Thromboembolism in Muscle-Invasive Bladder Cancer. A Population-based Nationwide Study
Abstract
BACKGROUND:
Routine VTE prophylaxis within 30 days of radical cystectomy (RC) for urinary bladder cancer (UBC) is used to protect from venous thromboembolism (VTE). However, randomized studies and nationwide population-based studies are lacking.
OBJECTIVE:
To study VTE and risk factors for VTE in muscle-invasive UBC in a nationwide population-based series, with a focus on the association with RC with and without chemotherapy.
MATERIALS AND METHODS:
We studied all patients with clinical stage T2-T4 UBC diagnosed 1997 to 2014 in the Bladder Cancer Data Base Sweden (BladderBaSe). Previous VTE events and risk factors for VTE were registered from 1987. Cox regression analyses and Kaplan-Meier curves were performed to study risk factors for VTE and cumulative incidence of VTE.
RESULTS:
In 9720 patients (71%males) with a median age of 74 years 546 (5.6%) had VTE after diagnosis. In Cox analyses controlling for patient’s and tumour characteristics, and risk factors for VTE, VTE after diagnosis and first treatment date were associated with chemotherapy with or without RC. Cumulative incidence of VTE increased during 24 months after diagnosis and first treatment date. VTE were less common in patients with previous cardiovascular disease.
CONCLUSIONS:
VTE was commonly observed after 30 days from diagnosis and from first treatment date in patients with T2-T4 UBC, particularly after chemotherapy. The findings suggest that long-term intervention studies of benefit and possible harms of VTE prophylaxis after UBC should be undertaken.
LIST OF ABBREVIATIONS
VTE | – thromboembolism |
UBC | – urinary bladder cancer |
SNRUBC | – Swedish National Registry of Urinary Bladder Cancer |
RT | – radiotherapy |
RC | – radical cystectomy |
Chemo-RC | – combination of chemotherapy and radical cystectomy |
OCT | – Other curative treatment |
Chemo-only | – chemotherapy as the only treatment |
CI | – confidence interval |
BACKGROUND
Venous thromboembolism (VTE) is commonly seen in malignancy [1–4]. Patients with locally advanced urinary bladder cancer (UBC) are treated aggressively with radical cystectomy (RC) with or without chemotherapy, increasing the risk of treatment-related VTE [5–7]. In an analysis of nine series of open RCs without VTE prophylaxis, the 30-day cumulative incidences of VTE were 2.9%, 5.6%and 11.6%in patients with low, intermediate or high risk for VTE, respectively [7]. These risk groups are defined by factors such as age >75 years, previous cardiovascular disease, previous VTE and in the Khorana score high body mass index (BMI) and blood cell counts are also included [1, 5, 8]. Using screening for VTE, Shomburg, et al. [9] found 14%subclinical VTE before RC and Clement et al. [10] found 7%subclinical VTE seven days after RC in spite of standard prophylaxis including heparin, demonstrating the particularly high risk of VTE in these patients.
A randomised study of patients undergoing abdominal or pelvic oncological surgery showed a 30-day cumulative incidence of VTE of 4.8%if 30 days prophylaxis was given compared to 12%in a group given 6–10 days VTE prophylaxis [11]. Similarly, for patients with UBC treated with RC, observational studies have indicated a decrease in VTE with a 30-day low molecular weight heparin (LMWH) prophylaxis [12–15].
During the last decade, neoadjuvant chemotherapy preceding RC has become increasingly used in stages T2-T4 UBC, in accordance with treatment guidelines [16]. Such treatment must include cisplatin, which may induce damage to the vascular endothelium, in conjunction with central venous catheters and other tumour-related factors, which may all increase the risk of VTE [17, 18]. This might explain some of the pathophysiology behind the reported 90- or 180-day cumulative incidences for VTE between 9.6%–26%related to such treatment [19–23].
We investigated the cumulative incidence of VTE and risk factors for VTE in a population-based nationwide series including all patients diagnosed with clinical stage T2-T4 UBC (stage II-IV) [24] in Sweden during 1997–2014, with the hypothesis that treatment with chemotherapy or RC and particularly the combination of these treatment modalities are clinically important risk factors for VTE maybe in combination with other known risk factors.
MATERIALS AND METHODS
The bladder cancer data base Sweden (BladderBaSe)
The BladderBaSe was initiated in 2015 through linkage of the Swedish National Register for Urinary Bladder Cancer (SNRUBC) to a number of health care and demographic registers [25]. The project was approved by the Research Ethics Board at Uppsala University, Uppsala, Sweden (File No 2015/277).
We included all patients registered with primary tumours localized in the urinary bladder and with clinical stage T2-T4 UBC (stages II-IV) diagnosed from January 1, 1997 to December 31, 2014. The tumour, node and metastatic (TNM) classification, tumour grade and detailed information about Charlson Comorbidity Index (CCI), marital and educational status have been presented elsewhere [25, 26].
The patients’ ages were stratified by the median age ≤74 years and >74 years. Although most patients had cisplatin-based treatment, information about the type of chemotherapy and the curative intention, was not always available and therefore patients treated with any modality of chemotherapy were analysed together. Primary treatment was grouped as follows: radiotherapy with curative intent (RT), radical cystectomy (RC), chemotherapy in combination with radical cystectomy (Chemo-RC), other curative treatment (OCT), chemotherapy only (Chemo-only) and supportive management with best supportive care only.
We used all diagnoses of hospitalisations from 1987 until the end of 2014 in the previously validated In-Patient Registry [27]. VTE events and risk factors for VTE were registered at least 10 years before the diagnosis of UBC. Peripheral arterial thrombosis, considered to be dependent on local factors such as localized arterial stenosis were not included in the VTE group. To study background risk factors for VTE, the following codes in the hospital records were considered; coronary heart disease (IC10 codes I210-I259), stroke (ICD-10 codes I610-I649, I740-I749), hypertension (ICD 10 codes I110-I159). In case of registration of one or more of these conditions, the patient was considered to have a previous cardiovascular disease. Furthermore, diabetes mellitus (ICD-10 codes E109-E159) was analysed as a risk factor. Registered VTE events were pulmonary embolism (ICD-10 codes I260-I269) and venous thrombosis (ICD-10 codes I800-I809). Screening for VTE was not recommended during the studied period. Death and cause of death were registered until 31 December 2014 according to data from the National Causes of Death Register.
