Systematic Review and Meta-Analysis of Cisplatin Based Neoadjuvant Chemotherapy in Muscle Invasive Bladder Cancer
Abstract
BACKGROUND:
Cisplatin-based neoadjuvant chemotherapy is the standard of care for muscle invasive bladder cancer (MIBC).
OBJECTIVE:
To compare the efficacy and safety of the two most commonly used cisplatin-based regimens; gemcitabine, and cisplatin (GC) vs. accelerated (dose-dense: dd) or conventional methotrexate, vinblastine, adriamycin, and cisplatin (MVAC).
METHODS:
We searched MEDLINE, Embase, Scopus and other sources. Outcomes of interest included overall survival, downstaging to pT≤1, pathologic complete response (pCR), recurrence, and toxicity. Meta-analysis was conducted using the random-effects model.
RESULTS:
We identified 24 studies. Efficacy outcomes were comparable between MVAC and GC for MIBC. dd-MVAC was associated with favorable efficacy compared to GC in terms of downstaging (OR 1.45; 95%CI 1.15–1.82) and all-cause mortality at longest follow-up (OR 0.63; 95%CI 0.44–0.81). However, GC was associated with a better safety profile in terms of febrile neutropenia (OR 0.32; 95%CI 0.13–0.80), anemia (OR 0.32; 95%CI 0.18–0.54), nausea and vomiting (OR 0.27; 95%CI 0.12–0.65) compared to dd-MVAC. Compared to MVAC, patients receiving GC had an increased risk of developing grade 3–4 thrombocytopenia (OR 4.70; 95%CI 1.59–13.89) and a lower risk of nausea and vomiting (OR 0.05; 95%CI 0.01–0.31). Certainty in the estimates was very low for most outcomes.
CONCLUSIONS:
Efficacy and safety outcomes were comparable between MVAC and GC for MIBC. Including non-peer-reviewed studies showed higher efficacy with dd-MVAC. A phase III randomized trial comparing the two regimens is needed to guide clinical practice.
INTRODUCTION
Bladder cancer remains the a common cancer worldwide with at least half a million new cases annually [1]. While 75%of patients have a non-mus-cle invasive disease at diagnosis, the rest will pre-sent with muscle invasive or advanced disease [2]. Following initial endoscopic removal of bladder tumors, further treatment is often required for muscle-invasive bladder cancer (MIBC) [3], which includes cystectomy (partial or radical), neoadjuvant or adjuvant therapy.
Cisplatin-based neoadjuvant chemotherapy (NAC) has been shown to provide a 5-year disease-free survival (DFS) benefit of close to 10%in patients with MIBC who underwent cystectomy [4–10]. Therefore, it has become a category 1 recommendation for MIBC in patients who are cisplatin-eligible and have operable disease, but there is lack of consensus with regard to the optimal cisplatin regimen [11–13]. Conventional methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) was among the first developed NAC regimens for MIBC with a pathologic complete response (pCR) rate of 38%[7].
Accelerated (or dose-dense: dd) MVAC with pegfilgrastim support was later developed to shorten time to surgery and was shown to be safe and effective [4, 14]. In the metastatic setting, the “newer regimen” gemcitabine and cisplatin (GC) was shown to be effective yet less toxic as compared to MVAC [15–17]. Similar to reasons behind developing dd MVAC, dd GC has been tested as NAC in MIBC and showed comparable pCR rates [6, 18]. Of note, grade 3/4 vascular events occurred in 9%of the first dd GC study [6] and precluded, delayed, or increased the risk of surgery for 23%of patients in the other dd GC study resulting in its early closure [18]. In light of a recent reported clinical trial comparing the dd-MVAC and conventional GC NAC regimens [19], we performed a systematic review and meta-analysis to compare GC with MVAC (including dd-MVAC) in the neoadjuvant setting.
MATERIALS AND METHODS
The reporting of this systematic review follows the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement [20]. As a systematic review of the literature, and as no animal or human research was involved, our study is exempt from any requirement for Institutional Review Board approval.
Eligibility criteria
We only included studies that (1) assessed patients with MIBC, (2) directly compared GC with MVAC (including dd MVAC) as neoadjuvant chemotherapy, and (3) reported at least one of the following outcomes: overall and relapse-free survival, pathologic response, and toxicity. We excluded noncomparative studies, studies without original data, mixed pop-ulation, intervention or comparison not of interest or those that did not provide sufficient data for meta-analysis. Prior systematic reviews were used for cross-referencing. Abstracts without a full text article were included if they met the inclusion criteria.
Data sources and search strategies
A comprehensive search of several databases from inception to March 2, 2020, limited to English language and excluding animal studies, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by a medical reference librarian with input from the study investigators. Controlled vocabulary supplemented with keywords was used to search for studies of interest. The actual strategy listing all search terms used and how they are combined is available in the appendix (Table s1).
Study selection
Three independent reviewers (RB, TN, SP) scr-eened all the titles and abstracts based on the chosen inclusion and exclusion criteria. Relevant references were retrieved in full text and were further evaluated against the eligibility criteria. Disagreements were resolved by consensus.
Data extraction
Three reviewers (RB, TN, SP) independently extracted data using standardized, pilot-tested forms created in Microsoft Excel. Disagreements were resolved by discussion between the reviewers. We extracted the following variables from each study: study characteristics, participants’ characteristics, intervention details, and outcomes of interest (total sample size and number of events in each group).
Outcomes
Outcomes of interest included overall survival (OS) at 1 year, 2 years, and at the longest follow-up, recurrence, pathologic complete response (pCR), downstaging, and toxicities (neutropenia, febrile neutropenia, anemia, thrombocytopenia, cardiac, nausea/vomiting and mucositis). OS was calculated from therapy start date till death. Patients with no confirmed death date were censored at last contact date. pCR was defined as pT0pN0 or pTispN0 in pathologic assessment. Downstaging was defined as pT≤1pN0 in cystectomy pathologic assessment.
Methodologic quality and risk of bias
We used the Newcastle-Ottawa scale [21] to assess risk of bias, in non-randomized studies focusing on cohort selection, outcome ascertainment, controlling for age and sex, and adequacy of follow-up. Cochrane risk-of-bias tool was used to evaluate randomized trials by examining: generation of allocation, concealment of allocation, blinding of participants, caregivers, data collectors, and outcome assessors, incomplete outcome data, selective outcome reporting and any other potential source of bias. Based on these factors, the risk of bias for each study was low, moderate, or high. Three reviewers (RB, TN, SP) independently assessed the risk of bias. Any conflicts were resolved by consensus.
Statistical analysis
For overall survival at 1, 2 years and at the lon-gest follow-up, pCR, downstaging, treatment-related death and toxicity outcomes, we calculated or extracted odds ratio (OR). For overall survival, we also calculated or extracted hazard ratio (HR). The DerSimonian and Laird random effects methods [22] were used to pool outcomes across studies. We conducted the analysis accounting for the intention-to-treat (ITT) principle. We conducted subgroup analyses to explore heterogeneity between studies based on whether the publication is peer-reviewed (i.e., journal article vs conference abstract). Heterogeneity across the included studies was estimated using I2 statistic, in which≥50%suggests substantial heterogeneity. Publication bias was assessed using funnel plots and Egger’s test [23]. When Egger’s test yielded a statistically significant result, we used Duval & Tweedie’s trim-and-fill procedure to adjust for funnel plot asymmetry. The trim-and-fill procedure estimates the effect size of missing small studies and adds them into the funnel plot until symmetry is reached [24]. Statistical analyses were completed using R version 3.6.3 (R Core Team, 2020).
Grading the Certainty of Evidence
We applied the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to rate the certainty of evidence (CoE). High initial certainty is assigned to the evidence resulting from randomized controlled trials, while evidence from observational studies and nonrandomized clinical trials start at low initial certainty. Then CoE is rated down on outcome bases for risk of bias, inconsistency (i.e., heterogeneity), indirectness, imprecision or publication bias [25].
