Longitudinal Analysis of Bladder Cancer-Specific Mortality Trends in the United States
Abstract
BACKGROUND:
Bladder cancer is the tenth leading cause of cancer death in the United States (US). Advances in diagnosis, imaging, and treatments have led to improvements in bladder cancer management.
OBJECTIVE:
To evaluate longitudinal bladder cancer mortality trends from 1999–2020 in the US by gender, race, ethnicity, age, geographic region, and urbanization category.
METHODS:
Age-adjusted bladder cancer death and incidence rates of individuals in the US of all ages between 1999–2020 were obtained using the CDC WONDER and NAACCR databases. Trends and average annual percent changes (AAPC) in age-adjusted Bladder Cancer-Specific Mortality (BCSM) and incidence rates were estimated. Data were analyzed from May 2023 to October 2023.
RESULTS:
From 1999–2020, overall BCSM decreased by 0.4% annually, with a dramatic decrease in deaths between 2015–2020 (AAPC: –2.0% [95% CI: –2.6,–1.3]). However, BCSM rates and metastatic malignant bladder cancer incidence rates from 1999–2020 increased for individuals≥85 years old (AAPC for BCSM: 0.8% [95% CI:0.5,1.1]; AAPC for metastatic malignant incidence: 2.5% [95% CI: 2.0,2.9]). Increases in BCSM were found for certain years in the South, in rural areas, and for Non-Hispanic White and Asian or Pacific Islander individuals.
CONCLUSIONS:
Overall mortality from bladder cancer has been decreasing in the US over two decades. Upon disaggregation, increasing trends were found for BCSM and for metastatic malignant bladder cancer incidence for individuals≥85 years old from 1999–2020. Further evaluation of these trends is essential to understand how to target specific populations to improve patient outcomes.
INTRODUCTION
An estimated 16,710 people in the United States (US) will die from bladder cancer in 2023, comprising 2.7% of all cancer deaths [1]. There will be an estimated 82,290 new cases of bladder cancer in 2023, representing 4.2% of all new cancer cases [1]. Compared to other cancers, bladder cancer is the sixth most common cancer and the tenth leading cause of cancer death in the US [1]. The 5-year survival rate in the US has risen over the past five decades from 71.9% for diagnoses in 1975 to 77.9% for diagnoses in 2023 [2], likely due to advances in diagnosis, imaging, and treatments [3].
Bladder cancer is more common in men than women and is most frequently diagnosed among people aged 65–74 [1]. The percent of bladder cancer deaths is highest among people aged 85 + [1]. Although men are more likely to be diagnosed with bladder cancer, among patients with bladder cancer, women are more likely to die from their disease [4–7]. Previous studies have found that among those with bladder cancer, overall and bladder cancer-specific survival is lower among Black patients, compared to White patients [6–9]. One study found that Black women had a higher risk of dying from bladder cancer compared to other race-sex subgroups [10]. Black patients were less likely to undergo appropriate cancer surveillance, radical cystectomy, or lymph node dissection [11, 12].
Recent data demonstrate decreasing bladder cancer-specific mortality (BCSM) rates between 2014–2018 for both men and women [13], however, these trends have not been further examined by race, ethnicity, age group, geographical region, or urbanization. The Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research database (CDC-WONDER) is a publicly available information system that allows researchers to access mortality data and disaggregate deaths based on cause of death and demographic factors [14]. The North American Association of Central Cancer Registries (NAACCR) database provides cancer incidence for North America and can disaggregate data based on stage at diagnosis and other factors [15]. Using these comprehensive datasets of bladder cancer mortality and incidence over two decades, we sought to evaluate differences in rates overall and by gender, race, ethnicity, age group, location, and urbanization category in the US.
MATERIALS AND METHODS
The study was considered exempt from guidelines on human participation in research by the Mass General Brigham institutional review board because it is a secondary analysis of publicly available deidentified data. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed. Age-adjusted mortality rates for bladder cancer (ICD-10 code 67) were obtained for individuals of all ages in the US from the Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research database (CDC-WONDER) from 1999–2020 [14]. BCSM trends were evaluated by gender, ethnicity (Hispanic and Non-Hispanic), race (Non-Hispanic American Indian or Alaska Native, Non-Hispanic Asian or Pacific Islander, Non-Hispanic Black, and Non-Hispanic White), census region, and urbanization category (eTables 1 and 2). For trends evaluated by 5-year age groups, crude rates of BCSM were gathered from 1999–2020, and age-adjusted rates per 100,000 population were calculated from the crude rates (count/population x 100,000) and then weighted by the proportion of the persons in the corresponding age groups to the standard population (2000 US standard population).
Age-adjusted incidence rates for bladder cancer (ICD-10 code 67) were gathered for individuals of all ages in the US from 1999–2020 from the North American Association of Central Cancer Registries (NAACCR) database [15]. Cancer incidence was evaluated by gender, ethnicity (Hispanic and Non-Hispanic), race (Non-Hispanic American Indian or Alaska Native, Non-Hispanic Asian or Pacific Islander, Non-Hispanic Black, Non-Hispanic White and Non-Hispanic Unknown), and stage at diagnosis (in situ malignant, localized malignant, regional malignant, and metastatic malignant). Crude incidence rates were collected for 5-year age groups and age-adjusted rates per 100,000 population were calculated as described above.