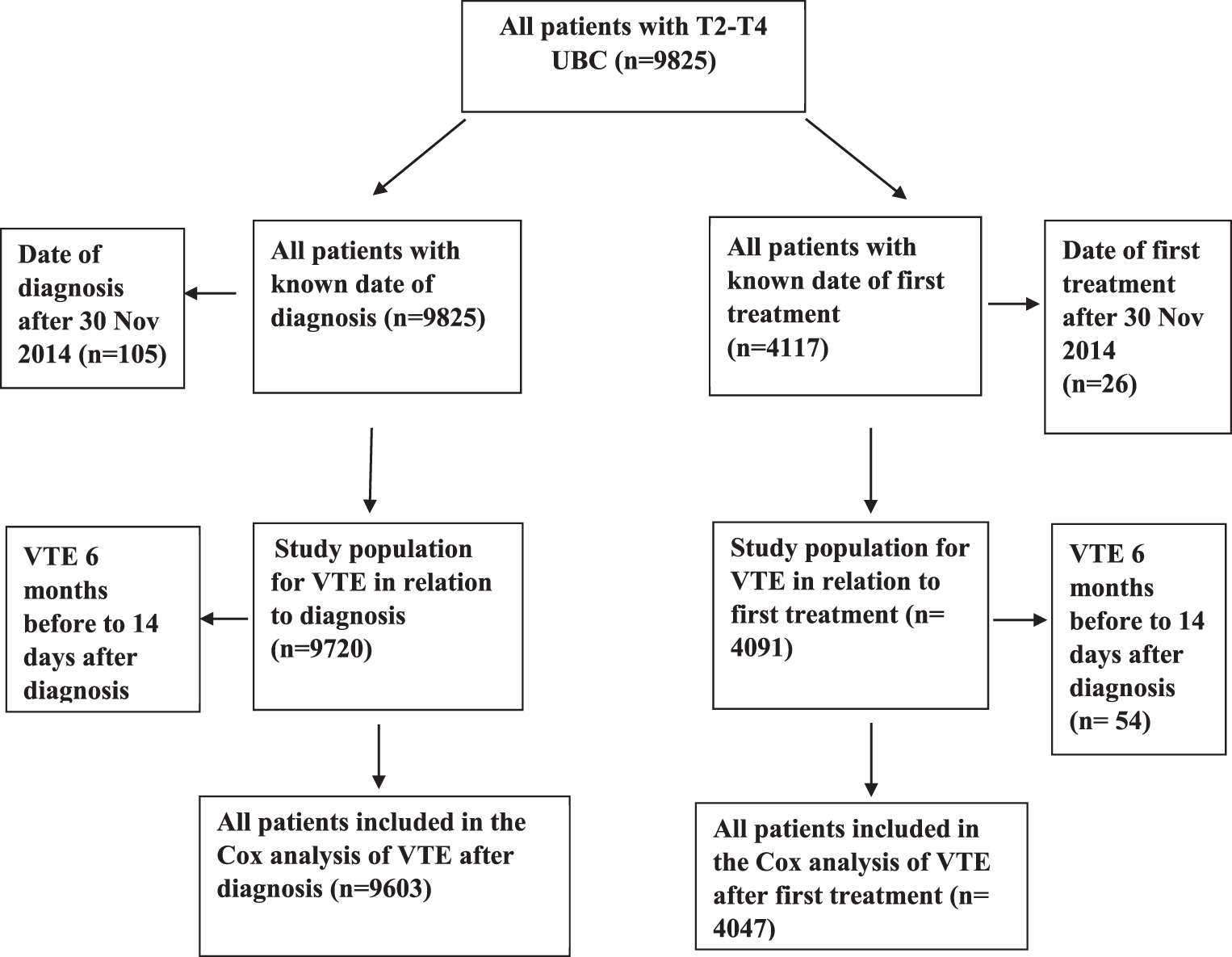
The national guidelines recommended prophylaxis against VTE with LMWH the day before RC and 14 days thereafter during the first part of the studied period, and later this recommendation was extended to 30 days. Information about the exact length of this prophylaxis for each patient was not available. At chemotherapy, no prophylaxis against VTE was recommended during the study period. A sub-group analysis, including only patients treated with RT, RC, Chemo-RC or Chemo-only with a known first treatment date, was used to relate VTE event to the date of treatment. In this analysis, patients with a treatment date after 30 November 2014 were excluded as the observation time from treatment was 31 days or less (Fig. 1).
Fig. 1
Diagram of studied groups of patients in those analysed for VTE after diagnosis and VTE after first treatment date, respectively, in all patients with stage T2-T4 urinary bladder cancer in Sweden 1997–2014.
Definitions of VTE occurrence
VTE before diagnosis was defined as all VTE events occurring prior to six months before diagnosis.
VTE at diagnosis was defined as all VTE events occurring from six months before diagnosis to 14 days after diagnosis.
VTE after diagnosis was defined as all VTE events occurring from 15 days after diagnosis until 24 months after diagnosis.
Late VTE was defined as all VTE events occurring from 24 months after diagnosis and onwards.
A second VTE event was defined as a VTE event occurring six months or more after the first VTE event.
Statistics
We investigated VTE events, patients and tumour characteristics, primary management and outcome. Differences between groups were studied using the chi squared test. A Cox Proportional Hazards analysis was used to study time to VTE, where patients were censored at the date of death or date of end of follow-up, if no VTE had occurred before this date. In a sub-group with a known first treatment date, a similar Cox analysis was carried out. A p-value <0.05 was considered to be statistically significant. Kaplan-Meier curves were used to illustrate the evolution of the cumulative incidence of VTE in the different treatment groups. Differences between groups were studied using the Log-rank test, and a p-value <0.05 was considered to be statistically significant.
The 117 patients with VTE events from six months before to 14 days after diagnosis were not included in the analyses of treatment-related VTE. All time to event analyses were limited to 24 months after diagnosis, restricting the study period to the most relevant one for treatment-associated VTE. Models were specified by using an a priori Diagnostic Acyclic Graph (DAG) displaying how different co-variates may influence the association between type of treatment and risk of VTE [28].
RESULTS
Out of 9720 included patients, with median age 74 years, Interquartile Range (IQR) 67–82 years, 6857 (71%) were men. VTE was observed in 1003 (10.3%of all patients) patients during the observation period. Details of VTE observation time were as follows: in 208 (20.7%) before diagnosis, in 117 (11.7%) at diagnosis, in 546 (54.4%) after diagnosis and 132 (13.2%) patients had later VTE (Table 1). The observation time from diagnosis to death or last date of follow-up was median 13 months (IQR 5–37 months).
