Detection of Muscle-Invasive Bladder Cancer on Biparametric MRI Using Vesical Imaging-Reporting and Data System and Apparent Diffusion Coefficient Values (VI-RADS/ADC)
Abstract
BACKGROUND:
Vesical Imaging-Reporting And Data System (VI-RADS) was proposed to detect muscle-invasive bladder cancer (MIBC).
OBJECTIVE:
To evaluate the performance of VI-RADS and additional value of apparent diffusion coefficient (ADC) values measured on diffusion-weighted magnetic resonance imaging (MRI) for detecting MIBC.
METHODS:
A total of 176 patients undergoing MRI (multiparametric in 97 [55%] and biparametric in 79 [45%]) before transurethral resection of bladder tumor for primary bladder cancer were retrospectively identified. MRI findings were scored according to VI-RADS. The standardized tumor ADC (sT-ADC: tumor ADC/gluteus maximus ADC) was calculated and used to account for the incompatibility among different MRI protocols. The accuracy of VI-RADS, sT-ADC and their combination to detect MIBC was assessed using the AUC of the ROC curve.
RESULTS:
MIBC was pathologically confirmed in 46 patients (26%). AUC of VI-RADS to detect MIBC was 0.86. When cut-off of VI-RADS was set at≥3 and≥4, sensitivity/specificity were 78% /70% and 63% /96%, respectively. A lower sT-ADC (≤0.894) was significantly associated with muscle invasion (p < 0.01, AUC 0.79) with sensitivity 78% and specificity 79%. Combination of VI-RADS and sT-ADC improved the accuracy (AUC 0.94); sensitivity was 100% when VI-RADS≥3 or sT-ADC≤0.894 was considered positive, and specificity was 99% when VI-RADS≥4 and sT-ADC≤0.894 was considered positive. Incorporation of sT-ADC reduced under-staging of MIBC as VI-RADS < 3 by 100% and over-staging of non-MIBC as VI-RADS≥4 by 80%.
CONCLUSIONS:
Incorporation of ADC values into VI-RADS improves accuracy to detect MIBC in primary bladder cancer patients.
INTRODUCTION
Clinical management of bladder cancer (BC) is determined primarily on the basis of distinguishing non-muscle-invasive BC (NMIBC) from MIBC. NMIBC can be treated conservatively with transurethral resection of bladder tumor (TURB) and intravesical instillation. On the other hand, patients with MIBC require more intensive therapies, including radical cystectomy [1]. As radical cystectomy could compromise patients’ quality of life [2], correct staging is mandatory in the management of BC.
Magnetic resonance imaging (MRI) has been reported to be useful for the correct diagnosis of MIBC [3, 4]. However, there are no standardized criteria of MRI findings for the diagnosis of MIBC. Recently, the Vesical Imaging-Reporting And Data System (VI-RADS) was proposed to define a standardized approach to imaging and reading MRI for BC, and to evaluate the possibility of MIBC [5]. Recently, several validation studies of VI-RADS demonstrated its accuracy in differentiating MIBC from NMIBC and good to excellent interobserver agreement [6–9].
In addition to anatomical and morphological information obtained by T1- and T2-weighted imaging, MRI provides functional and qualitative information of tissues using diffusion-weighted imaging (DWI). The image contrast of DWI is due to differences in the diffusion of water molecules in tissues and the degree of diffusion is quantified as an apparent diffusion coefficient (ADC) value. Tissues with restricted water diffusion exhibit high signal intensity on DWI and low ADC values [10]. Therefore, malignant lesions characterized by dense cellularity, tissue disorganization, and decreased extracellular space, all of which restrict water diffusion, often demonstrate high signal on DWI and low ADC values. Several studies found that MIBC exhibit a more intense signal and lower ADC values than NMIBC [10, 11]. As the ADC value is a functional biomarker independent of morphological features evaluated by VI-RADS, ADC values may play a complemental role to VI-RADS in detecting MIBC.
In this study, we validated the accuracy of VI-RADS to detect MIBC in a single-institutional cohort of 176 treatment-naïve patients with primary BC and assessed whether the incorporation of ADC improved the predictive accuracy.
MATERIALS AND METHODS
Study population
An institutional review board approved this retrospective study (IRB protocol nr. 2233). Between January 2013 and September 2018, 399 treatment-naïve patients underwent TURB for the primary (not recurrent) bladder tumor. Of them, 223 patients were excluded for the following reasons: no MRI before TURB (159 patients, most of whom had small and pedunculated papillary tumors presumed as pTa disease), invisibility on MRI due to small tumors, including carcinoma in situ (59 patients), and pathological diagnosis other than urothelial carcinoma (5 patients). The remaining 176 patients, who had undergone MRI for primary BC prior to TURB, were subjects for analysis. All BC were histologically confirmed urothelial carcinoma. An index tumor, defined as a tumor with the highest clinical T stage or the largest tumor among tumors with the same T stage, was evaluated as a representative in patients with multiple tumors. Tumor configuration was based on cystoscopy findings. Tumor location was determined according to a schematic map of the bladder modified by the Japanese Urological Association schema [12]. Histological grade was determined according to the 1973 World Health Organization classification [13]. Pathological T stage was determined using TURB specimens according to the 2017 TNM system [14]. Re-TURB was performed for patients with T1 or greater disease without sampling the muscular layer at the first TURB.
Image analysis
MRI was performed at either our institution, our affiliated clinic or others. At our institution and affiliated clinic, the images were acquired under free breathing conditions using a 3.0-Tesla scanner (Magnetom Skyra; Siemens, Berlin, Germany) with an 18-channel sensitivity-encoding body coil. The imaging parameters at our institution and affiliated clinic were set as described in Supplemental Table 1. No intramuscular antispasmodic agent or catheter was used as preparation. Detailed information on MRI protocols at the other institutions was not available.
