Venous Thromboembolism and Peri-Operative Chemotherapy for Muscle-Invasive Bladder Cancer: A Population-based Study
Abstract
Background:
Chemotherapy and major pelvic surgery are established risk factors for venous thromboembolism (VTE). We evaluate the incidence rate, timing, and factors associated with VTE in patients with bladder cancer who underwent radical cystectomy and peri-operative chemotherapy in routine clinical practice.
Methods:
Electronic records of treatment were linked to the population-based Ontario Cancer Registry to identify all patients who underwent cystectomy for bladder cancer in Ontario 1994–2013. VTE events within 6 months of before or after cystectomy were identified using diagnostic codes recorded on hospital admissions and emergency department visits. Multivariable logistic regression was used to analyze factors associated with VTE prior to surgery, within 90-days of cystectomy, and 120-days after the start of adjuvant chemotherapy.
Results:
4205 patients had cystectomy and 26% (1084/4205) received peri-operative chemotherapy. The overall incidence rate of VTE within 6 months of cystectomy was 9% (363/4205). VTE rate was highest among those patients treated with neoadjuvant chemotherapy (NACT) compared to patients treated with no chemotherapy or only adjuvant chemotherapy (ACT) (12% vs 8% vs 9%, p = 0.002). Among all VTE events, 10%, 28%, and 61% occurred before, during, and after hospitalization for cystectomy. Pre-operative VTE rate was highest among cases treated with NACT (4%) compared to patients with no chemotherapy (<1%) or ACT (<1%) (p < 0.001). VTE within 90 days of surgery was associated with greater length of hospital admission (p < 0.001) across all treatment groups.
Conclusions:
A substantial proportion of patients treated with peri-operative chemotherapy will develop VTE. The majority of these occur after discharge from hospital following cystectomy. Extended thromboprophylaxis treatment in high-risk patients including those who receive peri-operative chemotherapy should be considered.
INTRODUCTION
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in patients with cancer [1]. Patients with bladder cancer have multiple risk factors for VTE. While several reports have described an increased risk of VTE due to major pelvic surgeries, such as cystectomy, [2–5] the risk of VTE during peri-operative chemotherapy has not been well characterized. Existing studies present conflicting results regarding the association between chemotherapy and VTE, have not been restricted to curative intent patients, or include only a small number of patients treated with chemotherapy [6–15]. Several studies conclude that neoadjuvant chemotherapy (NACT) is not associated with an increased risk of VTE after RC, [6, 13, 14] though these studies included only a small number of patients with NACT (sample size range 78–216). Conversely, a multi-institutional study of 761 patients receiving NACT followed by radical cystectomy (RC) report a VTE rate of 11% [16]. Fifty-eight percent of events occurred before RC. Our previous work did not identify an association between delivery of adjuvant chemotherapy (ACT) and VTE [13]. A systematic review and meta-analysis of 5082 of patients with bladder cancer treated with chemotherapy for any stage of disease describe an overall VTE rate of 7% [17].
A growing body of evidence supports the use of extended thromboprophylaxis treatment after cystectomy [6, 13–15]. Thromboprophylaxis treatment is not without risks, and therefore identifying patients at highest risk of VTE may guide decision-making. To address existing gaps in the literature we undertook a population-based study to describe VTE rates and the factors associated with VTE among patients treated with chemotherapy and cystectomy.
METHODS
Study design and population
This is a population-based, retrospective cohort study to describe the rate, timing, and factors associated with VTE among patients treated with chemotherapy and cystectomy for muscle-invasive bladder cancer (MIBC) in the Canadian province of Ontario. Ontario has a population of 13.5 million people and universal healthcare. All incident cases of muscle-invasive urothelial bladder cancer in Ontario who underwent cystectomy 1994–2013 were identified using the Ontario Cancer Registry and linked treatment records.
Data sources
The Ontario Cancer Registry (OCR) is a passive, population-based cancer registry that captures diagnostic and demographic information on at least 98% of all incident cases of cancer in the province of Ontario [18]. Records of hospitalization from the Canadian Institute for Health Information (CIHI) provided information about surgical procedures. Provincial chemotherapy treatment records were used to identify chemotherapy utilization. Pathology reports were obtained from the OCR and reviewed to collect information about extent of disease.
