Non-Muscle Invasive Bladder Cancer: Many More Patients Die With It Than Of It
Abstract
BACKGROUND:
The National Cancer Institute SEER Program regularly publishes bladder-cancer specific survival statistics. However, this data is for all bladder cancers, and information for non-muscle invasive bladder cancer (NMIBC) is difficult to obtain.
OBJECTIVE:
To quantify 5-year overall and bladder cancer-specific survival in a cohort of Department of Veterans Affairs (VA) patients diagnosed with NMIBC.
METHODS:
We identified VA patients diagnosed with NMIBC who underwent a transurethral resection from 2003-2013. The patient demographics and Charlson Comorbidity Index were categorized. We acquired the patients’ date of death from the Veterans Health Administration’s Death Ascertainment File and their cause of death from the Mortality Data Repository. We calculated Kaplan Meier estimates of survival.
RESULTS:
A total of 27,008 patients were included; median age was 69 and almost all were male (99%). The median comorbidity score was 4. The most prevalent comorbidity indicators included Chronic Pulmonary Disease (48%), cancer other than Bladder (41%), and diabetes (40%). This cohort was found to have a 5-year overall survival of 68% (99% CI 67% –69%) and a 5-year bladder cancer-specific survival of 93% (99% CI 92% –94%).
CONCLUSIONS:
The 5-year bladder cancer-specific survival in patients diagnosed with non-muscle invasive bladder cancer is substantially higher than the 5-year overall survival. This difference may be related to the severity and number of comorbidities that patients in this population must manage. This warrants further research into the necessity of currently recommended high-intensity cancer surveillance for individuals with NMIBC.
INTRODUCTION
It is estimated that more than 82,000 patients will be diagnosed with bladder cancer in the United States in 2023, making it the 6th most prevalent cancer [1]. Bladder cancer can be further categorized as non-muscle-invasive bladder cancer (NMIBC) or muscle-invasive bladder cancer (MIBC) according to the extent of invasion into the bladder wall; treatment options and prognosis vary widely with respect to these designations [2]. The National Cancer Institute Surveillance Epidemiology and End Results (SEER) Program regularly publishes bladder cancer-specific survival data according to factors such as age, race, sex, and summary stage. However, the “localized” summary stage includes both NMIBC and MIBC. This makes it difficult to understand the survival of patients with the less aggressive non-muscle invasive disease, [3] which represents about three quarters of all diagnoses. Therefore, we sought to quantify the 5-year overall and bladder cancer-specific survival in a cohort of Department of Veterans Affairs (VA) patients diagnosed with NMIBC. These survival data provide important information for NMIBC trial planning and can provide perspective on cancer- and non-cancer related risks for clinicians and their patients.
METHODS
We used a previously validated Natural Language Processing (NLP) algorithm to identify 47,595 VA patients from the Corporate Data Warehouse that were diagnosed with NMIBC from 2000–2020 [4]. The NLP classified those who progressed from NMIBC to MIBC during this timeframe as MIBC; thus, these were excluded from the cohort. We began monitoring patient survival after their first TURBT that occurred between 2003-2013. TURBT was identified by CPT codes [52224, 52234, 52235, 52240, 52204]. We followed patients through 2018, thus, minimum length of follow-up was 5 years unless a patient died prior to 5 years. Maximum length of follow-up was 16 years. We acquired the patients’ dates of death from the Veterans Health Administration’s Death Ascertainment File and their cause of death from the Mortality Data Repository. We summarized demographics, and each patient was assigned a Charlson Comorbidity score. Utilizing the date and cause of death data, we calculated Kaplan Meier estimates for bladder-cancer specific survival and overall survival. These estimates were generated with 99% confidence intervals.
We performed sensitivity analyses among a subset of 10,270 patients for whom grade information was available from pathology reports from their index TURBT. Grade information was obtained using prior validated NLP algorithms [5]. Among this subset, we calculated Kaplan-Meier estimates of bladder cancer-specific and overall survival with 99% confidence intervals. We then calculated Kaplan-Meier estimates for these outcomes with 99% confidence intervals by grade (high versus low grade). The study was deemed exempt from institutional review board review, because it was secondary research for which consent was not required. A waiver of HIPAA authorization was granted.
