Review of SWOG S1314: Lessons from a Randomized Phase II Study of Co-Expression Extrapolation (COXEN) with Neoadjuvant Chemotherapy for Localized, Muscle-Invasive Bladder Cancer
Abstract
SWOG1314 is a randomized phase II study of co-expression extrapolation (COXEN) with neoadjuvant chemotherapy for localized, muscle-invasive bladder cancer. COXEN is a biomarker approach in which predictive biomarkers are developed using in vitro data, which may then be applied directly into a clinical testing application. Two separate Gene Expression Models (GEMs), one for the Methotrexate, Vinblastine, Doxorubicin, Cisplatin (MVAC) regimen and another for gemcitabine plus cisplatin (GC) were tested in S1314. The lessons learned from the development and operationalization of the S1314 clinical trial are described in detail, which may help to inform the future trials of predictive biomarkers for urothelial carcinoma in the neoadjuvant setting. Specific areas addressed include: The need for broad support from the bladder cancer community, planning for non-evaluable subjects, defining adequate neoadjuvant treatment, defining adequate tissue collection, setting expectations in phase II clinical studies of predictive biomarkers, and maximizing the impact of the samples collected in these studies for broader biomarker development. With a large number of newly available treatments in advanced urothelial carcinoma in the last few years, more investigations of these agents in the neoadjuvant setting is anticipated. There will be a great need for the development of predictive biomarkers in conjunction with the use of these agents in the preoperative setting. Insights from S1314 may provide useful information and lessons learned in this development.
INTRODUCTION
It is estimated that there will be 80,470 new cases and 17,670 deaths from bladder cancer in the United States in 2019 [1]. Predominately affecting males, bladder cancer has an average age of onset of 73 years. Worldwide it represents the 4th most common cause of cancer in men and the 11th most common cause in women [1].
The majority of bladder cancers are pathologically characterized as urothelial carcinoma. The stage of bladder cancer can be divided into 3 main categories: non-muscle invasive, muscle invasive disease or metastatic disease. Current guidelines recommend that patients with non-muscle invasive bladder carcinoma (NMIBC) should be initially treated with transurethral resection and a single dose of intravesical chemotherapy followed by either further intravesical chemotherapy or immunotherapy depending on their risk of recurrence and progression [2]. For those with muscle invasive bladder carcinoma (MIBC), approximately 25% of all patients, definitive local treatment is recommended. Acceptable treatment options for MIBC include neo-adjuvant cisplatin-based therapy followed by cystectomy or chemoradiotherapy, as well as other options based on patient eligibility and clinical stage [2]. In metastatic disease, cisplatin based regimens are effective and have long been considered first line options for systemic treatment. Methotrexate, Vinblastine, Doxorubicin, Cisplatin (MVAC) and gemcitabine plus cisplatin (GC) are two cisplatin-based regimens commonly used in the setting of MIBC and metastatic bladder cancer. Though many patients inially respond to medical therapy, the majority of patients with advanced disease on cisplatin-based regimens will ultimately experience cancer progression. In the last few years, the therapies available to treat bladder cancer have begun to diversify. Most prominently, the results of recent clinical trials have added a number of PD-1/PD-L1 immune checkpoint inhibitors (ICIs) as treatment options in non-muslce invasive, locally advanced and metastatic urothelial carcinoma. A recent example is pembrolizumab, which was FDA-approved on the basis of an open label, phase 3 clinical trial consisting of 542 patients diagnosed with advanced urothelial carcinoma, randomly assigned to receive either pembrolizumab or chemotherapy as second line therapy. Investigators found that the median overall survival was approximately 3 months longer in patients treated with pembrolizumab (10.3 months [95% confidence interval, 8.0 to 11.8]) than those treated with chemotherapy (7.4 months [95% CI, 6.1 to 8.3]) [3]. Another ICI, atezolizumab, was approved on the basis of a phase II clinical trial that enrolled 316 patients with metastatic urothelial carcinoma [4]. Interestingly, an open label phase three clinical trial that randomly assigned patients with metastatic carcinoma to receive either atezolizumab or chemotherapy found no difference in overall survival [5]. Nevertheless, IMvigor 130, a phase III study evaluating the efficacy and safety of atezolizumab combined with platinum based chemotherapy or as a monotherapy in patients with locally advanced or metastatic urothelial carcinoma, found that the combination of ICI+chemotherapy prolonged progression-free survival (8.2 months [95% CI, 6.5 to 8.3]) compared to chemotherapy alone (6.3 months [95% CI, 6.2 to 7.0]). No difference in overall survival was reported in the interim analysis [6].