Two readers, a radiologist with 9-year experience (reader 1, SI) and a urologist with 15-year experience (reader 2, MI) who were blinded to clinicopathological data independently interpreted the imaging data and recorded MRI findings according to VI-RADS [5]. Briefly, VI-RADS scores were defined to suggest the probability of muscle invasion as follows: 1, highly unlikely; 2, unlikely; 3, equivocal; 4, likely; 5, very likely (Fig. 1). When VI-RADS scores were different between the two readers, consensus on the final VI-RADS score was obtained. ADC values were measured by another researcher (KS) who was blinded to clinicopathological data, as described previously [15–17]. Briefly, the ADC values of the index tumor and the gluteus maximus were measured on an ADC map. A region of interest (ROI) was manually set in each index tumor to maximally cover the index tumor on a transverse ADC map at the MRI section of the largest tumor diameter. Another ROI with an area of 5 cm2 was set within the gluteus maximus. The ADC values of all pixels within each ROI were quantified and their mean value was obtained using View R software, version 1.20 (Yokogawa Medical Solutions Corporation, Tokyo, Japan). A standardized tumor ADC (sT-ADC) was calculated by dividing the mean tumor ADC value by the mean gluteus maximus ADC value to account for the incompatibility between different MRI protocols [18]. To assess intraobserver reproducibility of the sT-ADC values, ADC values were measured intertemporally by KS in 50 randomly selected patients who had taken MRI at our institution. To assess interobserver reproducibility, another researcher (MI) completed the same series of measurement.
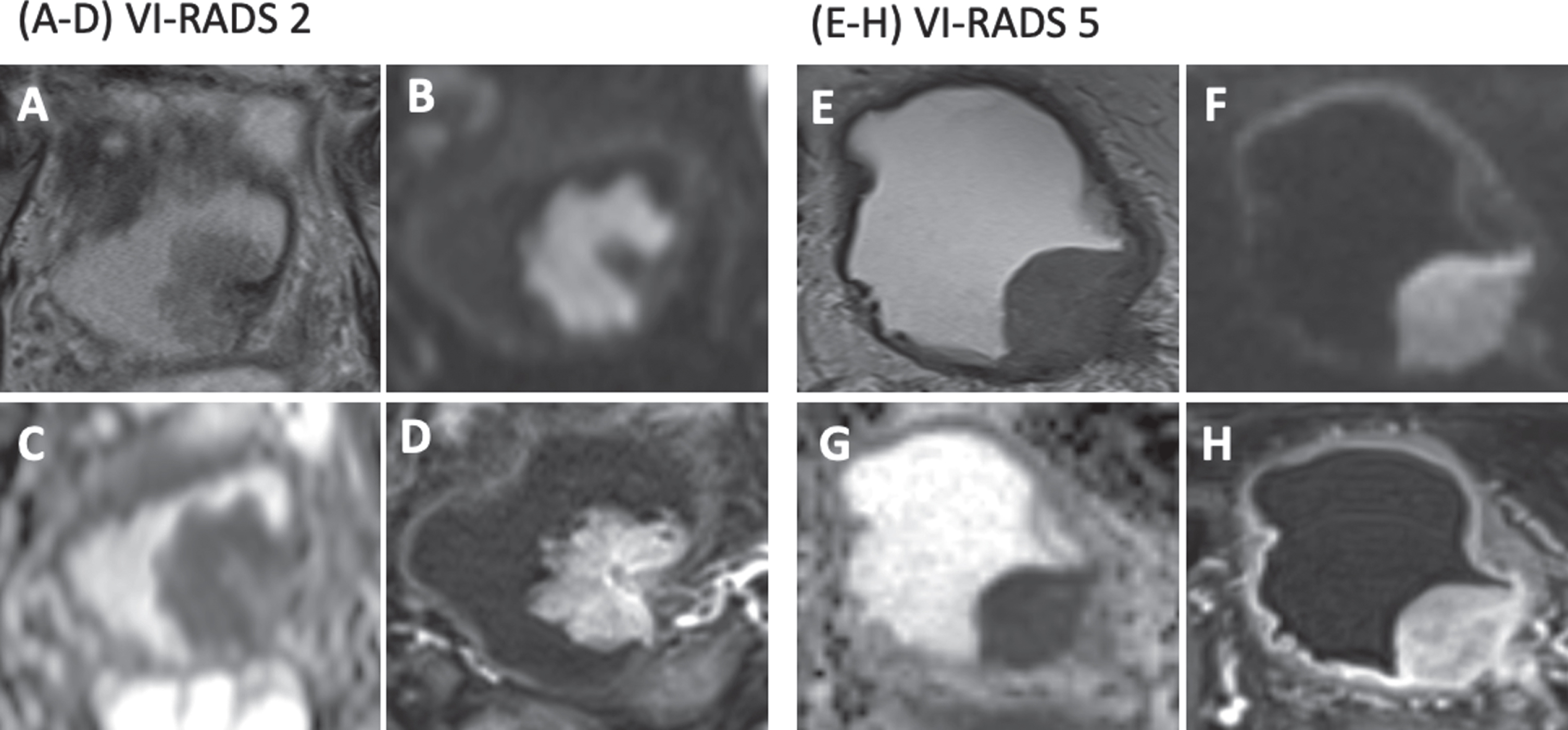
Fig. 1
Representative MR images of bladder cancer with VI-RADS 2 and 5. A-D, MRI pictures in an 84-year-old man with pT1, G2 urothelial carcinoma. The VI-RADS score is 2. (A) An axial T2-weighted image shows a mass near the left ureteral orifice. (B) An axial b1000 DWI shows a hyper-intense mass with a hypo-intense central stalk, resembling an inchworm. (C) An ADC map. The mean absolute tumor ADC and sT-ADC were 1.25×10-3 mm2/s and 0.96, respectively. (D) A DCE-MRI shows early enhancement of the tumor but not the muscular layer. E-H, MRI pictures in a 79-year-old man with≥pT2, G3 urothelial carcinoma. The VI-RADS score is 5. (E) An axial T2-weighted image shows a mass arising from the left ureteral orifice. (F) An axial b1000 DWI shows a hyper-intense mass involving the muscular layer. (G) An ADC map. The mean absolute tumor ADC and sT-ADC were 0.84×10-3 mm2/s and 0.54, respectively. (H) A DCE MRI shows early enhancement of a tumor involving the muscular layer.
