The Impact of Dose Reduction of Bacillus Calmette–Guerin on Oncological Outcomes and Toxicity in Non-Muscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis
Abstract
BACKGROUND:
Bacillus Calmette–Guerin (BCG) is the standard adjuvant treatment for intermediate and high-risk non-muscle invasive bladder cancer (NMIBC) following transurethral resection of the bladder (TURB). However, the optimal dose, strain, and schedule of BCG remain unclear.
OBJECTIVE:
To evaluate the impact of BCG dose reduction on oncological outcomes and toxicity in patients with non-muscle invasive bladder cancer.
METHODS:
We performed a systematic review of the literature in PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases. Selected studies were analyzed for Meta Analysis using PRISMA criteria. The study focused on disease recurrence, progression, and toxicity. We also compared the oncological outcomes of the different BCG strains.
RESULTS:
A total of 2963 patients in 13 randomized controlled trials were included. In recurrence analysis, we found a non-significant difference between the full dose and any dose reduction of BCG (RR = 1.17, [1.06–1.28], I2 = 0%, p = 0.7). In terms of progression, the difference was also non-statistically significant (RR: 1.12 [0.89 - 1.41], I2 = 0%, p = 0.93). In the toxicity analysis, there were more local (RR: 0.81 [0.67–0.99] I2 = 76%; p < 0.01) and systemic (RR: 0.53 [0.34–0.82] I2 = 83%; p < 0.01) side effects in the full dose group than in the dose reduction group. There were no statistically significant differences in oncological outcomes between the analyzed BCG strains.
CONCLUSIONS:
Dose reduction did not affect the oncological outcomes of patients with NMIBC who received adjuvant therapy with BCG. On the other hand, dose reduction showed a significant trend towards fewer systemic and local side effects. Further studies comparing oncological and toxicity outcomes using different strains are needed.
BACKGROUND
Bladder cancer is the second most common malignancy of the urinary tract, with 549,393 new cases reported worldwide in 2018. Approximately 75% to 85% of patients present with non-muscle invasive bladder cancer (NMIBC) [1]. Most of these patients require adjuvant treatment following transurethral resection of the bladder (TURB). Since Morales et al. first reported on it in 1976, several clinical trials have confirmed the value of bacillus Calmette-Guerin (BCG) in treating NMIBC [2, 3]. BCG instillation is the standard therapy for intermediate-risk (IR) and high-risk (HR) NMIBC, and various studies have demonstrated its long-term efficacy in delaying recurrence and reducing the risk of progression, particularly when maintenance therapy is utilized [4].
Regarding treatment schedules, it is widely accepted that BCG instillations should be administered according to the maintenance scheme described by Lamm et al. in 2000 [5]. Despite this, multiple efforts have been made to explore shorter or less intensive regimens, driven by concerns about BCG toxicity and shortages, among other reasons. In 2020, Grimm et al. published the results of the NIMBUS trial, comparing a standard BCG instillation scheme (6 induction doses followed by 3 weekly doses at 3, 6, and 12 months, totaling fifteen instillations within a year) with a reduced frequency scheme consisting of induction at weeks 1, 2, and 6, followed by 2 weekly doses of maintenance at 3, 6, and 12 months (nine instillations) [6]. They concluded that the reduced frequency of BCG instillations was inferior to the standard treatment in terms of time-to-first-recurrence.
Another controversial aspect of BCG treatment is the use of different strains without direct comparison thus far. A prospective randomized trial with 142 patients found a significantly greater 5-year recurrence-free survival in patients treated with Connaught compared to those who received TICE BCG (p = 0.01) [7]. However, maintenance BCG was not administered in this trial, and the superiority of one strain over the other may have been mitigated by the use of maintenance BCG. The ongoing randomized phase III clinical trial S1602, comparing TICE versus Tokyo-172 strains, may provide answers to these questions, but results are pending [8].
Indeed, there is another element that makes it challenging to compare BCG treatments with the same or different strains [9]. A recent publication demonstrates that there is considerable variability in the amount of colony forming units (CFU) among vials of the same BCG strain. It is normal for BCG vials to contain different ranges of CFU, which means that the dose each patient receives can vary from one instillation to another. Since this variability can result in a wide range of dosage modifications, it seems reasonable to consider that reducing the dose should not significantly impact the oncological prognosis.
The main disadvantages of BCG treatment are its local and systemic side effects, which depend on the dose and number of instillations. Several studies have compared the effectiveness and toxicity of different doses of BCG using the same strain. Martinez Piñeiro et al. suggested that a one-third dose of intravesical BCG is as effective as the standard dose in high-risk NMIBC patients, with a significant decrease in the progression rate as well as in local and systemic side effects [10].
In other words, the optimal dose of BCG is currently unclear, and published trial results have not been able to influence current practice. The aim of this meta-analysis is to compare the oncological and toxicity outcomes of dose reduction versus standard dose in BCG treatment for NMIBC.
METHODS
Database search
A systematic literature search was conducted between April and May 2022 using the PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases. Various combinations of the following search terms were used in a free text protocol: “bladder cancer,” “NMIBC,” “BCG,” “low dose,” “recurrence,” “progression,” “toxicity,” “Bacillus Calmette-Guerin,” and “maintenance.” Filters were applied for language (English) and full-text availability.
Study selection
The study eligibility criteria were defined according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria [11]. Studies were considered eligible if they included patients with intermediate and high-risk NMIBC (according to EAU classification) who underwent TURB and received intravesical BCG treatment with any schedule, comparing outcomes between full dose and any dose reduction. Retrospective studies were excluded.
The titles of the articles were initially screened to determine their potential eligibility. After assessing the abstracts, full-text articles underwent a more comprehensive evaluation. Studies without primary data (e.g., reviews, commentaries, and letters) were excluded; however, relevant citations within these studies were examined for possible inclusion (Fig. 1).
