MHC I Expression Predicts Response to Checkpoint Inhibitors in Metastatic Urothelial Carcinoma but Lacks Prognostic Value in Localized Disease
Abstract
BACKGROUND:
Loss of MHC I expression is a tumoral escape mechanism, part of the process of immunoediting. MHC expression patterns and their prognostic and predictive value have not been studied in urothelial carcinoma of the bladder (UC) so far.
OBJECTIVE:
To correlate the expression of MHC I and MHC II with prognosis after curative treatment, response to chemotherapy and checkpoint inhibition.
PATIENTS AND METHODS:
We analyzed different patient cohorts for their expression of MHC I(HLA-A/B/C) and II (HLA-DR/DP/DQ) and examined potential correlations with prognosis and response to cisplatin-based chemotherapy or PD-1/PD-L1 directed immunotherapy.
RESULTS AND LIMITATIONS:
Overall, MHC expression was analyzed in 246 patients, and complete MHC I loss was seen in 29.7% of patients. In 35% of patients aberrant tumoral expression of MHC II was observed. In a homogeneous cohort of 149 patients with cystectomy with curative intent there were no significant differences in survival between the MHC expression groups. MHC I+ and MHC II+ patients had higher infiltration densities with CD8+ T effector cells.
An analysis of 77 additional patients (cohort II) with neoadjuvant chemotherapy revealed no associations of MHC status with response defined as < pT2 pN0 in the cystectomy specimen. Lastly, we analyzed 26 patients with metastatic disease treated with PD-1/PD-L1 directed immunotherapy (cohort III, best response: 11 PD, 5 SD, 10 OR) and observed responses exclusively in MHC I+ patients (10/19 patients, 52.6). All four MHC I+ /MHC II+ /PD-L1+ patients had a progression-free interval of at least 12 months.
CONCLUSIONS:
Tumoral MHC I expression is frequently lost in UC. We found no association with prognosis or response to cisplatin-based chemotherapy but response to checkpoint inhibitors was limited to MHC I+ patients.
INTRODUCTION
Harnessing the immune system is a major constituent in the treatment of urothelial carcinoma (UC). In recent years immune-modulating antibodies directed against PD-1 or PD-L1 have become the second most important treatment for metastatic disease after cisplatin-containing chemotherapy [1–5]. Although long-lasting responses can be seen in a minority of patients, response rates do not exceed 30% in an unselected patient population. The rate of patients with an objective response can be augmented by grouping according to the intratumoral PD-L1 status [6]. Nevertheless, reliable and widely accepted response markers are lacking.
Table 2
Frequency of objective responses in patients with PD-1/PD-L1-directed treatment dependent on their MHC and PD-L1 status in cohort III
| Marker | Status | Responders | Fraction |
| MHC I | Positive | 10/19 | 52.6% |
| negative | 0/7 | 0% | |
| MHC II | positive | 5/10 | 50% |
| negative | 5/16 | 31% | |
| PD-L1 | positive | 4/6 | 66% |
| negative | 6/20 | 30% |
PD-L1 antibodies deploy their efficacy by reactivating CD8-positive cytotoxic T cells which recog-nize tumor-associated or tumor-specific antigenic structures on the surface of tumor cells [7, 8]. For recognition and finally elimination of cancer cells cytotoxic T cells need the interaction of CD 8 and MHC I, on which intracellular antigenic fragments are presented. MHC I loss is a well-described and accepted escape mechanism in the model of cancer immunoediting [9– 11]. A better prognosis of patients with MHC I+ tumors has been shown in a plenty of tumor entities [12]. In bladder cancer there has been only one analysis published so far showing a trend to better outcome but lacking statistical significance because of the low number of patients [13, 14].
It has been shown recently that a subset of human tumors atypically expresses MHC II, which is usually present on professional antigen-presenting cells like macrophages or dendritic cells to prime CD4 T cell responses. In the majority of published series this MHC II expression has been shown to be associated with a better prognosis or increased likelihood of response to PD1/PDL1 antibodies [10, 15, 16].
Furthermore, there is an increasing body of evidence that links the efficacy of conventional cytotoxic chemotherapy with modulation of the immune system [17– 19].
To comprehensively analyze the impact of MHC expression in urothelial carcinoma we wanted to address the prognostic role in localized bladder cancer and the predictive role for both conventional cytotoxic chemotherapy and PD-1/PD-L1 based immunotherapy.
PATIENTS AND METHODS
Patients
All patients were treated for urothelial carcinoma in a single university hospital (Department of Urology, Klinikum rechts der Isar, Munich, Germany). Different patient populations without overlap were analyzed to approach different questions.