Statistical analysis
The predictive accuracy was evaluated using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The best cut-off ADC value was determined as that at the point on the ROC curve with minimum distance from the left-upper corner of the unit square. Interobserver agreement was evaluated using the kappa statistics. Intra- and interobserver reproducibility of sT-ADC values were assessed using intraclass correlation coefficient (ICC). Differences in continuous variables between groups were assessed using the Wilcoxon’s rank sum test. The Fisher’s exact test or chi-square test was applied for comparisons between categorical variables. Statistical analysis was performed using JMP software, version 14 (SAS Institute, Cary, NC, USA). p < 0.05 were considered significant.
RESULTS
Patient and tumor demographics
The demographics of the 176 patients and their index lesions are summarized in Table 1. Seventy-three patients had multiple BC (41%). MRI was performed at our institution for 101 patients (57%), at our affiliated clinic for 56 (32%) and at others for 19 (11%). T2-weighted imaging, DWI, and ADC maps were available for all patients, whereas dynamic contrast enhancement (DCE)-MRI was performed for 97 (55%). MIBC was diagnosed in 46 patients (26%).
Table 1
Demographics of 176 patients with primary bladder cancer and their index tumors
| Variables | Categories | Number (%) | P-values | |||||
| Total | VI-RADS | |||||||
| 1 | 2 | 3 | 4 | 5 | ||||
| Total | 176 | 15 (8) | 84 (48) | 43 (24) | 18 (10) | 16 (9) | ||
| Age [median (range)] | 73 (30–95) | 69 (41–95) | 73 (30–92) | 77 (46–92) | 71 (45–84) | 70 (43–88) | 0.70 | |
| Sex | Male | 125 (71) | 13 (87) | 58 (69) | 29 (67) | 13 (72) | 12 (75) | 0.62 |
| Female | 51 (29) | 2 (13) | 26 (31) | 14 (33) | 5 (28) | 4 (25) | ||
| History of UTUC | No | 174 (99) | 15 (100) | 83 (99) | 42 (98) | 18 (100) | 16 (100) | 0.82 |
| Yes | 2 (1) | 0 (0) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | ||
| Tumor multiplicity | Solitary | 103 (58) | 10 (67) | 52 (62) | 23 (53) | 10 (56) | 8 (50) | 0.77 |
| Multiple | 73 (41) | 5 (33) | 32 (38) | 20 (47) | 8 (44) | 8 (50) | ||
| Tumor configuration | Papillary | 144 (82) | 14 (93) | 74 (88) | 40 (93) | 9 (50) | 7 (44) | <0.01 |
| Non-papillary | 32 (18) | 1 (7) | 10 (12) | 3 (7) | 9 (50) | 9 (56) | ||
| Tumor size | <1 cm | 22 (13) | 15 (100) | 0 (0) | 7 (16) | 0 (0) | 0 (0) | <0.01 |
| 1–3 cm | 107 (60) | 0 (0) | 58 (69) | 35 (81) | 8 (44) | 6 (38) | ||
| ≥3 cm | 47 (27) | 0 (0) | 26 (31) | 1 (2) | 10 (56) | 10 (63) | ||
| Tumor location | Bladder neck | 11 (6) | 0 (0) | 8 (10) | 2 (5) | 0 (0) | 1 (6) | 0.57 |
| Trigone | 10 (6) | 2 (13) | 1 (1) | 3 (7) | 2 (1) | 2 (12) | ||
| Posterior wall | 17 (10) | 1 (7) | 7 (8) | 6 (14) | 2 (1) | 1 (6) | ||
| Lateral wall | 46 (26) | 3 (13) | 21 (25) | 11 (26) | 5 (28) | 6 (38) | ||
| Dome | 5 (3) | 1 (7) | 1 (1) | 2 (5) | 0 (0) | 1 (6) | ||
| Anterior wall | 22 (12) | 2 (13) | 10 (12) | 6 (14) | 3 (17) | 1 (6) | ||
| Ureteral orifice | 65 (37) | 6 (40) | 36 (43) | 13 (30) | 6 (3) | 4 (25) | ||
| Institution of MRI performance | Our institution | 101 (57) | 11 (73) | 42 (50) | 30 (70) | 11 (61) | 7 (44) | 0.09 |
| Affiliated clinic | 56 (32) | 3 (20) | 34 (40) | 8 (19) | 5 (28) | 6 (38) | ||
| Others | 19 (11) | 1 (7) | 8 (10) | 5 (12) | 2 (11) | 3 (19) | ||
| sT-ADC | >0.894 | 113 (64) | 14 (93) | 54 (64) | 35 (81) | 6 (33) | 4 (25) | <0.01 |
| ≤0.894 | 63 (36) | 1 (7) | 30 (36) | 8 (19) | 12 (67) | 12 (75) | ||
| Pathological T stage | Ta | 85 (48) | 10 (67) | 51 (61) | 20 (47) | 4 (22) | 0 (0) | <0.01 |
| T1 | 45 (26) | 5 (33) | 25 (30) | 14 (33) | 1 (6) | 0 (0) | ||
| ≥T2 | 46 (26) | 0 (0) | 8 (10) | 9 (21) | 13 (72) | 16 (100) | ||
| Histological grade | 1 | 14 (8) | 2 (13) | 8 (10) | 4 (9) | 0 (0) | 0 (0) | <0.01 |
| 2 | 89 (51) | 9 (60) | 49 (58) | 23 (53) | 5 (28) | 3 (19) | ||
| 3 | 70 (40) | 4 (27) | 25 (30) | 16 (37) | 12 (67) | 13 (81) | ||
| Unknown | 3 (2) | 0 (0) | 2 (2) | 0 (0) | 1 (6) | 0 (0) | ||
VI-RADS: Vesical Imaging-Reporting And Data System, UTUC: upper tract urothelial carcinoma, sT-ADC: standardized tumor ADC.
