Histologic Variants of Urothelial Carcinoma: Morphology, Molecular Features and Clinical Implications
Abstract
Bladder cancer is a heterogeneous disease including conventional urothelial carcinoma (UC) and its histologic variants, and non-urothelial carcinoma, including squamous and glandular neoplasms. Urothelial carcinoma accounts for the majority of bladder cancer cases, but morphologic variants are common and include nested, microcystic, micropapillary, lymphoepithelioma-like, plasmacytoid, sarcomatoid, giant cell, undifferentiated, clear cell and lipoid. Certain variants of UC tend to be associated with a poor prognosis and have diagnostic and potential treatment implications that make the identification of variant histology crucial to clinical decision making. While there is still uncertainty regarding the prognostic implications of many of these variants, identifying and reporting variant histology is important to develop our understanding of their biology. Unique molecular features accompany many of these morphologic variants and to better understand these tumors, we review the molecular and clinical implications of histologic variants of bladder cancer. Major efforts are underway to include variant histology and divergent differentiation of UC in clinical trials to develop evidence based approaches to treatment. The purpose of this article is to review the current literature on variant histology of urothelial cancer and to highlight molecular findings and the clinical relevance of these tumors.
INTRODUCTION
Bladder cancer is the sixth most common cancer in the US with an estimated 80,470 new cases and 17,670 deaths in 2019 [1]. The incidence of bladder cancer is higher in men than women, with approximately three-quarters of all bladder cancer cases being diagnosed in men. UC may arise anywhere within the urinary tract, with the majority of tumors originating in the bladder and the rest occurring in the ureter, renal pelvis and proximal urethra. Risk factors for bladder cancer include tobacco use, occupational exposures and various chemicals, urinary tract infections, genetic factors, chronic inflammation, irradiation and pharmaceutical drug use.
Bladder cancer is divided into three clinical disease states - non-muscle invasive, muscle-invasive, and metastatic –each differing in tumor biology, clinical phenotype, management, and prognosis. In addition to the clinical disease states, bladder cancer demonstrates numerous histologic variants that also provide important insights into biology, phenotype, treatment and prognosis. In the United States, approximately 90% of urinary tract tumors are urothelial in origin, with 1–7% of tumors represented by primary squamous cell carcinoma and another 2% being primary bladder adenocarcinomas.
Of the 90% of urothelial-derived tumors, up to 33% of cystectomy specimens show some component of divergent differentiation [2]. Divergent differentiation includes squamous, glandular, small cell, trophoblastic and Mullerian features. Additionally, based on the 2016 WHO categorization, 10 histologic variants are recognized: nested including large nested, microcystic, micropapillary, lymphoepithelioma-like, plasmacytoid, sarcomatoid, giant cell, poorly differentiated, lipid-rich and clear cell [3]. Squamous cell neoplasms make up a separate category to include pure squamous cell carcinoma, but should be distinguished from urothelial carcinoma with squamous differentiation. Similarly, the category of glandular neoplasms includes primary adenocarcinoma, but does not include urothelial carcinoma with glandular differentiation. Urachal carcinoma is its own category and will be discussed briefly. Other WHO categories not covered in this review include tumors of Mullerian origin, melanocytic tumors, mesenchymal neoplasms, hematopoietic and lymphoid neoplasms. A separate WHO group of urothelial tumors is the “non-invasive urothelial neoplasms” which will not be discussed in this review.
This review will focus on the major histologic variants of bladder cancer including their molecular characterization and clinical implications.
HISTOLOGIC VARIANTS OF INFILTRATING UROTHELIAL CARCINOMA
Histologic variants are defined as distinctively different histomorphologic phenotypes of a particular neoplasm [4]. In addition to histomorphologic variation, a large body of literature is evolving around the diverse molecular subtypes of bladder cancer. Numerous groups were responsible for the first attempts at a molecular characterization schema for urothelial cancer; as a result, various classification terminology emerged [5–10]. Recently, an international group proposed a consensus molecular classification to standardize the use of these molecular groups in clinical trials. They identified six transcriptome-based consensus groups: luminal papillary, luminal nonspecified, luminal unstable, stroma-rich, basal/squamous and neuroendocrine-like. Subset analysis of histologic variants was incorporated and some variants were overrepresented in specific consensus classes [11]. Overall, the consensus classes were strongly associated with overall survival, with luminal papillary showing the best overall survival and used as the referent. Stroma-rich and luminal non-specified had similar outcomes to luminal papillary; whereas luminal unspecified had a moderately worse outcome. Basal/squamous tumors were associated with worse outcomes than luminal papillary and neuroendocrine-like had the overall worst prognosis.
When compared to conventional UC, some variant histologies of UC are associated with a poor prognosis, which may be related to more biologically aggressive disease and/or a poorer response to therapy. Early work on molecular subtypes shows that basal subtypes are associated with increased response to cisplatin-based neoadjuvant chemotherapy [12]. The presence of variant histology can influence the diagnosis, treatment and prognosis of a given patient; thus, it is critically important that any variant histology is accurately diagnosed and reported. It is important to note that the vast majority of cases with variant histology co-exist with conventional UC and do not usually arise in pure form. Below, we describe examples of histologic variants and discuss potential clinical implications.
Nested variant of urothelial carcinoma
Nested variant of urothelial carcinoma (NVUC) is a rare variant of urothelial carcinoma with a deceptively bland appearance and a reported incidence of 0.3% of invasive bladder tumors [13]. This variant’s low-grade cytology introduces a number of benign entities into the differential diagnosis including von Brunn nests, nephrogenic adenoma, or cystitis cystica. In addition to the small nested architecture, the diagnostic features include minimal cytologic atypia with mild pleomorphism and occasionally prominent nucleoli. The large nested variant shows similar bland cytology, but the nests are larger and may mimic inverted growth pattern of a non-invasive urothelial carcinoma [14]. In both small and large nested urothelial carcinoma, benign mimickers can be excluded if the urothelial nests invade the muscularis propria; however, the absence of invasion leads to diagnostic difficulties for the pathologist [15].
NVUC may be distinguished from benign urothelial processes by the presence of a TERT promoter mutation [16]. The finding of a TERT promoter mutation strongly supports a nested carcinoma, although a negative result does not entirely exclude the diagnosis. TERT mutation is not entirely specific, as they have also been identified in a subset of inverted papillomas, which have morphologic overlap with NVUC [17]. This variant’s transcriptional phenotype is most consistent with the luminal subtype, which is associated with better outcomes. This finding is supported by expression of FOXA1 and GATA3, with no expression of basal markers CK5/6 and CK14 [18]. Warrick et al. also showed nested variant emerging in the urothelial-like cluster [18].
Despite its innocuous morphology, NVUC has definite malignant behavior and often presents with locally advanced or metastatic disease [19, 20]. At the time of presentation, NVUC is usually at an advanced stage [15]. This may be due to under diagnosis of malignancy in a neoplasm with bland cytology, where a diagnosis of carcinoma is not made until muscle invasion is identified. However, patients with NVUC did not have worse outcomes or an increased rate of recurrence when stage matched to patients with conventional UC [21]. The optimal management for this variant has not yet been determined because NVUC is rare, and has not been the subject of prospective studies [22]. Further studies are needed to determine an effective multimodal approach for this variant; however, it should be considered a high-risk aggressive tumor and early cystectomy may be warranted [23, 24].
Microcystic urothelial carcinoma
The microcystic variant is another deceptively bland variant of UC characterized by microcysts, macrocysts, or tubular structures that range in size from microscopic to 2 cm in diameter and are characteristically round to oval [25, 26]. Tumors with microcystic histology represent 1.2% of bladder tumors and have a variable clinical course [27]. As seen in conventional UC, microcystic variant demonstrates GATA3, CK7, CK20, and p63 expression. Microcystic UC does express MUC5AC, which is a potential diagnostic problem as its expression is also seen in cystitis glandularis, a benign lesion with similar morphology. Microcystic UC may also be confused with bladder adenocarcinoma, as tubules and cysts mimic glandular structures [28].
The molecular characterization of this variant has not been well studied. Limited prognostic and treatment data exist for this rare variant, which also has morphologic overlap with the nested variant. There is no apparent difference in survival between microcystic carcinoma and conventional UC [28].
Micropapillary urothelial carcinoma
The micropapillary (MP) variant of urothelial carcinoma is a rare histologic variant characterized by multiple small nests of tumor cells lacking true fibrovascular cores. Multiple clusters of tumor cells are often present within a single lacunar space, resembling papillary serous carcinoma of the ovary. This variant accounts for 0.7–8% of bladder cancer, is seen predominantly in men and frequently co-exists with conventional urothelial carcinoma.
On a molecular level, MP is associated with downregulation of miRNA-296, which plays a crucial role in the regulation of the inflammatory response. This downregulation of miRNA is also observed as a late event of carcinogenesis and is associated with aggressive behavior. In addition, activation of the chromatin-remodeling complex RUVBL1 appears to drive the expression signature of MP and contribute to its development [29]. The activation of RUVBL1 is also associated with aggressive behavior in other cancers [30]. MP shows widespread dysregulation of its expression profile, which affects about 30% of the protein-coding genome. The change in the expression pattern affects different oncogenic pathways that are focused on transformation, cell cycle regulation, DNA damage repair, and signal transduction [29]. Therefore, RUVBL1 and miRNA-296 appear to play important roles in the pathogenesis of MP and may represent potential opportunities for targeted therapies.
HER2/ERBB2 overexpression and amplification is found at a higher rate in MP compared to conventional UC [31, 32]. In breast cancer, amplification or overexpression of HER2 is found in aggressive subtypes, including micropapillary. Despite the fact that HER2 amplification is associated with worse cancer-specific survival, it may also provide an opportunity for ERBB2-targeted therapy [33, 34]. The majority of MP cancers are of urothelial-like or luminal subtype, which is confirmed by the expression of FOXA1, a biomarker of luminal phenotype [18, 31]. A subset of luminal MP bladder cancers defined as being p53-like are the most aggressive variant of the disease, as they are associated with chemo-resistance to cisplatin-based neoadjuvant chemotherapy [29]. However, patients with luminal tumors tend to have better prognosis but may not respond that well to neoadjuvant cisplatin-based chemotherapy [12]. The consensus classification found an overrepresentation of micropapillary tumors in the luminal nonspecified category [35].
MP demonstrates aggressive clinical behavior with features including lymphovascular invasion (LVI), early lymph node metastases, and wide metastatic spread [29, 36]. Interestingly, MP histology is also associated with poor prognosis in cases of lung, breast, pancreas, colon/rectum, and salivary gland carcinoma with this histologic pattern [33]. In patients with micropapillary UC stage matched to patients with conventional urothelial carcinoma, one study showed no differences in recurrence or disease specific survival [37]. The presence of a moderate or extensive MP component is associated with a high risk of advanced stage at presentation, and early cystectomy in non-muscle invasive cases has been advocated in the past. However, more recent data has shown that patients with clinical non-muscle invasive (cT1) micropapillary urothelial carcinoma do not have adverse outcomes when managed conservatively [38]. A survey of the Society of Urologic Oncology members in 2014 showed no consensus on the treatment of MP, although the majority agreed it should be treated differently than conventional UC [39].
Intravesical BCG therapy appears to be ineffective in patients with non-muscle-invasive MP tumor. Radical cystectomy offers the best chance of cure in patients and many clinicians advocate for early radical cystectomy in patients with surgically resectable disease [40]. However, opinions on the management of MP are diverse among members of the Society of Urologic Oncology. There is currently no consensus on the incorporation of neoadjuvant chemotherapy with radical cystectomy based on this survey [41]. Conversely, a consensus panel of the European Association of Urology (EAU) and the European Society for Medical Oncology (ESMO) bladder cancer experts showed consensus (86% agreement) for treating high-grade pT1 MP with immediate radical cystectomy and lymphadenectomy [42]. The optimal treatment strategy for MP urothelial carcinoma requires more investigation.
Lymphoepithelioma-like carcinoma
The lymphoepithelioma-like carcinoma (LELC) variant is another rare variant of bladder cancer which resembles lymphoepithelioma of the nasopharynx, a tumor defined by both a prominent lymphoid infiltrate and ubiquitous Epstein-Barr Virus (EBV) positivity [43]. However, unlike lymphoepithelioma of the nasopharynx, LELC are universally EBV negative and designated as lymphoepithelioma-like [44]. LELC is characterized by undifferentiated, malignant epithelial cells with a syncytial appearance within a dense, mixed inflammatory infiltrate [45]. The cytoplasmic borders are poorly defined and the epithelial cells are often difficult to detect in the dense inflammation; they can be highlighted by expression of pan-keratin AE1/AE3, CK7, and GATA3 [46]. LELC cases often have p53 accumulation, which supports a similar pathogenesis to high grade UC [47]. LELC does not appear to show evidence of DNA mismatch repair protein deficiency, which suggests a microsatellite stable phenotype. PD-L1 expression is present in LELC, which creates a potential for the use of immunotherapy [48]. A case series detailing the RNA expression profiling of 14 LELC tumors showed a basal-like phenotype in 12 of the cases [48].