Cohort I included 149 patients who received cystectomy for invasive urothelial carcinoma (pT1-pT4) in curative intent without neoadjuvant treatment and lack of distant metastases as described earlier [20]. Adjuvant treatment was permitted based on the decision of the treating physicians. Patients with non-urothelial tumours, perioperative death within 60 days, lack of pelvic lymphadenectomy, and older than 85 years at the time of surgery were excluded from the study. Survival data were obtained retrospectively. The follow-up time was defined as the time from radical cystectomy to either death or the time of the last follow-up contact. Detailed patient characteristics are shown in Table 1. Tissue analysis was performed using tissue microarrays with a diameter of 1 mm as published previously [20].
Table 1
Characteristics of 149 patients in cohort I
| Category | ||
| T Stage | T1 | 17 (11.4%) |
| T2 | 54 (36.2%) | |
| T3 | 56 (37.6%) | |
| T4 | 22 (14,8%) | |
| N stage | N0 | 104 (69.8%) |
| N+ | 45 (30.2%) | |
| Mean age | 68 years (35– 85) | |
| Gender | 114 male | 35 female |
Cohort II included 77 patients who received pre-operative chemotherapy before cystectomy with curative intent. The indication for the use of preoperative chemotherapy were hints in the imaging studies for either locally advanced tumor growth (cT3/cT4) or lymph node metastases (cN+). Patients with distant metastases or non-urothelial histology were excluded. The combination of gemcitabine and cisplatin was used in all patients. Tissue specimens were taken from both TURB-T before the initiation of chemotherapy and from cystectomy afterchemotherapy.
Cohort III included 26 patients with metastatic urothelial carcinoma treated with immunotherapy with a PD-1/PD-L1 antibody in a palliative intention between 2015 and 2019 (14 pembrolizumab, 5 nivolumab, 4 atezolizumab, 3 durvalumab). 20 of the patients were male, six patients were female, mean age of the patients was 69 years. Response to the treatment was assessed using irRECIST criteria. For the analysis of MHC expression available tissue was either used from the primary tumor or metastases. In case of availability of more than one tissue sample we used the one retrieved directly before the initiation of immunotherapy.
Patient samples were assembled in tissue microarrays with a core diameter of 1 mm (MTBIO, Tissuebank of the Technical University of Munich). Three representative cores per patient were taken from vital regions of the tumor center to address potential intratumoral heterogeneity. In case of sparse tumor tissue whole slides were analyzed (due to the judgment of JS and KS).
The local ethics committee approved all analyses (number 321/19), all patients signed written informed consent.
Immunohistochemsistry
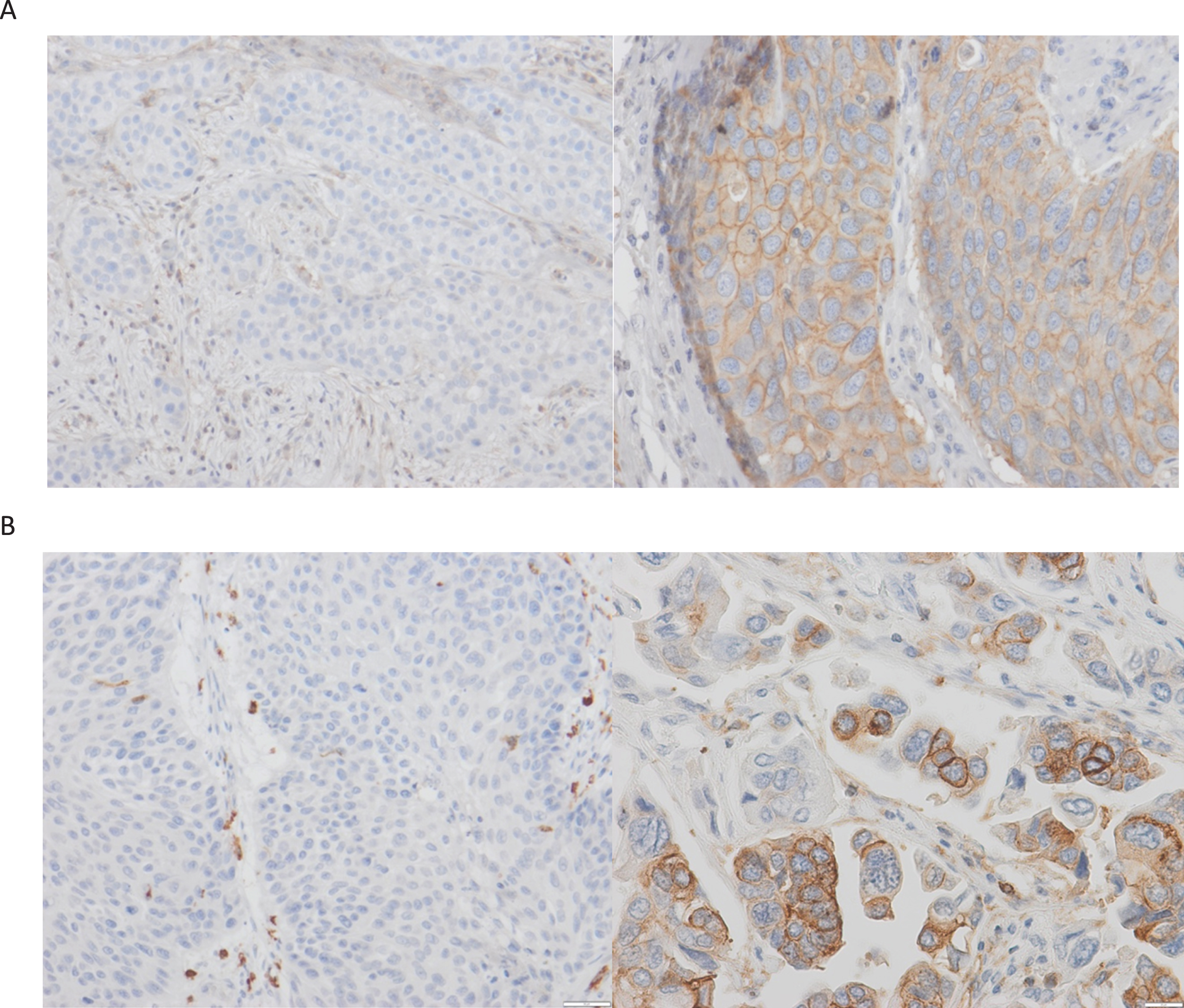
Immunohistochemical staining was performed using an automated stainer (Ventana XT, Ventana Medical Systems) to evaluate the expression of MHC I, MHC II and PD-L1. Monoclonal antibodies were used to detect the expression of MHC I (HLA-A/-B/-C, clone EMR8-5, BD Pharmingen), MHC II (HLA-DR/-DP/-DQ, clone CR3/43, Santa Cruz Biotechnology) and PD-L1 (clone E1L3N, Cell Signaling) according to the manufacturer’s instructions. Single staining per slide was performed. Exemplary images are shown in Fig. 1. Human tonsil was used as positive control. FFPE material was used in all cases. For the evaluation of MHC expression only membranous staining was considered positive. The MHC I antibody detects only the heavy chain and not the whole functional complex with beta2-microglobulin or even antigen.
