A Multicenter Study of 2-year Outcomes Following Hyperthermia Therapy with Mitomycin C in Treating Non-Muscle Invasive Bladder Cancer: HIVEC-E
Abstract
INTRODUCTION:
High grade, non-muscle invasive bladder cancer (NMIBC) is usually treated with intravesical Bacillus Calmette–Guérin. Chemohyperthermia therapy (CHT) may be a novel alternative therapy for the treatment of NMIBC.
OBJECTIVE:
To evaluate the recurrence-free survival (RFS) of patients treated with CHT using the Combat bladder recirculation system (BRS) for NMIBC.
METHODS:
This was a prospective multi-institutional study of 1,028 consecutive patients with NMIBC undergoing CHT between 2012 and 2020. A total of 835 patients were treated with CHT with Mitomycin C (MMC). Disease was confirmed on transurethral resection of bladder tumor (TURBT) prior to starting CHT. Follow-up included cystoscopy and subsequent TURBT if recurrence/progression was suspected. The primary endpoint was RFS. Secondary endpoints were progression-free survival (PFS) and adverse events from CHT.
RESULTS AND LIMITATIONS:
Median follow up was 22.4 months (Interquartile range (IQR): 12.8 –35.8). Median age was 70.4 years (IQR: 62.1 –78.6). A total of 557 (66.7%), 172 (20.6) and 74 (8.9%) of patients were classified to BCG naïve, BCG unresponsive and BCG failure, respectively. The RFS at 12 months and 24 months for BCG naïve was 87.6% (95% CI 85.0% - 90.4%) and 75.0% (95% CI 71.3% - 78.8%), respectively. The RFS at 12 months and 24 months for BCG unresponsive cohort was 78.1% (95% CI 72.0% - 84.7%) and 57.4% (95% CI 49.7% - 66.3%), respectively. The RFS at 24 months for the BCG unresponsive cohort for CIS with/without papillary disease and papillary only disease were 43.6% (95% CI 31.4% –60.4%) and 64.5% (95% CI 55.4% - 75.1%), respectively. Minor adverse events occurred in 216 (25.6%) patients and severe events occurred in 17 (2.0%) patients.
CONCLUSIONS:
CHT with MMC using the Combat BRS is effective in the medium term and has a favorable adverse event profile.
INTRODUCTION
Non-muscle invasive bladder cancer (NMIBC) represents 75% of bladder cancer. Adjuvant treatment with an induction course of intravesical therapy (with or without maintenance) is the current standard of care for intermediate and high risk NMIBC [3]. High risk NMIBC is typically treated with adjuvant intravesical chemotherapy or bacillus Calmette-Guerin (BCG) [1, 2]. There is currently a worldwide shortage of BCG and this shortage is unlikely to resolve in the near future. Hence, there is a clear clinical need for novel and effective therapies to manage NMIBC, with the aim of reducing tumor recurrences, treatment cost and requirement for radical cystectomy, while simultaneously improving patient life expectancy and quality-of-life.
Hyperthermia therapy for the treatment of NMIBC is a promising therapy. Hyperthermia therapy improves drug delivery to cancer cells, kills malignant urothelial cells directly, improve cancer cell sensitivity to therapeutic agents and radiotherapy, and trigger anti-cancer immune responses [4–7]. Three methods of clinically heating the bladder currently exist [8]: (1) deep regional hyperthermia administered via an external array of tunable radiofrequency antennae (e.g. BSD-2000 device), (2) intravesical radiofrequency antennae (e.g. Synergo SB-TS 101.1 device), and (3) intravesical circulating fluid convection heating (e.g. Combat Bladder Recirculation System [BRS] device). Many of these devices are large, expensive and somewhat cumbersome. The Combat BRS system is the cheapest available platform in the market that allows hyperthermia therapy to be administered, using conductive heating to deliver hyperthermia and intravesical chemotherapy into the bladder via a closed loop system [9]. The device is small, portable and very simple to operate, it does not require supervision during operation.
In this multi-institutional study, we aim to evaluate the medium-term cancer control outcomes and adverse event rate of patients treated with chemohyperthermia (CHT) therapy with Mitomycin C (MMC).
PATIENTS AND METHODS
Cohort
This is an IRB exempted study and informed consent was not obtained as this study was conducted as part of a prospective registry to audit treatment outcomes. Clinical information obtained was recorded by the investigator in such a manner that the identity of the human subjects cannot readily be ascertained directly or through identifiers linked to the subjects. We reviewed the Hyperthermic Chemotherapy registry (HIVEC-E) to identify patients who were treated with CHT Therapy between 2012 and 2020 for clinically localized, histologically confirmed bladder cancer. CHT using the Combat BRS was offered to patients diagnosed with localized NMIBC of grade 1 –3, with/ without isolated Carcinoma in situ (CIS) and in the primary and recurrent setting. Patients were classified into European Association of Urology (EAU) risk category low, intermediate and high-risk groups [10]. We excluded patients treated with any other chemotherapy agent other than MMC and patients with less than 90 days of follow up.