RESULTS
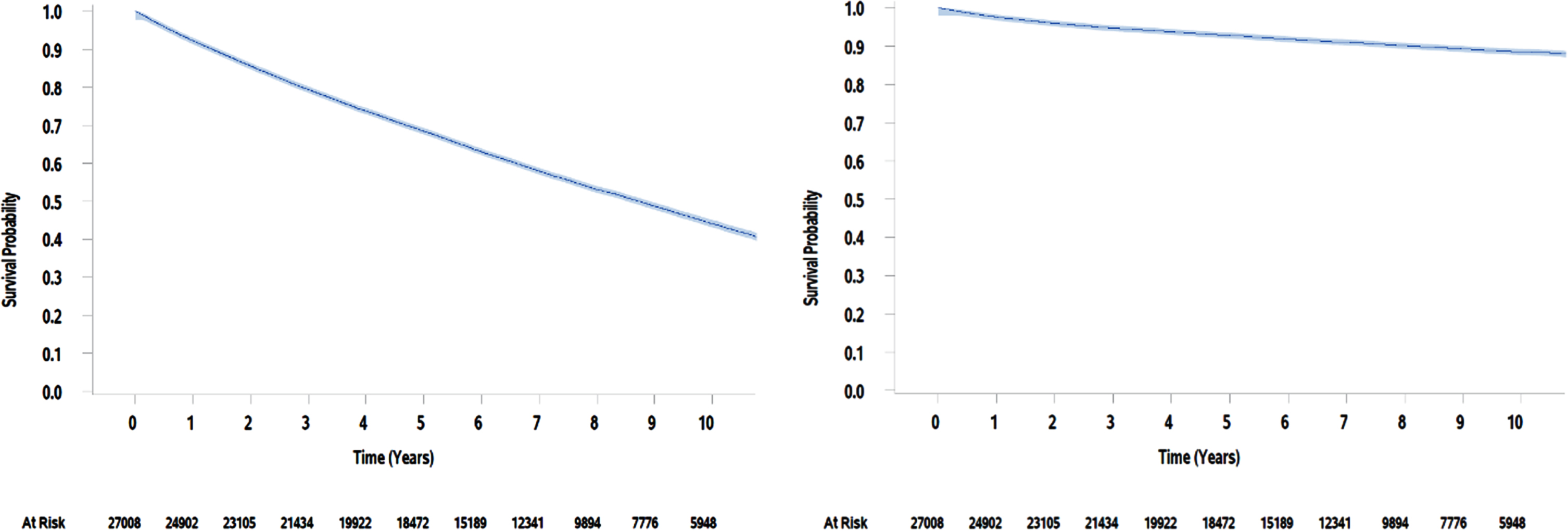
A total of 27,008 patients who underwent their first TURBT between 2003 and 2013 were included in this analysis. Demographic characteristics of this cohort are presented in Table 1. The median age was 69 (range, 21 to 99) and nearly all the patients were male (n = 26,683, 99%). The patients in this cohort were diagnosed with many comorbidities; almost all the patients carried a diagnosis of hypertension (n = 21,586; 80%). The Charlson Comorbidity Index had a median score of 4 and mean of 5 (range, 0 to 21). The most common comorbidity indicators included COPD (n = 13,074, 48%), cancer other than bladder (n = 11,082; 41%), and diabetes with or without chronic complications (n = 10,724; 40%). This cohort was found to have a 5-year overall survival of 68% (99% CI 67% -69%) and a 5-year bladder cancer-specific survival of 93% (99% CI 92% -94%, Fig. 1).
