Early Bone Metastases are Associated with Worse Outcomes in Metastatic Urothelial Carcinoma
Abstract
BACKGROUND:
Outcomes of patients with metastatic urothelial carcinoma (mUC) with early bone metastases (eBM) vs no early bone metastases (nBM) have not thoroughly been described in the age of immuno-oncology.
OBJECTIVE:
To compare survival and other clinical outcomes in patients with eBM and nBM.
METHODS:
We used a multi-institutional database of patients with mUC treated with systemic therapy. Demographic, metastatic site, treatment patterns, and clinical outcomes were recorded. Wilcoxon rank-sum, chi-square tests were performed. Survival was estimated by Kaplan-Meier method; multivariable Cox analysis was performed.
RESULTS:
We identified 270 pts, 67% men, mean age 69±11 years. At metastatic diagnosis, 27% had≥1 eBM and were more likely to have de novo vs. recurrent metastases (42% vs 19%, p < 0.001). Patients with eBM had shorter overall survival (OS) vs. those with nBM, (6.1 vs 13.7 months, p < 0.0001). On multivariable analysis, eBM independently associated with higher risk of death, HR = 2.52 (95% CI: 1.75–3.63, p < 0.0001). OS was shorter for patients with eBM who received initial immune checkpoint inhibitor vs platinum-based chemotherapy, (1.6 vs 9.1 months, p = 0.02). Patients with eBM received higher opioid analgesic doses compared to patients with nBM and received quantitatively more palliative radiation.
CONCLUSIONS:
Patients with mUC and eBM have poorer outcomes, may benefit less from anti-PD-1/PD-L1 therapy and represent an unmet need for novel therapeutic interventions. Dedicated clinical trials, biomarker validation to assist in patient selection, as well as consensus on reporting of non-measurable disease are required.
INTRODUCTION
Patients with urothelial carcinoma (UC) have substantial morbidity and the five-year overall survival (OS) of patients with UC who have distant metastases is unfortunately very low at approximately 5% [1]. In metastatic urothelial carcinoma (mUC), tissue specific tropism has been recognized, and common sites of metastatic spread include both lymph node, visceral organs and bone [2, 3]. Bone metastases may occur in almost 50% of cases and may confer significant morbidity [3]. Hypercalcemia, bone pain, pathologic fractures, and spinal cord compression are well known complications of bone metastases, and unfortunately many patients with mUC require hospitalization to manage related complications [4]. Visceral metastases have classically been defined for clinical trial data collection, as well as for clinical care, collectively to include liver, lung or bone metastases, the presence of metastases to any of these sites has been considered a negative prognostic clinical biomarker [5–8].
Disparate phenotypic metastatic progression patterns, such as the bone-only phenotype, may correspond with UC molecular subtypes and possibly suggest distinctive responses to systemic chemotherapy [2, 9–12]. For most patients, we continue to rely largely on clinical characteristics reflected in available trial data to inform treatment decision making. An elderly patient with mUC and de-novo bone metastases may have preexisting hearing or renal dysfunction and/or poor performance status secondary to bone pain and may not be a candidate for standard first-line cisplatin-based chemotherapy. With the approval of immune checkpoint inhibitors (ICI), there are now additional therapy options available for the non-cisplatin candidates, however little is known about how systemic chemotherapy and immunotherapy agents may differentially impact metastatic sites. Patients with early bone metastases (eBM), which include those patients with bone metastases identified prior to initial systemic therapy for metastatic disease, have been classically grouped with patients with visceral disease and an absence of early bone metastases (nBM). The optimal care and therapy sequence in patients with and without eBM who cannot tolerate cisplatin has not been definitively clarified in a prospective trial.
We sought to evaluate the impact of eBM on OS and other clinical outcomes of patients with mUC treated with systemic therapy at three large academic cancer centers. We hypothesized that eBM would be associated with poorer outcomes vs nBM.
MATERIALS AND METHODS
Patient selection
This retrospective cohort study was conducted at three academic cancer centers in the US, after approval of the institutional review board according to principles by the Declaration of Helsinki (University Hospitals Seidman Cancer Center, #02-14-36; Medical College of Wisconsin, #PRO00035649; University of Washington, #0005690). Patients with mUC diagnosed between March 2005 and August 2019 who received at least one dose of systemic therapy in the metastatic setting were retrospectively identified from the electronic medical record (EMR). All pure urothelial and/or histologic variants were included. Records were reviewed; demographics, clinicopathologic factors, treatment patterns, and OS data were recorded. Imaging studies chosen by the treating physician (CT, bone scan, MRI, or PET) completed prior to initiation of systemic therapy were reviewed, sites of baseline metastatic disease were recorded. The presence of bone lesions on imaging suggestive and/or concerning for metastases as determined by the reading radiologist or those felt to be metastatic by the treating physician were considered positive for bone metastases. Early bone metastases (eBM) was defined as the identification of ≥1 bone metastasis prior to initiation systemic treatment in the metastatic setting; patients who developed bone metastases after the initiation of systemic treatment in the metastatic setting were not included in this definition. Patients with mUC and the absence of early bone metastatic sites were categorized as having nBM. Patients were additionally categorized as having baseline bone only, bone and other non-bone visceral (liver and/or lung), non-bone visceral only, and neither bone nor non-bone visceral metastases. Initial systemic treatment was defined as the first systemic therapy administered in the metastatic setting and recorded and categorized as platinum-based chemotherapy (either cisplatin or carboplatin), ICI, or other (i.e. taxane, gemcitabine, FGFR inhibitor). Patients treated with systemic therapy as part of a clinical trial protocol were included. For patients who received additional lines of systemic therapy, the rationale for changes in treatment were recorded as indicated by the treating physician. Clinical progression was defined as a decline in performance status or development of clinical cancer-related symptoms necessitating a change or hold of systemic treatment. If radiographic progression was identified by the treating physician, the site of progression was recorded as bone, lymph node, lung, liver, CNS or other. Sites of palliative radiation were recorded. Outpatient medication records were reviewed, and maximum prescribed doses of opioid analgesic medications were recorded as morphine milligram equivalents (MME) at the defined oncology visits.