Table 1
VTE before diagnosis, at diagnosis, after diagnosis and later VTE after urinary bladder cancer in relation to patients’ characteristics, tumour characteristics and treatment variables in all T2-T4 bladder cancer in Sweden 1997–2014. Figures represent for the five left columns number of patients (%of the column). Treatment groups were: radiotherapy with curative intent (RT), radical cystectomy (RC), chemotherapy with RC (Chemo-RC), other curative treatment (OCT), chemotherapy only (Chemo-only) and supportive management
| No VTE*(n = 8717) | Before diagnosis (n = 208) | At diagnosis (n = 117) | After diagnosis (n = 546) | Later VTE (n = 132) | All pat. (n = 9720) | |
| Treatment | ||||||
| -RT | 557(6) | 11(5) | 4(3) | 30(6) | 13(10) | 615(6.3) |
| -RC | 2747(32) | 55(26) | 14(12) | 223(41) | 74(56) | 3113(32) |
| -Chemo-RC | 584(7) | 3(1) | 7(6) | 74(14) | 9(7) | 677(7.0) |
| -OCT | 51(0.6) | 0 | 1(1) | 5(1) | 1(1) | 58(0.6) |
| -Chemo-only | 196(2) | 2(1) | 5(4) | 20(4) | 1(1) | 224(2.3) |
| -Supportive | 4582(53) | 137(6) | 86(74) | 194(36) | 34(26) | 5033(52) |
| Age groups | ||||||
| -≤74 years | 4053(47) | 64(31) | 51(44) | 338(62) | 76(58) | 4582(47)** |
| ->74 years | 4661(53) | 144(69) | 66(56) | 208(38) | 56(42) | 5135(53)** |
| Gender | ||||||
| -men | 6141(70) | 145(70) | 76(65) | 400(73) | 95(72) | 6857(71) |
| -women | 2576(30) | 63(30) | 41(35) | 146(27) | 37(28) | 2863(29) |
| T category | ||||||
| -T2 | 5803(67) | 145(70) | 59(50) | 357(65) | 96(73) | 6460(66) |
| -T3 | 1770(20) | 44(21) | 28(24) | 119(22) | 28(21) | 1989(21) |
| -T4 | 1144(13) | 19(9) | 30(26) | 70(13) | 8(6) | 1271(13) |
| N category | ||||||
| -N0 | 3082(35) | 66(32) | 34(30) | 235(43) | 70(53) | 3813(39) |
| -N1-3 | 1039(12) | 17(8) | 18(15) | 80(15) | 6(5) | 1160(12) |
| -NX | 4270(49) | 125(60) | 65(56) | 231(42) | 56(42) | 4747(49) |
| M category | ||||||
| -M0 or MX | 7783(89) | 187(90) | 100(85) | 496(91) | 132(100) | 8697(89) |
| -M1 | 934(11) | 21(10) | 17(15) | 50(9) | 0 | 1023(11) |
| Marital | ||||||
| -non-married | 3947(45) | 96(46) | 54(46) | 206(38) | 50(38) | 4353(45) |
| -married | 4770(55) | 112(54) | 63(54) | 340(62) | 82(62) | 5367(55) |
| CCI | ||||||
| -0 | 4860(56) | 58(28) | 60(51) | 349(64) | 91(69) | 5418(56) |
| -1 | 1581(18) | 43(21) | 18(15) | 89(16) | 21(16) | 1752(18) |
| -2 | 1155(17) | 32(15) | 10(9) | 48(9) | 16(12) | 1261(13) |
| -3 or more | 1121(8713) | 75(36) | 29(25) | 60(11) | 4(3) | 1289(13) |
| Education | ||||||
| -compulsory | 4375(50) | 114(55) | 60(51) | 254(47) | 67(51) | 4870(50) |
| -secondary | 2719(31) | 59(28) | 32(27) | 203(37) | 44(33) | 3057(32) |
| -university | 1146(13) | 21(11) | 13(11) | 75(14) | 21(18) | 1276(13) |
| -missing | 477(6) | 14(7) | 12(11) | 14(3) | 0 | 517(5) |
| Previous cardiovascular disease | ||||||
| -no | 5829(67) | 64(31) | 45(39) | 406(74) | 97(74) | 6441(66) |
| -yes | 2888(33) | 144(69) | 72(61) | 140(26) | 35(26) | 3279(34) |
| Previous diabetes mellitus | ||||||
| -no | 7838(90) | 174(84) | 107(91) | 501(92) | 127(96) | 8747(90) |
| -yes | 879(10) | 34(16) | 10(9) | 45(8) | 5(4) | 973(10) |
| VTE before treatment | ||||||
| -no | 8717(100) | 0 | 17(15) | 530(97) | 126(95) | 9390(97) |
| -yes | 0 | 208(100) | 100(85) | 16(3) | 6(5) | 330(3.4) |
| Years of diagnosis | ||||||
| -1997–2005 | 4362(50) | 93(45) | 57(49) | 271(50) | 65(49) | 4848(50) |
| -2006–2014 | 4355(50) | 115(55) | 60(51) | 317(50) | 25(51) | 4872(50) |
*VTE before (within 6 months prior to UBC diagnosis), at diagnosis (from 6 months before to 14 days after UBC diagnosis), after diagnosis (from 15 days after date of diagnosis to 24 months after UBC diagnosis) or later from 24 months after UBC diagnosis and onwards. **Information for 3 patients is lacking.
The characteristics of the study population are detailed in Table 1. Due to high age and comorbidity, most patients had supportive management (52%). Among the other groups, RC was most common (29%of all patients) followed by RT (7%) and Chemo –RC (7%). Chemo-only and OCT were provided to small groups of patients: 2.3%and 0.6%of all, respectively. In the supportive management group, 67%of the patients died within three months from the VTE event. In the sub-group with known first treatment date, 55–63%of all VTE events were observed three months or later after this date.