Measures and outcomes
VTE events were identified using the International Classification of Disease (ICD) version 9 and 10 codes for pulmonary embolism and deep vein thrombosis (Supplemental eTable 1). ICD codes listed on CIHI records for admissions to hospitals and visits to emergency departments six months before, and after, cystectomy were measured.
Indicators of the socioeconomic status (SES) of the community in which patients resided at time of diagnosis were linked to the OCR as described previously [19]. Co-morbidity was classified using the modified Charlson Index [20]. Each case was assigned a surgeon volume index as previously described [21].
Patients were considered to have been treated with NACT if there was an chemotherapy administered within 16 weeks prior to surgery; NACT could have started up to 6 months before surgery. ACT was defined as any chemotherapy administered within 16 weeks after surgery. Pathological variables are not reported for NACT patients since they are derived from surgical pathology reports and would not reflect the stage of disease at the time of NACT initiation.
Statistical analysis
Comparisons of proportions between study groups and across the study period were made using the chi-square test and the Cochran-Armitage test for trend respectively. Factors associated with VTE were evaluated by logistic regression stratified by treatment groups; variables included in the models were selected by the study team a priori. For those patients treated with NACT, we describe rates of VTE and risk factors in the 180-day pre-operative and 30-day/90-day post-operative settings (Table 2). For patients treated with either cystectomy alone or cystectomy and ACT, we describe 180-day pre-operative, and 30-day/90-day post-operative VTE rates (Table 3). Finally, to understand the rate of VTE during active chemotherapy, among those patients treated with ACT we describe rates of VTE within 90 and 120 days after the start of chemotherapy (Supplemental eTable 2). Factors associated with VTE were evaluated by logistic regression stratified by treatment groups.
As per institutional policy, data that relate to <6 patients are not reported due to small size. Results were considered statistically significant at p-value <0.05. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The study was approved by the Research Ethics Board of Queen’s University, Kingston, Canada (IRB number ONGY-335-07). This study was designed, analyzed, and reported in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement [22].
RESULTS
Study population
During 1994–2013, 5582 patients in Ontario underwent cystectomy for bladder cancer; 658 cases had non-urothelial histology and 674 cases had non-muscle-invasive disease. Patients with pT0/pT1 disease were not excluded if they had been treated with NACT (Supplemental eFigure 1). Forty-five (1%) of patients were treated with pre-operative chemotherapy for longer than 6 months; these cases were excluded as it was felt they might represent palliative chemotherapy with salvage cystectomy. The final study population contained 4205 patients (Table 1). Twenty-six percent (1084/4205) of patients received peri-operative chemotherapy; 6%, 18%, and 1% received NACT, ACT, NACT/ACT respectively. Chemotherapy regimen was identifiable for 58% (634/1084) of treated patients and included cisplatin or carboplatin in 85% (540/634) and 11% (68/634) of patients respectively. The most common regimens were Gemcitabine-Cisplatin (63%, 400/634), Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC)(16%, 103/634) and Gemcitabine-Carboplatin (9%, 59/634).