Disease was localized using cystoscopy and confirmed on transurethral resection of bladder tumor (TURBT). Patients who underwent CHT therapy received four to six once-weekly instillations of 40 mg MMC in 40 ml of sterile water as part of induction treatment for 60 minutes depending on standard practice of the local institution. Depending on the practice pattern around Europe, some patients were offered maintenance therapy with three weekly sessions of CHT therapy at month three and six, followed by every six months, after induction therapy. MMC was heated to 43°C using an aluminum heat exchanger that enables efficient heat transfer and accurate temperature control within±0.5 °C (Supplementary Figure 1). [11, 12] The detailed protocol is listed in Appendix 1.
Aim
Primary outcomes were recurrence free survival (RFS) of patients with BCG naïve and BCG unresponsive disease. Secondary outcomes were complete response rate at 3 and 6 months, progression-free survival (PFS), and adverse event from CHT using the common terminology criteria for adverse events (CTCAE). RFS was defined by absence of disease on cystoscopic and radiographic evaluation or death/mortality. PFS was defined as the absence of disease progression from NMIBC to muscle invasive bladder cancer, evidence of metastasis on radiographic evaluation or death/mortality from bladder cancer.
Clinical and demographic data
Variables of interest were patient age, gender (Male/Female), smoking history (non-smoker, ex-smoker, current smoker), primary or recurrent disease, history of MMC use (yes, no), history of BCG use (yes, no), BCG unresponsive (yes, no), BCG Refractory/Relapsing/Intolerant (yes, no), date of TURBT prior to CHT (DD/MM/YY), pT stage (CIS, pTa, pT1), grade (G1, G2, G3), number of tumors (solitary tumor, 2-7 tumors, >8), size of tumor (<3 cm, ≥3 cm), concurrent CIS (yes, no), second TURBT (yes, no), tumor on repeat TURBT (yes, no), number of induction instillation with CHT (1 –6), number of maintenance instillation (1 –12), adverse event to CHT (No adverse events, Grade 1-2, Grade 3), consequences of adverse events (no consequences, short term duration of instillation, discontinuation of CHT), status of bladder on cystoscopy following CHT (disease free, bladder tumor recurrence, progression to T2, death, others), date of last follow up, cancer stage if recurrence found on cystoscopy following CHT (CIS, pTa, pT1,≥pT2), cancer grade if recurrence found following CHT (G1, G2, G3) and concurrent CIS if recurrence found following CHT (yes, no).
BCG unresponsive disease was defined by persistent or recurrent CIS alone or with recurrent Ta/T1 (noninvasive papillary disease/tumor invades the subepithelial connective tissue) disease within 12 months of completion of adequate BCG therapy, recurrent high-grade Ta/T1 disease within 6 months of completion of adequate BCG therapy or T1 high-grade disease at the first evaluation following an induction BCG course as defined by the FDA [13]. BCG failure was defined as BCG refractory, BCG relapsing or BCG intolerant disease as defined by Kamat et al. [14].
The Combat Medical HIVEC-E registry is a retrospectively collected prospective online database for academic hospitals and community urologists to aggregate real-world CHT data using the Combat Medical system. The registry is supervised by board members comprising of practicing urologists. Data collection was performed by each enrolling institution. All participating sites had approval from their institutional review board if required before submitting their data to the registry.
Statistical analysis
Data is presented as medians, 1st and 3rd quartile, and counts or frequencies with percentages or proportions. Categorical variables were assessed by nonparametric methods using the chi-square test. Survival analysis was depicted using Kaplan-Meier plots. A multi-variable cox proportionate regression model was used to determine variables affecting the likelihood of patient having recurrence and progression following CHT. Variables were included in the multi-variable model if they were statistically significant on univariate analysis. Statistical significance was defined as p < 0.05. Missing data was omitted for the analysis as missing rate of 5% or less is inconsequential [15]. R 3.5.1 for Rstudio 1.1.456 was used for statistical analyses, with the key packages dplyr, ggplot2, reshape2, survivor and VIM installed [16].
RESULTS
Baseline characteristics
A total of 1,028 patients with NMIBC were treated with the Combat BRS device, of whom 835 patients received MMC and reached at least 3-months follow up. Median follow up was 22.4 months (IQR: 12.8 –35.8). Median age was 70.4 years (IQR: 62.1 –78.6). Baseline patient characteristics are listed in Table 1.
