tests assessing pattern separation used behavioral tasks involving positive (food reward) or negative (electric shock) reinforcement strategies to generate detectable behavioral output. The important contribution of emotions would be consistent with anatomical data, showing extensive hippocampal connectivity with subcortical structures like dorsal raphe, VTA, locus coeruleus, amygdala and nucleus accumbens [19]. Therefore, it is unclear whether improvement in pattern separation is restricted to memory items which are emotionally charged, or whether it generally applies for hippocampus-dependent learning tasks.
For example, it is unknown, whether pattern separation in spontaneous behavior such as Novel Object Recognition (NOR) is also affected by changes in adult neurogenesis. This test is based on the spontaneous tendency of mice to preferentially explore a novel, previously unknown object relative to familiar objects. Thus, no reward or punishment is necessary to be associated with the behavior and the emotional content is minimal. The effect of increasing or decreasing adult neurogenesis on NOR is controversial. Whereas some studies could not detect any impairment in NOR memory after reduction of adult neurogenesis [20–22], there was a disruption of NOR memory reported by others [14, 23, 24].
To test effects of physical exercise on pattern separation in a less emotional task, we used a NOR paradigm with different types of objects. We analyzed exploration time of objects which were either similar or very distinct to familiar sample objects. Animals with free access to running wheels were compared to sedentary control animals, showing significant differences in the recognition of similar objects but not distinct objects. This indicates that running significantly improves pattern separation even if the emotional chargeis minimal.
1MATERIALS AND METHODS
Animals and housing conditions
Female 8 week-old C57BL/6-mice were purchased from Harlan Laboratories (Switzerland). Animals were placed for 3 weeks into different housing conditions using a 12:12 h light/dark cycle. Control mice were kept in eurostandard type II cages (267×207×140 mm), three animals per cage. Mice with free access to a running wheel (RW) were kept either in eurostandard type III cages (RW1, 425×266×185 mm) or environmentally enriched eurostandard type IV cages (RW2, 595×380×200 mm), with three or six animals per cage, respectively. All animals received some tissue paper to allow nest building. The behavioural studies were performed when the animals were ∼11 weeks old. The experiments were approved by the animal advisory committee of the Kanton Basel (Kantonales Veterinäramt BS, Switzerland).
Novel object recognition
To assess learning and long-term memory a Novel Object Recognition (NOR) task was used. Two identical objects were placed into the arena during a 6 min sample phase. Subsequently, one of the objects was exchanged by a new object and memory was assessed by comparing the time spent exploring the novel object as compared with the time spent exploring the familiar object during a 5 min test phase.
One week before the NOR experiments, the animals experienced handling by the experimenter and habituation to the arena for 2 and 3 consecutive days, respectively. The handling procedure included exposure to a transparent plexiglas tunnel with a length of 12 cm and a diameter of 6 cm. Using this tunnel, an animal was transferred into a new cage and after a few minutes back again to home cage. This type of handling apparently reduced anxiety as described previously [25]. Animals which were still jumping in the NOR test were excluded from the analysis (8 out of 59 mice). For habituation, mice were placed into the empty arena (38×38×30 cm, PVC) for 5 min. During all experiments the arena was illuminated with 60–90 Lux.
For NOR experiments custom-built plastic pieces (Polyoxymethylen, POM), were used with different shapes (cones: 4 cm diameter, 6 cm height, pyramids: 4×4×4–6 cm) and different color (white, black, red). The objects were cleaned thoroughly with 40% ethanol followed by distilled water between trials to remove olfactory cues. During the sample phase on the first day of the NOR test, the mice were allowed to explore the two identical white or black objects (either two cones or two pyramids) for 6 min. For the short-delay test phase (1.5 h) one of the sample objects was replaced by a new one (cone by pyramid or vice versa) and exploration was measured for 5 min. For the long-delay test phase (24 h) the new object was again exchanged by another new object. The location of the novel object at 24 h was always different from that at 1.5 h, either first left then right, or vice versa. Consequently, the location of the familiar object also switched between the two test phases. Objects with the same color but different shapes were considered to be similar to sample object. Objects with both, different shape and different colour (red, black, white) were considered to be distinct from the original sample objects.
The behaviour of the animals was recorded with a Logitech HD Pro Webcam C920 and running tracks of the animals during NOR were traced with Videomot2 (TSE Systems, Germany). To analyse strictly active exploration the time was measured manually using a digital stopwatch (Silva, Sweden). Active exploration was defined as direct sniffing or whisking towards the objects or direct nose contact. Climbing over the objects was not counted as exploration. The relative exploration was quantified by normalizing the difference between the exploration time of the novel (Tn) and familiar object (Tf) by the total time of exploration (Ttot) to calculate the NOR discrimination index: NOR index = (Tn–Tf)/Ttot. With identical sample objects the NOR index was always less than 0.2 (average NOR index = –0.01 ± 0.01, n = 45, p = 0.888) indicating that there was no side preference in the mice used for the study. Furthermore, as tested on a separate cohort of animals, neither cones nor pyramids are preferred by the mice (NOR index = –0.02 ± 0.07, n = 6, p = 0.571).
Immunohistochemistry
Three to four days after behavioral testing, the animals were anaesthetized with isoflurane (4% in O2, Vapor, Draeger) and killed by decapitation, in accordance with national and institutional guidelines. Transverse hippocampal brain slices were cut in a sucrose-based solution using a Leica VT1200 vibratome [26] and slices were fixed overnight with 4% paraformaldehyde at 4°C. After washing with PBS, tissue sections were incubated overnight at 4°C with the primary antibody Goat-anti-DCX (1:200–500, c-18, sc-8066, Santa Cruz, IgG) in PBS including 0.3% Triton X-100 and 5% Normal Donkey Serum. The secondary antibody Donkey-anti-goat Alexa 488 (1:500, A11055, Molecular Probes, Invitrogen) and DAPI (1:10000, Roche) were applied at the second day in PBS with 0.3% Triton X-100 and kept overnight by 4°C. The following day after washing, the slices were embedded in Prolong Gold (Molecular Probes, Invitrogen).
Fluorescence microscopy and image analysis
Fluorescence images were acquired using a Zeiss LSM 700 confocal microscope. For counting DCX+ cells in the hippocampus we used a 20× objective (NA 0.75). To measure the number of DCX+ cells in different animals, we first counted the number of DCX+ cells within 3μm thick confocal sections, which were obtained in all cut slices from one hemisphere at a depth of 10μm below the surface. DCX+ cells were counted manually in one optical section per slice with ImageJ using the plugin Cellcounter. For analysis of density of DCX+ cells in dorsal and ventral sub-regions, the number of cells per section was normalized by the length of the granule cell layer measured in the DAPI staining.