Table 1
Characteristics of all patients with muscle-invasive urothelial cancer treated with cystectomy in Ontario during 1994–2013 (n = 4205)
| Characteristic | All patients N = 4,205 | No VTE N = 3,842 | VTE N = 363 |
| Patient-related | |||
| Year of Surgery | |||
| 1994–1998 | 697 (17%) | 651 (17%) | 46 (13%) |
| 1999–2003 | 959 (23%) | 880 (23%) | 79 (22%) |
| 2004–2008 | 1,265 (30%) | 1,146 (30%) | 119 (33%) |
| 2009–2013 | 1,284 (31%) | 1,165 (30%) | 119 (33%) |
| Age, years | |||
| 20–49 | 136 (3%) | 122 (3%) | 14 (4%) |
| 50–59 | 502 (12%) | 460 (12%) | 42 (12%) |
| 60–69 | 1,085 (26%) | 999 (26%) | 86 (24%) |
| 70–79 | 1,652 (39%) | 1,498 (39%) | 154 (42%) |
| 80+ | 830 (20%) | 763 (20%) | 67 (18%) |
| Sex | |||
| Female | 1,023 (24%) | 930 (24%) | 93 (26%) |
| Male | 3,182 (76%) | 2,912 (76%) | 270 (74%) |
| Socioeconomic status by quintile1 | |||
| 1 | 847 (20%) | 765 (20%) | 82 (23%) |
| 2 | 948 (23%) | 873 (23%) | 75 (21%) |
| 3 | 907 (22%) | 829 (22%) | 78 (21%) |
| 4 | 773 (18%) | 715 (19%) | 58 (16%) |
| 5 | 691 (16%) | 624–629 (16%) | 63–68 (18%) |
| Unknown | 39 (1%) | 30–35 (1%) | ≤5 (1%) |
| Charlson comorbidity score | |||
| 0 | 2,880 (68%) | 2,644 (69%) | 236 (65%) |
| 1-2 | 1,101 (26%) | 994 (26%) | 107 (29%) |
| 3+ | 224 (5%) | 204 (5%) | 20 (6%) |
| Pathology-related2 | |||
| Tstage | |||
| <T3 | 1,135–40 (29%) | 1,073–78 (30%) | 62 (20%) |
| T3-T4 | 2,738 (71%) | 2,483 (70%) | 255 (80%) |
| Unstated | ≤5 (0%) | ≤5 (0%) | 0 (0%) |
| Nstage | |||
| Node negative | 1,944 (50%) | 1,812 (51%) | 132 (42%) |
| Node positive | 1,171 (30%) | 1,060 (30%) | 111 (35%) |
| NX | 763 (20%) | 689 (19%) | 74 (23%) |
| Lymphovascular invasion | |||
| No | 1,069 (28%) | 1,005 (28%) | 64 (20%) |
| Yes | 1,991 (51%) | 1,797 (50%) | 194 (61%) |
| Unstated | 818 (21%) | 759 (21%) | 59 (19%) |
| Treatment-related | |||
| Length of stay tertiles, days3 | |||
| <9 | 1,266 (30%) | 1,175 (31%) | 91 (25%) |
| 9–13 | 1,535 (37%) | 1,426 (37%) | 109 (30%) |
| 14+ | 1,404 (33%) | 1,241 (32%) | 163 (45%) |
| Peri-operative chemotherapy | |||
| Neoadjuvant | 270 (6%) | 237 (6%) | 33 (9%) |
| Adjuvant | 757 (18%) | 692 (18%) | 65 (18%) |
| Both neoadjuvant and adjuvant | 57 (1%) | 44 (1%) | 13 (4%) |
| No chemotherapy | 3,121 (74%) | 2,869 (75%) | 252 (69%) |
| System-related4 | |||
| Surgeon Volume by quartile | |||
| Q1 | 1,068 (25%) | 976 (25%) | 92 (25%) |
| Q2 | 1,172 (28%) | 1,092 (28%) | 80 (22%) |
| Q3 | 1,005 (24%) | 914 (24%) | 91 (25%) |
| Q4 | 952 (23%) | 850–855 (22%) | 96–101 (25%) |
| Unknown | 8 (0%) | 5–10 (0%) | ≤5 (1%) |
1Quintile 1 represents the communities where the poorest 20% of the Ontario population resided. Income data is missing for 39 patients. 2T stage, N stage, and LVI are not reported for NACT patients since these pathological variables (which are derived from surgical pathology reports) would not reflect the stage of disease at the time of initiation of NACT. Tstage data is unstated for <6 patients. 3Length of stay of hospital admission for cystectomy. 4Surgeon volume quartile 1 represents the lowest surgeon volumes. Surgeon volume data is missing for 8 patients.