RESULTS
Study characteristics
A total of 620 titles and abstracts were identified by screening the references retrieved from the electronic search strategy, of which 96 full-text articles were screened for eligibility. Twenty-four studies [19, 26–48] reporting on 3,591 patients were included in the qualitative synthesis (Fig. 1). Of which, 7 were international conference abstracts [19, 34–37, 44, 46] including a phase 3 randomized-controlled clinical trial recently presented by Culine et al. [19] at the annual American Society of Clinical Oncology Genitourinary Symposium 2020, San Francisco, CA. Eighteen studies [26–33, 38–47] evaluated GC versus MVAC. One study [42] was found to have three arms comparing GC versus MVAC versus dd-MVAC. The rest [19, 34–37, 42, 48] were comparing GC versus dd-MVAC. All of the included peer-reviewed studies [26–33, 38–43, 45, 47, 48] had a retrospective cohort design. We were not able to assess risk of bias for the international conference abstracts due to the unavailability of the required information. Baseline characteristics and quality of all included studies are fully detailed in Table 1 and Tables s2-s3 in the appendix.
Fig. 1
Flowchart demonstrating the process of study selection.
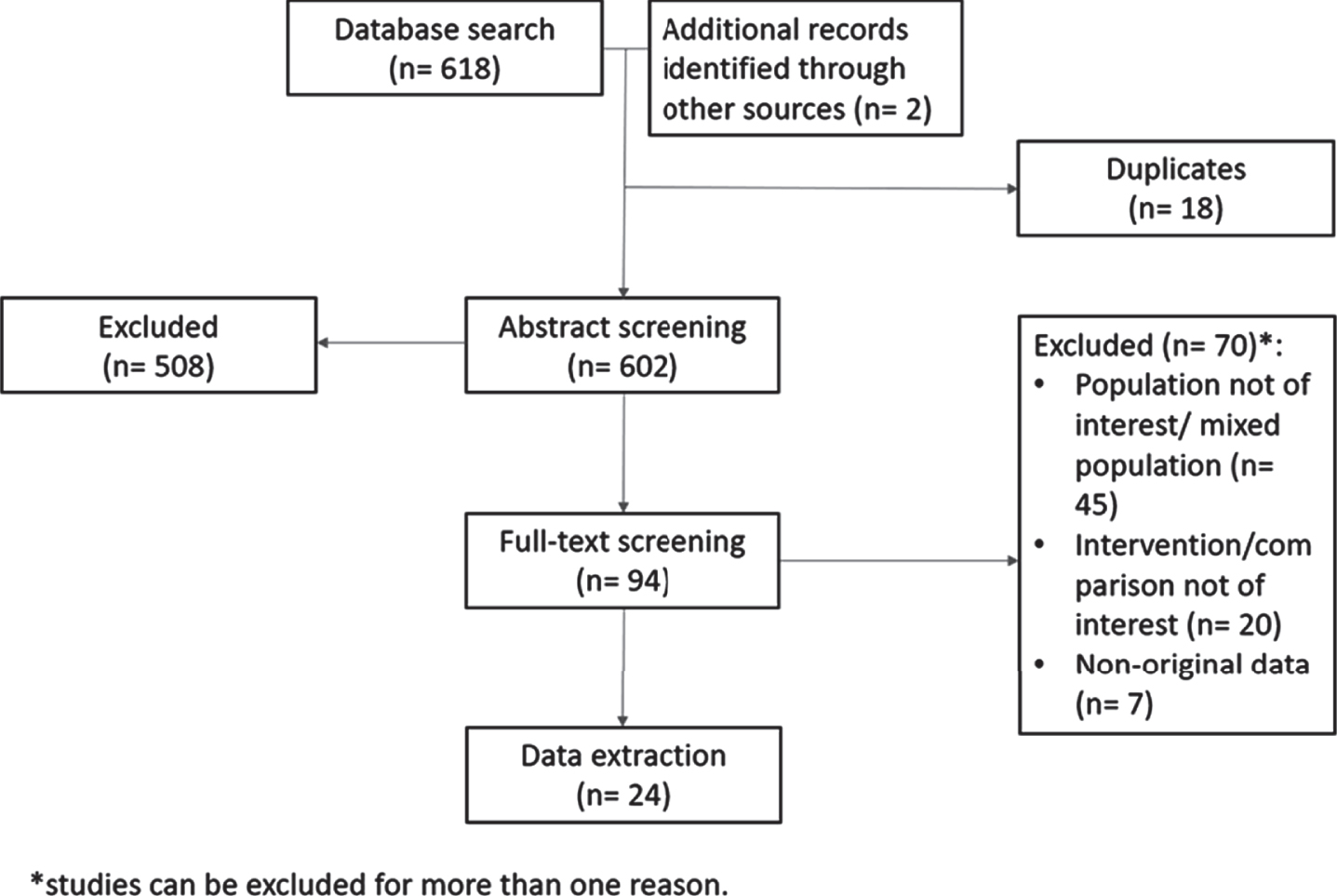
Table 1
Baseline characteristics of included patients; * median
| Author last name, year (country) | Number of patients | Age (mean±SD, years) | Males n (%) | Clinical staging n (%) | ||||||||
| MVAC | GC | dd-MVAC | MVAC | GC | dd-MVAC | MVAC | GC | dd-MVAC | MVAC | GC | dd-MVAC | |
| Conference abstracts | ||||||||||||
| Mitra, 2011 (USA) | NR | 23 | 15 | NR | NR | NR | NR | NR | NR | NR | T2-4 23(100) | T2-4 15(100) |
| Wright, 2013 (USA) | 32 | 46 | NR | NR | NR | NR | NR | NR | NR | T2-4 32(100) | T2-4 46 (100) | NR |
| Yokomizo, 2013 (Japan) | 55 | 46 | NR | NR | NR | NR | NR | NR | NR | T2- T4 55(100) | T2- T4 46(100) | NR |
| Matulay, 2019 (USA) | NR | 88 | 265 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lee, 2019 (South Korea) | NR | 176 | 41 | NR | NR | NR | NR | NR | NR | NR | T2-4 176(100) | T2-4 41(100) |
| Miron, 2019 (USA) | NR | 24 | 34 | NR | NR | NR | NR | NR | NR | NR | T2-4 24(100) | T2-4 34(100) |
| Culine, 2020 (France) | NR | 245 | 248 | NR | 63* | 63* | NR | 206(84) | 201(81) | NR | T2 210(85.7) | T2 200(80.6) |
| > T2 34(13.9) | > T2 47(19) | |||||||||||
| Peer-review journal articles | ||||||||||||
| Dash, 2008 (USA) | 54 | 42 | NR | 63* | 64* | NR | 43(79.6) | 32(76.2) | NR | T2 32(59.3) | T2 19(45.2) | NR |
| T3 15(27.7) | T3 19(45.2) | |||||||||||
| T3 7(13) | T4 4 (9.6) | |||||||||||
| Weight, 2009 (USA) | 4 | 20 | NR | NR | NR | NR | NR | NR | NR | T2-4 4(100) | T2-4 20(100) | NR |
| Kaneko, 2011 (Japan) | 9 | 22 | NR | 62 | 69 | NR | 8(88.9) | 16(72.7) | NR | T1 1(11.1) | T2 15(68.2) | NR |