Fig. 1
MHC immunohistochemistry.Image A: A case with complete lack of MHC I expression (left) and a case with >50% of MHC I-positive tumor cells (right). Image B: Same for MHC-II expression (400x magnification in positive cases to show membranous expression.
MHC expression was quantified using the percentage of positive tumor cells. Any expression exceeding five percent positivity was considered positive. The intensity of MHC staining was classified in relation to the staining intensity of accompanying lymphocytes (0: no tumoral MHC expression, 1: less intense than lymphocytes, 2: same intensity, 3: higher intensity). The frequency of positive cells (5– 100%) and the intensity of staining (1– 3) were multiplied to gain an H score between 5and 300.
For the analysis of PD-L1 expression both IC (immune cell) score and CPS (combined positivity score) were evaluated and an individual patient considered positive with either CPS > 10 or IC > 5% [21].
CD8 and FOXP3 expression data were used from a prior publication of cohort 1 [20].
Statistical analysis
Survival curves for cancer-specific survival were estimated using Kaplan-Meier analyses and compared between subgroups using log-rank tests.
All analyses were performed using two-tailed tests with a significance level of 5%. Comparing non-normally distributed data between groups a Mann-whitney-U-test was used.
RESULTS
MHC I expression is frequently lost, MHC II is expressed mainly in MHC I+ patients
Taken all the patients from cohorts I-III together we examined tumoral MHC expression in 246 patients with UC. MHC I was expressed in 70.7% (174 patients) while MHC II expression occurred in only 35% (86 patients). Thus, MHC I expression was lost in 29.3% of patients. Complete negativity for both MHC I and MHC II was observed in 66 patients (26.8%). Sole MHC II expression without coexpression of MHC I was a rare event and happened in only six patients (2.4%).
The frequency of positively stained cells varied in positive patients between 5% and 100% of tumor cells with a median of 30% in MHC I positive patients and 20% in MHC II positive patients.
MHC I/II expression has no prognostic role in UC
We examined 149 patients who received a cystectomy in curative intent without neoadjuvant treatment (cohort 1). 42 patients (28%) were negative for both MHC I and MHC II (MHC I -/MHC II –), 58 (39%) were positive for MHC I only (MHC I+ /MHC II–) and 46 patients (31%) were positive for both (MHC I+ /MHC II+). Only three patients (2%) were positive for MHC II only (MHC I -/MHC II+). In MHC I+ patients the fraction of MHC I+ tumor cells varied between 5% and 100% with a median of 30% positivity. 72 patients (48.3%) died during the follow-up period, the median follow-up duration of surviving patients was 59 months. No significant differences regarding MHC expression were found dependent on lymph node status or pT stage (data not shown).