Fig. 1
Study selection flow.
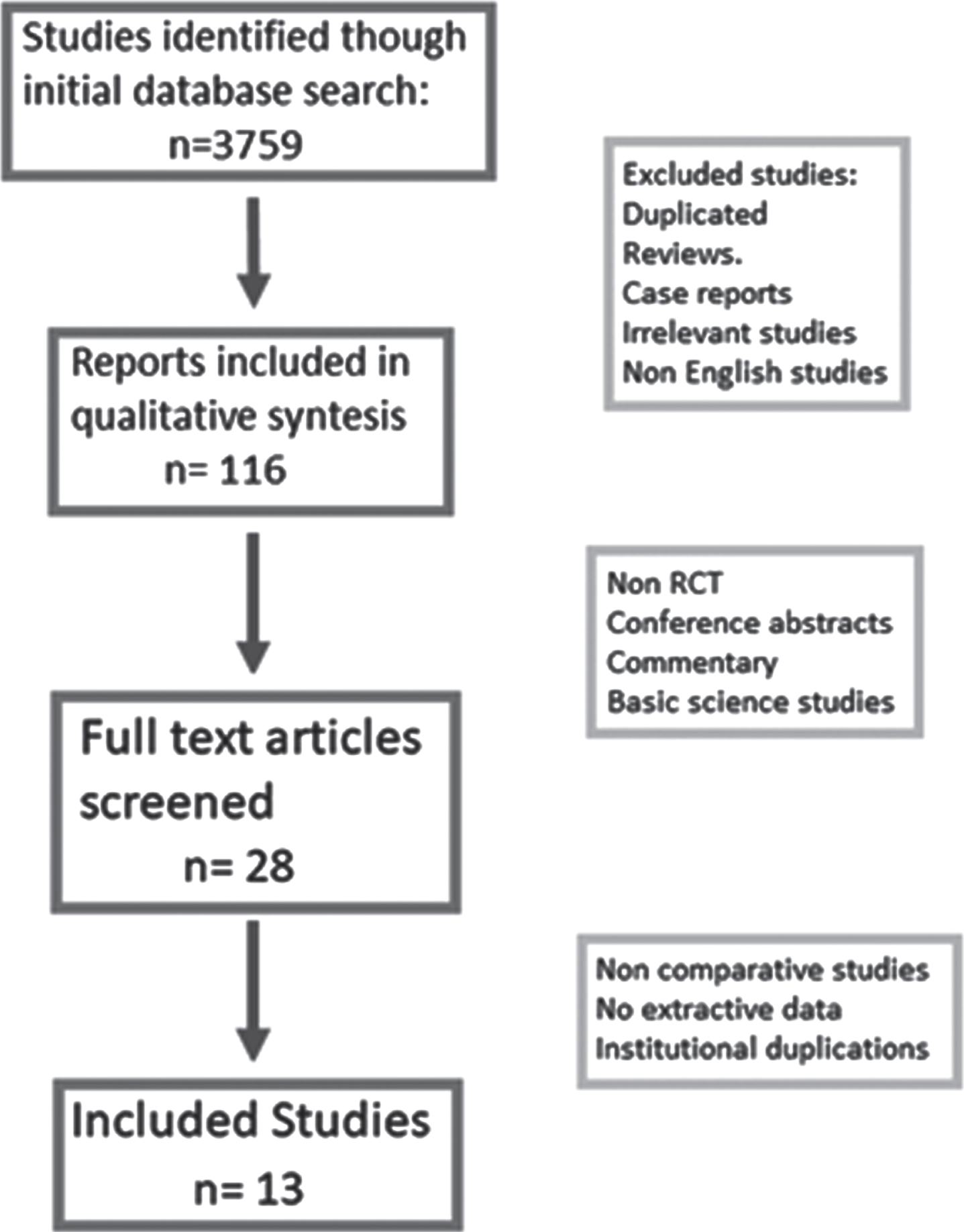
The patients included in the analysis underwent TURB and received intravesical BCG treatment. The control group received the full dose, while the experimental group received dose reduction according to various study protocols and authors’ preferences. All schemes of dose reduction were accepted since all the included studies compared the standard dose for the respective strain with a reduced dose.
Outcomes and definitions
The primary endpoint was treatment efficacy, measured by disease recurrence and/or progression. Tumor recurrence was defined as the first occurrence of recurrence after intravesical BCG treatment. Tumor progression was defined as progression to a higher stage than the initial stage or to muscle-invasive disease (T2). A subgroup analysis was performed to compare treatment efficacy among different BCG strains (see Supplemental Table 1).
The secondary endpoint was treatment toxicity. A subgroup analysis was conducted to describe the proportion of patients experiencing local adverse effects such as frequency, urgency, dysuria, and bladder pain, as well as systemic adverse events such as fever, sepsis, and liver toxicity.
Statistical analysis
For each selected study, the following information was recorded: first author’s name, year of publication, study design, country of origin, study period, patient characteristics (age and gender), tumor characteristics (stage, grade, size), BCG strain, treatment schedule, grade of progression (intermediate or high risk), reports of recurrent and progressed disease, and toxicity reports in the control and experimental groups.
The effects were presented as risk ratios (RR) with their corresponding 95% confidence intervals (95% CI). A random-effects model was used to pool the studies to account for between-study variability. Heterogeneity and inconsistency (i.e., differences between studies beyond chance) were assessed using Cochran’s Q test and the I2 statistic, respectively. Publication bias was explored using a funnel plot and formally assessed using Egger’s test. All statistical analyses were conducted using R version 4.0.1 (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The study selection process is illustrated in Fig. 1, and the funnel plot heterogeneity analysis of all included studies is presented in Fig. 2. After excluding 3746 studies, a total of 13 studies met the inclusion criteria and are listed in Table 1 [1, 10, 12–22]. Table 1 in the supplementary material provides further details on the included studies. The meta-analysis included 2963 patients with intermediate and high-risk NMIBC.
