Etiology of Treatment Delays in Patients Receiving Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer
Abstract
BACKGROUND:
Neoadjuvant chemotherapy (NAC) prior to radical cystectomy (RC) improves overall survival in muscle-invasive bladder cancer (MIBC), but successful completion rates of NAC are low. A retrospective analysis was undertaken to determine the etiology of deviations of NAC administration for MIBC.
METHODS:
We performed a retrospective review of MIBC patients in an institutional database who received NAC followed by RC from 2008 to 2016. Patients were characterized as having completed NAC without deviation (“No Deviation”) or with deviation (“Deviation”). Factors associated with “Deviation” were assessed with logistic regression models.
RESULTS:
172 MIBC patients received NAC followed by RC; 49 were excluded due to incomplete NAC data. Of the remaining 123 patients, 80 (65%) received Gemcitabine and Cisplatin (GC) and 25 (20%) received dose-dense MVAC (ddMVAC). In all, 85 (69%) patients had “Deviation” in planned NAC administration, while the remaining 38 (31%) patients had “No Deviation.” Twenty-six (33%) of GC patients experienced delays (mean = 21.5±17.0 days) and 6 (24%) ddMVAC patients experienced delays (mean = 10.5±9.5 days). Receipt of GC was associated with higher likelihood of “Deviation” in comparison to ddMVAC (OR = 15.4; 95% CI 4.43–53.72, p < 0.01), and administration of NAC at our institution was associated with lower likelihood of “Deviation” in comparison to receipt in the community (OR = 0.25; 95% CI 0.25–0.72, p = 0.01).
CONCLUSIONS:
Deviations in administration of NAC were common in our cohort (69%) and were associated with receipt of GC and administration of NAC at an outside institution.
INTRODUCTION
Bladder cancer is a common and morbid disease, with an estimated 17,000 attributable deaths in the United States in 2018 [1]. Additionally, the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database indicates that the incidence of bladder cancer has increased by 10% since 1975 [2]. As bladder cancer exists on a continuum of disease, muscle-invasive bladder cancer (MIBC)—representing around 50% of all bladder cancer patients [3] —is the driver of this observed morbidity and mortality. Management of MIBC is complex, as the current standard of care—neoadjuvant chemotherapy (NAC) with radical cystectomy (RC)—requires a multispecialty and multimodality approach.
Appropriate timing in the management of MIBC is complex. When RC was the only standard of care, a delay of greater than 12 weeks after initial diagnosis of MIBC was found to be associated with local tumor progression and inferior progression-free, cancer-specific, and overall survival [4–6]. However, since NAC has become standard [7–10], the impact of time to chemotherapy and surgical extirpation has been challenged [11–13]. While NAC and RC represent the standard of care in MIBC, with improvements in overall survival and an apparent attenuation of the impact of time to RC on overall survival, the administration of guideline-concordant NAC is fraught with unique challenges. Indeed, in Grossman’s landmark study, more than one-third of patients had severe (defined as National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE] grade 4) adverse effects leading to deviations in standard NAC regimens [7]. Similarly, in a multicenter assessment of NAC in MIBC, at least 10% of patients failed to complete the proposed NAC regimen due to toxicities [14]. Dose-limiting toxicities are commonly managed with dose reductions, delays, or discontinuation of chemotherapy [15]. While these modifications play an important role in mitigating short- and long-term toxicity of treatment, they come at a price. Park et al. demonstrated that patients who completed 2 or fewer cycles of NAC had a significantly worse overall survival compared to those receiving 3 or more [16]. Experts have postulated that a loss of dose intensity or deviations from planned NAC regimens may also be associated with inferior overall survival [17].
Currently, there are no published data regarding the etiology of either discontinuation of or delays in NAC in MIBC. Meanwhile, there have been a number of studies in the breast cancer literature identifying factors associated with longer time to treatment (TTT), including but not limited to race (African-Americans with longer TTT), insurance status (uninsured with longer TTT), and facility type (community practices with longer TTT) [18, 19]. While these data raise important questions about practice patterns and health equity and provide a framework for quality improvement and outreach, these identified factors are static factors. A number of questions still exist in multimodal treatment—including MIBC management—about modifiable, symptom- or treatment-related factors more amenable to intervention at the individual patient or provider level. Given the critical need to improve the administration of standard-of-care therapy to patients with MIBC, we conducted an analysis of factors associated with deviations in MIBC patients treated with standard NAC and RC.
METHODS
Human and animal rights
This type of study did not involve patients, as it was a retrospective chart review. For this type of study, formal consent was not required, as this was an institutional review board (IRB)-approved study, #140149.
Informed consent
No individual participants were included in this study. Data from this study was obtained retrospectively from an IRB-approved database without patient identifiers.