There was no significant association of MHC expression status with both cancer-specific and overall survival (see Supplementary Figure 1, p = 0.95). The lack of correlation between MHC expression and survival persisted after exclusion of 17 patients with a T1 tumor, in which interpretation of immunohistochemistry and selection of a tumor area for tissue microarray construction may be challenging (p = 0.95). Also limiting the analysis to patients with an expression of MHC I in more than 50% of the tumor cells (42 of 104 MHC-I-positive patients, 48%) did not change the results (p = 0.81, for all graphs see Supplementary Figure 1).
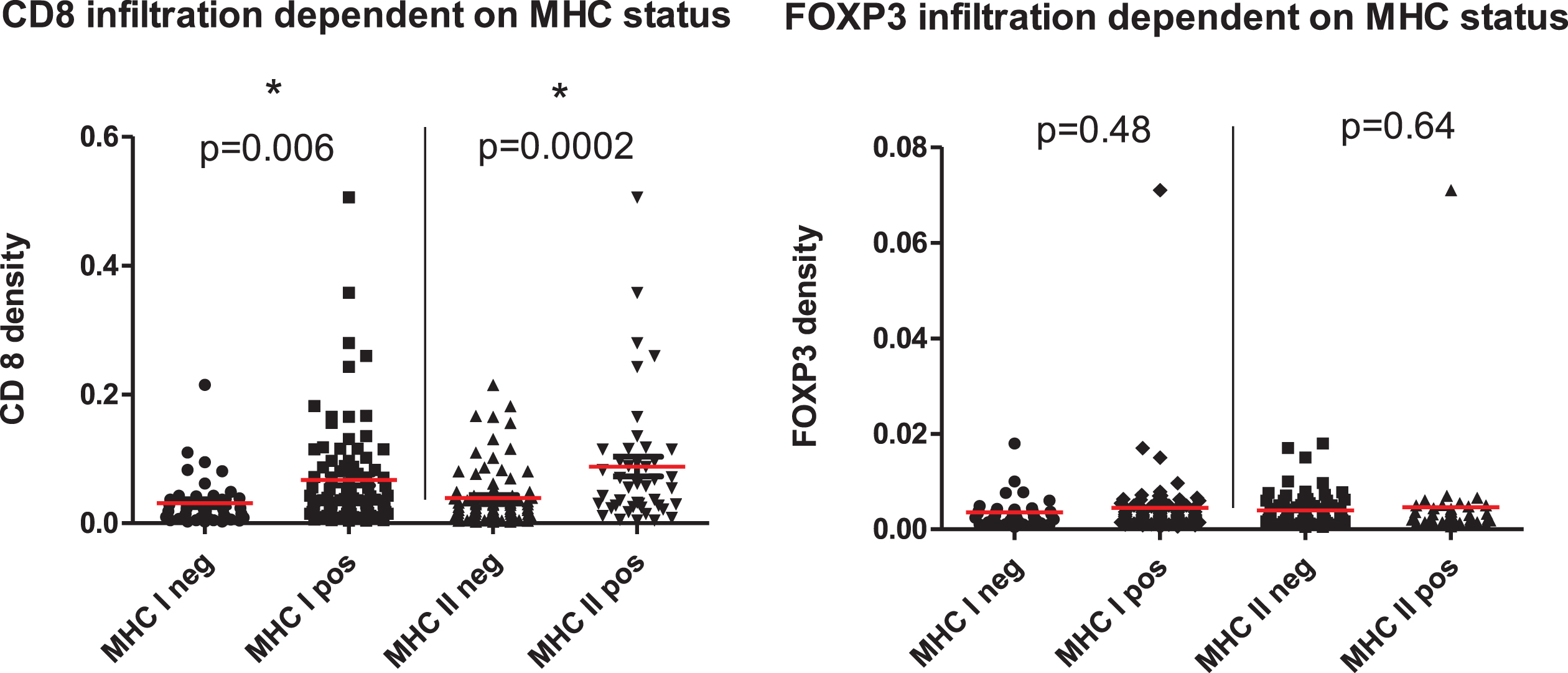
Both MHC I+ and MHC II+ tumors show a higher density of intratumoral CD8+ T effector cells, but not FOXP3+ T cells
We looked at the infiltration density with intratumoral CD8+ T effector and FOXP3+ regulatory T cells dependent on MHC expression. Therefore 149 patients from cohort 1 were evaluated. We did not find an MHC-dependent difference in the density of FOXP3+ regulatory T cells (p = 0.48 for MHC I, p = 0.64 for MHC II). On the other hand, both MHC I+ and MHC II+ tumors showed a statistically significant increase in the infiltration density with CD8+ T effector cells compared to tumors negative for MHC I or MHCII, respectively (p = 0.006 for MHC I, p = 0.0002 for MHC II, see Fig. 2).