Interobserver agreement of VI-RADS
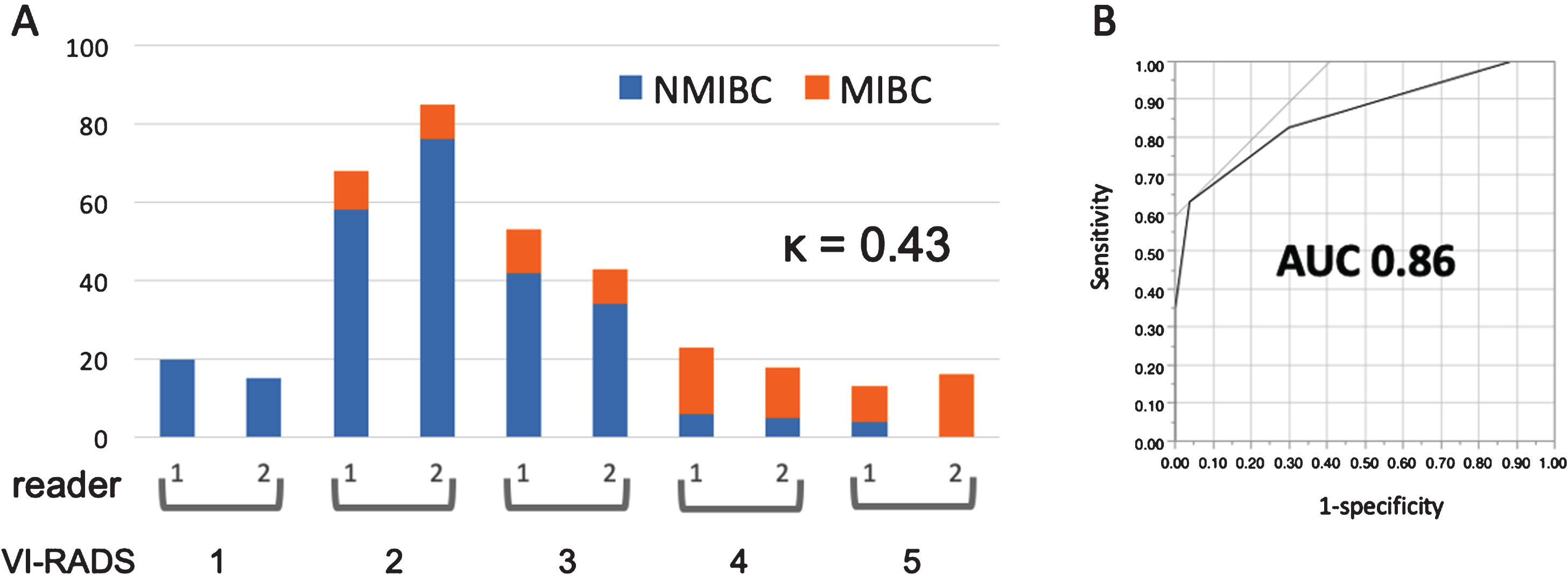
The interobserver agreement was moderate between two readers (κ score 0.43, Fig. 2A). Consensus on VI-RADS score was obtained for 72 patients (41%) in which the score was discordant between the readers. Allocation of VI-RADS score after consensus is listed in Table 1.
Fig. 2
Validation of the Vesical Imaging-Reporting And Data System (VI-RADS) to detect muscle-invasive bladder cancer (MIBC). (A) Interobserver agreement of the VI-RADS scores between two readers and the pathological results of non-muscle-invasive bladder cancer (NMIBC) or MIBC according to the VI-RADS. (B) The ROC curve of VI-RADS to detect MIBC.
Accuracy of VI-RADS to detect MIBC
MIBC was pathologically diagnosed in 0 (0%) / 8 (10%) / 9 (21%) / 13 (72%) / 16 (100%) patients for VI-RADS 1/2/3/4/5, respectively (p < 0.01, Table 1). The AUC of VI-RADS to detect MIBC was 0.86 (Fig. 2B). When cut-off of VI-RADS was set at≥3 and≥4, the sensitivity/ specificity/ positive predictive value (PPV)/ negative predictive value (NPV) to detect MIBC were 78% /70% /48% /90% and 63% /96% /85% /88%, respectively (Table 2). The AUC of VI-RADS was 0.88 and 0.84 in the 97 patients receiving and the remaining 79 not receiving DCE-MRI, respectively. Among the 97 patients receiving DCE-MRI, VI-RADS score changed from 3 to 4 in 2 (2%, pTa and≥pT2) and from 2 to 3 in 1 (1%, pTa) by considering the DCE-MRI category. The AUC of VI-RADS in the 97 patients was 0.84 when the DCE-MRI category was not considered.