An increased risk for VTE after diagnosis, in a Cox Proportional Hazards analysis, was associated with Chemo-RC and Chemo-only compared to RT (Table 2). A higher T and N category as well as married civil status were also associated with higher risks of VTE and in the latter group there was a higher incidence of curative treatment compared to patients with other marital status (53%versus 42%, data not shown). Age over 74 and previous cardiovascular disease were associated with lower risk (Table 2). In the sub-group with a known first treatment date, a Cox analysis showed that Chemo-RC was associated with increased cumulative incidence of VTE compared to RT. A lower risk of VTE was found for patients with previous cardiovascular disease compared to those without it (Table 3).
Table 2
Cox proportional hazards analysis of VTE between 15 days to 24 months after diagnosis of bladder cancer in relation to patients' characteristics, tumour characteristics and treatment variables in all T2-T4 bladder cancer (n = 9603). Treatment groups were: radiotherapy with curative intent (RT), radical cystectomy (RC), chemotherapy with RC (Chemo-RC), other curative treatment (OCT), chemotherapy only (Chemo-only) and supportive management
| Variable | HR Univariate (95%CI) | p-value p-value | HR Multivariate (95%CI) | p-value |
| Treatment | ||||
| -RT | 1.0 | 1.0 | ||
| -RC | 1.37(0.94–2.00) | 0.11 | 1.21(0.81–1.77) | 0.35 |
| -Chemo-RC | 2.13(1.39–3.26) | <0.001 | 1.58(1.01–2.49) | 0.046 |
| -OCT | 1.61(0.62–4.14) | 0.33 | 1.36(0.53–3.59) | 0.53 |
| -Chemo-only | 2.53(1.43–4.45) | 0.001 | 1.56(0.85–2.85) | 0.15 |
| -Supportive | 1.29(0.88–1.88) | 0.19 | 1.23(0.84–1.83) | 0.29 |
| Age group | ||||
| -<74 years | 1.0 | 1.0 | ||
| ->74 years | 0.71(0.60–0.84) | <0.001 | 0.79(0.64–0.98) | 0.03 |
| Gender | ||||
| -men | 1.0 | 1.0 | ||
| -women | 0.96(0.79–1.16) | 0.67 | 0.99(0.81–1.20) | 0.89 |
| Marital | ||||
| -non-married | 1.0 | 1.0 | ||
| -married | 1.20(1.01–1.43) | 0.04 | 1.21(1.01–1.44) | 0.047 |
| CCI | ||||
| -0 | 1.0 | 1.0 | ||
| -1 | 0.92(0.73–1.16) | 0.40 | 1.15(0.89–1.49) | 0.29 |
| -2 | 0.68(0.51–0.92) | 0.01 | 0.81(0.59–1.11) | 0.19 |
| -≥3 | 1.12(0.84–1.46) | 0.43 | 1.39(1.00–1.93) | 0.05 |
| Education | ||||
| -mandatory | 1.0 | 1.0 | ||
| -secondary | 1.17(0.97–1.41) | 0.10 | 1.08(0.90–1.31) | 0.41 |
| -university | 0.96(0.75–1.25) | 0.78 | 0.88(0.67–1.14) | 0.33 |
| -missing | 0.72(0.42–1.23) | 0.23 | 0.81(0.47–1.39) | 0.44 |
| Tumour category | ||||
| -T2 | 1.0 | 1.0 | ||
| -T3 | 1.25(1.03–1.56) | 0.024 | 1.30(1.05–1.62) | 0.018 |
| -T4 | 1.58(1.22–2.04) | <0.001 | 1.48(1.14–1.93) | 0.004 |
| N category | ||||
| -N0 | 1.0 | 1.0 | ||
| -N1-3 | 1.63(1.26–2.10) | <0.001 | 1.34(1.02–1.75) | 0.04 |
| -NX | 1.01(0.83–1.20) | 0.99 | 1.10(0.91–1.34) | 0.34 |
| M category | ||||
| -M0MX | 1.0 | 1.0 | ||
| -M1 | 1.62(1.21–2.17) | 0.001 | 1.27(0.93–1.76) | 0.14 |
| Previous cardiovascular disease | ||||
| -no | 1.0 | 1.0 | ||
| -yes | 0.79(0.64–0.95) | 0.01 | 0.75(0.60–0.95) | 0.017 |
| Previous diabetes | ||||
| -no | 1.0 | 1.0 | ||
| -yes | 0.89(0.65–1.20) | 0.43 | 0.91(0.65–1.28) | 0.59 |
| VTE before diagnosis | ||||
| -no | 1.0 | 1.0 | ||
| -yes | 1.42(0.87–2.34) | 0.17 | 1.57(0.94–2.60) | 0.08 |
| Year of diagnosis | ||||
| -1997–2005 | 1.0 | 1.0 | ||
| -2006–2014 | 1.19(1.01–1.41) | 0.04 | 1.26(1.05–1.52) | 0.014 |
Table 3
Cox proportional hazards analysis of VTE within 24 months from first treatment day in bladder cancer in relation to patients’ characteristics, tumour characteristics and treatment variables in all T2-T4 bladder cancer (n = 4047). Treatment groups were: radiotherapy with curative intent (RT), radical cystectomy (RC), chemotherapy with RC (Chemo-RC) and chemotherapy only (Chemo-only)