| T2 4(44.4) | T3 6(27.3) | |||||||||||
| T3 1(11.1) | T4 1(4.5) | |||||||||||
| T4 3(33.3) | ||||||||||||
| Alva, 2011 (USA) | 12 | 20 | NR | NR | NR | NR | NR | NR | NR | T2 -4 12(100) | T2-4 20(100) | NR |
| Pal, 2012 (USA) | 22 | 24 | NR | 60* | 68.6* | NR | 20(90.9) | 19(79.2) | NR | T2 18(81.8) | T2 22(91.7) | NR |
| T3 1(4.5) | T3 2(8.3) | |||||||||||
| T4 2(9.1) | T4 0(0) | |||||||||||
| Yeshchina, 2012 (USA) | 45 | 16 | NR | NR | NR | NR | NR | NR | NR | T2-T4a 45(100) | T2-T4a 16(100) | NR |
| Shindo, 2012 (Japan) | 17 | 10 | NR | 64* | 61.5* | NR | 16(94) | 8(80) | NR | T2-4 17 (100) | T2-4 10 (100) | NR |
| Fairey, 2013 (USA) | 58 | 58 | NR | 63 | 67 | NR | 45(77.6) | 44(75.9) | NR | T2 28(48.3) | T2 28(48.3) | NR |
| T3 14(24.1) | T3 18(31) | |||||||||||
| T4 16(27.6) | T4 12(20.7) | |||||||||||
| Lee, 2013 (USA) | 31 | 41 | NR | NR | NR | NR | 28(90.3) | 31(75.6) | NR | T2 14(45.2) | T2 23(56.1) | NR |
| T3 9(29) | T3 11(26.8) | |||||||||||
| T4 8(25.8) | T4 7(17.1) | |||||||||||
| Kawamura, 2013 (Japan) | 44 | 14 | NR | 64±10.25 | 68±8.75 | NR | 37(84.1) | 10 (71.4) | NR | T2a/b 11 (25) | T2a/b 6 (42.9) | NR |
| T3a 6 (13.6) | T3a 0 (0), | |||||||||||
| T3b 21 (47.7) | T3b 7 (50) | |||||||||||
| T4a 5 (11.4), | T4a 0 (0), | |||||||||||
| T4b 1 (2.3) | T4b 1 (7.1) | |||||||||||
| Zargar, 2015 (International) | 183 | 602 | NR | 62±8.9 | 65±10.4 | NR | 145(79.2) | 472(78.4) | NR | T2-4 183(100) | T2-4 602(100) | NR |
| Van De Putte, 2015 (Netherlands) | 35 | 51 | 80 | 59±9 | 63±8 | 57±8 | 26(74.3) | 36(70.6) | 60(75) | T2 15(42.9) | T2 11(21.6) | T2 25(31.3) |
| T3 6(17.1) | T3 25(49) | T3 30(37.4) | ||||||||||
| T4 14(40) | T4 15(29.4) | T4 25(31.3) | ||||||||||
| Galsky, 2015 (International) | 66 | 146 | NR | 63* | 63* | NR | NR | NR | NR | T2 41(62.1) | T2 90(61.6) | NR |
| T3 17(25.8) | T3 40(27.4) | |||||||||||
| T4 8(12.1) | T4 16(11) | |||||||||||
| Fukui, 2016 (Japan) | 21 | 37 | NR | NR | NR | NR | NR | NR | NR | T2-4 21(100) | T2-4 37 | NR |
| Zargar, 2018 (International) | NR | 219 | 100 | NR | 67±10.4 | 61±7.4 | NR | 171(78.1) | 74(74) | NR | T2-4 219(100) | T2-4 100(100) |
| Nguyen, 2018 (France) | 23 | 4 | NR | 62* | 70* | NR | 20(87) | 3(75) | NR | T1 2(8.7) | T2 3(75) | NR |
| T2 20(87) | T3 1(25) | |||||||||||
| T3 1(4.3) | ||||||||||||
| T4 0(0) | ||||||||||||
| Okabe, 2018 (Japan) | 74 | 58 | NR | 59.4±9 | 68±8.6 | NR | 65(87.8) | 51(87.9) | NR | T2 24(32.4) | T2 22(37.9) | NR |
| T3 36(48.6) | T3 29(50) | |||||||||||
| T4 14(19) | T4 7(12.1) | |||||||||||
Results of all performed analyses are summarized in Table 2. Forest plots of the remaining outcomes including subgroup analyses can be found in Figures s1-22 in the appendix. Tables s4-s5 in the appendix contain details of CoE of all reported outcomes.
Table 2
