Incidental Prostate Cancer in Radical Cystoprostatectomy Specimens is Associated with Worse Overall Survival
Abstract
BACKGROUND:
The impact of incidental prostate cancer (IPC) on oncological outcomes after radical cystoprostatectomy (RCP) specimens from patients with bladder cancer (BC) remains controversial. This relationship has not been well elucidated in Asian countries, where the incidence of prostate cancer has recently shown dramatic increases.
OBJECTIVES:
This study retrospectively compared pathological features and oncological outcomes between BC patients with and without IPC in the RCP specimens.
METHODS:
This study included 142 men who underwent RCP for BC. Men who were previously diagnosed with prostate cancer were excluded. Each prostate gland and seminal vesicle was processed as whole mounts and 4-mm close-step sectioning was performed. A single genitourinary pathologist diagnosed IPC. The pathological features and oncological outcomes such as overall survival (OS), bladder cancer-specific survival (BCSS), and progression-free survival (PFS) were compared between patients with IPC (IPC+group, n = 45) and without IPC (IPC- group, n = 97). P values less than 0.05 considered to indicate statistical significance for patients’ characteristics. Because of multi-primary endpoint, P values less than 0.0167 was considered statistical significance for oncological outcomes.
RESULTS:
We detected IPC in 45 RCP specimens (31.6%). Patients in the IPC- group were significantly younger at surgery than those in the IPC+group (P < 0.001). The pathological features of the RCP specimens did not differ significantly. In multivariable analyses, presence of IPC was significantly associated with worse OS (P = 0.005), but not with either BCSS or PFS (P = 0.038 and 0.326, respectively). In Kaplan–Meier analyses, OS tended to be longer in the IPC- group than that in the IPC+group (NR vs 65 months, P = 0.0017).
CONCLUSIONS:
Our results suggested significantly better OS in patients without IPC than that in those with IPC.
ABBREVIATIONS
BC | bladder cancer |
BCSS | bladder cancer-specific survival |
BMI | body mass index |
GS | Gleason score |
IPC | incidental prostate cancer |
OS | overall survival |
PC | prostate cancer |
PFS | progression-free survival |
RPC | radical cystoprostatectomy |
INTRODUCTION
Bladder cancer (BC), the second most common genitourinary malignancy worldwide, is an aggressive malignancy that causes significant mortality [1]. In comparison, prostate cancer (PC) is the second most frequently diagnosed cancer and the fifth leading cause of cancer-related deaths among men worldwide [1]. As PC is often slow-growing and clinically insignificant, it is generally detected as subclinical forms such as latent and incidental cancer. Incidental prostate cancer (IPC) is incidentally detected during the histopathological examination of radical cystoprostatectomy (RCP) specimens from patients with BC. An association between PC and BC has long been suspected [2]. There have been several reports indicating that patients with BC is are at increased risk for primary PC [3, 4]. The causes of this increased risk are suggested to be exposure to common carcinogens, genetic predisposition and frequent examination during followup. The reported prevalence of IPC in the literature varies from 4 to 61% [5–7]. The discrepancies are possibly due to differences in histopathologic sampling technique such as slice thickness of step sections as well as the ethnicities of the study population. A recent meta-analysis of 13,140 patients reported an incidence of IPC of 24.4% [8], while a multi-institutional retrospective study including 2114 patients reported an incidence of 24.3% [9]. The reported incidences of IPC in Japanese populations were relatively low, from 12.3 to 26.1% [10–12]. However, reports from Japan included relatively old cohorts and our previous study on IPC in 148 RCP specimens in Japan from 2009 to 2017 observed an incidence of 31.6% [13]. The incidence of both clinical and subclinical PC is increasing, particularly in Asian countries [7]. We previously reported increasing prevalence and size of latent prostate cancer tumors in the last 25 years in Japan [14]. In addition, the prevalence and pathological characteristics did not differ significantly between latent and IPC [13].
The impact of IPC on oncological outcomes after RCP remains controversial. The above meta-analysis reported IPC to be significantly associated with lower 5-year overall survival (OS) [8]. However, the multi-institutional retrospective study, which was not included in the above meta-analysis, reported that IPC was not significantly associated with cancer-specific and overall survival [9]. However, the retrospective study had limitations, including a lack of no central pathological review and non-standardized embedding technique, such that 79.2% of the specimens underwent partial embedding.