To obtain the total number of cells per hemisphere, the number of cells in 3-μm sections was extrapolated to the number of cells in the 3D volume of the150μm thick slices. First, we performed control experiments, counting all DCX+ cells in the 3D volume of 45μm thick confocal stacks (n = 4) by using a grid-based analysis in ImageJ. Each stack consisted of 30 sections with a thickness of 3μm obtained at an interval of 1.5μm. We then compared this 3D-cell number with the number of cells visible in 15 non-overlapping, adjacent 3-μm confocal sections of the corresponding stack. The ratio of these two numbers was 1.65 ± 0.15 (n = 4), indicating that a single cell is on average visible on 1.65 adjacent 3-μm sections. Second, we considered the thickness of embedded slices (150μm), the thickness of a single confocal section (3μm) and the fact that a single cell is visible on 1.65 adjacent sections to calculate a scaling factor f = 150μm/(3μm* 1.65) = 30.3. Finally, we multiplied the number of DCX+ cells per section in each slice with this scaling factor to extrapolate from section to slice and to calculate the sum of all DCX+ cells per hemisphere. For example, a number of 405 cells counted in imaged sections, thus leads to 12 272 DCX+ cells per dentate.
This method allowed to use thick slices, to preserve the dendritic tree of cells for further analysis (see below). Furthermore, as f was the same for all slices and for all animals, the relative comparison between animals in the different cohorts was independent of this stereological simplification.
For analysis of dendrites, image stacks were acquired with a 40× oil-immersion objective (NA 1.4). 20–50 images were taken at an interval of 0.5μm with a slice thickness of 1μm. Maximum intensity projections were calculated and dendrites were counted in the inner (IML) and outer molecular layer (OML) at a relative distance from the granule cell layer of 15% and 50% of the total molecular layer, respectively.
In addition to directly counting the number of dendrites, we determined DCX fluorescence as an independent measure of dendrite outgrowth. The average background-subtracted fluorescence intensity was measured within rectangular areas of about 40 × 250μm in the IML close to the granule cell layer, as well as in the middle of the OML. Background intensity was measured from areas devoid of DCX-positive dendrites in the outer border of the OML. For analysis of dorsal versus ventral hippocampus, data from the dorsal and ventral half of the slices were pooled and displayed separately.
Statistics and data presentation
All data are presented as mean ± SEM. All statistical analysis was done with GraphPad Prism6. For all comparisons, a two-tailed Wilcoxon-Mann-Whitney Test was used. The significance level was set to P < 0.05.
RESULTS
Running increases number and dendritic length of newly generated young neurons
To study the impact of voluntary running on learning and memory, adult 8-week-old female mice were housed in different conditions for 2-3 weeks prior to behavioral analysis (Fig. 1). To analyze the impact of running wheels on adult neurogenesis, young granule cells were stained with an antibody against doublecortin (DCX, Fig. 2) [27]. Voluntary running significantly increased the number of DCX+ young granule cells (Fig. 2C, 15 593 ± 663, n = 8) relative to control (11 506 ± 500, n = 8, P < 0.05). The effect was most pronounced in the dorsal hippocampus with an about 2-fold increase in the number of young neurons (Fig. 2D, E). Furthermore, we analyzed the dendritic growth by counting dendrites in the inner (IML) and outer molecular layer (OML) of the dentate gyrus. In sedentary animals, most of the DCX+ dendrites grow into the IML, the region where associative fibers from mossy cells terminate (Fig. 2A). By contrast, in running mice the dendrites of DCX+ cells are longer with more dendrites in the outer molecular layer, where fibers from the entorhinal cortex terminate (Fig. 2B). As the number of DCX+ neurons was larger with running wheels, we normalized the number of long dendrites by calculating the ratio of the number of OML (50%) to IML (15%) dendrites (Fig. 2A, B). As shown in Fig. 2F, the dendrites of young granule cells are on average longer with running wheels, with 34 ± 4% of the dendrites extending well into the OML, significantly more than in control animals (21 ± 4% , P < 0.05, n = 10 each). Again this effect was largely constrained to the dorsal half of the hippocampus showing 3 times more dendrites in the OML in running animals (Fig. 2G, 34 ± 5% versus 11 ± 4% in control, P = 0.0038, n = 7 and n = 5, respectively). Similarly, calculating the ratio of DCX-fluorescence in the OML relative to the fluorescence in the IML revealed a 2-fold increase in dorsal DCX fluorescence with running (61 ± 10% versus 34 ± 6% , P = 0.0294, n = 11 and n = 10, respectively, Fig. 2H). The running-induced increase in dendritic outgrowth is consistent with a recent report, showing that dendritic growth in newly generated 2-week-old granule cells is accelerated by exercise [28]. Together with the 2-times larger number of young granule cells in running mice in the dorsal hippocampus, this corresponds to a 4-fold increase in number of young GC dendrites in the molecular layer. As a consequence there might be a 4-times larger chance for the formation of new synapses of young cells with axon terminals projecting from the entorhinal cortex.
Taken together, the data not only show that the animals make use of running wheels, leading to the well-known increase in the number of newly generated granule cells. It further strengthens the notion that also dendritic morphology is changed by wheel running with more dendrites in the termination zone of entorhinal input fibers.
Running improves pattern separation during novel object recognition
To study hippocampus-dependent learning andmemory we used a novel object recognition task with a 6 min sample phase, followed by a 5 min test phase either 1.5 h (Fig. 3) or 24 h later (Fig. 4). In the sample phase animals were always exposed to two identical objects, either cones or pyramids with the same color (Fig. 3B). During the sample phase animals used on average 29.5 ± 1.4 s (n = 45) for active object exploration. The exploration time of the two identical objects was very similar leading to a NOR-discrimination index of –0.02 ± 0.02 in control (n = 20) and 0.00 ± 0.02 with running wheels (n = 25, Fig. 3A). This suggests that the animals do not show a bias towards the right or left side. Exchanging one of the objects by a novel object, however, induced a significant side preference towards the novel object in the 1.5 h-delay test phase. This was independent of whether the new object was similar to (Fig. 3C, D) or distinct from the sample object (Fig. 3E, F). Using a similar object with the same color (but different shape) the running animals showed a discrimination index of 0.18 ± 0.07 (n = 9) comparable to control animals (0.15 ± 0.06, n = 9, P = 0.932). Similar results were obtained with distinct objects having both, different color and different shape for running (0.21 ± 0.04, n = 16) and sedentary mice (0.23 ± 0.08, n = 11, P = 0.846). This shows that the animals are perfectly able to distinguish sample objects from similar test objects 1.5 h after the sample phase, independent of enhanced adult neurogenesis.
Furthermore, we tested the animals at a later time point 24 h after the sample phase presenting again the familiar object as well as a further novel object, with switched locations relative to the 1.5 h test (Fig. 4). Animals with previously similar objects at 1.5 h delay were exposed to a novel distinct object at 24 h delay (Fig. 4A, B), whereas animals with previously distinct objects were exposed to a novel object similar to the sample (Fig. 4C, D).
During this long-term retention test, there was a significant difference between running and control mice. With distinct objects, sedentary mice are able to remember which of the objects is familiar and which is novel, leading to a discrimination index of 0.21 ± 0.06 (n = 9) comparable to running mice (0.30 ± 0.05, n = 9, p = 0.398). With similar novel objects, however, the running animals remembered the familiar object significantly better (0.21 ± 0.04, n = 16) than sedentary mice (0.07 ± 0.05, n = 11, p < 0.05). This difference is further exemplified in Fig. 4E, F showing representative 2 min example tracks of a control (Fig. 4E) and a runner mouse (Fig. 4F) during the 24 h-delay NOR test. The differential recognition of similar and distinct objects clearly shows that the control animals remember the familiar sample object 24 h after the sample exposure. However, the memory trace apparently contains less detail as compared to the runner mice with enhanced neurogenesis, indicating that there is a difference in neuronal patternseparation.
