Prognostic Role of mRNA-Expression of Aquaporins (AQP) 3, 4, 7 and 9 in Stage pT1 Non-Muscle-Invasive Bladder Cancer
Abstract
BACKGROUND:
AQP proteins show a variety of functions in human cell metabolism. The role of different AQP subtypes in tumor metabolism and prognosis are subject of ongoing research.
OBJECTIVE:
To investigate the mRNA expression of Aquaporin (AQP) 3, 4, 7 and 9 in pT1 non-muscle-invasive bladder cancer (NMIBC) and its prognostic value in therapeutic decision making.
METHODS:
Formalin-fixed-paraffin-embedded (FFPE) tissues from transurethral resection of the bladder (TURB) from 112 patients with initial diagnosis of stage pT1 NMIBC were analyzed retrospectively together with clinical data and therapeutic approaches. mRNA expression of AQP3, 4, 7 and 9 was measured and quantified using RT-qPCR.
RESULTS:
Of the 112 patients (83.9%male, median age 72 years), 40 had a recurrence (35.7%), 16 a progression (14.3%) and 14 patients (12.5%) died tumor-related. mRNA expression for AQP3 was detected in 99.1%, AQP4 in 46.4%, AQP7 in 86.6%and AQP9 in 97.3%. Spearman analysis revealed statistically significant correlations between AQP3, AQP7 and AQP9 mRNA expression with adverse clinical and histopathological parameters (WHO1973 grade 3, concomitant Cis or multifocality). High AQP9 mRNA expression was associated with worse PFS in the total cohort (p = 0.034) and in Grade 3 tumors (p = 0.003) in Kaplan-Meier analysis. In patients with bladder sparing approach, high AQP3 mRNA expression was significantly associated with worse CSS in patients receiving BCG therapy (p = 0.029).
CONCLUSIONS:
mRNA expression of AQP3, 7 and 9 correlates with adverse clinical and pathological parameters. AQP3 and 9 may help to identify a subgroup of highest risk patients who may be considered for early cystectomy.
INTRODUCTION
The exchange of water molecules and fluids through the lipid bilayer of the cell membrane is a very important factor for the cell balance in a variety of human tissue cells. The mechanism of water exchange and regulation of osmotic gradients from different molecules is a complex and dynamic process that involves a multitude of components within the cell structure. The protein family of aquaporine water channels (aquaporines, AQP) play an important role in this process. These transmembrane transporter proteins were first discovered in 1993 by Peter Agre et al. and revolutionized the understanding of the permeability and restriction of water in the cell balance [1]. AQPs form channels or pores within the cell membrane enabling water and other small molecules like glycerol or urea to pass along osmotic or hydrostatic gradients without electric charge or antiporter mechanisms. First discovered in erythrocytes and cells of the renal tubule, currently 13 different AQPs in mammalian cells are currently known and can be divided in two subgroups: Aquaglyceroporins, which are capable of facilitating the transport of water and other small molecules (AQP3 and AQP6-12), and classical AQPs, which seem to be only permeable to water (AQP0, 1, 2, 4 and 5) [2].
Though mainly regulating water transport, AQPs are also involved in several physiological processes and also in pathological steps of tissue change like cell migration or tumor angiogenesis [3].
According to current cancer reports, urothelial carcinoma of the bladder remains the ninth most common malignancy worldwide [4]. While a quarter of patients is initially diagnosed with muscle-invasive bladder cancer, the majority of nearly 75%is detected in the non-muscle-invasive stage [5]. The first diagnostic and therapeutic step is transurethral resection of the bladder (TURB). In case of histopathologically proven non-muscle-invasive bladder cancer (NMIBC) the TURB is followed by a second resection, instillation therapy of either Mitomycin C (MMC) or Bacillus-Calmette-Guérin (BCG), and/or follow-up with regular cystoscopy, depending on clinical and histopathological features [6]. Strict follow-up is essential since recurrence of NMIBC occurs in 50–70%and the risk of tumor progression after primary TURB can add up to 20%[7].
A central question remains the therapy of pT1 NMIBC. A risk of tumor progression or cancer related death of up to 30%urges to utilize all appropriate therapeutic options, from BCG maintenance therapy to early cystectomy [8]. The chance of improved oncological outcome vs. overtreatment therefore remains difficult in each individual case [9]. With indicators for the benefit of an early cystectomy still lacking in general patient or tumor characteristics, individual decisions of the most convenient treatment have to be made for patients with high risk pT1 NMIBC.
AQP expression as potential indicator to identify highest risk patients consequently deserves further study. The role of different AQPs in carcinogenesis and metabolism in tumor cells of the urothelial carcinoma (UC) is currently investigated. Previous studies showed varying levels of expression of AQP3, 4, 7, 9 and 11 in urothelial cells as well as in UC cells [10, 11].
Downwards regulation of AQP3 seems to correlate with tumor invasiveness and differentiation, as AQP3 is highly expressed in urothelial cells, pTa UC and Carcinoma in situ (Cis), whereas the expression recedes in pT1 and WHO1973 G2 or G3 UC as well as MIBC [12, 13]. High AQP3 expression therefore significantly correlated with an improved progression free survival (PFS) in a retrospective study [14]. A possible influence on disease or therapy prognosis of the other mentioned AQPs has not been systematically investigated yet.
The aim of the present study was to quantify mRNA expression of AQP3, 4, 7 and 9 in pT1 UC cells and to investigate a potential influence on grading, staging and prognosis.