Fig. 2
Funnel plot showing heterogeneity between included studies.
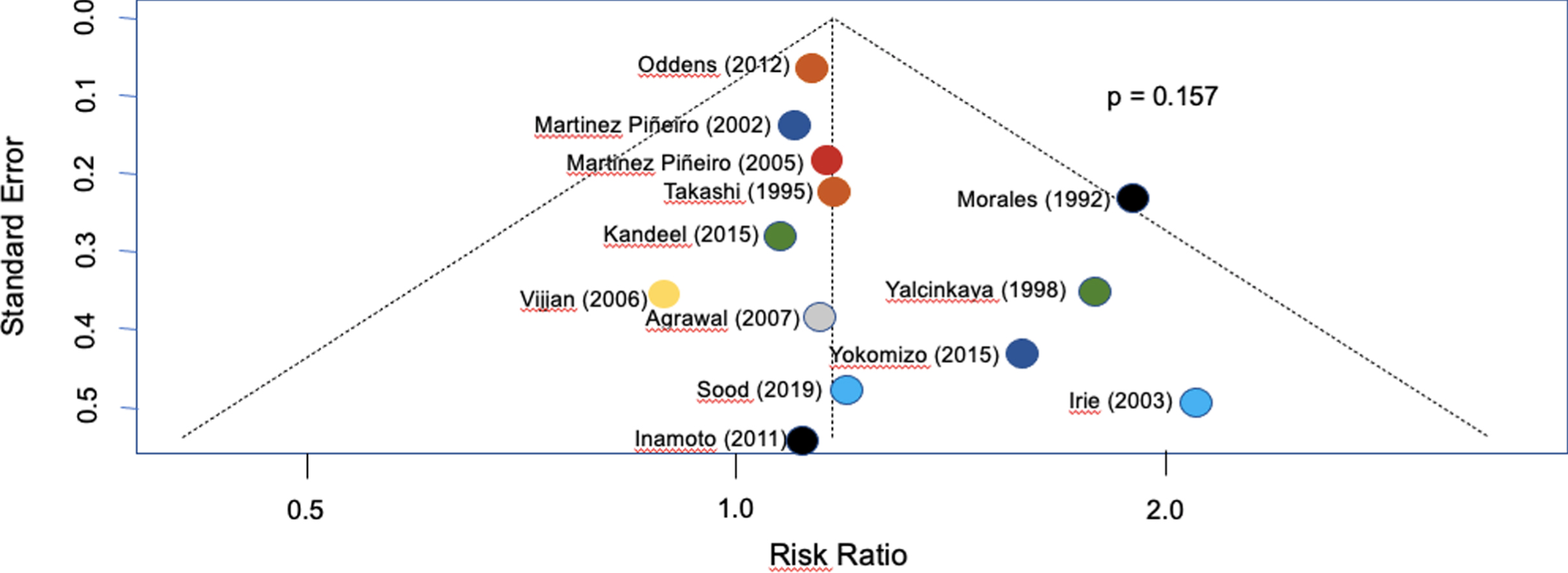
Table 1
Studies included in Meta-analysis
| Study | Institution | Year | Study Type | Arms | Inclusion Criteria | LE |
| Morales | Department of Urology, Queen’s University, Kingston, Ontario, Canada | 1992 | qRCT | 2 | NMIBC | 2b |
| Takashi | Nagoya University School of Medicine (Japan) | 1995 | RCT | 2 | NMIBC without CIS | |
| Yalcinkaya | Social Security Council, Ankara Hospital, Ankara. (Turkey) | 1998 | RCT | 2 | NMIBC | |
| Martinez-Pineiro | CUETO (Spain) | 2002 | RCT | 2 | NMIBC | 2b |
| Irie | Kitasato University school of Medicine | 2003 | qRCT | 2 | NMIBC | 2b |
| Martinez-Pineiro | CUETO (Spain) | 2005 | RCT | 2 | T1G3 and CIS NMIBC | 1b |
| Vijjan | Sanjay Gandhi Institute of Medical Sciences (India) | 2006 | RCT | 3 | NMIBC High risk | 2b |
| Agrawal | Medical College Agra (India) | 2007 | RCT | 3 | NMIBC without CIS | 2b |
| Inarnoto | Osaka Medical College (Japan) | 2011 | RCT | 2 | NMIBC | 2b |
| Oddens | EORTC-GU (Europe) | 2012 | RCT | 4 | Intermediate or high risk NMIBC | 1b |
| Kandeel | Urology Department, Benha Faculty of Medicine. Benha University. (Egypt) | 2015 | RCT | 2 | Intermediate risk NMIBC | |
| Yokomizo | Department of Urology, Graduate School of Medical Sciences, Kyushu University, Fukuoka. (Japan) | 2015 | RCT | 2 | NMIBC and/or CIS | |
| Sood | Dr Ram Manohar Lohia Hospital (India) | 2019 | RCT | 2 | NMIBC |
qRCT: quasi randomized controlled trial. RCT: randomized controlled trial. NMIBC: non muscle-Invasive bladder cancer. CIS: carcinoma In situ.