Exercise inhibits temporal decay of pattern separation after learning
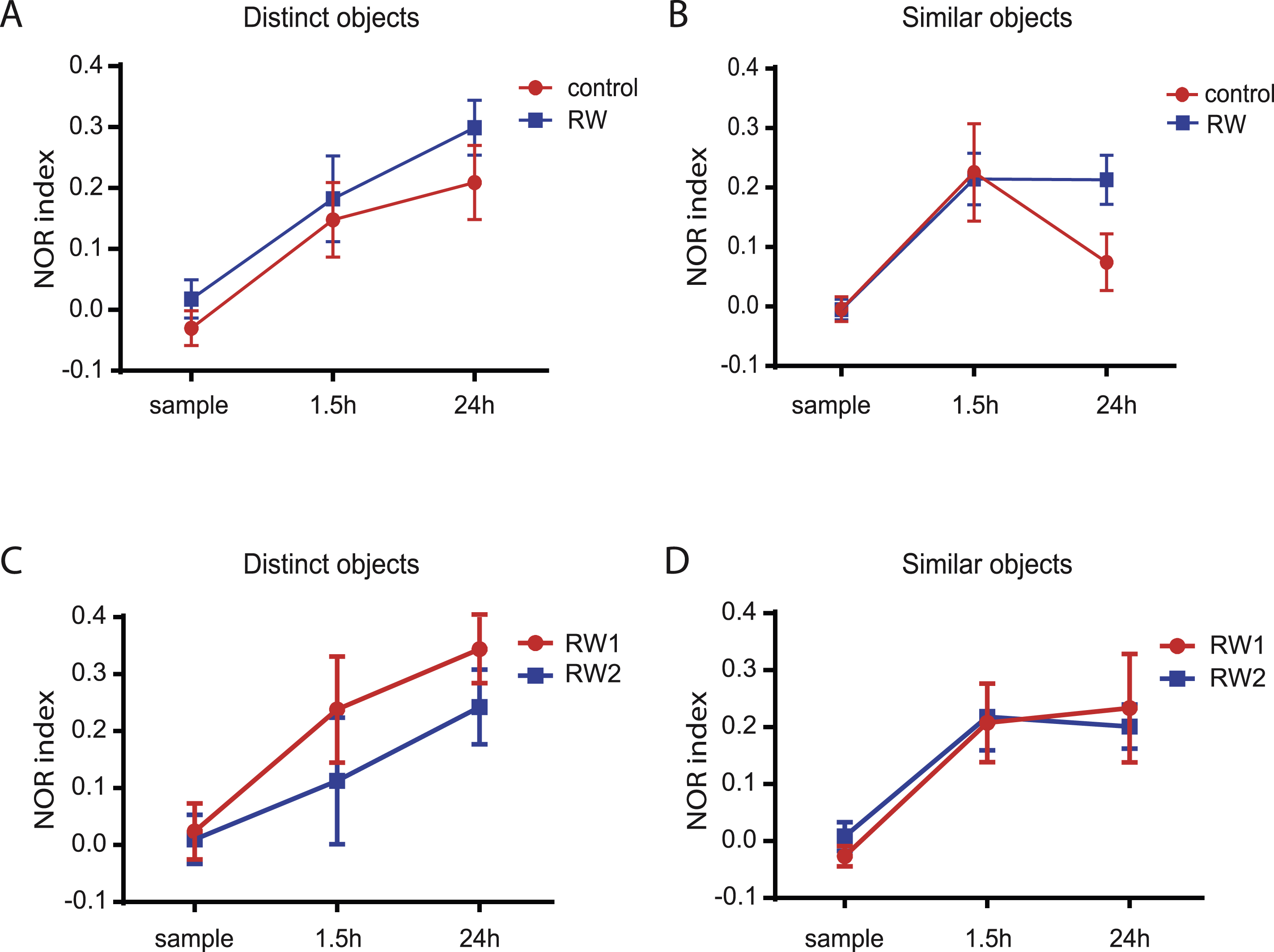
To compare differences between the 1.5 h-delay and the 24 h-delay NOR test we plotted the discriminationindex against memory retention time (Fig. 5). Whereas both, runners and control animals show a similar discrimination index for distinct objects of about 0.2 at 1.5 h and 24 h (Fig. 5A) after learning, there is a pronounced temporal gradient for the retention with similar objects in control but not in running mice (Fig. 5B). The clear distinction of similar objects at 1.5 h in control mice suggests that there is no difference in sensory perception of details. Also it seems that details can be transiently encoded and retrieved 1.5 h after the sample phase, suggesting no difference in short-term memory. However, in control animals, the recognition memory clearly decays within the next 24 h with a NOR index close to chance level. By contrast, this decay is prevented in running mice, potentially by an increased number of newly formed synapses in the OML with 4-times more young dendrites in running mice.
We also compared memory retention in mice housing with only a running wheel (RW1) to mice with additional enrichment with tunnels and small houses (RW2), which did not lead to significant differences in the NOR index at 1.5 h and 24 h delay (Fig. 5C, D). The temporal decay in pattern separation is prevented with both paradigms. This suggests that the most important factor for improving pattern separation in our study is physical exercise by voluntary wheel running. This would be consistent with previous findings, showing that enhanced physical exercise is the most power ful neurogenic factor of environmental enrichment [29, 30].
Taken together, these data indicate that physical exercise improves pattern separation by preserving the hippocampal processing of memory details over time, which might decay rapidly otherwise.
DISCUSSION
We have studied the effect of voluntary running on adult neurogenesis, dendritic growth and learning behavior. Using NOR, we show that running improves pattern separation in a learning task, which does not involve negative or positive reinforcement strategies. This conclusion is based on several important findings. First, testing NOR memory at 24 h after the sample phase shows that only running mice could distinguish familiar objects from similar novel objects, whereas sedentary animals could not. Second, running did not affect NOR memory of highly distinct objects, indicating that sedentary animals still remember the familiar object 24 h after sample phase, although less precise. Third, similar objects could be clearly distinguished by sedentary mice with a 1.5 h delay, showing that perception of details and short-term memory was not affected by running. Thus, the data show that running improves hippocampal pattern separation during spontaneous behavior, to generate unique and detailed long-term representations of similar but nevertheless different memoryitems.
Running–induced increase in hippocampal neurogenesis and dendritic growth
It is well known that physical exercise like voluntary running in running wheels substantially increases proliferation of adult neural stem cells leading to increased adult hippocampal neurogenesis [15, 29, 30]. However, the dorso-ventral distribution was only rarely investigated. We have found that running wheels preferentially increase neurogenesis in the dorsal hippocampus. A similar preferential increase in adult neurogenesis in dorsal hippocampus was also shown previously [31]. Conversely, chronic mild stress and fluoxetine, selectively decrease and increase neurogenesis in the ventral hippocampus, respectively [32].