PATIENTS AND METHODS
Study population
Tissue samples of 130 patients with pT1 NMIBC were examined retrospectively. Tissue samples were acquired through transurethral resection of the bladder (TURB) between 2007 and 2015 at the Department of Urology of the University of Regensburg at the Caritas St. Josef Medical Center in Regensburg. All tumor samples were primary NMIBC cases and in each case a secondary resection or early cystectomy was performed. The tissue samples were stored as formalin-fixed paraffin-embedded blocks (FFPE).
The histopathological parameters of all cases were ascertained at the Institute of Pathology at the University Hospital Regensburg, including grading according to WHO1973 and 2004/2016 classification. All the findings, data acquisition and processing in this study comply with the ethical standards laid down in the latest declaration of Helsinki. The study was approved by the local ethics committee of the University of Regensburg (Nr. 16-321-101). Informed consent was obtained from all patients included in the study.
Isolation of tumor RNA
Whole tissue specimen sections without macrodissection were used for RNA extraction. Following pathologic review of the specimens, only those with at least 30%tumor cells were used for mRNA-extraction and further analysis. To extract the tumor tissue from the FFPE blocks, 5 sections of 10μm thickness and a maximum square size of 250 mm2 were cut out of every block using a microtome. For tumor RNA isolation the sections were processed according to a commercially available extraction method (RNeasy Mini Kit, Qiagen, Netherlands) after incubation with proteinase K for 60 minutes. The measurement of RNA quantity was performed using a Nanodrop spectrometer (Nanodrop 2000c, Thermo Scientific, USA).
RNA expression by RT-qPCR
RT-qPCR expression levels of AQP3, 4, 7 and 9 and of Calmodulin (CALM2) as reference gene (REF) were determined. Tumor RNA from the NMIBC tissue was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, USA) according to the manufacturer’s protocol. The RT-qPCR was performed using the iTaq Universal Probes One Step Kit, the CFX Connect (both Bio-Rad, USA) and Taqman Primer and Probes (Applied Biosystems, USA). The experiments were run in accordance with the following protocol: 10 min at 50°C, 3 min at 95°C, followed by 50 cycles of 10 s at 95°C and 45 s at 60°C. Fifty amplification cycles were exercised and the median cycle quantification threshold (Cq) values of the four AQPs and the REF were identified for each sample. The results were normalized against the median REF expression. Therefore, it was ensured that normalized gene expression obtained by the test is proportional to the corresponding levels of mRNA expression.
Statistical analysis
All statistical tests were performed using SPSS Version 26 (IBM, Germany). The mRNA expression levels were stratified by quartiles. Strength and direction of the mutual linear relationships of clinical and histopathological features with mRNA expression levels were examined by means of Spearman’s rank correlation. Recurrence-free survival (RFS), progression-free survival (PFS) and cancer-specific survival (CSS) were evaluated using Kaplan-Meier analysis. A p-value of < 0.05 was considered statistically significant.
RESULTS
Patient population
From 2007 to 2015 a total of 130 patients received the first diagnosis of a pT1 NMIBC through TURB. All cases were confirmed in a histopathological reassessment after a secondary TURB or underwent immediate cystectomy and 4 samples with muscle-invasive disease at secondary TURB or at immediate cystectomy were excluded. 14 samples (10.8%) had to be excluded as mRNA analysis could not be performed due to the small amount of tissue. Clinical and histopathological data of 112 patients (83.9%male, median age 72 years) were available for follow-up and further analysis. The patient characteristics are shown in Table 1. 34 cases (30.4%) were graded G2 according to the WHO1973 grading system and 78 cases (69.6%) were graded G3. According to WHO2004/2016 grading, 6 low grade (5.4%) and 105 high grade (93.7%) tumors occurred in the cohort. For 1 case (0.9%) no WHO2004/2016 grading was reported. A concomitant Carcinoma in situ (Cis) was diagnosed in 45 cases (40.2%) and multifocal tumor growth was present in 67 cases (59.8%). Median follow-up was 41.5 months. Recurrence was detected in 40 cases (35.7%), tumor progress was confirmed histopathologically in 16 cases (14.3%) and 14 patients (12.5%) died tumor-related.
Table 1
Patient characteristics in stage pT1 NMIBC study cohort
| Parameter | n (%) |
| Patient data | |
| Total stage pT1 2007–2015 | 112 (100) |
| Female patients | 18 (16.1) |
| Male patients | 94 (83.9) |
| Median age (years) | 72 [IQ range: 63–80] |
| Clinical and pathological parameters | |
| Grading WHO1973 | |
| G1 | 0 (0) |
| G2 | 34 (30.4) |
| G3 | 78 (69.6) |
| Grading WHO2016 | |
| low grade | 6 (5.4) |
| high grade | 105 (93.7) |
| n.a. | 1 (0.9) |
| Tumor diameter | |
| < 30 mm | 54 (48.2) |
| ≥30 mm | 58 (51.8) |
| Concomitant Cis | |
| yes | 45 (40.2) |
| no | 67 (59.8) |
| Focality | |
| unifocal | 45 (40.2) |
| multifocal | 67 (59.8) |
| Treatment | |
| Instillation therapy | 85 (75.9) |
| MMC | 19 (17.0) |
| BCG | 66 (58.9) |
| Cystectomy for NMIBC | 17 (15.2) |
| Secondary cystectomy | 7 (6.3) |
| Follow-up information | |
| Median follow-up (months) | 41.5 [IQ range: 26–74] |
| Maximum follow-up (months) | 136 |
| Recurrence≤pT1 | 40 (35.7) |
| Progression | 16 (14.3) |
| Death | 30 (26.8) |
| Death of disease | 14 (12.5) |
Abbreviations: IQ: interquartile, n.a.: not available.