Joinpoint regression modeling was used to determine average annual percent changes (AAPCs) in mortality and incidence rates for bladder cancer with a maximum of four join points [16]. Two-sided hypothesis tests were performed using the Joinpoint Regression Program, Version 4.9.1.0 [17]. The Joinpoint Regression Program algorithm starts with the simplest model (one line) and tests whether adding another line significantly improves the fit. This process is repeated until no additional lines significantly improve the fit, and the number of join points and years of the segments are determined by the final model. AAPCs with p-values<0.05 after correcting for multivariate tests were classified as statistically significant [18].
RESULTS
319,285 people died from bladder cancer in the US from 1999–2020. The age adjusted BCSM rate was 4.3 per 100,000 population overall, and 7.5 per 100,000 population for men and 2.2 per 100,000 population for women (Table 1).
Table 1
Age-Adjusted mortality rates per 100,000 population and Annual Percentage Changes in Bladder Cancer Death Rates by Gender From 1999–2020 in the US
| Gender | Age-adjusted mortality rates (95% CI) | Deaths | Average APC from 1999–2020(95% CI) | p value | Segment 1 | p value | Segment 2 | p value | ||
| All | 4.3 (4.3–4.3) | 319285 | –0.4**(–0.6,–0.3) | <0.001 | 1999–2015 | 0.1(0.0,0.2) | 0.25 | 2015–2020 | –2.0**(–2.6,–1.3) | <0.001 |
| Men | 7.5 (7.5–7.5) | 224774 | –0.5**(–0.6,–0.3) | <0.001 | 1999–2013 | 0.1(–0.1,0.2) | 0.28 | 2013–2020 | –1.6**(–2.0,–1.2) | <0.001 |
| Women | 2.2 (2.2–2.2) | 94511 | –0.9**(–1.3,–0.5) | <0.001 | 1999–2017 | –0.5**(–0.7,–0.3) | <0.001 | 2017–2020 | –3.1*(–5.8,–0.4) | 0.027 |
** indicates the p-value is significant after Holm-Bonferroni correction. * indicates the p-value is < 0.05, but not significant after Holm-Bonferroni correction.
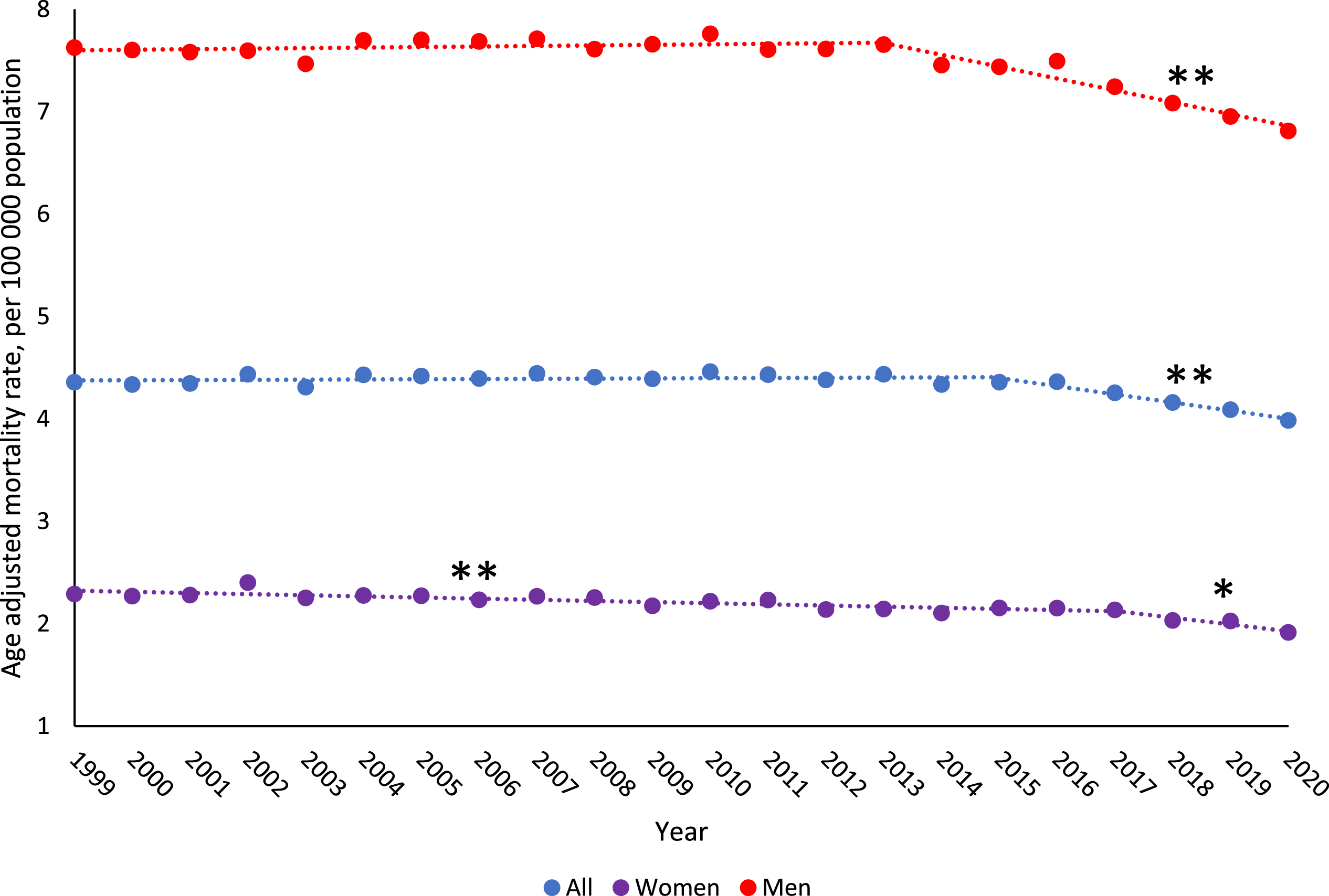
From 1999–2020, age-adjusted BCSM rate overall decreased by 0.4% per year (95% CI:–0.6,–0.3), with the largest decrease in 2015–2020 (AAPC: –2.0% [95% CI:–2.6,–1.3]) (Table 1, Fig. 1). Evaluating by gender, women had a slightly larger decrease in BCSM rates from 1999–2020 than men (AAPC, women: –0.9[95% CI:–1.3,–0.5]; AAPC, men: –0.5[95% CI:–0.6,–0.3]). The age-adjusted incidence rate increased from 1999–2020 for both men and women with metastatic malignant bladder cancer (AAPC, men: 1.7% [95% CI:1.1,2.2]; AAPC, women: 1.4% [95% CI: 1.1,1.7]) (eTable 3).