Current research has turned to examine the role of ICI in neoadjuvant therapy for MIBC. To date, a number of clinical trials are underway in this vein (NCT02812420, NCT02845323, NCT02365766, NCT02690558), although dose-dense MVAC (ddMVAC) and GC remain the standard of care treatments in this setting. These studies examining ICIs either in combination with cisplatin-based chemotherapy or alone in the neoadjuvant setting will provide important information on the optimal treatment of MIBC. PURE-01, a study examining pembrolizumab in the neoadjuvant setting, was reported last year and demonstrated encouraging results [7].
A notable aspect of contemporary and historical treatment of MIBC is the underutilization of neo-adjuvant chemotherapy [8–10]. This underutilization exists despite the support of two randomized, phase III clinical trials. Initiated in 1987, SWOG 8710 randomized 317 subjects with MIBC to either cystectomy alone or three cycles of standard dose MVAC prior to cystectomy [11]. Investigators found that median survival was 77 months in the patients treated with MVAC compared to 44 months for those treated with no chemotherapy (P = 0.06). BA06 30894, a large European trial initiated in 1989, randomized 976 patients with MIBC to receive either no chemotherapy or three cycles of cisplatin, methotrexate and vinblastine (CMV) prior to cystectomy and/ or radiotherapy [12]. Long term results showed a statistically significant 16% reduction in risk of death (hazard ratio, 0.84; 95% CI, 0.72 to 0.99; P = 0.037) in those treated with CMV [13].
Despite the clear survival benefit with neoadjuvant therapy, further optimization and improvements in its application are needed. Given that a notable number of patients in both phase III trials were observed to be unresponsive to neoadjuvant chemotherapy, how can we identify patients who have disease that is unlikely to respond to neoadjuvant treatment and who might be better served by proceeding directly to surgery or receiving another treatment strategy entirely? Secondly, with multiple chemotherapy regimens (e.g. ddMVAC and GC) as acceptable standards of care, how can we evaluate whether a given patient is more likely to respond to one particular regimen as opposed to another? CoeXpression ExtrapolatioN (COXEN) presents the potential to answer both of these questions.
COXEN
COXEN (CO-eXpression ExtrapolatioN) is an algorithm developed by Dan Theodorescu, Jae Lee and colleagues at the University of Virginia that predicts the drug sensitivity of cancer cells based on in vitro drug sensitivity of a previously analyzed panel of diverse cancer cell models. COXEN was originally developed using the National Cancer Institute’s Development Therapeutics program’s NCI-60 Human Tumor Cell Line Screen, which included 60 cancer cell lines that have been used to screen over 100,000 anti-cancer compounds [14]. The algorithm uses a comparison of correlation matrices to identify gene expression patterns that are concordantly expressed in both cell lines of interest in relation to drug sensitivity (e.g. NCI-60) and relevant human samples (e.g. human bladder cancer samples). The result of this comparison (known as the COXEN coefficient) allows for the development of gene expression models (GEM) which can potentially provide prognostic and therapeutic benefit on the individual tumor level [15]. Thus, COXEN provides an a priori approach based on in vitro results that differs from the traditional process of biomarker discovery which oftentimes requires multiple cohorts of human samples with clinical outcome data. Since the signature is derived in the preclinical arena, COXEN allows for the immediate validation of the gene expression profile in human trials and rapid clinical translation.