The median mRNA-expression of AQP3, 4, 7 and 9 were normalized against the median REF-expression and quartiles were determined, particular cut-off values are itemized in Table 2. Valid mRNA-expression of AQP3 was verified in 111 tissue samples (99.1%), of AQP4 in 52 samples (46.4%), of AQP7 in 97 samples (86.6%) and AQP9 in 109 samples (97.3%). The median expression values of AQP mRNA in the present tumor tissue samples were measured in the ensuing scales: AQP3 45.81, AQP4 33.76, AQP7 37.96, AQP9 40.19.
Table 2
mRNA expression of AQP3, 4, 7 and 9 in the total T1 NMIBC cohort (n = 112)
| AQP3 | AQP4 | AQP7 | AQP9 | |
| n.a. | 1 | 60 | 15 | 3 |
| 25th percentile | 44.18049 | 32.62950 | 35.99372 | 39.00336 |
| 50th percentile | 45.80687 | 33.75500 | 37.96374 | 40.19502 |
| 75th percentile | 47.22441 | 35.07864 | 39.22685 | 41.64838 |
Abbreviations: n.a.: not available.
Correlation of APQ mRNA expression with clinical and histopathological features
Spearman analysis revealed statistically significant negative correlation between AQP3 mRNA-expression and higher grade according to WHO1973 classification (ρ: –0.257; p = 0.007) and concomitant Cis (ρ: –0.251; p = 0.008). AQP4 mRNA expression correlated significantly with mRNA expression of AQP7 (ρ: 0.358; p = 0.010). AQP7 mRNA expression further showed a statistically significant positive correlation with higher grade according to WHO1973 classification (ρ: 0.217; p = 0.033). AQP9 mRNA expression showed positive correlations to multifocal tumors (ρ: 0.190; p = 0.048) and concomitant Cis (ρ: 0.302; p = 0.001).
High AQP9 mRNA-expression correlates with worse PFS
High AQP9 mRNA-expression (cut-off: median AQP9 mRNA-expression 40.19) showed statistically significantly worse PFS in the total cohort (p = 0.034) (Fig. 1). In high-risk tumors with WHO1973 grade 3 tumors high APQ9 mRNA-expression showed a statistically significantly worse RFS (p = 0.044) and PFS (p = 0.003) (Fig. 1), and a tendency towards a worse CSS (p = 0.052). In high grade tumors, high AQP9 mRNA-expression was also statistically significantly associated with worse PFS (p = 0.047) and high AQP7 mRNA-expression (above median) was statistically significantly associated with worse CSS (p = 0.048).