| Variable | HR Univariate (95%CI) | p-value | HR Multivariate (95%CI) | p-value |
| Treatment | ||||
| -RT | 1.0 | 1.0 | ||
| -RC | 1.50(0.94–2.50) | 0.12 | 1.56(0.92–2.65) | 0.10 |
| -Chemo-RC | 2.34(1.37–4.02) | 0.002 | 2.28(1.27–4.10) | 0.006 |
| -Chemo-only | 2.72(0.99–7.44) | 0.053 | 2.26(0.79–6.46) | 0.13 |
| Age | ||||
| -≤74 years | 1.0 | 1.0 | ||
| ->74 years | 0.92(0.71–1.90) | 0.53 | 1.13(0.85–1.49) | 0.40 |
| Gender | ||||
| -men | 1.0 | 1.0 | ||
| -women | 0.78(0.59–1.04) | 0.10 | 0.81(0.62–1.06) | 0.13 |
| Marital | ||||
| -non-married | 1.0 | 1.0 | ||
| -married | 1.08(0.85–1.38) | 0.52 | 1.13(0.89–1.43) | 0.32 |
| CCI | ||||
| -0 | 1.0 | 1.0 | ||
| -1 | 1.00(0.72–1.40) | 0.99 | 1.32(0.92–1.88) | 0.13 |
| -2 | 0.75(0.48–1.16) | 0.19 | 0.93(0.61–1.42) | 0.74 |
| -≥3 | 1.04(0.62–1.72) | 0.89 | 1.58(0.91–2.72) | 0.11 |
| Education | ||||
| -mandatory | 1.0 | 1.0 | ||
| -secondary | 1.25(0.97–1.61) | 0.09 | 1.19(0.93–1.52) | 0.16 |
| -university | 0.93(0.66–1.31) | 0.67 | 0.85(0.61–1.19) | 0.35 |
| -missing | 0.30(0.04–2.13) | 0.23 | 0.29(0.04–2.08) | 0.22 |
| Tumour category | ||||
| -T2 | 1.0 | 1.0 | ||
| -T3 | 1.03(0.75–1.40) | 0.88 | 1.07(0.79–1.44) | 0.66 |
| -T4 | 1.19(0.77–1.85) | 0.43 | 1.09(0.72–1.67) | 0.68 |
| N category | ||||
| -N0 | 1.0 | 1.0 | ||
| -N1-3 | 1.30(0.88–1.91) | 0.18 | 1.14(0.79–1.64) | 0.48 |
| -NX | 1.16(0.89–1.50) | 0.27 | 1.16(0.90–1.49) | 0.25 |
| M category | ||||
| -M0MX | 1.0 | 1.0 | ||
| -M1 | 1.70(0.93–3.10) | 0.09 | 1.36(0.74–2.49) | 0.32 |
| Previous cardiovascular disease | ||||
| -no | 1.0 | 1.0 | ||
| -yes | 0.71(0.56–0.98) | 0.036 | 0.69(0.50–0.96) | 0.03 |
| Previous diabetes | ||||
| -no | 1.0 | 1.0 | ||
| -yes | 0.62(0.37–1.03) | 0.07 | 0.62(0.35–1.07) | 0.09 |
| VTE before diagnosis | ||||
| -no | 1.0 | 1.0 | ||
| -yes | 1.01(0.42–2.44) | 0.98 | 1.15(0.47–2.83) | 0.76 |
| Year of diagnosis | ||||
| 1997–2005 | 1.0 | 1.0 | ||
| 2006–2014 | 1.22(0.97–1.53) | 0.09 | 1.23(0.96–1.58) | 0.11 |
In a Kaplan-Meier curve displaying the cumulative incidence of VTE (Fig. 2), Chemo-RC and Chemo-only had a rapidly increased cumulative incidence of VTE up to about six months after diagnosis, and continued also to rise thereafter at a higher level than for the other groups (p < 0.001). For all other treatment groups, the cumulative incidence also increased over 24 months, but at a steady pace (Fig. 2). In the sub-group with a known first treatment date, the pattern was similar, with the most pronounced increase during the first months for the Chemo-RC group (Fig. 3). None of the groups displayed a definitive plateau of the incidence curve. This is further shown in Table 4, where the cumulative incidence of VTE continues to increase beyond the one and three month time points.
Fig. 2
VTE after diagnosis (n = 9603) in different management groups in all patients with T2-T4 urinary bladder cancer in Sweden 1997–2014 within 24 months. Treatment groups were: radiotherapy with curative intent (RT), radical cystectomy (RC), chemotherapy with RC (Chemo-RC), other curative treatment (OCT), chemotherapy only (Chemo-only), and supportive management.
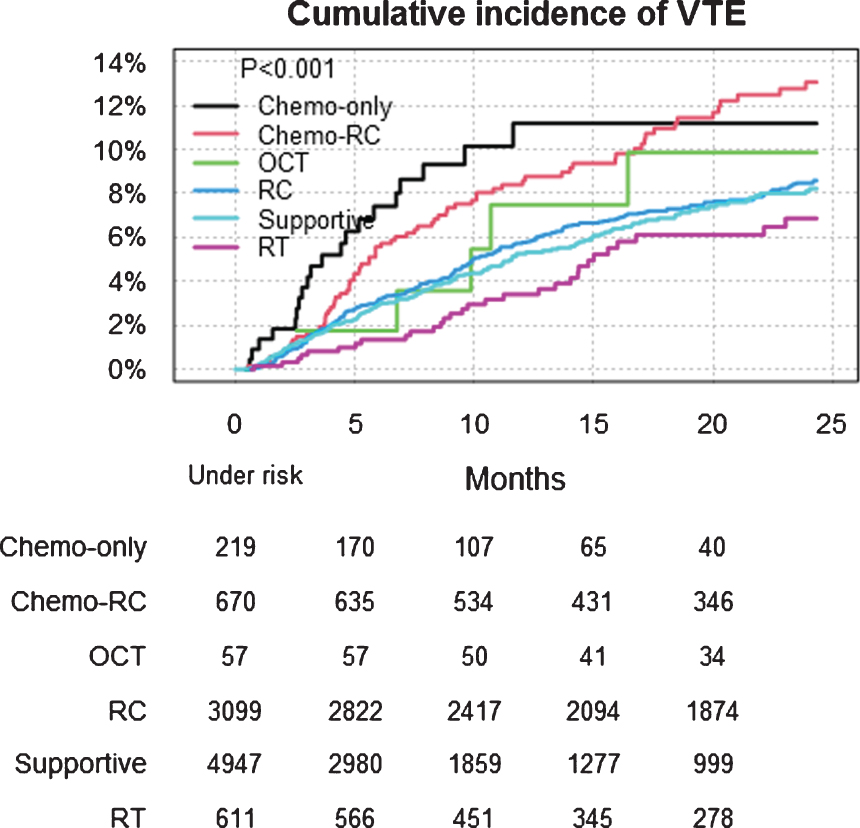
Fig. 3
VTE after first treatment date (n = 4047) in different management groups in all patients with T2-T4 urinary bladder cancer in Sweden 1997–2014 within 24 months. Treatment groups were: radiotherapy with curative intent (RT), radical cystectomy (RC), chemotherapy with RC (Chemo-RC) and chemotherapy only (Chemo-only).