Results of analyses on all studied outcomes
| Outcome | subgroup | Comparison | Number of studies | Number of patients | Effect measure (95%Cl) | Heterogeneity (P; I2(%)) |
| All- cause mortality | At 1 year | GC vs MVAC | 3 (28, 39, 40) | 140 vs 154 | OR 0.84 (0.43–1.67) | 0.25; 28 |
| At 2 years | GC vs MVAC | 3 (28, 39, 40) | 140 vs 154 | OR 0.80 (0.50–1.30) | 0.57; 0 | |
| Longest follow up | GC vs MVAC | 6(26, 28, 30, 32, 39, 40) | 320 vs 276 | OR 0.67 (0.35–1.32) | 0.02; 63 | |
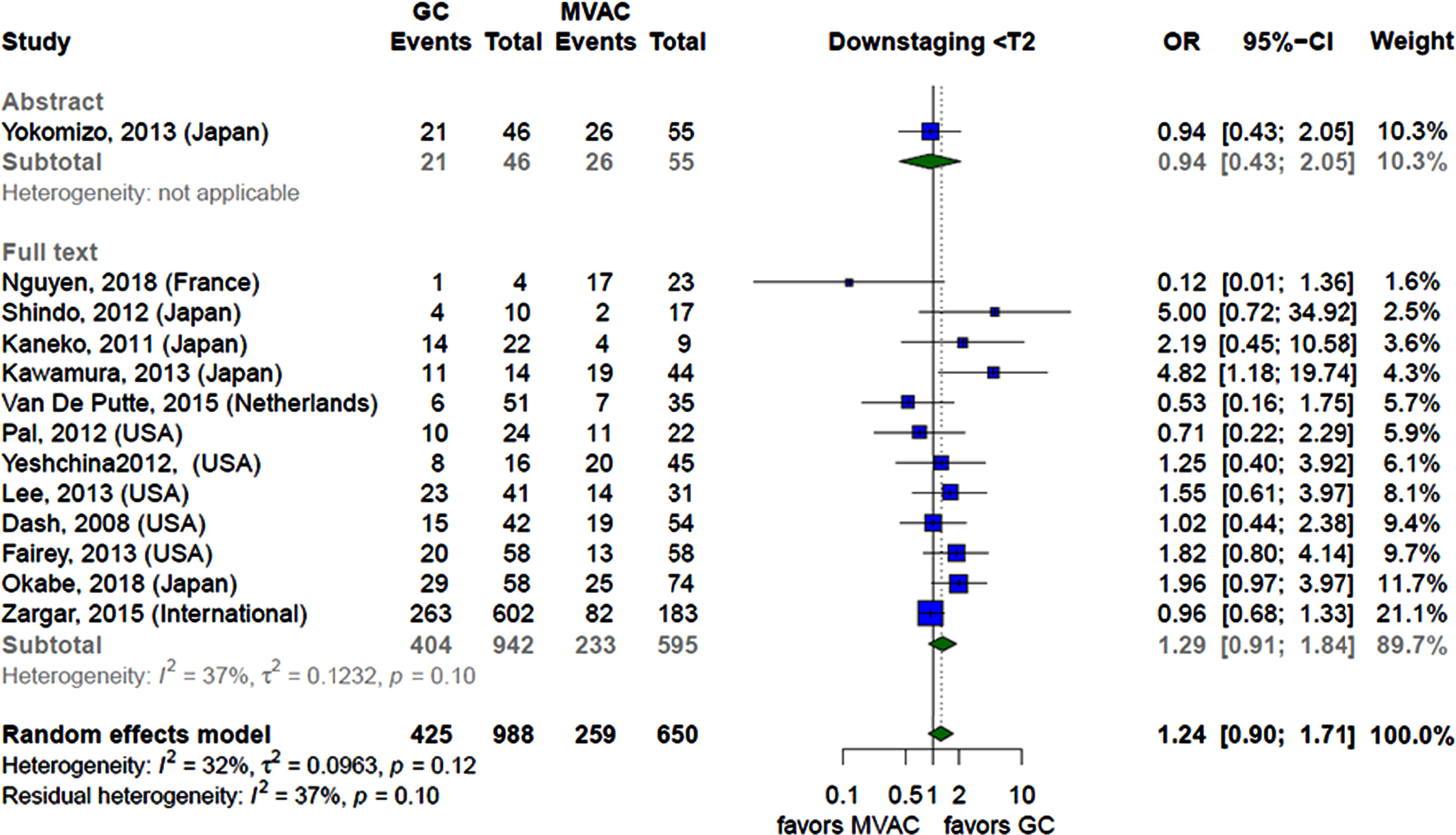
| GC vs dd-MVAC | 4(34–36, 48) | 507 vs 440 | OR 1.68 (1.23–2.28) | 0.53; 0 | ||
| Overall survival | GC vs MVAC | 5(26, 28, 38, 45, 47) | 700 vs 321 | HR 0.97 (0.43–2.19) | 0.87; 0 | |
| Recurrence | At 1 year | GC vs MVAC | 3(27, 28, 39) | 158 vs 186 | OR 1.13 (0.62–2.03) | 0.30; 16 |
| At 2 years | 3(27, 28, 39) | 158 vs 186 | OR 0.92 (0.57–1.46) | 0.41; 0 | ||
| Longest follow up | 3(27, 28, 39) | 158 vs 186 | OR 0.75 (0.32–1.74) | 0.09; 58 | ||
| pCR | GC vs MVAC | 15(27, 28, 30–33, 39–47) | 1196 vs 729 | OR 1.20 (0.95–1.51) | 0.58; 0 | |
| GC vs dd-MVAC | 6(19, 34, 35, 37, 42, 48) | 802 vs 749 | OR 0.81 (0.59–1.12) | 0.19; 33 | ||
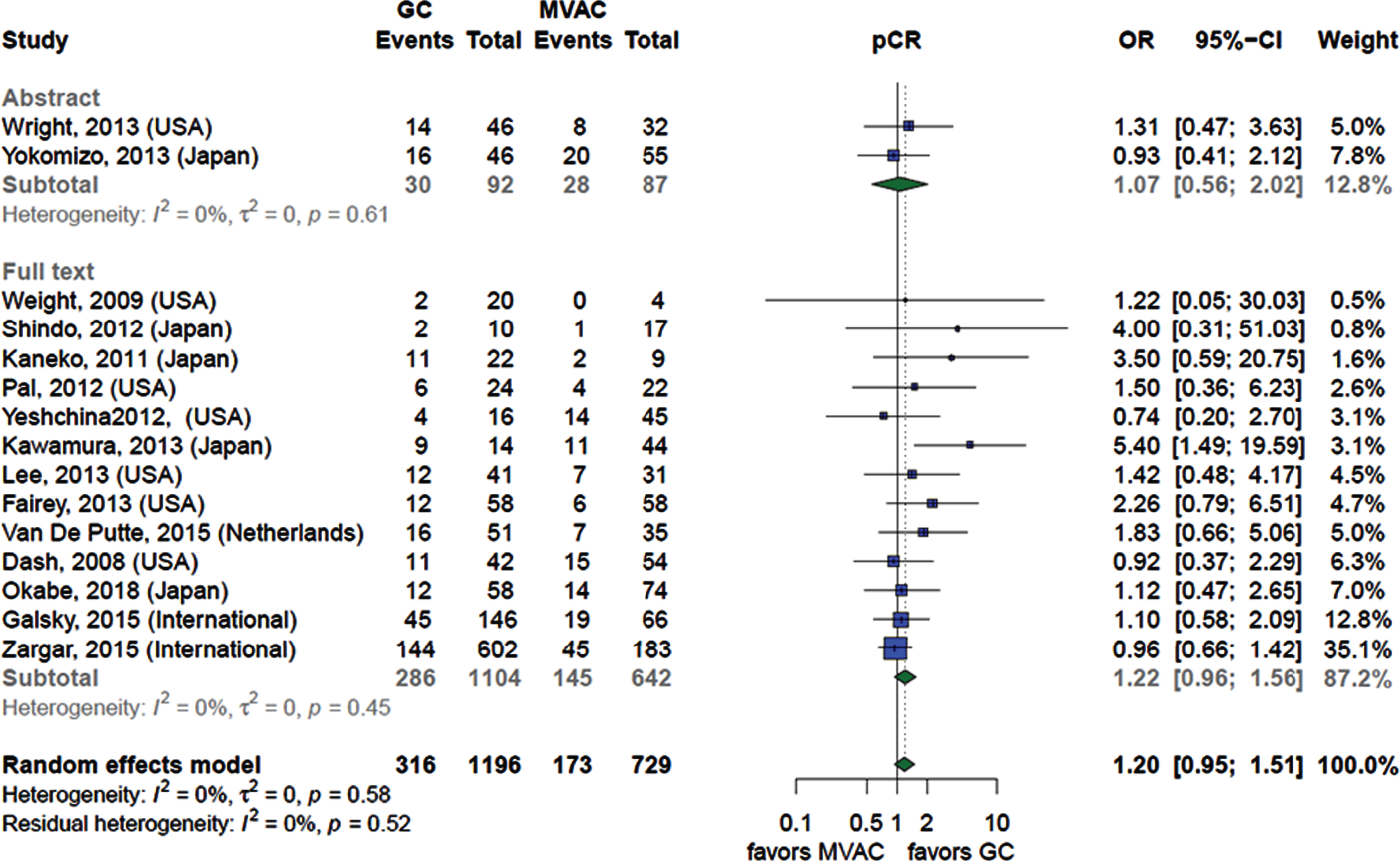
| Downstaging | GC vs MVAC | 13(27, 28, 31–33, 38–42, 45–47) | 988 vs 650 | OR 1.24 (0.90–1.71) | 0.12; 32 | |
| GC vs dd-MVAC | 6(19, 34, 35, 37, 42, 48) | 803 vs 749 | OR 0.69 (0.55–0.87) | 0.87; 0 | ||
| Febrile Neutropenia | GC vs MVAC | 4(31, 32, 41, 42) | 97 vs 105 | OR 0.35 (0.07–1.75) | 0.29; 21 | |
| GC vs dd-MVAC | 2(19, 42) | 296 vs 328 | OR 0.32 (0.13–0.80) | 0.44; 0 | ||
| Neutropenia | GC vs MVAC | 3(31, 32, 41) | 46 vs 70 | OR 1.31 (0.43–3.98) | 0.28; 21 | |
| GC vs dd-MVAC | 1(19) | 245 vs 248 | OR 1.33 (0.93–1.91) | – | ||
| Anemia | GC vs MVAC | 4(31, 32, 41, 42) | 97 vs 105 | OR 0.81 (0.20–3.22) | 0.39; 1 | |
| GC vs dd-MVAC | 2(19, 42) | 296 vs 328 | OR 0.32 (0.18–0.54) | 0.46; 0 | ||
| Thrombocytopenia | GC vs MVAC | 4(31, 32, 41, 42) | 97 vs 105 | OR 4.70 (1.59–13.89) | 0.66; 0 | |
| GC vs dd-MVAC | 2(19, 42) | 296 vs 328 | OR 0.80 (0.51–1.26) | 0.53; 0 | ||
| Cardiac Toxicity | GC vs dd-MVAC | 1(19) | 245 vs 248 | OR 1.08 (0.53–2.19) | – | |
| Nausea/Vomiting | GC vs MVAC | 2(31, 32) | 36 vs 53 | OR 0.05 (0.01–0.31) | 0.28; 13 | |
| GC vs dd-MVAC | 1(19) | 245 vs 248 | OR 0.27 (0.12–0.65) | – | ||
| Mucositis | GC vs MVAC | 2(31, 32) | 36 vs 53 | OR 0.24 (0.02–2.50) | 0.71; 0 |
Findings
GC vs MVAC
Overall Survival. Six retrospective peer reviewed studies [26, 28, 30, 32, 39, 40] compared overall survival at different time points between GC and MVAC. The analysis showed that neither of the two regimens was significantly associated with reduction in mortality at one year, at two years and at the longest follow up. The CoE was very low due to study design and severe imprecision.