Table 1
Demographics
| Variable | BCG Naïve | BCG Unresponsive | BCG Failure | Unknown |
| Age, median (IQR) | 70.6 (62.8 –78.7) | 68.9 (59.8 –76.24) | 72.6 (61.5 –80.2) | |
| Gender, n (%) | ||||
| Male | 450 (66.5) | 139 (20.5) | 62 (9.2) | 26 (3.8) |
| Female | 107 (67.7) | 33 (20.9) | 12 (7.6) | 6 (3.8) |
| Missing Data | 0 | 0 | 0 | 0 |
| Smoking History, n (%) | ||||
| Non-smoker | 114 (63.7) | 40 (22.3) | 10 (5.6) | 6 (3.4) |
| Ex-smoker | 283 (64.8) | 92 (21.1) | 48 (11.0) | 14 (3.2) |
| Current smoker | 134 (71.7) | 35 (18.7) | 10 (5.3) | 8 (4.3) |
| Missing data | 26 | 5 | 6 | 4 |
| Tumor, n (%) | ||||
| Primary | 336 (100) | 0 | 0 | 0 |
| Recurrence≤1/year | 141 (49.5) | 83 (29.1) | 48 (16.8) | 13 (4.6) |
| Recurrence > 1/year | 80 (41.2) | 75 (38.7) | 25 (12.9) | 14 (7.2) |
| Missing data | 0 | 14 | 1 | 5 |
| cT stage, n (%) | ||||
| CIS | 7 (13.7) | 27 (52.9) | 15 (29.4) | 2 (3.9) |
| Ta | 415 (76.4) | 68 (12.5) | 38 (7.0) | 22 (4.1) |
| T1 | 126 (55.0) | 77 (33.6) | 21 (9.2) | 5 (2.2) |
| Missing Data | 11 | 0 | 0 | 2 |
| Grade, n (%) | ||||
| Low | 186 (84.9) | 17 (7.8) | 10 (4.6) | 6 (2.7) |
| High | 358 (62.7) | 136 (23.8) | 53 (9.3) | 24 (4.2) |
| Missing Data | 13 | 19 | 11 | 2 |
Values are rounded to the nearest whole number and may exceed or be under 100%. BCG: Bacillus Calmette Guerin; IQR: Interquartile range; cT stage: Clinical T stage; G: Grade.
There was a total of 273 recurrences. Of the 273 recurrence, 62 patients had low grade disease, 158 patients had high grade disease and 53 patients did not have a grade classified. A total of 130 had pTa disease, 27 patients had pT1, 29 patients had pT2 disease or greater, 54 patients had CIS and 33 patients did not have the stage classified.
3.2Primary outcome
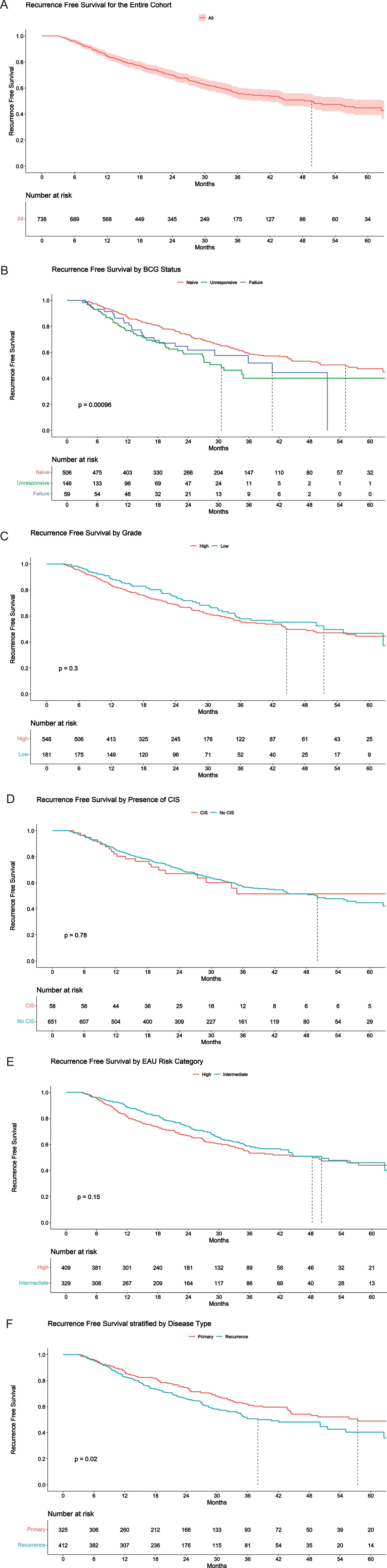
The RFS at 1 year and 2 years was 85.0% (95% CI 82.5% - 87.6%) and 70.0% (95% CI 66.4% - 73.3%), respectively (Fig. 1A). The RFS at 12 months and 24 months for BCG naïve was 87.6% (95% CI 85.0% - 90.4%) and 75.0% (95% CI 71.3% - 78.8%), respectively. The RFS at 12 months and 24 months for BCG unresponsive cohort was 78.1% (95% CI 72.0% - 84.7%) and 57.4% (95% CI 49.7% - 66.3%), respectively (Fig. 1B).
Fig. 1
Recurrence free survival stratified by a) Entire cohort; b) BCG status; c) Grade; d) CIS; e) European Urological Association Risk Category; f) Disease type.