Fig. 2
Infiltration density with CD8+ T effector cells (upper image) and FOXP3+ regulatory T cells (lower image) dependent on MHC expression. 149 patients from cohort 1 were grouped according to their MHC status and the density of infiltrating immune cells determined by IHC. A significantly higher infiltration density in MHC + patients was found.
MHC expression has no predictive potential in neoadjuvant chemotherapy
In the next step we analyzed 77 patients with urothelial carcinoma of the bladder who received neoadjuvant chemotherapy before cystectomy in curative intent (cohort II). 26 patients (34%) showed a response defined as reaching a pathological tumor stage < pT2 pN0 in the cystectomy specimen.
We did not find a difference in MHC expression between responders and non-responders (p = 0.24 for MHC I, p = 0.51 for MHC II, supplementary Fig. 2). Thus, MHC expression status failed as a prediction marker for response to cisplatin-containing neoadjuvant chemotherapy.
PD-L1 positive patients are clustered in the MHC I+ /MHC II+ group
We were able to coanalyze PD-L1 expression and MHC expression in a total of 97 patients (from cohorts II and III). PD-L1 positivity was found in 3/24 (12.5%) MHC I -/MHC II – patients, 5/36 (13.9%) MHC I+ /MHC II - patients and in 1/3 MHC I -/MHC II+ patients. In contrast, 18 of 34 MHC I+ /MHC II+ patients (52.9%) were found to be positive for PD-L1. Thus, 51.4% (19/37) of MHC II+ patients, but only 13.3% (8/60) of MHC II –patients were positive also for PD-L1.
Responses to PD-1/PD-L1 antibodies were limited to MHC I+ patients
We were able to correlate both PD-L1 expression and MHC expression with response to PD-1/PD-L1 inhibition in 26 patients with metastatic urothelial carcinoma in cohort 3. Ten of 26 patients (38.5%) had either a complete or partial response. We found that objective radiological responses were exclusively seen in MHC I+ patients (10/19 patients, 52.6%) and that responders showed a statistically significantly increased MHC I expression (p = 0.025, Fig. 3). Moreover, MHC I+ patients had longer progression-free survival in comparison to MHC I negative patients (median 7 vs 3 months, p = 0.069, Supplementary Fig. 3).
Fig. 3
Responders to PD-1/PD-L1 directed immunotherapy display a higher expression of MHC I (left image, p = 0.025). Responders are exclusively found in the MHC I + group (right image, responders in green).
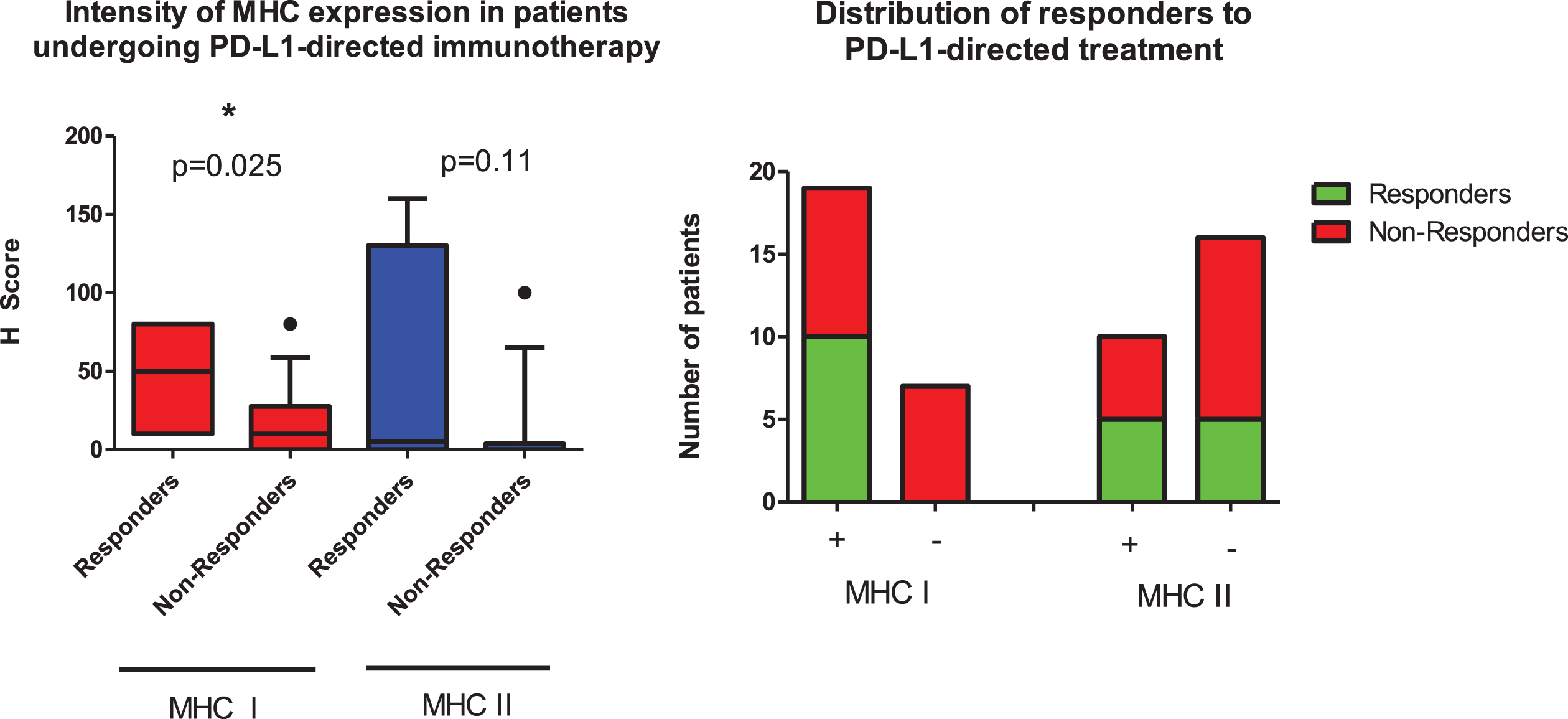
In addition, both MHC II+ and PD-L1+ patients had a higher likelihood of response (see Fig. 3 and Table 1). Four patients were MHC I+, MHC II+ and PD-L1+. All these patients showed an objective response lasting at least for 12 months. Three of these patients received pembrolizumab, one patient nivolumab. Remarkably, all these four patients showed an H score of 80 for MHC I and at least 40 for MHC II, representing high expression (Supplementary Table 1).