Statistical analysis
Demographic and cancer characteristics were summarized using descriptive statistics and compared between the cohorts using Wilcoxon rank-sum test for continuous and ordinal measures and chi-square tests for categorical outcomes. Patients were followed for OS from the time of the initial systemic treatment in the metastatic setting to death or last follow-up. Follow-up was administratively censored at 36 months due to the small number of patients under follow-up beyond that point. OS curves were estimated using Kaplan-Meier methods, and compared between groups via the log-rank test. Cox regression was used for the multivariable analysis of survival. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics
Characteristics of the patient cohorts are listed in Table 1. Overall, 270 patients were identified, 67% men (n = 181), and mean age was 69±11 years at the start of initial systemic therapy in the metastatic setting. Patients received a median of two lines of systemic treatment. Most patients, 75% (n = 203) had localized disease at the time of initial cancer diagnosis, and 26% (n = 70), had received neoadjuvant chemotherapy (NAC). Almost half (n = 139) of patients received platinum-based chemotherapy (54% cisplatin and 46% carboplatin) and a third (n = 92) received an ICI as initial systemic treatment in the metastatic setting. Patients who had received prior NAC were more likely to receive an ICI in the initial metastatic setting (p < 0.001). A quarter of patients (n = 67) had de-novo metastatic disease and these patients were more likely to be treated with platinum-based chemotherapy (p < .001), 61% received cisplatin and 39% received carboplatin.
Table 1
Demographic, Baseline and Treatment Characteristics
| Total N = 270 (%) | Early Bone Metastases (eBM) N = 72 (%) | No Early Bone Metastases (nBM) N = 198 (%) | P-Value | |
| Sex | ||||
| Male | 181 (67) | 52 (72) | 129 (65) | 0.274 |
| Female | 89 (33) | 20 (28) | 69 (35) | |
| Mean Age±SD | 66.8±10.7 | 68.0±11.0 | 69.1±10.7 | 0.432 |
| Smoking History | 193 (72) | 54 (76) | 139 (71) | 0.407 |
| Primary Site | ||||
| Bladder | 211 (78) | 60 (83) | 151 (76) | 0.214 |
| Upper Tract | 59 (22) | 12 (17) | 47 (24) | |
| Histology | ||||
| Urothelial | 214 (80) | 65 (90) | 149 (76) | 0.008 |
| Mixed / Variant | 55 (20) | 7 (10) | 48 (24) | |
| NAC∗ | 69 (34) | 14 (33) | 55 (34) | 0.920 |
| Cisplatin | 63 (90) | 12 (80) | 51 (93) | 0.302 |
| Carboplatin | 6 (9) | 3 (20) | 3 (6) | |
| Adjuvant Chemotherapy | 28 (11) | 3 (4) | 25 (13) | 0.040 |
| Definitive Surgery | 147 (54) | 28 (39) | 119 (60) | 0.002 |
| Median TTM (range)† | 10.6 (1.1 –91.3) | 17.0 (1.1 –76.3) | 10.0 (1.5 –91.3) | 0.459 |
| De-novo metastatic | 67 (25) | 30 (42) | 37 (19) | <0.001 |
| Metastatic Sites‡ | ||||
| Lymph Node | 154 (57) | 35 (49) | 119 (60) | 0.092 |
| Lung | 95 (35) | 23 (32) | 72 (36) | 0.501 |
| Liver | 59 (22) | 19 (26) | 40 (20) | 0.277 |
| Lymph Node Only | 81(30) | 16(22) | 65(33) | 0.093 |
| ECOG PS§ | 0.057 | |||
| 0 | 63 (28) | 13 (21) | 50 (31) | |
| 1 | 102 (46) | 27 (44) | 75 (46) | |
| ≥2 | 58 (26) | 21 (34) | 37 (23) | |
| Initial Treatment | 0.041 | |||
| Platinum-based | 139 (52) | 45 (63) | 94 (48) | |
| Immune Checkpoint Inhibitor | 92 (34) | 16 (22) | 76 (38) | |
| Other | 39 (14) | 11 (15) | 28 (14) | |
| Initial Treatment: Platinum | 0.532 | |||
| Cisplatin | 75 (54) | 26 (58) | 49 (52) | |
| Carboplatin | 64 (46) | 19 (42) | 45 (48) | |
| Initial Treatment: ICI | 0.296 | |||
| Pembrolizumab | 50 (54) | 11 (69) | 39 (51) | |
| Atezolizumab | 35 (38) | 5 (31) | 30 (40) | |
| Nivolumab | 7 (8) | 0 | 7 (9) |
∗NAC=Neoadjuvant Chemotherapy; †TTM = Time from definitive surgery to metastases development in months; ‡Metastatic disease may have been identified in more than one site with exception of lymph node only; §ECOG Performance Status at start of initial treatment, missing 17% of data
Patterns of metastatic progression
At the time of metastatic disease diagnosis, 27% (n = 72) of patients were identified as having eBM. In patients who had non-metastatic disease at initial diagnosis, eBM occurred at similar rates for those who had received NAC vs those who had not (20% vs 21%, p = 0.92). Patients with eBM were more likely to have de-novo metastatic disease, compared to those who progressed from localized to metastatic disease (45% vs 21%, p < 0.001) (Table 1). Disease progression after initial systemic therapy in the metastatic setting occurred within the bones in 31% of patients with eBM vs 11% of patients with nBM (p < 0.001). Patients with eBM were less likely to have progression within lymph nodes (22% vs 41%, p = 0.005) and lung (13% vs 26%, p = 0.020) (Table 2). Patients with eBM were more likely to have a change or stop in initial systemic therapy due to clinical progression (31% vs 16%, p = 0.006).