Patient population
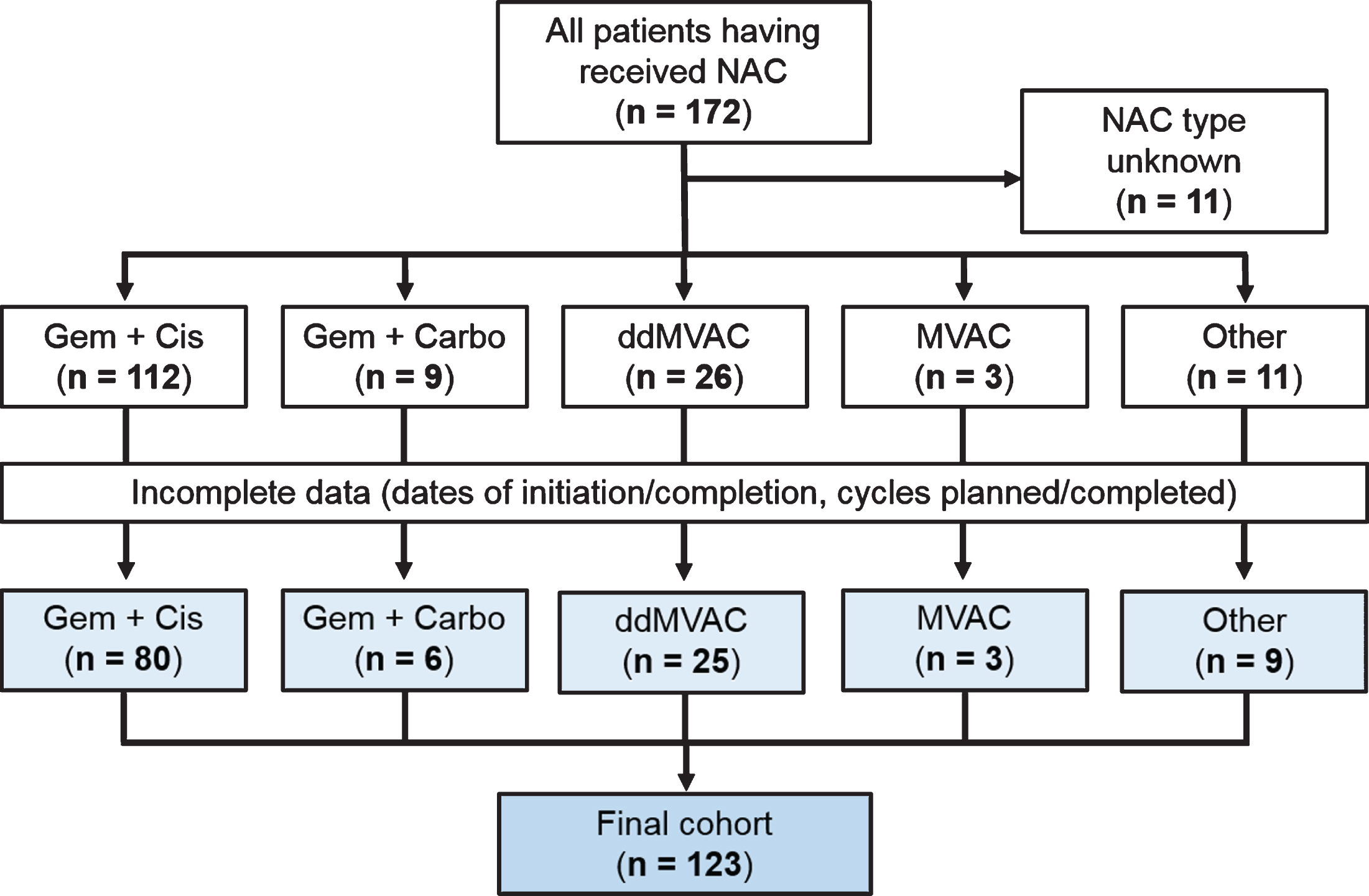
After obtaining the approval of the institutional review board, patients treated with NAC and RC were identified from an established radical cystectomy database. Patients treated with definitive chemoradiation therapy, those without documentation of NAC regimen, and those with metastatic disease at the time of presentation were excluded from the study. Cohort selection is outlined in Fig. 1.
Fig. 1
CONSORT (Consolidated Standards of Reporting Trials) diagram shows study cohort of 123 patients who received NAC and underwent RC for MIBC.
Data collection
Patient characteristics including age, sex, race and insurance status were abstracted. Comorbid conditions prior to initiation of NAC were assessed, and age-adjusted Charlson comorbidity index (ACCI) scores were calculated by the method previously reported by Charlson [20]. Diagnostic and staging data were obtained by chart review, including American Joint Committee on Cancer (AJCC) 2010 TNM staging prior to NAC delivery (clinical stage) and after RC (pathologic stage), histology, and the presence of carcinoma in-situ (CIS).