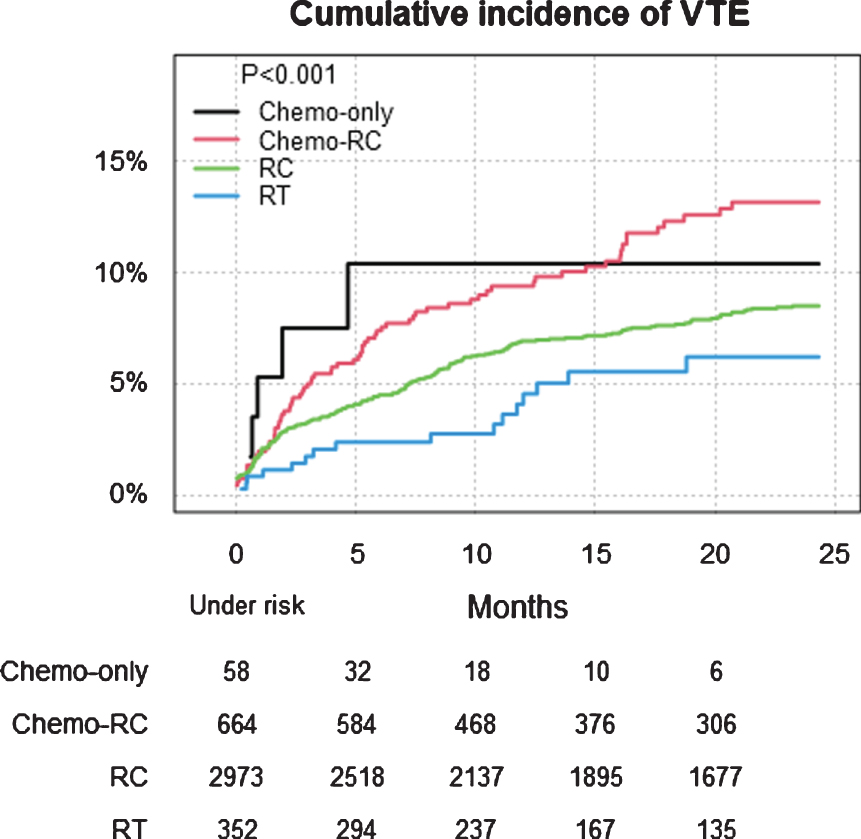
Table 4
Cumulative incidence and 95%confidence interval (CI) of VTE at one month, three months and 24 months in the different treatment groups
| a) After diagnosis | |||
| Treatment | VTE cumulative incidence (95%CI) | ||
| 1 month | 3 months | 24 months | |
| RT | 0.16(0.0–0.48) | 0.66(0.01–1.30) | 6.9(4.4–9.3) |
| RC | 0.13(0.0–0.26) | 1.4(0.98–1.8) | 8.5(7.4–9.6) |
| Chemo-RC | 0.15(0.0–0.44) | 1.6(0.67–2.6) | 13.1(10.2–15.9) |
| OCT | 0.0(0.0-0.0) | 1.8(0.0–5.1) | 9.9(1.2–17.8) |
| Chemo-only | 1.4(0.0–2.9) | 3.7(1.1–6.2) | 11.2(6.2–15.9) |
| Supportive | 0.23(0.1–0.37) | 1.3(1.0–1.7) | 8.1(6.9–9.3) |
| b) After first treatment date | |||
| Treatment | VTE cumulative incidence (95%CI) | ||
| 1 month | 3 months | 24 months | |
| RT | 0.85(0.0–1.8) | 1.7(0.35–3.1) | 6.2(3.1–9.2) |
| RC | 1.8(1.3–2.3) | 3.3(2.6–3.9) | 8.5(7.4–9.6) |
| Chemo-RC | 1.9(0.89–3.0) | 4.9(3.3–6.6) | 13.0(10.1–15.8) |
| Chemo-only | 5.2(0.0–10.8) | 7.4(0.1–14.1) | 10.2(1.1–18.4) |
DISCUSSION
The cumulative incidence of VTE after diagnosis continued to increase up to 24 months in all treatment groups without a discernible tendency to plateau. The majority of all VTE events were observed more than three months after the first treatment date. The risk for VTE was highest after Chemo-RC and Chemo-only. A lower cumulative incidence of VTE was observed in patients with previous cardiovascular disease compared to those without it, while no association with other risk factors for VTE was observed. Around ten percent of all VTE occurred at diagnosis in the entire cohort. In patients with supportive management, a substantial proportion of all VTE occurred close to the date of death.
The main study limitation is that despite using multivariate adjusted models, there may be other factors associated with the risk of VTE that were not accounted for, and which could have been unevenly distributed among the treatment groups. Another study limitation is the lack of individual information about VTE prophylaxis. During the studied period, recommended LMWH prophylaxis was extended from 14 to 30 days for all patients undergoing RC. Although we adjusted for year of diagnosis and treatment, our analysis may not discern if the lower risk for VTE in older patients and in those with previous cardiovascular disease is a consequence of higher compliance with the guidelines and/or longer periods of prophylaxis. Older patients may have a higher proportion of anti-thrombotic medications for cardiovascular comorbidities and also a lower proportion of chemotherapy, both resulting in lower rate of VTE. Likewise, information about body mass index (BMI) as well as blood cell count was lacking, despite being an important part of the Khorana score for risk assessment of VTE [8], and the distribution of these factors may have influenced the use of prophylaxis.
Using register-data, the VTE diagnosis is likely to have high specificity and be to the great majority indicating clinically relevant diagnoses, but on the other hand have a low sensitivity to detect subclinical VTE. Screening for VTE with e.g. ultrasound or biomarkers have not been recommended in Sweden during the studied period. Thus, the occurrence of VTE may be even higher than estimated in this study.
Although recommended chemotherapy protocols were cisplatin-based, lack of detailed information about the protocols and given doses in relation to cystectomy or VTE prophylaxis were other limitations. Accordingly, we cannot separately analyse VTE risk in relation to interventions such as picc lines.
Results of the study in relation to previous findings
In this study a substantial number of patients with UBC had VTE shortly before or at the time of diagnosis and sometimes leading to diagnosis of UBC [2]. Therefore, all VTE events from six months before diagnosis until two weeks after diagnosis were considered to be related to the disease per se and not to treatment. In contrast, in most comparable series such stratification was not reported [5, 14, 15, 19, 20, 22, 23].
We considered the time period for VTE after diagnosis up to 24 months after the start of first treatment, which is a longer period than in other series [19, 22–23, 29, 30]. In contrast to our findings of increased cumulative incidence during 24 months after treatment, others have found the incidence of VTE to decrease after six months [20, 29, 30], possibly due to differences in how VTE was reported. In our series the diagnosis of VTE came from all hospitalisations and most outpatient care services covering all hospital facilities in the country, while other studies may have reported only from to the institution providing the bladder cancer treatment.