VTE events
Nine percent (363/4205) of the study population experienced VTE within 6 months before or after cystectomy. The incidence rate of pre-operative and post-operative VTE was 1% and 8%, respectively. The incidence rate of VTE increased slightly over the study period (7% in 1994–1998 to 9% in 2009–2013, p = 0.019) (Supplemental eFigure 2).
The incidence rates of pre-operative and post-operative VTE in patients by treatment groups are shown in Fig. 1. Patients treated with NACT had a substantially higher rate of pre-operative VTE (4%) compared to patients not treated with pre-operative chemotherapy (0.5%, p <0.001). Post-operative rates of VTE within 6 months of cystectomy were comparable between patients treated with ACT and patients who did not receive chemotherapy (8% vs 8% p = 0.624). The cumulative rate of VTE within 6 months before and after cystectomy was substantially higher among those patients treated with NACT compared to patients with no chemotherapy or only ACT (12% vs 8% vs 9%, p = 0.002).
Fig.1
Crude incidence rates and timing of venous thromboembolism in 4205 patients with muscle-invasive bladder cancer treated with radical cystectomy by peri-operative chemotherapy groups in Ontario from 1994–2013. Abbreviations: VTE, venous thromboembolism NACT neoadjuvant chemotherapy, ACT, adjuvant chemotherapy, mos, Months. Post-cystectomy VTE rates are not cumulative and include VTEs that occurred during the hospital admission for cystectomy. Patients' earliest VTE is presented and those with pre-operative VTE are excluded from post-cystectomy VTE rates. Ranges are presented to prevent small cells from being calculated. The 57 patients who received neoadjuvant and adjuvant chemotherapy have been excluded from this figure to prevent numbers ≤6 from being calculated.
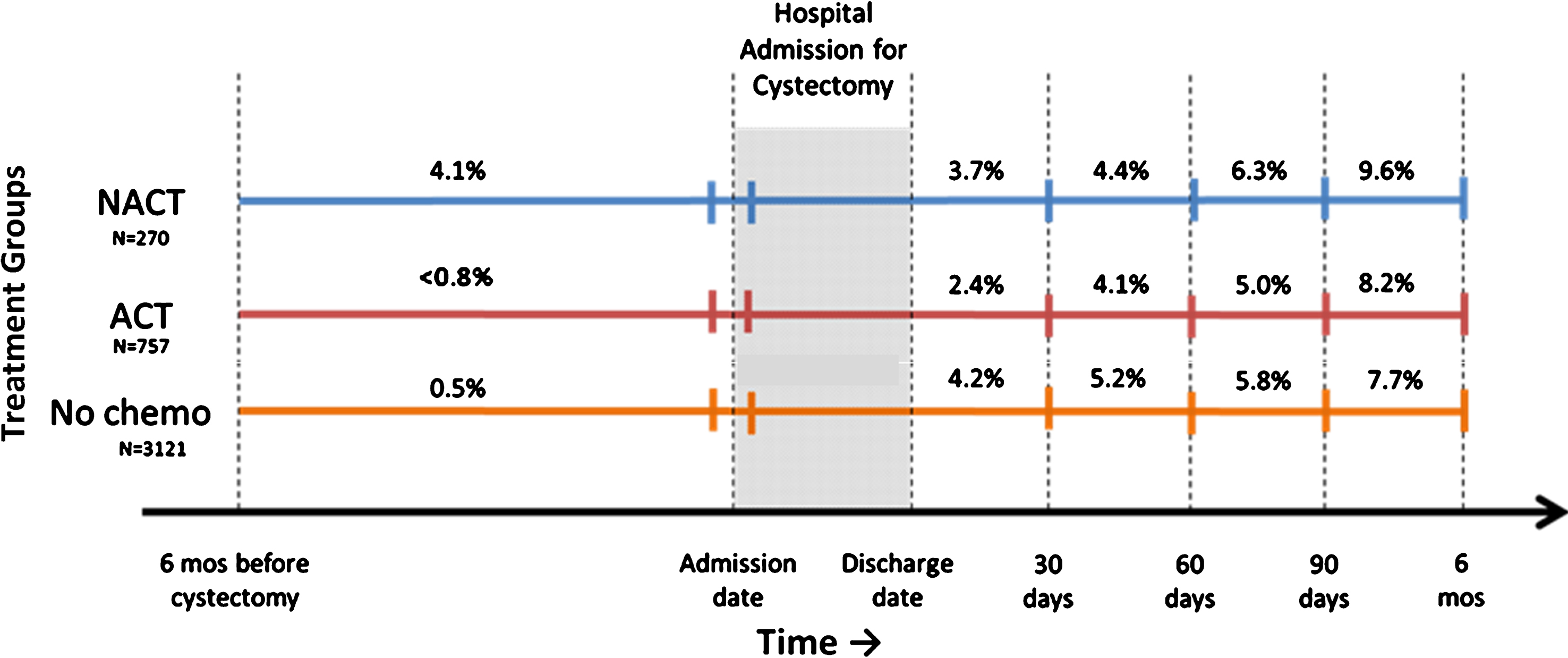
Timing of VTE relative to surgery is shown in Supplemental eFigure 3. Overall, 10%, 28% and 61% of VTEs were experienced before, during, and after the admission to hospital for cystectomy. Among patients treated with ACT, 51% of VTEs were experienced within 8 weeks and 82% within 16 weeks of starting chemotherapy (Supplemental eFigure 4).
Factors associated with VTE