NAC data encompassed the following: site of chemotherapy delivery (University of Kansas Health System or other facility—clinic or hospital not associated with University of Kansas Health System), NAC regimen (see below), date of initial medical oncology appointment, number of cycles planned, number of cycles completed, date of initiation and completion of NAC, and the time interval between commencement of NAC and RC.
NAC regimens
For analysis, NAC was classified as: 1) gemcitabine and cisplatin (GC) [21], 2) gemcitabine and carboplatin (GCa), 3) dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) [22, 23], 4) conventional methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) [26], or 5) other. The expected duration was based on the length of a standardized regimen for each therapy (e.g. eight weeks for ddMVAC and 12 weeks for GC) as described in randomized control trials and guidelines [7, 26]. Clinical documentation from oncology providers was used to confirm the time period during which the patients were expected to undergo therapy including start date and expected end date.
Characterization of deviations
The interval from the first and last day of NAC administration was used to calculate the duration of NAC. Patients were characterized as either having completed NAC without deviation (“No Deviation”) or with deviation (“Deviation”). Those who were classified as having “Deviation” were further characterized as either having completed NAC with delay (“Delayed”), completed all cycles of NAC but with skipped doses (“Skipped Dose”), or not completed all planned cycles of NAC (“Incomplete”). “Delayed” was defined as a patient having completed all expected cycles of NAC greater than 5 days after the expected time to completion. Data regarding chemotherapy-induced thrombocytopenia demonstrates that patients with thrombocytopenia have delays of therapy until the platelet count recovers, typically at least 5 days [25]. Therefore, five days was chosen as a surrogate for the shortest duration of a delay that would encompass all clinically relevant factors rather than scheduling issues or holidays.
Statistical analysis
Categorical variables were summarized with frequencies and percentages while continuous variables were summarized with medians and interquartile ranges (IQR). Multivariable logistic regression was performed to identify factors independently associated with deviation from standard NAC delivery adjusting for age, type of chemotherapy, Charlson comorbidity index, and location of chemotherapy receipt. Models were summarized using odds ratios (OR) and 95% confidence intervals. Analyses were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) with 2-tailed p-values reported.
RESULTS
Patient demographics and clinicopathologic parameters
A total of 172 patients having received neoadjuvant chemotherapy were identified for analysis, of which 49 patients were excluded due to incomplete data. Of the 525 patients in the cystectomy database, 123 (23%) patients underwent NAC followed by RC with sufficient NAC data for analysis as per the methodology.
Table 1 summarizes the cohort demographics and Table 2 summarizes the cohort clinical and pathological parameters. Median age of the cohort was 64 (IQR: 57–71) years with 82% of patients being male and a majority being Caucasian (92%). Most patients had either Medicare with or without supplement (53%) or commercial insurance (36%). The ACCI score was ≤2 in 40% of patients, corresponding to patients who are younger than 60 years of age with few or no comorbidities, whereas 47% of patients had an ACCI score of 3–5 and 13% with ACCI score of >5.
Table 1
Demographics of the entire cohort (n = 123)
| Number of patients (%) | |
| Age (years) (mean±SD) | 63.7±9.8 |
| Gender | |
| Male | 101 (82) |
| Female | 22 (18) |
| Race | |
| Caucasian | 113 (92) |
| African-American | 7 (6) |
| Hispanic | 1 (1) |
| Other | 2 (1) |
| Insurance status | |
| Medicaid | 2 (2) |
| Medicare | 41 (33) |
| Medicare + supplement | 25 (20) |
| Uninsured | 11 (9) |
| Private insurance plan | 44 (36) |
| Age-Adjusted Charlson Comorbidity Index | |
| ≤2 | 49 (40) |
| 3–5 | 58 (47) |
| > 5 | 16 (13) |
| NAC administration site | |
| KU Hospital | 75 (61) |
| Outside hospital | 48 (39) |
Table 2
Clinicopathologic parameters of the entire cohort (n = 123)
| Number of patients (%) | |
| Clinical nodal stage | |
| Nx | 68 (55) |
| N0 | 37 (30) |
| N+ | 18 (15) |
| Pathologic stage | |
| Tumor classification | |
| T0 | 31 (25) |
| Ta | 1 (1) |
| Tis | 13 (10) |
| T1 | 8 (7) |
| T2 | 26 (22) |
| T3 | 28 (22) |
| T4 | 16 (13) |
| Lymph node status | |
| N0 | 96 (78) |
| N+ | 27 (22) |
| Margin | |
| Negative | 117 (95) |
| Positive | 4 (3) |
| N/A | 2 (2) |
| Histology at cystectomy | |
| Urothelial | 81 (66) |
| Mixed urothelial | 12 (10) |
| Squamous cell carcinoma | 3 (2) |
| Other | 1 (1) |
| NA | 26 (21) |
| Carcinoma in-situ at cystectomy | |
| No | 98 (80) |
| Yes | 25 (20) |
The median time to RC from the initiation of NAC was 98 (IQR 84–112) days (Table 3). The median time to RC from the termination of NAC was 28 (IQR 21–42) days.