One group has proposed that LELC be classified into three different categories: pure, predominant, and focal [49]. There is limited case series data showing that pure and predominant LELC are associated with better outcomes and show better responses to chemotherapy than focal LELC [49–53]. More recently, a study of 30 cases of LELC showed that 5 year survival after cystectomy was equivalent in pure LELC when compared to mixed LELC and conventional UC (62% versus 57%) [54]. At the time of presentation, most LELCs have invaded the muscularis propria, but have not metastasized outside of the bladder. Larger prospective studies are warranted to determine if there is an optimal treatment regimen for these tumors based on amount of LELC component present.
Plasmacytoid urothelial carcinoma
The plasmacytoid variant of urothelial carcinoma is a rare and aggressive variant characterized by discohesive, single cells with eccentric nuclei that resemble plasma cells. The WHO also includes the terminology “signet ring cell” and “diffuse” for this entity. There is sufficient morphologic overlap with lymphoma, plasmacytoma, melanoma and metastatic carcinomas of breast and gastric origin that immunohistochemical workup is often necessary in the absence of a conventional urothelial carcinoma component. In addition to morphologic similarities to plasma cells, these tumors also express CD138, which is a diagnostic pitfall.
The molecular hallmark of plasmacytoid tumors is a nonsense mutation in CDH1, the gene coding for E-cadherin, a cellular adhesion molecule. Truncating CDH1 mutations were the only unique mutations identified in plasmacytoid variant when compared to other urothelial cancers, making them the defining molecular feature of the variant [55]. Mutations in CDH1 have been shown to lead to increased cellular migration and this may explain its propensity for peritoneal spread and ability to cross fascial planes. A biomarker for CDH1 mutation is loss of immunohistochemical expression of E-cadherin. In a study of molecular subtypes of histologic variants, plasmacytoid carcinoma was classified as urothelial-like, or luminal, with a subset of tumors in the genomically unstable groups [18].
Patients with plasmacytoid variant typically present at an advanced stage and have a high mortality rate [33]. Compared to patients with pure urothelial carcinomas, patients with the plasmacytoid variant are more likely to have nodal metastases and higher pT3/pT4 stage [56]. Some studies suggest that there is no difference in survival between patients with plasmacytoid variant treated with neoadjuvant chemotherapy plus surgery compared to surgery alone, with recommendations for early radical cystectomy whenever possible [56, 57]. Further studies addressing the unique biology of plasmacytoid variant, including novel treatment approaches are needed for this aggressive variant.
Sarcomatoid urothelial carcinoma
Sarcomatoid carcinoma of the urinary bladder is an uncommon malignancy that is composed of epithelial-derived malignant cells that may exhibit both epithelial and sarcomatoid morphology [58–60]. The sarcomatoid component may demonstrate non-specific malignant spindle cells, leiomyosarcoma-like, or other heterologous component such as osteosarcoma or chondrosarcoma [61]. These tumors are extremely rare and only represent 0.1% to 0.3% of all carcinomas [58]. The epithelial component is most commonly conventional urothelial carcinoma, but squamous cell carcinoma may also be present. The sarcomatoid components of the tumors tend to occupy more than 50% of the tumor area; however, it is possible for these tumors to lack any epithelial component, complicating the diagnosis. Immunohistochemistry for urothelial or epithelial markers (p63, GATA3, pan-keratin, cytokeratin 903, cytokeratin 7, and cytokeratin 5/6) may be useful in this setting to support a diagnosis of sarcomatoid carcinoma [62]. Although rare, sarcomatoid differentiation is more common than a primary sarcoma; therefore, sarcomatoid carcinoma should be considered in any tumor with sarcomatoid features.
The sarcomatoid divergence can be explained by loss of cell-to-cell and cell matrix adhesion, which enables the development of metastasis [61]. In a series of 28 cases of sarcomatoid carcinoma compared with conventional UC, sarcomatoid carcinomas showed relative increase in mutations in TP53, RB1 and PIK3CA [63]. The authors also showed that epithelial-mesenchymal transition (EMT) was either partial or complete, with the complete EMT showing a worse prognosis. The tumors with complete EMT showed an entirely mesenchymal phenotype (negative for epithelial markers), rather than a mixture of epithelial and mesenchymal components (focal retention of epithelial markers). An immunohistochemical analysis of 28 sarcomatoid carcinomas showed frequent expression of the epithelial-to-mesenchymal markers vimentin, FoxC2, SNAIL and ZEB1. The authors suggest the identification of these biomarkers may drive aggressive behavior [62]. Wang et al. found TERT C228T mutations in 35% of patients with sarcomatoid UC that resulted in mortality for all patients, indicating that the presence of TERT mutation may be indicative of poor prognosis [64]. In a cohort of variant histology tumors, sarcomatoid carcinoma clustered in both the basal-squamous and genomically unstable groups [18]. The consensus molecular classification found that sarcomatoid carcinomas were overrepresented in the basal/squamous group [35].
Patients with sarcomatoid carcinoma usually present with advanced stage and have worse disease specific and overall survival compared to conventional UC [65, 66]. These neoplasms should be considered high-grade carcinomas, but it is not clear whether they should be treated the same way as high-grade urothelial carcinoma. There is no optimal treatment for this variant of UC, as many patients develop metastasis after surgery [61]. However, a number of patients have experienced prolonged survival with the combination of radical cystectomy and radiation; the role of neoadjuvant chemotherapy is unclear [65].
Giant cell variant of urothelial carcinoma
Giant cell variant of urothelial carcinoma resembles giant cell carcinoma of the lung and pancreas, with bizarre pleomorphic cells [67, 68]. The giant cell component of the tumor varies from 20–100% and is usually admixed with conventional UC [69]. Lopez-Beltran et al. found that both conventional and giant cell UC were positive for CK7, CAM 5.2 and AE1/AE3, and epithelial membrane antigen by immunohistochemical staining. Appropriate immunohistochemical studies can be useful to distinguish giant cell UC from its mimickers, including secondary involvement of bladder by another primary carcinoma or pleomorphic sarcoma [69]. These tumors are exceptionally rare, and little is known about their molecular characterization. The universal clinical outcome for these aggressive tumors is poor.
Lipid-rich variant of urothelial carcinoma
The lipid-rich variant of urothelial carcinoma is extremely rare, with fewer than 40 cases reported [70]. Lipid-rich UC is characterized by eccentrically placed nuclei and clear cytoplasmic vacuoles resembling large lipoblasts or signet-ring cells [71]. Tumors with this morphology are usually comprised of 10–50% lipid-rich morphology mixed with either conventional UC or other variants of UC [70]. The majority of neoplastic cells have nuclei of intermediate nuclear grade with occasional pleomorphism [72]. Diffuse staining with cytokeratin AE1/AE3 supports an epithelial phenotype of the lipid cell component [67]. There is a need for pathologists to be aware of this rare variant and to be able to distinguish it from conventional UC, liposarcoma, signet-ring cell carcinoma, and metastatic carcinoma from other organs [71].
The behavior of lipid-rich morphology is not well understood, however it appears to be associated with a poor prognosis [70]. It tends to present as an advanced stage high-grade UC [72]. It is suggested that patients with aggressive variants of UC could benefit from early radical cystectomy [73].
Clear cell urothelial carcinoma
Clear cell UC is characterized by extensive areas of clear cells, demonstrates distinctive glycogen-rich cytoplasm and presence of extensive clear cell carcinoma in more than 30% of tumor cells [74, 75]. CK7 and CK20 expression in clear cell carcinomas makes it harder to differentiate between clear cell carcinoma and UC and suggests substantial immunoprofile overlap with UC [76, 77]. Positive uroplakin III immunostaining is present in 50% of clear cell UC cases [77]. PAX-8 is positive in clear cell carcinomas of renal or gynecologic origin and may be useful in the differential diagnosis. Due to the rarity of this tumor, little is known about its prognosis or optimal treatment.
Neuroendocrine carcinoma
Neuroendocrine tumors of the bladder include small cell carcinoma, large cell neuroendocrine carcinoma, well-differentiated neuroendocrine tumor and paraganglioma. Small cell and large cell neuroendocrine carcinomas may arise from urothelial neoplasms and frequently are admixed with conventional urothelial carcinoma or other variant histologies.
Small cell carcinoma comprises <1% of all bladder tumors, with large cell neuroendocrine carcinoma representing even fewer cases. Morphologically, small cell carcinoma shows small cells with high nuclear to cytoplasmic ratio, nuclear molding, abundant mitotic figures and necrosis. Large cell neuroendocrine carcinoma has larger cells with evident cytoplasm, finely stippled chromatin and prominent nucleoli. Both tumors express some combination of neuroendocrine biomarkers, e.g. synaptophysin, chromogranin and CD56. Small cell carcinomas are highly aggressive with over one-third (43.2%) of tumors in a series of 44 presenting as metastatic disease and a median overall survival of 1.7 years [78]. Additional biomarkers were identified in a study of 63 small cell bladder cancers, with DLL3, PD-L1, CD56 and ASCL1 differentially expressed by gene expression profiling and IHC. Multivariate analysis in this study showed that overall survival was shorter in patients with DLL3 and CD56 overexpression, and suggest a possible target for anti-DLL3 therapy [79].
In the consensus molecular classification, small cell and neuroendocrine carcinomas unsurprisingly fall into the neuroendocrine-like category [35]. The neuroendocrine-like group is characterized by TP53 and RB1 mutations. Almost three-quarters of the tumors within the neuroendocrine-like category were confirmed to have neuroendocrine morphology. A single-patient classifier was developed in a testing set of 175 cases of urothelial cancer, which identified a specific transcriptomic profile for neuroendocrine bladder cancer. This classifier was then tested on a cystectomy set and identified cases with transcriptome-level neuroendocrine features, which lacked specific neuroendocrine morphology (neuroendocrine-like tumors.) The neuroendocrine-like cases showed poor overall survival, consistent with the aggressive behavior of neuroendocrine carcinoma [80]. Validation of this neuroendocrine classifier in a subsequent study supported its ability to identify neuroendocrine-like tumors, with the need to manage these tumors similar to morphologically apparent neuroendocrine carcinomas [81].
The established therapeutic regimen for small cell carcinoma is platinum-based chemotherapy (carboplatin or cisplatin) with etoposide. However, a recent study showed increased overall survival and progression-free survival in patients treated with atezolizumab (Tecentriq, F. Hoffmann–La Roche/Genentech) plus carboplatin and etoposide [82].
Urothelial carcinoma with divergent differentiation
Squamous, glandular, trophoblastic and other morphologies are frequently identified in high-grade urothelial carcinomas and should be distinguished from pure squamous cell carcinomas and bladder adenocarcinomas, along with other malignancies. Any component of urothelial carcinoma, even surface involvement of urothelial carcinoma in situ, is sufficient to rule out a pure squamous cell carcinoma or adenocarcinoma. In the absence of any conventional urothelial component, a pure squamous cell carcinoma or adenocarcinoma should be considered. The percentage of squamous or glandular features may provide additional information regarding these risks, and should be reported.
Lopez-Beltran reported a series of tumors with histologic variants and found approximately 20% of tumors exhibited squamous features [67]. Multiple case series have demonstrated decreased response to therapy and increased risk of progression in urothelial carcinomas with squamous features [83–85]. Multiple studies have shown that squamous tumors show basal molecular expression profiles, which is not surprising given that basal markers and squamous markers overlap. However, the morphologic identification of squamous features did not always cluster with basal group and tumors in the basal group did not always have squamous features [86]. RNA expression analysis of tumors with both urothelial and squamous components showed divergent molecular subtypes in 25% of cases, indicating that morphologic variation indicates molecular variation [87]. A study of urothelial cancer variants and PD-L1 expression demonstrated high tumor cell staining in squamous differentiation when compared to other variants [88].
Glandular features are less commonly identified in urothelial carcinoma and usually present as intestinal type glands with mucinous secretion or malignant cells within pools of extracellular mucin. Similar to squamous features, a glandular component in urothelial carcinoma also portends worse prognosis [67, 89]. Despite these findings, mixed histologic features (including glandular or squamous differentiation) did not confer worse response with neoadjuvant MVAC chemotherapy in a secondary analysis of the Southwest Oncology Group study S8710 [90].