Even though there are several reports about the prevalence of IPC [7], the influence of IPC on the oncological outcomes of invasive BC in recent Asian populations has not been well elucidated. Thus, we compared pathological features and oncological outcomes between patients with invasive BC with and without IPC in RCP specimens.
MATERIALS AND METHODS
This study was approved by the Ethics Committee Institutional Review Board (19–157 [5088]). We reviewed a total of 148 men who underwent RCP for invasive BC at Jikei University Hospital and Jikei University Kashiwa Hospital between 2009 and 2017. We excluded six cases with insufficient medical records; thus, this study included 142 cases.
Each prostate gland and seminal vesicle were fixed en-block in 10% neutral buffered formalin, and subsequently sliced in step sections vertical to the urethra at 4-mm intervals. Each section was embedded in paraffin and 5-μm sections were stained with hematoxylin and eosin. When PC was difficult to diagnose, MA903, P63, and AMACR immunohistochemical staining were performed. An experienced genitourinary pathologist performed the histological evaluations. The index tumor, which was identified as the tumor with the largest tumor volume, was evaluated in detail [15]. The tumor volume was calculated using the following formula: 4/3π×(length/2)×(width/2)×(height/2). We assessed Gleason grade and score according to standard criteria. We defined clinically significant PC as the presence of≥T3 and/or index tumor volume≥500 mm3 and/or Gleason score (GS)≥7 (Epstein’s Criteria) [16].
We compared pathological features and oncological outcomes between patients with IPC (IPC+group) and without IPC (IPC- group). Data were analyzed using the Student’s t and chi-square tests. Progression-free survival (PFS), bladder cancer-specific survival (BCSS), and OS from surgery were calculated and analyzed using the Kaplan–Meier method and log-rank tests. OS and BCSS were defined as the time from surgery to all-cause or bladder cancer specific death confirmed by hospital records and death certificates. PFS was defined as the time from surgery to local recurrence and/ or distant metastasis of BC. Generally, assessment of progression was conducted by computed tomography once every 3 months for 2 years, semi-annually for 3 years, annually thereafter. Univariable and multivariable Cox proportional hazard models were used to explore the predictors of OS. The covariates included age, pT stage of BC (≤2 or 3≤), IPC, body mass index (BMI), neoadjuvant and adjuvant chemotherapy, variant status, grade (≤2 or 3), resect margin (RM) status, pN stage, and lymphovascular invasion (LVI) status. Because age was significantly different between IPC+and IPC- groups, age and IPC were included as covariates in the multivariable analyses. Statistical analyses were performed using GraphPad Prism (version 5, GraphPad Software, La Jolla, CA, USA) and Stata (version 13, StataCorp, College Station, TX, USA), with P values less than 0.05 considered to indicate statistical significance for patients’ characteristics. Because of multi-primary endpoint, P values less than 0.0167 was considered statistical significance by Bonferroni correction for oncological outcomes.
RESULTS
Prevalence and pathological features of IPC
The clinical backgrounds and pathological features of BC are shown in Table 1. We detected IPC in 45 of 142 (31.6%) RCP specimens. Measurement of prostate-specific antigen (PSA) in 75 patients (29 in IPC+and 46 in IPC- group) showed levels above 4 ng/mL in 12 patients (16.0%). Neoadjuvant chemotherapy was administrated in 30.3% (43/142) patients. Eighteen patients (12.7%) were pathologically diagnosed with pT0 disease. The pathological features of 45 IPC cases are shown in Table 2. Thirty-one patients (68.9%) were GS 6 and no patients were GS≥8. Four patients (8.9%) were pT3. Fourteen patients (31.1%) had clinically significant cancer based on Epstein’s Criteria. No patients had biochemical recurrence or underwent additional therapy for PC during the observation period.
