Changes in Lean Muscle Mass Associated with Neoadjuvant Platinum-Based Chemotherapy in Patients with Muscle Invasive Bladder Cancer
Abstract
Background:
Baseline sarcopenia or severe lean muscle deficiency is independently associated with increased mortality after cystectomy for muscle-invasive urothelial carcinoma of the bladder (MIUC). The impact of chemotherapy on muscle mass in MIUC patients remains undefined
Objectives:
To describe preoperative changes in body composition in MIUC patients receiving platinum-based neoadjuvant chemotherapy (NC).
Methods:
Patients with cT2-4 N0-1 M0 UC of the bladder who received NC were identified. Lumbar skeletal muscle index (SMI, cm2/m2), visceral adipose index (VAI, cm2/m2), and the subcutaneous and intramuscular adipose index (SAI, cm2/m2) were calculated using validated methodology (cross sectional area of skeletal muscle/height2 at L3) from measurement of soft tissue areas on pre- (pre-NC) and post-NC (post-NC) computed tomography. Patients were classified as sarcopenic according to consensus definitions: Male: SMI <55 cm2/m2, Female: SMI <38.5 cm2/m2. Pre-NC and post-NC median body mass index (BMI kg/m2), SMI, and adipose indices were compared.
Results:
The study cohort consisted of 26 patients, with a median age 70 years, including 7 females (27%). Chemotherapy regimens included dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (31%), gemcitabine/cisplatin (62%) and gemcitabine/carboplatin (3.8%) with a median of 3.5 (range 2–6) cycles. Median pre- and post-NC BMI were 27.1 kg/m2 and 27.2 kg/m2 (p = 0.36). Median pre- and post-NC SMI were 49.1 cm2/m2 and 44.5 (p < 0.001) respectively. Median percent change in SMI was –6.4% (range –30% to 10%). Pre-NC, 18 (69%) patients were sarcopenic vs. 21 (81%, p = 0.002) post-NC. Median time between initiation of chemotherapy and cystectomy was 110 days.
Conclusions:
We observed a significant decrease in lean muscle mass among MIUC patients treated with platinum-based NC prior to cystectomy, with an associated increase in the prevalence of sarcopenia. Patients undergoing NC may benefit from pre-habilitative interventions to mitigate lean muscle loss prior to cystectomy.
INTRODUCTION
In the United States in 2017, 79,030 individuals are projected to receive a new diagnosis of bladder cancer and 16,870 patients are projected to die of the disease [1]. Bladder cancer disproportionally affects older patients with diminished performance status and significant medical comorbidities [2–4]. Radical cystectomy (RC) with bilateral extended pelvic lymphadenectomy and urinary diversion following neoadjuvant cisplatin-based chemotherapy is the standard of care for muscle-invasive urothelial carcinoma (MIUC) of the bladder. However, even with the addition of neoadjuvant chemotherapy in modern series, 5-year overall survival (OS) rates following RC remain low, at 42 –58% [5, 6]. Given these poor outcomes, there is a need to identify potentially modifiable factors contributing to inferior OS.
Recently, sarcopenia or a severe deficiency of lean muscle mass, has been identified as a prognostic factor that is independently associated with both cancer-specific and all-cause mortality following RC for MIUC [7–10]. Specifically, in patients with urothelial carcinoma of the bladder treated with RC, those with sarcopenia had a significantly inferior 5-year cancer-specific survival and OS with a median overall follow-up of 6.7 years [9]. On multivariable analysis, sarcopenia remained associated with a greater than two-fold increase in the risk of bladder cancer death [9]. Concurrently, sarcopenia is a well established prognostic factor in other malignancies, including melanoma, breast, pancreatic, colorectal, and hepatobiliary carcinoma [11–16].
Interestingly, recent research efforts have demonstrated that low lean muscularity is also associated with increased treatment-associated toxicity in patients receiving chemotherapy and other systemic treatments for malignancy [12, 15]. There is conflicting evidence, however, regarding how chemotherapy may alter body composition and lean muscle mass during administration. Cytotoxic chemotherapy has been associated with substantial lean muscle mass loss in colorectal [16] and pancreatic cancers [17], but a paradoxical increase in lean muscle mass has been reported in patients with advanced lung cancer receiving platinum-based chemotherapy [18].