Fig. 1
Trends in age-adjusted bladder cancer death rates from 1999–2020 among US population by gender. Observed rates are presented per 100,000 population and represented by “•”. Modeled trends are represented by “—”. ** indicates the p-value is significant after Holm-Bonferroni correction. * indicates the p-value is < 0.05, but not significant after Holm-Bonferroni correction.
By ethnicity, overall, Non-Hispanic patients had a decrease in BCSM from 1999–2020 (AAPC, Non-Hispanic: –0.3[95% CI:–0.5,–0.2]), while the decrease for Hispanic patients was not significant by Holm-Bonferroni correction (Table 2). Non-Hispanic patients experienced a slight increase in BCSM rates from 1999–2015 (AAPC: 0.2% [95% CI:0.1,0.3]) followed by a larger decrease from 2015–2020 (AAPC: –1.9% [95% CI:–2.5,–1.3]). The increase from 1999–2015 was driven by Non-Hispanic men, who had a significant increase in BCSM rates from 1999–2013 (Table 2). For Non-Hispanic women of all races, BCSM decreased by 0.4% annually from 1999–2016, which then rapidly reduced to –2.4% annually from 2016–2020. For Hispanic women, BCSM showed a decreasing trend from 2001–2020, however this was not significant by Holm-Bonferroni correction (Table 2). From 1999–2020, metastatic malignant bladder cancer incidence increased for both Hispanic and Non-Hispanic individuals (eTable 4). By race, BCSM rates significantly decreased for Non-Hispanic Asian or Pacific Islander individuals and Non-Hispanic Black individuals from 1999 to 2020 (AAPC, Non-Hispanic Asian or Pacific Islander: –0.4[95% CI:–0.6,–0.2]; AAPC, Non-Hispanic Black: –0.8[95% CI:–1.1,–0.6]). From 1999–2013, Non-Hispanic Asian or Pacific Islander individuals had a significant increase in BCSM, which then decreased from 2013 to 2020. Non-Hispanic White individuals had an increasing BCSM rate from 1999–2015, followed by a decline from 2015–2020 (Table 2). Metastatic malignant bladder cancer incidence increased from 1999–2020 for Non-Hispanic Asian or Pacific Islander men, Non-Hispanic Black men, and Non-Hispanic White women. In situ malignant bladder cancer incidence increased from 1999–2020 for Non-Hispanic American Indian or Alaska Native men and Non-Hispanic Black men and women (eTable 5).