Table 1
Demographic data and Charlson Comorbidity Score for this cohort of patients. N = number of patients. SD = Standard Deviation
| Total | |
| Age (years), mean (SD) | 69.7 (10.32) |
| Age by decade, N (%) | 27,008 (100.0) |
| 20–29 | 22 (0.1) |
| 30–39 | 74 (0.3) |
| 40–49 | 465 (1.7) |
| 50–59 | 3,659 (13.5) |
| 60–69 | 9,533 (35.3) |
| 70–79 | 7,899 (29.2) |
| 80–89 | 4,987 (18.5) |
| 90–99 | 369 (1.4) |
| Gender, N (%) | 27,008 (100.0) |
| Male | 26,683 (98.8) |
| Female | 325 (1.2) |
| Race, N (%) | 27,008 (100.0) |
| White | 22,130 (81.9) |
| Black or African American | 2,423 (9.0) |
| American Indian or Alaska Native | 119 (0.4) |
| Asian | 57 (0.2) |
| Native Hawaiian / Pacific Islander | 185 (0.7) |
| Unknown / Declined | 2,094 (7.8) |
| Ethnicity, N (%) | 27,008 (100.0) |
| Not Hispanic or Latino | 24,600 (91.1) |
| Hispanic or Latino | 924 (3.4) |
| Unknown / Declined | 1,484 (5.5) |
| BMI, mean (SD) [was available for 8,587 patients] | 29.1 (5.94) |
| TURBTs during 5-year follow-up, mean (SD) | 0.88 (1.63) |
| Specific Comorbidities, N (%) | |
| Obesity | 3,280 (38.2) |
| Autoimmune disease | 4,297 (15.9) |
| COPD | 9,463 (35.0) |
| Chronic kidney disease | 3,496 (12.9) |
| Liver cirrhosis | 440 (1.6) |
| Hearing loss | 9,528 (35.3) |
| Hypertension | 21,586 (79.9) |
| Charlson Comorbidity Score, mean (SD) | 5 (3.6) |
| Charlson Comorbidity Indicators, N (%) | |
| Myocardial infarction | 4,093 (15.2) |
| Congestive heart failure | 6,893 (25.5) |
| Peripheral vascular disease | 8,676 (32.1) |
| Cerebrovascular disease | 7,053 (26.1) |
| Dementia | 3,230 (12.0) |
| Chronic pulmonary disease | 13,074 (48.4) |
| Rheumatologic disease | 1,199 (4.4) |
| Peptic ulcer disease | 1,933 (7.2) |
| Diabetes without chronic complications | 4,625 (17.1) |
| Diabetes with chronic complications | 6,099 (22.6) |
| Hemiplegia paraplegia | 1,069 (4.0) |
| Renal disease | 8,703 (32.2) |
| Cancer other than bladder | 11,082 (41.0) |
| Mild liver disease | 3,113 (11.5) |
| Moderate severe liver disease | 734 (2.7) |
| Metastatic solid tumor | 3,024 (11.2) |
| AIDS HIV | 126 (0.5) |
Fig. 1
Kaplan Meier estimates of overall survival and bladder cancer-specific survival for VA patients over 10 years.
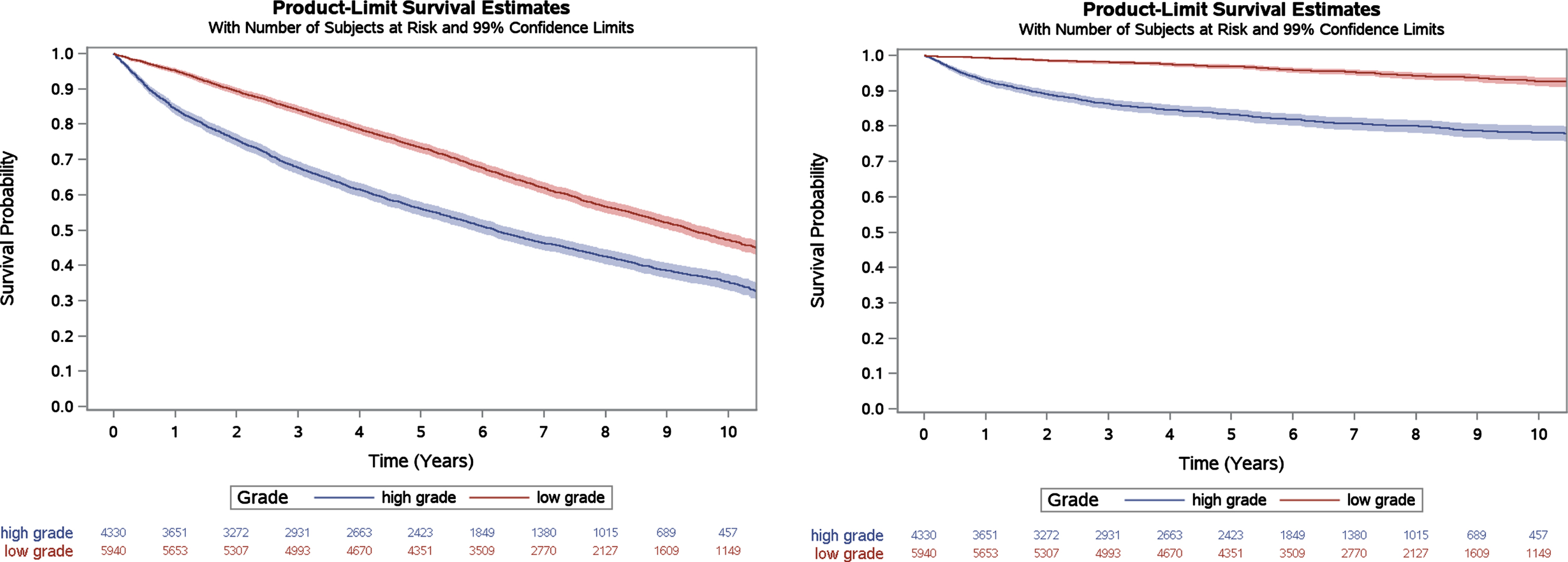
In sensitivity analyses among the subset of patients with grade information available from their index TURBT (n = 10,270), 5-year overall and bladder cancer-specific survival were similar to the overall cohort at 66% (99% CI 65% -67%) and 91% (99% CI 90% -92%), respectively. When stratified by grade, patients with high grade versus low grade NMIBC had significantly lower overall survival (56%, 99% CI 54% -58% versus 73%, 99% CI 72% -75%) and bladder cancer-specific survival (83%, 99% CI 82% -85% versus 97%, 99% CI 96% -97%; Fig. 2).
Fig. 2
Kaplan Meier estimates of overall survival and bladder cancer-specific survival for VA patients with grade information available, stratified by grade. Log-rank p < 0.001 for both outcomes.
DISCUSSION