Fig. 1
Kaplan-Meier analysis of AQP9 mRNA-expression (cut-off: median expression (quartile 1–2 (Q1–2) vs. quartile 3–4 (Q3–4)) with regard to progression-free survival (PFS) in the total cohort (A; n = 109; Q1–2:54 patients, 4 progressions; Q3–4:55 patients, 12 progressions) and in patients with WHO1973 Grade 3 tumors (B; n = 76; Q1–2:34 patients, 0 progressions; Q3–4:42 patients, 11 progressions). p-value < 0.05 indicates significant results.
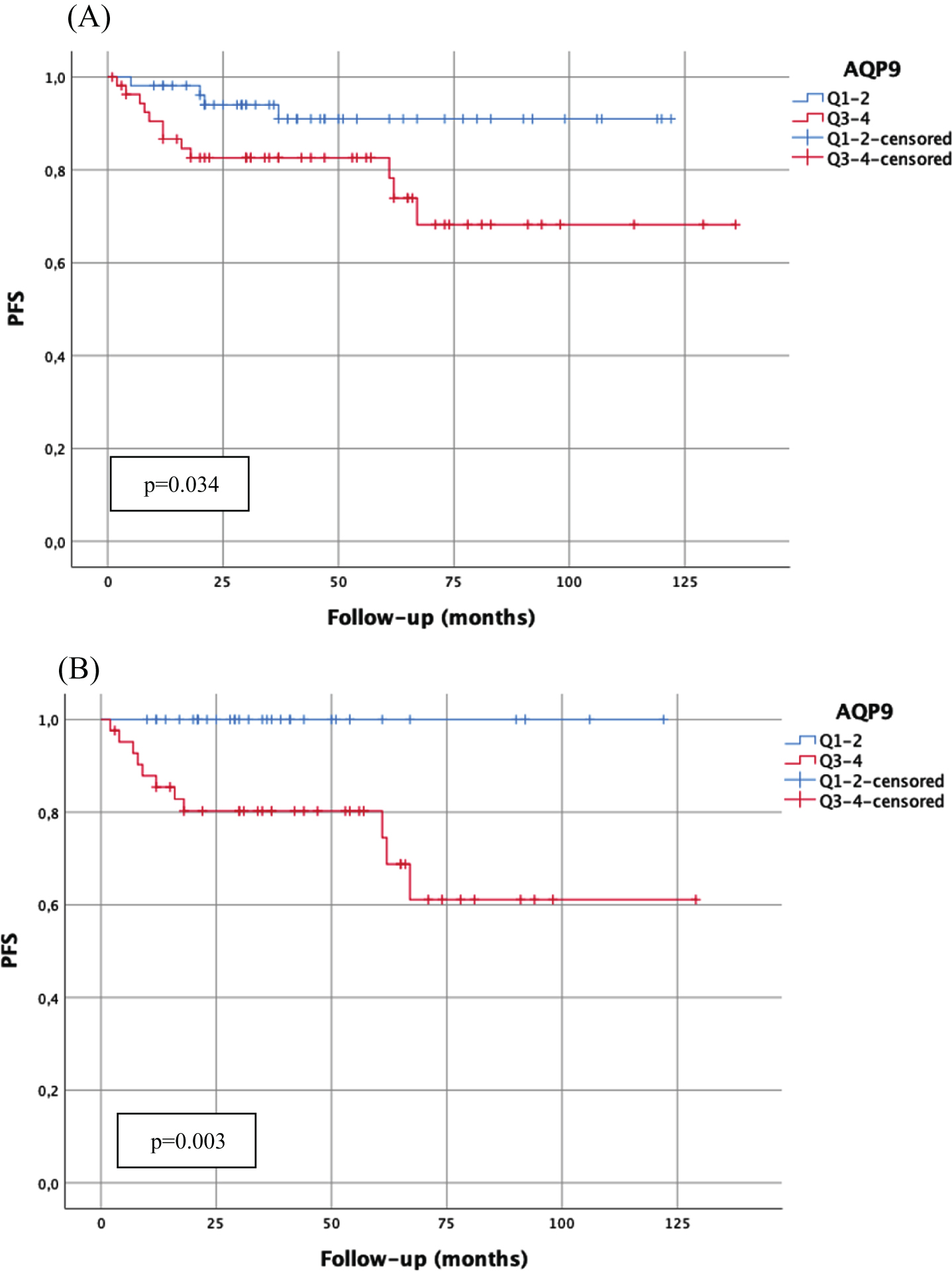
There was no correlation with AQP9 mRNA-expression and RFS or CSS in the total cohort or in other high-risk subgroups (concomitant Cis or multifocal tumors).
Furthermore, no significant correlation of AQP3, 4 and 7 mRNA-expression with recurrence, progression or disease-specific death was observed.
Of the clinical and pathological parameters, multifocal tumors were significantly associated with worse RFS (p = 0.031) and PFS (p = 0.025) in the total cohort. Concomitant Cis was significantly associated with worse PFS (p = 0.021). None of the clinical and pathological parameters showed a statistically significant correlation with CSS.
On multivariable Cox-regression analysis none of the significant parameters in univariable analysis (multifocal tumors, concomitant Cis, high AQP9 mRNA-expression) was predictive for disease progression.
Cohort with bladder-sparing approach
In patients with a primary bladder-sparing approach (n = 95; without cystectomy for NMIBC), multifocality was the only significant parameter for RFS (p = 0.031), PFS (p = 0.015) and CSS (p =0.027), respectively. No significant correlation of AQP3, 4, 7 and 9 mRNA-expression with recurrence, progression or disease-specific death could be observed.
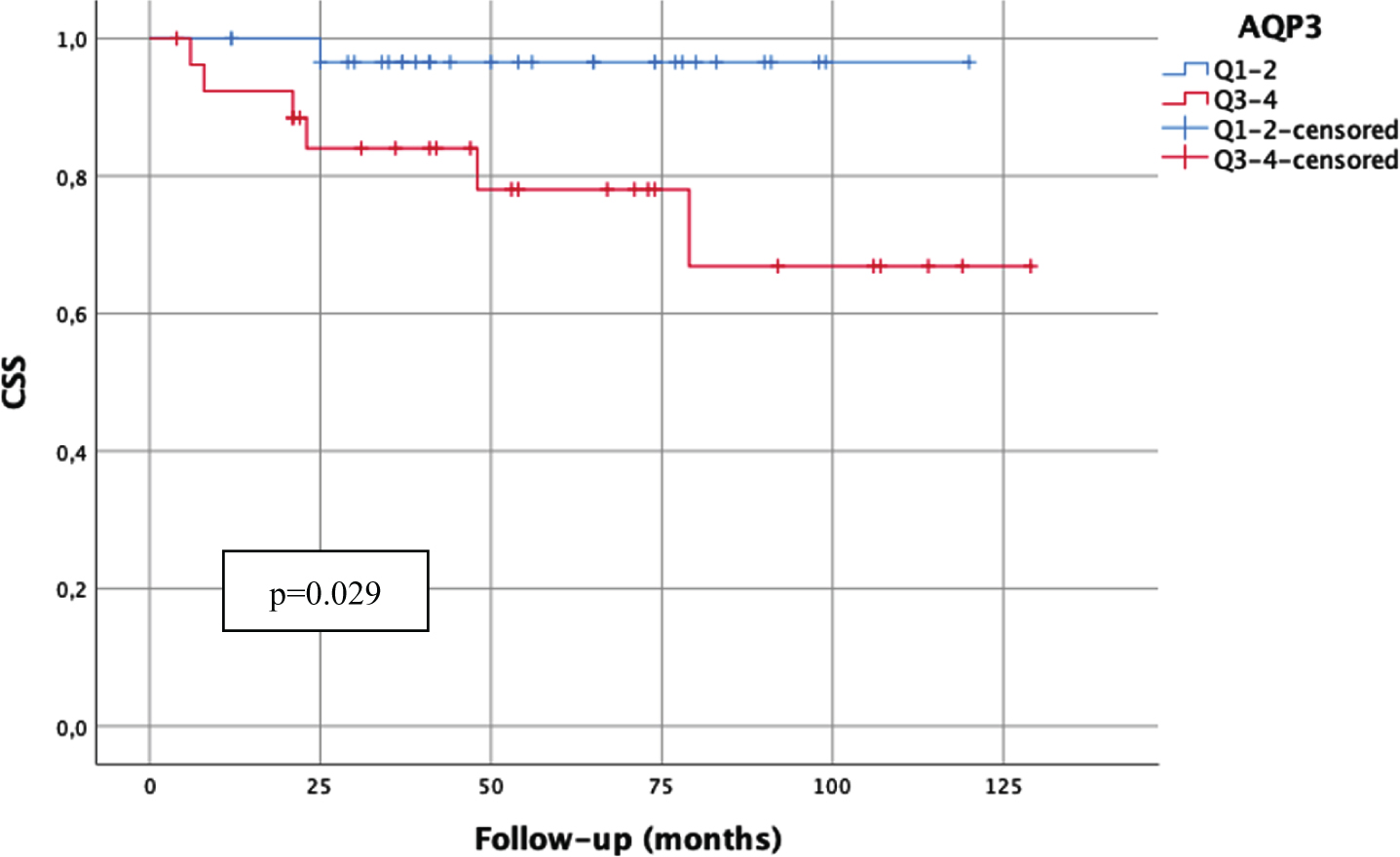
However, high AQP9 mRNA-expression (above median) correlated significantly with worse PFS (p = 0.018) and CSS (p = 0.041) in the high-risk subgroup of WHO1973 G3 tumors (Fig. 2). Furthermore, high AQP3 mRNA-expression (above median) was significantly associated with worse CSS in patients undergoing BCG therapy (p = 0.029, Fig. 3).