What are the mechanisms underlying the running-induced neurogenesis in the dorsal hippocampus? Physical exercise increases BDNF release in the hippocampus in a dose dependent manner [33]. Adult hippocampal stem cells express TrkB receptors, which are activated by NMDA-receptor dependent BDNF release from mature neurons [34]. Mice with genetic deletion of NR2A-NMDA receptors neither show exercise-induced BDNF-release nor running-induced increase in neurogenesis [35]. Furthermore, CamKII-Cre-dependent conditional knock-out of BDNF or deletion of TrkB-receptors in GFAP+ hippocampal stem cells abolished running-induced increase in proliferation and neurogenesis [36, 37]. Finally, wheel running stimulates excitatory synaptic inputs from the entorhinal cortex and induce hippocampal synaptic plasticity [38, 39]. So far, this wheel-running-induced excitation was measured only in the dorsal hippocampus. However, running in an open field induces theta oscillations with about 10-fold smaller amplitudes in ventral as compared to dorsal hippocampus [40]. Thus, the preferential stimulation of dorsal hippocampal neurogenesis could be due to a potentially lower activation of the ventral hippocampus due to different grid-cell activity and other functional differences along the dorso-ventral axes [19].
In addition to the increased number of neurons we found increased dendritic growth of DCX+ young granule cells leading to about 2- to 3-times more dendrites per cell extending into the OML of the dorsal dentate gyrus. This is consistent with Golgi-impregnation studies showing an increased dendritic length in granule cells and other hippocampal neurons in running mice [41, 42]. However, our data together with a recent study showing that running specifically accelerates dendritic growth in 2-week-old immature neurons [28], indicates that young granule cell dendrites are most sensitive. The enhanced dendritic growth could be mediated by running induced activation of GABAergic and glutamatergic synapses,which were shown to regulate dendritic growth [43–45]. By contrast, dendrites of 4-week-old cells are not affected anymore by exercise [28]. In contrast to dorsal hippocampus, we could not find enhanced dendritic outgrowth in ventral regions. However, this does not exclude running-induced effects on spine formation or synapse formation, which were reported in ventral hippocampus by others [46, 47].
Overall, this activity-dependent increase in proliferation and dendritic growth leads to a 4-fold increase of young dendrites in the OML, where entorhinal axons terminate. These new dendrites can exquisitely compete for new synapses with preexisting granule cells [48], and may account for the pronounced enhancement of long-term potentiation (LTP) observed in dentate gyrus of running mice [16, 49].
Running increases pattern separation during NOR
Using similar objects we have found a strong effect of running on NOR memory at 24 h delay after sample phase. As running specifically increased adult neurogenesis in the dorsal hippocampus, this would be consistent with a previous report showing that NOR memory is disrupted after lesion of the dorsal dentate gyrus [50]. The data are also consistent with several studies showing running improves NOR memory [32, 51–54]. Conversely, disruption of NOR memory was reported after ablation of adult neurogenesis by anti-mitotic agents or X-ray irradiation [23, 24] as well as after inhibition of synaptic plasticity specifically in the newly generated neurons younger than 6 weeks post mitosis [14]. However, these findings are in contradiction with other previous studies claiming that the reduction of adult neurogenesis does not affect NOR memory [20, 21, 55].
Our data provide a potential solution for this apparent discrepancy, as we have found that the effects of running on NOR memory is dependent on the relative similarity of the used objects. The difference we have found between similar and distinct objects at 24 h suggests that the sedentary animals do not remember exactly the shape of objects they have seen one day before. Therefore, they do not consider the similar novel object as novel. By contrast, with very different objects NOR memory is comparable to running mice and therefore probably independent of adult neurogenesis. Only if objects are relatively similar, young neurons may specifically contribute to pattern separation during NOR, similar to what was shown for other hippocampus-dependent tasks as for example navigation in an 8-arm maze or context-fear conditioning [6, 7, 9]. Therefore, the usage of different types of objects would explain the contradictory results of the different NOR studies mentioned above.
We have found a strong effect of running on pattern separation during NOR memory at 24 h delay. However, with 1.5 h delay there was no significant difference in control animals compared to running mice. Sedentary animals could nicely distinguish the novel objects, which were relatively similar to familiar objects. A temporal gradient of running/enrichment dependent enhancement of NOR memory was also observed in other studies [23, 52]. The fact that memory retention at about 1 h after sample phase is not affected by running (our study) and after 50% decrease of neurogenesis via antimitogenic agents [23] would indicate that neurogenesis is not required for short term memory but more important for long-term representations of memory items.
Contribution of enhanced neurogenesis to running induced increase in pattern separation during NOR
Although running induces both, substantial changes in number and morphology of young neurons as well as significant changes in learning behavior, this does not prove a causal relationship. Instead, these two phenomena could occur in parallel without direct functional interconnection. Nevertheless, there are several arguments, suggesting that increased adult neurogenesis during running indeed contributes to the enhanced pattern separation during NOR.
First, our data indicate that long-term memory was improved at 24 h without an obvious change in sensory processing because of (similar NOR at) 1.5 h, pointing towards a change in hippocampal function. Second, acquisition of NOR memory was shown to be strongly reduced after blocking synaptic plasticity specifically in a cohort of newly generated young granule cells (<6 weeks post mitosis) by genetic deletion of NR2B receptors [14]. Third, newly generated granule cells contribute to pattern separation in context fear conditioning, as context discrimination is decreased after inhibition of hippocampal proliferation [9] or after functionally silencing a population of young neurons [10]. Finally, DCX+ positive young granule cells are critically important for consolidation of object memories, as NOR at 24 h delay was disrupted by ablation of DCX+ neurons 1 h after sample phase [24]. Taken together, these data converge to the notion that increased neurogenesis may significantly contribute to running-induced improvement of pattern separation during NOR.
The mechanisms underlying the neurogenesis-dependent consolidation of object memories are largely unclear. The available data would indicate that young neurons probably form new synapses during learning with competitive advantage relative to preexisting mature cells [48]. However, this might not directly impact on learning behavior. The main associative memory-storage unit in the hippocampus is the CA3 network, which receives direct inputs from the entorhinal cortex and rapidly adjusts recurrent synaptic connections in an activity-dependent manner [2, 9, 56]. However, temporal stability of synaptic plasticity like long-term potentiation (LTP) is known to be activity dependent and weak stimuli induce LTP, which decays back to baseline within a few hours. Granule cells not only form powerful excitatory mossy-fiber synapses onto CA3 pyramidal cells, but even more numerously, synapses with GABAergic interneurons generating efficient feed-forward inhibition in CA3 [57, 58]. Inhibitory mossy-fiber synapses as well as feed-forward inhibition are well developed already at 4 weeks after mitosis, much earlier than feedback inhibition [10, 59]. As a consequence, newly generated granule cells might increase firing in some, but decrease firing in other CA3 pyramidal cells, thereby enhancing contrast between the activity of different cells, to shape population coding of similar objects within the CA3 network [5].