PRIMARY SQUAMOUS CELL CARCINOMA
Falling under the squamous cell neoplasms categorization, pure squamous cell carcinoma (SCC) represents 1–7% of all newly diagnosed bladder cancer cases in the United States [91]. It is important to note that pure SCC differs from UC with squamous cell features, which accounts for 40–60% of cases of urothelial carcinoma [92]. The diagnosis of squamous cell carcinoma is reserved for tumors that are solely composed of keratin-forming squamous cells, lacking any identifiable urothelial component in patients without a history of conventional urothelial carcinoma [93]. General risk factors for squamous cell carcinoma include smoking, urinary tract infections, schistosomiasis, and chronic irritation from catheterization. Histologic markers for the development of squamous cell carcinoma include keratinizing squamous metaplasia, squamous carcinoma in situ, and verrucous squamous hyperplasia [94].
Cyclooxygenase (COX)-2 is a protein that has been found to be important in carcinogenesis. COX-2 is undetectable in normal bladder tissue, but is expressed in SCC, which suggests that chronic inflammation leads to production of COX-2, and in turn, induces the development of SCC. Therefore, inhibiting the peroxidase activity of COX-2 may help reduce the incidence of SCC [95]. In addition, EGFR is a type I tyrosine kinase growth factor receptor that transduces signals controlling aspects like cell growth and differentiation [96]. The presence of EGFR outside of the urine is extremely rare. Guo et al. found expression of EGFR in all invasive squamous cell carcinomas (n = 16), which suggests that EGFR may play a role in this variant [93]. There may be potential for targeted therapeutics that inhibit EGFR signaling in squamous cell carcinoma.
Most squamous cell carcinomas present with advanced, muscle-invasive disease [93]. It is two times more likely that squamous cell carcinomas (84%) will present with advanced disease when compared to UC (42%) [85, 97]. Radical cystectomy provides better outcomes compared with radiation therapy and chemotherapy, although neoadjuvant radiation may benefit a number of patients with locally advanced disease. There is no clear role for neoadjuvant chemotherapy in primary squamous cell carcinoma. Preliminarily data are emerging that show neoadjuvant pembrolizumab has activity in squamous cell carcinoma of the bladder [98]. A better understanding of the morphological variations associated with SCC tumors is needed in order to develop a more concrete therapeutic approach [93].
PRIMARY BLADDER ADENOCARCINOMA
Included in the WHO category of glandular neoplasms, primary adenocarcinoma is an extremely rare bladder malignancy with an incidence of 0.5–2% [99]. Adenocarcinoma of the bladder is a tumor that is composed entirely of malignant glandular epithelium, without any conventional urothelial carcinoma present [100]. Differential diagnosis is extremely difficult on small biopsies with poorly differentiated tumors [101]. Risk factors include urinary bladder exstrophy, intestinal metaplasia resulting from chronic irritation and obstruction. Metastatic adenocarcinoma, especially from the colon and gynecologic sites, should be excluded before making a diagnosis of primary bladder adenocarcinoma. Full work-up can be achieved by extensive clinical, endoscopic and radiologic evaluation [101].