To the authors’ knowledge, there are currently no studies that have evaluated the change in body composition in patients with bladder cancer following neoadjuvant chemotherapy utilizing clinically validated measures. Given that current guidelines include neoadjuvant platinum-based chemotherapy as the standard of care prior to RC in patients with MIUC, it is essential to understand both the impact of chemotherapy on body composition, and the interaction between chemotherapy-associated changes in body composition and the tolerability of chemotherapy and oncologic outcomes.
Therefore, the hypothesis tested herein was that the receipt of neoadjuvant chemotherapy prior to radical cystectomy for MIUC is associated with a decrease in cross-sectional muscle area (CSMA) and an increase in the proportion of patients meeting criteria for sarcopenia. A secondary exploratory objective was to assess whether lean muscle mass was associated with chemotherapy-related toxicity and tolerance.
MATERIALS AND METHODS
We retrospectively identified all patients with MIUC, cT2-4, N0-1, M0 who received neoadjuvant platinum-based chemotherapy prior to planned RC between 1/2011 and 10/2016 at a single institution (n = 59). Inclusion criteria included available digitized cross-sectional axial imaging with computed tomography (CT) of the abdomen and pelvis within the month prior to initiation and following completion of chemotherapy [19]. This study received institutional review board (IRB) approval following a standard assessment from the Northwestern University IRB and the Northwestern University Scientific Review Committee, the IRB number is STU00203694.
From these studies, the pre- and post-chemotherapy skeletal muscle index (SMI, cm2/m2) was calculated according to previously described, validated methodology [20–22]. Briefly, a single DICOM image was extracted from pre- and post-chemotherapy CT imaging at the midpoint of the third lumbar vertebrae. These DICOM images were then exported to a image analysis software (SliceOmatic v5.0TM TomoVision, Montreal, Quebec, Canada) from which the cross-sectional lumbar soft tissue areas (muscle, visceral fat, subcutaneous and intramuscular adipose tissue, Supplementary Figure 1) were measured by a single investigator (KJR) in cm2 according to standardized methodology [19]. Image analysis was performed in a blinded fashion to all clinical data to minimize introduction of bias. The SMI was calculated (cross-sectional area of skeletal muscle at L3/height2). Similarly, the visceral adipose index (VAI) and subcutaneous and intramuscular adipose index (SAI) were calculated by normalizing the relevant adipose areas by height squared. Patients were classified as being sarcopenic according to sex-specific consensus definitions derived from the cancer cachexia literature: Male: SMI <55 cm2/m2, Female: SMI <39 cm2/m2 [22].
Clinicopathologic variables were obtained prior to and following NC, including age, sex, race, Charlson co-morbidity score, Eastern Cooperative Oncology Group (ECOG) score, American Society of Anesthesia (ASA) score, smoking status, hemoglobin, albumin, and clinical tumor stage at diagnosis. Continuous features were summarized with medians while categorical features were summarized with proportions.
Pre- and post-NC median body mass index (BMI), VAI, SAI, and SMI were compared using paired Wilcoxon signed rank tests. Secondary outcomes of interest included change in sarcopenia designation following NC, time from NC to receipt of radical cystectomy, need for dose reduction, incomplete number or delay of chemotherapeutic cycles, grade 3 toxicity or greater, and response to chemotherapy.
Statistical analysis were performed using the JMP Pro software package (version 12.2.0). All tests were 2-sided with a P-value of <0.05 considered to be statistically significant.
RESULTS
Clinicopathologic characteristics
The study cohort consisted of 26 patients (7 female [27% ], 19 male [73%]). The majority of patients were Caucasian (77%), with a median age of 70 years (IQR 61.75–76.5). Median Charlson Comorbidity Index was 5 (IQR 5–8). The majority of patients were current or former smokers (80.7%).
All tumors were all histologically consistent with urothelial carcinoma, although some histologic variants were noted (2 plasmacytoid [7.7% ], 1 sarcomatoid [3.8% ], 1 micropapillary [3.8% ]). Prior to chemotherapy, the majority of patients were considered to have clinical T2 disease (69%). Eight patients (30.7%) presented with evidence of lymph node involvement. Clinicopathologic characteristics are summarized in Table 1.