Pathological complete response (pCR). Fifteen retrospective cohort studies [27, 28, 30–33, 39–47] compared pCR between GC and MVAC. The analysis showed no significant difference in achieving pCR between the two groups. The analysis was consistent when comparing the results of the thirteen peer-[27, 28, 30–33, 39–43, 45, 47] and two non-peer reviewed studies [44, 46] (Fig. 2).
Fig. 2
Forest plot comparing the rates of achieving pathological complete response between patients receiving GC and MVAC stratified by the type of publication.
We assessed the publication bias using funnel plot and Egger’s test. The funnel plot (Figure s23) showed asymmetry suggesting the presence of publication bias which was confirmed when Egger’s test yielded a statistically significant result (p-value < 0.02). We ran Duval & Tweedie’s trim-and-fill procedure to estimate the effect size of the missing small studies. The trim-and-fill procedure added 6 studies to the funnel plot (Figure s24) to achieve symmetry and the overall effect size became less prominent with the same statistical insignificance OR (1.03; 95%CI 0.79–1.36; very low CoE due to study design, methodological limitations, imprecision and publication bias).
Downstaging. Thirteen retrospective cohort studies [27, 28, 31–33, 38–42, 45–47) compared downstaging between GC and MVAC. The analysis showed no significant difference between the two groups. The analysis was consistent when comparing the results of the twelve peer-27, 28, 31–33, 38–42, 45, 47] and one non-peer reviewed studies [46] (Fig. 3).
Fig. 3
Forest plot comparing the rates of achieving a pT≤1 stage of between patients receiving GC and MVAC stratified by the type of publication.
We assessed the publication bias using funnel plot and Egger’s test. The funnel plot (Figure s25) was symmetric excluding the presence of publication bias which was confirmed when Egger’s test yielded a statistically insignificant result (p-value > 0.05).
The CoE was very low due to study design, methodological limitations and imprecision.
Recurrence probability. Three retrospective peer reviewed studies [27, 28, 39] compared the probability of recurrence at different timepoints between GC and MVAC. The analysis did not show any significant difference between the two groups at one year, at two-year intervals as well as at the longest follow-up. The CoE was very low due to study design, methodological limitations and severe imprecision.
Grade 3–4 toxicity analysis. Grade 3–4 neutropenia, febrile neutropenia, anemia, thrombocytopenia, nausea/vomiting, mucositis, and cardiac toxicity were assessed in six retrospective peer reviewed studies [19, 31, 32, 39, 41, 42].
The analysis showed that receiving GC significantly increased the risk of developing grade 3–4 thrombocytopenia (OR 4.70; 95%CI 1.59–13.89) but decreased the risk of nausea and vomiting (OR 0.05; 95%0.01–0.31) when compared to MVAC. There was no statistical difference between the two regimens in developing mucositis, neutropenia, febrile neutropenia and anemia. The CoE is very low due to study design, methodological limitations and imprecision.
GC vs dd-MVAC
Overall Survival. Four retrospective cohort studies [34–36, 48] compared overall survival between GC and dd-MVAC at the longest follow-up. Compared with GC, dd-MVAC was associated with reduction in mortality (OR 0.63; 95%CI 0.44–0.81). The analysis was consistent when comparing the results of the one peer-[48] and the three non-peer reviewed studies [34–36] (Fig. 4).The CoE was very low due to study design and methodological limitations.
Fig. 4
Forest plot comparing all-cause mortality rates at the longest follow up between patients receiving GC and dd-MVAC stratified by the type of publication.
Pathological complete response (pCR). Six studies [19, 34, 35, 37, 42, 48] compared pCR between GC and dd-MVAC. The analysis showed no significant difference between the two regimens. The analysis was consistent when comparing the results of the two peer-[42, 48] and the four non-peer reviewed studies [19, 34, 35, 37]. Of the included studies, only one RCT [19] was found to report the outcome of interest, the results of which were consistent with those of the remaining five retrospective cohort studies [34, 35, 37, 42, 48]. The CoE was very low due to study design, methodological limitations and imprecision.
Downstaging. Six studies [19, 34, 35, 37, 42, 48] compared downstaging between GC and dd-MVAC. The analysis favored dd-MVAC over GC for this outcome (OR 0.69; 95%CI 0.55–0.87; very low CoE due to study design and methodological limitations) (Fig. 5). The analysis showed different results when assessing the outcome based on the type of publication. Four of the included studies [19, 34, 35, 37] were non-peer reviewed and reported consistent findings, also favoring dd-MVAC over GC (OR 0.68; 95%CI 0.53–0.89). The analysis of the remaining two peer reviewed studies [42, 48] showed no significant difference between the two regimens. The results were significant and consistent when assessing the outcome based on study design. Only one RCT [19] was found to report on downstaging and favored dd-MVAC over GC (OR 0.65; 95%CI 0.45–0.92; moderate CoE due to methodological limitations). The remaining five retrospective cohort studies [34, 35, 37, 42, 48] yielded similar results, also favoring dd- MVAC (OR 0.72; 95%CI 0.54–0.97; very low CoE due to study design and methodological limitations).
Fig. 5
Forest plot comparing the rates of achieving a pT≤1 stage between patients receiving GC and dd-MVAC stratified by the type of publication.