Factors associated with VTE are shown in Tables 2, 3. In patients treated with NACT, the only factor identified that was associated with VTE within 90 days of surgery was length of hospital stay [OR 7.03 (95% CI 1.88–26.32) for 14+ days vs<9 days] (Table 2). Among patients who did not receive NACT, VTE within 30 days of surgery was independently associated with length of hospital admission (p < 0.001) and greater surgeon volume (p = 0.005) (Table 3). VTE within 90 days of surgery was associated with later years of surgery (p = 0.025), higher T stage (p = 0.024), greater length of hospital admission (p < 0.001) and greater surgeon volume (p = 0.006). Receipt of ACT was not associated with VTE within 90 days of cystectomy. Among the 772 patients treated with cystectomy and adjuvant chemotherapy we did not identify any patient- or disease-related factors that were associated with VTE within 90 or 120 days of starting chemotherapy (Supplemental eTable 2).
Table 2
Factors associated with VTE within 90 days of cystectomy among 3111 patients with bladder cancer treated with cystectomy and NACT in Ontario 1994–2013
| Characteristic | Proportion With VTE | Univariable2 analysis | Multivariable2 analysis | ||
| N = 311 | OR (95% CI) | P value | OR (95% CI) | P value | |
| Patient-related | |||||
| Year of Surgery | 0.057 | 0.308 | |||
| Unit = 1 year | – | 0.93 (0.86–1.00) | 0.96 (0.88–1.04) | ||
| Age, years | 0.229 | 0.186 | |||
| Unit = 1 year | – | 0.97 (0.93–1.02) | 0.97 (0.92–1.02) | ||
| Sex | 0.763 | 0.760 | |||
| Female | 7% | 1.18 (0.41–3.38) | 1.20 (0.38–3.75) | ||
| Male | 6% | Ref | Ref | ||
| Charlson comorbidity score | 0.654 | 0.833 | |||
| 0 | 6% | Ref | Ref | ||
| 1+ | 5% | 0.77 (0.25–2.40) | 1.15 (0.33–4.01) | ||
| Treatment-related | |||||
| Length of stay tertiles, days3 | 0.005 | 0.003 | |||
| <9 | 3% | Ref | Ref | ||
| 9–13 | 4% | 1.40 (0.37–5.32) | 1.04 (0.25–4.26) | ||
| 14+ | 15% | 5.68 (1.71–18.90) | 7.03 (1.88–26.32) | ||
| Adjuvant chemotherapy | 0.254 | 0.217 | |||
| No | 5% | Ref | Ref | ||
| Yes | 10% | 1.86 (0.64–5.42) | 2.08 (0.65–6.65) | ||
| Surgeon volume by quartile4 | 0.614 | 0.378 | |||
| Q1 | 8% | Ref | Ref | ||
| Q2 | 3% | 0.33 (0.06–1.69) | 0.33 (0.06–1.88) | ||
| Q3 | 6% | 0.74 (0.20–2.77) | 1.61 (0.36–7.30) | ||
| Q4 | 7% | 0.81 (0.26–2.51) | 1.37 (0.38–5.00) | ||
116 patients who had a previous VTE event during the 6 months preceding cystectomy were excluded. 2Logistic regression was used. 3Length of stay of hospital admission for cystectomy. 4Surgeon volume quartile 1 represents the lowest surgeon and hospital volumes.