Table 1
Patient demographics
| Variable | Mean (range) or n (%) |
| Age (years) | 67 (40–82) |
| Sex | |
| Male | 19 (73) |
| Female | 7 (27) |
| Race | |
| Caucasian | 21 (80) |
| Other | 5 (20) |
| Median Charlson Co-morbidity Index | 5 (2–10) |
| Median ECOG | 0 (0–2) |
| Median ASA | 1 (1–3) |
| Smoker | |
| yes | 21 (80) |
| Hemoglobin | 13.1 (8.2–16.8) |
| Albumin | 3.8 (2.7–4.8) |
| Clinical Tumor Stage | |
| T2 | 18 (69) |
| ≥T3 | 8 (31) |
| N1 | 8 (31) |
| Chemotherapy Regimen | |
| MVAC | 8 (31) |
| Gem/Cis | 17 (65) |
| Gem/Carbo | 1 (4) |
| Median # Cycles | 3.5 (3–6) |
Abbreviations: ECOG (Eastern Cooperative Oncology Group); ASA (American Society of Anesthesiologists); MVAC (methotrexate, vinblastine, doxorubicin, cisplatin); Gem/Cis (gemcitabine, cisplatin); Gem/Carbo (gemcitabine, carboplatin).
Treatment characteristics
Chemotherapy regimens included dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC) in 8 (31%), gemcitabine and cisplatin in 17 (65%) and gemcitabine and carboplatin in 1 (3.8%). Median number of cycles was 3.5 (range 2–6). Hematologic (3.8%) and non-hematologic (3.8%) complications requiring dose reduction were rare.
The median time from initiation of chemotherapy to cystectomy was 110 days (IQR 78, 148) overall. Median time to cystectomy according to sarcopenic status approached, but did not achieve a statistically significant difference (sarcopenic: 114 days vs. non-sarcopenic 84 days, p = 0.07). However, no significant differences were observed in the incidence of dose reduction (17% vs. 25%, p = 0.62), delay in administration of chemotherapy (22% vs. 13%, p = 0.55), grade 3 or greater toxicity (17% vs. 0%, p = 0.12), or treatment response (p = 0.66) in this cohort, by sarcopenia status (Table 2). Post-cystectomy complications occurred in 14/26 patients including post-operative ileus (n = 3), candidemia (n = 1), C. Difficile infection (n = 1), deep abscess (n = 1), ventral hernia necessitating mesh closure (n = 1), distal ureteral stricture (n = 1), urinary leak (n = 1), DVT (n = 2). There was no association between individual complication types and sarcopenic status.
Table 2
Chemotherapeutic information and outcomes
| Variable | Overall (%) | Sarcopenic (%) | Non-Sarcopenic (%) | p-value |
| Time from chemotherapy to radical cystectomy, median number of days (Interquartile range, IQR) | 110 (IQR 78,148) | 128 (IQR 93, 153) | 84 (66,106) | 0.07 |
| Dose reduction | 5 (19) | 3 (17) | 2 (25) | 0.62 |
| Incomplete # of cycles | 5 (19) | 3 (17) | 2 (25) | 0.62 |
| Delay in # of cycles | 5 (19) | 4 (22) | 1 (13) | 0.55 |
| ≥Grade 3 Toxicity | 3 (12) | 3 (17) | 0 (0) | 0.12 |
| Response | 0.66 | |||
| Complete | 8 (31) | 6 (33) | 2 (25) | |
| Partial | 4 (15) | 2 (11) | 2 (25) | |
| Progression | 14 (54) | 10 (56) | 4 (50) |
Abbreviations: RC (radical cystectomy).
Baseline anthropometric data and change in body composition
Body composition data pre- and post-NC is summarized in Table 3. The median time between the initial pre-chemotherapy CT scan and the post-chemotherapy CT scan for the entire cohort was 110 days (IQR 73, 125). At baseline, the prevalence of sarcopenia was 69.2% (n = 18). Following chemotherapy, 21 patients met criteria for sarcopenia (80.7%). There were no significant differences between median pre- and post-NC BMI (27.1 kg/m2 vs. 27.2 kg/m2, p = 0.36), VAI (61.9 cm2/m2 vs. 56.7 cm2/m2, p = 0.63), or SAI (61.1cm2/m2 vs. 77.8 cm2/m2, p = 0.85) (Table 3).