An initial evaluation of the COXEN approach sought to predict the drug sensitivity of the BLA-40, a panel of 40 human urothelial bladder carcinomas, none of which were included in NCI-60. Using the NCI-60 gene expression and in vitro drug sensitivity data, investigators were able to apply the COXEN algorithm to accurately predict bladder cancer cell line sensitivity to paclitaxel and cisplatin [16]. Investigators subsequently used two cohort-based breast cancer clinical trials to show that COXEN can accurately predict clinical responses in a retrospective application of the approach [16]. Subsequently, researchers applied COXEN methodology to develop GEMs to evaluate tumor response and/or patient survival in seven independent cohorts of patients with a number of different cancer types, who underwent a number of different treatments [17].
SWOG 1314 TRIAL
Prior to utilizing COXEN to direct treatment and select which patients should receive a particular neoadjuvant regimen, we first needed to prospectively validate its use, showing that a treatment-specific COXEN score predicts response to chemotherapy. We endeavored to gain this prospective data by randomizing patients between two standard and acceptable treatments. Subsequently, we evaluated the association of the COXEN marker with the patient’s pathologic outcome. If the marker’s predictive properties were found to be adequate from a clinical perspective, this information would then inform and support the next trial, which would test whether assigning treatment based on the COXEN scores is more advantageous than just randomly assigning treatment.
SWOG 1314, a National Clinical Trial Network (NCTN) sponsored trial, sought to evaluate COXEN in the setting of two specific standard of care neoadjuvant regimens: ddMVAC and GC (Fig. 1). The phase II study included 227 eligible patients diagnosed with T2-T4aN0M0 urothelial carcinoma who were planning to undergo cystectomy (Fig. 2) [18]. These patients were randomized to receive 4 cycles of either GC or ddMVAC. At the time of enrollment, mandatory cancer tissue collection was required to enable gene expression profiling and COXEN score generation on the transurethral bladder tumor resection sample. Two distinct COXEN biomarkers were assessed, corresponding to each regimen (GC and MVAC). Setting a goal of enrolling 92 patients per treatment arm and applying a one-sided alpha of 0.05, investigators calculated that there would be “a 99% (82%) statistical power to detect an absolute difference in pT0 rates of 50% (30%) between patients with a favorable vs. unfavorable Coxen score (NCT02177695).”
Fig. 1
Schema. S1314 enrolled patients with T2 to T4a N0 M0 urothelial carcinoma (mixed histology which included a component of urothelial carcinoma was acceptable). There was mandatory tissue collection from the TURBT at the time of randomization, as well as tumor sampling of those with residual disease at cystectomy.