Table 3
Duration of neoadjuvant chemotherapy treatments
| Therapy Span | Gem/Cis, Median Days (IQR) | ddMVAC, Median Days (IQR) | Other NAC, Median Days (IQR) |
| n = 80 | n = 25 | n = 18 | |
| Start of chemotherapy to cystectomy | 103 (95–121) | 82 (76–90) | 110 (80–128) |
| Start to end of chemotherapy | 77 (62–90) | 49 (42–60) | 76 (54–90) |
| End of chemotherapy to cystectomy | 30 (20–41) | 32 (20–38) | 32 (28–39) |
NAC regimens
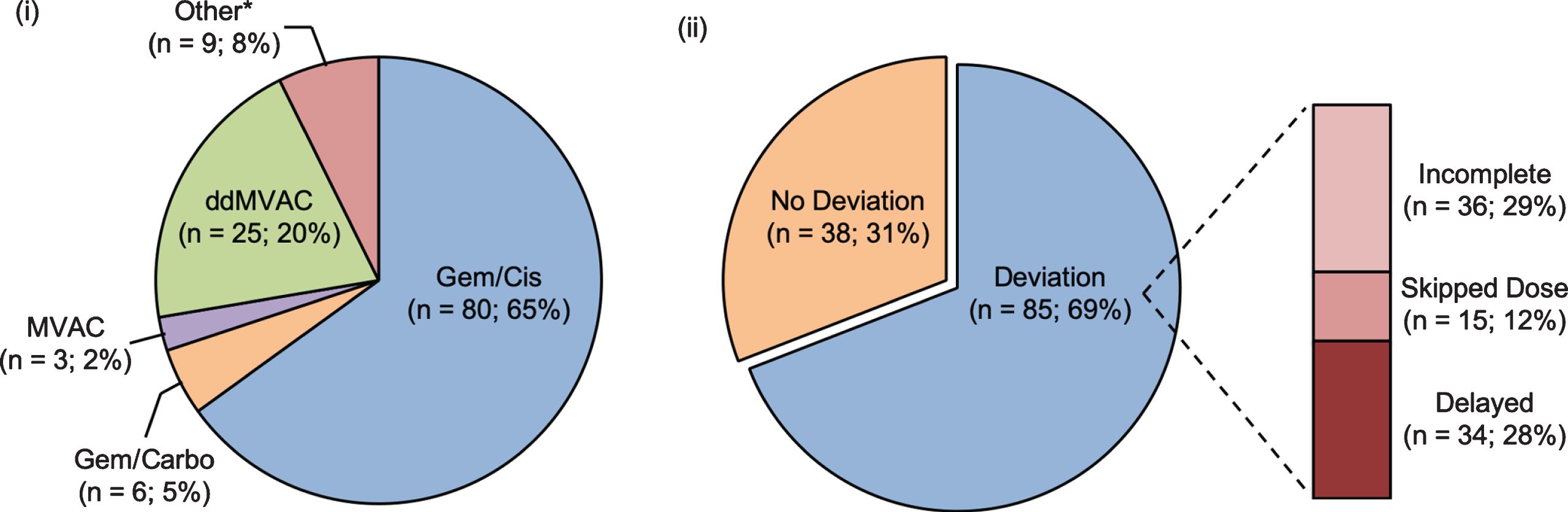
In total, 61% of patients received NAC at the study institution. Regimens are outlined in Fig. 2, with patients most commonly receiving GC (n = 80; 65%), whereas 25 (20%) received ddMVAC, 6 (5%) received GCa, 3 (3%) received conventional MVAC and 9 (7%) received other chemotherapy regimens.
Fig. 2
Breakdown of all patients (n = 123) by (i) neoadjuvant chemotherapy type and (ii) deviation in administration of NAC. * Other NAC type: Gem + Taxol, Gem + Carbo + Taxol.
Pathologic outcomes
A pathologic complete response to NAC (ypT0) was observed in 25% of the cohort, and another 18% demonstrated non-muscle-invasive bladder cancer at RC. Only 4 (3%) patients had positive surgical margins at time of RC, and 22% of patients had lymph node invasion. All tumors analyzed in this study were high grade. Most tumors were pure urothelial carcinoma histology but 13% had variant histology, including squamous and adenomatous differentiation as well as micropapillary and sarcomatoid variants.