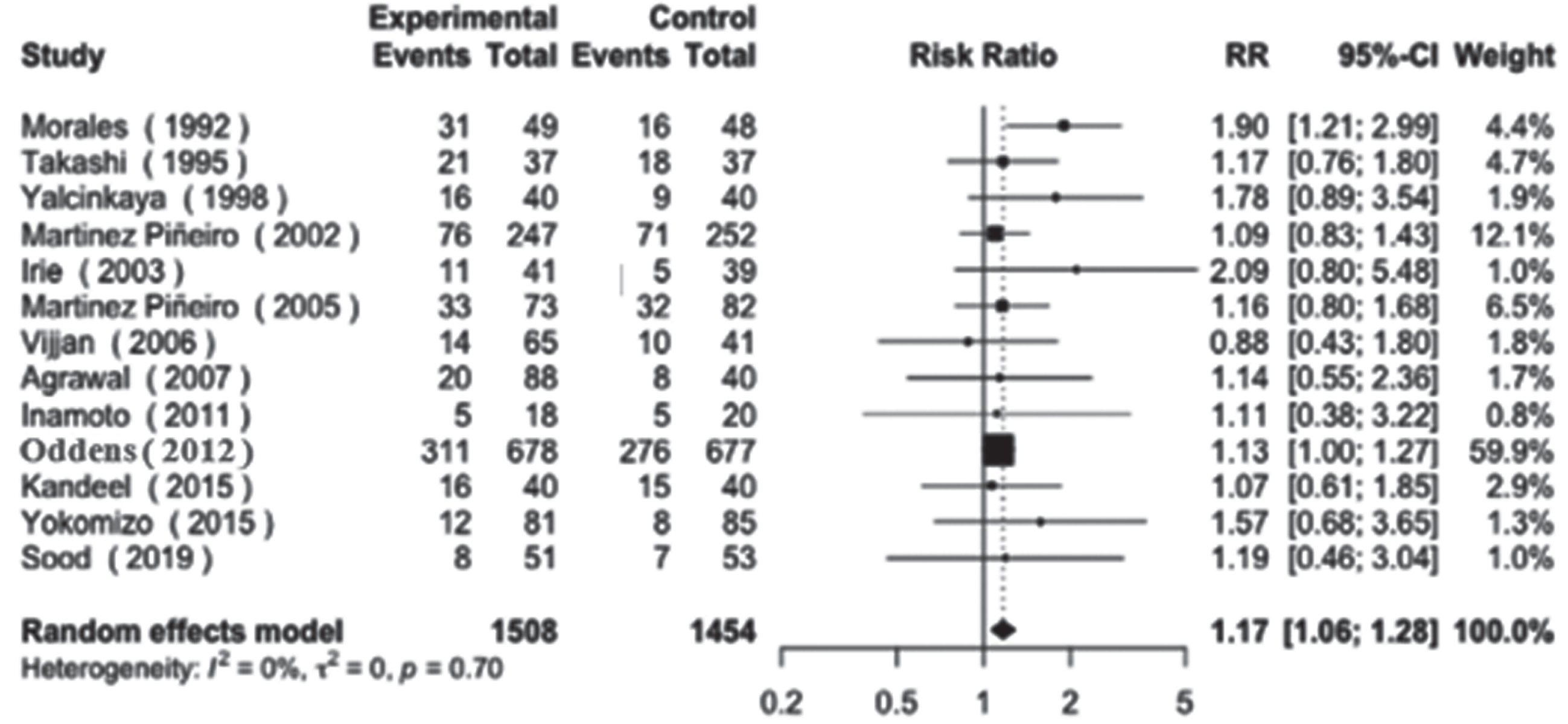
Regarding disease recurrence, our analysis revealed a non-statistically significant trend towards higher recurrence rates in the low-dose groups (RR = 1.17, 95% CI: [1.06–1.28], I2 = 0%, p = 0.7). This finding was consistent across both smaller and larger studies, as depicted in Fig. 3.
Fig. 3
Disease recurrence.
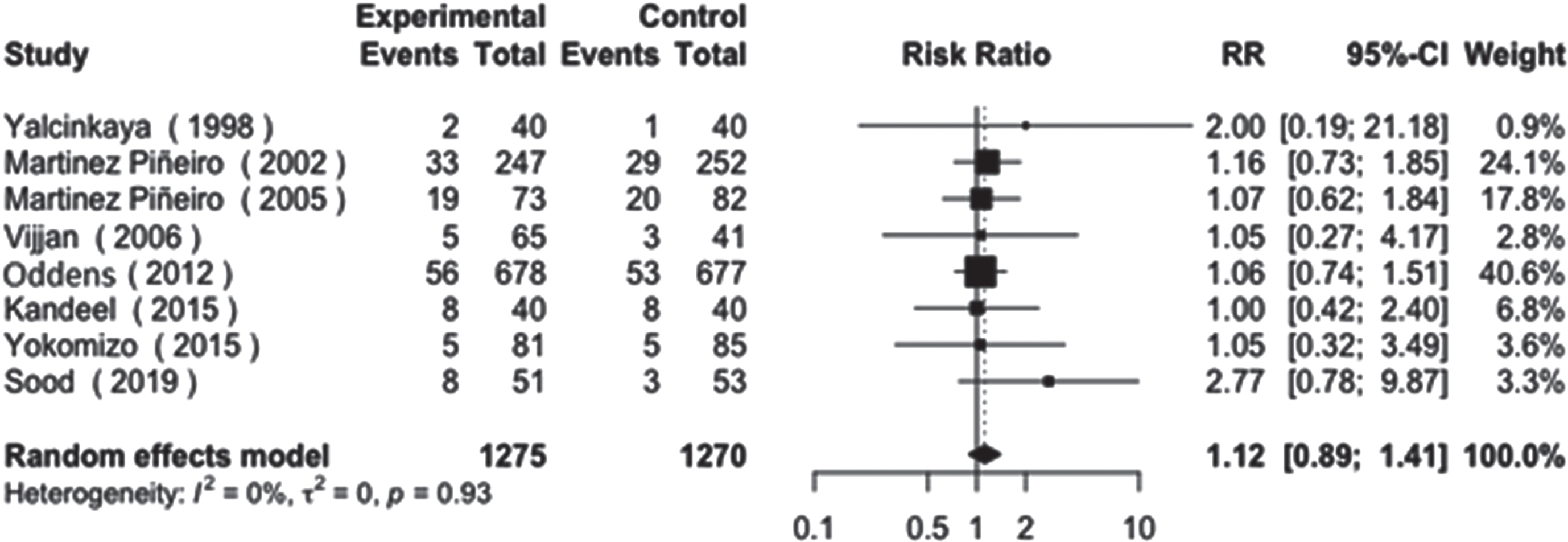
For disease progression, a total of 8 studies were analyzed. The difference between the control and experimental arms was also non-statistically significant, with a similar trend observed (RR: 1.12, 95% CI: [0.89 - 1.41], I2 = 0%, p = 0.93), as shown in Fig. 4.