Table 2
Detectability of muscle invasion by VI-RADS, sT-ADC, and VI-RADS/ADC
| Models | Sensitivity | Specificity | PPV | NPV |
| VI-RADS≥3 | 78% | 70% | 48% | 90% |
| VI-RADS≥4 | 63% | 96% | 85% | 88% |
| sT-ADC≤0.894 | 78% | 79% | 57% | 91% |
| VI-RADS/ADC | ||||
| VI-RADS≥3 or sT-ADC≤0.894 | 100% | 53% | 43% | 100% |
| VI-RADS≥3 &sT-ADC≤0.894 | 61% | 97% | 88% | 88% |
| VI-RADS≥4 or sT-ADC≤0.894 | 91% | 76% | 58% | 96% |
| VI-RADS≥4 &sT-ADC≤0.894 | 50% | 99% | 96% | 85% |
VI-RADS: Vesical Imaging-Reporting And Data System, sT-ADC: standardized tumor ADC, PPV; positive predictive value, NPV; negative predictive value.
Reproducibility of ADC values
Intra- and interobserver reproducibility of sT-ADC values was excellent with respective ICC of 0.903 and 0.921 (data not shown).
Accuracy of ADC values to detect MIBC
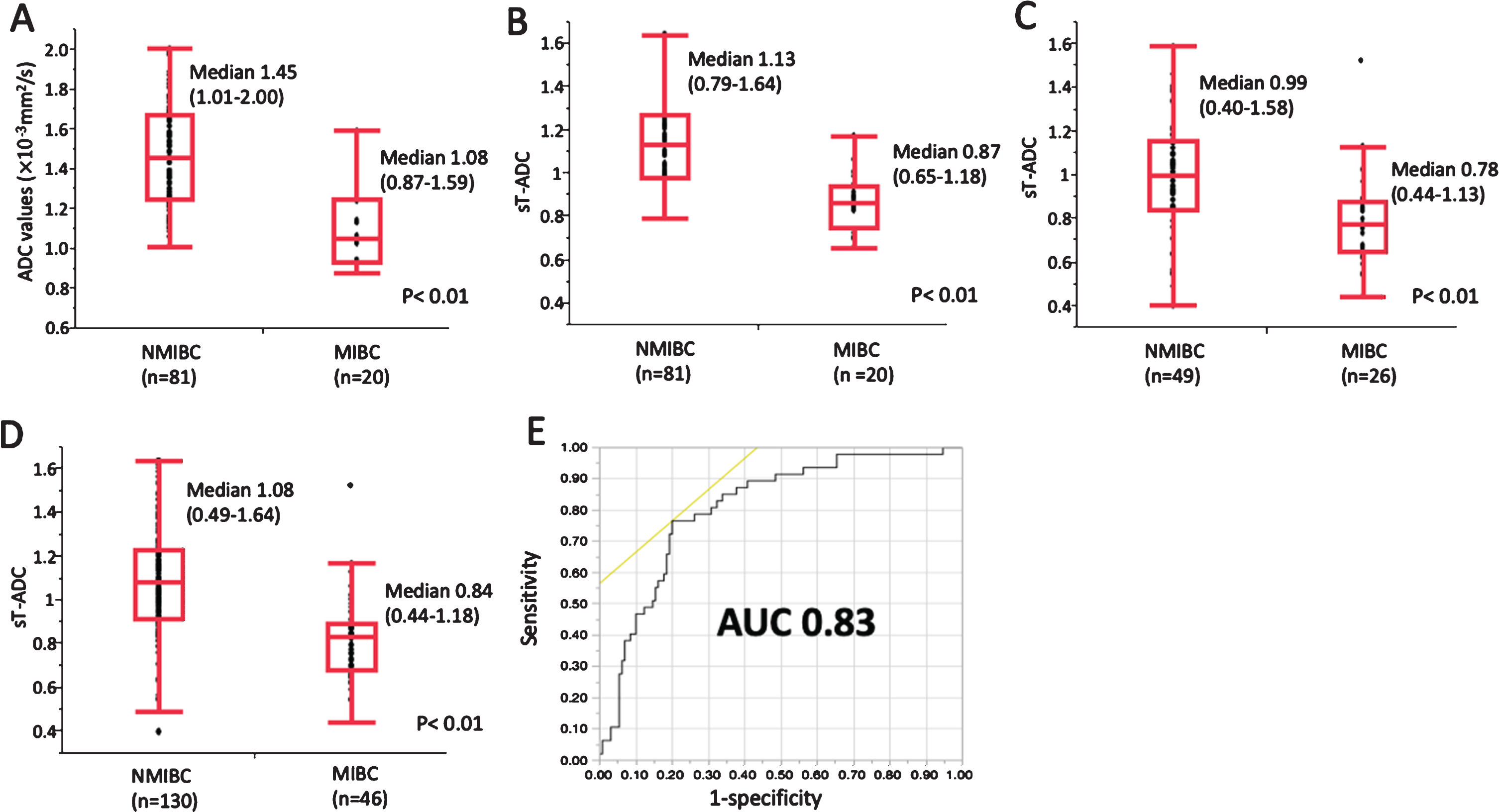
Tumor ADC values according to the presence of muscle invasion are shown in Fig. 3. In 101 patients who underwent MRI at our institution, absolute tumor ADC values ranged from 0.87 to 2.00×10-3mm2/s (median 1.38×10-3 mm2/s). The absolute tumor ADC values were significantly lower in 20 MIBC patients (median 1.08×10-3 mm2/s, range 0.87 –1.59×10-3 mm2/s) than in 81 NMIBC patients (median 1.45×10-3 mm2/s, range 1.01 –2.00×10-3 mm2/s, p < 0.01; Fig. 3A). In this subpopulation, sT-ADC values ranged from 0.65 to 1.64 (median 1.08). The difference in sT-ADC values was significant between NMIBC and MIBC (p < 0.01) and the distribution was graphically almost identical to that of absolute tumor ADC values (Fig. 3B). Similar results were obtained for 56 patients who underwent MRI at our affiliated clinic (Supplemental Figure 1). When 19 patients who underwent MRI at other institutions were combined to the 56, the sT-ADC values were significantly lower in 26 MIBC patients (median 0.78, range 0.44 –1.13) than in 49 NMIBC patients (median 0.99, range 0.49 –1.58, p < 0.01; Fig. 3C). In a total of 176 patients, sT-ADC values ranged from 0.44 to 1.64 (median 1.00). The sT-ADC values were significantly lower in 46 MIBC patients (median 0.84, range 0.44 –1.18) than in 130 NMIBC patients (median 1.08, range 0.49 –1.64, p < 0.01; Fig. 3D). The ROC curve of sT-ADC to detect MIBC is shown in Fig. 3E. The AUC was 0.79 and the best cut-off value of sT-ADC was 0.894. When this cut-off value was used, the sensitivity, specificity, PPV, and NPV to detect MIBC were 78%, 79%, 57%, and 91%, respectively (Table 2).