Table 3
Body Composition Pre- and Post-Chemotherapy
| All (n = 26) | Male (n = 19) | Female, n = 7 | |||||||
| Variable (median, IQR or N, %) | Pre-NAC | Post-NAC | p-value | Pre-NAC | Post-NAC | p-value | Pre-NAC | Post-NAC | p-value |
| Body Mass Index (BMI, kg/m2) | 27.1 | 27.2 | 0.36 | 26.3 (23.9, 31,2) | 28.4 (23.4, 32.3) | 0.95 | 28 (25.1, 30.1) | 27 (25.9,28.9) | 0.81 |
| Skeletal Muscle Index (SMI, cm2/m2) | 49.1 | 44.5 | <0.001 | 50.5 (46.1, 54.9) | 47.3 (42.7, 50.7) | 0.15 | 35.2 (32.2, 40.5) | 34.9 (31, 40) | 0.53 |
| Visceral Adipose Index (VAI, cm2/m2) | 61.9 | 56.7 | 0.63 | 71.1 (52.9, 114.2) | 61.9 (47.1, 123.1) | 0.82 | 39.2 (26.1, 57.4) | 54.1 (35.6, 59.7) | 0.62 |
| Subcutaneous/Intramuscular adipose (SIAI, cm2/m2) | 61.1 | 77.8 | 0.85 | 57.8 (37.9, 87.0) | 59.9 (39.3, 89.4) | 0.91 | 107.9 (80.4, 108.1) | 107.3 (76.9, 131.4) | 0.71 |
| Baseline Sarcopenia (N, %) | 18 (69) | 21 (31) | 0.002 | 14 (74%) | 17 (89%) | 0.21 | 4 (57.1%) | 4 (57.1%) | 1.0 |
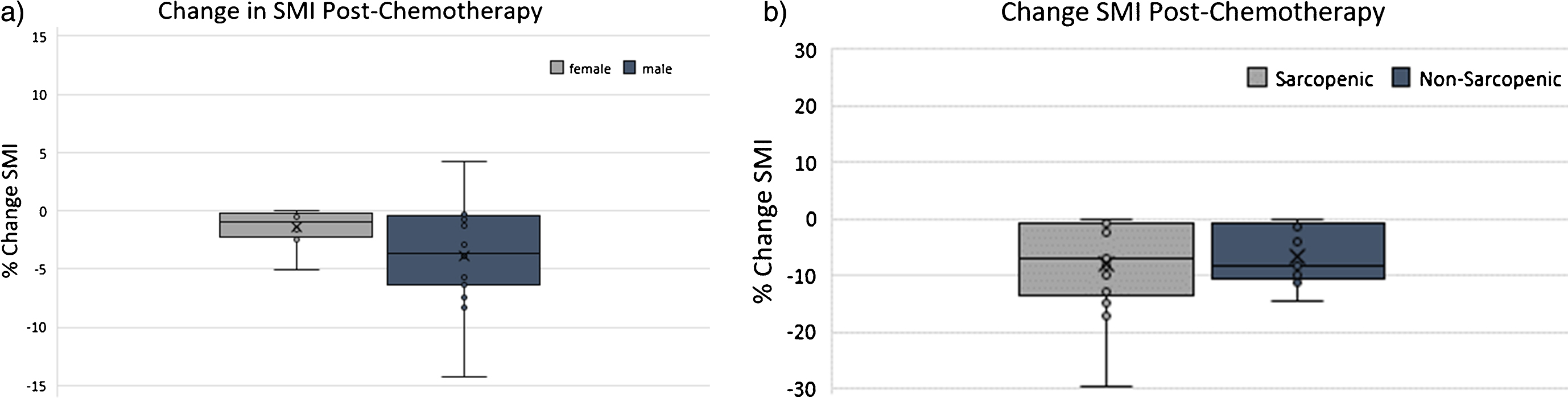
With respect to measurement of lean muscularity, the median pre- and post-NC cross-sectional lumbar muscle areas were 141 cm2 and 129.4 cm2 (p < 0.001) overall. Median pre- and post-NC SMI were 49.2 cm2/m2 and 44.5 (p < 0.001) (Table 3). Stratified by sex, among males, median pre-and post-NC SMI were 50.5 vs. 47.3 cm2/m2 (p = 0.15), while among females, median pre-and post-NC SMI were 35.2 vs. 34.9 cm2/m2 (p = 0.53). The median percent change in SMI overall was –6.4% (range –30% to 10%, Fig. 1). Percent change in SMI did not differ according to baseline sarcopenic status (p = 1.00) (Fig. 2) or number of chemotherapy cycles (p = 0.38).