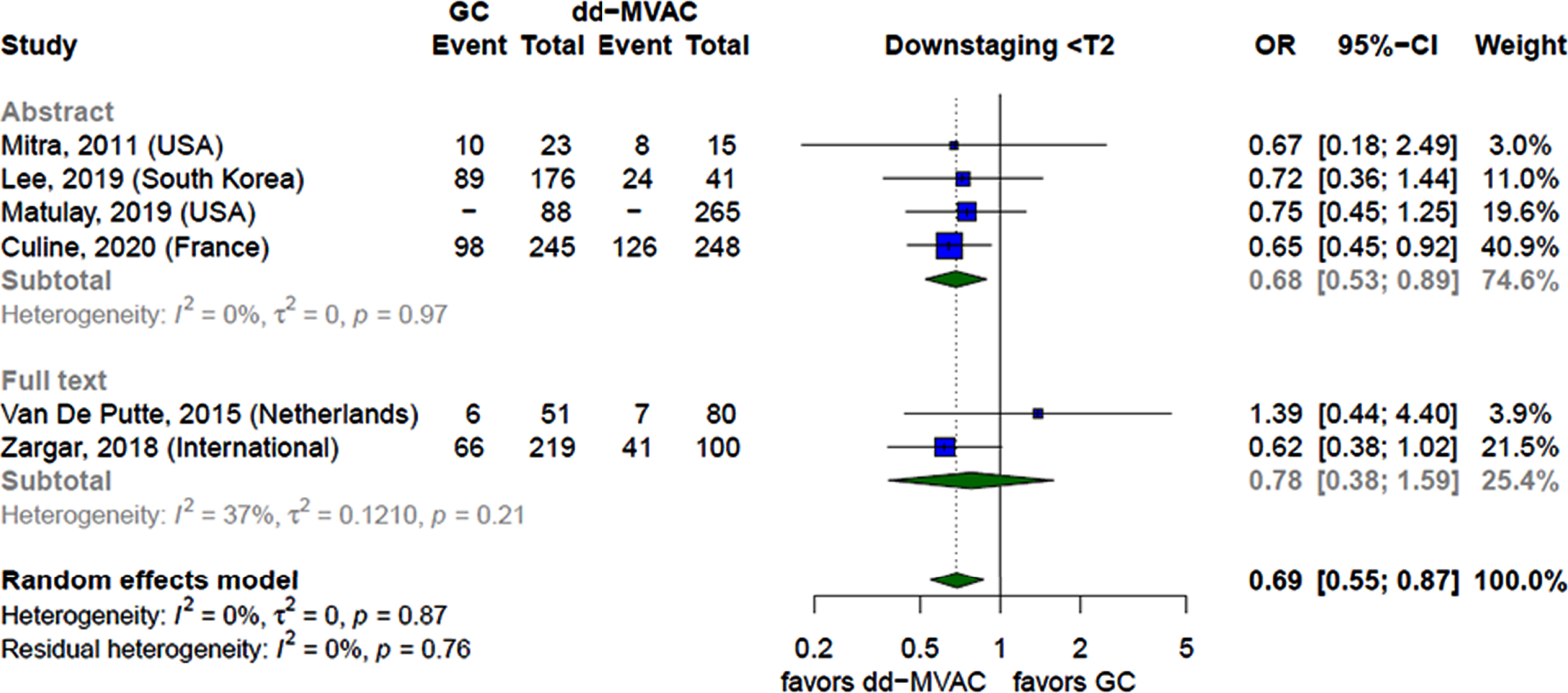
Grade 3–4 toxicity analysis. Overall, two studies [19, 42] evaluated the risk of developing grade 3–4 toxicities between GC and dd-MVAC. The analysis showed that receiving GC significantly reduced the risk of developing febrile neutropenia (OR 0.32; 95%CI 0.13–0.80), anemia (OR 0.32; 95%CI 0.18–0.54) and nausea and vomiting (OR 0.27; 95%CI 0.12–0.65) compared to dd-MVAC. CoE was low due to methodological limitations and imprecision. Even after the addition of prophylactic Granulocyte Colony-Stimulating Factor (G-CSF) to dd- MVAC, the incidence of febrile neutropenia remained significantly lower in the group of patients who received GC (p = 0.004) [34]. No regimen demonstrated a favorable safety profile over the other in terms of developing neutropenia, thrombocytopenia or cardiac toxicity. CoE was very low due to methodological limitations and severe imprecision. Of the included studies, one non-peer reviewed RCT [19] reported significant and consistent results favoring GC over dd-MVAC in terms of developing febrile neutropenia (OR 0.36; 95%CI 0.14–0.80), anemia (OR 0.30; 95%CI 0.17–0.54) and nausea and vomiting (OR 0.27; 95%CI 0.12–0.65). Neither of the two regimens demonstrated a favorable safety profile over the other in terms of developing neutropenia, thrombocytopenia or cardiac toxicity. The other one was a peer reviewed retrospective cohort study [42], the results of which were insignificant and inconsistent with the results of the included RCT [19].
DISCUSSION
In this systematic review and meta-analysis of patients with MIBC treated with the two most common cisplatin-based neoadjuvant chemotherapies, we demonstrate that MVAC and GC may have comparable efficacy and safety outcomes. Non-peer-reviewed studies showed higher downstaging rates with dd-MVAC at the expense of febrile neutropenia, anemia, nausea and vomiting. Higher downstaging rates have been linked to survival advantage [8], and this is consistent with our findings that showed favorable survival outcomes with dd-MVAC when we analyzed non-peer-reviewed studies.
Our study included 24 studies that met the eligibility criteria with a total patient population of 3591. In 2016, Yin et al. [49] assessed a total of 1,766 patients from 13 retrospective studies and found no significant difference in pCR between MVAC and GC. On the other hand, a later meta-analysis done by Yu et al. [50], which included 2174 patients from 13 retrospective studies found that pCR rates were higher among patients receiving GC as compared to those receiving MVAC. Our review included a higher number of studies and patients; however, we did not find a significant difference between MVAC and GC in terms of pCR. Additionally, our review shed light on studies comparing dd-MVAC to GC which has not been published previously.
Our study faces several limitations, the most important of which is the lack of randomized com-parisons. Therefore, the results are subject to confounding and selection bias. Some studies were not peer reviewed and were only available as abstracts, with minimal details about risk of bias. In addition, the included studies had some differences in their chemotherapy protocols (dd-MVAC vs conventional MVAC), patient selection and follow up time. Moreover, evidence on the addition of G-CSF was lacking. Lee et al. [34] was the only study that addressed this question. And finally, the CoE of most of the outcomes was very low, hence caution is advised when interpreting the results.
The phase III CETUG/AFU V05 VESPER trial (NCT01812369) compares dd-MVAC to GC with the primary outcome of progression-free survival (PFS) at 3 years, and the secondary outcomes of pCR and safety. Preliminary results from this trial showed higher pCR and downstaging rates in the dd-MVAC arm, which was at the expense of higher gastrointestinal grade≥3 toxicities in this arm. PFS data was not mature at this interim analysis. Findings from our study agree with the preliminary results from this trial; however, our findings remain based on retrospective data. Therefore, awaiting the final analysis of NCT01812369 is essential to help guide clinical practice.
CONCLUSION
The available literature comparing MVAC and GC neoadjuvant therapy is retrospective in nature. Efficacy and safety outcomes may be comparable between the two regimens for MIBC. Analysis of non-peer-reviewed studies suggested higher efficacy with dd-MVAC but increased toxicity. A Large randomized trial comparing dd-MVAC to GC is ongoing and will help provide more definitive evidence to guide clinical practice. Future research should explore post NAC cystectomy completion rates and the possible association between the different components of NAC (number of completed chemotherapy cycles, dosages, co-administered medications, etc.) and the outcomes of interest.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Raed Benkhadra, MD: conception; performance of work; interpretation of data; writing the article.
Tarek Nayfeh, MD: conception; performance of work; interpretation of data; writing the article.
Sai Krishna Patibandla, MD: conception; performance of work; interpretation of data; writing the article.
Chelsea Peterson, DO: conception; performance of work; interpretation of data; writing the article
Larry Prokop, MLS: conception; performance of work; interpretation of data; writing the article
Omar Alhalabi, MD: conception; performance of work; interpretation of data; writing the article
M. Hassan Murad, MD, MPH: conception; performance of work; interpretation of data; writing the article
Shifeng S Mao, MD, PhD: conception; performance of work; interpretation of data; writing the article
ETHICAL CONSIDERATIONS
As a systematic review of the literature, and as no animal or human research was involved, our study is exempt from any requirement for Institutional Review Board approval.
CONFLICT OF INTEREST
Raed Benkhadra, Tarek Nayfeh, Sai Krishna Patibandla, Chelsea Peterson, Larry Prokop, Omar Alhalabi, M. Hassan Murad and Shifeng S. Mao declare that they have no conflict of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-201511.