Fig. 4
Disease progression.
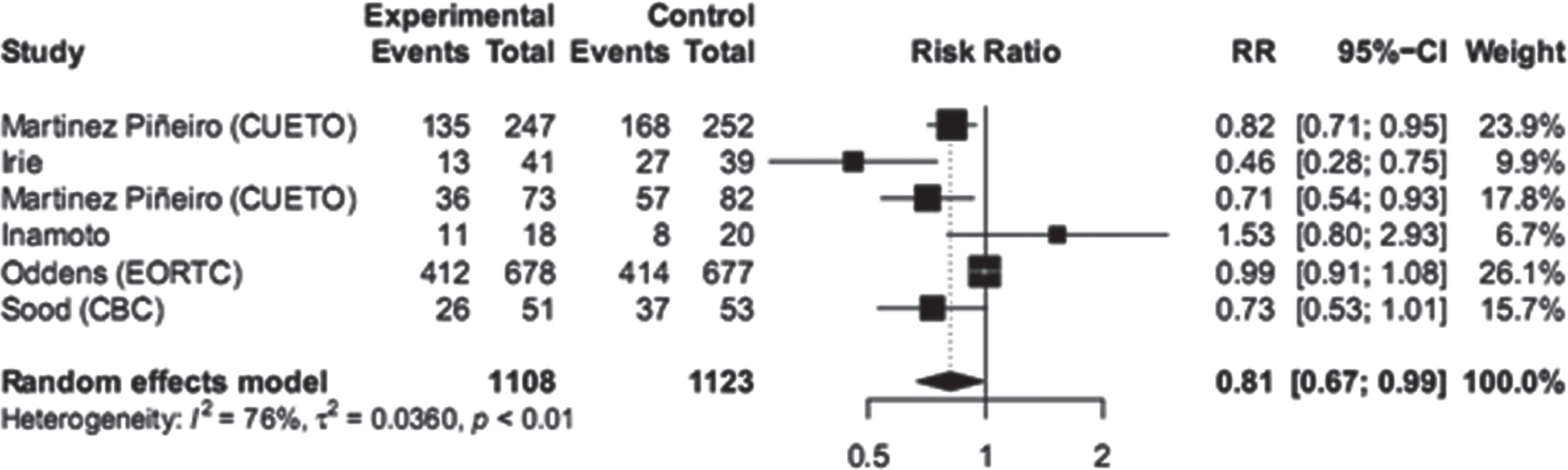
The analysis of toxicity in the dose reduction group showed a significantly lower number of events compared to the full dose group. Both systemic and local side effects were more frequent in the full dose group. The RR for systemic side effects was 0.53 with a CI of [0.34 - 0.82], and the RR for local side effects was 0.81 with a CI of [0.67 - 0.99]. These results indicate a lower risk of side effects in the dose reduction group compared to the full dose group (Figs. 5 and 6).
Fig. 5
Systemic toxicity.
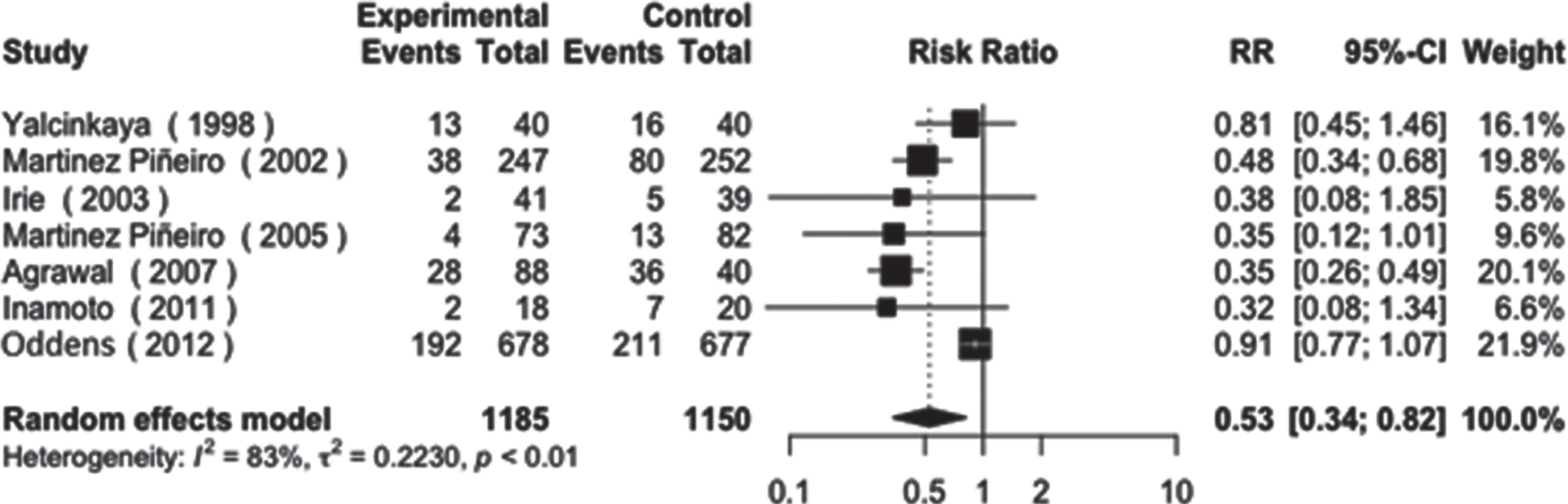
Fig. 6
Local toxicity.