Table 2
Annual Percentage Changes in Bladder Cancer Death Rates by Race, Ethnicity and Gender From 1999–2020 in the US
| Race /Ethnicity | Average APC from 1999–2020(95% CI) | p value | Segment 1 | p value | Segment 2 | p value | ||
| All | ||||||||
| Hispanic | –0.4*(–0.7,–0.1) | 0.023 | 1999–2020 | –0.4*(–0.7,–0.1) | 0.023 | NA | NA | NA |
| Non-Hispanic | ||||||||
| All Races | –0.3**(–0.5,–0.2) | <0.001 | 1999–2015 | 0.2**(0.1,0.3) | 0.003 | 2015–2020 | –1.9**(–2.5,–1.3) | <0.001 |
| American Indian or Alaska Native | 0.8(–0.6,2.1) | 0.25 | 1999–2020 | 0.8(–0.6,2.1) | 0.25 | NA | NA | NA |
| Asian or Pacific Islander | –0.4**(–0.6,–0.2) | <0.001 | 1999–2013 | 0.2**(0.0,0.4) | 0.014 | 2013–2020 | –1.5**(–2.0,–1.1) | <0.001 |
| Black | –0.8**(–1.1,–0.6) | <0.001 | 1999–2020 | –0.8**(–1.1,–0.6) | <0.001 | NA | NA | NA |
| White | –0.1(–0.3,0.0) | 0.11 | 1999–2015 | 0.4**(0.2,0.5) | <0.001 | 2015–2020 | –1.8**(–2.5,–1.0) | <0.001 |
| Men | ||||||||
| Hispanic | –0.6**(–1.0,–0.2) | 0.008 | 1999–2020 | –0.6**(–1.0,–0.2) | 0.008 | NA | NA | NA |
| Non-Hispanic | ||||||||
| All Races | –0.4**(–0.6,–0.2) | <0.001 | 1999–2013 | 0.2**(0.0,0.4) | 0.014 | 2013–2020 | –1.5**(–2.0,–1.1) | <0.001 |
| American Indian or Alaska Native | NA | NA | NA | NA | NA | NA | NA | NA |
| Asian or Pacific Islander | 0.0(–0.7,0.6) | 0.9 | 1999–2020 | 0.0(–0.7,0.6) | 0.9 | NA | NA | NA |
| Black | –0.5**(–0.8,–0.1) | 0.011 | 1999–2020 | –0.5**(–0.8,–0.1) | 0.011 | NA | NA | NA |
| White | –0.2**(–0.4,–0.1) | 0.007 | 1999–2013 | 0.3**(0.2,0.5) | 0.001 | 2013–2020 | –1.4**(–1.9,–0.9) | <0.001 |
| Women | ||||||||
| Hispanic | 0.6(–1.5,2.6) | 0.59 | 1999–2001 | 14.6(–8.1,43.0) | 0.21 | 2001–2020 | –0.8*(–1.5,–0.2) | 0.018 |
| Non-Hispanic | ||||||||
| All Races | –0.7**(–1.1,–0.4) | <0.001 | 1999–2016 | –0.4**(–0.5,–0.2) | 0.001 | 2016–2020 | –2.4**(–4.0,–0.8) | 0.006 |
| American Indian or Alaska Native | NA | NA | NA | NA | NA | NA | NA | NA |
| Asian or Pacific Islander | –0.9*(–1.7,–0.2) | 0.016 | 1999–2020 | –0.9*(–1.7,–0.2) | 0.016 | NA | NA | NA |
| Black | –1.5**(–1.9,–1.2) | <0.001 | 1999–2020 | –1.5**(–1.9,–1.2) | <0.001 | NA | NA | NA |