Fig.1
Percent Change in skeletal muscle index (SMI) following neoadjuvant chemotherapy (NAC). Waterfall plot demonstrating percent change in SMI following NAC. Each bar represents one patient. Horizontal line represents median % change in SMI (–6.4%).
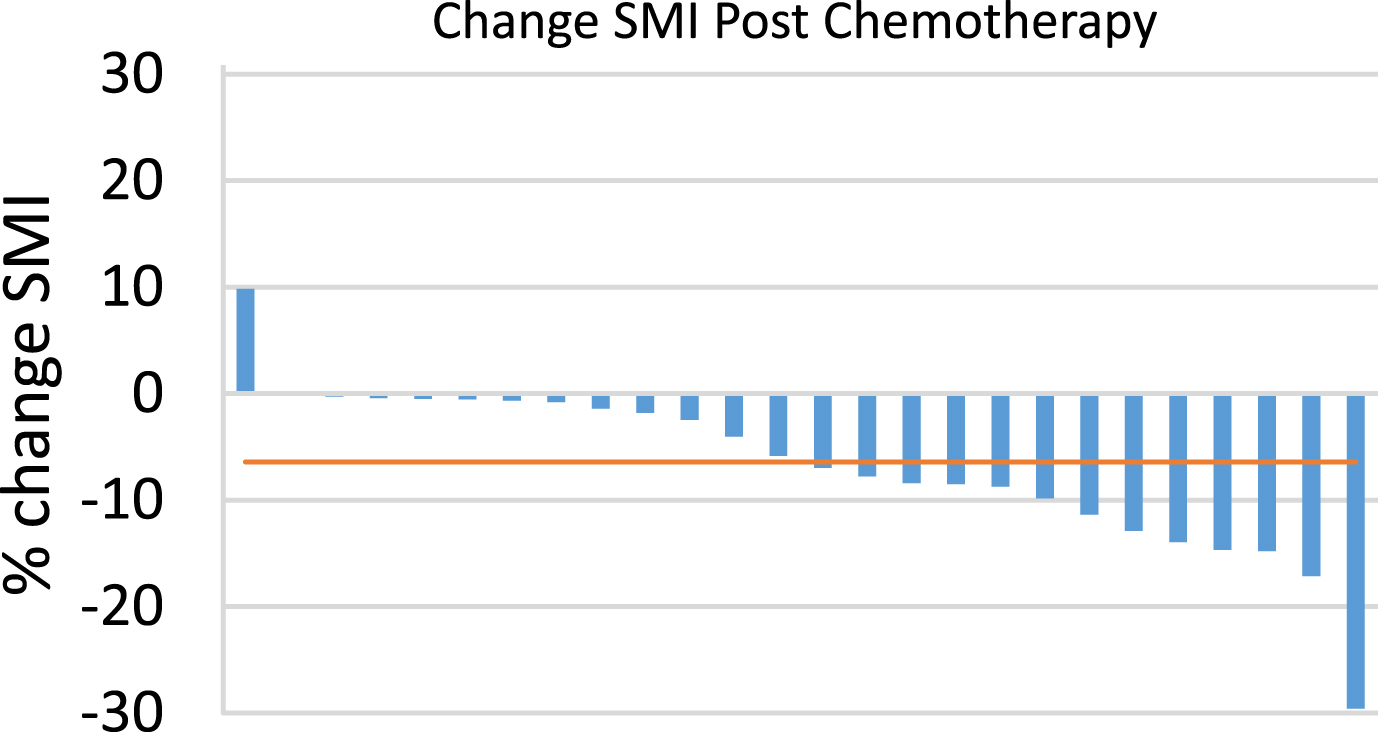
Fig.2
Percent Change in skeletal muscle index (SMI) following neoadjuvant chemotherapy (NAC) according to (a) sex and (b) sarcopenia status pre-NAC.