Together, these findings would indicate that the increased number and dendritic growth of young neurons after running contribute to increased activation of a ‘specific’ set of CA3 pyramidal cells, to induce long-lasting late LTP in distinct CA3 cell assemblies, coding for similar but nevertheless distinct memory items. Without synaptic inputs from young granule cells, synapses in CA3 cell assemblies are less specific and less strongly activated. Thus early LTP decays back to baseline with time, generating a degraded representation of memories. Therefore, exercise-induced increase in neurogenesis improves pattern separation by supporting unique and detailed long-term representations of similar but nevertheless different memory items to finally preserve hippocampal processing of memory details over time.
ACKNOWLEDGMENTS
We would like to thank Theresa M. Ballard for helpful discussions about initial NOR experiments and Selma Becherer for histochemical stainings and technical assistance. Supported by the Swiss National Science Foundation (SNSF, Project 31003A 13301).
REFERENCES
1 | McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S(2007) Dentate gyrus NMDA receptors mediate rapid pattern separation inthe hippocampal networkScience317: 9499 |
2 | Rolls ET(2013) The mechanisms for pattern completion and pattern separation in the hippocampusFront Syst Neurosci7: 74 |
3 | Deng W, Aimone JB, Gage FH(2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory?Nat Rev Neurosci11: 339350 |
4 | Sahay A, Wilson DA, Hen R(2011) Pattern separation: a common function for new neurons in hippocampus and olfactory bulbNeuron70: 582588 |
5 | Niibori Y, Yu TS, Epp JR, Akers KG, Josselyn SA, Frankland PW(2012) Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 regionNat Commun3: 1253 |
6 | Clelland CD, Choi M, Romberg C, Clemenson GDJr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ(2009) A functional role for adult hippocampal neurogenesis in spatial pattern separationScience325: 210213 |
7 | Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R(2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separationNature472: 466470 |
8 | Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN(2012) Adult-born neurons are necessary for extended contextual discriminationHippocampus22: 292298 |
9 | Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S(2012) Young dentate granule cells mediate pattern separation, whereasold granule cells facilitate pattern completionCell149: 188201 |
10 | Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S(2012) Optical controlling reveals time-dependent roles for adult-born dentate granule cellsNat Neurosci15: 17001706 |
11 | Snyder JS, Kee N, Wojtowicz JM(2001) Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrusJ Neurophysiol85: 24232431 |
12 | Schmidt-Hieber C, Jonas P, Bischofberger J(2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampusNature429: 184187 |
13 | Ge S, Yang CH, Hsu KS, Ming GL, Song H(2007) A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brainNeuron54: 559566 |
14 | Kheirbek MA, Tannenholz L, Hen R(2012) NR2B-dependent plasticity of adult-born granule cells is necessary for context discriminationJ Neurosci32: 86968702 |
15 | van Praag H, Kempermann G, Gage FH(1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrusNat Neurosci2: 266270 |
16 | van Praag H, Christie BR, Sejnowski TJ, Gage FH(1999) Running enhances neurogenesis, learning, and long-term potentiation in miceProc Natl Acad Sci USA96: 1342713431 |
17 | van Praag H, Shubert T, Zhao C, Gage FH(2005) Exercise enhances learning and hippocampal neurogenesis in aged miceJ Neurosci25: 86808685 |
18 | Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ(2010) Running enhances spatial pattern separation in miceProc Natl Acad Sci USA107: 23672372 |
19 | Strange BA, Witter MP, Lein ES, Moser EI(2014) Functional organization of the hippocampal longitudinal axisNat Rev Neurosci15: 655669 |
20 | Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernández-Lamo I, García-Verdugo JM, Bernal J, Guadaño-Ferraz A(2006) Modulation of adult hippocampal neurogenesis by thyroid hormones: implications indepressive-like behaviorMol Psychiatry11: 361371 |
21 | Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C(2010) Young hippocampal neurons are critical for recent andremote spatial memory in adult miceNeuroscience171: 769778 |
22 | Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR(2012) 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioningHippocampus22: 11881201 |
23 | Bruel-Jungerman E, Laroche S, Rampon C(2005) New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichmentEur J Neurosci21: 513521 |
24 | Suárez-Pereira I, Canals S, Carrión AM(2015) Adult newborn neurons are involved in learning acquisition and long-term memory formation: The distinct demands on temporal neurogenesis of different cognitive tasksHippocampus25: 5161 |
25 | Hurst JL, West RS(2010) Taming anxiety in laboratory miceNat Methods7: 825826 |
26 | Bischofberger J, Engel D, Li L, Geiger JRP, Jonas P(2006) Patch-clamprecording from mossy fiber terminals in hippocampal slicesNatureProtocols1: 20752081 |
27 | Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG(2003) Transient expression of doublecortin during adult neurogenesisJ Comp Neurol467: 110 |
28 | Steib K, Schäffner I, Jagasia R, Ebert B, Lie DC(2014) Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brainJ Neurosci34: 66246633 |
29 | Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H(2011) Running is the neurogenic and neurotrophic stimulus in environmental enrichmentLearn Mem18: 605609 |
30 | Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS(2012) Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J miceNeuroscience219: 6271 |
31 | Tanti A, Rainer Q, Minier F, Surget A, Belzung C(2012) Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampusNeuropharmacology63: 374384 |