Secondary outcome
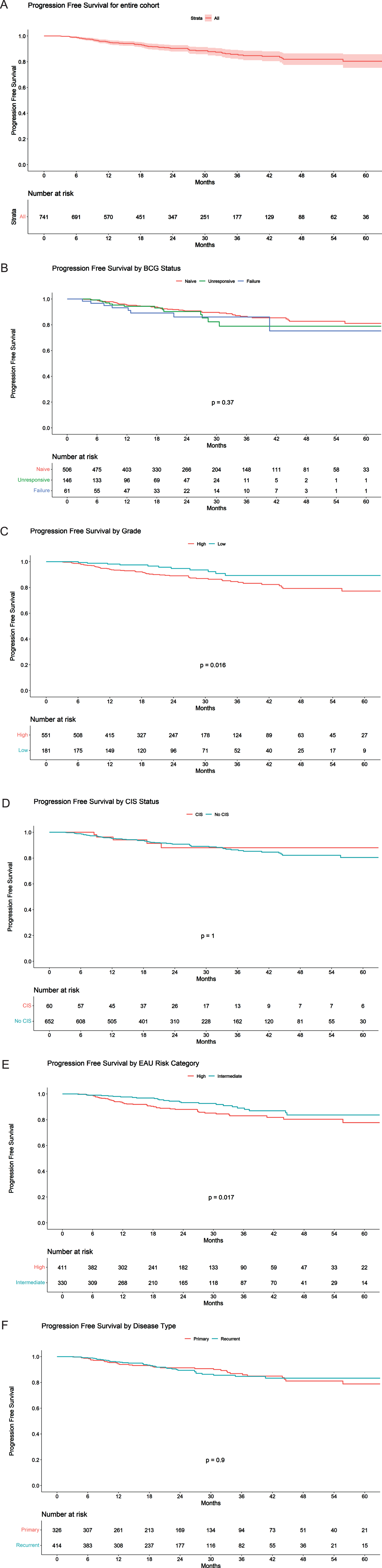
The complete response rate at 3 months and 6 months was 95.9% and 90.4%, respectively. The PFS at 12 months and 24 months was 95.5% (95% CI 94.0% - 97.0%) and 90.8% (95% CI 88.5% - 93.1%) (Fig. 2A). Kaplan-Meier for PFS estimates at 24 months for BCG Naïve and BCG unresponsive were 91.3% (95% CI 88.8% - 93.9%) and 90.1% (95% CI 84.8% and 95.8%), respectively (Fig. 2B). Figure 1C, 1D, 1E and 1F represents RFS by grade, presence of CIS, EAU risk category and disease type, respectively. Figure 2C, 2D, 2E and 2F represents PFS by grade, presence of CIS, EAU risk category and disease type, respectively. RFS for primary disease, recurrent disease and BCG status is listed in Supplementary Figure 2, 3 and 4, respectively.
Fig. 2
Progression free survival stratified by a) Entire cohort; b) BCG status; c) Grade; d) CIS; e) European Urological Association Risk Category; f) Disease type.
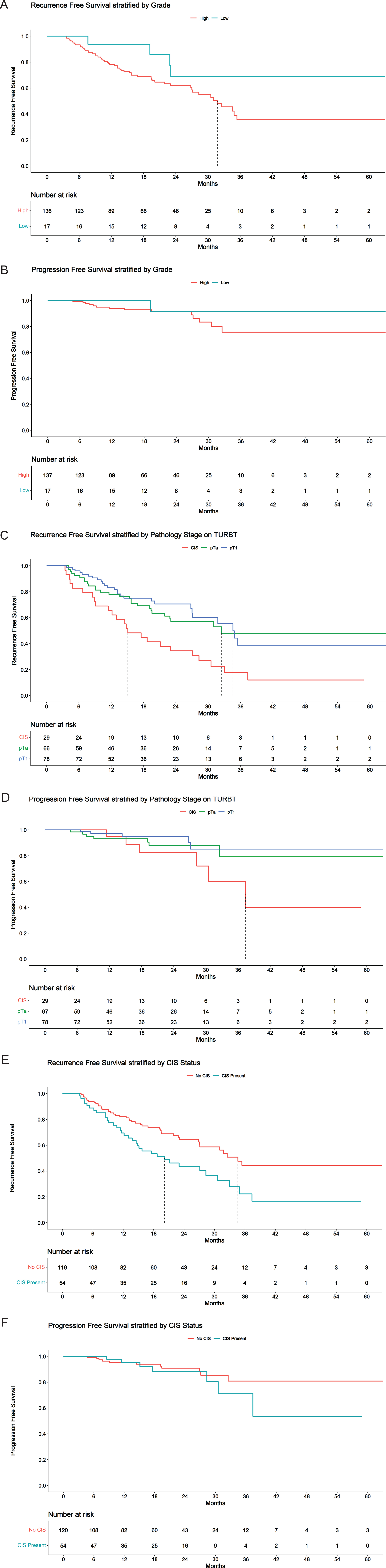
Subgroup analysis of the BCG unresponsive cohort by the presence of CIS suggest that RFS at 12 months was 69.5% (95% CI 58.1% - 83.2%) and 82.2% (95% CI 75.4% - 89.6%) in the CIS with/without papillary group and papillary only group respectively (Fig. 3). The RFS at 24 months was 43.6% (95% CI 31.4% –60.4%) and 64.5% (95% CI 55.4% - 75.1%) in the CIS with/without papillary group and papillary only group respectively.
Fig. 3
BCG unresponsive disease: Kaplan-Meier plot of a) recurrence free survival and b) progression free survival stratified by grade; Kaplan-Meier plot of c) recurrence free survival and d) progression free survival stratified by pathological stage on transurethral resection of bladder cancer; Kaplan-Meier plot of e) recurrence free survival and f) progression free survival stratified by CIS status.
Following CHT, 44 patients underwent a radical cystectomy, 6 required a nephroureterectomy, 64 died of other causes, 4 patients had metastasis at last follow up, 3 received chemotherapy and 4 received chemoradiation to the bladder.