Table 2
Patterns of Disease Progression
| Reason for change in Initial Treatment* | Total N = 270 (%) | Early Bone Metastases (eBM) N = 72 (%) | No Early Bone Metastases (nBM) N = 198 (%) | P-Value |
| Bone Progression | 43(16) | 22(31) | 21(11) | <0.001 |
| Liver Progression | 56(21) | 14(19) | 42(21) | 0.751 |
| Lung Progression | 60(22) | 9(13) | 51(26) | 0.020 |
| Nodal Progression | 97(36) | 16(22) | 81(41) | 0.005 |
| Clinical Progression | 53(20) | 22(31) | 31(16) | 0.006 |
∗Progressive disease may have been identified in more than one site
Opioid and palliative radiation outcomes
Patients with eBM were prescribed higher opioid analgesic doses compared to patients with nBM at the time of their first vs last outpatient oncology visit: MME of 60 mg±91 mg vs 28 mg±65 mg (p = 0.004) and 171 mg±214 mg vs. 94 mg+229 mg (p < 0.001). There was no difference in the mean increase in MME usage between the two groups (p = 0.151).
Sixty-three percent (n = 45) of patients with eBM received palliative radiation to any site compared to 34% (n = 66) of patients with nBM (p < 0.001); those with bone metastases received palliative radiation to quantitatively more sites (p < 0.001). Fifty-eight percent (n = 42) of patients with eBM received radiation to bone vs 11.6% (n = 23) of patients with nBM (p < 0.001).
Overall survival (OS)
The median OS from the start of initial systemic therapy in the metastatic setting was 11.5 months (95% CI: 9.3–12.9) for the entire cohort, median length of follow up was 18.4 months (0.5 –36). Patients with eBM had significantly shorter OS than those with nBM (median 6.1 vs 13.7 months, p < 0.0001) (Fig. 1B). Comparison of OS between subgroups with different metastatic patterns demonstrated patients with metastatic disease at sites other than bone had longer OS compared to those with only bone metastases or both bone and visceral metastases (p < 0.0001) (Fig. 1C).
Fig. 1
Kaplan- Meier Overall Survival. A. Overall survival for the entire cohort was 11.5 months; B. Overall survival for patients with early bone metastases versus no bone metastases. Median overall survival was 6.1 versus 13.7 months (p < 0.0001), respectively; C. Overall survival subgroup comparisons amongst those with bone-only, mixed, and non-bone shows, C1 Bone without other visceral sites OS was 5.7 months, C2 Bone and other visceral sites OS was 10.3 months, C3 Non bone visceral sites OS was 13.8 months and C4 Neither visceral nor bone OS was 12.5 months; grouping includes one patient with only CNS metastases.
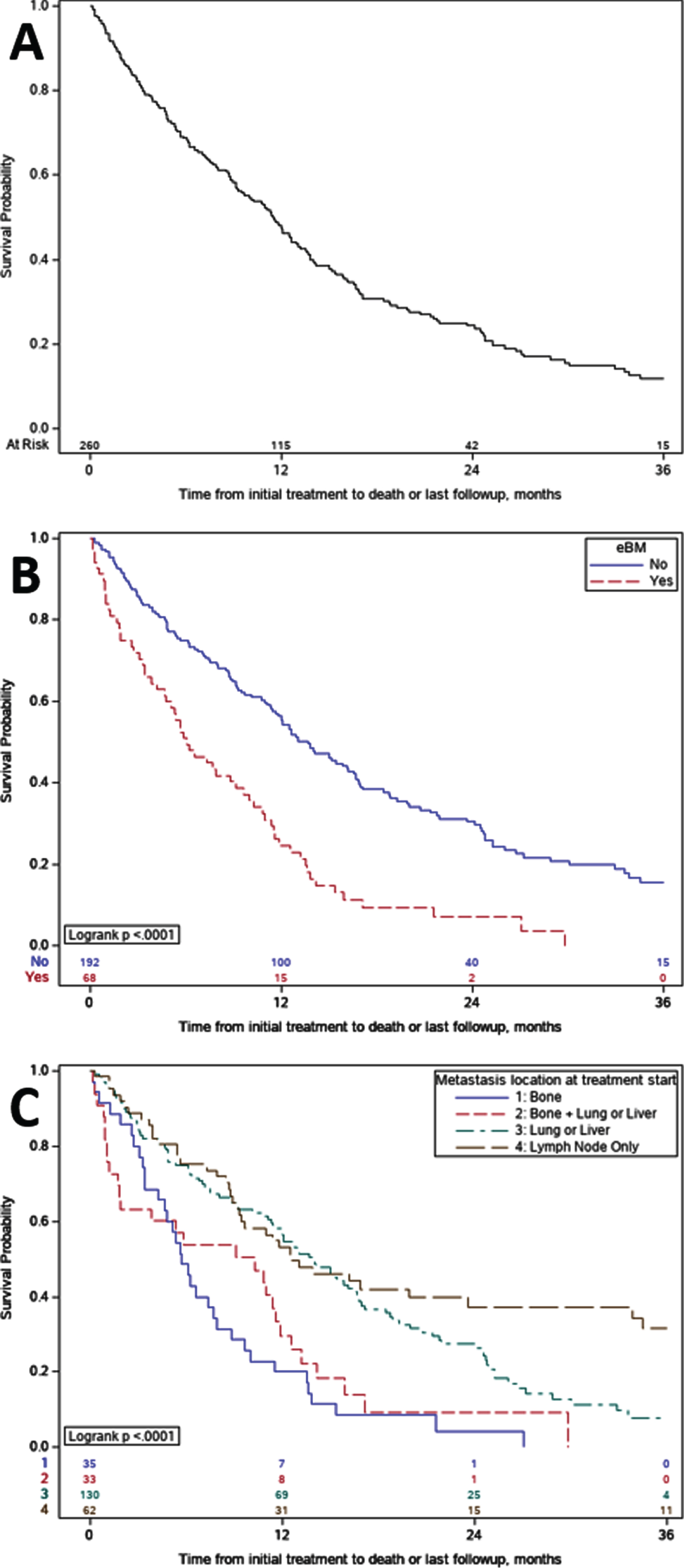