| White | –0.5**(–0.9,–0.2) | 0.001 | 1999–2016 | –0.2*(–0.4,0.0) | 0.027 | 2016–2020 | –2.0**(–3.5,–0.4) | 0.015 |
** indicates the p-value is significant after Holm-Bonferroni correction. * indicates the p-value is < 0.05, but not significant after Holm-Bonferroni correction.
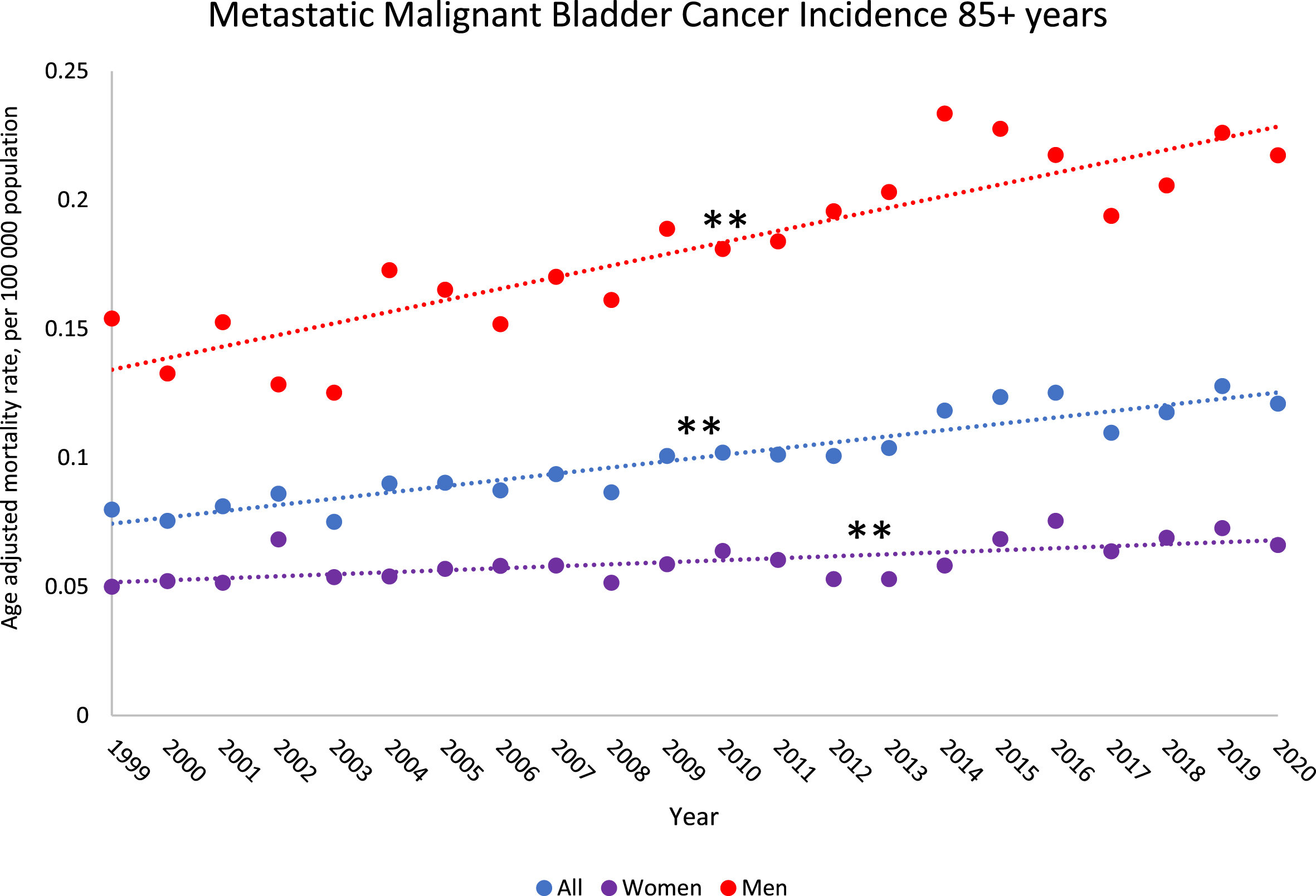
All age groups had a significant decline in BCSM rates from 1999–2020, except for individuals older than 85 years, which had an increase in BCSM rates from 1999–2020 (AAPC: 0.8% [95% CI:0.5,1.1]) (eTable 6, Fig. 2). Individuals 80–84 years old had a significant increase in BCSM rates from 1999–2014 (AAPC: 0.7% [95% CI:0.4,1.0]) (eTable 6). By gender, women older than 85 years did not have a significant increase in BCSM from 1999–2020, while men older than 85 years had a significant increase overall from 1999–2020 (AAPC: 0.6% [95% CI:0.2,0.9]), driven by an increase from 1999–2016. The largest decreases in BCSM rates were in the 35–39 and 40–44 years age groups (eTable 6). Incidence rates for metastatic malignant bladder cancer increased from 1999–2020 for most age groups above 55 years old (except the 70–74 year age group). In particular, metastatic malignant bladder incidence increased significantly for the 85 + year age group for both men and women (AAPC, men: 2.6% [95% CI:1.9,3.2], AAPC, women: 1.3% [95% CI:0.6,2.0]) (Fig. 3, eTable 7).
Fig. 2
Trends in bladder cancer death rates from 1999–2020 among US population 85 years and older by gender. Observed rates are presented per 100,000 population and represented by “•”. Modeled trends are represented by “—”. ** indicates the p-value is significant after Holm-Bonferroni correction. * indicates the p-value is < 0.05, but not significant after Holm-Bonferroni correction.
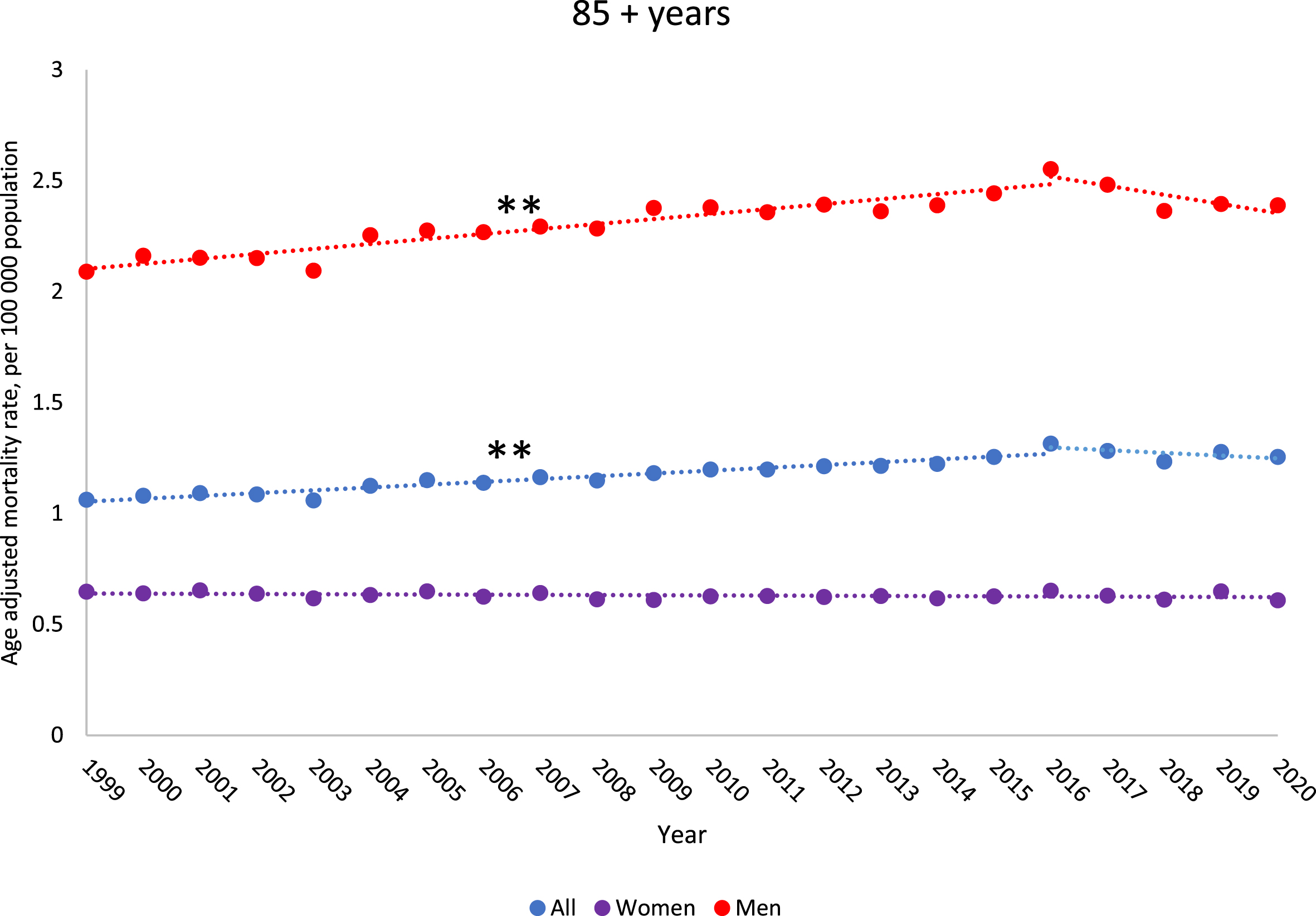
Fig. 3
Trends in metastatic malignant bladder cancer incidence rates from 1999–2020 among US population 85 years and older by gender. Observed rates are presented per 100,000 population and represented by “•”. Modeled trends are represented by “—”. ** indicates the p-value is significant after Holm-Bonferroni correction. * indicates the p-value is < 0.05, but not significant after Holm-Bonferroni correction.