A total of 574 patients (68.1%) did not experience any adverse events. Minor events (Grade 1 and Grade 2) occurred in 216 patients (25.6%) (Table 2). Grade 1 symptoms were self-limiting. Grade 2 symptoms consisted of urinary discomfort and were controlled with symptomatic relieves such as anti-cholinergic medication and phenazopyridine. Three patients with G2 adverse events developed a urinary tract infection requiring antibiotics. Grade 3 events occurred in 17 patients (2.0%) which comprised of pain (n = 4), hematuria (n = 3), severe irritative voiding symptoms (n = 2), allergic reaction (n = 1), urethral stenosis (n = 1) and not listed (n = 6). Of the 233 patients who experienced adverse events following CHT, 7 (3.0%) patients had a shortened duration of instillation, and 6 (2.6%) patients had a prolonged time between instillation. Only 42 (5%) statistically patients had to be discontinued from CHT using the BRS system due to adverse events.
Table 2
Adverse events
| Variable | BCG Naïve (%) | BCG Unresponsive | BCG Failure | Unknown | Total |
| No Adverse Events | 394 | 134 | 43 | 3 | 574 (68.1%) |
| Grade 1/Grade 2 adverse events | 147 | 33 | 31 | 5 | 216 (25.6%) |
| Grade 3 adverse events | 10 | 2 | 4 | 1 | 17 (2.2%) |
| Unknown | 0 | 0 | 0 | 36 | 36 (4.3%) |
Values are rounded to the nearest whole number and may exceed or be under 100%. BCG: Bacillus Calmette Guerin.
Both univariable and multivariable analyses report that the presence of tumor on second TURBT were significant in relation to disease recurrence (HR 3.47, 95% CI1.92–6.24) (Table 3). Similarly, in both univariable and multivariable analyses, only the presence of tumor on second TURBT were significant in relation with disease progression (HR 2.80, 95% CI: 1.15–6.82) (Table 4).
Table 3
Univariate and multivariable model for recurrence free survival
| Univariate Model | 1st Multivariable model | 2nd Multivariable model | ||||
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
| Received BCG | 1.81 (1.44 –2.28) | <0.01* | 1.37 (0.90 –2.08) | 0.14 | 1.44 (0.95 –1.28) | 0.09 |
| Received MMC | 0.86 (0.64 –1.15) | 0.31 | ||||
| Non-smoker | – | – | ||||
| Ex-smoker | 1.14 (0.79 –1.65) | 0.49 | ||||
| Current smoker | 0.95 (0.63 –1.54) | 0.95 | ||||
| Age (per year) | 1.01 (1 –1.02) | 0.14 | ||||
| Stage | ||||||
| pTa/T1 | – | – | – | – | ||
| pT2 | 1.07 (0.78 –1.48) | 0.32 | 1.23 (0.81 –1.89) | 0.85 | ||
| CIS | 2.03 (1.43 –2.89) | <0.01* | 1.83 (0.73 - 4.46) | 0.52 | ||
| Grade | ||||||
| 1 | – | – | – | – | ||
| 2 | 1.00 (0.68 –1.47) | 1.0 | 0.80 (0.45 –1.34) | 0.58 | ||
| 3 | 1.37 (1.03 –1.82) | 0.03* | 0.66 (0.38 –1.16) | 0.22 | ||
| Tumor on 2nd TURBT | 2.31 (1.44 –3.70) | <0.01* | 3.47 (1.92 –6.24) | <0.01* | 3.28 (1.81 –5.91) | <0.01* |
| EAU | ||||||
| Intermediate | – | – | ||||
| High | 1.13 (0.89 –1.42) | 0.32 | 0.79 (0.53 –1.18) | 0.24 | ||
| CIS Status | 1.44 (1.09 –1.89) | <0.01* | 0.93 (0.44 –1.98) | 0.73 | 0.96 (0.45 –2.08) | 0.71 |
BCG: Bacillus Calmette Guerin; MMC: Mitomycin C; pT: pathological T stage; CIS: Carcinoma in situ; TURBT: Transurethral resection of bladder tumor; EAU: European Association of Urology; CI: Confidence interval.
Table 4
Univariate and multivariable model for progression free survival
| Univariate Model | Multivariable model | |||
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
| Received BCG | 1.24 (0.74 –2.07) | 0.41 | ||
| Received MMC | 1.63 (0.87 –3.05) | 0.13 | ||
| Non-smoker | – | – | ||
| Ex-smoker | 2.15 (0.91 –5.08) | 0.08 | ||
| Current smoker | 1.74 (0.66 –4.59) | 0.26 | ||
| Age (per year) | 1.04 (1.12 –1.07) | <0.01 | 1.03 (1.0 –1.07) | 0.08 |
| Stage | ||||
| pTa/T1 | – | – | ||
| pT2 | 1.59 (0.92 –2.75) | 0.10 | ||
| CIS | 2.37 (1.13 –4.97) | 0.02* | ||
| Grade | ||||
| 1 | – | – | ||
| 2 | 2.06 (0.87 –4.85) | 0.1 | ||
| 3 | 4/86 (2.25 –10.53) | <0.01* | ||
| Tumor on 2nd TURBT | 3.02 (1.27 –7.16) | 0.01* | 2.8 (1.15 –6.82) | <0.02* |
| EAU | ||||
| Intermediate | – | – | – | – |
| High | 2.08 (1.16 –3.72) | 0.01 | 1.42 (0.57 –3.59) | 0.45 |
| CIS Status | 0.56 (0.17 –1.78) | 0.32 |
BCG: Bacillus Calmette Guerin; MMC: Mitomycin C; pT: pathological T stage; CIS: Carcinoma in situ; TURBT: Transurethral resection of bladder tumor; EAU: European Association of Urology; CI: Confidence interval.