Table 1
Patients’ characteristics and pathological features of bladder cancer
| All | IPC+ | IPC- | P | ||||
| Number | 142 | 45 | 97 | ||||
| Age (years), madlan (range) | 69 | (42–89) | 74 | (53–89) | 66 | (42–85) | 0.0002 |
| PSA(ng/ml), median (range)* | 1.76 | (0.01–21.6) | 2.44 | (0.59–6.1) | 13 | (0.01–21:5) | 0.64 |
| BMI, median (range) | 23 | (16.6–33.1) | 22.9 | (16.8–30.3) | 23 | (16.6–33.1) | 0.68 |
| Neoadjuvant chemotherapy | |||||||
| Yes (%) | 43 | (30.3) | 11 | (24.4) | 32 | (33.0) | 0.33 |
| No (%) | 99 | (69.7) | 34 | (75.6) | 65 | 67.0) | |
| Adjuvant chemotherapy | |||||||
| Yes (%) | 14 | (9.9) | 4 | (8.9) | 10 | (10.3) | 1.00 |
| No (%) | 128 | (90.1) | 41 | (91.1) | 87 | (89.7) | |
| pT stage | |||||||
| T0 (%) | 18 | (12.7) | 4 | (8.9) | 14 | (14.4) | 0.65 |
| Ta,1,is (%) | 40 | (28.2) | 16 | (35.6) | 24 | (24.7) | |
| T2 (%) | 26 | (18.3) | 6 | (13.3) | 20 | (20.6) | |
| T3–4 (%) | 58 | (40.8) | 19 | (42.2) | 39 | (40.2) | |
| Grade | |||||||
| 0 (%) | 18 | (12.7) | 4 | (8.9) | 14 | (14.4) | 0.52 |
| 1 (%) | 1 | (0.7) | 0 | (0.0) | 1 | (1.0) | |
| 2 (%) | 31 | (21.8) | 9 | (20.0) | 22 | (22.7) | |
| 3 (%) | 92 | (64.8) | 32 | (71.1) | 60 | (61.9) | |
| LVI | |||||||
| 0 (%) | 82 | (57.7) | 27 | (60.0) | 55 | (56.7) | 0.85 |
| 1 (%) | 60 | (42.3) | 18 | (40.0) | 42 | (43.3) | |
| pN | |||||||
| 0 (%) | 107 | (75.4) | 32 | (71.1) | 75 | (77.3) | 0.79 |
| 1 (%) | 21 | (14.8) | 7 | (15.6) | 14 | (14.4) | |
| x (%) | 14 | (9.9) | 6 | (13.3) | 8 | (8.2) | |
| RM | |||||||
| 0 (%) | 121 | (85.2) | 41 | (91.1) | 80 | (82.5) | 0.21 |
| 1 (%) | 21 | (14.8) | 4 | (8.9) | 17 | (17.5) | |
* PSA was measured in 75 patients (29 in IPC + and 46 in IPC–group). IPC: incidental prostate cancer, PSA: prostate specific antigen, BMI: body mass index, LVI: lymphovascular invasion, RM: resection margin.
Table 2
Pathological features of incidental prostate cancer
| Number of cases | 45 | |
| Gleason score (%) | ||
| 6> | 31 | 68.9 |
| 7 | 14 | 31.1 |
| pT stage (%) | ||
| 2 | 41 | 91.1 |
| 3 | 4 | 8.9 |
| Index tumor volume | ||
| Median volume (mm3, range) | 42.2 | (0.091–19367) |
| 500 mm3> | 38 | 84.4 |
| 500 mm3≥ | 7 | 15.6 |
| Clinicallysignificant (%) | 14 | 31.1 |
Associations between IPC and the pathological features of BC
Comparisons between patients with and without IPC showed a significantly lower age at surgery in the IPC- group than that in the IPC+group (median 66 vs. 74 years, P = 0.0002, Table 1). Although the number of measured patients was limited, PSA level did not differ significantly between the groups. Neoadjuvant chemotherapy did not significantly influence the presence of IPC. Finally, the pathological features of RCP specimens, including pT stage, grade, LVI status, pN stage, and RM status, also did not differ significantly between the groups.