32 | Tanti A, Belzung C(2013) Neurogenesis along the septo-temporal axis of the hippocampus: are depression and the action of antidepressants region-specific?Neuroscience252: 234252 |
33 | Cotman CW, Berchtold NC(2002) Exercise: A behavioral intervention to enhance brain health and plasticityTrendsNeurosci25: 295301 |
34 | Babu H, Ramirez-Rodriguez G, Fabel K, Bischofberger J, Kempermann G(2009) Synaptic Network Activity Induces Neuronal Differentiation of Adult Hippocampal Precursor Cells through BDNF SignalingFront Neurosci3: 49 |
35 | Kitamura T, Mishina M, Sugiyama H(2003) Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunitNeurosci Res47: 5563 |
36 | Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF(2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatmentNeuron59: 399412 |
37 | Choi SH, Li Y, Parada LF, Sisodia SS(2009) Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNFMol Neurodegener4: 52 |
38 | Czurkó A, Hirase H, Csicsvari J, Buzsáki G(1999) Sustained activation of hippocampal pyramidal cells by ‘space clamping’ in a running wheelEur J Neurosci11: 344352 |
39 | Pastalkova E, Itskov V, Amarasingham A, Buzsáki G(2008) Internally generated cell assembly sequences in the rat hippocampusScience321: 13221327 |
40 | Patel J, Fujisawa S, Berényi A, Royer S, Buzsáki G(2012) Travelingtheta waves along theentire septotemporal axis of the hippocampusNeuron75: 410417 |
41 | Redila VA, Christie BR(2006) Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrusNeuroscience137: 12991307 |
42 | Stranahan AM, Khalil D, Gould E(2007) Running induces widespread structural alterations in the hippocampus and entorhinal cortexHippocampus17: 10171022 |
43 | Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming G, Song H(2006) GABA regulates synaptic integration of newly generated neurons in the adult brainNature439: 589593 |
44 | Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH(2006) NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrusNature442: 929933 |
45 | Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC(2009) GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampusJ Neurosci29: 79667977 |
46 | Piatti VC, Davies-Sala MG, Espósito MS, Mogiat LA, Trinchero MF, Schinder AF(2011) The timing for neuronal maturation in the adult hippocampus is modulated by local network activityJ Neurosci31: 77157728 |
47 | Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann KK, Schinder AF, Götz M, Berninger B(2015) A critical period for experience-dependent remodelingof adult-born neuron connectivityNeuron85: 710717 |
48 | Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH(2007) Synapse formationon neurons born in the adult hippocampusNat Neurosci10: 727734 |
49 | Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR(2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo Neuroscience124: 7179 |
50 | Dees RL, Kesner RP(2013) The role of the dorsal dentate gyrus in object and object-context recognitionNeurobiol Learn Mem106: 112117 |
51 | O’Callaghan RM, Ohle R, Kelly AM(2007) The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learningBehav Brain Res176: 362366 |
52 | Thuret S, Toni N, Aigner S, Yeo GW, Gage FH(2009) Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ miceHippocampus19: 658669 |
53 | Hopkins ME, Nitecki R, Bucci DJ(2011) Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levelsNeuroscience194: 8494 |
54 | Bechara RG, Kelly ÁM(2013) Exercise improves object recognition memory and induces BDNF expression and cellproliferation in cognitively enriched ratsBehav Brain Res245: 96100 |
55 | Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK(2009) New hippocampal neurons are not obligatory formemory formation; cyclin D2 knockout mice with no adult brainneurogenesis show learningLearn Mem16: 439451 |
56 | Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S(2002) Requirement for hippocampal CA3 NMDAreceptors in associative memory recallScience297: 211218 |
57 | Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G(1998) GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampusJ Neurosci18: 33863403 |
58 | Bischofberger J, Engel D, Frotscher M, Jonas P(2006) Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal networkPflugers Arch453: 361372 |
59 | Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF(2015) Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cellsNeuron85: 116130 |
Figures and Tables
Fig.1
Housing conditions and experimental design. A, Timeline of the experimental procedure. 8-week-old animals were placed into different housing conditions for a period of 3 weeks, which ended with a novel object recognition test including sample phase, a 1.5 h-delay and a 24 h-delay memory test. B, Control condition in eurostandard type II cages, three animals per cage. C, Mice housed in the eurostandard type III cage with a plastic running wheel (RW1), three mice per cage. D, Mice housed in the eurostandard type IV cage with a running wheel, two houses and a tunnel (RW2), six mice per cage. B-D, Scale bars, 10 cm.
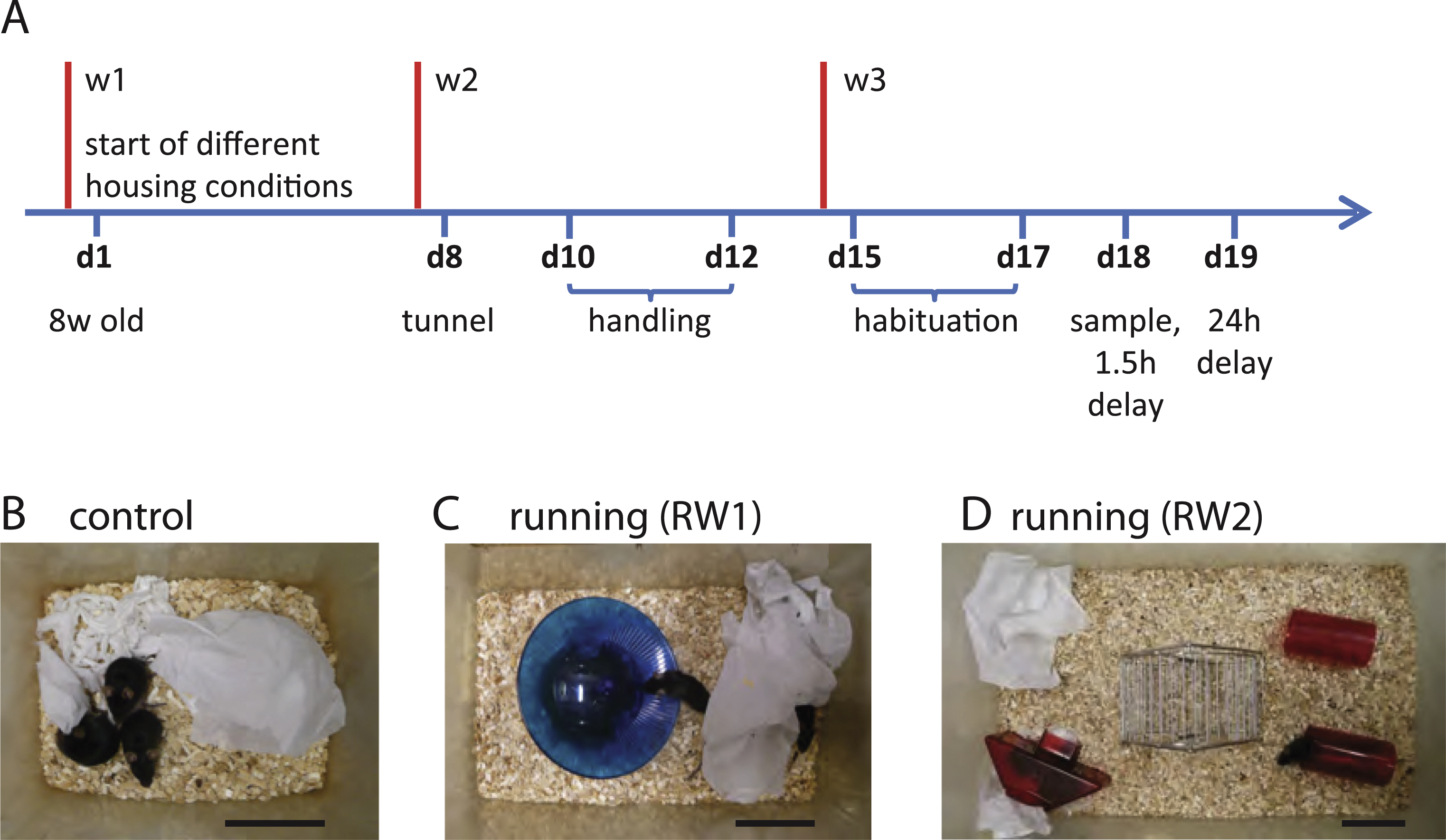
Fig.3
Running does not change novel object recognition memory with short delays. A, B, Animals do not show preference for left or right side during the exploration of two identical sample objects (cones) shown in (B) (animals:n = 20 control and n = 25 with running wheels). C, D, One sample object (cone) was replaced by a similar novel object (white pyramid) shown in (D). The animals spent significantly more time exploring the novel object tested 1.5 h after sample phase as measured by the NOR index in both, control (n = 9) and running mice (n = 9). E, F, In a different cohort of animals, the sample object (cone) was replaced by a distinct novel object (black pyramid) shown in (F). Again the animals spent significantly more time exploring the novel object in both, control (n = 11) and running mice (n = 16).