DISCUSSION
Hyperthermia therapy for NMIBC is gaining traction as an exciting alternative to BCG therapy. In this multi-center study, we report that patients treated with CHT have a 24-month RFS of 70% and a PFS of 90.8%. In patients with BCG unresponsive disease, RFS and PFS at 24 months were 57.4% and 90.1%, respectively.
RFS at 24 months was 43.6% for patients with BCG unresponsive disease with CIS, compared to 64.5% in BCG unresponsive patients with papillary only disease. Only 2.2% of patients had severe adverse events. We found that the presence of bladder cancer on second TURBT was associated with an increased risk of both disease recurrence as well as progression.
Device assisted therapies in the form of CHT for NMIBC has been gaining popularity particularly in Europe. The mechanism of action of hyperthermia is postulated to synergistically improve the efficacy of chemotherapy by enhancing cell membrane permeability, denaturing of cellular proteins and release of heat shock proteins during cell necrosis, particularly HSP70. This results in the increase of circulating tumor antigen which stimulates an adaptive T cell response to induce both adaptive and innate immune system [11]. Hence, hyperthermia represents an attractive treatment option due to solid preclinical and clinical data, excellent tolerability and relatively cost effectiveness.
The current data would suggest that CHT may have a role in patients with BCG naive and BCG unresponsive disease. It is anticipated that there will be a shortage of BCG for the presumable future, suggesting that alternative adjuvant treatment options for high risk NMIBC is urgently needed. Our study reports a 24-month RFS of 70%, which is comparable to patients treated with induction BCG [17, 18]. The role of CHT for BCG unresponsive disease is more dire as there remains an unmet need for a bladder sparing treatment. Recently, the FDA approved pembrolizumab for the treatment of BCG unresponsive CIS with/without papillary NMIBC who were not suitable or refuse radical cystectomy. In a single arm phase 2 trial of 96 patients, while 3-month complete response rate was 40.6%, only 18.8% of patients of the total cohort were disease free > 12 months [19]. We report that CHT achieved a 12 and 24-month RFS rate of 78.1% and 57.4% in this same patient cohort. This efficacy is beyond the threshold of what is deemed as effective treatment by the International Bladder Cancer Group [20]. This compares favorably to better than other novel treatments such as intravesical adenovirus treatment- nadofaragene firadenovec gene therapy (12-month RFS 45·5%) [21] and Gem/ Doce (12-month RFS 60%, 24-month RFS 46%) [22]. However, our data should be interpreted with caution given this is a retrospective series, whereas data for pembrolizumab and nadofaragene firadenovec were derived from prospective clinical trials.
Our study shows that the most important factor when it comes to recurrence and progression of NMIBC is the presence of tumor on second TURBT. Many other factors such as pathological stage, grade, BCG status and CIS status were no longer statistically significant on multi-variable analysis, indicating that presence of tumor on the second TURBT is the driving factor for both recurrence and progression. We believe that this is likely due to the inability to completely eliminate residual tumor from the bladder, resulting an incomplete resection prior to initiating CHT [23, 24].
In this study, we showed that majority of patients experienced minimal adverse events. Only 5% of patients had to discontinue CHT treatment due to adverse events which is reassuring. This compared to 8.1% of patients failing to complete induction BCG in SWOG study 8795 [25]. Further, in the BCG unresponsive cohort, 13% and 4% of serious adverse events (Grade≥3) were reported following pembrolizumab and nadofaragene firadenovec gene therapy, respectively.
Our study has several limitations that should be interpreted within context. First, this single arm study is limited by the absence of a direct comparator group, hence no comparison with patients who underwent BCG therapy can be evaluated. The HYMN trial highlights the importance of a control arm as hyperthermia treatment in patients with disease recurrence following intravesical BCG was not superior to control treatment which comprised predominantly of further intravesical BCG [26]. Difference in treatment dose and delivery of CHT may explain differences in the results of the current study. Second, selection bias is present due to the study’s retrospective nature. CHT was performed at the discretion of the treatment physician, hence higher risk disease might have bene offered cystectomy, whereas lower risk disease offered CHT. Third, many of the patients were lost to follow-up after the 3 years mark, hence the shorter follow up. Finally, the different treatment regimes, induction only vs with maintenance and various follow up protocol, which was dependent on each respective institution might have confounded the findings.