Fig. 2
Kaplan-Meier analysis of AQP9 mRNA-expression (cut-off: median expression (quartile 1–2 (Q1–2) vs. quartile 3–4 (Q3–4)) in WHO1973 G3 tumors with bladder-sparing approach (n = 63) with regard to progression-free survival (A; PFS; Q1–2:29 patients, 0 progressions; Q3–4:34 patients, 7 progressions) and carcinoma-specific survival (B; CSS; Q1–2:29 patients, 0 cancer-related death; Q3–4:34 patients, 6 cancer-related deaths). p-value < 0.05 indicates significant results.
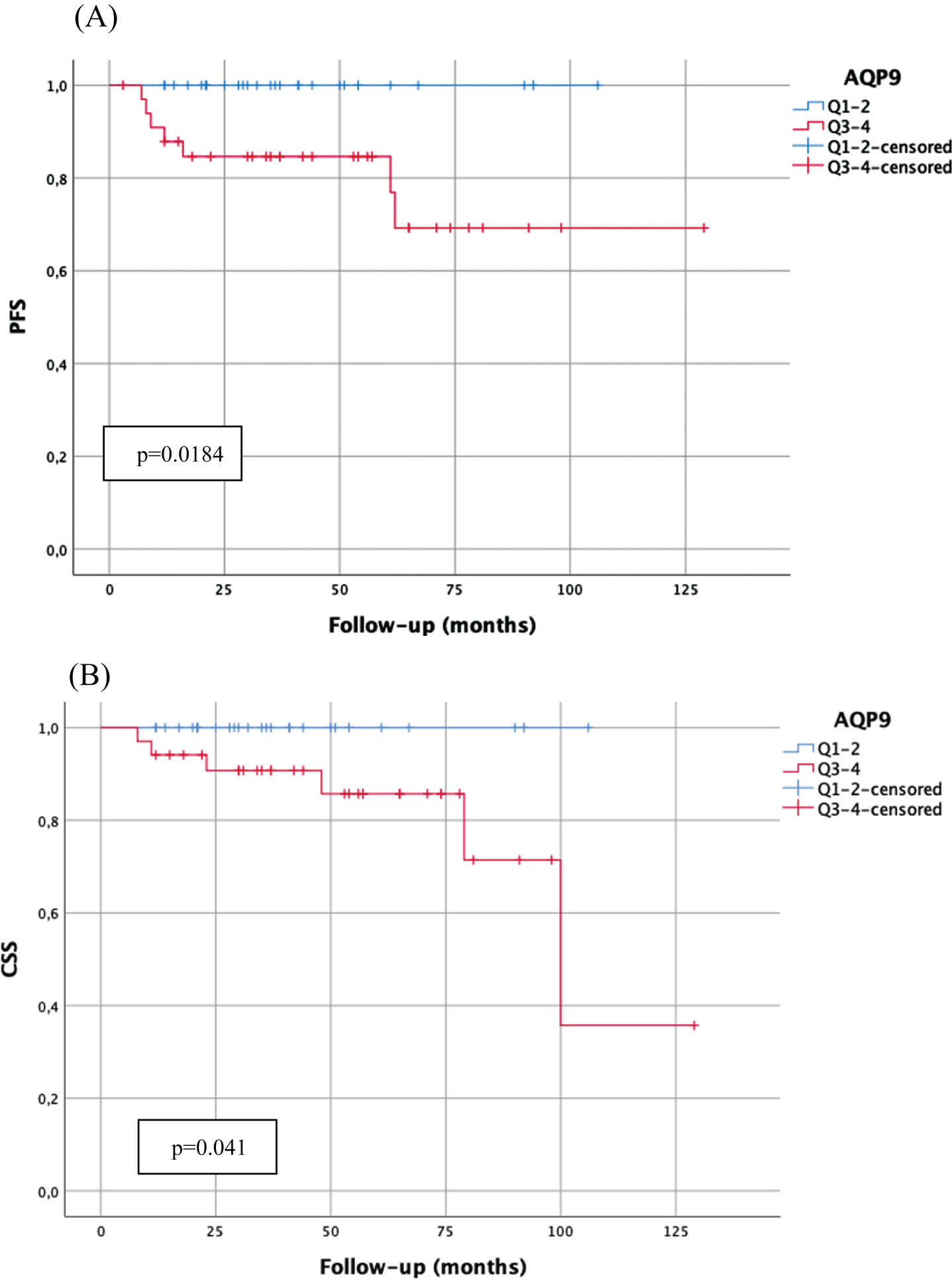
Fig. 3
Kaplan-Meier analysis of AQP3 mRNA-expression (cut-off: median expression (quartile 1–2 (Q1–2) vs. quartile 3–4 (Q3–4)) in patients treated with BCG and a bladder-sparing approach (n = 58; Q1–2:31 patients, 1 cancer-related death; Q3–4:27 patients, 6 cancer-related deaths) with regard to carcinoma-specific survival (CSS). p-value < 0.05 indicates significant results.