There is a range of morphologic appearances in adenocarcinoma of the bladder. The enteric type adenocarcinoma displays similar histology as colorectal adenocarcinoma. This pattern is composed of intestinal-type glands with pseudostratified columnar cells and cellular atypia. Intracellular or extracellular mucin is often present. Mucinous adenocarcinoma is characterized by abundant extracellular mucin with floating carcinoma cells. Mixed adenocarcinomas are comprised of a mix of more than one pattern of growth [102]. The presence of signet ring cells has been linked to a worse prognosis [89].
Limited data exist on the molecular profile of bladder adenocarcinoma. A molecular analysis of 15 primary bladder adenocarcinomas identified alterations in numerous genes within the MAPK, mTOR, Wnt, and Tp53/Rb1 pathways, with TP53, PIK3CA and KRAS being the most frequently mutated genes [103]. Adenocarcinoma is less likely to demonstrate high tumor mutation burden when compared to conventional UC [98]. These molecular findings show a close genetic relationship to colorectal adenocarcinomas and suggest options for possible targeted therapy trials.
Principles for chemotherapy are derived from the management of mucinous adenocarcinomas arising in other sites, most notably the colon using fluoropyrimidine-based regimens. There is a need for more effective chemotherapy for these carcinomas [104].
URACHAL CARCINOMA
Urachal carcinomas represent a subset of primary adenocarcinomas of the bladder, but are designated separately in the WHO classification [105]. These tumors may consist of mucinous, enteric, not otherwise specified, and signet ring cell types [106]. Urachal carcinomas arise from the urachal remnant and involve the dome of the bladder. For patients with surgically resectable disease, a partial cystectomy with en-bloc resection of the urachal ligament with the bladder dome and umbilicus and lymph node dissection is performed.
Table 1
Summary of Urothelial Variants and Their Key Diagnostic, Molecular and Clinical Features
| WHO Recognized Urothelial Variant Histology | Diagnostic Features | Molecular Features | Clinical Features |
Nested, including large nested 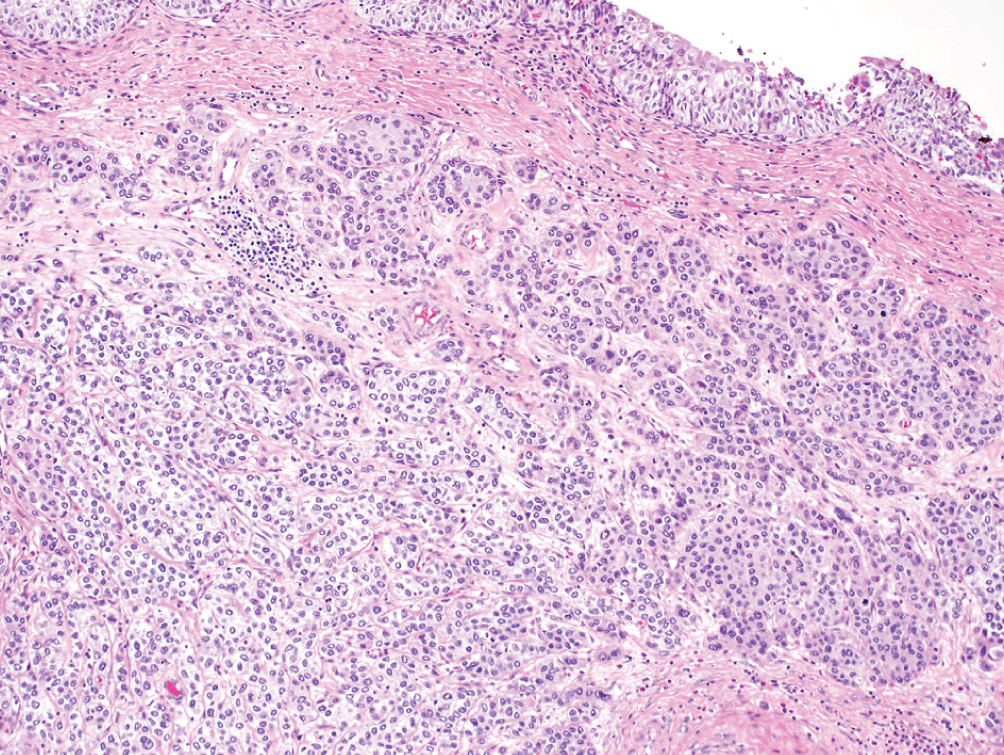 | Small or large nests of “low-grade” appearing urothelial cells with irregular infiltrating pattern | TERT promoter mutations; Luminal or urothelial-like molecular subtype | Often present at higher stage; however, stage for stage prognosis is similar to conventional urothelial carcinoma |
Microcystic 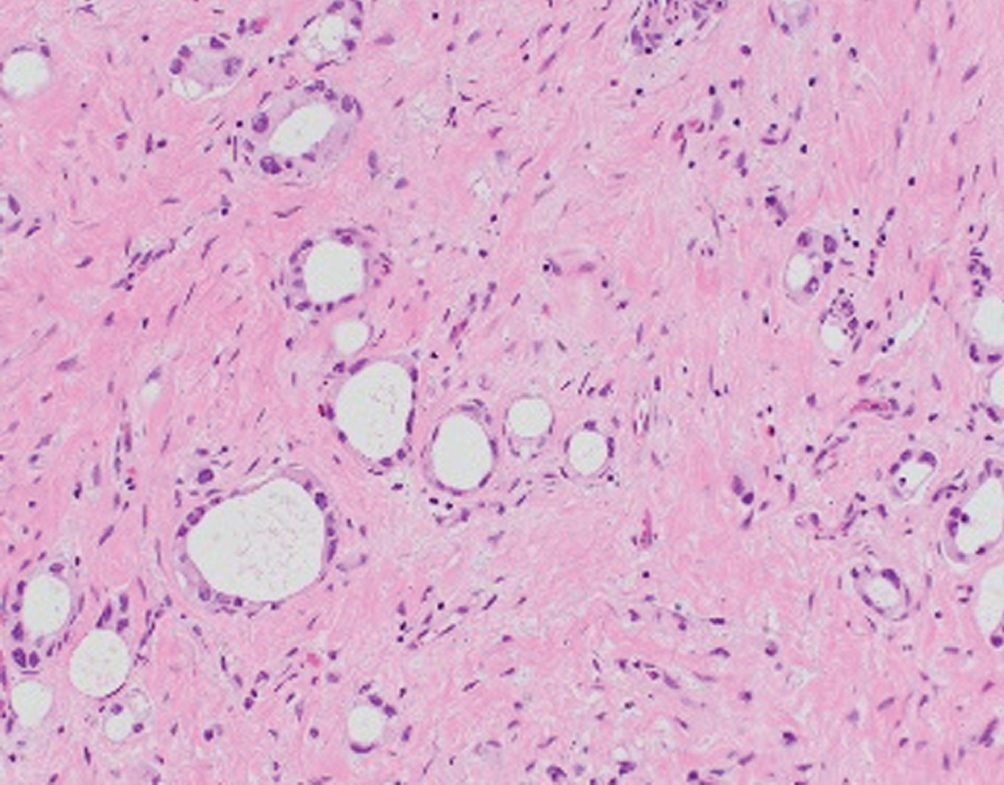 | Round micro- or macrocysts with thin lining of low-grade urothelial cells, irregular infiltrating pattern; may be related to nested pattern | Unknown | Often presents at higher stage; behaves like conventional urothelial carcinoma |
Micropapillary 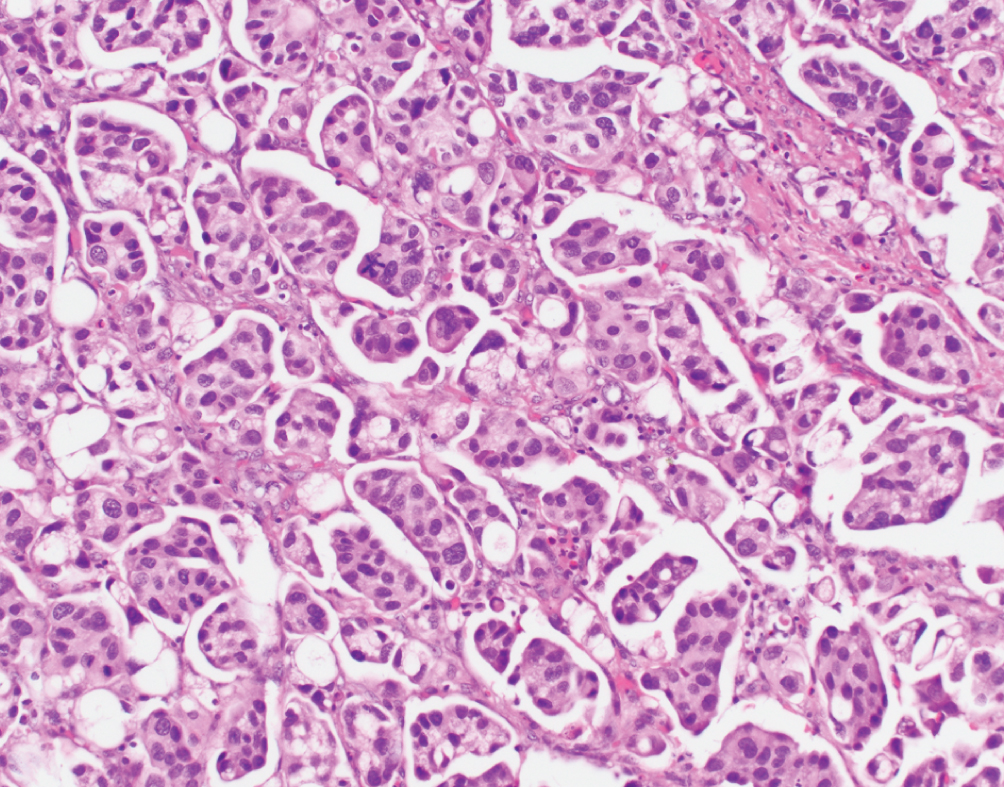 | Nests of tumor without fibrovascular cores, multiple nests within single lacunar space, reverse polarity of nuclei | Downregulation of miRNA-296, activation of RUVBL1, overexpression of HER2; Luminal nonspecified molecular subtype | Aggressive disease; lymphovascular invasion. Stage for stage similar prognosis to conventional UC. Possible HER2 targeted therapy. No consensus on optimal treatment. |
Lymphoepithelioma-like 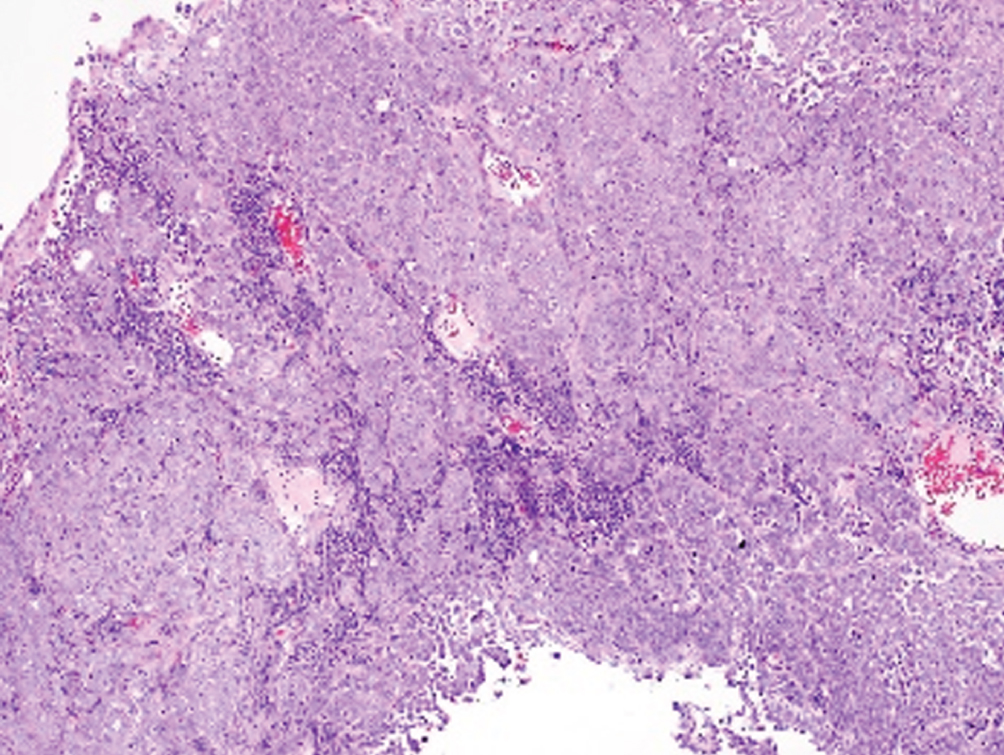 | Undifferentiated syncytial growth of epithelial cells within dense mixed inflammatory cell infiltrate; EBV negative | Basal-like molecular subtype | Older data showing pure/predominant LELC may have better response to chemotherapy; no consensus on optimal treatment |