The studies included in the analysis were organized based on the strain of BCG used. Group 3 consisted of 1355 patients from the EORTC study using the TICE strain. Group 2 included 234 patients with the Danish 1331 strain. Group 1 consisted of 735 patients from three studies using the Connaught strain. Lastly, group 0 included 521 patients from multiple studies using different strains (Armand Frapier, Moscow, Vacera, and Tokyo).
When analyzing recurrence rates, only Group 0 showed a tendency towards better results in the full dose arm, but the difference was not statistically significant. The other groups did not show statistically significant differences between the experimental and control arms (Figure 1, supplementary material). There were no significant differences observed between the different BCG strains in terms of progression (Figure 2, supplementary material).
DISCUSSION
The present study aimed to compare oncologic and toxicity outcomes of standard dose BCG and any dose reduction in intermediate and high-risk NMIBC. Statistical analysis showed significant heterogeneity between studies due to various factors such as different classifications of NMIBC, strains of BCG, doses, and schedules of instillations used. Despite the heterogeneity, the analysis sought to identify similarities among the studies.
The CUETO study 90.008 was the first Spanish study to compare the efficacy and toxicity of intravesical BCG (Connaught strain) at full dose (81 mg) or reduced dose (
Two subsequent studies by the same group, CUETO 95.011 and CUETO 95.012, further investigated the efficacy and toxicity of reduced doses of BCG [23]. CUETO 95.011 focused on intermediate-risk patients and compared BCG 27 mg (
The CUETO 95.012 study compared the standard 81 mg dose of BCG (full dose) with a reduced dose of 27 mg (
The EORTC 30962 study was conducted to assess whether the toxicity of BCG maintenance therapy could be decreased without compromising its efficacy [1]. The study was designed as a non-inferiority 2x2 factorial clinical trial, with two planned comparisons for each of the two null hypotheses.
The first null hypothesis compared the efficacy of
However, the study did not reach the prespecified decrease of 10% in the 5-year disease-free rate in any of the primary and secondary objectives or in the preplanned stratification. Only a non-planned stratification comparing
The study’s most relevant conclusions regarding dose reduction were derived from non-planned stratifications, and the results regarding the percentage of events recorded in the different groups were debatable. The data on toxicity showed no difference between reduced dose BCG and full dose BCG. Brausi and Oddens published data on local and systemic toxicity in 1316 patients from the same EORTC 30962 database who received
Several single-arm studies evaluated dose reduction with BCG Pasteur strain or Connaught strain and showed similar results compared to other series at that time [25–32]. These studies also highlighted the superior effect of full dose BCG in patients with multiple risk factors, such as multifocal tumors and higher recurrence rates, suggesting the potential for risk-adapted dosing.
A meta-analysis published in 2017 evaluated the efficacy of different BCG strains compared to other intravesical therapies [33]. It found that BCG Tokyo-172 was associated with significantly better recurrence-free survival compared to all other BCG strains, while the Connaught strain showed a non-significant increase in disease recurrence compared to Pasteur, TICE, and Tokyo-172 strains. The analysis concluded that BCG was superior to chemotherapy in preventing recurrence, but no superiority was found among different BCG strains.
Another BCG dose reduction meta-analysis was published in 2023 [34]. The authors found that dose reduction did not affect progression, metastasis nor survival. Although the recurrence results initially appeared better in the full-dose group, these findings were disregarded after excluding studies that had only provided BCG induction but no maintenance. These findings align with those published by Malmstrom et al. in 2009 and Chou et al. in 2015, which showed that BCG treatment results improve significantly with maintenance [35, 36]. The authors also suggest the need to evaluate the results of BCG dose reduction by comparing different strains of BCG.
In the present study, when analyzing recurrence stratified by strains, only group 0 showed a slightly higher recurrence rate in the dose reduction arm, but the difference was not statistically significant. These trials (Morales and Irie) had small sample sizes (97 and 80 patients), which may require a larger number of events to detect significant differences. In Morales’ study, there was no statistical difference in recurrence in the 1/2 dose arm of Tokyo BCG in patients with Ta or T1 disease, but a significant difference was observed in patients with CIS+Ta or CIS disease. High-risk patients represented a significant proportion of the study population. The results of recurrence for other strains were similar and not statistically significant.
Based on the current evidence, it is not possible to determine the optimal BCG dose for intermediate and high-risk NMIBC, nor can we determine if any BCG strain is superior in terms of efficacy and toxicity. Prospective, randomized studies such as S1602 are needed to provide more conclusive answers to these questions [8]. S1602 is a three-arm trial comparing different BCG strains and instillation strategies, and its results are still pending.
The study presents certain limitations that are important to highlight. Firstly, the heterogeneity of the included studies, partly due to the wide range of publication dates, different definitions used to classify patient risk, varying BCG administration schemes, differences between strains, and treatment durations. However, it is crucial to acknowledge that this also constitutes a strength of this study. This heterogeneity makes the results more similar to real-life scenarios found worldwide. As for strengths, we must emphasize the multiplicity of studies included from various regions across the globe, the diversity of strains examined, and the meta-analysis comparing recurrence and progression among different strains, which contributes to the originality of this research.