Associations between IPC and oncological outcomes of BC
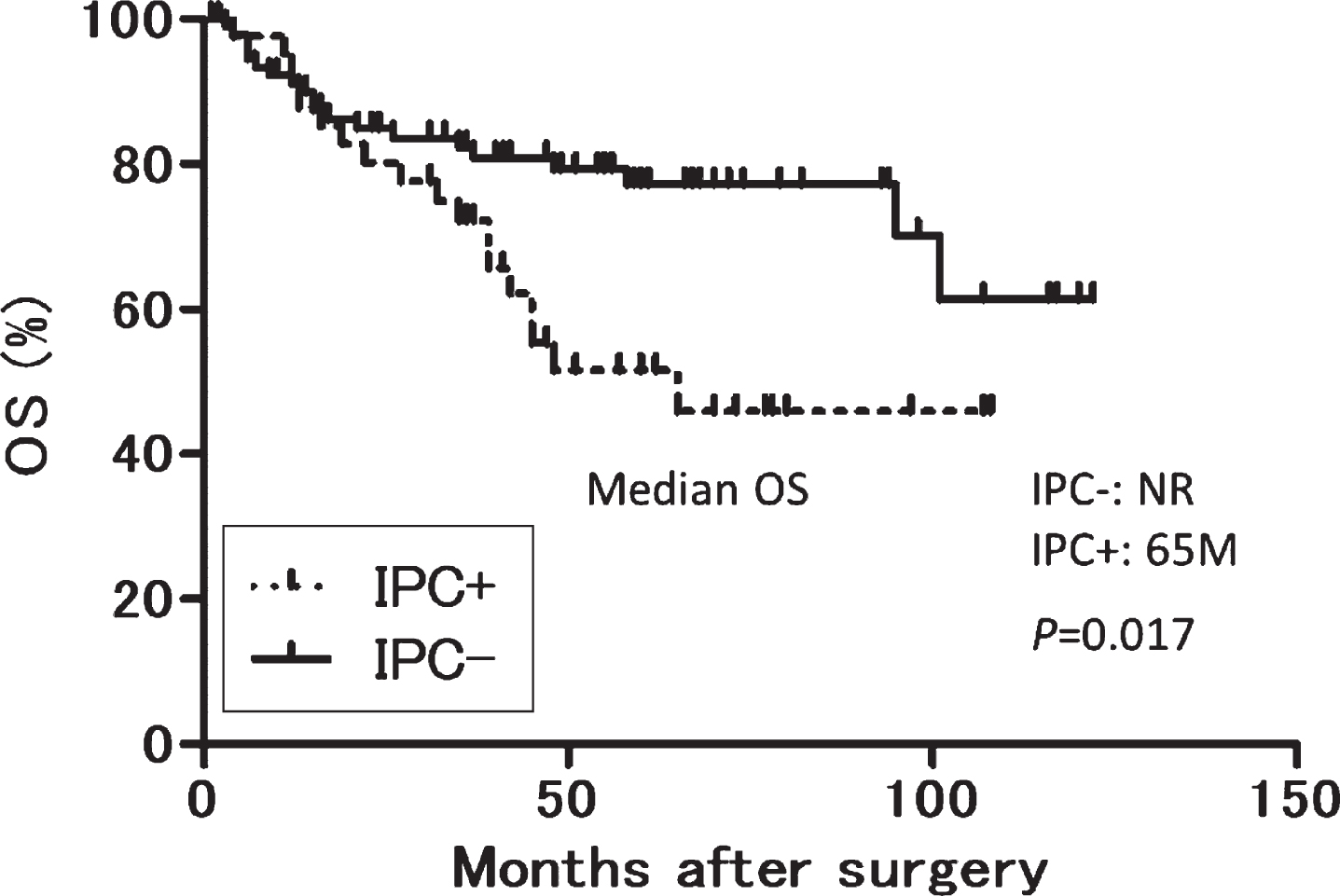
The median observation period was 45 months (interquartile range, 15–64.3 months). Twenty-nine, 19 and 20, and 15, 13 and 18 patients experienced disease progression, bladder cancer specific and all-cause dearth in the IPC- and IPC+groups, respectively. In multivariable analyses, presence of IPC was significantly associated with worse OS (P = 0.005), whereas no variables were significantly associated with either BCSS or PFS (Table 3). Kaplan–Meier analyses showed that OS tended to be longer in the IPC-group than that in the IPC + group although it was not statistically significant (NR vs 65 months, P = 0.0017, log-rank test. Fig. 1).