Deviations
In all, 38 (31%) patients were considered “No Deviation”, while the remaining 85 (69%) patients had “Deviation” in planned NAC administration. Of those who were classified as “Deviation”, 36 (42%) patients were “Incomplete”, 15 (18%) patients were “Skipped Dose” and 34 (40%) patients were “Delayed”.
We further performed analysis of patients in the “Deviation” group. Determinants for “Incomplete”, “Skipped Dose,” or “Delayed” were abstracted from the patient record (Table 4).
Table 4
Etiologies of deviation of administration of neoadjuvant chemotherapy
| Total (%) | Gem/Cis (%) | ddMVAC (%) | Other (%) | |
| n = 123 | n = 80 | n = 25 | n = 18 | |
| No Deviation | 38 (31) | 13 (16) | 15 (60) | 10 (55) |
| Deviation | ||||
| Delayed | 34 (28) | 26 (33) | 6 (24) | 2 (11) |
| Cytopenia | 10 (8) | 9 (11) | – | 1 (6) |
| Symptom control | 5 (4) | 4 (5) | 1 (4) | – |
| Organ dysfunction | 5 (4) | 2 (2.5) | 3 (12) | – |
| Decline in functional status | – | – | – | – |
| Infection | 5 (4) | 4 (5) | 1 (4) | – |
| Psychosocial issues | 3 (2.5) | 3 (4) | – | – |
| Other | 6 (5) | 4 (5) | 1 (4) | 1 (6) |
| Skipped Dose | 15 (12) | 13 (16) | 1 (4) | 1 (6) |
| Cytopenia | 11 (9) | 11 (14) | – | – |
| Symptom control | – | – | – | – |
| Organ dysfunction | 3 (2.5) | 1 (1) | 1 (4) | 1 (6) |
| Decline in functional status | – | – | – | – |
| Infection | 1 (<1) | 1 (1) | – | – |
| Psychosocial issues | – | – | – | – |
| Other | – | – | – | – |
| Incomplete | 36 (29) | 28 (35) | 3 (12) | 5 (28) |
| Cytopenia | 8 (6.5) | 7 (9) | – | 1 (6) |
| Symptom control | 4 (3) | 3 (4) | 1 (4) | – |
| Organ dysfunction | 5 (4) | 5 (6) | – | – |
| Decline in functional status | 3 (2.5) | 2 (2.5) | 1 (4) | – |
| Infection | 3 (2.5) | 1 (1) | – | 2 (11) |
| Psychosocial issues | 1 (<1) | 1 (1) | – | – |
| Other | 12 (10) | 9 (11) | 1 (4) | 2 (11) |
Incomplete
In all, 36 (29%) patients did not complete all expected cycles of NAC. Of the 28 patients in the GC cohort who did not complete all expected cycles of NAC, the most common reasons for discontinuation of chemotherapy were cytopenias (n = 7) and acute renal failure/azotemia (n = 5). By comparison, 3 patients in the ddMVAC group were unable to complete NAC. Reasons for discontinuation included fatigue, decline in functional status, and gastrointestinal bleed secondary to thrombocytopenia.
Skipped dose
Fifteen (12%) patients in the total cohort skipped one or more doses during NAC administration, with the most common etiology being cytopenias (n = 12).
Delayed
Of the 80 patients who received GC, 26 (33%) experienced a delay of more than 5 days, with the most common reason for delay being cytopenias (n = 9). In the ddMVAC group, 6 (24%) patients had a delay in administration of NAC, most commonly for acute renal failure/azotemia (n = 3).
For those patients who experienced “Delay” in NAC administration, the median delay experienced was 20 (IQR 13–28) days. The delay in the ddMVAC group was 6.5 (IQR 6–17.5) days compared to a median of 22 (IQR 14–29.5) days among GC patients (p = 0.045).
On multivariable analysis, receipt of GC was associated with higher likelihood of “Deviation” in comparison to ddMVAC (OR = 15.4; 95% CI 4.43–53.72, p < 0.01). On the other hand, administration of NAC at our institution was strongly associated with not having a “Deviation” in comparison to receiving NAC at an institution outside of KU (OR = 0.25; 95% CI 0.08–0.72, p = 0.01). Other factors such as age and ACCI did not demonstrate any significance (Table 6). Despite its lack of significance, ACCI does show close association with the aforementioned “Deviation” to NAC administration (OR = 1.39, 95% CI 0.97–2.00, p = 0.07) (Table 6).