DISCUSSION
Different subtypes of AQP proteins have been detected in multiple mammalian tissues [15]. There are also various research results that indicate a potential influence of down-regulation or increased expression of AQP proteins in different cancer cells on metabolism, cell migration and division or cell adhesion mechanisms of the underlying tumor entity [16]. To our knowledge this is the first systematical investigation of AQP mRNA-expression in pT1 NMIBC. The intention of the study was to analyze the possible value of AQP-expression levels as diagnostic tool to identify different risk groups within the heterogeneous cluster of pT1 NMIBC patients. Of note, the current study is the first to investigate AQP7 and AQP9 expression in urothelial carcinoma tissue.
As AQP3 and AQP 9 mRNA-expression were verified in 99.1%and 97.4%, respectively, these AQPs qualified for further analysis.
Earlier studies have shown various correlations of different AQP3 levels of both immunohistochemical and mRNA-expression levels in features of urothelial carcinoma and different other malignant tumors [17].
The reduction or loss of AQP3 has been found to be statistically significant associated with urothelial carcinoma of higher stage and grade, indicating a potential role of the protein in tumor progression [11, 13]. In muscle-invasive urothelial carcinoma, reduction of AQP3 was associated with worse PFS and CSS [12].
Similar effects on tumor progression for the down-regulation of AQP3 were found in other tumor entities. In prostate cancer cells, reduction of AQP3 was associated with higher preoperative PSA values, higher risk according to the D’Amico classification and a higher Gleason-/ISUP-grade [18]. Furthermore, gastric adenocarcinoma cell lines showed increased migration and proliferation after in-vitro knockdown of AQP3 expression [19].
In contrast to these effects of AQP3 down-regulation or loss, high AQP3-expression was observed in tumor areas of human primary squamous cell carcinoma such as esophageal and lingual cancers and lymph node metastases of these tumors [20].
In ovarian cancer cell lines in-vitro AQP3 upregulation has been demonstrated to promote cell migration [21]. Moreover, high AQP3 expression was also associated with lower post-surgical survival rates and cell migration in breast cancer as well as promotion of angiogenesis and invasiveness in lung adenocarcinoma [22, 23].
In our study, AQP3 mRNA-expression was associated significantly with different prognostic parameters. Similar to earlier results, we found prognostic favorable correlations of high AQP3 mRNA-expression in regard to lower WHO1973 grading and more infrequent concomitant Cis. High AQP3 mRNA-expression also was found negatively associated with higher age, indicating a potential age-based loss or down-regulation of AQP3 mRNA-expression.
Conflicting an advantageous conclusion, high AQP3 mRNA-expression was also statistically significantly associated with worse CSS in patients undergoing BCG instillation therapy. These findings might be of therapeutic interest as patients with high AQP3 expression might rather benefit from early cystectomy than BCG instillation therapy. Of course, this issue has to be investigated and confirmed in further studies before drawing definite conclusions.
In contrast to AQP3 AQP9 expression in urothelial carcinoma tissue has not been analyzed previously. With regard to other tumor entities, predominantly negative correlations of high AQP9 expression rates were reported [17]. While hardly expressed in glia and neurons, a higher AQP9 expression in glioma cells showed associations with pathological grade and potential promotion of cell invasiveness in this aggressive type of brain cancer [24–26]. Morover, patients with adenocarcinoma of the colon which were non-responsive to adjuvant chemotherapy displayed a higher rate of low AQP9 expression compared to responders [27, 28].
Conversely, low AQP9 expression was found statistically significantly associated with liver neoplasm stage, metastasis, tumor differentiation and rapid tumor development in hepatocellular carcinoma [29, 30]. In-vivo experiments with transfected mice showed a tumor suppressive effect of AQP9 upregulation on hepatocellular carcinoma [31].
Our findings suggest a prognostic unfavorable role of high AQP9 expression in NMIBC. AQP9 mRNA-expression rates above the median were significantly associated with worse PFS in our cohort. The increased risk of rapid tumor progression in these patients may indicate a benefit from early cystectomy. AQP9 expression therefore should be considered as a potential diagnostic parameter to identify patients with higher risk of tumor progression in T1 NMIBC.
Interestingly, the cohort of patients with NMIBC pT1G3 according to WHO1973 grading showed a statistically significant worse PFS and also an inferior CSS in the subgroup with primary bladder sparing approach. The combination of higher grading according to WHO1973 and an increased AQP9 expression rate may be of prospective value to identify a subgroup of highest-risk patients within this already high-risk NMIBC constellation. These highest-risk patients should either be introduced to a more frequent and intense surveillance or be offered an early cystectomy to avoid further risk by progression and increased cancer-specific mortality.