Plasmacytoid/signet ring cell/diffuse 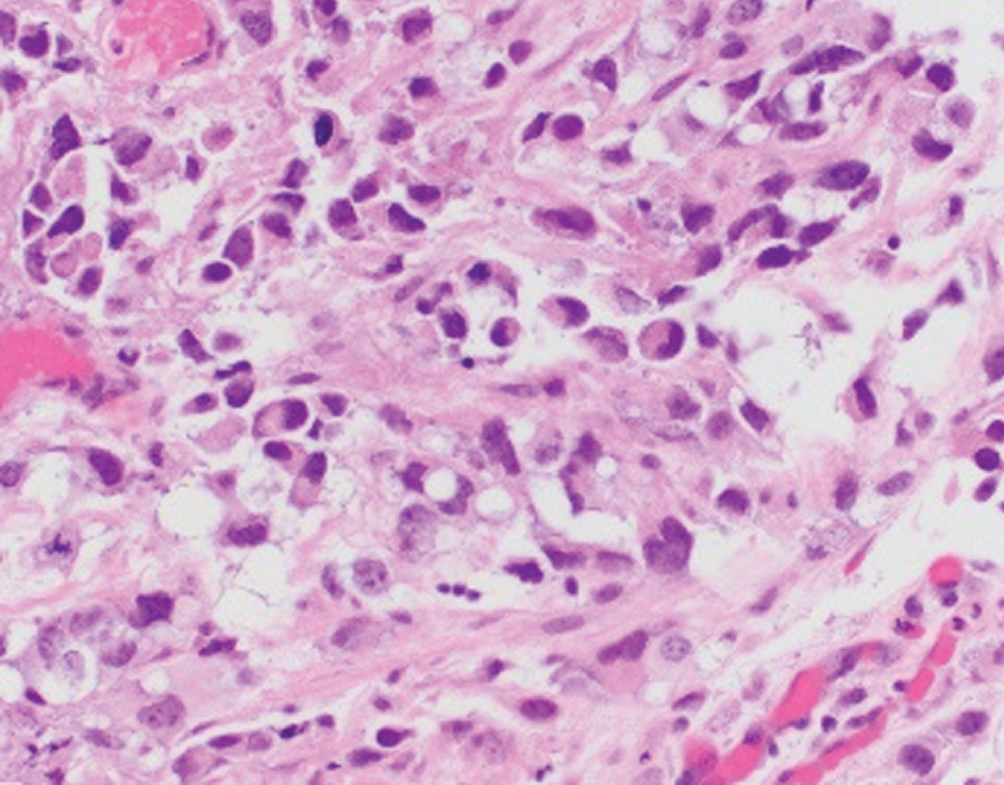 | Single discohesive cells infiltrating within myxoid background; morphologic overlap with plasma cells. Differential diagnosis includes plasmacytoma, melanoma, metastasis from GI or breast | Nonsense mutation in CDH1, e-cadherin loss by immunohistochemistry. Urothelial-like molecular subtype | Aggressive disease with frequent perivesical and peritoneal involvement; higher rates of recurrence when compared to conventional urothelial carcinoma |
Sarcomatoid 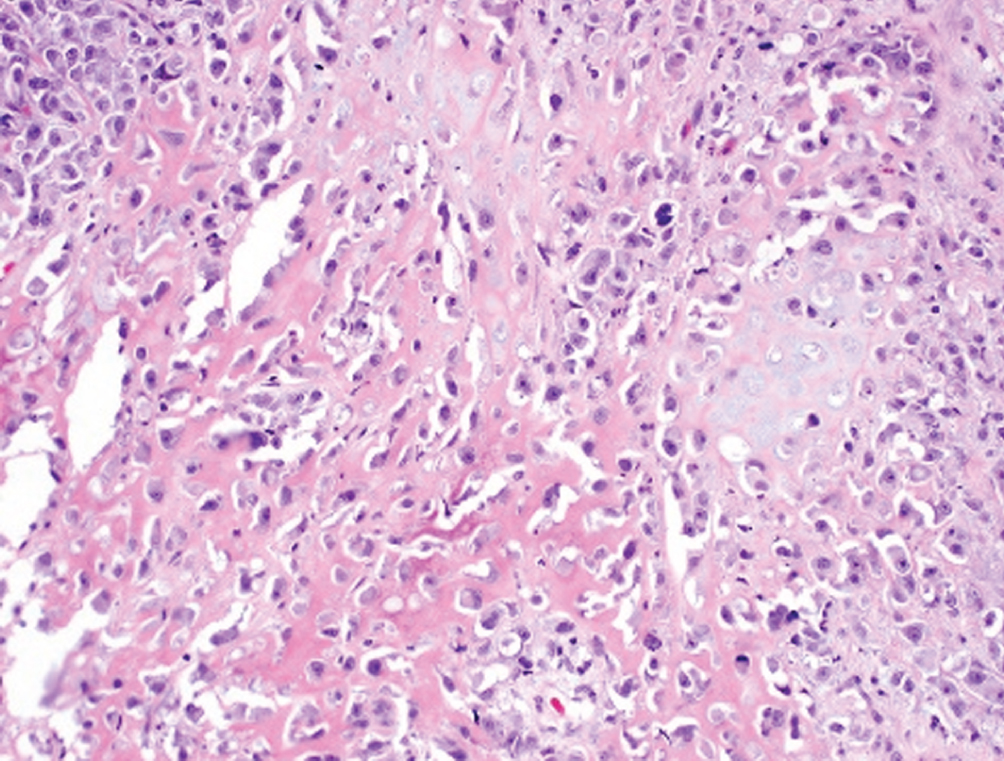 | Biphasic malignant epithelioid and mesenchymal neoplasm; may have heterologous sarcomatous elements (osteosarcoma, chondrosarcoma) | Basal-squamous subtype | Aggressive, often presents with nodal or visceral metastatic disease. Cystectomy recommended. Heterologous elements may impart worse prognosis. |
Giant cell 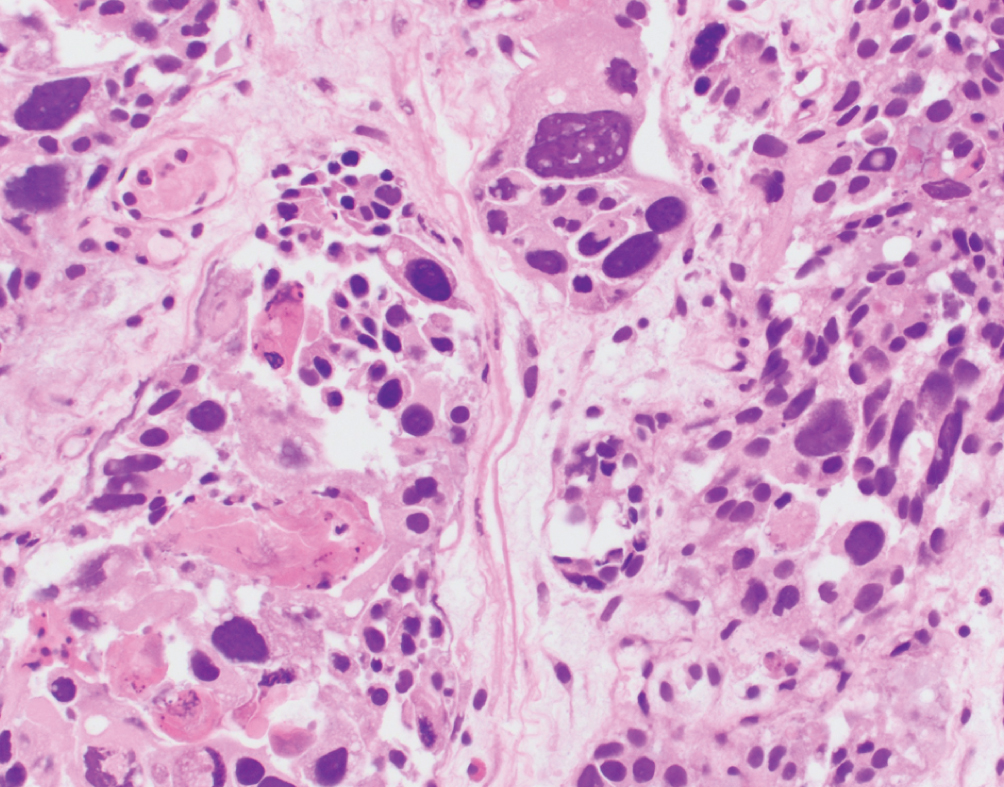 | Pleomorphic giant cells often admixed with poorly differentiated urothelial carcinoma; more common in renal pelvis | Unknown | Highly aggressive with uniformly poor outcomes |
Lipid-rich 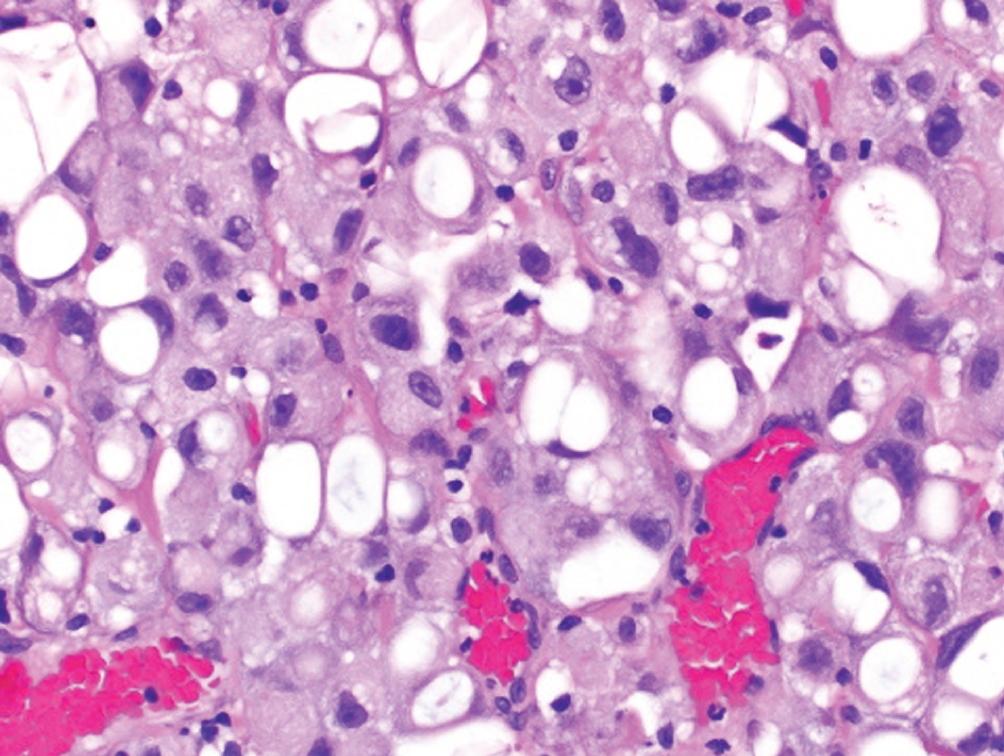 | Urothelial cells with numerous cytoplasmic vacuoles which indent the nucleus resulting in a “lipoblast-like” appearance | Unknown | Presents at higher stage with high mortality |
Clear cell 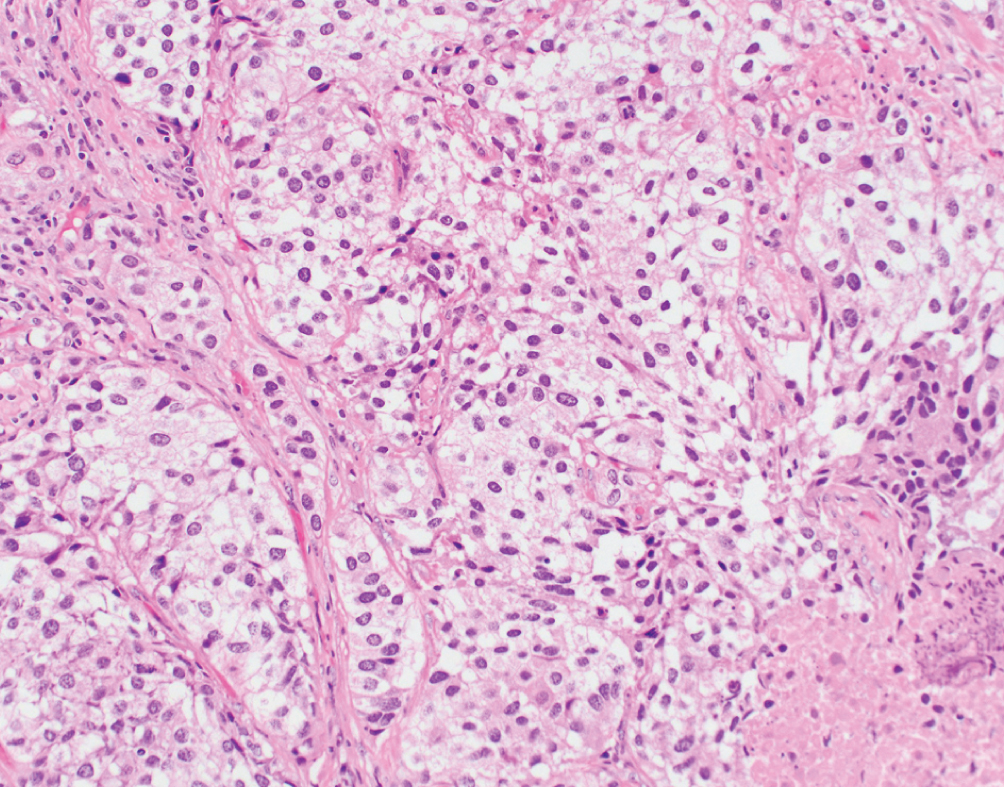 | Voluminous, clear glycogen-rich cytoplasm; differential diagnosis includes clear cell adenocarcinoma | Unknown | Rare variant, prognosis uncertain. Radical surgery with adjuvant chemotherapy suggested |
Neuroendocrine Carcinoma 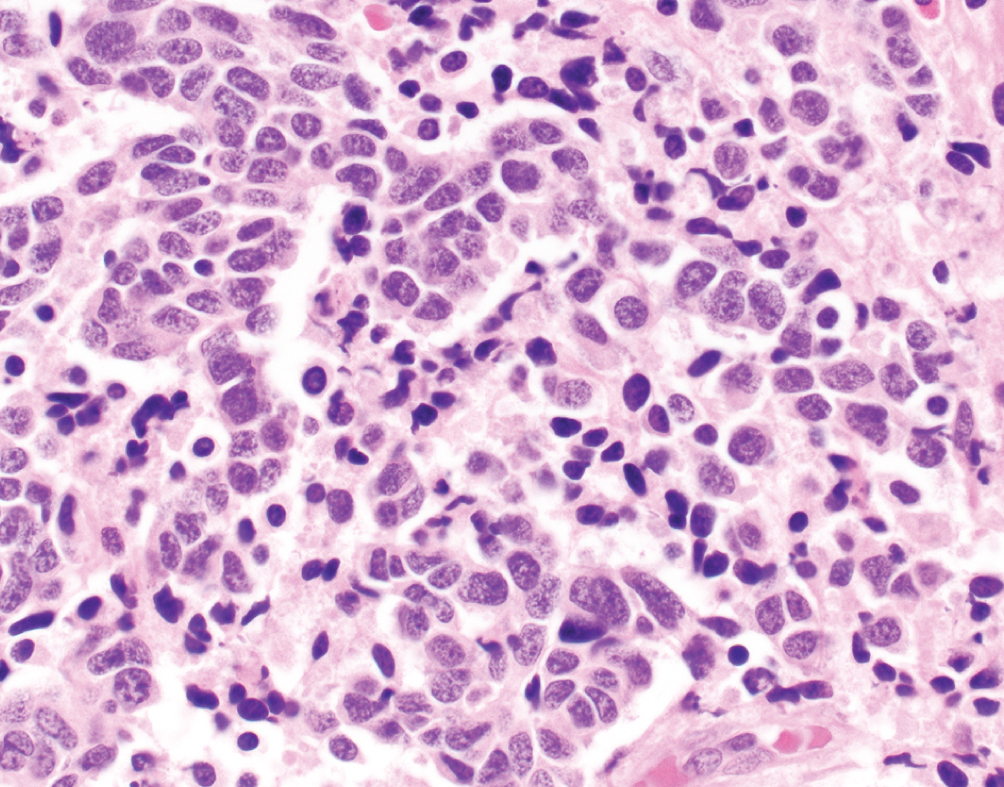 | Small cell carcinoma demonstrates high nuclear to cytoplasmic ratio with nuclear molding, frequent mitotic figures and necrosis | Neuroendocrine-like group, TP53 and RB1 mutations | Aggressive disease often presenting with metastatic disease; platinum-based chemotherapy with etoposide; atezolizumab in trials |
Urothelial Carcinoma with Squamous Features 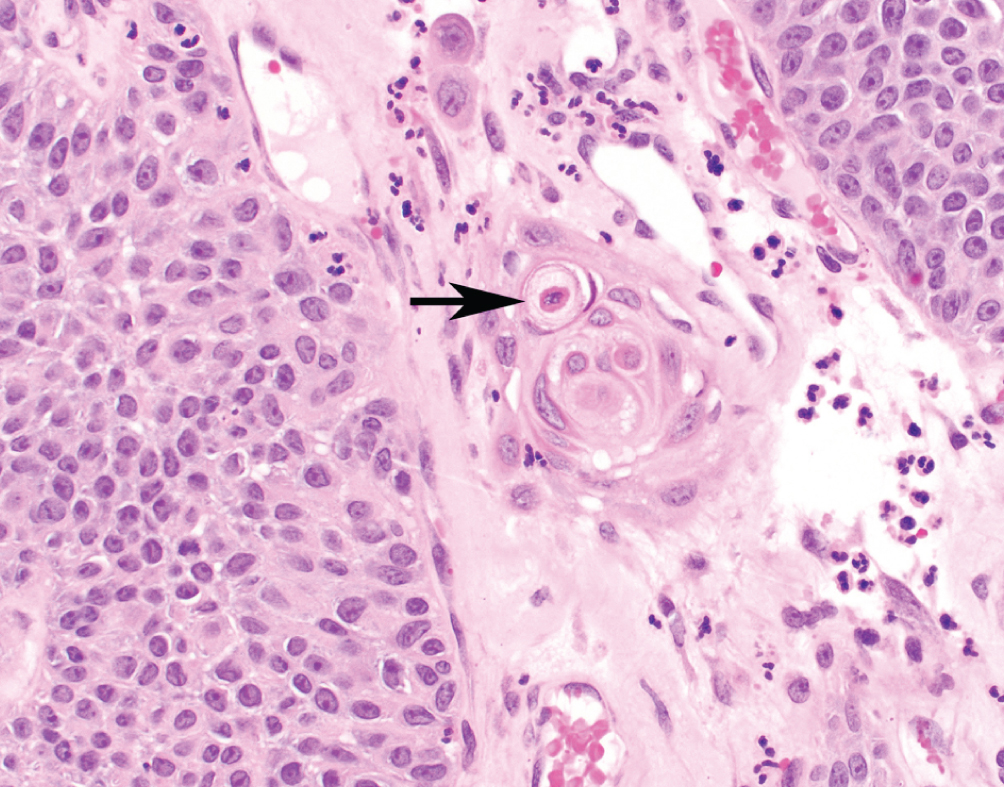 | Keratinizing cells (present at arrow) or intracellular bridges consistent with squamous derivation. Must be admixed with conventional urothelial carcinoma | Basal-squamous subtype; high PD-L1 expression | Worse prognosis than conventional urothelial carcinoma without squamous features. Increasing amounts of squamous features may drive behavior |
Urothelial Carcinoma with Glandular Features 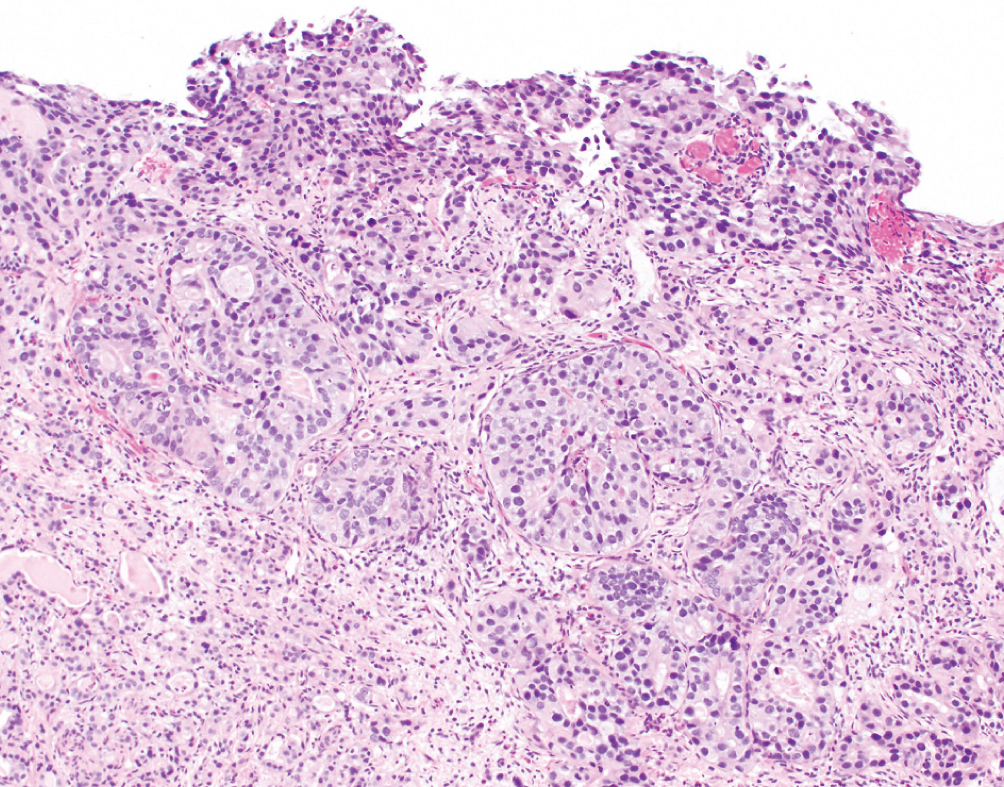 | Intestinal type glands with mucinous secretions or extracellular mucin containing malignant cells. Must be admixed with conventional urothelial carcinoma. | Unknown | Worse prognosis than conventional urothelial carcinoma. Increasing amounts of glandular features may drive behavior. Fluoropyrimidine-based chemotherapy often used for advanced disease. No clear role for neoadjuvant chemotherapy |
Primary Squamous Cell Carcinoma 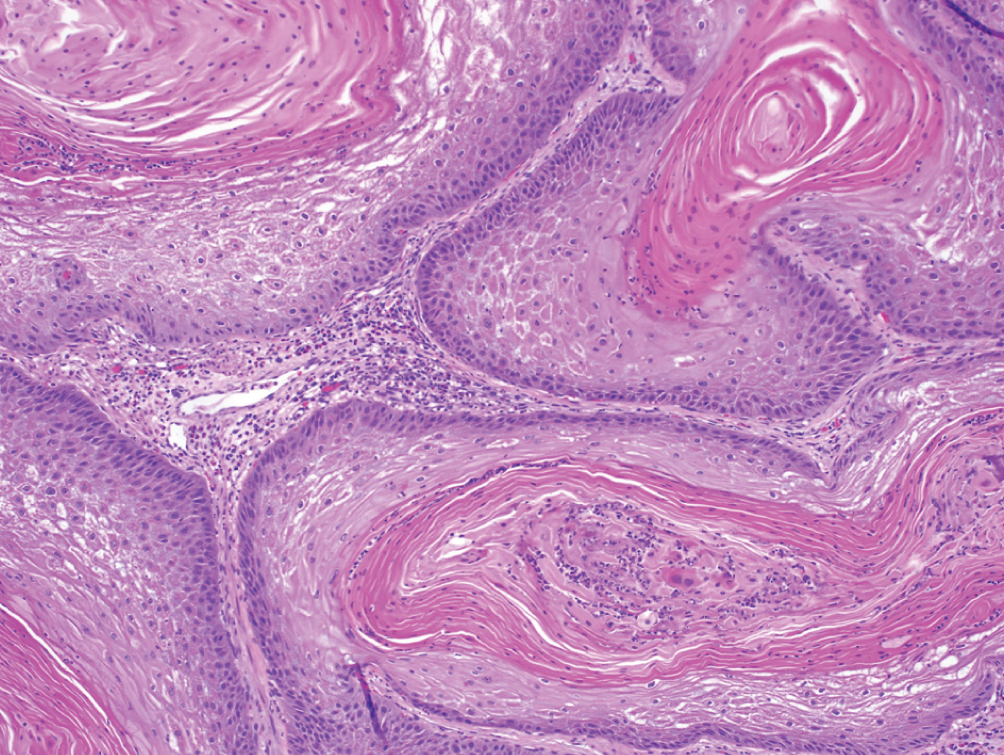 | Exclusively composed of keratinizing squamous cell carcinoma without conventional urothelial carcinoma. Must exclude secondary involvement by gynecologic squamous cell in women. | EGFR expression; high PD-L1 expression | Presents at locally advanced stage, metastatic presentation rare. Cystectomy may lead to better outcomes than radiation or chemotherapy. No clear role for neoadjuvant chemotherapy |
Primary Adenocarcinoma 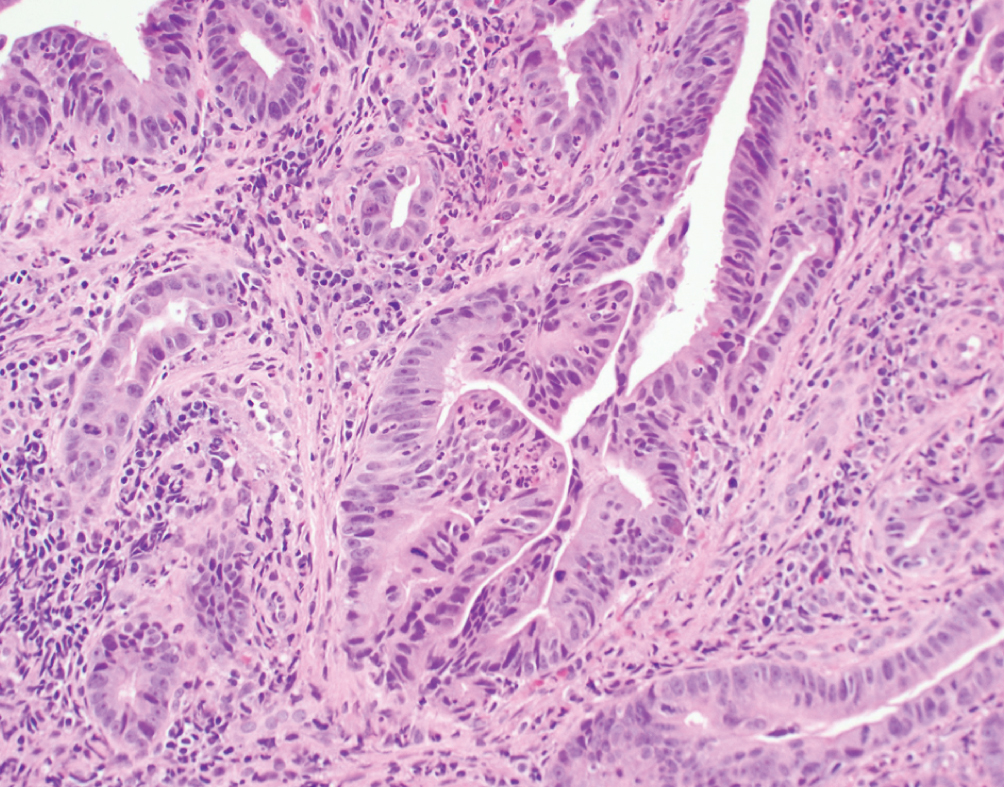 | Exclusively composed of malignant glandular neoplasm without conventional urothelial carcinoma. Primary bladder adenocarcinoma is a diagnosis of exclusion once all other potential primary sites have been excluded | TP53, PIK3CA and KRAS mutations | Clinically aggressive presenting at advanced stage with nodal metastasis. No established gold standard for therapy, typically use standard adenocarcinoma chemotherapy regimens (fluoropyrimidine-based) |