COMMENT
Sarcopenia, or severe deficiency of lean muscle mass, is thought to be multifactorial in etiology [23], reflecting nutritional deficiency, systemic inflammation and catabolism related to both tumor- and host-related factors, in the context of a stereotypic process associated with aging. The association between sarcopenia and inferior oncologic outcomes across a wide array of different malignancies including MIUC is increasingly recognized [11]. There is also evidence that receipt of chemotherapy may be associated with changes in body composition, notably loss of lean muscle mass [16, 17, 24]. Given the poor outcomes associated with sarcopenia in patients with MIUC and the current recommendations that patients with MIUC receive cytotoxic neoadjuvant chemotherapy prior to radical cystectomy, we endeavored to describe changes in body composition of patients with MIUC who received neoadjuvant platinum-based chemotherapy. We utilized validated methodology [20, 21] to quantify changes in both lean muscle mass using the lumbar skeletal muscle index before and after treatment with NC. We also explored changes in visceral, subcutaneous, and intramuscular adipose tissue indices, following NC. While no universally accepted SMI-based thresholds exist to define sarcopenia specifically, we utilized the thresholds defined according to the sex-specific consensus definitions for cancer cachexia proposed by Fearon et al. which are most commonly utilized (absolute muscularity below the fifth percentile for healthy young adults) [22]. Following NC, in this contemporary case series, we observed that the patient population experienced a significant decline in skeletal muscle mass, with an increase in the prevalence of sarcopenia increased from 69% to 81% over a median of less than 4 months. Interestingly, there were no synchronous consistent changes in adiposity, nor BMI during the same time period.
Our results with regard to skeletal muscle loss following platinum-based chemotherapy are consistent with findings in other non-urothelial malignancies, however much of this work has been in the setting of adjuvant treatment or advanced/metastatic disease [16, 18]. In the neoadjuvant setting, Rutten et al. utilized the same methodology described in this paper in 123 patients with International Federation of Gynecology and Obstetrics (FIGO) stage IIb-IV ovarian cancer who received neoadjuvant platinum-based chemotherapy. Treatment with neoadjuvant chemotherapy resulted in a significant decrease in SMI. Subjects who were able to maintain or gain skeletal muscle during chemotherapy were also noted to have increased OS (p = 0.004) [24].
Recently, Zargar et al. utilized psoas muscle volume (PMV) in patients with MIUC before and after NC as a proxy for determining skeletal muscle loss in 60 patients, demonstrating a significant decrease in post-chemotherapy PMV. The authors did not observe any association between changes in PMV and pathologic response to chemotherapy, post-operative complications, or survival. However, they did report that PMV loss was associated with NAC dose reduction/delay [25]. Unlike measurements of SMI as used in the current study, PMV measurements have yet to be validated against DEXA scans, the gold standard for quantification of lean muscle mass and the evidence supporting their use as surrogates of total body fat-free mass is limited [20, 21, 26–28]. In the current study, while there were trends towards a slight increase in the median time to cystectomy (114 vs. 104 days, p = 0.07) and an increased incidence of grade 3 toxicity (17% vs. 0%, p = 0.12) in patients with sarcopenia, these findings were not statistically significant. However, given the small study population, our study may have been underpowered to detect these differences. These results are not surprising as sarcopenia has been associated with increased treatment related toxicity as well as inferior oncologic outcomes following chemotherapy in other malignancies, though this has primarily been in the advanced setting [16, 17, 29]. Though scarce, the available data of patients treated in the neoadjuvant setting appears to support our findings.
The mechanism by which cytotoxic chemotherapy may potentiate changes in body composition, specifically with respect to acceleration of muscle mass depletion, is poorly understood and is an burgeoning area of research. The literature is inconsistent with respect to observations regarding how cytotoxic chemotherapy impacts body composition across cancers and chemotherapy regimens with some studies reporting loss of muscle mass or muscle quality [30–32] while others demonstrate changes in adipose tissue only [33], in the absence of changes in muscularity. In general, muscle mass loss is throught to be mediated predominantly by pro-inflammatory cytokines including IL-6, IL-10, and TNF-alpha [34]. The concept of chemotherapy-induced muscle wasting was first introduced by Damrauer and colleagues in 2008 [35]. In animal models, cisplatin, which is routinely utilized in neoadjuvant chemotherapy regimens for urothelial carcinoma, has been observed to promote muscle loss, even in mice who do not harbor malignancy. Proposed mechanisms for cisplatin-induced muscle atrophy include induction of the NF-κB signaling pathway [35] which, interestingly, impacts both the muscle fibers and muscle stem cells [36]. In a colon cancer mouse model (C26), exposure to Folfiri resulted in a proteomic signature consistent with activation of mechanisms associated with necrosis, as well as muscle cell death, weakness and damage [36]. Further work is needed to understand the complex interplay between tumor- and chemotherapy-associated muscular atrophy among bladder cancer patients undergoing treatment with chemotherapy to aid in the development of strategies to mitigate these losses.