Multivariate analysis controlling for age, sex, smoking history, site of primary tumor, histology, presence of de-novo metastases, presence of liver and lung metastases, lymph node only metastases, ECOG PS, and initial systemic therapy, demonstrated a 2.5 fold increase in the risk of death for patients with eBM at the time of metastatic diagnosis, HR = 2.52 (95% CI: 1.75–3.63, p < 0.0001) (Table 3). The presence of liver metastases was also associated with increased risk of death, but not as much as that of eBM, HR = 1.47 (95% CI: 1.01–2.12, p = 0.04). Interestingly, lung metastases were not significantly associated with increased risk of death, HR = 0.91 (95% CI: 0.64–1.30, p = 0.61). Primary site of upper tract was also associated with longer OS when compared to bladder primary, HR = 0.66 (95% CI: 0.45–0.95, p = 0.03), as was treatment with initial platinum-based chemotherapy in the metastatic setting, HR of 0.60 versus ICI (95% CI: 0.54–0.85, p = 0.004). Neither variant histology, nor the presence of de-novo metastatic disease significantly impacted OS, HR = 0.76 (95% CI: 0.53–1.10, p = 0.15) and HR = 0.94 (95% CI: 0.65–1.36, p = 0.74) respectively.
Table 3
Multivariable Cox Regression Analysis for Overall Survival
| Factor | HR | 95% CI | P-Value |
| eBM (Yes vs No) | 2.52 | (1.75 –3.63) | <0.0001 |
| Liver Metastases (Yes vs No) | 1.47 | (1.01 –2.12) | 0.042 |
| Lung Metastases (Yes vs No) | 0.91 | (0.64 –1.30) | 0.610 |
| Lymph Node Only Metastases (Yes vs No) | 0.84 | (0.56 –1.27) | 0.416 |
| De-Novo Metastases (Yes vs No) | 0.94 | (0.65 –1.36) | 0.736 |
| Age | 1.00 | (0.99 –1.02) | 0.982 |
| Sex (Male vs Female) | 0.91 | (0.66 –1.26) | 0.563 |
| Smoking (Yes vs No) | 1.21 | (0.86 –1.71) | 0.267 |
| Upper vs Bladder Primary | 0.66 | (0.45 –0.95) | 0.027 |
| Histology (Urothelial Ca vs. Variant) | 0.76 | (0.53 –1.10) | 0.147 |
| ECOG PS 1 vs 0 | 1.56 | (1.05 –2.32) | 0.027 |
| ECOG PS≥2 vs 0 | 2.33 | (1.48 –3.69) | <0.001 |
| ECOG PS Missing | 0.89 | (0.53 –1.49) | 0.662 |
| Initial Treatment: Platinum vs CPI | 0.60 | (0.42 –0.85) | 0.004 |
| Initial Treatment: Other vs CPI | 0.85 | (0.54 –1.33) | 0.474 |
eBM, early bone metastases; CPI, checkpoint inhibitor; HR, hazard ratio; CI, confidence interval
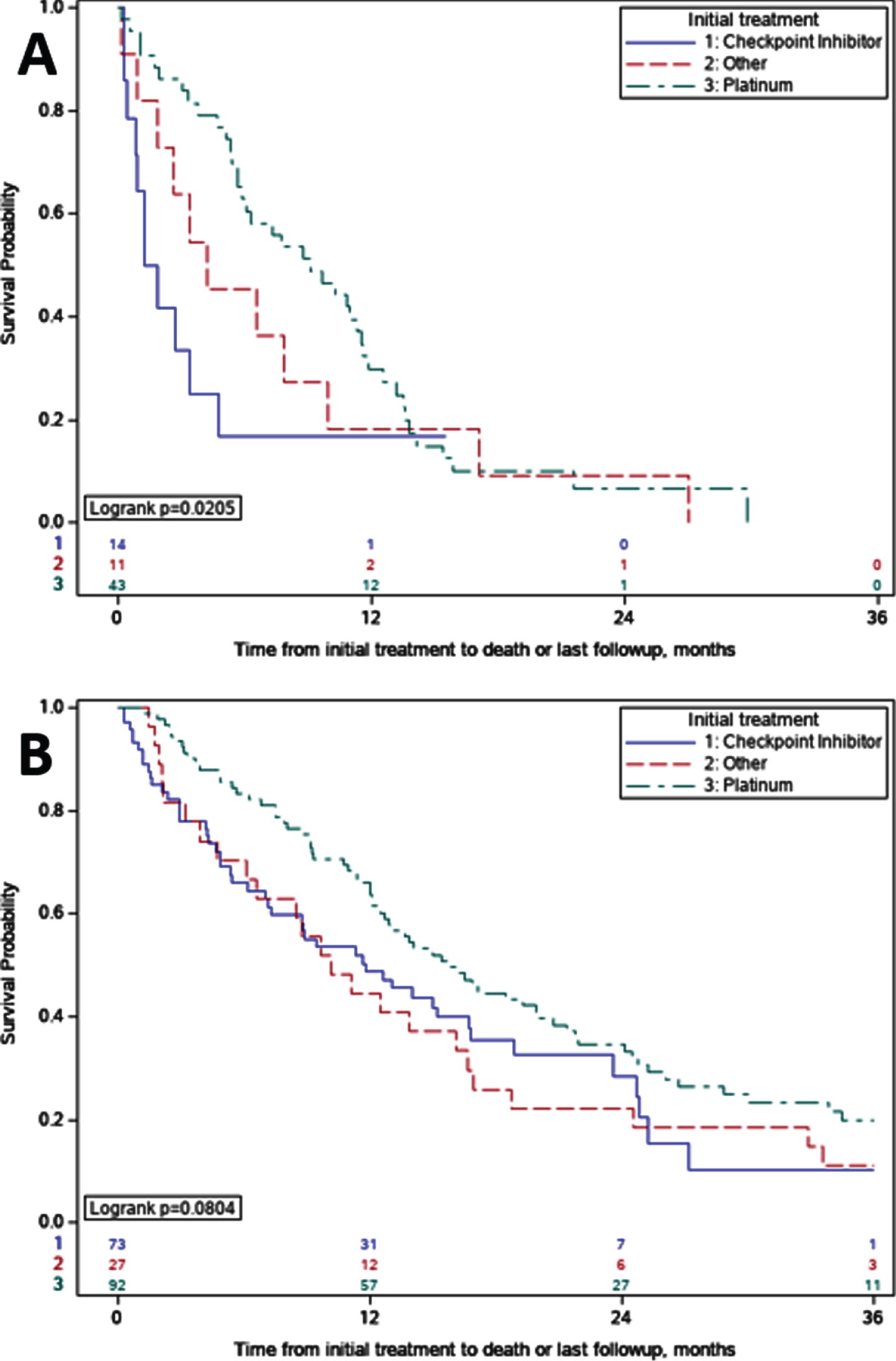
OS comparisons between initial systemic therapy regimens for metastatic disease in patients with eBM demonstrated shorter OS for those who received ICI (n = 14) compared to patients who received platinum-based chemotherapy regimens (n = 43), median OS of 1.6 vs 9.1 months (p = 0.02) (Fig. 2A). Pairwise comparison of OS with stratified log-rank test of ICI versus platinum-based chemotherapy supported this finding (p = 0.02). In patients with nBM who received initial ICI, median OS was also shorter when compared to patients who received platinum-based chemotherapy, but this difference was non-significant on pairwise comparison with stratified log-rank test (11.8 vs 15.8 months; p = 0.11) (Fig. 2B).