Next-generation sequencing was performed on 70 urachal carcinomas and showed mutations in TP53, KRAS, BRAF and PIK3CA, which are commonly mutated in colon cancer [107]. In a series of 12 urachal adenocarcinomas analyzed by targeted exon sequencing and transcriptome profiling, investigators found that urachal adenocarcinoma closely resembles colorectal cancer with a subset of cases showing a microsatellite unstable phenotype. A single patient in this series underwent treatment with immune checkpoint blockade, resulting in stabilization of their metastatic disease [107–109].
Although there is no standard chemotherapy, 5-fluorouracil-based chemotherapy regimens are generally used. Based on the mutational profile with mutations in the RAS pathway, anti-EGFR therapy may be effective in these tumors. The possibility of including immune checkpoint inhibitors is also promising for this rare disease and a clinical trial of chemotherapy (fluorouracil, leucovorin calcium, gemcitabine, and cisplatin) is underway to evaluate efficacy in adenocarcinoma of the bladder (NCT00082706); this study is no longer recruiting patients, however.
CLINICAL TREATMENT IMPLICATIONS
Evidence based guidance for clinical treatment of histologic variants of UC is lacking, given that variants have largely been excluded from clinical trials in the past. Recently, an expert panel from a FDA/NCI workshop on eligibility for bladder cancer adjuvant clinical trials concluded that patients with UC with predominant (≥50%) urothelial carcinoma with a component of variant histology may be enrolled in adjuvant trials [110]. If sufficient numbers of patients are enrolled with specific histologic variants, subset analyses should be performed; however, pure non-UC tumor such as small cell carcinomas should be analyzed separately. The selection of≥50% (predominant) urothelial histology component is arbitrary but a reasonable consensus-based cut off point; however, the impact of the specific non-urothelial histology proportion on tumor biology, treatment response and clinical trial outcomes is still unclear.
A recently launched and important National Clinical Trials Network (NCTN) Alliance phase 2 trial A031702 (ICONIC) is evaluating a novel combination regimen (ipilimumab, cabozantinib and nivolumab) in rare genitourinary cancers comprising unusual bladder tumors. These tumors will include adenocarcinoma, squamous cell carcinoma and small cell carcinoma, as well as variants of urothelial carcinoma including plasmacytoid, sarcomatoid and others (NCT03866382).
CONCLUSION
Variant and divergent histology is common in bladder cancer and must be recognized, quantified and reported accurately by an expert pathologist. There is still uncertainty regarding the biological, predictive, prognostic and treatment implications of UC histologic variants. Therefore, the identification and consistent reporting of variant histology in UC is essential. Additional dedicated prospective clinical trials with defined criteria and endpoints, translational research, registries, databases and biorepositories are needed to better define treatment strategies and biomarkers for patients with distinct histologic variants of urinary tract cancer.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
MA: Performance and interpretation of data; PG: Interpretation of data; MM: Conception, and interpretation of data; SW: Conception, performance and interpretation of data.