Fig. 3
Distribution of tumor ADC values according to the presence of muscle invasion. (A) Absolute tumor ADC values for 101 patients who underwent MRI at our institution. (B) Standardized tumor ADC (sT-ADC) values for 101 patients who underwent MRI at our institution. (C) sT-ADC values for 75 patients who underwent MRI at other institutions (56 patients at our affiliated clinic). (D) sT-ADC values for all 176 patients. NMIBC, non-muscle-invasive bladder cancer; MIBC, muscle-invasive bladder cancer. The box and bars indicate a range from 25th to 75th percentile and the minimum, median, and maximum values, respectively. (E) The ROC curve of sT-ADC to detect MIBC.
Combination of VI-RADS and ADC values to detect MIBC
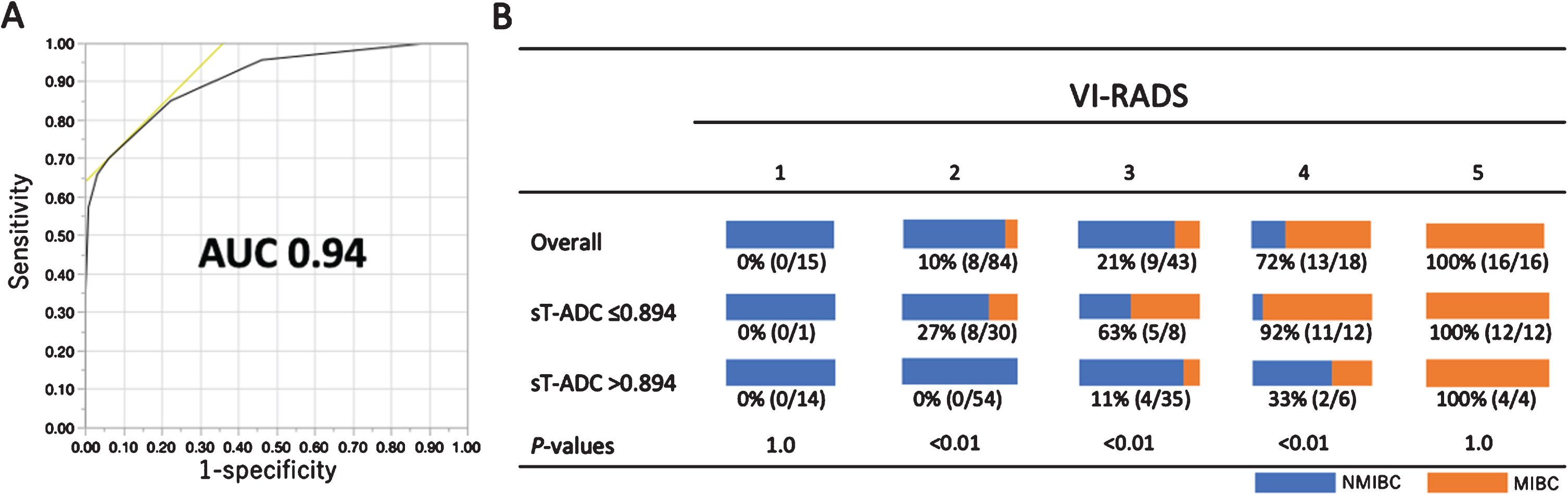
Incorporation of sT-ADC into VI-RADS improved accuracy to detect MIBC from AUC 0.86 to 0.94 (Fig. 4A). The improvement of the accuracy was evident when assessed for the two readers separately (AUC 0.80 to 0.91 and 0.82 to 0.93 for reader 1 and 2, respectively; Supplemental Figure 2).
When VI-RADS≥3 or sT-ADC≤0.894 were considered positive, the sensitivity (100%) and NPV (100%) were highest among the models. When VI-RADS≥4 and sT-ADC≤0.894 were considered positive, the specificity (99%) and PPV (96%) were highest among the models (Table 2).
Eight MIBC (17%) and five NMIBC (4%) were under-staged as VI-RADS≤2 and over-staged as VI-RADS≥4, respectively (Fig. 4B). Incorporation of sT-ADC reduced under-staging of MIBC by 100% (8/8) and over-staging of NMIBC by 80% (4/5, Fig. 4B).
Fig. 4
Incorporation of standardized tumor ADC (sT-ADC) values into the Vesical Imaging-Reporting And Data System (VI-RADS) to detect muscle invasion. (A) The ROC curve of VI-RADS/ADC. (B) Distribution of muscle-invasive disease (MIBC) according to sT-ADC for each VI-RADS score.