Table 3
Uni- and multi-variable analyses for OS, BCSS and PFS
| OS | BCSS | PFS | |||||||||||||||||||||||
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | ||||||||||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||||||
| Age | 1.03 | 0.99 | 1.07 | 0.102 | 1.02 | 0.97 | 1.07 | 0.482 | 1.02 | 0.98 | 1.06 | 0.415 | 1.00 | 0.95 | 1.05 | 0.947 | 1.02 | 0.98 | 1.05 | 0.311 | 1.00 | 0.96 | 1.05 | 0.977 | |
| (continuous) | |||||||||||||||||||||||||
| pT stage | 6.23 | 3.01 | 12.90 | <0.001 | 3.12 | 1.13 | 8.67 | 0.029 | 9.42 | 3.86 | 22.99 | <0.001 | 3.87 | 1.16 | 12.91 | 0.027 | 5.32 | 2.71 | 10.47 | <0.001 | 2.66 | 0.98 | 7.24 | 0.055 | |
| (0–2 or 3–4) | |||||||||||||||||||||||||
| IPC (no or yes) | 2.15 | 1.13 | 4.08 | 0.019 | 3.23 | 1.42 | 7.34 | 0.005 | 1.58 | 0.78 | 3.21 | 0.206 | 2.58 | 1.06 | 6.32 | 0038 | 1.18 | 062 | 2.25 | 0624 | 1.52 | 066 | 3.54 | 0.326 | |
| BMI | 0.91 | 0.80 | 1.04 | 0.166 | 0.98 | 0.85 | 1.13 | 0.806 | 104 | 0.92 | 1.17 | 0.525 | |||||||||||||
| (continuous) | |||||||||||||||||||||||||
| NAC (no o ryes) | 1.54 | 0.78 | 3.04 | 0.212 | 1.54 | 0.74 | 3.24 | 0.249 | 1.01 | 0.50 | 2.02 | 0.988 | |||||||||||||
| AC (no or yes) | 3.63 | 1.58 | 8.37 | 0.002 | 1.75 | 0.62 | 4.49 | 0.292 | 4.42 | 1.88 | 10.42 | 0.001 | 1.65 | 0.56 | 4.89 | 0.368 | 4.13 | 1.95 | 8.76 | <0.001 | 2.02 | 0.78 | 5.20 | 0.147 | |
| Variant | 0.77 | 0.24 | 2.53 | 0.672 | 0.61 | 0.15 | 2.56 | 0.500 | 0.21 | 003 | 1.53 | 0.123 | |||||||||||||
| (no or yes) | |||||||||||||||||||||||||
| Grade | 1.44 | 0.72 | 2.91 | 0.306 | 1.49 | 0.68 | 3.32 | 0.313 | 1.70 | 0.85 | 3.39 | 0.113 | |||||||||||||
| (0–2 or 3) | |||||||||||||||||||||||||
| RM (0 or l) | 2.58 | 1.22 | 5.48 | 0.013 | 1.44 | 0.44 | 4.78 | 0.547 | 2.66 | 1.19 | 5.95 | 0.017 | 1.05 | 0.26 | 4.29 | 0.947 | 3.49 | 1.77 | 6.87 | <0.001 | 1.23 | 0.37 | 4.07 | 0.731 | |
| pN (0 or l) | 4.78 | 2.15 | 10.61 | <0.001 | 2.07 | 0.78 | 5.51 | 0.145 | 6.03 | 2.62 | 13.86 | <0.001 | 2.22 | 0.80 | 6.15 | 0.127 | 4.83 | 2.23 | 10.45 | <0.001 | 1.61 | 0.63 | 4.09 | 0.322 | |
| LVI (0 or l) | 3.35 | 1.73 | 6.50 | <0.001 | 1.95 | 0.72 | 5.30 | 0.189 | 4.86 | 2.24 | 10.54 | (0.001 | 2.46 | 0.77 | 7.83 | 0.128 | 3.93 | 2.05 | 7.53 | <0.001 | 2.93 | 1.09 | 7.87 | 0.033 | |
OS: overall survival, BCSS: bladder cancer specific survival, PFS: progression free survival, HR: hazard ratio, CI: confidence Interval, IPC: incidental prostate cancer, BMI: body mass index, NAC: neoadjuvant chemotherapy, AC: neoadjuvant chemotherapy, RM: resection margin, LVI: lymphovascular invasion.
Fig. 1
OS according to IPC after radical cystoprostatectomy. OS: overall survival, IPC: incidental prostate cancer. OS after cystoprostatectomy.