Table 5
Univariate associations for deviation in neoadjuvant chemotherapy administration
| Variable | OR | CI | P-value |
| Age | 1.01 | 0.97–1.05 | 0.50 |
| NAC Type (ref: ddMVAC) | |||
| Gem/Cis | 7.73 | 2.85–20.94 | <0.01 |
| Other | 1.20 | 0.35–4.09 | 0.77 |
| Gender (ref: Male) | 0.94 | 0.35–2.56 | 0.92 |
| Insurance (ref: Medicare) | |||
| Medicare + Supp | 1.23 | 0.43–3.52 | 0.09 |
| Medicaid | Unevaluable | Unevaluable | – |
| Private | 1.38 | 0.56–3.41 | 0.49 |
| Uninsured | 2.60 | 0.49–1.36 | 0.26 |
| NAC at KU | 0.71 | 0.33–1.54 | 0.39 |
| CCI | 1.15 | 0.93–1.41 | 0.19 |
Table 6
Multivariate analysis of factors associated with deviation in administration of neoadjuvant chemotherapy
| Variable | OR | CI | P-value |
| Age | 0.95 | 0.89–1.01 | 0.16 |
| NAC Type (ref: ddMVAC) | |||
| Gem/Cis | 15.4 | 4.43–53.72 | <0.01 |
| Other | 1.49 | 0.37–6.02 | 0.57 |
| NAC at KU | 0.25 | 0.08–0.72 | 0.01 |
| CCI | 1.39 | 0.97–2.00 | 0.07 |
DISCUSSION
Our data demonstrate a significant rate of deviation (69%) in the expected administration of NAC in all comers, with the most common cause of deviation being cytopenias. Additionally, the use of GC carried with it a unique set of risk factors for deviations in NAC administration (OR 15.4) secondary to toxicity and disease progression. Conversely, administration of NAC at our institution was associated with lower likelihood of “Deviation” in comparison to receipt of NAC in the community (OR 0.25).
Level 1 evidence has demonstrated a substantial survival benefit conferred by MVAC administered before radical cystectomy and pelvic lymph node dissection [7, 10, 26–28]. Although such evidence does not exist for GC or ddMVAC in the NAC setting, there has been a shift in practice away from MVAC due to the desire to minimize toxicity while maintaining similar oncologic efficacy [29]. GC has become the most commonly used regimen [30], presumably based on extrapolation of data from patients with metastatic bladder cancer and owing to a better toxicity prolife [33]. Similarly, ddMVAC has also become increasingly utilized in conjunction with associations with improved OS and limited toxicities [32]. These shifts in practice patterns have been confirmed in our series, in which 65% of patients and 20% of patients received GC and ddMVAC, respectively, whereas only 2% received conventional MVAC.
There has been a plethora of studies exploring the pathological response and survival outcomes of these various NAC regimens, but one knowledge gap persists –the need for comprehensive data regarding the non-completion of NAC and deviation from the expected and planned administration of NAC. The limited data currently available on the non-completion rate of NAC is mixed. In a real-world analysis of pathologic response rates to NAC, Zargar et al. found 13% and 10% non-completion rates in patients who received MVAC and GC, respectively [14]. A later study by Park et al. revealed that nearly 57% of patients given MVAC or GC received 2 or fewer cycles of chemotherapy [12]. As previously mentioned, these patients had poor overall survival in comparison to the cohort who completed at least 3 cycles of NAC. While neither of these studies delineated the reasons for treatment delays or discontinuation, they do underscore the clinical relevance of these data, which would allow clinicians to address contributing factors and improve delivery of care in this population.
Despite originally being chosen over traditional MVAC for its better safety and lower toxicity profiles [29, 33], GC was associated with more deviations due to toxicity and progression of disease (Table 3). Furthermore, the numbers of delayed, skipped and incomplete courses were also increased in patients receiving GC compared to those having received ddMVAC. There are a number of possible population-related explanations for these contradictory findings. One such explanation is patient age. Patients receiving GC were on average 6 years older than those receiving ddMVAC (64 versus 58 years, respectively). It is possible that the increased age in the GC population led to increased medical frailty and treatment-related toxicity. Medical complexity is another possible factor, as patients receiving GC had a median CCI of 3.2 whereas those receiving ddMVAC had a median CCI of 2.2. Therefore, even though the CCI was not predictive of delays during NAC, this could have indirectly contributed to the differences in toxicity. Indeed, pretreatment patient characteristics, as opposed to the GC regimen itself, may have been responsible for the increased number of deviations within this group. Although it is true that delays may be due to predetermined patient characteristics, our data reveal that the deviations themselves exist, which is the first step to resolving them.
Another possible explanation for the increased toxicity of GC could relate to the administration of the regimens themselves, specifically to the dose-dense scheduling of ddMVAC. A recent study by Bamias et al. showed that not only was dose-dense Gemcitabine/Cisplatin (ddGC) associated with improved OS, but it was also associated with a significantly higher rate of course completion compared to ddMVAC (85% versus 63%, respectively, p = 0.01) [34]. Another phase II trial found similar results, with patients in the ddGC arm receiving double the dose of cisplatin compared to the standard MVAC [35]. Furthermore, ddGC was associated with limited toxicity and improved pathological response rates in comparison to other dose-dense regimens. The difference between GC and ddMVAC could be mainly contributable to the lack of dose densification, but Phase III data are needed to fully elucidate the impact of dose-dense scheduling of NAC in MIBC.