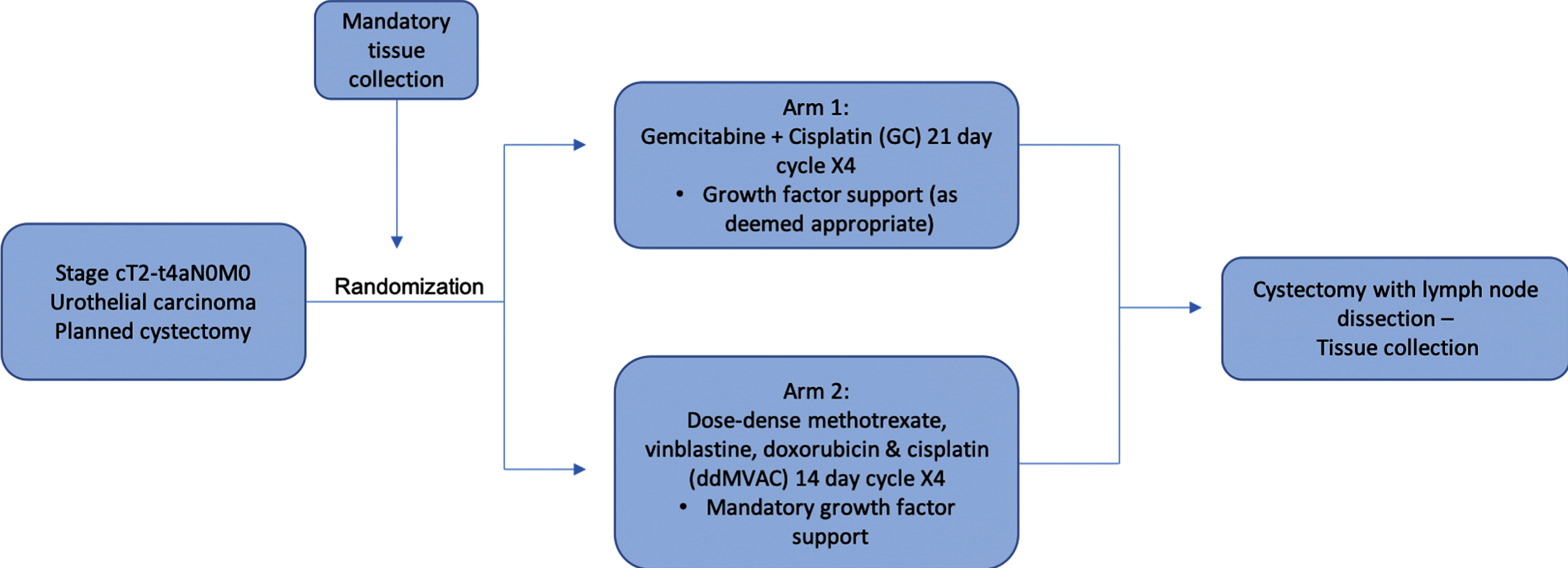
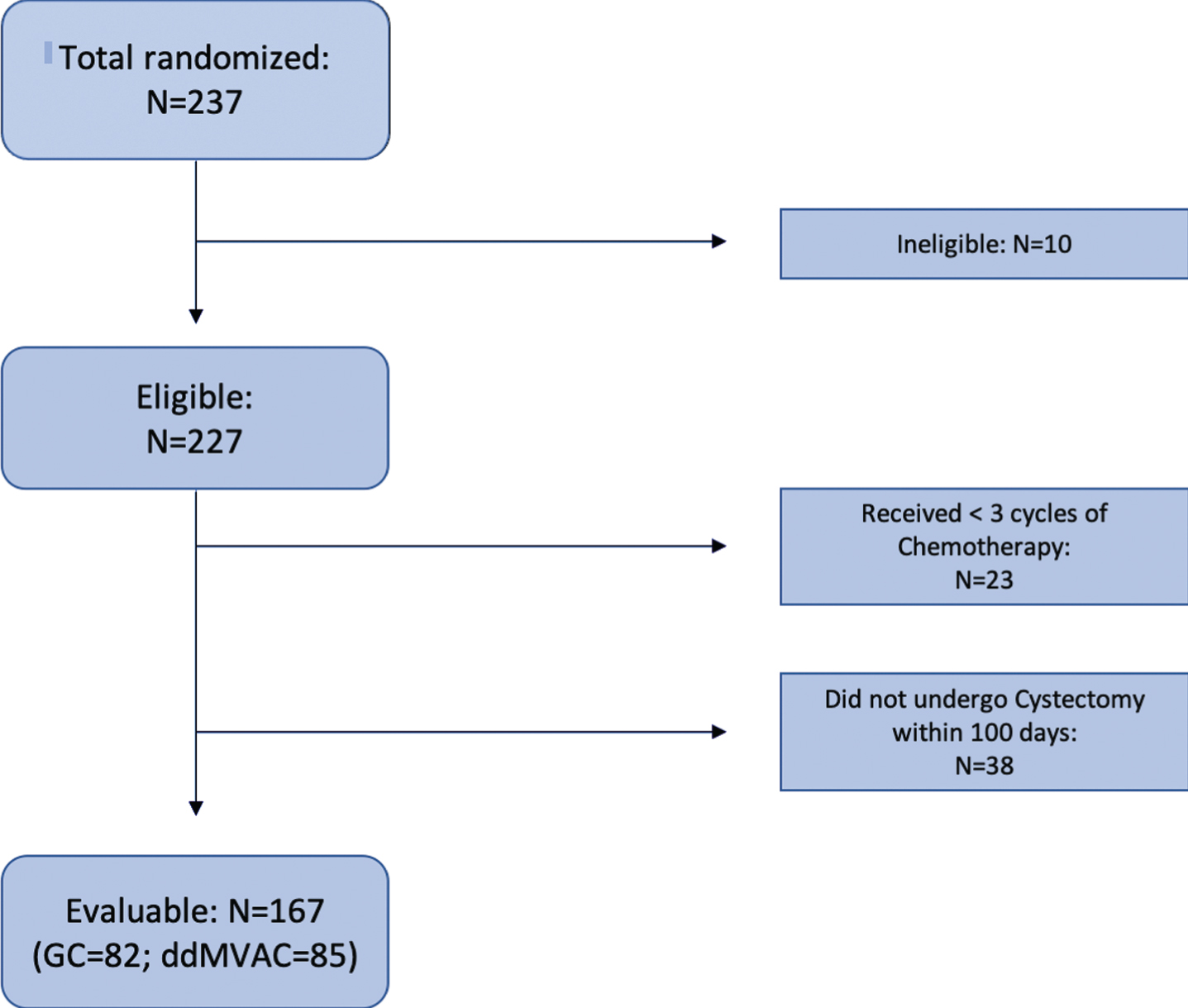
Fig. 2
Consort Diagram. 237 Patients were included for randomization in this Phase II trial. Nine patients were found ineligible. Of eligible patients, exclusions left 167 evaluable randomized patients.
The Primary aim of SWOG 1314 was to characterize the relationship of DDMVAC- and GC-specific COXEN scores in terms of pT0 rate at cystectomy in patients treated with neoadjuvant chemotherapy. This was done in two ways. First, by assessing whether the treatment-specific COXEN score is prognostic of pT0 rate or≤pT1 in this patient population and to assess in a preliminary fashion whether the COXEN score is a predictive factor distinguishing between these two chemotherapy regimens. Second, by evaluating the correlation between the GC- and the DDMVAC-COXEN score [18].
DISCUSSION
SWOG 1314 was conducted at an intriguing moment during which bladder cancer therapeutic development entered a new and more productive era. In the past two decades both excessive accrual times and a discouragingly high number of prematurely terminated trials have led to a pervading negativity when it came to conducting clinical trials in bladder cancer [11, 19]. As a fully accrued, contemporary neoadjuvant bladder cancer biomarker trial, SWOG 1314 presents a rejoinder to this mentality in achieving its accrual goal, although the study did have more non-evaluable patients than anticipated. Coupled with the FDA’s approval of multiple new bladder specific therapies since 2016, the most recent being that of enfortumab vedotin (EV) and the targeted agent Erdafitinib [20, 21], SWOG 1314’s successful completion provides encouragement to pursue additional trials in this area. It is also notable that SWOG 1314 represents a novel example of translating an in vitro biomarker directly into a clinical application. With a larger array of therapies now available to treat bladder cancer there is a clear need for more investigations to identify predictive biomarkers in this setting. Encouragingly, Iyer et al. in their examination of neoadjuvant chemosensitivity in patients harboring DNA damage response and repair alterations have already begun this work (NCT03609216) [22].