CONFLICT OF INTEREST
PG: https://coi.asco.org/share/AM6-Y2LD/Petros%20Grivas
MM: https://coi.asco.org/share/7UQ-6ARQ/Matthew%20Milowsky
SW: https://coi.asco.org/share/TZY-BL52/Sara%20Wobker
MA has no conflict of interest to declare.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. (2019) ;69: (1):7–34. |
[2] | Humphrey PA , Moch H , Cubilla AL , Ulbright TM , Reuter VE . The WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. (2016) ;70: (1):106–19. |
[3] | Moch H , Cubilla AL , Humphrey PA , Reuter VE , Ulbright TM . The WHO Classification of Tumours of the Urinary System and Male Genital Organs Part A: Renal, Penile, and Testicular Tumours. European Urology. (2016) ;70: (1):93–105. |
[4] | Amin MB . Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Modern Pathology. (2009) ;22: (2):S96–S118. |
[5] | Mo Q , Nikolos F , Chen F , Tramel Z , Lee YC , Hayashi K , et al. Prognostic Power of a Tumor Differentiation Gene Signature for Bladder Urothelial Carcinomas. J Natl Cancer Inst. (2018) ;110: (5):448–59. |
[6] | Damrauer JS , Hoadley KA , Chism DD , Fan C , Tiganelli CJ , Wobker SE , et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. (2014) ;111: (8):3110–5. |
[7] | Choi W , Porten S , Kim S , Willis D , Plimack ER , Hoffman-Censits J , et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. (2014) ;25: (2):152–65. |
[8] | Robertson AG , Kim J , Al-Ahmadie H , Bellmunt J , Guo G , Cherniack AD , et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. (2018) ;174: (4):1033. |
[9] | Rebouissou S , Bernard-Pierrot I , de Reyniès A , Lepage M-L , Krucker C , Chapeaublanc E , et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Science Translational Medicine. (2014) ;6: (244):244ra91. |
[10] | Marzouka N-a-d , Eriksson P , Rovira C , Liedberg F , Sjödahl G , Höglund M . A validation and extended description of the Lund taxonomy for urothelial carcinoma using the TCGA cohort. Scientific Reports. (2018) ;8: (1):3737. |
[11] | Kamoun A , de Reyniès A , Allory Y , Sjödahl G , Robertson AG , Seiler R , et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. European Urology. |
[12] | Seiler R , Ashab HAD , Erho N , van Rhijn BWG , Winters B , Douglas J , et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. European Urology. (2017) ;72: (4):544–54. |
[13] | Dhall D , Al-Ahmadie H , Olgac S . Nested Variant of Urothelial Carcinoma. Archives of Pathology & Laboratory Medicine. (2007) ;131: (11):1725–7. |
[14] | Cox R , Epstein JI . Large Nested Variant of Urothelial Carcinoma: 23 Cases Mimicking von Brunn Nests and Inverted Growth Pattern of Noninvasive Papillary Urothelial Carcinoma. The American Journal of Surgical Pathology. (2011) ;35: (9):1337–42. |
[15] | Venyo AK-G . Nested Variant of Urothelial Carcinoma. Advances in Urology. (2014) ;2014: :24. |
[16] | Zhong M , Tian W , Zhuge J , Zheng X , Huang T , Cai D , et al. Distinguishing nested variants of urothelial carcinoma from benign mimickers by TERT promoter mutation. Am J Surg Pathol. (2015) ;39: (1):127–31. |
[17] | Cheng L , Davidson DD , Wang M , Lopez-Beltran A , Montironi R , Wang L , et al. Telomerase reverse transcriptase (TERT) promoter mutation analysis of benign, malignant and reactive urothelial lesions reveals a subpopulation of inverted papilloma with immortalizing genetic change. Histopathology. (2016) ;69: (1):107–13. |
[18] | Warrick JI , Sjödahl G , Kaag M , Raman JD , Merrill S , Shuman L , et al. Intratumoral Heterogeneity of Bladder Cancer by Molecular Subtypes and Histologic Variants. European Urology. (2019) ;75: (1):18–22. |
[19] | Drew PA , Furman J , Civantos F , Murphy WM . The nested variant of transitional cell carcinoma: an aggressive neoplasm with innocuous histology. Mod Pathol. (1996) ;9: (10):989–94. |
[20] | Sten Holmäng SLJ . The Nested Variant of Transitional Cell Carcinoma - A Rare Neoplasm with Poor Prognosis. Scandinavian Journal of Urology and Nephrology. (2001) ;35: (2):102–5. |
[21] | Linder BJ , Frank I , Cheville JC , Thompson RH , Thapa P , Tarrell RF , et al. Outcomes Following Radical Cystectomy for Nested Variant of Urothelial Carcinoma: A Matched Cohort Analysis. The Journal of Urology. (2013) ;189: (5):1670–5. |
[22] | Venyo AK-G . Nested variant of urothelial carcinoma. Advances in urology. (2014) ;2014: :192720. |
[23] | Lin O , Cardillo M , Dalbagni G , Linkov I , Hutchinson B , Reuter VE . Nested Variant of Urothelial Carcinoma: A Clinicopathologic and Immunohistochemical Study of 12 Cases. Modern Pathology. (2003) ;16: (12):1289–98. |
[24] | Wasco MJ , Daignault S , Bradley D , Shah RB . Nested variant of urothelial carcinoma: a clinicopathologic and immunohistochemical study of 30 pure and mixed cases. Human Pathology. (2010) ;41: (2):163–71. |
[25] | Young RH , Zukerberg LR . Microcystic Transitional Cell Carcinomas of the Urinary Bladder: A Report of Four Cases. American Journal of Clinical Pathology. (1991) ;96: (5):635–9. |
[26] | Young RH , Eble JN . Unusual forms of carcinoma of the urinary bladder. Human Pathology. (1991) ;22: (10):948–65. |
[27] | Paz A , Rath-Wolfson L , Lask D , Koren R , Manes A , Mukamel E , et al. The clinical and histological features of transitional cell carcinoma of the bladder with microcysts: analysis of 12 cases. Br J Urol. (1997) ;79: (5):722–5. |
[28] | Lopez Beltran A , Montironi R , Cheng L . Microcystic urothelial carcinoma: morphology, immunohistochemistry and clinical behaviour. Histopathology. (2014) ;64: (6):872–9. |
[29] | Guo CC , Dadhania V , Zhang L , Majewski T , Bondaruk J , Sykulski M , et al. Gene Expression Profile of the Clinically Aggressive Micropapillary Variant of Bladder Cancer. European Urology. (2016) ;70: (4):611–20. |
[30] | Taniuchi K , Furihata M , Iwasaki S , Tanaka K , Shimizu T , Saito M , et al. RUVBL1 directly binds actin filaments and induces formation of cell protrusions to promote pancreatic cancer cell invasion. Int J Oncol. (2014) ;44: (6):1945–54. |
[31] | Zinnall U , Weyerer V , Compérat E , Camparo P , Gaisa NT , Knuechel-Clarke R , et al. Micropapillary urothelial carcinoma: evaluation of HER2 status and immunohistochemical characterization of the molecular subtype. Human Pathology. (2018) ;80: :55–64. |
[32] | Ching CB , Amin MB , Tubbs RR , Elson P , Platt E , Dreicer R , et al. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: analysis by dual-color in situ hybridization. Modern Pathology. (2011) ;24: (8):1111–9. |
[33] | Lopez-Beltran A , Henriques V , Montironi R , Cimadamore A , Raspollini MR , Cheng L . Variants and new entities of bladder cancer. Histopathology. (2019) ;74: (1):77–96. |
[34] | Schneider SA , Sukov WR , Frank I , Boorjian SA , Costello BA , Tarrell RF , et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Modern Pathology. (2014) ;27: (5):758–64. |
[35] | Kamoun A , de Reyniès A , Allory Y , Sjödahl G , Robertson AG , Seiler R , et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. European Urology. 2019 Sep 26. pii:S0302-2838(19)30695-5. |
[36] | Mendiratta P , Barata PC , Koshkin VS , Belcher A , McKenney J , Schach K , et al. Clinicopathologic factors, treatment patterns, and outcomes in micropapillary urothelial carcinoma (UC). Journal of Clinical Oncology. (2018) ;36: (6_suppl):439. |
[37] | Wang JK , Boorjian SA , Cheville JC , Kim SP , Tarrell RF , Thapa P , et al. Outcomes following radical cystectomy for micropapillary bladder cancer versus pure urothelial carcinoma: a matched cohort analysis. World Journal of Urology. (2012) ;30: (6):801–6. |
[38] | Spaliviero M , Dalbagni G , Bochner BH , Poon BY , Huang H , Al-Ahmadie HA , et al. Clinical outcome of patients with T1 micropapillary urothelial carcinoma of the bladder. The Journal of urology. (2014) ;192: (3):702–7. |
[39] | Willis DL , Flaig TW , Hansel DE , Milowsky MI , Grubb RL , Al-Ahmadie HA , et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urologic oncology. (2014) ;32: (6):826–32. |
[40] | Kamat AM , Dinney CPN , Gee JR , Grossman HB , Siefker-Radtke AO , Tamboli P , et al. Micropapillary bladder cancer. Cancer. (2007) ;110: (1):62–7. |
[41] | Willis DL , Flaig TW , Hansel DE , Milowsky MI , Grubb RL , Al-Ahmadie HA , et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol Oncol. (2014) ;32: (6):826–32. |
[42] | Witjes JA , Babjuk M , Bellmunt J , Bruins HM , De Reijke TM , De Santis M , et al. EAU-ESMO Consensus Statements on the Management of Advanced and Variant Bladder Cancer—An International Collaborative Multistakeholder Effort: Under the Auspices of the EAU-ESMO Guidelines Committees. Eur Urol. 2019 Nov 16. pii:S0302-2838(19)30763-8. |
[43] | Yang AW , Pooli A , Lele SM , Kim IW , Davies JD , LaGrange CA . Lymphoepithelioma-like, a variant of urothelial carcinoma of the urinary bladder: a case report and systematic review for optimal treatment modality for disease-free survival. BMC Urology. (2017) ;17: (1):34. |
[44] | Gulley ML , Amin MB , Nicholls JM , Banks PM , Ayala AG , Srigley JR , et al. Epstein-barr virus is detected in undifferentiated nasopharyngeal carcinoma but not in lymphoepitheliomalike carcinoma of the urinary bladder. Human Pathology. (1995) ;26: (11):1207–14. |
[45] | Raphael V , Jitani AK , Sailo SL , Vakha M . Lymphoepithelioma-like carcinoma of the urinary bladder: A rare case report. Urol Ann. (2015) ;7: (4):516–9. |
[46] | Lopez-Beltran A , Paner G , Blanca A , Montironi R , Tsuzuki T , Nagashima Y , et al. Lymphoepithelioma-like carcinoma of the upper urinary tract. Virchows Archiv. (2017) ;470: (6):703–9. |
[47] | Williamson SR , Zhang S , Lopez-Beltran A , Shah RB , Montironi R , Tan PH , et al. Lymphoepithelioma-like carcinoma of the urinary bladder: clinicopathologic, immunohistochemical, and molecular features. The American journal of surgical pathology. (2011) ;35: (4):474–83. |
[48] | Manocha U , Kardos J , Selitsky S , Zhou M , Johnson SM , Breslauer C , et al. RNA Expression Profiling of Lymphoepithelioma-Like Carcinoma of the Bladder Reveals a Basal-like Molecular Subtype. Am J Pathol. Jan. (2020) ;190: (1):134–144. |
[49] | Amin MB , Ro JY , Lee KM , Ordonez NG , Dinney CP , Gulley ML , et al. Lymphoepithelioma-like carcinoma of the urinary bladder. Am J Surg Pathol. (1994) ;18: (5):466–73. |
[50] | Cohen RJ , Stanley JC , Dawkins HJ . Lymphoepithelioma-like carcinoma of the renal pelvis. Pathology. (1999) ;31: (4):434–5. |
[51] | Lopez-Beltran A , Luque RJ , Vicioso L , Anglada F , Requena MJ , Quintero A , et al. Lymphoepithelioma-like carcinoma of the urinary bladder: a clinicopathologic study of 13 cases. Virchows Arch. (2001) ;438: (6):552–7. |
[52] | Porcaro AB , Gilioli E , Migliorini F , Antoniolli SZ , Iannucci A , Comunale L . Primary lymphoepithelioma-like carcinoma of the urinary bladder: report of one case with review and update of the literature after a pooled analysis of 43 patients. Int Urol Nephrol. (2003) ;35: (1):99–106. |
[53] | Yoshino T , Ohara S , Moriyama H . Lymphoepithelioma-like carcinoma of the urinary bladder: a case report and review of the literature. BMC Res Notes. (2014) ;7: :779. |
[54] | Tamas EF , Nielsen ME , Schoenberg MP , Epstein JI . Lymphoepithelioma-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. Mod Pathol. (2007) ;20: (8):828–34. |
[55] | Al-Ahmadie HA , Iyer G , Lee BH , Scott SN , Mehra R , Bagrodia A , et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat Genet. (2016) ;48: (4):356–8. |
[56] | Li Q , Assel M , Benfante NE , Pietzak EJ , Herr HW , Donat M , et al. The Impact of Plasmacytoid Variant Histology on the Survival of Patients with Urothelial Carcinoma of Bladder after Radical Cystectomy. European Urology Focus. (2019) ;5: (1):104–8. |
[57] | Klaile Y , Schlack K , Boegemann M , Steinestel J , Schrader AJ , Krabbe L-M . Variant histology in bladder cancer: how it should change the management in non-muscle invasive and muscle invasive disease? Transl Androl Urol. (2016) ;5: (5):692–701. |