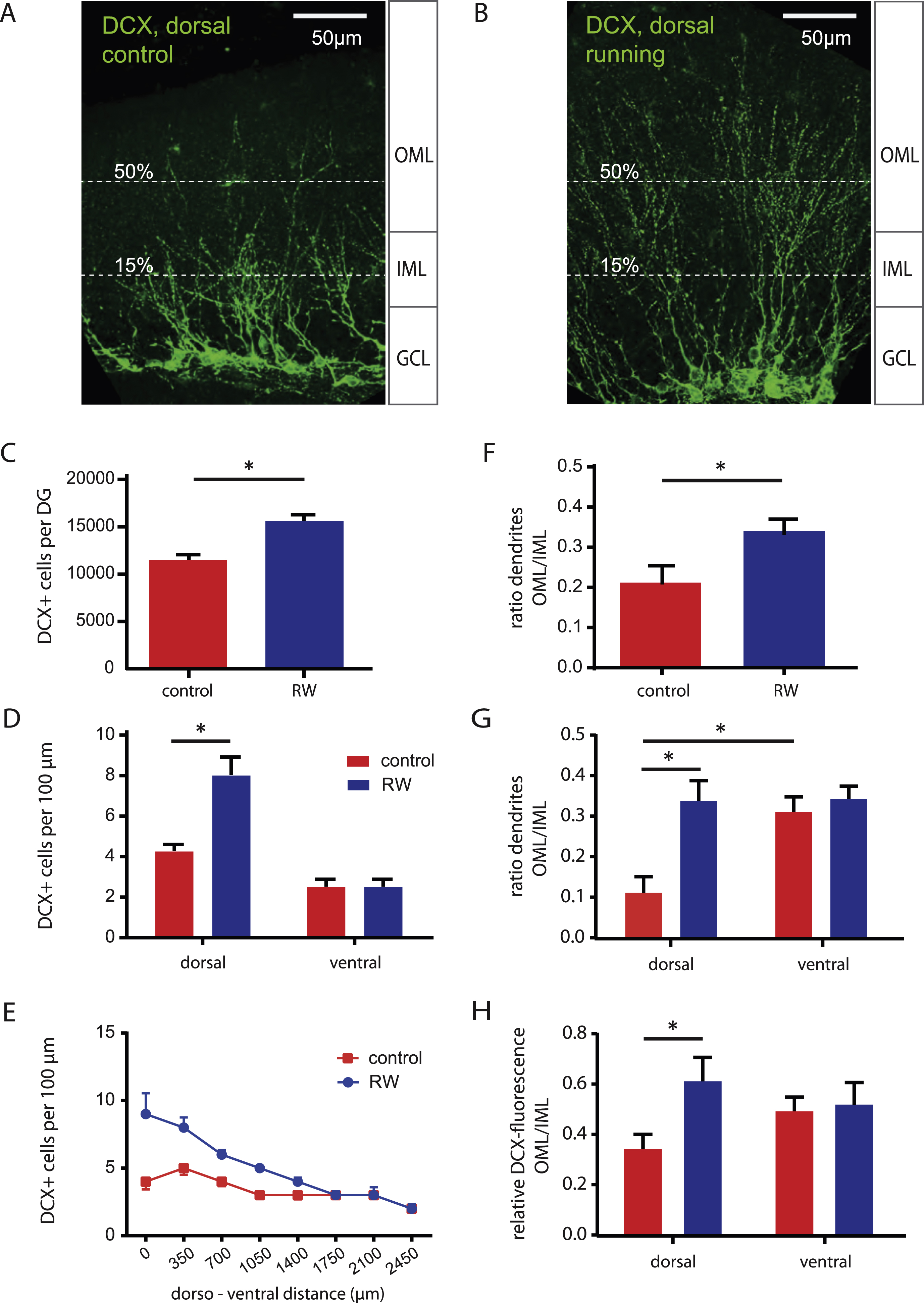
Fig.2
Running increases number and dendritic length of newly generated young granule cells. A, B, Immunohistochemical staining for doublecortin (DCX) in hippocampal slices from adult animals housed in control cages (A) or with running wheels (B). Extension of granule cell dendrites into the molecular layer was analysed by counting the crossings of dendrites with the dashed lines placed in the inner molecular layer (IML) and outer molecular layer (OML), corresponding to about 15% and 50% of the total molecular layer extent. C, Total number of newly generated DCX+ neurons is increased in running mice (n = 8) relative to control (n = 8). D, E, Mice with running wheels show significantly more DCX+ cells in the dorsal hippocampus (each group, n = 4 mice). The bars in D represent data from the 3 first (dorsal) and the 3 last (ventral) slices along the dorso-ventral axis. F, The ratio of dendrites reaching the middle of the molecular layer is significantly increased in running mice (n = 10) relative to control (n = 10). G, H, Along the dorso-ventral axis, the running-dependent increase in dendritic outgrowth is restricted to the dorsal half of the DG. This was analysed either by directly counting the number of dendrites crossing the 15% and 50% lines shown in A and B (G: n = 3 to 7), or calculating the ratio of the DCX fluorescence intensity, measured in rectangular 40 × 250μm subfields in the OML and IML oriented in parallel to the GCL (H: n = 9 to 11).