DISCUSSION
VI-RADS was proposed in 2018 to define a standardized approach to imaging and reporting MRI for BC, particularly to accurately detect MIBC on MRI. Very recently, several studies demonstrated the validity of VI-RADS to differentiate MIBC from NMIBC in BC patients [6–9]. This study confirmed the validity of VI-RADS. More importantly, incorporation of ADC into VI-RADS improved the accuracy to detect MIBC, particularly reducing under-staging of MIBC as VI-RADS < 3 and over-staging of NMIBC as VI-RADS≥4 by 100% and 80%, respectively.
Several studies have demonstrated the ADC value to be a quantitative biomarker associated with pathological phenotypes, including histological grade and T stage in BC [11, 19, 20]. Kobayashi et al. reported an inverse correlation of ADC values with the invasive and proliferative potential of BC [10]. The present study confirmed that ADC values serve as a functional biomarker to differentiate MIBC from NMIBC. ADC values corroborate the morphological properties of VI-RADS from the qualitative aspect. Indeed, despite the low incidence of MIBC (8%) and NMIBC (15%) among BC with VI-RADS < 3 and≥4, respectively, incorporation of ADC values avoided under-staging of MIBC and over-staging of NMIBC in the majority of cases. In the similar concept to ours, an Egyptian group is conducting a prospective study to evaluate if incorporation of ADC values improves the MIBC-predictive accuracy of VI-RADS [21].
Unlike men with suspected prostate cancer who undergo pre-biopsy MRI, BC patients cannot avoid TURB for the therapeutic purpose regardless of the results of pre-TURB MRI. What does pre-TURB MRI substantially benefit BC patients? First, assessment of the depth of invasion may reduce the risk of bladder perforation during TURB. Patients with low probability of MIBC may avoid unnecessary resection of the deeper muscle layer, consequently reducing the risk of perforation. Second, pre-TURB MRI can provide prognostic information in NMIBC patients. Lower ADC values were associated with recurrence after TURB in NMIBC patients [22]. In addition, the absence of an inchworm sign on DWI was significantly associated with progression of T1 BC [23]. Third, ADC values may be useful for therapeutic decision making of MIBC patients who desire bladder preservation. Lower ADC values of MIBC were predictive of favorable response to chemoradiation, which is a prerequisite for successful bladder preservation [16]. Thus, pre-TURB MRI with ADC measurement may have potential roles in the management of BC patients.
A major limitation of the clinical utility of ADC values is incompatibility among different MRI protocols. As ADC values depend on the MRI systems and imaging protocols, comparison of absolute ADC values across different MRI protocols is impossible. Nishizawa et al. proposed a concept of standardized ADC values to overcome this incompatibility and demonstrated that differences in absolute tumor ADC values among different MRI protocols were negated by using sT-ADC for BC [18]. The present study confirmed the validity of sT-ADC for detecting MIBC independent of MRI protocols. Conceptually, the cut-off sT-ADC value of 0.894 determined in this study is applicable irrespective of MRI protocols. However, external validation studies are needed to assess the general applicability.
There are several limitations in this study. First, this was a single-institutional retrospective study. A multi-institutional prospective study with an independent larger patient cohort is required to confirm and validate the predictive accuracy of the VI-RADS/ADC. Second, although we used sT-ADC to overcome the incompatibility of different MRI protocols, absolute tumor ADC values may give better results than sT-ADC. However, such a difference was not observed in our study. Considering the practical use of ADC values across MRI systems and protocols, standardization of ADC values is needed to overcome the incompatibility among different protocols. Third, there was a possible selection bias because all patients with primary BC did not undergo pre-TURB MRI. However, the proportion of MIBC patients in our cohort (26%) represented that of the general population of primary BC patients [24]. Fourth, DCE-MRI was not performed for approximately half of the patients in the study population. According to the original VI-RADS criteria, VI-RADS scores can change in some instances by considering the DCE-MRI category. However, such changes in the VI-RADS score were observed only in 3 patients (3%) among the 97 patients receiving DCE-MRI. In addition, the accuracy of VI-RADS to detect MIBC was comparable whether the DCE-MRI category was considered or not among the 97 patients. Fifth, in the present study, the interobserver agreement of VI-RADS score was not better than that of the published validation studies [6–9] in most of which experienced radiologists read MRI. A urologist scored VI-RADS 4/5 in less patients than a radiologist (Supplemental Table 2) and tended to diagnose large NMIBCs more correctly. The difference in the background and experience of readers could be attributed to our lower κ score. However, our study may reflect the real-world practice of using VI-RADS. Fifth, pathological T stage was evaluated using TURB but not cystectomy specimens. For correct T staging, Re-TURB was routinely performed for patients with T1 or greater disease without sampling the muscular layer at the first TURB.
CONCLUSIONS
The incorporation of ADC values improves the accuracy of VI-RADS to detect MIBC. External validation is needed to confirm the accuracy of the VI-RADS/ADC.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
KS: drafting the manuscript, data acquisition, statistical analysis and interpretation of the data; MI: drafting the manuscript, data acquisition, statistical analysis and interpretation of the data; SI: data acquisition and interpretation of the data; YN: critical revision of the manuscript for scientific and factual content; MK: critical revision of the manuscript for scientific and factual content; KT: drafting the manuscript, statistical analysis and interpretation of the data; HS: drafting the manuscript and interpretation of the data; KT: supervision; TK: supervision; FK: supervision, conception and design, drafting the manuscript, statistical analysis and interpretation of the data.
CONFLICT OF INTEREST
No potential conflicts of interest are disclosed.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-190267.