In the RC treatment group, we observed the majority of VTE events more than 30 days after surgery, favouring a longer LMWH prophylaxis in line with other authors. James et al. [14] found in 1581 patients the cumulative incidence of VTE to be 2.9%during hospitalisation and 3.3%from the end of hospitalisation up to 90 days after surgery. VanDlac et al. [15] found in 1307 patients a 30-day cumulative incidence of VTE in 6%and the majority occurred after hospital discharge. Schomburg et al. [20] observed, in a small randomised study comparing 30-day prophylaxis with prophylaxis during hospitalisation, a 90-day cumulative incidence of VTE of 5%and 17.6%, respectively. No increased risk of bleeding complications was observed with 30 days of LMWH prophylaxis [7, 20].
The results in the present and other series indicate that neoadjuvant or adjuvant chemotherapy might have a further prolonged effect on the occurrence of VTE [20–23, 29–31], possibly due to cisplatin-induced apoptosis and vascular necrosis mediated by the cytotoxic effect of caspases and calpain or vascular damage from central venous catheters or other mechanisms of hypercoagulability [17, 18]. Similar results have been found with carboplatin and gemcitabine [19]. However, due to a lack of complete data on date of initiation of chemotherapy, a comparison between occurrence of VTE after neoadjuvant vs adjuvant chemotherapy in conjunction with radical cystectomy was not possible.
The lower risk of VTE in patients with previous cardiovascular disease stands in contrast with previous findings [8, 23]. Reasons for this, besides the possible differences in compliance with guidelines by the risk group, might be that in Sweden a systematic approach to the treatment and prophylaxis of other cardiovascular events has been adopted since the nineties, including Warfarin medication on wide indications [32, 33]. Many patients with previous cardiovascular disease may have had Warfarin or LMWH medication throughout the observation period. The observed increased risk with later years of diagnosis might be due to a trend over time for wider indications for radical treatment including increased use of neoadjuvant chemotherapy [34].
The reported 9.6–26%cumulative incidence of VTE in the case of palliative chemotherapy [19, 22], and the observed high risk for VTE in our small Chemo-only group, might be dependent on factors such as progressive disseminated disease, immobilisation and comorbidity besides the given systemic chemotherapy, as seen in our supportive management group, where 67%of the VTE events occurred within three months before death as part of the progressive disease. A higher risk among married patients cannot be fully explained by insufficient adjustment for the known higher treatment intensity in this patient group [35] since the higher risk is still obvious in the adjusted analyses. A higher propensity to seek advice for symptoms and thus a higher diagnostic activity is a more likely explanation.
CONCLUSION
Our findings suggest there is a need for a randomised study of prolonged VTE prophylaxis than currently used, for two reasons. First, a large majority of the VTE events were observed more than 30 days after the first treatment date, with no clear cut-off point of decrease. Secondly, increased long-term use of Warfarin or LMWH medication might explain the lower risk of VTE events in patients with previous cardiovascular disease. Endpoints should include both risk of VTE as well as risk of bleeding and other potential side-effects.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
FUNDING
This work was supported by the Swedish Cancer Society (grant numbers CAN 2016/470 and CAN 2017/278). The funding source have no influence on study design, data collection, analyses, interpretation or manuscript writing.
AUTHOR’S CONTRIBUTION
Staffan Jahnson, conception; performance of work; interpretation or analysis of data; writing the article. Truls Gårdmark, conception; writing the article. Abolfazl Hosseini, conception; writing the article. Tomas Jerlström, conception; writing the article. Fredrik Liedberg, conception; performance of work; interpretation or analysis of data; writing the article. Per-Uno Malmström, conception; writing the article. Oskar Hagberg, conception; performance of work; interpretation or analysis of data; writing the article. Amir Sherif, conception; writing the article. Viveka Ströck, conception; writing the article. Karin Söderkvist conception; writing the article. Anders Ullen, conception; writing the article. Christel Häggström, conception; performance of work; writing the article. Lars Holmberg, conception; performance of work; interpretation or analysis of data; writing the article. Firas Aljabery, conception; performance of work; interpretation or analysis of data; writing the article.
CONFLICT OF INTEREST
SJ, TG, AH,TJ, FL, PUM, OH, AS, VS, KS, AU, CH, LH and FA have no conflicts of interest to declare.
REFERENCES
[1] | Heit JA , Silverstein MD , Mohr DN , et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med (2000) ;160: :809–15. |
[2] | Khan F , Rahman A , Carrier M Occult cancer detection in venous thromboembolism: the past, the present, and the future. Res Pract Thromb Haemost (2017) ;1: :9–13. |
[3] | LymanGH The incidence of venous thromboembolism in cancer patients: A real-world analysis. Clin Adv Hematol Oncol (2012) ;10: :40–2. |
[4] | Chew HK , Wun T , Harvey D , et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med (2006) ;166: :458–64. |
[5] | Moore RA , Adel N , Riedel E , et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J Clin Oncol (2011) ;29: :34,66–73. |
[6] | Ording AG , Nielsen ME , Smith AB , et al. Venous thromboembolism and effect of comorbidity in bladder cancer: A danish nationwide cohort study of 13,809 patients diagnosed between 1995 and 2011. Urol Oncol (2016) ;34: 292–8. doi: 10.1016/j.urolonc.2016.02.014 |
[7] | Tikkinen KAO , Craigie S , Agarwal A , et al. Procedure-specific risks of thrombosis and bleeding in urological cancer surgery: Systematic review and meta-analysis. Eur Urol 2017. |