REFERENCES
[1] | Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. (2018) ;68: (6):394–424. |
[2] | Kamat AM , Hahn NM , Efstathiou JA , Lerner SP , Malmström P-U , Choi W , Guo CC , Lotan Y , Kassouf W . Bladder cancer. The Lancet. (2016) ;388: (10061):2796–810. |
[3] | Herr HW , Sogani PC . Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? The Journal of Urology. (2001) ;166: (4):1296–9. |
[4] | Plimack ER , Hoffman-Censits JH , Viterbo R , Trabulsi EJ , Ross EA , Greenberg RE , Chen DYT , Lallas CD , Wong YN , Lin JQ , Kutikov A , Dotan E , Brennan TA , Palma N , Dulaimi E , Mehrazin R , Boorjian SA , Kelly WK , Uzzo RG , Hudes GR . Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin Is Safe, Effective, and Efficient Neoadjuvant Treatment for Muscle-Invasive Bladder Cancer: Results of a Multicenter Phase II Study With Molecular Correlates of Response and Toxicity. J Clin Oncol. (2014) ;32: (18):1895–901. |
[5] | Griffiths G , Canc MRCAB , Grp NBCS , Tratamiento CUE . International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 4 Trial. J Clin Oncol. (2011) ;29: (16):2171–7. |
[6] | Iyer G , Balar AV , Milowsky MI , Bochner BH , Dalbagni G , Donat SM , Herr HW , Huang WC , Taneja SS , Woods M , Ostrovnaya I , Al-Ahmadie H , Arcila ME , Riches JC , Meier A , Bourque C , Shady M , Won H , Rose TL , Kim WY , Kania BE , Boyd ME , Cipolla CK , Regazzi AM , Delbeau D , McCoy AS , Vargas HA , Berger MF , Solit DB , Rosenberg JE , Bajorin DF . Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer. J Clin Oncol. (2018) ;36: (19):1949–56. |
[7] | Grossman HB , Natale RB , Tangen CM , Speights VO , Vogelzang NJ , Trump DL , deVere White RW , Sarosdy MF , Wood DP Jr. , Raghavan D , Crawford ED . Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. (2003) ;349: (9):859–66. |
[8] | Sonpavde G , Goldman BH , Speights VO , Lerner SP , Wood DP , Vogelzang NJ , Trump DL , Natale RB , Grossman HB , Crawford ED . Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. (2009) ;115: (18):4104–9. |
[9] | Rosenblatt R , Sherif A , Rintala E , Wahlqvist R , Ullen A , Nilsson S , Malmstrom PU , Grp NUC . Pathologic Downstaging Is a Surrogate Marker for Efficacy and Increased Survival Following Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Urothelial Bladder Cancer. Eur Urol. (2012) ;61: (6):1229–38. |
[10] | Advanced Bladder Cancer Meta-analysis C. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. (2005) ;48: (2):202–5; discussion 5-6. |
[11] | Network NCC . Clinical Practice Guidelines in Oncology: Bladder Cancer 2019 [updated 7/101/9. Version 4.2019:[NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer, 2019]. Available 533 from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. |
[12] | Bellmunt J , Orsola A , Leow JJ , Wiegel T , De Santis M , Horwich A , Grp EGW . Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2014) ;25: (suppl_3):40–8. |
[13] | Witjes J.A. , (Chair) MB , Cathomas A , Compérat E , Cowan NC , Gakis G , Hernández V , Lorch A , Ribal (Vice-chair) MJ , Thalmann GN , van der Heijden AG , Veskimäe E , Guidelines Associates: E. Linares Espinós MR, Y. Neuzillet. EAU Guidelines. Edn. presented at the EAU Annual Congress Barcelona 2019. : EAU Guidelines Office, Arnhem, The Netherlands.; 2019 [Available from: http://uroweb.org/guidelines/compilations-of-all-guidelines/. |
[14] | Choueiri TK , Jacobus S , Bellmunt J , Qu A , Appleman LJ , Tretter C , Bubley GJ , Stack EC , Signoretti S , Walsh M , Steele G , Hirsch M , Sweeney CJ , Taplin M-E , Kibel AS , Krajewski KM , Kantoff PW , Ross RW , Rosenberg JE . Neoadjuvant Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin With Pegfilgrastim Support in Muscle-Invasive Urothelial Cancer: Pathologic, Radiologic, and Biomarker Correlates. J Clin Oncol. (2014) ;32: (18):1889–94. |
[15] | von der Maase H , Hansen SW , Roberts JT , Dogliotti L , Oliver T , Moore MJ , Bodrogi I , Albers P , Knuth A , Lippert CM , Kerbrat P , Sanchez Rovira P , Wersall P , Cleall SP , Roychowdhury DF , Tomlin I , Visseren-Grul CM , Conte PF . Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J Clin Oncol. (2000) ;18: (17):3068–77. |
[16] | Bamias A , Dafni U , Karadimou A , Timotheadou E , Aravantinos G , Psyrri A , Xanthakis I , Tsiatas M , Koutoulidis V , Constantinidis C , Hatzimouratidis C , Samantas E , Visvikis A , Chrisophos M , Stravodimos K , Deliveliotis C , Eleftheraki A , Pectasides D , Fountzilas G , Dimopoulos MA . Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol. (2013) ;24: (4):1011–7. |
[17] | von der Maase H , Sengelov L , Roberts JT , Ricci S , Dogliotti L , Oliver T , Moore MJ , Zimmermann A , Arning M . Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. (2005) ;23: (21):4602–8. |
[18] | Anari F , O’Neill J , Choi W , Chen DYT , Haseebuddin M , Kutikov A , Dulaimi E , Alpaugh RK , Devarajan K , Greenberg RE , Bilusic M , Wong Y-N , Viterbo R , Hoffman-Censits JH , Lallas CD , Trabulsi EJ , Smaldone M , Geynisman DM , Zibelman M , Lin J , Kelly WK , Uzzo R , McConkey D , Plimack ER . Neoadjuvant Dose-dense Gemcitabine and Cisplatin in Muscle-invasive Bladder Cancer: Results of a Phase 2 Trial. Eur Urol Oncol. (2018) ;1: (1):54–60. |
[19] | Culine S , Gravis G , Flechon A , Soulie M , Guy L , Laguerre B , Mottet N , Joly F , Allory Y , Harter V , Pfister C . Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (dd-MVAC) or gemcitabine and cisplatin (GC) as perioperative chemotherapy for muscle invasive urothelial bladder cancer (MIUBC): Preliminary results of the GETUG/AFU V05 VESPER trial on toxicity and pathological responses. J Clin Oncol. (2020) ;38: (6_suppl):437. |
[20] | Moher D , Liberati A , Tetzlaff J , Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) ;339: :b2535. |
[21] | Wells G , Shea B , O’Connell D , Peterson J , Welch V , Losos M , Tugwell P . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2009 [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
[22] | DerSimonian R , Laird N . Meta-analysis in clinical trials. Control Clin Trials. (1986) ;7: (3):177–88. |
[23] | Egger M , Smith GD , Schneider M , Minder C . Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) ;315: (7109):629–34. |
[24] | Duval S , Tweedie R . Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) ;56: (2):455–63. |
[25] | Guyatt G , Oxman AD , Akl EA , Kunz R , Vist G , Brozek J , Norris S , Falck-Ytter Y , Glasziou P , deBeer H , Jaeschke R , Rind D , Meerpohl J , Dahm P , Schünemann HJ . GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. (2011) ;64: (4):383–94. |
[26] | Alva AS , Tallman CT , He C , Hussain MH , Hafez K , Montie JE , Smith DC , Weizer AZ , Wood D , Lee CT . Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer: a multidisciplinary approach. Cancer. (2012) ;118: (1):44–53. |
[27] | Dash A , Pettus JAt , Herr HW , Bochner BH , Dalbagni G , Donat SM , Russo P , Boyle MG , Milowsky MI , Bajorin DF . A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. (2008) ;113: (9):2471–7. |