Fig. 2
Overall Survival Comparisons by Initial Treatment. A. Early Bone Metastases (eBM); For patients with eBM treated with immune checkpoint inhibitors versus platinum, overall survival was 1.6 vs 9.1 months (p = 0.02). B. No Early Bone Metastases (nBM). For patients with nBM treated with immune checkpoint inhibitors versus platinum, overall survival was 11.8 vs 15.8 months (p = 0.11).
DISCUSSION
Multiple prognostic models have identified the presence of visceral metastases as a negative prognostic biomarker, and historically, bone metastases have been included in the definition of visceral metastases along with liver and lung metastases [3, 5–7]. Our study found that the presence of eBM, independent of other sites of metastases, was associated with a 7.6-month decrease in median OS when compared to patients with nBM and a 2.5-fold increase in the risk of death. Lung metastases were not associated with increased risk, a finding which has previously been reported by other groups [13, 14]. These results suggest that eBM is a biomarker of poor prognosis. Lung, liver, and bone metastatic sites may represent differing underlying biology or tumor microenvironment, and therapeutic responses may differ across sites of metastases; a hypothesis which should be tested in future prognostic models.
Additionally, we demonstrated that those patients with eBM had longer OS when treated with initial platinum-based chemotherapy regimens in the metastatic setting when compared to those who received ICI. Although the number of patients within this subset is small, and there are selection and confounding biases in this retrospective cohort study, this represents an intriguing hypothesis which deserves further investigation. It is also interesting to investigate whether or not ICI therapy is beneficial in patients with tumor progression within the bone microenvironment. In other tumor types, such as non-small cell lung cancer, patients with bone metastases when compared to patients without bone metastases have also demonstrated inferior responses to ICI [15, 16]. The efficacy of ICI in patients with mUC and bone metastases is not well understood, and our data support the need for further research on the bone marrow niche, host immune-tumor microenvironment, and molecular mechanisms.