Despite the mounting data about the impact of choice and dose-density of NAC regimen on MIBC outcomes, to the best of our knowledge there is no literature exploring the prevalence of deviations in administration of NAC and the association of these delays with MIBC outcomes. Identifying risk factors for treatment delays or discontinuation of NAC is therefore of paramount importance to urologists and medical oncologists, as this could allow for implementation of interventions to minimize delays and possibly improve survival. Basch et al. has found that prospective monitoring of patient-reported outcome (PRO) measures while receiving chemotherapy led to improvements in quality of life, less frequent emergency department visits, less frequent hospital admissions, and a longer duration of chemotherapy. Correspondingly, the findings of this study could be used to create PROs tailored for specific NAC regimens with the intent of targeting the symptom control issues responsible for delays and/or dose modifications of each regimen. For instance, our data showed that patients receiving ddMVAC were commonly “Delayed” secondary to azotemia, and therefore future PRO interventions could target modifiable causes of azotemia. For example, questionnaires could be implemented to target the early identification and subsequent intervention of symptom control issues that could worsen azotemia (e.g. nausea, vomiting, and dehydration).
This study has several limitations, such as the inherent selection bias of retrospective review, lack of randomization, and lack of standardization of NAC administration between institutions. Selection bias in the choice of chemotherapy regimen is plausible, as not all factors could be controlled for in the multivariate analysis. There is additional bias introduced in the variability between outside and institutional documentation from which our data were obtained. Furthermore, since data were collected retrospectively, we could not assess the outcome in patients who received NAC but were not treated with RC due to disease progression, toxicity or performance status deterioration.
Additionally, comparison of patients who received dose reductions was not included in this analysis as we were unable to corroborate from outside records the degree of dose reduction. Although it will be critical to better characterize the effect of NAC deviation on outcomes, this study was not designed to address this question. Due to the heterogenous nature of this patient population, loss of follow-up, and limited sample size, a robust analysis of oncologic outcomes could not be performed. Ideally, patients with similar baseline characteristics and clinical stage may be compared to meaningfully evaluate for any effect of NAC on oncologic outcomes.
MIBC is an aggressive systemic disease that requires a multidisciplinary team to coordinate systemic and local treatment. Significant improvements in MIBC outcomes were achieved by the advent of cisplatin-based combination chemotherapy regimens. Similar strides have been made to increase the tolerability of these systemic regimens, but unfortunately the utilization of NAC for the treatment of MIBC by the oncology and urology communities remains low [37]. Our data demonstrate that, even when guideline-concordant NAC is attempted, the real-world experience is marked by deviations from expected NAC administration. Identification of risk factors and tendencies as discussed above may allow for circumvention of such toxicities.
ACKNOWLEDGMENTS
The authors have no acknowledgments
FUNDING
The authors report no funding
AUTHOR CONTRIBUTIONS
AM El-Arabi: Protocol Development, Data Collection, Data Analysis, Manuscript Writing and Editing; SM Alam: Data Analysis, Manuscript Writing and Editing; G Sherman: Data Collection, Data Analysis, Man-uscript Writing; J Thompson: Data Analysis, Manuscript Editing; WP Parker: Data Analysis, Manuscript Editing; JM Holzbeierlein: Protocol Development, Manuscript Editing; EK Lee: Protocol Development, Manuscript Writing and Editing; EM Wulff-Burchfield: Protocol Development, Manuscript Writing and Editing.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, CA. Cancer J Clin. (2018) ;68: :7–30. |
[2] | Cancer Statistics Review, 1975-2014 - SEER Statistics. Available at: https://seer.cancer.gov/csr/1975 2014/s. (Accessed: 18th January 2018) |
[3] | Amin MB , et al. ICUD-EAU International Consultation on Bladder Cancer Pathology. Eur Urol. (2013) ;63: :16–35. |
[4] | Chang SS , Hassan JM , Cookson MS , Wells N , Smith JA . Delaying Radical Cystectomy for Muscle Invasive Bladder Cancer Results in Worse Pathological Stage. J Urol. (2003) ;170: :1085–7. |
[5] | May M , Nitzke T , Helke C , Vogler H , Hoschke B . Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. (2004) ;38: :231–5. |
[6] | Lee CT , et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol. (2006) ;175: :1262–7; discussion 1267. |