DISCUSSION
The recognition of tumor-specific antigenic structures by cytotoxic CD8-positive T cells depends on the interaction of the T cell receptor with functionally intact MHC I. Thus, loss of MHC I expression on the surface of tumor cells has consequences for the immunogenicity of the tumor and likely for prognosis and the response to immunotherapy [9–12, 22–24]. We observed complete MHC I loss in almost 30% of patients in our series of patients with urothelial carcinoma, in line with other studies [22]. Moreover, we found 86 of 246 patients (35%) positive for MHC II. Almost all of these patients were also positive for MHC I. This is in line with the findings of another study with 17/131 MHC II+ UC patients all of which were also positive for MHC I [14]. Usually limited to professional antigen-presenting cells MHC II expression by cancer cells is described in many tumor entities [10, 15]. Its clinical significance is not completely understood, but MHC II expression has often been linked to a favorable outcome[16].
Somehow unexpected, we were unable to show a survival benefit for patients with MHC I+ or MHC II+ tumors, which was also true when we limited the definition of MHC I positivity to an expression of more than 50% of tumor cells or excluded T1 tumors (Supplementary Figure 1). Analyzing 149 patients with invasive urothelial carcinoma receiving a cystectomy in curative intent we deem the size and the homogeneity of our cohort as strengths of our study. Another trial has correlated MHC expression with survival in 77 patients, comprised of both invasive and non-invasive cancers, and did not find a clear association [14]
Potential reasons why patients with MHC I-deficient tumors do not have a shortened survival are decreased inhibition of NK cells by reduced MHC expression [25] and the fact that loss of MHC I expression obviously does not confer a facilitation of metastatic tumor growth. All of our patients in cohort I still had localized disease and it will be of interest in the future to look for the prognostic relevance of MHC expression in metastatic patients as well as the evolution of MHC expression over time. Remarkably, the frequency of MHC I loss was 30% in localized disease (cohort I) and 27% in metastatic disease, (26 patients in cohort 3). This is a hint is not necessarily an integral part of metastatic evolution but an earlier event. Looking at MHC loss it needs to be kept in mind that there are multiple ways of MHC I loss besides complete lack of expression, among others epigenetic downregulation, loss of allelic diversity and loss of beta2-microglobulin expression [14, 23, 26, 27].