The 5-year bladder cancer-specific survival of patients diagnosed with non-muscle invasive bladder cancer is substantially higher than the 5-year overall survival. Although this study focused solely on VA patients, these data closely align with the 5-year NMIBC-specific survival (90%) and the 5-year overall survival (71%) noted in a recent study based on SEER data [6]. The abundance and severity of comorbidities may explain why overall survival is much lower than bladder cancer-specific survival in this population.
Regarding our sensitivity analyses stratified by grade, it should be noted that more than half of the patients included in the main analyses did not have grade information available. This is likely due to (1) some sites not reliably depositing pathology reports into the Text Integration Utility domain in the Corporate Data Warehouse, (2) inability to access pathology reports that were done in VA-contracted pathology laboratories, and (3) inability of the NLP to abstract information from all available pathology reports as first determinations needed to be made whether those reports were related to urothelial carcinoma. Thus, we caution readers to rely on specific point estimates of survival by grade. Rather, our results demonstrate that both overall and bladder cancer-specific survival were worse in high- versus low-grade disease and that patients with high grade NMIBC still had substantially higher bladder cancer-specific survival than overall survival.
As is typical for studies from large administrative datasets, there are a number of limitations worth discussing. First, we did not have access to VA pharmacy data. Thus, we were unable to ascertain use of BCG and other intravesical treatments among the NMIBC patients included in this cohort. However, in prior work, we examined use of BCG in a similar cohort of patients diagnosed with NMIBC within VA and found that about a quarter of patients with high grade disease received BCG [7]. This proportion was similar to what was found within SEER-Medicare (20% received BCG) [8]. Thus, the rate of BCG use within VA appears to be similar to what was found in other settings and inclusion of these data is unlikely to change our conclusions. Second, we acknowledge that we did not have certain patient-level covariates such as cancer stage and tumor size. However, the purpose of our analyses was to provide a big picture assessment of survival among NMIBC patients treated within the VA. Third, patients who progressed from NMIBC to muscle-invasive disease between 2000 and 2020 were excluded from our NMIBC cohort due to the prior design of the NLP algorithms [4]. However, transitions from NMIBC to muscle-invasive disease were quite rare when this was examined as part of the prior NLP development among patients included in the NLP validation set, with only three of 100 patients transitioning to muscle-invasive disease [4]. Thus, this limitation is unlikely to have a major impact on our bladder-cancer specific survival estimates. Finally, we used a sizable cohort from the largest equal access health care system in the U.S., the VA. However, our findings may not be generalizable to other bladder cancer patient populations with a lower prevalence ofcomorbidities.