[7] | Grossman HB , et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. (2003) ;349: :859–66. |
[8] | Vale CL , Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. (2005) ;48: :202–6. |
[9] | Griffiths G . Neoadjuvant cisplatin,methotrexate,and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. The Lancet. (1999) ;354: :533–40. |
[10] | International Collaboration of Trialists et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol Off J Am Soc Clin Oncol. (2011) ;29: :2171–7. |
[11] | Alva AS , et al. Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer. Cancer. (2012) ;118: :44–53. |
[12] | Park JC , et al. A Retrospective Analysis of the Effect on Survival of Time from Diagnosis to Neoadjuvant Chemotherapy to Cystectomy for Muscle Invasive Bladder Cancer. J Urol. (2015) ;195: :880–5. |
[13] | Gin G , et al. PD06-11 Factors affecting delays in neoadjuvant chemotherapy and radical cystectomy: An analysis of the national cancer database cohort. J Urol. (2017) ;197: :e126. |
[14] | Zargar H , et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. (2015) ;67: :241–9. |
[15] | Ozer H . et al. Update of Recommendations for the Use of Hematopoietic Colony-Stimulating Factors: Evidence-Based, Clinical Practice Guidelines. J Clin Oncol. (2000) ;18: :3558–85. |
[16] | Park JC , et al. A Retrospective Analysis of the Effect on Survival of Time from Diagnosis to Neoadjuvant Chemotherapy to Cystectomy for Muscle Invasive Bladder Cancer. J Urol. (2015) ;195: :880–5. |
[17] | Gallagher DJ , Bajorin DF . Neoadjuvant chemotherapy for the treatment of muscle-invasive bladder cancer: argument in favor. Nat Clin Pract Urol. (2008) ;5: :484–5. |
[18] | Sanford RA , et al. Impact of delayed neoadjuvant systemic chemotherapy on survival outcomes in breast cancer patients. J Clin Oncol. (2016) ;34: :1038. |
[19] | Khanna S , et al. Impact of patient demographics, tumor characteristics, and treatment type on treatment delay throughout breast cancer care at a diverse academic medical center. Int J Womens Health. (2017) ;9: :887–96. |
[20] | Charlson M , Szatrowski TP , Peterson J , Gold J . Validation of a combined comorbidity index. J Clin Epidemiol. (1994) ;47: :1245–51. |
[21] | Dash A , et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder. Cancer. (2008) ;113: :2471–7. |
[22] | Plimack ER , et al. Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin Is Safe, Effective, and Efficient Neoadjuvant Treatment for Muscle-Invasive Bladder Cancer: Results of a Multicenter Phase II Study With Molecular Correlates of Response and Toxicity. J Clin Oncol. (2014) ;32: :1895–901. |
[23] | Choueiri TK , et al. Neoadjuvant Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin With Pegfilgrastim Support in Muscle-Invasive Urothelial Cancer: Pathologic, Radiologic, and Biomarker Correlates. J Clin Oncol. (2014) ;32: :1889–94. |
[24] | Sternberg CN , et al. Randomized Phase III Trial of High-Dose-Intensity Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC) Chemotherapy and Recombinant Human Granulocyte Colony-Stimulating Factor Versus Classic MVAC in Advanced Urothelial Tract Tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol. (2001) ;19: :2638–46. |
[25] | Dose Reductions and Delays: Limitations of Myelosuppressive Chemotherapy | Cancer Network. Available at: http://www.cancernetwork.com/ovarian-cancer/dose-reductions-and-delays-limitations-myelosuppressive-chemotherapy. (Accessed: 31st May 2018) |
[26] | Stenzl A , et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. (2011) ;59: :1009–18. |
[27] | Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet Lond Engl. (2003) ;361: :1927–34. |
[28] | Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. (2005) ;48: :202–5; discussion 205-206. |
[29] | von der Maase H , et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol Off J Am Soc Clin Oncol. (2000) ;18: :3068–77. |
[30] | Porter MP , Kerrigan MC , Donato BMK , Ramsey SD . Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol. (2011) ;29: :252–8. |
[31] | von der Maase H , et al. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J Clin Oncol. (2005) ;23: :4602–8. |
[32] | Zargar H , et al. Neoadjuvant Dose Dense MVAC versus Gemcitabine and Cisplatin in Patients with cT3-4aN0M0 Bladder Cancer Treated with Radical Cystectomy. J Urol. (2018). doi:10.1016/j.juro.2017.12.062 |
[33] | von der Maase H , et al. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J Clin Oncol. (2005) ;23: :4602–8. |
[34] | Bamias A , et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol Off J Eur Soc Med Oncol. (2013) ;24: :1011–7. |
[35] | Iyer G , et al. Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer. J Clin Oncol. JCO.2017.75.0158 (2018). doi:10.1200/JCO.2017.75.0158 |
[36] | Basch E , et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol. (2016) ;34: :557–65. |
[37] | Reardon ZD , et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol. (2015) ;67: :165–70. |