As the roles of AQP3 and AQP9 in metabolism and progression of pT1 NMIBC remain not completely understood, further research with larger patient cohorts will be necessary to draw a concluding statement about their prognostic role in this complex tumor entity.
Limitations
Data assessment was performed in a retrospective single-center setting. The cohort size of 130 patients does not suffice to generate universally valid conclusions, so confirmation and reproduction of the presented results are necessary. Therefore, a multicenter and prospective study with a larger cohort is essential. As technical limitation, mRNA analysis as only indication of AQP expression should be confirmed by immunohistochemical validation of the described expression rates in NMIBC. Furthermore, there are a few barriers that limit the use of AQP mRNA-expression in clinical routine. Tumor tissue selection requires the reevaluation by an experienced pathologist and there will be a substantial drop-out rate of tissues that will not be suitable for mRNA-analysis (i.e. not enough tissue in small tumors). In the study at hand this resulted in over 10%of tissue samples that could not be analyzed. Moreover, the ideal cut-off has to be evaluated and determined in a multicenter setting before application in clinical routine.
CONCLUSIONS
Our study was the first to analyze AQP mRNA expression in NMIBC, and also contained AQP7 and AQP9 analysis in UC for the first time. We could verify associations to predominantly adverse clinical and pathological parameters for high mRNA expressions of AQP3, 4, 7 and 9, with AQP3 and 9 as potential valuable indicators for highest-risk patients. This may be of worth in therapeutic decision making in order to identify NMIBC patients that benefit from an early cystectomy. AQP3 and 9 show the highest mRNA expression rates in NMIBC and should be subjects of further research.
ACKNOWLEDGMENTS
We would like to thank Stefanie Goetz for excellent technical support.
FUNDING
No funding was obtained for this study.
AUTHOR CONTRIBUTIONS
JR: writing the article, interpretation of data; SB: performance of work; JB (Bründl): conception, writing the article; BR: writing the article, performance of work; MG: writing the article, performance of work; FW: performance of work, writing the article; ME: performance of work, writing the article; RMW: conception, analysis of data; SD: performance of work, writing the article; MB: conception, writing the article; WO: conception, performance of work, writing the article; JB (Breyer): conception, analysis and interpretation of data, writing the article.
CONFLICT OF INTEREST
RMW is founder and employee of STRATIFYER Molecular Pathology GmbH. JR, SK, JB (Bründl), BR, MG, FW, ME, SD, MB, WO and JB (Breyer) have no conflict of interest to declare.
REFERENCES
[1] | Agre P . The aquaporin water channels. Proc Am Thorac Soc. (2006) ;3: (1):5–13. |
[2] | Magni F , Sarto C , Ticozzi D , Soldi M , Bosso N , Mocarelli P , Kienle MG . Proteomic knowledge of human aquaporins. Proteomics. (2006) ;6: (20):5637–49. |
[3] | Jeyaseelan K , Sepramaniam S , Armugam A , Wintour EM . Aquaporins: a promising target for drug development. Expert Opin Ther Targets. (2006) ;10: (6):889–909. |
[4] | Antoni S , Ferlay J , Soerjomataram I , Znaor A , Jemal A , Bray F . Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. (2017) ;71: (1):96–108. |
[5] | Babjuk M , Burger M , Compérat EM , Gontero P , Mostafid AH , Palou J , van Rhijn BWG , Rouprêt M , Shariat SF , Sylvester R , Zigeuner R , Capoun O , Cohen D , Escrig JLD , Hernández V , Peyronnet B , Seisen T , Soukup V . European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol. (2019) ;76: (5):639–57. |
[6] | Chang SS , Boorjian SA , Chou R , Clark PE , Daneshmand S , Konety BR , Pruthi R , Quale DZ , Ritch CR , Seigne JD , Skinner EC , Smith ND , McKiernan JM . Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. (2016) ;196: (4):1021–9. doi: 10.1016/j.juro.2016.06.049. Epub 2016 Jun 16. |
[7] | Prout GR Jr , Barton BA , Griffin PP , Friedell GH . Treated history of noninvasive grade 1 transitional cell carcinoma. The National Bladder Cancer Group. J Urol. (1992) ;148: (5):1413–9. doi: 10.1016/s0022-5347(17)36924-0. |
[8] | Shahin O , Thalmann GN , Rentsch C , Mazzucchelli L , Studer UE . A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade 3 bladder cancer: recurrence, progression and survival. J Urol. (2003) ;169: (1):96–100; discussion 100. |
[9] | Denzinger S , Fritsche HM , Otto W , Blana A , Wieland WF , Burger M . Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol. (2008) ;53: (1):146–52. |
[10] | Rubenwolf PC , Georgopoulos NT , Kirkwood LA , Baker SC , Southgate J . Aquaporin expression contributes to human transurothelial permeability in vitro and is modulated by NaCl. PLoS One. (2012) ;7: (9):e45339. |