CONCLUSION
In conclusion, BCG dose reduction did not affect the oncological outcomes of NMIBC patients, even when comparing different BCG strains. While there was a tendency towards lower recurrence and progression rates in the full dose groups, these differences were not statistically significant. Dose reduction was associated with fewer systemic and local side effects. Further randomized controlled trials using standardized schedules and follow-up are necessary to confirm whether BCG dose reduction can reduce toxicity without compromising oncological outcomes. BCG dose reduction may be a useful strategy in cases of BCG shortage or in patients with BCG toxicity.
ACKNOWLEDGMENTS
The authors wish to thank Dr Javier Mariani MD, for his support in the statistical analysis.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Dr. Azuri: performance of work; interpretation of data; writing the article.
Dr. Jaunarena: conception; performance of work; interpretation of data; writing the article.
Dr. Camean: conception; interpretation of data.
Dr. Chemi: conception; performance of work.
Dr. Villaronga: conception; interpretation of data.
Dr. Daneshmand: conception; interpretation of data.
Dr. Villoldo: conception; interpretation of data; writing the article.
CONFLICTS OF INTEREST
Dr. Azuri has no conflicts of interest to report.
Dr. Jaunarena has no conflicts of interest to report.
Dr. Camean has no conflicts of interest to report.
Dr. Chemi has no conflicts of interest to report.
Dr. Villaronga has no conflicts of interest to report.
Dr. Daneshmand is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. Apart from that, has no conflicts of interest to report.
Dr. Villoldo has no conflicts of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and/or its supplementary material.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-230044.
REFERENCES
[1] | Oddens J , Brausi M , Sylvester R , Bono A , van de Beek C , van Andel G , et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. (2013) ;63: (3):462–72. |
[2] | Morales A , Eidinger D , Bruce AW . Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. (2002) ;167: (2 Pt 2):891–3; discussion 893–5. |
[3] | Morales A , Eidinger D , Bruce AW . Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J Urol. (2017) ;197: (2S):S142–5. |
[4] | Kapoor R , Vijjan V , Singh P . Bacillus Calmette-Guerin in the management of superficial bladder cancer. Indian J Urol. (2008) ;24: (1):72–6. |
[5] | Lamm DL , Blumenstein BA , Crissman JD , Montie JE , Gottesman JE , Lowe BA , et al. Maintenance bacillus Calmette-Guerin immuno therapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. (2000) ;163: (4):1124–9. |
[6] | Grimm MO , van der Heijden AG , Colombel M , Muilwijk T , Martínez-Piñeiro L , Babjuk MM , et al. Treatment ofHigh-grade Non-muscle-invasive Bladder Carcinoma by Standard Numberand Dose of BCG Instillations Versus Reduced Number and StandardDose of BCG Instillations: Results of the European Association ofUrology Research Foundation Randomised Phase III Clinical Trial“NIMBUS. ” Eur Urol. (2020) ;78: (5):690–8. |
[7] | Rentsch CA , Birkhäuser FD , Biot C , Gsponer JR , Bisiaux A , Wetterauer C , et al. Bacillus Calmette-Guérin strain difference shave an impact on clinical outcome in bladder cancer immuno therapy. Eur Urol. (2014) ;66: (4):677–88. |
[8] | Svatek RS , Tangen C , Delacroix S , Lowrance W , Lerner SP . Background and Update for S“A Phase III Randomized Trial to Evaluate the Influence of BCG Strain Differences and T Cell Priming with Intradermal BCG Before Intravesical Therapy for BCG-naïve High-grade Non-muscle-invasive Bladder Cancer. Eur Urol Focus. (2018) ;4: (4):522–4. |
[9] | Kamat AM , Lobo N , Lerner SP , Li R , Matulay JT , Palou J , et al. Reduced dose intravesical Bacillus Calmette-Guérin: Why it might not matter. Bladder Cancer. (2022) ;8: (2):113–7. |
[10] | Martínez-Piņeiro JA , Martínez-Piņeiro L , Solsona E , Rodríguez RH , Gómez JMF , Martín MG , et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumorsthan the standard dose? Results of a prospective randomized trial. J Urol. (2005) ;174: (4):1242–7. |
[11] | Moher D , Liberati A , Tetzlaff J , Altman DG . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. (2010) ;8: (5):336–41. |
[12] | Morales A , Nickel JC , Wilson JW . Dose-response of bacillus Calmette-Guerin in the treatment of superficial bladder cancer. J Urol. (1992) ;147: (5):1256–8. |
[13] | Takashi M , Wakai K , Ohno Y , Murase T , Miyake K . Evaluation of alow-dose intravesical bacillus Calmette-Guérin (Tokyo strain) therapy for superficial bladder cancer. Int Urol Nephrol. (1995) ;27: (6):723–33. |
[14] | Yalçinkaya F , Kamiş L , Ozteke O , Günlüsoy B , Yigitbaşi O , Unal S . Prospective randomized comparison ofintravesical BCG therapy with standard dose versus low doses insuperficial bladder cancer. Int Urol Nephrol. (1998) ;30: (1):41–4. |
[15] | Martínez-Piñeiro JA , Flores N , Isorna S , Solsona E , Sebastián JL , Pertusa C , et al. Long-term follow-up of a randomized prospective trial comparing a standard 81mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27mg in superficial bladder cancer. BJU Int. (2002) ;89: (7):671–80. |