Recently Khaki et al. demonstrated that patients with poor performance status (ECOG 2-3) may have shorter OS when treated with ICI in the first-line setting vs. those with ECOG PS 0-1; however, differences in ECOG PS at the time of initiation of systemic therapy between patients with eBM and those with nBM approached but did not reach statistical significance in our study [17]. The presence of eBM was associated with a similar increase in risk of death as that of ECOG performance status ≥2, demonstrating the significant prognostic impact of early bone metastases. After adjusting for ECOG performance status and other factors, eBM remained a significant independent negative prognostic factor in multivariate analysis.
This study is the first to report on survival and pain outcomes of patients with eBM who received immune checkpoint inhibitors as an initial systemic therapy in the metastatic setting. Few publications exist in the pre-immune checkpoint era which address outcomes of patients with mUC and early bone metastases. A population-based study utilizing the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) database demonstrated similarly poor median OS of just 4 months in patients with bone metastases at diagnosis [18]. Necchi et al. evaluated outcomes in the subgroup of patients with mUC with exclusive bone metastases compared to those with no bone metastases and both bone and other sites of metastases [2]. Similar to our findings, patients with bone metastasis had worse prognosis irrespective of other sites of disease. For patients with bone-only metastatic sites, median OS was 14.4 months, those with bone and other metastatic sites had median OS of 12 months [2]. In patients who received first-line platinum-based chemotherapy, the presence of bone metastases was associated with lower response rate to chemotherapy [2].
Patients with mUC have multiple sources of morbidity due to prior chemotherapy, past surgeries, as well as metastatic disease burden, all of which may impact a patient’s functional status and, ultimately, treatment options. Bone metastases specifically may lead to bone-related pain, pathologic fractures, hypercalcemia, and epidural spinal cord compression. We demonstrated that patients with eBM were more likely to present with de- novo metastatic disease in bone and more likely to develop progressive or new metastases within bone sites. Patients with eBM required higher doses of opioid analgesics and more palliative radiation (to bone, or to any site) than their nBM counterparts. Overall, these results emphasize that patients with eBM suffer higher disease-related morbidity and emphasize the need for clinical care approaches that adopt early, integrated palliative care.
These inferior pain and symptom control findings amongst those with eBM impact quality of life, performance status, and ability to participate in clinical trials especially whose criteria restrict candidate patients who have escalating pain or analgesia requirements, radiation washout periods, or concurrent radiation. Prospective trials that include palliative care intervention and patient reported outcome endpoints in bladder cancer in the era of immuno-oncology and antibody-drug conjugates are needed. Intervention trials have often excluded patients with mUC who have bone progression due to non-measurable disease status, poor prognosis, escalating pain, or poor performance status. An urgent need amongst the bladder cancer research community is for development of consensus for inclusion criteria that reconciles the current knowledge- and access-gaps for those with non-measurable disease, as well as for reporting outcomes of patients with non-measurable disease.
This study reports on a multi-institutional dataset and large patient cohort outside the filter of clinical trials. Our study analyzed OS from initiation of systemic therapy in the metastatic setting and thus is not confounded by prior therapies in the metastatic setting. The specific effect of subsequent treatments is hard to quantify in this analysis. The recent FDA approval of switch maintenance avelumab after platinum-based chemotherapy, current FDA approved salvage therapies after chemotherapy and ICI (enfortumab-vedotin and erdafitinib), as well as other non-FDA approved therapies may impact survival and/or other clinical outcomes [19–25].
Limitations of our study include its retrospective design in only three academic centers with moderate sample size, potential heterogeneity in data extraction, missing data, selection and confounding biases, as well as lack of randomization, standardized baseline imaging and centralized radiographic review. There was also variability in therapy selection, intervals of assessment and follow up times. There was no assessment of PD-L1 status, other molecular or laboratory biomarkers. We did not identify those patients who were cisplatin eligible vs. ineligible. We did not measure progression-free survival, cancer-specific survival, toxicities and overall response rate to a particular therapy, bone fractures and other skeletal related events. Despite these certain limitations, this study generated several intriguing hypotheses and supports further assessment of this important patient population.
In conclusion, we define eBM as the presence of at least one identified metastatic bone lesion before initiation of systemic therapy in the metastatic setting. The presence of eBM was associated with poor response to treatment, need for escalated palliative measures, and early death compared to patients with nBM. It is likely that this clinical phenotype will be further understood with molecular profiling and subtyping. Tumor tissue correlatives and incorporation of stratification based on osseous involvement in trials can further enhance our understanding of the mechanisms underlying bone and other metastatic site tropism. Consensus for inclusion and reporting of patients’ non-measurable disease, as well as palliative care endpoints, should be developed and incorporated into clinical trial design.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
AN: Conception, performance of data collection, interpretation of data and writing the article; RC: Performance of data collection; EL: Performance of data collection; AS: Analysis of data; AK: Performance of data collection; LD: Performance of data collection; PG: Writing the article; BN: Performance of data collection and writing the article; GM: Performance of data collection and writing the article; TZ: Writing the article; CH: Conception, interpretation of data and writing the article.