[28] | Fairey AS , Daneshmand S , Quinn D , Dorff T , Dorin R , Lieskovsky G , Schuckman A , Cai J , Miranda G , Skinner EC . Neoadjuvant chemotherapy with gemcitabine/cisplatin vs. methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive urothelial carcinoma of the bladder: a retrospective analysis from the University of Southern California. Urol. (2013) ;31: (8):1737–43. |
[29] | Fukui T , Matsui Y , Umeoka S , Inoue T , Kamba T , Togashi K , Ogawa O , Kobayashi T . Predictive value of radiological response rate for pathological response to neoadjuvant chemotherapy and post-cystectomy survival of bladder urothelial cancer. Jpn J Clin Oncol. (2016) ;46: (6):560–7. |
[30] | Galsky MD , Pal SK , Chowdhury S , Harshman LC , Crabb SJ , Wong YN , Yu EY , Powles T , Moshier EL , Ladoire S , Hussain SA , Agarwal N , Vaishampayan UN , Recine F , Berthold D , Necchi A , Theodore C , Milowsky MI , Bell-munt J , Rosenberg JE , Retrospective International Study of Cancers of the Urothelial Tract I. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. (2015) ;121: (15):2586–93. |
[31] | Kaneko G , Kikuchi E , Matsumoto K , Obata J , Nakamura S , Miyajima A , Oya M . Neoadjuvant gemcitabine plus cisplatin for muscle-invasive bladder cancer. Jpn J Clin Oncol. (2011) ;41: (7):908–14. |
[32] | Kawamura N , Matsushita M , Okada T , Ujike T , Nin M , Tsujihata M . [Relative efficacy of neoadjuvant gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management for muscle-invasive bladder cancer]. Hinyokika Kiyo. (2013) ;59: (5):277–81. |
[33] | Lee FC , Harris W , Cheng HH , Shenoi J , Zhao S , Wang J , Champion T , Izard J , Gore JL , Porter M , Yu EY , Wright JL . Pathologic Response Rates of Gemcitabine/Cisplatin versus Methotrexate/Vinblastine/Adriamycin/Cisplatin Neoadjuvant Chemotherapy for Muscle Invasive Urothelial Bladder Cancer. Adv. (2013) ;2013: :317190. |
[34] | Lee Y , Kim YS , Hong B , Cho YM , Lee JL . Comparing clinical outcomes between patients with urothelial carcinoma who treated neoadjuvant chemotherapy by gemcitabine-cisplatin and dose dense MVAC. J Clin Oncol. (2019) ;37: (7). |
[35] | Matulay JT , Campbell MT , Narayan VM , Seif MA , Lim AH , Shah AY , Msaouel P , Gao J , Siefker-Radtke AO , Dinney CPN , Kamat AM , Navai N . Pathologic outcomes after neoadjuvant chemotherapy for high-risk muscle invasive bladder cancer. Ann Oncol. (2019) ;30: :370-. |
[36] | Miron B , Ross EA , Anari F , O’Neill J , Hoffman-Censits JH , Zibelman MR , Kutikov A , Viterbo R , Greenberg RE , Chen D , Lallas CD , Trabulsi EJ , Alpaugh RK , Dulaimi E , Golemis E , Uzzo R , Plimack ER . Defects in DNA repair genes and long-term survival in cisplatin-based neoadjuvant chemotherapy for muscle invasive bladder cancer (MIBC). J Clin Oncol. (2019) ;37: (15). |
[37] | Mitra N , Monk JP , Pohar KS , Shabsigh A , Sharp DS , Abaza R , Box GN , Zynger DL , Clinton SK , Mortazavi A . Early outcomes with neoadjuvant high-dose intensity methotrexate, vinblastine, doxorubicin, and cisplatin (HD-MVAC) or gemcitabine and cisplatin (GC) in muscle-invasive urothelial carcinoma of the bladder: A single-institution experience. Journal of Clinical Oncology Conference. (2011) ;29: (7 SUPPL. 1). |
[38] | Nguyen TT , Huillard O , Dabi Y , Anract J , Sibony M , Zerbib M , Xylinas E . Neoadjuvant Chemotherapy in Patients With Muscle-Invasive Bladder Cancer and Its Impact on Surgical Morbidity and Oncological Outcomes: A Real-World Experience. Front. (2018) ;5: :58. |
[39] | Okabe K , Shindo T , Maehana T , Nishiyama N , Hashimoto K , Itoh N , Takahashi A , Taguchi K , Tachiki H , Tanaka T , Masumori N . Neoadjuvant chemotherapy with gemcitabine and cisplatin for muscle-invasive bladder cancer: multicenter retrospective study. Jpn J Clin Oncol. (2018) ;48: (10):934–41. |
[40] | Pal SK , Ruel NH , Wilson TG , Yuh BE . Retrospective analysis of clinical outcomes with neoadjuvant cisplatin-based regimens for muscle-invasive bladder cancer. Clin Genitourin Cancer. (2012) ;10: (4):246–50. |
[41] | Shindo T , Kitamura H , Masumori N , Tsukamoto T . [The effect of presurgical gemcitabine cisplatin chemotherapy for urothelial carcinoma]. Hinyokika Kiyo. (2012) ;58: (8):391–4. |
[42] | van de Putte EE , Mertens LS , Meijer RP , van der Heijden MS , Bex A , van der Poel HG , Kerst JM , Bergman AM , Horenblas S , van Rhijn BW . Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World J Urol. (2016) ;34: (2):157–62. |
[43] | Weight CJ , Garcia JA , Hansel DE , Fergany AF , Campbell SC , Gong MC , Jones JS , Klein EA , Dreicer R , Stephenson AJ . Lack of pathologic down-staging with neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder: a contemporary series. Cancer. (2009) ;115: (4):792–9. |
[44] | Wright JL , Lee F , Harris WP , Cheng HH , Zhao S , Champion T , Gore JL , Dean J , Porter MP , Yu EY . Pathologic response rates between gemcitabine-cisplatin (GC) and methotrexate, vinblastine, doxorubicin hydrochloride, and cisplatin (MVAC) neoadjuvant chemotherapy in muscle-invasive urothelial cell carcinoma of the bladder. J Clin Oncol. (2013) ;31: (6). |
[45] | Yeshchina O , Badalato GM , Wosnitzer MS , Hruby G , RoyChoudhury A , Benson MC , Petrylak DP , McKiernan JM . Relative efficacy of perioperative gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. (2012) ;79: (2):384–90. |
[46] | Yokomizo A , Wada Y , Fujimoto N , Koga H , Tomoda T , Sato Y , Wakeda H , Nomura T , Irie S , Naito S . The comparison of GC vs. M-VAC regimen as neoadjuvant chemotherapy for muscle invasive bladder cancer. Urology. (2013) ;(1):S128. |
[47] | Zargar H , Espiritu PN , Fairey AS , Mertens LS , Dinney CP , Mir MC , Krabbe LM , Cookson MS , Jacobsen NE , Gandhi NM , Griffin J , Montgomery JS , Vasdev N , Yu EY , Youssef D , Xylinas E , Campain NJ , Kassouf W , Dall’Era MA , Seah JA , Ercole CE , Horenblas S , Sridhar SS , McGrath JS , Aning J , Shariat SF , Wright JL , Thorpe AC , Morgan TM , Holzbeierlein JM , Bivalacqua TJ , North S , Barocas DA , Lotan Y , Garcia JA , Stephenson AJ , Shah JB , van Rhijn BW , Daneshmand S , Spiess PE , Black PC . Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. (2015) ;67: (2):241–9. |
[48] | Zargar H , Shah JB , van Rhijn BW , Daneshmand S , Bivalacqua TJ , Spiess PE , Black PC , Kassouf W . Neoadjuvant Dose Dense MVAC versus Gemcitabine and Cisplatin in Patients with cT3-4aN0M0 Bladder Cancer Treated with Radical Cystectomy. J Urology. (2018) ;199: (6):1453–9. |
[49] | Yin M , Joshi M , Meijer RP , Glantz M , Holder S , Harvey HA , Kaag M , Fransen van de Putte EE , Horenblas S , Drabick JJ . Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist. (2016) ;21: (6):708–15. |
[50] | Yu C , Hequn C , Jinbo C , Feng Z , Xiongbing Z , Jian D . Gemcitabine/cisplatin versus methotrexate/vinb-lastine/doxorubicin/cisplatin for muscle-invasive bladder cancer: A systematic review and meta-analysis. Journal of Cancer Research and Therapeutics. (2018) ;14: (6):1260–5. |