CONCLUSIONS
Our results suggest that CHT is an attractive treatment option where BCG supplies are limited or in patients with BCG unresponsive disease. Treatment tolerability is excellent with a good adverse event profile. The strength of this study lies in its large multi-center nature, with follow-up data prospectively collected in an internationally mandated registry. Further prospective studies are warranted to validate our findings.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conception: Wei Phin Tan, Wei Shen Tan
Performance of work: Ana Plata Bello, Juan Carlos Garcia Alvarez, Félix Guerrero-Ramos, Daniel A. González-Padilla, Cajetan Nzeh, Jose Manuel de la Morena, Ignacio Gonzalez Valcarcel de Torres, Kees Hendricksen, Francisco Javier Díaz Goizueta, Fernandez J. Del Alamo, Francesco Chiancone, Paolo Fedelini, Massimiliano Poggio, Francesco Porpiglia, Victoria C. Gonzalo Rodríguez, Javier Montero Torres, Daniel Wilby, Richard Robinson, Alejandro Sousa-Escandón, Juan León Mata, Jose L. Pontones Moreno, Francisco Delgado Molina, Miguel A. Adriazola Semino, Andrew Townsley Stemberger, Jesús Calleja-Escudero, Joan Palou Redorta, Wei Shen Tan
Interpretation of data: Wei Phin Tan, Wei Shen Tan
Writing of manuscript: Wei Phin Tan
Review of manuscript: Ana Plata Bello, Juan Carlos Garcia Alvarez, Félix Guerrero-Ramos, Daniel A. González-Padilla, Cajetan Nzeh, Jose Manuel de la Morena, Ignacio Gonzalez Valcarcel de Torres, Kees Hendricksen, Francisco Javier Díaz Goizueta, Fernandez J Del Alamo, Francesco Chiancone, Paolo Fedelini, Massimiliano Poggio, Francesco Porpiglia, Victoria C. Gonzalo Rodríguez, Javier Montero Torres, Daniel Wilby, Richard Robinson, Alejandro Sousa-Escandón, Juan León Mata, Jose L Pontones Moreno, Francisco Delgado Molina, Miguel A. Adriazola Semino, Andrew Townsley Stemberger, Jesús Calleja-Escudero, Joan Palou Redorta, Wei Shen Tan
Access to data: All authors had access to the data.
CONFLICTS OF INTEREST
Daniel A. González-Padilla has received payment for trial collaboration from Combat Medical. Félix Guerrero Ramos is a consultant and speaker for Combat Medical.
Wei Shen Tan is a consultant to Combat Medical.
Joan Palou Redorta is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Wei Phin Tan, Ana Plata Bello, Carlos Garcia Alvarez, Cajetan Nzeh, Jose Manuel de la Morena, Ignacio Gonzalez Valcarcel de Torres, Kees Hendricksen, Francisco Javier Díaz Goizueta, Fernandez J. Del Alamo, Francesco Chiancone, Paolo Fedelini, Massimiliano Poggio, Francesco Porpiglia, Victoria C. Gonzalo Rodríguez, Javier Montero Torres, Daniel Wilby, Richard Robinson, Alejandro Sousa-Escandón, Juan León Mata, Jose L. Pontones Moreno, Francisco Delgado Molina, Miguel A. Adriazola Semino, Andrew Townsley Stemberger and Jesús Calleja-Escudero declare no conflicts of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-220026.
Appendix 1 Hyperthermia therapy using Mitomycin C. Bladder hyperthermia was achieved using the Combat BRS device (Combat Medical, UK) (Supplemental Figure 1). The device uses an aluminum heat exchanged and peristaltic pump to heat and recirculate chemotherapy solutions in the bladder. We used the disposable package which contained a 16 F 3-way catheter with a temperature monitoring thermistor in its tip. Mitomycin C (MMC) was reconstituted and diluted using sterile technique in a laminar flow hood and stored in a capped 60 mL syringe in a double bag Ziploc bag transport system. Each treatment used 40 mg of MMC in 50 ml of water for a continuous 60 minutes. Institutions included in the study:< /tabcap>Hospital Country Number of Patients Hospital Universitario de Canarias Spain 163 Hospital Universitario 12 Octubre Spain 148 St. Barbara Hospital Germany 98 Hospital Universitario Infanta Sofía, Spain 92 Netherlands Cancer Institute Netherlands 91 Hospital Universitario de Torrejón Spain 84 Antonio Cardarelli Hospital Italy 77 San Luigi Hospital Orbassano Italy 57 Hospital Universitario de Burgos Spain 56 Queen Alexandra Hospital United Kingdom 56 Hospital Monforte de Lemos Spain 44 Hospital Universitario La Fe Spain 38 Hospital General Rio Carrion Spain 13 Fundació Puigvert, Autonomous University of Barcelona Spain 11.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, CA: A Cancer Journal for Clinicians (2018) ;68: :7–30. |
[2] | Soloway MS . It is time to abandon the “superficial” in bladder cancer, European Urology (2007) ;52: :1564–5. |
[3] | National Comprehensive Cancer Network. Bladder Cancer (Version 4.2021). |
[4] | Tan WP , Chang A , Brousell SC , Grimberg DC , Fantony JJ , Longo TA , et al. Safety and efficacy of intravesical chemotherapy and hyperthermia in the bladder: results of a porcine study, International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Grou (2020) ;37: :854–60. |
[5] | Hildebrandt B , Wust P , Ahlers O , Dieing A , Sreenivasa G , Kerner T , et al. The cellular and molecular basis of hyperthermia, Critical Reviews in Oncology/Hematology (2002) ;43: :33–56. |