While heartening, the completion of SWOG 1314 was not without challenges and many lessons learned.
Broad support
Foremost among these lessons was the importance of obtaining large scale buy-in from the medical oncologists and urologists, as well as the bladder cancer patient community. This was aided by the sponsorship of the NCTN which allowed the trial to be available at a large number of sites across the United States. Ultimately, 237 patients were enrolled at 85 NCTN sites across the country. When S1314 was initiated in 2013, there was much less optimism in our ability to design and successfully complete novel trials in bladder cancer. The fact that SWOG 1314 was designed as a randomized trial of DDMVAC- and GC-chemotherapy, rather than a marker-directed trial, certainly made obtaining this buy-in more difficult. As biomarkers are validated and allow for therapy selection in trials, more patient and provider engagement should be realized.
Non-evaluable subjects
From a practical perspective, SWOG 1314 highlighted the importance of having a conservative expectation for the number of evaluable patients when developing biomarkers in the neoadjuvant bladder cancer setting. Originally, the study was designed to randomize 184 evaluable patients for COXEN assessment. To account for ineligible patients, inadequate specimens and non-evaluable patients, the trial planned to over-accrue by 15% raising the total accrual to 212 patients. Due to a larger number of non-evaluable patients than expected, the protocol was amended to increase the accrual goal to 237 patients. Despite this, the trial continued to have a sizable number of non-evaluable patients (26.7% of eligible), due largely to inadequate chemotherapy (a failure to undergo at least three cycles of chemotherapy) or not receiving a timely cystectomy within 100 days from the last dose of neoadjuvant therapy (Fig. 2). These findings suggest that future trials of this nature may need to assume a non-evaluable rate of 30% or higher to account for this finding.
Defining adequate treatment
The trial was written with the chemotherapy regimen defined specifically to provide less variability of delivery. Patients were randomized to either 4 cycles of ddMVAC or GC chemotherapy. In the analysis, patients who received 3 or 4 cycles of chemotherapy were considered evaluable, and those with less than 3 cycles were considered non-evaluable. A substantial number of patients were deemed non-evaluable due to inadequate chemotherapy, which was oftentimes due to toxicity of the regimen. Depending on the therapeutic intervention, a determination of treatment adequacy will need to be established as part of the analysis plan. Additionally, the adjudication of patients with clear progression, which precludes the administration of adequate therapy, is not common, but could be considered evaluable for treatment failure depending on the trial intervention.
Adequate tissue collection
Having sufficient tissue to perform the COXEN analysis was key to the success of the trial. Therefore, in S1314 tissue submission was mandatory at enrollment. We initially included several eligibility requirements to support the collection of adequate tissue (e.g. Disease measuring at least 10 mm on cross-sectional imaging or by endoscopic assessment), although specific measurements could be difficult to confirm (e.g. bladder wall thickening). However, having the local pathologist certify the availability of≥0.5 cm of viable tumor (longest diameter) from the biopsy sample proved to be the most practical approach. While this certification did add to the complexity of the trial by requiring additional coordination with pathology, it proved an efficient and acceptable way of ensuring adequate tissue in most cases.
Tissue block versus slides
In tissue-based trials using Formalin-Fixed Paraffin-Embedded tissues, tumor blocks have traditionally been requested. Having the tumor block available is advantageous as it provides more flexibility with regard to central processing as well as allowing for additional samples or core samples to be obtained immediately if needed. In addition, there may be less degradation of tissue when it exists as a block, than as cut slides. Initially our trial required tissue blocks, but many institutions were not able to ship tumor blocks for central processing due to local institutional rules. Consequently, we transitioned to the submission of 20 (10 micron) slides of formalin-fixed paraffin embedded (FFPE) tissue, with 2 (5 micron) slides at the start and end of the 20 slides, for a total of 22 unstained slides. This approach proved workable both from the submitting centers’ point of view as well as that of the central processing facility. Nevertheless, utilization of tissue slides may increase the risk of tissue degradation. The study analysis plan should account for this, by for example, running samples from the same specimen in multiple cohorts.