[58] | Cheng L , Zhang S , Alexander R , MacLennan GT , Hodges KB , Harrison BT , et al. Sarcomatoid Carcinoma of the Urinary Bladder: The Final Common Pathway of Urothelial Carcinoma Dedifferentiation. The American Journal of Surgical Pathology. (2011) ;35: (5):e34–e46. |
[59] | Torenbeek R , Blomjous CE , de Bruin PC , Newling DW , Meijer CJ . Sarcomatoid carcinoma of the urinary bladder. Clinicopathologic analysis of 18 cases with immunohistochemical and electron microscopic findings. Am J Surg Pathol. (1994) ;18: (3):241–9. |
[60] | Torenbeek R , Hermsen M , Meijer GA , Baak J , Meijer CJ . Analysis by comparative genomic hybridization of epithelial and spindle cell components in sarcomatoid carcinoma and carcinosarcoma: histogenetic aspects. The Journal of Pathology. (1999) ;189: (3):338–43. |
[61] | Ikegami H , Iwasaki H , Ohjimi Y , Takeuchi T , Ariyoshi A , Kikuchi M . Sarcomatoid carcinoma of the urinary bladder: A clinicopathologic and immunohistochemical analysis of 14 patients. Human Pathology. (2000) ;31: (3):332–40. |
[62] | Sanfrancesco J , McKenney JK , Leivo MZ , Gupta S , Elson P , Hansel DE . Sarcomatoid Urothelial Carcinoma of the Bladder: Analysis of 28 Cases With Emphasis on Clinicopathologic Features and Markers of Epithelial-to-Mesenchymal Transition. Archives of Pathology & Laboratory Medicine. (2016) ;140: (6):543–51. |
[63] | Guo CC , Majewski T , Zhang L , Yao H , Bondaruk J , Wang Y , et al. Dysregulation of EMT Drives the Progression to Clinically Aggressive Sarcomatoid Bladder Cancer. Cell reports. (2019) ;27: (6):1781–93.e4. |
[64] | Wang X , Lopez-Beltran A , Osunkoya AO , Wang M , Zhang S , Davidson DD , et al. TERT promoter mutation status in sarcomatoid urothelial carcinomas of the upper urinary tract. Future Oncology. (2017) ;13: (8):705–14. |
[65] | Lopez-Beltran A , Pacelli A , Rothenberg HJ , Wollan PC , Zincke H , Blute ML , et al. Carcinosarcoma and Sarcomatoid Carcinoma of the Bladder: Clinicopathological Study of 41 Cases. The Journal of Urology. (1998) ;159: (5):1497–503. |
[66] | Wright JL , Black PC , Brown GA , Porter MP , Kamat AM , Dinney CP , et al. Differences in Survival Among Patients With Sarcomatoid Carcinoma, Carcinosarcoma and Urothelial Carcinoma of the Bladder. The Journal of Urology. (2007) ;178: (6):2302–7. |
[67] | Lopez-Beltran A , Cheng L . Histologic variants of urothelial carcinoma: differential diagnosis and clinical implications. Human Pathology. (2006) ;37: (11):1371–88. |
[68] | O’connor RC , Hollowell CMP , Laven BA , Yang XJ , Steinberg GD , Zagaja GP . Recurrent Giant Cell Carcinoma Of The Bladder. The Journal of Urology. (2002) ;167: (4):1784. |
[69] | Lopez-Beltran A , Blanca A , Montironi R , Cheng L , Regueiro JC . Pleomorphic giant cell carcinoma of the urinary bladder. Human Pathology. (2009) ;40: (10):1461–6. |
[70] | Patel A , Velilla RE , Shurbaji MS . Lipid-Rich Variant of Urothelial Carcinoma Presenting as the Dominant Morphology in a Recurrent Tumor After Local Therapy. Am J Case Rep. (2018) ;19: :478–81. |
[71] | Kojima Y , Takasawa A , Murata M , Akagashi K , Inoue T , Hara M , et al. A case of urothelial carcinoma, lipid cell variant. Pathology International. (2013) ;63: (3):183–7. |
[72] | Lopez-Beltran A , Amin MB , Oliveira PS , Montironi R , Algaba F , McKenney JK , et al. Urothelial carcinoma of the bladder, lipid cell variant: clinicopathologic findings and LOH analysis. Am J Surg Pathol. (2010) ;34: (3):371–6. |
[73] | Harshman LC , Preston MA , Bellmunt J , Beard C . Diagnosis of Bladder Carcinoma: A Clinician&#xs Perspective. Surgical Pathology Clinics. (2015) ;8: (4):677–85. |
[74] | Mai KT , Bateman J , Djordjevic B , Flood TA , Belanger EC . Clear Cell Urothelial Carcinoma: A Study of 10 Cases and Meta-Analysis of the Entity. Evidence of Mesonephric Differentiation. International Journal of Surgical Pathology. (2016) ;25: (1):18–25. |
[75] | Yamashita RYO , Yamaguchi R , Yuen K , Niwakawa M , Tobisu K . Urothelial carcinoma (clear cell variant) diagnosed with useful immunohistochemistry stain. International Journal of Urology. (2006) ;13: (11):1448–50. |
[76] | Oliva E , Amin MB , Jimenez R , Young RH . Clear cell carcinoma of the urinary bladder: a report and comparison of four tumors of mullerian origin and nine of probable urothelial origin with discussion of histogenesis and diagnostic problems. Am J Surg Pathol. (2002) ;26: (2):190–7. |
[77] | Sung M-T , Zhang S , MacLennan GT , Lopez-Beltran A , Montironi R , Wang M , et al. Histogenesis of Clear Cell Adenocarcinoma in the Urinary Tract: Evidence of Urothelial Origin. Clinical Cancer Research. (2008) ;14: (7):1947. |
[78] | Choong NWW , Quevedo JF , Kaur JS . Small cell carcinoma of the urinary bladder. Cancer. (2005) ;103: (6):1172–8. |
[79] | Koshkin VS , Garcia JA , Reynolds JP , Elson P , Magi-Galluzzi C , McKenney JK , et al. Transcriptomic and protein analysis of small cell bladder cancer (SCBC) identifies prognostic biomarkers and DLL3 as a relevant therapeutic target. Clin Cancer Res. (2019) ;25: (1):210–21. |
[80] | Batista da Costa J , Gibb EA , Bivalacqua TJ , Liu Y , Oo HZ , Miyamoto DT , et al. Molecular Characterization of Neuroendocrine-like Bladder Cancer. Clinical Cancer Research. (2019) ;25: (13):3908. |
[81] | Grivas P , Bismar TA , Alva AS , Huang H-C , Liu Y , Seiler R , et al. Validation of a neuroendocrine-like classifier confirms poor outcomes in patients with bladder cancer treated with cisplatin-based neoadjuvant chemotherapy. Urol Oncol. 2019. pii: S1078-1439(19)30459-4. |
[82] | Horn L , Mansfield AS , Szczęsna A , Havel L , Krzakowski M , Hochmair MJ , et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. New England Journal of Medicine. (2018) ;379: (23):2220–9. |
[83] | Ro JY , Staerkel GA , Ayala AG . Cytologic and histologic features of superficial bladder cancer. Urol Clin North Am. (1992) ;19: (3):435–53. |
[84] | Lopez-Beltran A , Martin J , Garcia J , Toro M . Squamous and glandular differentiation in urothelial bladder carcinomas. Histopathology, histochemistry and immunohistochemical expression of carcinoembryonic antigen. Histol Histopathol. (1988) ;3: (1):63–8. |
[85] | Zahoor H , Elson P , Stephenson A , Haber G-P , Kaouk J , Fergany A , et al. Patient Characteristics, Treatment Patterns and Prognostic Factors in Squamous Cell Bladder Cancer. Clinical Genitourinary Cancer. (2018) ;16: (2):e437–e42. |
[86] | Gellert LL , Warrick J , Al-Ahmadie HA . Urothelial carcinoma with squamous differentiation—The pathologists—perspective. Urologic Oncology: Seminars and Original Investigations. (2015) ;33: (10):437–43. |
[87] | Hovelson DH , Udager AM , McDaniel AS , Grivas P , Palmbos P , Tamura S , et al. Targeted DNA and RNA Sequencing of Paired Urothelial and Squamous Bladder Cancers Reveals Discordant Genomic and Transcriptomic Events and Unique Therapeutic Implications. European Urology. (2018) ;74: (6):741–53. |
[88] | Reis H , Serrette R , Posada J , Lu V , Chen YB , Gopalan A , et al. PD-L1 Expression in Urothelial Carcinoma With Predominant or Pure Variant Histology: Concordance Among 3 Commonly Used and Commercially Available Antibodies. Am J Surg Pathol. (2019) ;43: (7):920–7. |
[89] | Sadaps M , Zahoor H , Elson P , Emamekhoo H , Schach K , Brey ND , et al. Evaluation of patient (Pt), treatment, and prognostic factors in urinary tract adenocarcinoma. Journal of Clinical Oncology. (2017) ;35: (6_suppl):408. |
[90] | Scosyrev E , Ely BW , Messing EM , Speights VO , Grossman HB , Wood DP , et al. Do mixed histological features affect survival benefit from neoadjuvant platinum-based combination chemotherapy in patients with locally advanced bladder cancer? A secondary analysis of Southwest Oncology Group-Directed Intergroup Study (SBJU International. (2011) ;108: (5):693–9. |
[91] | Kantor AF , Hartge P , Hoover RN , Fraumeni JF . Epidemiological Characteristics of Squamous Cell Carcinoma and Adenocarcinoma of the Bladder. Cancer Research. (1988) ;48: (13):3853–5. |
[92] | Gulmann C , Paner GP , Parakh RS , Hansel DE , Shen SS , Ro JY , et al. Immunohistochemical profile to distinguish urothelial from squamous differentiation in carcinomas of urothelial tract. Human Pathology. (2013) ;44: (2):164–72. |
[93] | Guo CC , Gomez E , Tamboli P , Bondaruk JE , Kamat A , Bassett R , et al. Squamous cell carcinoma of the urinary bladder: a clinicopathologic and immunohistochemical study of 16 cases. Human Pathology. (2009) ;40: (10):1448–52. |
[94] | Lagwinski N , Thomas A , Stephenson AJ , Campbell S , Hoschar AP , El-Gabry E , et al. Squamous Cell Carcinoma of the Bladder: A Clinicopathologic Analysis of 45 Cases. The American Journal of Surgical Pathology. (2007) ;31: (12):1777–87. |
[95] | Shirahama T , Sakakura C . Overexpression of Cyclooxygenase-2 in Squamous Cell Carcinoma of the Urinary Bladder. Clinical Cancer Research. (2001) ;7: (3):558–61. |
[96] | Røtterud R , Nesland JM , Berner A , Fosså SD . Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU International. (2005) ;95: (9):1344–50. |
[97] | Rogers CG , Palapattu GS , Shariat SF , Karakiewicz PI , Bastian PJ , Lotan Y , et al. Clinical Outcomes Following Radical Cystectomy for Primary Nontransitional Cell Carcinoma of the Bladder Compared to Transitional Cell Carcinoma of the Bladder. The Journal of Urology. (2006) ;175: (6):2048–53. |
[98] | Raggi D , Ross JS , Ali SM , Chung J , Schrock AB , Madison R , et al. 930PComparison of immuno-oncology (IO) biomarkers in adenocarcinoma (ACB), urothelial carcinoma (UCB) and squamous cell carcinoma (SCCB) of the bladder, with interim results from PURE01. Annals of Oncology. 2019;30(Supplement 5). |
[99] | Thomas DG , Ward AM , Path MRC , Williams JL . A Study of 52 Cases of Adenocarcinoma of the Bladder. British Journal of Urology. (1971) ;43: (1):4–15. |
[100] | Zhong M , Gersbach E , Rohan SM , Yang XJ . Primary adenocarcinoma of the urinary bladder: differential diagnosis and clinical relevance. Arch Pathol Lab Med. (2013) ;137: (3):371–81. |
[101] | Roy S , Parwani AV . Adenocarcinoma of the Urinary Bladder. Archives of Pathology & Laboratory Medicine. (2011) ;135: (12):1601–5. |
[102] | Dadhania V , Czerniak B , Guo CC . Adenocarcinoma of the urinary bladder. Am J Clin Exp Urol. (2015) ;3: (2):51–63. |
[103] | Roy S , Pradhan D , Ernst WL , Mercurio S , Najjar Y , Parikh R , et al. Next-generation sequencing-based molecular characterization of primary urinary bladder adenocarcinoma. Modern Pathology. (2017) ;30: (8):1133–43. |
[104] | Zaghloul MS , Nouh A , Nazmy M , Ramzy S , Zaghloul AS , Sedira MA , et al. Long-term results of primary adenocarcinoma of the urinary bladder: A report on 192 patients. Urologic Oncology: Seminars and Original Investigations. (2006) ;24: (1):13–20. |
[105] | Siefker-Radtke A . Urachal adenocarcinoma: a clinician’s guide for treatment. Semin Oncol. (2012) ;39: (5):619–24. |
[106] | Dhillon J , Liang Y , Kamat AM , Siefker-Radtke A , Dinney CP , Czerniak B , et al. Urachal carcinoma: a pathologic and clinical study of 46 cases. Hum Pathol. (2015) ;46: (12):1808–14. |
[107] | Reis H , van der Vos KE , Niedworok C , Herold T , Módos O , Szendrői A , et al. Pathogenic and targetable genetic alterations in 70 urachal adenocarcinomas. International Journal of Cancer. (2018) ;143: (7):1764–73. |
[108] | Kardos J , Wobker SE , Woods ME , Nielsen ME , Smith AB , Wallen EM , et al. Comprehensive Molecular Characterization of Urachal Adenocarcinoma Reveals Commonalities With Colorectal Cancer, Including a Hypermutable Phenotype. JCO Precision Oncology. (2017) (1):1–12. |
[109] | Collazo-Lorduy A , Castillo-Martin M , Wang L , Patel V , Iyer G , Jordan E , et al. Urachal Carcinoma Shares Genomic Alterations with Colorectal Carcinoma and May Respond to Epidermal Growth Factor Inhibition. European Urology. (2016) ;70: (5):771–5. |
[110] | Apolo AB , Milowsky MI , Kim L , Inman BA , Kamat AM , Steinberg G , et al. Eligibility and Radiologic Assessment in Adjuvant Clinical Trials in Bladder Cancer. JAMA Oncology. (2019) ;5: (12):1790–8. |