[6] | Frey B , Weiss EM , Rubner Y , Wunderlich R , Ott OJ , Sauer R , et al. Old and new facts about hyperthermia-induced modulations of the immune system, International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Grou (2012) ;28: :528–42. |
[7] | Schildkopf PJ , Ott O , Frey B , Wadepohl M , Sauer R , Fietkau R , et al. Biological rationales and clinical applications of temperature controlled hyperthermia-implications for multimodal cancer treatments, Current Medicinal Chemistry (2010) ;17: :3045–57. |
[8] | Stauffer PR , van Rhoon GC . Overview of bladder heating technology: matching capabilities with clinical requirements. International Journal of Hyperthermia : The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2016:1-10. |
[9] | Tan WP , Longo TA , Inman BA . Heated intravesical chemotherapy: biology and clinical utility, The Urologic Clinics of North America (2020) ;47: :55–72. |
[10] | Babjuk M , Burger M , Compérat EM , Gontero P , Mostafid AH , Palou J , et al. European Association of Urology Guidelines onNon-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) –Update, European Urology (2019) ;76: :639–57. |
[11] | Tan WS , Kelly JD . Intravesical device-assisted therapies for non-muscle-invasive bladder cancer, Nature Reviews Urology (2018) ;15: :667–85. |
[12] | Sousa A , Piñeiro I , Rodríguez S , Aparici V , Monserrat V , Neira P , et al. Recirculant hyperthermic IntraVEsical chemotherapy(HIVEC) in intermediate-high-risk non-muscle-invasive bladdercancer, International Journal of Hyperthermia: The Official Journalof European Society for Hyperthermic Oncology, North AmericanHyperthermia Grou (2016) ;32: :374–80. |
[13] | Administration USDoHaHSFaD. BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for TreatmentGuidance for Industry. 2018. |
[14] | Kamat AM , Sylvester RJ , Böhle A , Palou J , Lamm DL , Brausi M , et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer GrouJournal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology (2016) ;34: :1935–44. |
[15] | Schafer JL . Multiple imputation: a primer, Statistical Methods in Medical Research (1999) ;8: :3–15. |
[16] | Team. R. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc. 2015. |
[17] | Sylvester RJ , Brausi MA , Kirkels WJ , Hoeltl W , Calais Da Silva F , Powell PH , et al. Long-term efficacy results of EORTC genito-urinarygroup randomized phase 3 study 1 comparing intravesicalinstillations of epirubicin, bacillus Calmette-Guérin, andbacillus Calmette-Guérin plus isoniazid in patients withintermediate- and high-risk stage Ta T1 urothelial carcinoma of thebladder, European Urology (2010) ;57: :766–73. |
[18] | Sylvester RJ , van der Meijden AP , Witjes JA , Kurth K . Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. The Journal of Urology. (2005) ;174: ::86–91 discussion -2. |
[19] | Balar AV , Kamat AM , Kulkarni GS , Uchio EM , Boormans JL , Roumiguié M , et al. Pembrolizumab monotherapy for the treatmentof high-risk non-muscle-invasive bladder cancer unresponsive to BCG(KEYNOTE-057): an open-label, single-arm, multicentre, phase 2study, The Lancet Oncology (2021) ;22: :919–30. |
[20] | Kamat AM , Sylvester RJ , Böhle A , Palou J , Lamm DL , Brausi M , et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer GrouJournal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology (2016) ;34: :1935–44. |
[21] | Boorjian SA , Alemozaffar M , Konety BR , Shore ND , Gomella LG , Kamat AM , et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial, The Lancet Oncology (2021) ;22: :107–17. |
[22] | Steinberg RL , Thomas LJ , Brooks N , Mott SL , Vitale A , Crump T , et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer, The Journal of Urology (2020) ;203: :902–9. |
[23] | Brausi M , Collette L , Kurth K , van der Meijden P , Oosterlinck W , Witjes JA ,et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies, European Urology (2002) ;41: :523–31. |
[24] | Cumberbatch MGK , Foerster B , Catto JWF , Kamat AM , Kassouf W , Jubber I , et al. Repeat Transurethral Resection in Non–muscle-invasive Bladder Cancer: A Systematic Review, European Urology (2018) ;73: :925–33. |
[25] | Lamm DL , Blumenstein BA , David Crawford E , Crissman JD , Lowe BA , Smith JA , et al. Randomized intergroup comparison of bacillus calmette-guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a southwest oncology group study, Urologic Oncology: Seminars and Original Investigations (1995) ;1: :119–26. |
[26] | Tan WS , Panchal A , Buckley L , Devall AJ , Loubière LS , Pope AM , et al. Radiofrequency-induced Thermo-chemotherapy Effect Versus aSecond Course of Bacillus Calmette-Guérin or InstitutionalStandard in Patients with Recurrence of Non-muscle-invasive BladderCancer Following Induction or Maintenance BacillusCalmette-Guérin Therapy (HYMN): A Phase III, Open-label,Randomised Controlled Trial, European Urology (2019) ;75: :63–71. |