REFERENCES
[1] | Flaig TW , Spiess PE , Agarwal N , Bangs R , Boorjian SA , Buyyounouski MK , et al NCCN Guidelines Insights: Bladder Cancer, Version 5.2018. J Natl Compr Canc Netw. (2018) ;16: (9):1041–53. |
[2] | Asgari MA , Safarinejad MR , Shakhssalim N , Soleimani M , Shahabi A , Amini E . Quality of life after radical cystectomy for bladder cancer in men with an ileal conduit or continent urinary diversion: A comparative study. Urol Ann. (2013) ;5: (3):190–6. |
[3] | Rajesh A , Sokhi HK , Fung R , Mulcahy KA , Bankart MJ . Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol. (2011) ;66: (12):1140–5. |
[4] | Woo S , Suh CH , Kim SY , Cho JY , Kim SH . Diagnostic performance of MRI for prediction of muscle-invasiveness of bladder cancer: A systematic review and meta-analysis. Eur J Radiol. (2017) ;95: :46–55. |
[5] | Panebianco V , Narumi Y , Altun E , Bochner BH , Efstathiou JA , Hafeez S , et al Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol. (2018) ;74: (3):294–306. |
[6] | Ueno Y , Takeuchi M , Tamada T , Sofue K , Takahashi S , Kamishima Y , et al Diagnostic Accuracy and Interobserver Agreement for theVesical Imaging-Reporting and Data System for Muscle-invasive Bladder Cancer: A Multireader Validation Study. Eur Urol. 2019. |
[7] | Barchetti G , Simone G , Ceravolo I , Salvo V , Campa R , Del Giudice F , et al Multiparametric MRI of the bladder: inter-observer agreement and accuracy with the Vesical Imaging-Reporting and Data System(VI-RADS) at a single reference center. Eur Radiol. 2019. |
[8] | Wang H , Luo C , Zhang F , Guan J , Li S , Yao H , et al Multiparametric MRI for Bladder Cancer: Validation of VI-RADS for the Detection of Detrusor Muscle Invasion. Radiology. (2019) ;291: (3):668–74. |
[9] | Makboul M , Farghaly S , Abdelkawi IF . Multiparametric MRI in differentiation between muscle invasive and nonmuscle invasive urinary bladder cancerwith vesical imaging reporting and data system (VI-RADS) application. Br J Radiol. 2019:20190401. |
[10] | Kobayashi S , Koga F , Kajino K , Yoshita S , Ishii C , Tanaka H , et al Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging. (2014) ;39: (1):172–8. |
[11] | Kobayashi S , Koga F , Yoshida S , Masuda H , Ishii C , Tanaka H , et al Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. (2011) ;21: (10):2178–86. |
[12] | Japanese Urological Association/Japanese Society of Pathology, General Rule for Clinical and Pathological Studies on Renal Pelvic, Ureteral and Bladder Cancer of Japanese Urological Association. Tokyo: Kanehara Shuppan; 2011. pp. 16. |
[13] | Mostofi FK . Histological typing of urinary bladder tumours. Geneva: WHO; 1973. |
[14] | Paner GP , Stadler WM , Hansel DE , Montironi R , Lin DW , Amin MB . Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. (2018) ;73: (4):560–9. |
[15] | Yoshida S , Kobayashi S , Koga F , Ishioka J , Ishii C , Tanaka H , et al Apparent diffusion coefficient as a prognostic biomarker of upper urinary tract cancer: a preliminary report. Eur Radiol. (2013) ;23: (8):2206–14. |
[16] | Yoshida S , Koga F , Kobayashi S , Ishii C , Tanaka H , Komai Y , et al Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. (2012) ;83: (1):e21–7. |
[17] | Yoshida S , Masuda H , Ishii C , Tanaka H , Fujii Y , Kawakami S , et al Usefulness of diffusion-weighted MRI in diagnosis of upper urinary tract cancer. AJR Am J Roentgenol. (2011) ;196: (1):110–6. |
[18] | Nishizawa T , Yoshida S , Koga F , Tanaka H , Kaga M , Watanabe K , et al Standardization of the apparent diffusion coefficient value of bladder cancer across different centers: Applicability in predicting aggressive pathologic phenotypes. Clin Imaging. (2017) ;44: :121–6. |
[19] | Yoshida S , Koga F , Masuda H , Fujii Y , Kihara K . Role of diffusion-weighted magnetic resonance imaging as an imaging biomarker of urothelial carcinoma. Int J Urol. (2014) ;21: (12):1190–200. |
[20] | Padhani AR , Liu G , Koh DM , Chenevert TL , Thoeny HC , Takahara T , et al Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. (2009) ;11: (2):102–25. |
[21] | U.S. National Library of Medicine [homepage on the Internet]. Can Vesical Imaging-Reporting And Data System and Apparent Diffusion Coefficient Values (the VI-RADS/ADC) Accurately Predict Non-Muscle Invasive Bladder Cancer [updated 2019 November 22, cited 2020 February 11]. Available from: [https://clinicaltrials.gov/ct2/show/NCT04167631]. |
[22] | Funatsu H , Imamura A , Takano H , Ueda T , Uno T . Can pretreatment ADC values predict recurrence of bladder cancer after transurethral resection? Eur J Radiol. (2012) ;81: (11):3115–9. |
[23] | Yajima S , Yoshida S , Takahara T , Arita Y , Tanaka H , Waseda Y , et al Usefulness of the inchworm sign on DWI for predicting pT1 bladder cancer progression. Eur Radiol. (2019) ;29: (7):3881–8. |
[24] | Bladder cancer: diagnosis and management of bladder cancer: ©NICE (2015) Bladder cancer: diagnosis and management of bladder cancer. BJU Int. (2017) ;120: (6):755–65. |