CONFLICT OF INTEREST
AA Nelson has no conflicts of interest to report; RJ Cronk has no conflicts of interest to report; EA Lemke has no conflicts of interest to report; A Szabo has no conflicts of interest to report; AR Khaki has previously owned stocks in Merck and Sanofi, sold as of 4/28/20. LN Diamantopoulos has no conflicts of interest to report; P Grivas: (all unrelated in the last 3 years): consulting for AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis Oncology, Driver, EMD Serono, Exelixis, Foundation Medicine, GlaxoSmithKline, Genentech, Genzyme, Heron Therapeutics, Janssen, Merck, Mirati Therapeutics, Pfizer, Roche, Seattle Genetics, QED Therapeutics; participation in educational program for Bristol-Myers Squibb; and institutional research funding from AstraZeneca, Bavarian Nordic, Bayer, Bristol-Myers Squibb, Clovis Oncology, Debiopharm, Genentech, Immunomedics, Kure It Cancer Research, Merck, Mirati Therapeutics, Oncogenex, Pfizer, QED Therapeutics. BG Nezami has no conflicts of interest to report; GT MacLennan has no conflicts of interest to report; T Zhang: research funding (to Duke) from Pfizer, Janssen, Acerta, Abbvie, Novartis, Merrimack, OmniSeq, PGDx, Merck, Mirati, Astellas; consulting/speaking with Genentech Roche, Exelixis, Genomic Health, and Sanofi Aventis; and consulting with AstraZeneca, Bayer, Pfizer, Foundation Medicine, Janssen, Amgen, MJH Associates, and BMS. Stock ownership/employment (spouse) from Capio Biosciences, Archimmune Therapeutics, and Nanorobotics; CJ Hoimes Consult for Merck, Genentech, Seattle Genetics.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2019. CA Cancer J Clin. (2019) ;69: (1):7–34. |
[2] | Necchi A , Pond GR , Pal SK , Agarwal N , Bowles DW , Plimack ER , Yu EY , Ladoire S , Baniel J , Crabb S , Niegisch G , Srinivas S , Berthold DR , Rosenberg JE , Powles T , Bamias A , Harshman LC , Bellmunt J , Galsky MD . Bone Metastases as the Only Metastatic Site in PatientsWith Urothelial Carcinoma: Focus on a Special Patient Population. Clin Genitourin Cancer. (2018) ;16: (2):e483–e90. |
[3] | Shinagare AB , Ramaiya NH , Jagannathan JP , Fennessy FM , Taplin ME , Van den Abbeele AD . Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. (2011) ;196: (1):117–22. |
[4] | Coleman RE . Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clinical Cancer Research. (2006) ;12: (20):6243s–9s. |
[5] | Bajorin DF , Dodd PM , Mazumdar M , Fazzari M , McCaffrey JA , Scher HI , Herr H , Higgins G , Boyle MG . Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. (1999) ;17: (10):3173–81. |
[6] | Apolo AB , Ostrovnaya I , Halabi S , Iasonos A , Philips GK , Rosenberg JE , Riches J , Small EJ , Milowsky MI , Bajorin DF . Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst. (2013) ;105: (7):499–503. |
[7] | Necchi A , Sonpavde G , LoVullo S , Giardiello D , Bamias A , Crabb SJ , Harshman LC , Bellmunt J , DeGiorgi U , Sternberg CN , Cerbone L , Ladoire S , Wong YN , Yu EY , Chowdhury S , Niegisch G , Srinivas S , Vaishampayan UN , Pal SK , Agarwal N , Alva A , Baniel J , Golshayan AR , Morales-Barrera R , Bowles DW , Milowsky MI , Theodore C , Berthold DR , Daugaard G , Sridhar SS , Powles T , Rosenberg JE , Galsky MD , Mariani L . Nomogram-based Prediction of Overall Survival in Patients with Metastatic Urothelial Carcinoma Receiving First-line Platinum-based Chemotherapy: Retrospective International Study of Invasive/Advanced Cancer5 of the Urothelium (RISC). Eur Urol. (2017) ;71: (2):281–9. |
[8] | Galsky MD , Moshier E , Krege S , Lin CC , Hahn N , Ecke T , Sonpavde G , Godbold J , Oh WK , Bamias A . Nomogram for predicting survival in patients with unresectable and/or metastatic urothelial cancer who are treated with cisplatin-based chemotherapy. Cancer. (2013) ;119: (16):3012–9. |
[9] | McConkey DJ , Choi W , Shen Y , Lee IL , Porten S , Matin SF , Kamat AM , Corn P , Millikan RE , Dinney C , Czerniak B , Siefker-Radtke AO . A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-naïve Urothelial Cancer is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisp. European Urology. (2016) ;69: (5):855–62. |
[10] | Choi W , Porten S , Kim S , Willis D , Plimack ER , Hoffman-Censits J , Roth B , Cheng T , Tran M , Lee IL , Melquist J , Bondaruk J , Majewski T , Zhang S , Pretzsch S , Baggerly K , Siefker-Radtke A , Czerniak B , Dinney CP , McConkey DJ . Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer cell. (2014) ;25: (2):152–65. |
[11] | Rosenberg JE , Hoffman-Censits J , Powles T , van der Heijden MS , Balar AV , Necchi A , Dawson N , O’Donnell PH , Balmanoukian A , Loriot Y , Srinivas S , Retz MM , Grivas P , Joseph RW , Galsky MD , Fleming MT , Petrylak DP , Perez-Gracia JL , Burris HA , Castellano D , Canil C , Bellmunt J , Bajorin D , Nickles D , Bourgon R , Frampton GM , Cui N , Mariathasan S , Abidoye O , Fine GD , Dreicer R . Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England). (2016) ;387: (10031):1909–20. |