By census region, BCSM rates for all individuals significantly decreased from 1999–2020 for the Northeast and West (AAPC, Northeast: –1.0[95% CI:–1.4,–0.7]; AAPC, West: –0.4[95% CI:–0.6,–0.2]) (eTable 8). In the South, there was a significant increase in BCSM rates for all individuals from 1999–2014 (AAPC: 0.3% [95% CI:0.1,0.5]), driven by BCSM rates for men in the South from 1999–2013 (eTable 8).The largest decreases were in the Northeast from 2016–2020 (AAPC: –4.4% [95% CI:–6.0,–2.7]) and in the Midwest from 2013–2020 (AAPC: –1.4% [95% CI:–2.0,–0.8]), both driven by decreases in BCSM rates for women (eTable 8).
By urbanization, rural areas (non-core) had significantly increasing BCSM rates from 1999–2011 (AAPC: 0.9% [95% CI:0.6,1.3]) (eTable 9). By gender, men in rural areas had a significant increase in BCSM rates from 1999–2011 (AAPC: 0.8% [95% CI:0.3,1.3]), while women in rural areas had no significant change in BCSM rates from 1999–2020 (eTable 9). BCSM rates significantly decreased from 1999–2020 for individuals in large central metro, large fringe metro, and medium metro areas (AAPC, large central metro: –0.9[95% CI:–1.1,–0.6]; AAPC, large fringe metro: –0.5[95% CI:–0.8,–0.2]; AAPC, medium metro: –0.5[95% CI:–0.8,–0.2]). The largest decreases for these areas were in more recent years (eTable 9).
DISCUSSION
The overall rate of deaths due to bladder cancer in the US has steadily decreased from 1999–2020, driven by the last five years, indicating there may be recent improvements that have led to decreasing bladder mortality. However, bladder cancer death rates are increasing for individuals over 85 years old, particularly men. Metastatic malignant bladder cancer incidence rates are increasing for individuals in age groups over 55 years, particularly those over 85 years old. Individuals of certain ethnicities and races and from certain geographic regions and urbanization categories had increasing BCSM rates for earlier years, although the data showed improvements in bladder cancer mortality in more recent years.
Previous studies found similar trends in bladder cancer mortality by gender, as Islami et al. found a decline among men starting in 2013, and a steady decline among women throughout 2001–2018 [13]. The decline in mortality rates is paired with a decline in bladder cancer incidence. Siegel et al. found a decline in incidence since the mid-2000 s, which accelerated from –0.6% per year to –1.8% per year during 2015–2019, although this was not broken down by stage at diagnosis [19]. However, they noted variation in incidence by race and ethnicity, and an increase by 1.3% per year in American Indian and Alaskan Native individuals [13, 19]. Although there was an upward trend in bladder cancer mortality in Non-Hispanic American Indian or Alaska Native from 1999–2020 in our study, the AAPC was not significant. Among men, our findings of declines in BCSM rates from 1999–2020 may be attributed to declines in incidence of bladder cancer as noted by Islami et al. from 2013–2017 in White, Asian or Pacific Islander, and Hispanic men, potentially due to declines in cigarette smoking [13]. Incidence rates for bladder cancer were stable among Black men [13].
In men aged 80 years and older, bladder cancer was the fourth leading cause of cancer death in the United States in 2020 [13]. There may be gender differences in BCSM rates among the older age group due to the lower prevalence of smoking among women than men in the older birth cohorts [20]. Risk of mortality from smoking related diseases are comparable for men and women in more recent birth cohorts [21]. Age is now widely accepted as the greatest single risk factor for developing bladder cancer [22]. The increase in bladder cancer mortality rates from 1999–2020 for individuals older than 85 is concerning, as 85 years and older is the fastest growing segment of the population and is projected to increase to 18.2 million by 2050 [23]. Some groups have observed lower rates of disease recurrence and progression with better survival in younger patients, and these differences in outcomes may be due to more advanced stage at diagnosis and the administration of less aggressive and effective therapies in the elderly [22, 24]. Age-related declines in performance status and medical comorbidities significantly affect the risk of treatment-related toxicity [25]. For example, treatments with intravesical bacillus Calmette-Guerin, radical cystectomy, and perioperative chemotherapy are less well tolerated and have poorer response in elderly patients [25–27]. Proper selection of elderly patients for treatment may improve outcomes in this cohort.