[16] | Irie A , Uchida T , Yamashita H , Matsumoto K , Satoh T , Koh H , et al. Sufficient prophylactic efficacy with minor adverse effects by intravesical instillation of low-dose bacillus Calmette-Guérinfor superficial bladder cancer recurrence. Int J Urol. (2003) ;10: (4):183–9. |
[17] | Vijjan V , Mandhani A , Kapoor R , Dubey D , Srivastava A , Ansari MS , et al. A randomized trial comparing low dose (40 or 80mg) with standard dose (120mg) of bacillus Calmette-Guerin for superficial bladder cancer. Indian J Urol. (2006) ;22: (4):317. |
[18] | Agrawal MS , Agrawal M , Bansal S , Agarwal M , Lavania P , Goyal J . The safety and efficacy of different doses of bacillus CalmetteGuérin in superficial bladder transitional cell carcinoma. Urology. (2007) ;70: (6):1075–8. |
[19] | Inamoto T , Ubai T , Nishida T , Fujisue Y , Katsuoka Y , Azuma H . Comparable effect with minimal morbidity of low-dose Tokyo 172 strain compared with regular dose Connaught strain as an intravesical bacillus Calmette-Guérin prophylaxis in non muscleinvasive bladder cancer: Results of a randomized prospective comparison. Urol Ann. (2013) ;5: (1):7–12. |
[20] | Kandeel W , Abdelal A , Elmohamady BN , Sebaey A , Elshaaer W , ElbarkyE , et al. A comparative study between full-dose and half-dose intravesical immune bacille Calmette-Guérin injection in the management of superficial bladder cancer. Arab Journal of Urology. (2015) ;13: (4):233–7. |
[21] | Yokomizo A , Kanimoto Y , Okamura T , Ozono S , Koga H , Iwamura M , et al. Randomized Controlled Study of the Efficacy, Safety and Quality of Life with Low Dose bacillus Calmette-Guérin Instillation Therapy for Nonmuscle Invasive Bladder Cancer. J Urol. (2016) ;195: (1):41–6. |
[22] | Sood R , Sharma H , Sharma B , Parekh S , Pujari P , Shewale S . A prospective comparative study to assess the efficacy and tolerability of 2 different doses of intravesical bacillus Calmette-Guerin (BCG) in patients with non-muscle-invasive bladder cancer. Urol Oncol. (2020) ;38: (5):433–9. |
[23] | Ojea A , Nogueira JL , Solsona E , Flores N , Gómez JMF , Molina JR , et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27mg) versus very low-dose bacillus Calmette-Guerin (13.5mg) versus mitomycin C. Eur Urol. (2007) ;52: (5):1398–406. |
[24] | Brausi M , Oddens J , Sylvester R , Bono A , van de Beek C , van Andel G , et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. (2014) ;65: (1):69–76. |
[25] | Mack D , Frick J . Five-year results of a phase II study with low-dose bacille Calmette-Guerin therapy in high-risk superficial bladder cancer. Urology. (1995) ;45: (6):958–61. |
[26] | Lebret T , Gaudez F , Hervé JM , Barré P , Lugagne PM , Botto H . Low-dose BCG instillations in the treatment of stage T1 grade 3 bladder tumours: recurrence, progression and success. Eur Urol. (1998) ;34: (1):67–72. |
[27] | Lebret T , Bohin D , Kassardjian Z , Herve JM , Molinie V , Barre P , et al. Recurrence, progression and success in stage Ta grade 3 bladder tumors treated with low dose bacillus Calmette-Guerin instillations. J Urol. (2000) ;163: (1):63–7. |
[28] | Hurle R , Losa A , Ranieri A , Graziotti P , Lembo A . Low dose Pasteur bacillus Calmette-Guerin regimen in stage T1, grade 3 bladder cancer therapy. J Urol. (1996) ;156: (5):1602–5. |
[29] | Losa A , Hurle R , Lembo A . Low dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: long-term results. J Urol. (2000) ;163: (1):68–71; discussion 71–2. |
[30] | Mack D , Frick J . Low-dose bacille Calmette-Guérin (BCG) therapyin superficial high-risk bladder cancer: a phase II study with the BCG strain Connaught Canada. Br J Urol. (1995) ;75: (2):185–7. |
[31] | Mack D , Höltl W , Bassi P , Brausi M , Ferrari P , de Balincourt C , et al. The ablative effect of quarter dose bacillus Calmette-Guerin on a papillary marker lesion of the bladder. J Urol. (2001) ;165: (2):401–3. |
[32] | Pfister C , Kerkeni W , Rigaud J , Le Gal S , Saint F , Colombel M , et al. Efficacy and tolerance of one-third full dose bacillus Calmette-Guérin maintenance therapy every 3 months or 6 months: two-year results of URO-BCG-4 multicenter study. Int J Urol. (2015) ;22: (1):53–60. |
[33] | Boehm BE , Cornell JE , Wang H , Mukherjee N , Oppenheimer JS , Svatek RS . Efficacy of bacillus Calmette-Guérin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. J Urol. (2017) ;198: (3):503–10. |
[34] | Verri P , Baboudjian M , Diana P , Gallioli A , Territo A , Gaya JM , et al. Reduced- vs full-dose BCG in bladder cancer: A systematic review and meta-analysis. Actas Urol Esp. (2023) ;47: (1):4–14. |
[35] | Malmström PU , Sylvester RJ , Crawford DE , Friedrich M , Krege S , Rintala E , et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. (2009) ;56: (2):247–56. |
[36] | Chou R , Buckley D , Fu R , Gore JL , Gustafson K , Griffin J , et al. Emerging Approaches to Diagnosis and Treatment of Non-Muscle-Invasive Bladder Cancer. Rockville (MD): Agency for Healthcare Research and Quality (US); (2015) Oct. Report No.: 15(16)-EHC017-EF. |