[11] | Rubenwolf PC , Otto W , Denzinger S , Hofstädter F , Wieland W , Georgopoulos NT . Expression of aquaporin water channels in human urothelial carcinoma: correlation of AQP3 expression with tumour grade and stage. World J Urol. (2014) ;32: (4):991–7. |
[12] | Otto W , Rubenwolf PC , Burger M , Fritsche HM , Rößler W , May M , Hartmann A , Hofstädter F , Wieland WF , Denzinger S . Loss of aquaporin 3 protein expression constitutes an independent prognostic factor for progression-free survival: an immunohistochemical study on stage pT1 urothelial bladder cancer. BMC Cancer. (2012) ;12: :459. |
[13] | Breyer J , Otto W , Burger M , Hartmann A , Rubenwolf PC . Aquaporin 3 Expression Loss in Urothelial Carcinoma: Association with Tumor Invasion Depth, but not with Grading? Bladder Cancer. (2017) ;3: (1):31–4. doi: 10.3233/BLC-160082. |
[14] | Rubenwolf P , Thomas C , Denzinger S , Hartmann A , Burger M , Georgopoulos NT , Otto W . Loss of AQP3 protein expression is associated with worse progression-free and cancer-specific survival in patients with muscle-invasive bladder cancer. World J Urol. (2015) ;33: (12):1959–64. |
[15] | Rubenwolf PC , Georgopoulos NT , Clements LA , Feather S , Holland P , Thomas DF , Southgate J . Expression and localisation of aquaporin water channels in human urothelium in situ and in vitro . Eur Urol. (2009) ;56: (6):1013–23. |
[16] | Papadopoulos MC , Saadoun S . Key roles of aquaporins in tumor biology. Biochim Biophys Acta. (2015) ;1848: (10 Pt B):2576–83. |
[17] | Chow PH , Bowen J , Yool AJ . Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers. Cancers (Basel). (2020) ;12: (7):E1911. |
[18] | Bründl J , Wallinger S , Breyer J , Weber F , Evert M , Georgopoulos NT , Rosenhammer B , Burger M , Otto W , Rubenwolf P . Expression, localisation and potential significance of aquaporins in benign and malignant human prostate tissue. BMC Urol. (2018) ;18: (1):75. |
[19] | Huang Y , Zhu Z , Sun M , Wang J , Guo R , Shen L , Wu W . Critical role of aquaporin-3 in the human epidermal growth factor-induced migration and proliferation in the human gastric adenocarcinoma cells. Cancer Biol Ther. (2010) ;9: (12):1000–7. |
[20] | Kusayama M , Wada K , Nagata M , Ishimoto S , Takahashi H , Yoneda M , Nakajima A , Okura M , Kogo M , Kamisaki Y . Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. (2011) ;102: (6):1128–36. |
[21] | Ji C , Cao C , Lu S , Kivlin R , Amaral A , Kouttab N , Yang H , Chu W , Bi Z , Di W , Wan Y . Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother Pharmacol. (2008) ;62: (5):857–65. |
[22] | Kang S , Chae YS , Lee SJ , Kang BW , Kim JG , Kim WW , Jung JH , Park HY , Jeong JH , Jeong JY , Park JY . Aquaporin 3 Expression Predicts Survival in Patients with HER2-positive Early Breast Cancer. Anticancer Res. (2015) ;35: (5):2775–82. |
[23] | Liu YL , Matsuzaki T , Nakazawa T , Murata S , Nakamura N , Kondo T , Iwashina M , Mochizuki K , Yamane T , Takata K , Katoh R . Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. Hum Pathol. (2007) ;38: (1):171–8. |
[24] | Badaut J , Regli L . Distribution and possible roles of aquaporin 9 in the brain. Neuroscience. (2004) ;129: (4):971–81. |
[25] | Fossdal G , Vik-Mo EO , Sandberg C , Varghese M , Kaarbo M , Telmo E , Langmoen IA , Murrell W . Aqp 9 and brain tumour stem cells. ScientificWorldJournal. (2012) ;2012: :915176. |
[26] | Lv Y , Huang Q , Dai W , Jie Y , Yu G , Fan X , Wu A , Miao Q . AQP9 promotes astrocytoma cell invasion and motility via the AKT pathway. Oncol Lett. (2018) ;16: (5):6059–64. |
[27] | Huang D , Feng X , Liu Y , Deng Y , Chen H , Chen D , Fang L , Cai Y , Liu H , Wang L , Wang J , Yang Z . AQP9-induced cell cycle arrest is associated with RAS activation and improves chemotherapy treatment efficacy in colorectal cancer. Cell Death Dis. (2017) ;8: (6):e2894. |
[28] | Dou R , Deng Y , Huang L , Fu S , Tan S , Wang L , Lian L , Fang L , Fan X , Jin G , Liu H , Wang J . Multi-microarray identifies lower AQP9 expression in adjuvant chemotherapy nonresponders with stage III colorectal cancer. Cancer Lett. (2013) ;336: (1):106–13. |
[29] | Peng R , Zhao GX , Li J , Zhang Y , Shen XZ , Wang JY , Sun JY . Auphen and dibutyryl cAMP suppress growth of hepatocellular carcinoma by regulating expression of aquaporins 3 and 9 in vivo . World J Gastroenterol. (2016) ;22: (12):3341–54. |
[30] | Qian Y , Liu F , Zhang W , Zheng X , Liao S , Lv L , Mei Z . AQP9 suppresses hepatocellular carcinoma cell invasion through inhibition of hypoxia-inducible factor 1α expression under hypoxia. J Gastroenterol Hepatol. 2020 Mar 1. Doi: 10.1111/jgh.15023. Online ahead of print |
[31] | Liao S , Chen H , Liu M , Gan L , Li C , Zhang W , Lv L , Mei Z . Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/β-catenin pathway. Aging (Albany NY). (2020) ;12: (2):1527–1544. |