We discovered a remarkably high prevalence (69%) of sarcopenia at baseline in this population, though this is consistent with a prior study of the pre-cystectomy population as well as other malignancies [9]. For reference, the prevalence of sarcopenia in the general elderly population (age > 60 years) based on estimates from population based studies is roughly 10% in men and 10% in women [37]. Interestingly, the median BMI for our study population would place most patients in the category of “overweight’ according to the World Health Organization Criteria, and median BMI was not significantly changed following NC. This finding has a number of interesting implications. The decline in skeletal muscle mass observed does not appear to be consistent with definitions of cancer cachexia, which is defined as whole body wasting despite adequate nutritional intake [22]. On the contrary, herein we observed a consistent pattern of lean muscle loss among patients with MIUC being treated with neoadjuvant platinum-based chemotherapy without a clear pattern regarding changes in fat-mass. This finding further underscores the degree to which BMI is a poor proxy for skeletal muscle loss [38], and the degree to which high or “normal” BMI may mask underlying body composition changes, such as a deficiency in lean muscle mass, which have been associated with poor outcomes [9].
The high prevelance of sarcopenia in this population, the increased prevelance following neoadjuvant chemotherapy, in conjunction with the worse outcomes that have been shown to be associated with sarcopenia following radical cystectomy support the fact that this population may benefit from pre-habilatative intervention to mitigate skeletal muscle loss. Yamamoto et al. recently published their results of a pre-operative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. With a median program participation time of 16 days, they observed an increase in hand-grip strength (p = 0.022), and a decrease in the prevalence of sarcopenia (22 pre-intervention [100%] vs. 18 post-intervention [82%]) [39]. Though the study population was small, the authors demonstrated the feasibility of a pre-habilitation protocol prior surgery and the ability to potentially decrease the prevelance of a known risk factor for worse outcomes (sarcopenia). Though many institutions are adopting enhanced recovery after surgery protocols for patients following radical cystectomy, the findings of this small observational story suggest that there may be a role for earlier intervention at the time when neoadjuvant chemotherapy is initiated, to mitigate muscle loss, and potentially attempt to increase lean muscularity in the period leading up to radical cystectimy.
There are a number of limitations to the current study that must be acknowledged. Most notably, the current study is limited by the small sample size which was the result of stringent inclusion and exclusion criteria. Due to the quaternary referral nature of the practice, baseline digitized imaging was not always available for patients who received NAC at outside institutions. As such, there is the potential for selection bias influencing our results due to differences between the patients for whom imaging was available and those excluded. A larger sample size followed prospectively for a longer period of time will be required to further define the impact of cytotoxic chemotherapy on body composition, and how these changes correlate with chemotherapy tolerance as well as postoperative complications and oncologic outcomes in patients with MIUC. The lack of these outcomes measures is a clear limitation of this study. Future directions include the prospect pre-habilitative interventions involving nutrition and exercise to mitigate muscle mass loss in patients receiving neoadjuvant chemotherapy.
CONCLUSION
Although BMI and measurements of adiposity remained stable, we observed a significant decline in skeletal muscle mass among MIUC patients treated with platinum-based NC prior to cystectomy, with an associated increase in the prevalence of sarcopenia. These patients may benefit from pre-habilitative interventions to mitigate lean muscle loss prior to cystectomy. The association between changes in SMI and surgical and oncologic outcomes in this cohort is under investigation.
FUNDING AND FINANCIAL DISCLOSURES
None.
CONFLICT OF INTEREST DISCLOSURES
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: 10.3233/BLC-180188.