[12] | Seiler R , Ashab HAD , Erho N , van Rhijn BWG , Winters B , Douglas J , Van Kessel KE , Fransen van de Putte EE , Sommerlad M , Wang NQ , Choeurng V , Gibb EA , Palmer-Aronsten B , Lam LL , Buerki C , Davicioni E , Sjodahl G , Kardos J , Hoadley KA , Lerner SP , McConkey DJ , Choi W , Kim WY , Kiss B , Thalmann GN , Todenhofer T , Crabb SJ , North S , Zwarthoff EC , Boormans JL , Wright J , Dall’Era M , van der Heijden MS , Black PC . Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol. (2017) ;72: (4):544–54. |
[13] | Taguchi S , Nakagawa T , Matsumoto A , Nagase Y , Kawai T , Tanaka Y , Yoshida K , Yamamoto S , Enomoto Y , Nose Y , Sato T , Ishikawa A , Uemura Y , Fujimura T , Fukuhara H , Kume H , Homma Y . Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of survival in patients with metastatic urothelial carcinoma: A multi-institutional study. Int J Urol. (2015) ;22: (7):638–43. |
[14] | Taguchi S , Nakagawa T , Uemura Y , Matsumoto A , Nagase Y , Kawai T , Tanaka Y , Yoshida K , Yamamoto S , Enomoto Y , Nose Y , Sato T , Ishikawa A , Fujimura T , Fukuhara H , Kume H , Homma Y . Validation of major prognostic models for metastatic urothelial carcinoma using a multi-institutional cohort of the real world. World J Urol. (2016) ;34: (2):163–71. |
[15] | Landi L , D’Incà F , Gelibter A , Chiari R , Grossi F , Delmonte A , Passaro A , Signorelli D , Gelsomino F , Galetta D , Giannarelli D , Soto Parra H , Minuti G , Tiseo M , Migliorino MR , Cognetti F , Toschi L , Bidoli P , Piantedosi F , Calabro L , Cappuzzo F . Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. (2019) ;7: (1):316. |
[16] | Schmid S , Diem S , Li Q , Krapf M , Flatz L , Leschka S , Desbiolles L , Klingbiel D , Jochum W , Früh M . Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother. (2018) ;67: (12):1825–32. |
[17] | Khaki AR , Li A , Diamantopoulos LN , Bilen MA , Santos V , Esther J , Morales-Barrera R , Devitt M , Nelson A , Hoimes CJ , Shreck E , Assi H , Gartrell BA , Sankin A , Rodriguez-Vida A , Lythgoe M , Pinato DJ , Drakaki A , Joshi M , Isaacsson Velho P , Hahn N , Liu S , Alonso Buznego L , Duran I , Moses M , Jain J , Murgic J , Baratam P , Barata P , Tripathi A , Zakharia Y , Galsky MD , Sonpavde G , Yu EY , Shankaran V , Lyman GH , Grivas P . Impact of performance status on treatment outcomes: A real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer. (2020) ;126: (6):1208–16. |
[18] | Zhang C , Liu L , Tao F , Guo X , Feng G , Chen F , Xu Y , Li L , Han X , Baklaushev VP , Bryukhovetskiy AS , Wang X , Wang G . Bone Metastases Pattern in Newly Diagnosed Metastatic Bladder Cancer: A Population-Based Study. J Cancer. (2018) ;9: (24):4706–11. |
[19] | Powles T editor Maintenance avelumab+best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis2020; ASCO Virtual Scientific Program: American Society of Clinical Oncology. |
[20] | McCaffrey JA , Hilton S , Mazumdar M , Sadan S , Kelly WK , Scher HI , Bajorin DF . Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. (1997) ;15: (5):1853–7. |
[21] | Sweeney CJ , Roth BJ , Kabbinavar FF , Vaughn DJ , Arning M , Curiel RE , Obasaju CK , Wang Y , Nicol SJ , Kaufman DS . Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. (2006) ;24: (21):3451–7. |
[22] | Ko YJ , Canil CM , Mukherjee SD , Winquist E , Elser C , Eisen A , Reaume MN , Zhang L , Sridhar SS . Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol. (2013) ;14: (8):769–76. |
[23] | Rosenberg JE , O’Donnell PH , Balar AV , McGregor BA , Heath EI , Yu EY , Galsky MD , Hahn NM , Gartner EM , Pinelli JM , Liang SY , Melhem-Bertrandt A , Petrylak DP . Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. (2019) ;37: (29):2592–600. |
[24] | Loriot Y , Necchi A , Park SH , Garcia-Donas J , Huddart R , Burgess E , Fleming M , Rezazadeh A , Mellado B , Varlamov S , Joshi M , Duran I , Tagawa ST , Zakharia Y , Zhong B , Stuyckens K , Santiago-Walker A , De Porre P , O’Hagan A , Avadhani A , Siefker-Radtke AO . Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. (2019) ;381: (4):338–48. |
[25] | Apolo AB , Nadal R , Tomita Y , Davarpanah NN , Cordes LM , Steinberg SM , Cao L , Parnes HL , Costello R , Merino MJ , Folio LR , Lindenberg L , Raffeld M , Lin J , Lee MJ , Lee S , Alarcon SV , Yuno A , Dawson NA , Allette K , Roy A , De Silva D , Lee MM , Sissung TM , Figg WD , Agarwal PK , Wright JJ , Ning YM , Gulley JL , Dahut WL , Bottaro DP , Trepel JB . Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: an open-label, single-centre, phase 2 trial. Lancet Oncol. (2020) ;21: (8):1099–109. |