[8] | Khorana AA , Kuderer NM , Culakova E et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood (2008) ;111: :4902–7. |
[9] | Schomburg JL , Krishna S , Cotter KJ , et al. Preoperative Incidence of Deep Venous Thrombosis in Patients With Bladder Cancer Undergoing Radical Cystectomy. J Urol (2018) ;116: :120–4. doi: 10.1016/.2018.01.052 |
[10] | Clement C , Rossi P , Aissi K , et al. Incidence, risk profile and morphological pattern of lower extremity venous thromboembolism after urological cancer surgery. J Urol (2011) ;186: :2293–7. |
[11] | Bergqvist D , Agnelli G , Cohen AT , et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med (2002) ;346: :975–80. |
[12] | De Martino RR , Goodney PP , Spangler E , et al. Variation in thromboembolic complications among patients undergoing commonly performed cancer operations. J Vasc Surg (2012) ;55: :1035–40. |
[13] | Dyer J , Wyke S , Lynch C Hospital Episode Statistics data analysis of postoperative venous thromboembolus in patients undergoing urological surgery: A review of 126,891 cases. Ann R Coll Surg Engl (2013) ;95: :65–9. |
[14] | James AC , Holt SK , Wright JL , et al. Burden and timing of venothrombolic events in patients younger than 65 years undergoing radical cystectomy for bladder cancer. Urol Oncol (2014) ;32: :815–9. doi: 10.1016/j.urolonc.2014.02.016. Epub 2014 May 16. |
[15] | VanDlac AA , Cowan NG , Chen Y , et al. Timing, incidence and risk factors for venous thromboembolism in patients undergoing radical cystectomy for malignancy: a case for extended duration pharmacological prophylaxis. J Urol (2014) ;191: :943–7. doi: 10.1016/j.juro.2013.10.096. Epub 2013 Oct 29. |
[16] | Witjes A , Lebret T , Compérat EM , et al. Updated EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol (2016) ;71: :462–75. oi: 10.1016/j.eururo.2016.06.020 |
[17] | Dursun B , He Z , Somerset H , et al. Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol (2006) ;291: :578–87. |
[18] | Ottosson K , Pelander S , Johansson M , et al. The increased risk for thromboembolism pre—cystectomy in patients undergoing neoadjuvant chemotherapy for muscle—invasive urinary bladder cancer is mainly due to central venous access: a multicentre evaluation. International Urology and Nephrology (2020) ;52: :661–9. doi.org/10.1007/s11255-019-02338-4 |
[19] | Tully CM , Apolo AB , Zabor EC , et al. The High Incidence of Vascular Thromboembolic Events in Advanced Urothelial Cancer Treated with Platinum Chemotherapy Agents. Cancer (2016) ;122: :712–21. doi: 10.1002/cncr.29801 |
[20] | Schomburg J , Krishna S , Soubra A , et al. Extended outpatient chemoprophylaxis reduces venous thromboembolism after radical cystectomy. Urol Oncol (2018) ;36: :77.e9–77.e13. doi: 10.1016/j.urolonc.2017.09.029 |
[21] | Brennan K , Karima S , Doiron RC , et al. Venous Thromboembolism and Peri-Operative Chemotherapy for Muscle-Invasive Bladder Cancer: A Population-based Study. Bladder Cancer (2018) ;4: :419–28. doi: 10.3233/BLC-180184 IOS Press |
[22] | Ramos JD , Martin F Casey MF ,et al. Venous thromboembolism in metastatic urothelial carcinoma or variant histologies: incidence, associative factors, and effect on survival. Cancer Medicine (2017) ;6: :186–94. doi: 10.1002/cam4.986 |
[23] | Duivenvoorden WCM , Daneshmand S , Canter D , et al. Incidence, Characteristics and Implications of Thromboembolic Events in Patients with Muscle Invasive Urothelial Carcinoma of the Bladder Undergoing Neoadjuvant Chemotherapy. J Urol (2016) ;196: :1627–33. http://dx.doi.org/10.1016/j.juro.2016.06.017 |
[24] | TNM/UICC classification of malignant tumours 7th Edition. |
[25] | Haggstrom C , Liedberg F , Hagberg O , et al. Cohort profile: The Swedish National Register of Urinary Bladder Cancer (SNRUBC) and the Bladder Cancer Data Base Sweden (BladderBaSe). BMJ Open (2017) ;7: (9):e016606. |
[26] | Thorstenson A , Hagberg O , Ljungberg B , et al. Gender-related differences in urothelial carcinoma of the bladder: a population-based study from the Swedish National Registry of Urinary Bladder Cancer. SJU (2016) ;50: :292–7. doi: 10.3109/21681805.2016.1158207 |
[27] | Ludvigsson JF , Andersson E , Ekbom A , et al. External review and validation of the Swedish national inpatient register. BMC Public Health (2011) ;11: :450. http://www.biomedcentral.com/1471-2458/11/450. |
[28] | Greenland S , Pearls J , Robins JM . Causal Diagrams of Epidemiologic Research. Epidemiology (1999) ;10: :37–48. |
[29] | Ramos JD , Wingate JT , Gulati R , et al. Venous Throm-boembolism Risk in Patients With Locoregional Urothelial Tract Tumors. Clin Genitourin Cancer. 2017;24. pii: S1558-7673(17)30242-2. doi: 10.1016/j.clgc.2017.08.001 |
[30] | Bagrodia A , Sukhu R , Winer AG , et al. Incidence and Effect of Thromboembolic Events in Radical Cystectomy Patients Undergoing Preoperative Chemother-apy for Muscle-invasive Bladder Cancer. Clin Geni-tourin Cancer 2017;10. pii: S1558-7673(17)30232-X. doi: 10.1016/j.clgc.2017.07.022 |
[31] | Zareba P , Duivenvoorden WCM , Pinthus JH . Thromboembolism in Patients with Bladder Cancer: Incidence, Risk Factors and Prevention. Bladder Cancer (2018) ;4: :139–147,. DOI 10.3233/BLC-170146 IOS Press |
[32] | Jernberg T , Attebring MF , Hambraeus K , et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart (2010) ;96: 1617–21. doi: 10.1136/hrt.2010.198804. Epub 2010 Aug 27.Heart. 2010. |
[33] | Grip L , Lindahl B , Levin LÅ , et al. From European to National guidelines on heart disease. Scand Cardiovasc J (2011) ;45: :3–13. doi: 10.3109/14017431.2010.536566. Epub 2010 Dec 6.Scand Cardiovasc J. 2011. |
[34] | Jahnson S , Hosseini Aliabad A , Holmang S , et al. Swedish National Registry of Urinary Bladder Cancer: No difference in relative survival over time despite more aggressive treatment. SJU (2016) ;50: :14–20. doi: 10.3109/21681805.2015.1085089. PubMed PMID: 26382667. |
[35] | Macleod LC , Yabes JG , Yu M , et al. Trends and appropriateness of perioperative chemotherapy for muscle-invasive bladder cancer. Urol Oncol (2019) ;37: :462–9. doi: 10.1016/j.urolonc.2019.04.006. Epub 2019 Apr 30. |