Another reason could be that paracrine signaling in the tumor microenvironment, for example through IFNs, is potentially able to restore MHC I expression that we had missed at the time of our analyses [28]. Indeed, we were able to show an increased CD8 T effector cell infiltration in MHC I+ patients (Fig. 2), but in this observation it is unclear if MHC positivity of the tumor attracted tumor-specific T cells or if recruited T cells induced MHC expression via cytokine signaling. Additionally, considering T cell infiltration not only the mere number of T cells is important but also their location in terms of intratumoral or stromal invasion. Intratumoral T cells are linked with the expression of MHC I and MHC II and a strong antitumoral T cell response while the latter represents T cell exclusion and is linked to a poorer prognosis [14].
Conventional cytotoxic chemotherapy exerts its effects in part by modulation of the immune system [17–19]. Nevertheless, we did not find increased susceptibility to neoadjuvant chemotherapy in patients with MHC I/II+ tumors. It will be an interesting aspect to monitor changes in tumoral MHC expression conveyed by cisplatin-based chemotherapy because reinduction of MHC I expression by cisplatin has been described [29].
There is increasing preclinical evidence that loss of MHC I expression conveys resistance to checkpoint blockade [23, 30] while few clinical trials have highlighted this issue so far [24, 30, 31]. Our analysis included only 26 patients treated with checkpoint inhibitors, but we found responses limited to patients with MHC I+ tumors (Fig. 3). No patient with loss of MHC I expression showed a response to checkpoint inhibition.
The frequency of responders was also higher in the MHC II+ population compared to MHC II - patients but this difference was smaller than for MHC I. The observed clustering of patients with PD-L1 positivity, which is a weak predictive marker for response to immunotherapy, in the MHC I+ /MHC II+ group underlines the potential positive predictive role of MHC II expression. All four patients that were positive for MHC I and -II as well as PD-L1 had an objective response lasting for at least 12 months. Remarkably, all H scores for MHC I/II expression in these four patients were over 40 representing high MHC expression.
A limitation of our study is the use of TMA sections for MHC staining as the expression in inhomogeneous. Nevertheless, we tried to overcome this limitation by using triplicate cores in each case.
Thus, our data feed the hypothesis that MHC expression may serve as a biomarker for the success of checkpoint inhibition, while et seems to be neither prognostic in localized disease nor predictive for the success of cisplatin-based chemotherapy. In the future, deeper understanding of the underlying immunologic mechanisms may increase the efficacy of immunotherapy. MHC expression can be restored by different means, among them not only IFN as described earlier [28] but also other treatment modalities like chemotherapy or tyrosine kinase inhibitors [29, 32] Potentially, combination or sequencing of different treatment modalities with checkpoint inhibition, like already used in urothelial cancer [2] and renal cell cancer [33] among others, could reinduce MHC expression and turn tumors more susceptible to checkpoint inhibition.
ACKNOWLEDGMENTS
The Else-Kröner-Fresenius-Stiftung funded this work (grant number 2018/A43).
AUTHOR CONTRIBUTIONS
Slotta-Huspenina J.: conceptualization, formal analysis
Schwamborn K.: formal analysis, investigation
Steiger K.: methodology
Simon R.: investigation, data curation
Kirchhoff F.: investigatio.n, data curation
Büchler J.: investigation, data curation
Fiedler J.: Data curation
Retz M.: validation, resources
Nawroth R.: methodology
Ritschel C.: investigation, data curation
Gschwend J.: conceptualization, resources, supervision
Horn T.: conceptualization, funding acquisition, project administration, writing
CONFLICTS OF INTEREST
Nawroth R. is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Slotta-Huspenina J.: none
Schwamborn K.: lecture honoraria and advisory board participation for Roche, BMS, MSD, Merck
Steiger K.: none
Simon R.: none
Kirchhoff F.: none
Büchler J.: none
Fiedler J.: none
Retz M.: lecture honoraria from BMS
Ritschel C.: none
Gschwend J.: lecture honoraria from Amgen, Astellas, Bayer, Janssen, Merck, Roche, consultant for AAA, Amgen, Bayer, BMS, Janssen, MSD, Merck, Pfizer, Roche
Horn T.: lecture honoraria from Merck, medac GmbH and Pfizer, advisory board participation Merck and Bayer
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-211604.