We found significant increases in BCSM rates from 1999–2014 in the South, particularly among men from 1999–2013, as well as increases in BCSM rates from 1999–2011 in rural (non-core) areas. Our findings in the South may be related to the large burden of smoking-attributable cancer mortality in the Southern states [28]. Other researchers investigated how temporal, socioeconomic, and environmental factors associate with bladder cancer mortality in the US and found that an increase in bladder cancer mortality was associated with multiple environmental exposures, such as smoking, air pollution, well water, urban residence, and mining employment [29]. It is unclear why there was an increase in BCSM rates in rural (non-core) areas. A study comparing bladder cancer stage and mortality in urban vs. rural residency from 2004–2016 found that patients residing in rural areas were not diagnosed at a later stage or with higher bladder cancer grade, although the number of patients with rural residency was extremely low [30].
Study limitations include lack of information about cancer stage at diagnosis, age at diagnosis/death, treatments received, and stage at time of death. There may be inaccuracies in the race and ethnicity data, and we are unable to account for how race and ethnicity data is captured. Due to limitations of the dataset, the current study does not disaggregate broad racial and ethnic groups into subgroups. We were unable to account for the impact of individuals who migrate from one geographic location to another. Other factors that may influence bladder cancer mortality rates were not accounted for in CDC-WONDER, such as income, insurance, employment, smoking status, and exposure to air pollution.
Using comprehensive data on BCSM in the US over two decades, we demonstrate that the overall mortality rate from bladder cancer has been decreasing. However, when disaggregating BCSM by gender, race, ethnicity, age groups, census regions, and urbanization categories, increasing mortality trends were found from 1999–2020 for individuals older than 85. Increasing incidence trends were found for metastatic malignant bladder cancer from 1999–2020 for older individuals. This trend is particularly worrisome considering the aging US population, and the high morbidity and mortality of the standard treatment regimens in older patients. Increasing BCSM rates were found for certain years for Non-Hispanic Asian or Pacific Islander and Non-Hispanic White individuals and individuals in the South and in rural areas, however these trends demonstrated improvement in recent years. Further studies are needed to create solutions targeting specific populations to improve BCSM and overall outcomes for all patients with bladder cancer.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
Dr. Chino is funded in part through the NIH/NCI Support Grant P30 CA008748.
AUTHOR CONTRIBUTIONS
Isabella R. Pompa, David Qi, Anushka Ghosh, Saveli I. Goldberg, and Sophia C. Kamran had access to the data.
Conception: Isabella R. Pompa, Anushka Ghosh, Sophia C. Kamran, Fumiko Chino, Jason A. Efstathiou.
Performance of work: Isabella R. Pompa, David Qi, Saveli I. Goldberg, Sophia C. Kamran.
Interpretation of data: Isabella R. Pompa, Sophia C. Kamran.
Writing the article: Isabella R. Pompa, David Qi, Sophia C. Kamran.
CONFLICT OF INTEREST
Dr. Jason A. Efstathiou is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. Dr. Efstathiou reports consulting fees from Blue Earth Diagnostics, Boston Scientific, AstraZeneca, and Genentech. Dr. Efstathiou reports participation on a Data Safety Monitoring Board or Advisory Board at Merck, Roivant Pharma, Myovant Sciences, Janssen, Bayer Healthcare, Progenics Pharmaceuticals, Pfizer, Astellas, Gilead, Lantheus, Blue Earth Diagnostics, and Angiodynamics. Dr. Efstathiou reports board membership at Massachusetts Prostate Cancer Coalition (MPCC), American College of Radiation Oncology (ACRO), and Radiation Oncology Institute (ROI).
Isabella R. Pompa, David Qi, Anushka Ghosh, Saveli I. Goldberg, and Dr. Sophia C. Kamran have no conflicts of interest to report.
DATA AVAILABILITY
The data underlying this article are available via the publicly available Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research (CDC-WONDER) found here: https://wonder.cdc.gov/.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-230062.
REFERENCES
[1] | Cancer of the Urinary Bladder –Cancer Stat Facts [Internet]. SEER. [cited 2023 Jul 6]. Available from: https://seer.cancer.gov/statfacts/html/urinb.html |
[2] | Saginala K , Barsouk A , Aluru JS , Rawla P , Padala SA , Barsouk A Epidemiology of Bladder Cancer, Med Sci (Basel) [Internet]. (2020) ;8: (1). Available from: http://dx.doi.org/10.3390/medsci8010015 |
[3] | Cumberbatch MGK , Noon AP Epidemiology, aetiology and screening of bladder cancer, Transl Androl Urol. (2019) ;8: (1):5–11. |
[4] | Cárdenas-Turanzas M , Cooksley C , Pettaway CA , et al. Comparativeoutcomes of bladder cancer, Obstet Gynecol. (2006) ;108: :169–75. https://doi.org/10.1097/01.AOG.0000223885.25192.91. |
[5] | Mungan NA , Aben KK , Schoenberg MP , et al. Gender differences in stage-adjusted bladder cancer survival, Urology. (2000) ;55: :876–80. https://doi.org/10.1016/s0090-4295(00)00523-9. |