Setting expectations in phase II biomarker studies
As a large, randomized phase II study, S1314 will provide important data on predictive capabilities of the MVAC and GC COXEN predictors. A critical question in designing such trials is setting expectations. Specifically, what type and how strong of a signal is needed to proceed to a definitive phase III study? This raises the question: what is an adequate endpoint for a phase II biomarker? Is a statistically significant p value as opposed to a clinical predictive value more important? Ultimately the question becomes, is there a strong enough signal to proceed to a therapy-directive phase III study?
Maximizing the utility of the tissue collected as part of this study
Several criteria were put in place to ensure that adequate tissue was collected to perform the gene expression assessment necessary to test the COXEN biomarker. Beyond gene expression, consideration of other relevant and important types of tissue evaluation were also planned. These assessments included genetic, single nucleotide polymorphism (SNP) and circulating tumor cell (CTC) analyses. Plans to maximally utilize the tissues collected in a neoadjuvant study, including residual tumor and germline genetic assessment via peripheral blood, are an important consideration in trial planning.
CONCLUSION
In evaluating the prognostic and predictive capabilities of the MVAC and GC COXEN biomarkers in the neoadjuvant setting of bladder cancer, SWOG 1314 represents a novel example of translating an in vitro biomarker directly into clinical application. Furthermore, successful completion of S1314 as a large phase II clinical trial gives confidence that other neoadjuvant biomarker trials are likely to be successfully run in the setting of bladder cancer. Given the array of treatments now available in bladder cancer, there can be little argument that many important questions exist in this clinical setting. Beyond clinical outcomes and specific results of S1314, this trial provides lessons that may be useful to consider in the design of future trials. Chief amongst these is obtaining buy in from the research community –an important lesson that should not go underappreciated. Equally important is a careful consideration of sample collection, analysis plan and outcome expectations, given the time and effort to complete such trials. We are in an optimistic time in the development of new therapies for bladder cancer patients. Given the variety of existing therapeutic options, trials that seek to define predictive markers will only become more important.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180888 and U10CA180819. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
PB: Writing - Original Draft, Writing - Review & Editing, Conceptualization; MP: Writing - Original Draft, Writing - Review & Editing; TF: Writing - Original Draft, Writing - Review & Editing, Conceptualization.
ETHICAL CONSIDERATIONS
This paper is a literature review and discussion that does not present any primary results of the study it describes. As such, it is exempt from any requirement for Institutional Review Board approval.
CONFLICT OF INTEREST
TF: Stock and Ownership- Aurora Oncology; Speaker Honoraria- BN ImmunoTherapeutics; Consulting or Advisory Role- GTX; Research Funding- Novartis, Bavarian Nordic, Dendreon, GTX, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol-Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, astrazeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seattle Genetics, La Roche-Posay, Merck.
PB and MP have no conflicts of interest to report.