Table 3
Factors associated with VTE within 30 and 90 days of cystectomy among 38571 patients with bladder cancer treated with cystectomy and no neoadjuvant chemotherapy in Ontario 1994–2013
| Characteristic | VTE within 30 days of cystectomy | VTE within 90 days of cystectomy | ||||
| % with VTE | Multivariable2 analysis OR (95% CI) | P value | % with VTE | Multivariable2 analysis OR (95% CI) | P value | |
| Patient-related | ||||||
| Year of Surgery | 0.054 | 0.024 | ||||
| Unit = 1 year | – | 1.03 (1.00–1.07) | – | 1.03 (1.00–1.06) | ||
| Age, years | 0.750 | 0.137 | ||||
| Unit = 1 year | – | 1.00 (0.98–1.01) | – | 0.99 (0.98–1.00) | ||
| Sex | 0.754 | 0.680 | ||||
| Female | 4% | 1.06 (0.72–1.56) | 6% | 1.07 (0.78–1.47) | ||
| Male | 4% | Ref | 5% | Ref | ||
| Charlson comorbidity score | 0.738 | 0.673 | ||||
| 0 | 4% | Ref | 5% | Ref | ||
| 1+ | 4% | 0.94 (0.66–1.35) | 6% | 1.07 (0.79–1.43) | ||
| Pathology-related | ||||||
| Tstage3 | 0.366 | 0.024 | ||||
| <T3 | 3% | Ref | 4% | Ref | ||
| T3-T4 | 4% | 1.20 (0.81–1.78) | 6% | 1.49 (1.05–2.10) | ||
| Nstage | 0.549 | 0.161 | ||||
| Node negative | 4% | Ref | 5% | Ref | ||
| Node positive | 4% | 0.90 (0.61–1.34) | 6% | 1.06 (0.75–1.50) | ||
| NX | 4% | 1.20 (0.75–1.93) | 6% | 1.45 (0.99–2.15) | ||
| Treatment-related | ||||||
| Length of stay tertiles, days4 | <0.001 | <0.001 | ||||
| <9 | 2% | Ref | 4% | Ref | ||
| 9–13 | 3% | 1.40 (0.83–2.38) | 4% | 1.31 (0.87–1.99) | ||
| 14+ | 6% | 3.76 (2.32–6.08) | 9% | 2.89 (1.96–4.25) | ||
| Adjuvant chemotherapy5 | – | 0.616 | ||||
| Yes | – | – | 5% | 0.90 (0.60–1.35) | ||
| No | – | – | 6% | Ref | ||
| System-related | ||||||
| Surgeon Volume by quartile6 | 0.005 | 0.006 | ||||
| Q1 | 4% | Ref | 5% | Ref | ||
| Q2 | 2% | 0.59 (0.36–0.98) | 4% | 0.81 (0.54–1.23) | ||
| Q3 | 4% | 1.25 (0.80–1.97) | 7% | 1.40 (0.95–2.06) | ||
| Q4 | 5% | 1.46 (0.91–2.33) | 7% | 1.57 (1.05–2.35) | ||
1348 patients who had a previous VTE event during the 6 months preceding cystectomy or were treated with NACT were excluded. 2Logistic regression was used. 3T stage data missing for <6 patients. 4Length of stay of hospital admission for cystectomy. 5Adjuvant chemotherapy was included in the 90 day model only. 6Surgeon volume quartile 1 represents the lowest surgeon and hospital volumes. Surgeon volume data missing for 8 patients.