[6] | Scosyrev E , Noyes K , Feng C , Messing E Sex and racial differences in bladder cancer presentation and mortality in the US, Cancer. (2009) ;115: :68–74. https://doi.org/10.1002/cncr.23986 |
[7] | Ballas LK , Navarro S , Luo C , et al. Disparities in male versus female oncologic outcomes following bladder preservation: a population-based cohort study, Cancer Med. (2021) ;10: :3004–12. |
[8] | Kaye DR , Canner JK , Kates M , et al. Do African American patients treated with radical cystectomy for bladder cancer have worse overall survival? accounting for pathologic staging and patient demographics beyond race makes a difference, Bladder Cancer. (2016) ;2: :225–34. https://doi.org/10.3233/BLC-150041 |
[9] | Wang Y , Chang Q , Li Y Racial differences in urinary bladder cancer in the United States. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-29987-2 |
[10] | Shu TD , Schumacher FR , Conroy B , et al. Disparities in cause-specific mortality by race and sex among bladder cancer patients from the SEER database, Cancer Causes Control. (2023) ;34: :521–31. https://doi.org/10.1007/s10552-023-01679-x |
[11] | Schrag D , Hsieh LJ , Rabbani F , et al. Adherence to surveillance among patients with superficial bladder cancer, J Natl Cancer Inst. (2003) ;95: :588–97. https://doi.org/10.1093/jnci/95.8.588 |
[12] | Williams SB , Huo J , Kosarek CD , et al. Population-based assessment of racial/ethnic differences in utilization of radical cystectomy for patients diagnosed with bladder cancer, Cancer Causes Control. (2017) ;28: :755–66. https://doi.org/10.1007/s10552-017-0902-2 |
[13] | Islami F , Ward EM , Sung H , Cronin KA , Tangka FKL , Sherman RL , et al. Annual Report to the Nation on the Status of Cancer, Part National Cancer Statistics, J Natl Cancer Inst. (2021) ;113: (12):1648–69. |
[14] | CDC WONDER. Underlying Cause of Death [Internet]. [place unknown]: Centers for Disease Control and Prevention; [reviewed 2023 January 11; cited 2023 July 7]. Available from: https://wonder.cdc.gov/Deaths-by-Underlying-Cause.html |
[15] | NAACCR. North American Association of Central Cancer Registries [Internet]. [place unknown]; [reviewed 2023 September 25; cited 2023 October 9]. Available from: http://www.naaccr.org/ |
[16] | Division of Cancer Control and Population Sciences. Average Annual Percent Change (AAPC) and Confidence Interval [Internet]. [place unknown]: National Cancer Institute; [cited 2023 July 7]. Available from: https://surveillance.cancer.gov/help/joinpoint/settingparameters/method-and-parameters-tab/apc-aapc-tauconfidence-intervals/average-annual-percent-change-aapc |
[17] | Division of Cancer Control and Population Sciences. Joinpoint Trend Analysis Software [Internet]. [place unknown]: National Cancer Institute; [cited 2023 July 7]. Available from: https://surveillance.cancer.gov/joinpoint/ |
[18] | Holm S A simple sequentially rejective multiple test procedure, Scandinavian Journal of Statistics. (1979) ;6: (2):65–70. |
[19] | Siegel RL , Miller KD , Wagle NS , Jemal A Cancer statistics, CA Cancer J Clin. (2023) ;73: (1):17–48. |
[20] | Giovino GA Epidemiology of tobacco use in the United States, Oncogene. (2002) ;21: (48):7326–40. |
[21] | Thun MJ , Carter BD , Feskanich D , Freedman ND , Prentice R , Lopez AD , et al. 50-year trends in smoking-related mortality in the United States, N Engl J Med. (2013) ;368: (4):351–64. |
[22] | Shariat SF , Milowsky M , Droller MJ Bladder cancer in the elderly, Urologic Oncology: Seminars and Original Investigations. (2009) ;27: (6):653–67. |
[23] | Data and Statistics [Internet]. 2023 [cited 2023 Jul 7].Available from: https://www.cdc.gov/aging/data/index.htm |
[24] | Muss HB , Biganzoli L , Sargent DJ , Aapro M Adjuvant therapy in the elderly: making the right decision, J Clin Oncol. (2007) ;25: (14):1870–5. |
[25] | Guancial EA , Roussel B , Bergsma DP , Bylund KC , Sahasrabudhe D , Messing E , Mohile SG , Fung C Bladder cancer in the elderly patient: challenges and solutions, Clinical Interventions in Aging. (2015) ;10: :939–49. DOI: 10.2147/CIA.S74322. |
[26] | Nielsen ME , Shariat SF , Karakiewicz PI , Lotan Y , Rogers CG , Amiel GE , et al. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy, Eur Urol. (2007) ;51: (3):699–706; discussion 706-8. |
[27] | Joudi FN , Smith BJ , O’Donnell MA , Konety BR The impact of age on the response of patients with superficial bladder cancer to intravesical immunotherapy, J Urol. (2006) ;175: (5):1634–9; discussion 1639-40. |
[28] | Lortet-Tieulent J , Goding Sauer A , Siegel RL , Miller KD , Islami F , Fedewa SA , et al. State-Level Cancer Mortality Attributable to Cigarette Smoking in the United States, JAMA Intern Med. (2016) ;176: (12):1792–8. |
[29] | Smith ND , Prasad SM , Patel AR , Weiner AB , Pariser JJ , Razmaria A , et al. Bladder Cancer Mortality in the United States: A Geographic and Temporal Analysis of Socioeconomic and Environmental Factors, J Urol. (2016) ;195: (2):290–6. |
[30] | Deuker M , Stolzenbach LF , Collà Ruvolo C , Nocera L , Tian Z , Roos FC , et al. Bladder cancer stage and mortality: urban vs rural residency, Cancer Causes Control. (2021) ;32: (2):139–45. |