DISCUSSION
The incidence of PC is dramatically changing in Asian countries, particularly in northeast Asian countries. In Japan, the incidences of both clinical and latent PC have been increasing and are now similar to those of Western populations [7, 17]. Although the cause of this increase may be multifactorial, one possible explanation is changes in lifestyle due to Western diets. In the present study, the incidence of IPC was 31.6%, a rate relatively higher than that in previous studies in Japanese cohorts. Only one study including 349 men from 1995 to 2007 reported an incidence of 26.1% [11]; those in other studies ranged from 11.2 to 18.1% [9, 10, 12]. This discrepancy might be caused by differences in histopathologic sampling techniques as two of the studies used a partial embedding technique [9]. Another explanation may be differences in study periods. The study period of our cohort was relatively recent, from 2009 to 2017, while the other studies included subjects from the 1970s to the 1990s. However, clinically significant cancer was detected in 31.1% of the IPC in our study, a rate consistent with those in other reports from Japan, ranging from 25.3 to 47.0% [10–12].
Patients with IPC were significantly older than those without IPC. Several reports have indicated the positive association between age and the incidence of clinical PC and prevalence of latent PC [7, 14]. The other clinical and pathological characteristics were not significantly associated with the presence of IPC. The findings are mainly consistent with those of the meta-analysis, in which IPC was significantly associated with greater age, but no association was observed between IPC and pathological features except lymphovascular invasion of BC [8]. In addition, the correlation between IPC and lymphovascular invasion in the meta-analysis was limited by the inclusion of only two studies with high heterogeneity.
The most important finding of this study was that the OS was significantly better among patients without IPC than that among those with IPC in the multivariable analyses, although BCSS and PFS did not differ. To our knowledge, this is the first investigation to compare the prognosis of BC with and without PC in an Asian population with an increasing incidence of PC. The results were consistent with those of the meta-analysis in which IPC was significantly associated with lower 5-year OS [8]. However, the meta-analysis did not evaluate BCSS and PFS. In contrast, the multi-institutional retrospective study reported that IPC was not significantly associated with CSS and OS [9]. However, the incidence of IPC might be underestimated because about 80% of the subjects in that cohort were evaluated by partial embedding technique.
OS but not BCSS was significantly different between the IPC+and IPC- groups in the multivariable analyses. The discrepancy was possibly caused by the limited number of subjects and events. In the present study, 15 and 29 patients had disease progression, and 18 and 20 patients died in IPC+and IPC- groups, respectively. Among them, five of the 18 patients in the IPC+group died from causes other than BC including lung cancer, colon cancer, unknown primary cancer, and benign lung disease. In contrast, only one patient in the IPC- group died from other causes, namely, gastrointestinal perforation. No patient died from PC. It is unclear why the number of patients who died from other causes was higher in the IPC+group. Patients with double primary cancers might have higher risks of another primary cancer than the risks in patients with single primary cancer. Future investigation is necessary to validate the finding.
This study has several limitations. First, there are limitations due to its retrospective nature. The follow-up protocol and criteria for the administration of neoadjuvant and adjuvant chemotherapy were not standardized. Secondly, the sample size was small and the observation period might not have been sufficient. It might be a reason why factors reported as significant predictors of cancer specific mortality such as pT stage, lymph node metastases and resect margin status failed to show the association with the oncological outcomes in this study. In addition, the findings may not apply to other countries due to well-known racial differences in the incidence and biology of PC, and the dramatic increasing incidence in Japan compared to stable levels in Western countries over the last decade.
In conclusion, we detected IPC in 31.6% of RCP specimens in contemporary Japanese patients with invasive BC. The OS was significantly better among patients without IPC than that among those with IPC. The results suggested that existence of IPC might have influence on the prognosis of BC.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Takahiro Kimura: conception; interpretation or analysis of data; writing the article. Hajime Onuma: interpretation or analysis of data. Shun Sato: performance of work. Hiroyuki Inaba: performance of work. Wataru Fukuokaya: interpretation or analysis of data. Fumihiko Urabe: interpretation or analysis of data. Shoji Kimura: interpretation or analysis of data. Kojiro Tashiro: interpretation or analysis of data. Shunsuke Tsuzuki: interpretation or analysis of data. Jun Miki: interpretation or analysis of data. Akira Furuta: interpretation or analysis of data. Hiroyuki Takahashi: conception; interpretation or analysis of data. Shin Egawa: conception.