Clinical trial citations
1. https://www.clinicaltrials.gov/ct2/show/NCT02812420
2. https://www.clinicaltrials.gov/ct2/show/NCT02845323
3. https://www.clinicaltrials.gov/ct2/show/study/NCT02365766
4. https://www.clinicaltrials.gov/ct2/show/NCT02690558
5. https://clinicaltrials.gov/ct2/show/NCT02177695?term = S1314&rank = 1
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2019. CA Cancer J Clin. (2019) 69: :7–34, doi:10.3322/caac.21551 |
[2] | Flaig TW , et al NCCN Guidelines Insights: Bladder Cancer, Version 5.2018. J Natl Compr Canc Netw. (2018) ;16: :1041–53, doi:10.6004/jnccn.2018.0072 |
[3] | Bellmunt J , et al Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. New England Journal of Medicine. (2017) ;376: :1015–26, doi:10.1056/NEJMoa1613683 |
[4] | Hoffman-Censits JH , et al IMvigor 210, a phase II trial of atezolizumab (MPDL3280A) in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC). Journal of Clinical Oncology. (2016) ;34: :355, doi:10.1200/jco.2016.34.2_suppl.355 |
[5] | Powles T , et al Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. The Lancet. (2018) ;391: :748–57, doi:10.1016/S0140-6736(17)33297-X |
[6] | Galsky MD , et al IMvigor A randomized, phase III study evaluating first-line (1L) atezolizumab (atezo) as monotherapy and in combination with platinum-based chemotherapy (chemo) in patients (pts) with locally advanced or metastatic urothelial carcinoma (mUC). Journal of Clinical Oncology. (2018) ;36: :TPS4589–TPS4589, doi:10.1200/JCO.2018.36.15_suppl.TPS4589 |
[7] | Necchi A , et al Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. Journal of Clinical Oncology. (2018) ;36: :3353–60, doi:10.1200/jco.18.01148 |
[8] | Duplisea JJ , et al Trends and disparities in the use of neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma. Can Urol Assoc J. (2019) ;13: :24–8, doi:10.5489/cuaj.5405 |
[9] | David KA , Milowsky MI , Ritchey J , Carroll PR , Nanus DM . Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol. (2007) ;178: :451–4, doi:10.1016/j.juro.2007.03.101 |
[10] | Yafi FA , et al Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: a Canadian multicentre experience. BJU Int. (2011) ;108: :539–45, doi:10.1111/j.1464-410X.2010.09912.x |
[11] | Grossman HB , et al Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. New England Journal of Medicine. (2003) ;349: :859–66, doi:10.1056/NEJMoa022148 |
[12] | Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. (1999) ;354: :533–40. |
[13] | Griffiths G , Hall R , Sylvester R , Raghavan D , Parmar MK . International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2011) ;29: :2171–7, doi:10.1200/jco.2010.32.3139 |
[14] | Paull KD , et al Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J Natl Cancer Inst. (1989) ;81: :1088–92, doi:10.1093/jnci/81.14.1088 |
[15] | Smith SC , Baras AS , Lee JK , Theodorescu D . The COXEN principle: translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in cancer. Cancer Research. (2010) ;70: :1753–8, doi:10.1158/0008-5472.CAN-09-3562 |
[16] | Lee JK , et al A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proceedings of the National Academy of Sciences. (2007) ;104: :13086–91, doi:10.1073/pnas.0610292104 |
[17] | Williams PD , et al Concordant Gene Expression Signatures Predict Clinical Outcomes of Cancer Patients Undergoing Systemic Therapy. Cancer Research. (2009) ;69: :8302–9, doi:10.1158/0008-5472.Can-09-0798 |
[18] | Flaig TW , et al SWOG S1314: A randomized phase II study of co-expression extrapolation (COXEN) with neoadjuvant chemotherapy for localized, muscle-invasive bladder cancer. Journal of Clinical Oncology. (2019) ;37: :4506, doi:10.1200/JCO.2019.37.15_suppl.4506 |
[19] | Stensland KD , et al Premature termination of genitourinary cancer clinical trials. Journal of Clinical Oncology. (2014) ;32: :288, doi:10.1200/jco.2014.32.4_suppl.288 |
[20] | Petrylak DP , et al EV- Results of enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer previously treated with platinum and immune checkpoint inhibitors. Journal of Clinical Oncology. (2019) ;37: :4505, doi:10.1200/JCO.2019.37.18_suppl.LBA4505 |
[21] | Siefker-Radtke AO , et al First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt). Journal of Clinical Oncology. (2018) ;36: :4503, doi:10.1200/JCO.2018.36.15_suppl.4503 |
[22] | Iyer G , et al Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2018) ;36: :1949–56, doi:10.1200/jco.2017.75.0158 |