REFERENCES
[1] | American Cancer Society: Cancer Facts & Figures 2017, (2017) . |
[2] | Prout GR Jr , Wesley MN , Yancik R , et al. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: A population-based study. Cancer. (2005) ;104: :1638. |
[3] | Aziz A , May M , Burger M , et al. Prediction of 90-day Mortality After Radical Cystectomy for Bladder Cancer in a Prospective European Multicenter Cohort. Eur Urol. (2013) . |
[4] | Kluth LA , Rieken M , Xylinas E , et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: An international multi-institutional study of more than 8000 patients. Eur Urol. (2014) ;66: :913. |
[5] | Ploussard G , Shariat SF , Dragomir A , et al. Conditional Survival After Radical Cystectomy for Bladder Cancer: Evidence for a Patient Changing Risk Profile over Time. Eur Urol. (2013) . |
[6] | Shariat SF , Karakiewicz PI , Palapattu GS , et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res. (2006) ;12: :6663. |
[7] | Mayr R , Fritsche HM , Zeman F , et al. Sarcopenia predicts 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J Urol. (2018) . |
[8] | Mayr R , Gierth M , Zeman F , et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle. (2018) . |
[9] | Psutka SP , Carrasco A , Schmit GD , et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer. (2014) ;120: :2910. |
[10] | Wan F , Zhu Y , Gu C , et al. Lower skeletal muscle index and early complications in patients undergoing radical cystectomy for bladder cancer. World J Surg Oncol. (2014) ;12: :14. |
[11] | Shachar SS , Williams GR , Muss HB , et al. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. (2016) ;57: :58. |
[12] | Mir O , Coriat R , Blanchet B , et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. (2012) ;7: :e37563. |
[13] | Del Fabbro E , Parsons H , Warneke CL , et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. (2012) ;17: :1240. |
[14] | Sabel MS , Lee J , Cai S , et al. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. (2011) ;18: :3579. |
[15] | Antoun S , Borget I , Lanoy E . Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care. (2013) ;7: :383. |
[16] | Blauwhoff-Buskermolen S , Versteeg KS , de van der Schueren MA , et al. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol (2016) ;34: :1339. |
[17] | Choi Y , Oh DY , Kim TY , et al. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS One. (2015) ;10: :e0139749. |
[18] | Stene GB , Helbostad JL , Amundsen T , et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. (2015) ;54: :340. |
[19] | Martin L , Birdsell L , Macdonald N , et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. (2013) ;31: :1539. |
[20] | Mourtzakis M , Prado CM , Lieffers JR , et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. (2008) ;33: :997. |
[21] | Shen W , Punyanitya M , Wang Z , et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol. (2004) ;97: :2333. |
[22] | Fearon K , Strasser F , Anker SD , et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. (2011) ;12: :489. |
[23] | Vaughan VC , Martin P , Lewandowski PA . Cancer cachexia: Impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle. (2013) ;4: :95. |
[24] | Rutten IJ , van Dijk DP , Kruitwagen RF , et al. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. (2016) ;7: :458. |
[25] | Zargar H , Almassi N , Kovac E , et al. Change in Psoas Muscle Volume as a Predictor of Outcomes in Patients Treated with Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Bladder Cancer. (2017) ;3: :57. |
[26] | Prado CM , Birdsell LA , Baracos VE . The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. (2009) ;3: :269. |
[27] | Prado CM , Heymsfield SB . Lean tissue imaging: A new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. (2014) ;38: :940. |
[28] | Baracos VE . Psoas as a sentinel muscle for sarcopenia: A flawed premise. J Cachexia Sarcopenia Muscle. (2017) ;8: :527. |
[29] | Prado CM , Baracos VE , McCargar LJ , et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. (2009) ;15: :2920. |
[30] | Mazzuca F , Onesti CE , Roberto M , et al. Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget. (2018) ;9: :25714. |
[31] | Daly LE , Ni Bhuachalla EB , Power DG , et al. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. (2018) ;9: :315. |
[32] | Rier HN , Jager A , Sleijfer S , et al. Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. (2018) ;168: :95. |
[33] | Sandini M , Patino M , Ferrone CR , et al. Association Between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg. (2018) . |
[34] | Fearon KC , Glass DJ , Guttridge DC . Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. (2012) ;16: :153. |
[35] | Damrauer JS , Stadler ME , Acharyya S , et al. Chemotherapy-induced muscle wasting: Association with NF-kappaB and cancer cachexia. Eur J Transl Myol. (2018) ;28: :7590. |
[36] | Barreto R , Mandili G , Witzmann FA , et al. Cancer and Chemotherapy Contribute to Muscle Loss by Activating Common Signaling Pathways. Front Physiol. (2016) ;7: :472. |
[37] | Shafiee G , Keshtkar A , Soltani A , et al. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) ;16: :21. |
[38] | Psutka SP , Boorjian SA , Moynagh MR , et al. Mortality after radical cystectomy: Impact of obesity versus adiposity after adjusting for skeletal muscle wasting. J Urol. (2015) ;193: :1507. |
[39] | Yamamoto K , Nagatsuma Y , Fukuda Y , et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer. (2017) ;20: :913. |