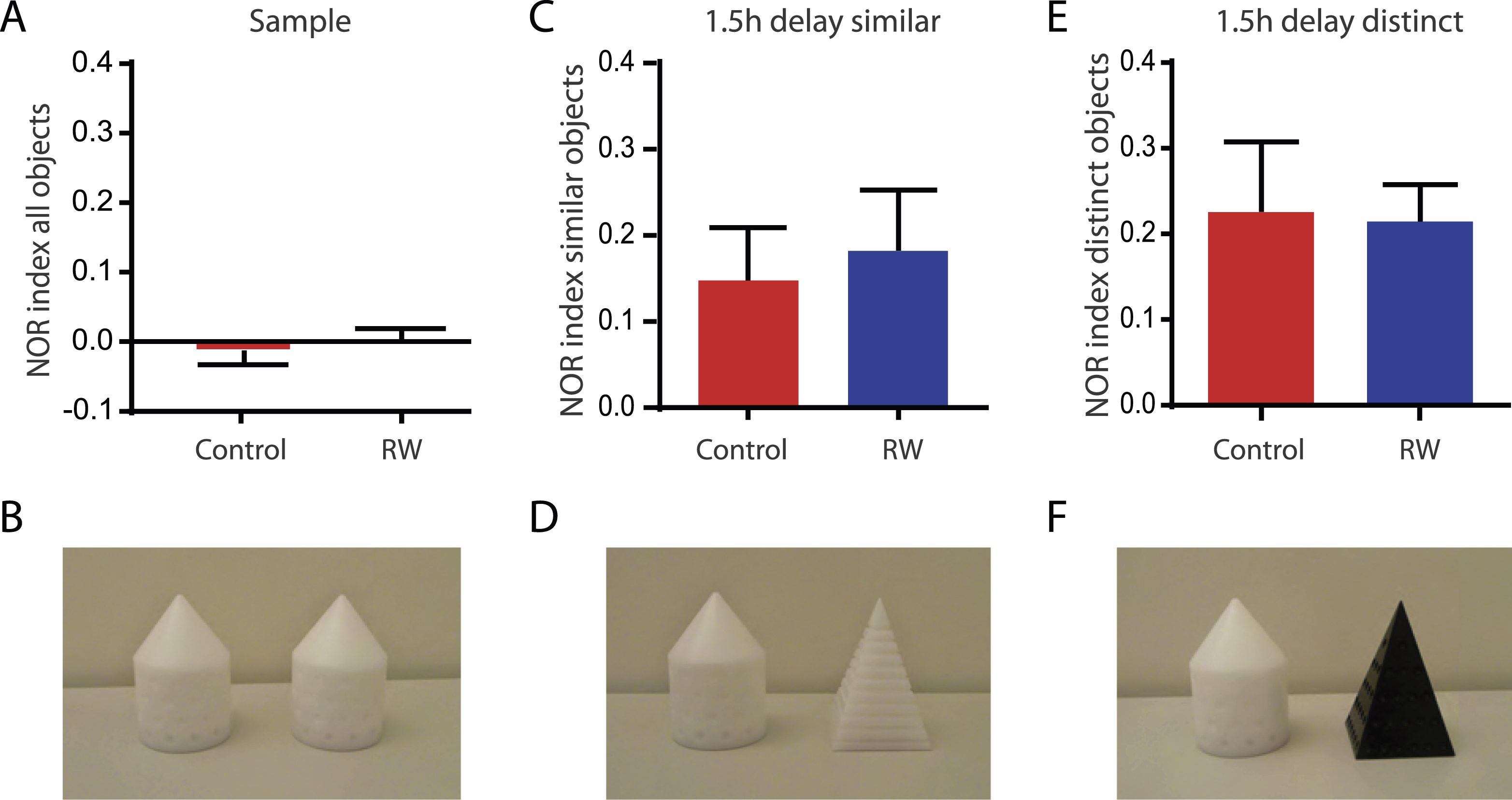
Fig.4
Running improves pattern separation and recognition memory at 24 h delay. A, B, After 24 h delay, one sample object (cone) was replaced by a distinct object (red pyramid) shown in (B). Animals spent significantly more time in exploring the novel object relative to the familiar object in both, control (n = 9) and running mice (n = 9). C, D, In a different cohort of animals one sample object (cone) was replaced by a similar object (white pyramid) shown in (D). At 24 h delay, running animals spent significantly more time in exploring the novel object (n = 11) relative to control animals (n = 16). Control animals did not show a significant recognition memory for the familiar object at 24 h delay. E, F, Example tracks showing a 2 min path of a control animal (E) and a running animal (F), during the 24 h delay novel object recognition test. The novel object in E and F was placed on the left.
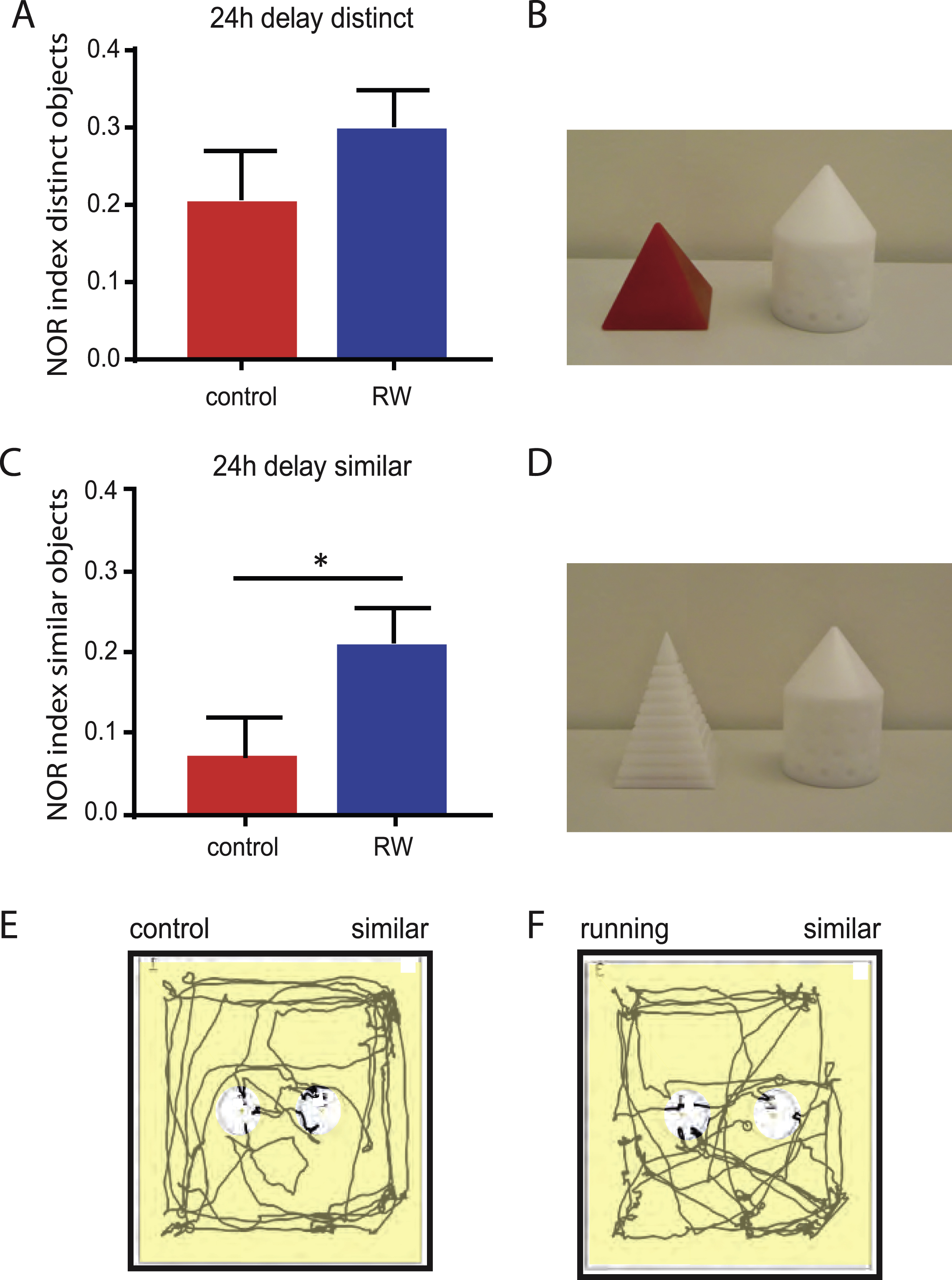
Fig.5
Exercise inhibits temporal decay of pattern separation after learning. A, B, NOR index during memory testing is plotted against the time after sample phase for similar (A) and distinct objects (B). Animals with running wheels (RW1 & RW2) are shown in blue. Control animal are shown in red. C, D, Similar to (A) and (B), NOR index during memory testing is plotted against the time after sample phase for similar (C) and distinct objects (D). Animals with running wheels, tunnels and houses (RW2, blue) are compared to animals with only running wheels (RW1, red).