DISCUSSION
In this population-based study, we describe incident rates of VTE among patients with bladder cancer treated with cystectomy and peri-operative chemotherapy in routine clinical practice. VTE events were identified using diagnostic codes from hospital admissions and/or emergency room visits. Accordingly, the events captured in this study and were likely symptomatic events and not incidental VTE identified on surveillance imaging. Several important findings have emerged. First, the incidence of any VTE within 6 months of surgery was 9% among all patients. Second, these rates have remained stable over the study period. Third, among patients treated with chemotherapy, the VTE rate was highest with NACT compared to ACT (12% vs 9%). Fourth, length of hospitalization and stage of disease are strongly associated with VTE risk independent of chemotherapy exposure. Finally, most VTEs were experienced after discharge for cystectomy.
Our results are consistent with several smaller studies reporting VTE rates of 9–18% [9, 11, 23–25]. These existing reports are limited by their small size, limited generalizability (i.e. single centre), often not describing in detail how VTEs were identified (symptom-driven vs incidental). Duivenoorden and colleagues have recently reported a multi-institutional study of 761 bladder cancer patients treated with NACT and cystectomy. In their study the VTE incidence rate was 11%; half of these events occurred before surgery which is very comparable to our study which reported a 4% VTE rate before cystectomy [9]. A retrospective chart review study of 202 patients treated with cystectomy at two centres in Canada reported an overall arterial/venous thrombosis rate of 8%; higher rates were observed with NACT(19%) [12].
In a retrospective cohort study of 1581 bladder cancer patients under age 65 by James et al., the incidence of VTE at three months post-RC was 10% and 45% of events occurred in the outpatient setting. Fourteen percent (216/1581) of patients received NACT, which was not found to be associated with VTE (OR1.57 95% CI 0.99–2.50) [6]. Vandlac et al. studied 1307 patients in a retrospective cohort study using chart data, and reported a VTE incidence of 6% (78/1307) after cystectomy with 55% diagnosed in the outpatient setting. Similar to the aforementioned study, NACT was not found to be associated with VTE, which the authors attributed to small numbers [14].
A systematic review and meta-analysis by Gopalakrishna et al., aimed to determine the rate of VTE in patients receiving systemic therapy for urothelial carcinoma. Included studies were published from 1985 to 2015 and a wide distribution of VTE rates was found across studies. 5082 patients were identified from 62 studies, with a median age of 65 and 55% of patients underwent surgery. The VTE rate was found to be 7%. Important limitations of this review were the heterogeneity in the study population, and incomplete information on several variables of interest and timing of systemic therapy and VTE in the included studies. Finally, rates of VTE did not significantly change over time, which the authors suggest may be related to poor development of guidelines and adherence for prophylaxis treatment [17].
Current guidelines recommend perioperative VTE prophylaxis for bladder cancer patients undergoing RC while in hospital [26–29]. Although there is a lack of consensus across all guidelines, recent guideline updates are support extended thromboprophylaxis [30, 31]. The 2012 American College of Chest Physicians guidelines [26], the 2017 European Association of Urology guidelines [32] and the 2018 NCCN guidelines [33] recommend 4 weeks of prophylaxis for patients undergoing major abdominal or pelvic surgery for cancer, while the American Urological Association [27] does not recommend extended duration prophylaxis. The Level 1 evidence supporting the use of extended thromboprophylaxis has largely been from studies of non-urologic cancer [34–36]. This combined with the variable recommendations for extended prophylaxis and provider concerns of post-operative hemorrhage might explain the lower compliance and slow uptake of extended thromboprophylaxis treatment for bladder cancer patients [37, 38].
Several studies have shown that a large proportion (45%–55%) of VTEs occur after discharge from hospital, [6, 14] including our previous work, [13]; this observation lends support to the role of extended thromboprophylaxis. In the present study, we found that 61% of VTE events occur after discharge. In 2017, a retrospective study by Pariser et al., of 402 RC patients, examined the post-RC VTE rate before and after the implementation of an extended enoxaparin prophylaxis regimen at their institution. The VTE rate before and after the regimen implementation was 28/234 (12%) and 9/168 (5%) (p = 0.024). Post-discharge VTE rates within 90 days of RC decreased from 6% to 2% (p = 0.039) and transfusion rates were similar across both time periods 44% vs 49% (p = 0.342) [15].