CONFLICT OF INTEREST
Takahiro Kimura is a paid consultant/advisor to Sanofi and Janssen. Shin Egawa is a paid consultant/advisor to Takeda, Astellas, AstraZeneca, Sanofi, Janssen, and Pfizer.
Hajime Onuma, Shun Sato, Hiroyuki Inaba, Wataru Fukuokaya, Fumihiko Urabe, Shoji Kimura, Kojiro Tashiro, Shunsuke Tsuzuki, Jun Miki, Akira Furuta and Hiroyuki Takahashi have nothing to disclose.
REFERENCES
[1] | Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) ;68: (6):394–424. |
[2] | Melicow MM , Uson AC . Multiple unrelated primary malignancies of the genitourinary tract. J Urol. (1957) ;77: (1):96–105. |
[3] | Kinoshita Y , Singh A , Rovito PM Jr , Wang CY , Haas GP . Double primary cancers of the prostate and bladder: a literature review. Clin Prostate Cancer. (2004) ;3: (2):83–6. |
[4] | Coyte A , Morrison DS , McLoone P . Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. (2014) ;14: :272. |
[5] | Babaian RJ , Troncoso P , Ayala A . Transurethral-resection zone prostate cancer detected at cystoprostatectomy. A detailed histologic analysis and clinical implications. Cancer. (1991) ;67: (5):1418–22. |
[6] | Lee SH , Chang PL , Chen SM , Sun GH , Chen CL , Shen BY , et al. Synchronous primary carcinomas of the bladder and prostate. Asian J Androl. (2006) ;8: (3):357–9. |
[7] | Kimura T , Egawa S . Epidemiology of prostate cancer in Asian countries. Int J Urol. (2018) ;25: (6):524–31. |
[8] | Fahmy O , Khairul-Asri MG , Schubert T , Renninger M , Stenzl A , Gakis G . Clinicopathological Features and Prognostic Value of Incidental Prostatic Adenocarcinoma in Radical Cystoprostatectomy Specimens: A Systematic Review and Meta-Analysis of 13,140 Patients. J Urol. (2017) ;197: (2):385–90. |
[9] | Malte R , Kluth LA , Kaushik D , Boorjian SA , Abufaraj M , Foerster B , et al. Frequency and prognostic significance of incidental prostate cancer at radical cystectomy: Results from an international retrospective study. Eur J Surg Oncol. (2017) ;43: (11):2193–9. |
[10] | Kurahashi T , Miyake H , Furukawa J , Kumano M , Takenaka A , Fujisawa M . Characterization of prostate cancer incidentally detected in radical cystoprostatectomy specimens from Japanese men with bladder cancer. Int Urol Nephrol. (2010) ;42: (1):73–9. |
[11] | Nakagawa T , Kanai Y , Komiyama M , Fujimoto H , Kakizoe T . Characteristics of prostate cancers found in specimens removed by radical cystoprostatectomy for bladder cancer and their relationship with serum prostate-specific antigen level. Cancer Sci. (2009) ;100: (10):1880–4. |
[12] | Tanaka T , Koie T , Ohyama C , Hashimoto Y , Imai A , Tobisawa Y , et al. Incidental prostate cancer in patients with muscle-invasive bladder cancer who underwent radical cystoprostatectomy. Jpn J Clin Oncol. (2017) ;47: (11):1078–82. |
[13] | Inaba H , Kimura T , Onuma H , Sato S , Kido M , Yamamoto T , et al. Tumor Location and Pathological Features of Latent and Incidental Prostate Cancer in Contemporary Japanese Men. J Urol. 2020:101097JU0000000000000804. |
[14] | Kimura T , Takahashi H , Okayasu M , Kido M , Inaba H , Kuruma H , et al. Time Trends in Histological Features of Latent Prostate Cancer in Japan. J Urol. (2016) ;195: (5):1415–20. |
[15] | van der Kwast TH , Amin MB , Billis A , Epstein JI , Griffiths D , Humphrey PA , et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group T2 substaging and prostate cancer volume. Mod Pathol. (2011) ;24: (1):16–25. |
[16] | Epstein JI , Walsh PC , Carmichael M , Brendler CB . Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. (1994) ;271: (5):368–74. |
[17] | Zlotta AR , Egawa S , Pushkar D , Govorov A , Kimura T , Kido M , et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. (2013) ;105: (14):1050–8. |