REFERENCES
[1] | Bellmunt J , de Wit R , Vaughn DJ et al,. Pembrolizumab as Second-Line Therapy for Advanced Urothelial CarcinomaThe New England journal of medicine 2017. |
[2] | Powles T , Park SH , Voog E et al,. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. The New England Journal of Medicine (2020) ; 383: (13): 1218–30. |
[3] | Balar AV , Galsky MD , Rosenberg JE et al,. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 3894) (2017) ; 389: (10064):67–76. |
[4] | Rosenberg JE , Hoffman-Censits J , Powles T et al,. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 3871) (2016) ;387: (10031)):1909–20. |
[5] | Sharma P , Retz M , Siefker-Radtke A et al,. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. The Lancet Oncology (2017) ; 18: (3): 312–22. |
[6] | Vuky J , Balar AV , Castellano D et al,. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology (2020) ; 38: (23): 2658–66. |
[7] | Sharma P , Allison JP Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol (2020) ; 20: (2): 75–6. |
[8] | Blank C , Gajewski TF , Mackensen A Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunology, Immunotherapy: CII (2005) ; 54: (4): 307–14. |
[9] | Schreiber RD , Old LJ , Smyth MJ Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, NY) 331 (2011) ; 331: (6024):1565–70. |
[10] | Sabbatino F , Liguori L , Polcaro G et al,. Role of Human Leukocyte Antigen System as A Predictive Biomarker for Checkpoint-Based Immunotherapy in Cancer Patients. Int J Mol Sci. 21(19) (2020) ;21: (19). |
[11] | Garrido F , Aptsiauri N Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology (2019) ; 158: (4): 255–66. |
[12] | Dhatchinamoorthy K , Colbert JD , Rock KL Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol (2021) ; 12: : 636568. |
[13] | Sharma P , Shen Y , Wen S et al,. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proceedings of the National Academy of Sciences of the United States of America. (2007) ; 104: (10): 3967–72. |
[14] | Gil-Julio H , Perea F , Rodriguez-Nicolas A et al,. Tumor Escape Phenotype in Bladder Cancer Is Associated with Loss of HLA Class I Expression, T-Cell Exclusion and Stromal Changes. Int J Mol Sci (2021) ; 22: (14). |
[15] | Axelrod ML , Cook RS , Johnson DB et al,. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin Cancer Res (2019) ; 25: (8): 2392–402. |
[16] | Johnson DB , Estrada MV , Salgado R et al,. Melanoma-specific MHC-II expression represents a tumour-auto-nomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. (2016) ; 7: : 10582. |
[17] | Ladoire S , Enot D , Andre F et al,. Immunogenic cell death-related biomarkers: Impact on the survival of breast cancer patients after adjuvant chemotherapy. Oncoimmunology (2016) ; 5: (2):e1082706. |
[18] | Pfirschke C , Engblom C , Rickelt S et al,. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity (2016) ; 44: (2): 343–54. |
[19] | Galluzzi L , Buque A , Kepp O et al,. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell (2015) ; 28: (6): 690–714. |
[20] | Horn T , Laus J , Seitz AK et al,. The prognostic effect of tumour-infiltrating lymphocytic subpopulations in bladder cancer. World Journal of Urology (2016) ; 34: (2): 181–7. |
[21] | Schwamborn K , Ammann JU , Knüchel R et al,. Multicentric analytical comparability study of programmed death-ligand 1 expression on tumor-infiltrating immune cells and tumor cells in urothelial bladder cancer using four clinically developed immunohistochemistry assays. Virchows Archiv: An International Journal of Pathology (2019) ; 475: (5): 599–608. |
[22] | Cabrera T , Pedrajas G , Cozar JM et al,. HLA class I expression in bladder carcinomas. Tissue Antigens (2003) ; 62: (4): 324–7. |
[23] | Sade-Feldman M , Jiao YJ , Chen JH et al,. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nature Communications (2017) ; 8: (1): 1136. |
[24] | Aptsiauri N , Carretero R , Garcia-Lora A et al,. Regressing and progressing metastatic lesions: resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunology, Immunotherapy: CII (2008) ; 57: (11): 1727–33. |
[25] | Thielens A , Vivier E , Romagné F NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Curr Opin Immunol (2012) ; 24: (2): 239–45. |
[26] | Zaretsky JM , Garcia-Diaz A , Shin DS et al,. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. The New England Journal of Medicine (2016) ; 375: (9): 819–29. |
[27] | McGranahan N , Rosenthal R , Hiley CT et al,. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell (1271) ; 171: (6): 1259–1271.e1211. |
[28] | Zhou F Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol (2009) ; 28: (3-4): 239–60. |
[29] | de Biasi AR , Villena-Vargas J , Adusumilli PS Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res (2014) ; 20: (21): 5384–91. |
[30] | Paulson KG , Voillet V , McAfee MS et al,. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nature communications (2018) ; 9: (1): 3868. |
[31] | Rodig SJ , Gusenleitner D , Jackson DG et al,. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med 10(450) (2018) ;10: (450). |
[32] | Im JS , Herrmann AC , Bernatchez C et al,. Immune-Modu-lation by Epidermal Growth Factor Receptor Inhibitors: Implication on Anti-Tumor Immunity in Lung Cancer. PLoS Onee (2016) ; 11: (7): e0160004. |
[33] | Powles T , Plimack ER , Soulières D et al,. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. The Lancet Oncology (2020) ; 21: (12): 1563–73. |