In spite of these limitations, our findings support the notion that for most patients with NMIBC, the biggest threat to their life comes from their comorbidities and not from their bladder cancer. Thus, intensive surveillance of NMIBC may not always be warranted for these patients. Surveillance is usually done with invasive cystoscopy procedures that are associated with substantial anxiety and discomfort for patients [9]. We believe that future studies are needed to examine whether there are oncologic benefits to high-intensity surveillance or whether patients can be spared some of these invasive surveillance cystoscopy procedures. A VA Cooperative Studies Program trial is in planning that will examine this question (VA CSP #2036).
ACKNOWLEDGMENTS
This study was supported using resources and facilities at the White River Junction VA Healthcare System, VT, the Perry Point VA Medical Center, MD, and the VA Informatics and Computing Infrastructure (VINCI), VA HSR Res 13-457.
FUNDING
Department of Veterans Affairs (VA) Cooperative Studies Program (CSP): planning funding for CSP #2036.
AUTHOR CONTRIBUTIONS
Conception: ZM, KB, FRS
Performance of work: EJD, ZM
Interpretation of data: all authors
Drafting the manuscript: KM, FRS
Critical review and editing of the manuscript: all authors
All authors had access to the data.
ETHICAL CONSIDERATIONS
This study was deemed to be exempt from review by the Veteran’s Institutional Review Board (IRB) of Northern New England (Study #1688491) and by the University of Maryland IRB (Study #HP-00101077).
Disclaimer: Opinions expressed are those of the authors and do not constitute official positions of the U.S. Federal Government or the Department of Veterans Affairs.
CONFLICT OF INTEREST
Kathryn McGonagle, Ellen J. Dematt, Zhibao Mi, and Kousick Biswas report no relevant conflicts of interest. Florian R. Schroeck reports research funding from the National Cancer Institute, Pacific Edge Ltd., Cepheid, and Nucleix.
DATA AVAILABILITY
The data supporting the findings of this article are available to qualified Department of Veterans Affairs researchers via the VA Informatics and Computing Infrastructure (VINCI). Potential researchers interested in accessing these data are encouraged to contact the corresponding author for further information on data access.
REFERENCES
[1] | Bladder Cancer — Cancer Stat Facts. Accessed August 22, 2023. https://seer.cancer.gov/statfacts/html/urinb.html |
[2] | Lenis AT , Lec PM , Chamie K . Bladder cancer a review. JAMA – Journal of the American Medical Association. (2020) ;324: (19):1980–1991. doi:10.1001/jama.2020.17598 |
[3] | SEER*Explorer Application. Accessed September 9, 2023. https://seer.cancer.gov/statistics-network/explorer/application.html?site=71&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&stage=104&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=1#resultsRegion0 |
[4] | Yang R , Zhu D , Howard LE , et al. Context-Based Identification of Muscle Invasion Status in Patients With Bladder Cancer Using Natural Language Processing. JCO Clin Cancer Inform. (2022) ;(6). doi: 10.1200/cci.21.00097. |
[5] | Schroeck FR , Patterson O V , Alba PR, et al. Development of a Natural Language Processing Engine to Generate Bladder Cancer Pathology Data for Health Services Research. Urology.. (2017) ;110: :84–91. doi: 10.1016/J.UROLOGY.2017.07.056 |
[6] | Ślusarczyk A , Zapała P , Zapała Ł , Borkowski T , Radziszewski P . Cancer-Specific Survival of Patients with Non-Muscle-Invasive Bladder Cancer: A Population-Based Analysis. Ann Surg Oncol. Published online. (2023) . doi: 10.1245/s10434-023-14051-9. |
[7] | Rezaee ME , Ismail AAO , Okorie CL , Seigne JD , Lynch KE , Schroeck FR . Partial Versus Complete Bacillus Calmette-Guérin Intravesical Therapy and Bladder Cancer Outcomes in High-risk Non–muscle-invasive Bladder Cancer: Is NIMBUS the Full Story? Eur Urol Open Sci. (2021) ;26: :35–43. doi: 10.1016/J.EUROS.2021.01.009. |
[8] | Chamie K , Saigal CS , Lai J , et al. Quality of care in patients with bladder cancer. Cancer. (2012) ;118: (5):1412–1421. doi:10.1002/CNCR.26402. |
[9] | Koo K , Zubkoff L , Sirovich BE , et al. The Burden of Cystoscopic Bladder Cancer Surveillance: Anxiety, Discomfort, and Patient Preferences for Decision Making. Urology. (2017) ;108: :122–128. doi: 10.1016/j.urology.2017.07.016. |