Although current guidelines have mixed recommendations, [26, 27] a growing body of literature exists to support the use of extended prophylaxis in patients undergoing RC for bladder cancer [6, 13, 14]. While an established threshold risk has not been agreed on, a VTE risk of at least 10% has been used for justification for primary thromboprophylaxis in several settings [39–43].
In the absence of a randomized trial to strengthen the quality of current evidence, and non-negligible bleeding risks accompanying prophylaxis treatment, identifying high risk VTE patients is one possible approach for selecting patients who would benefit most from extended thromboprophylaxis treatment. Our findings suggest there is a group of identifiable patients who are experiencing VTE after discharge with several key risk factors including advanced disease, extended hospital admissions and potentially receipt of chemotherapy. In addition, the association with higher surgeon volume likely reflects more complex patients receiving treatment at larger centers. It may also reflect differences in use of radiologic testing that may increase the proportion of patients diagnosed with low-volume VTE.
This study has limitations that merit comment. Incidence rates of VTE are potentially underestimated due to the requirement of a hospital interaction for data capture. However, it is worth noting that VTE events in our study were likely symptomatic since they were associated with a visit to the Emergency Room and/or admission to hospital. It is therefore likely that our data under-estimate the true risk as VTE managed in out-patient clinics is not captured. The available data-sets also do not allow us to identify those patients who were treated with anticoagulants or whom may have had a more remote VTE. We also did not have granular clinical information such as use of central venous catheters of laboratory indices (i.e. Khorana risk factors) to include in the models. As with all retrospective observational studies, unmeasured confounding variables could not be accounted for in the adjusted analyses. It is possible that some cases classified as having ACT were in fact being treated with palliative intent chemotherapy for early recurrent disease. Due to missing drug name/number of cycles we were also unable to explore the association between chemotherapy intensity/duration and VTE. In the present study, the incidence of any VTE within 6 months of cystectomy was found to be 10% in patients treated with chemotherapy and 8% in patients not treated with chemotherapy. Patients who do not receive chemotherapy are often older, have more comorbidity, and longer hospital admissions for cystectomy. The resulting higher baseline of rate of VTE in patients who do not receive chemotherapy, makes it difficult to demonstrate the additional increase in risk of VTE due to chemotherapy when comparing patient groups. Importantly, data regarding adherence to and methods of mechanical and pharmacological VTE prophylaxis and data regarding patients’ perioperative mobility were unavailable. It is unlikely during the study period (pre-2014) that there was widespread utilization of extended thromboprophylaxis among patients treated with RC. Although the rate of pre-operative VTE was much higher among those patients with NACT, it is not clear whether this is being driven by pro-thrombotic factors related to chemotherapy, the presence of central venous lines, or by the presence of more advanced disease. In this study we did not explore arterial thrombotic events; this is an area that required further study. Despite these limitations, in addition to the large sample size, a strength of this study is the fact that the study population included all cases of bladder cancer treated with curative intent within Ontario and was therefore unselected. By including the entire population of interest it is possible to minimise the selection biases that affect traditional institution-based observational studies.
In summary, our data suggest that a substantial proportion of patients with bladder cancer treated with peri-operative chemotherapy will develop VTE. The majority of these occur after discharge from hospital following cystectomy. Further work is needed to understand whether there is a role for thromboprophylaxis during neoadjuvant chemotherapy. Extended thromboprophylaxis after RC for high-risk patients including those who receive peri-operative chemotherapy should be considered.
FUNDING
Dr. Booth is supported as a Canada Research Chair in Population Cancer Care. This work was also supported by the Canada Foundation for Innovation and the Canadian Cancer Society Research Institute.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Parts of this material are based on data and information provided by Cancer Care Ontario and CIHI. However, the analysis, conclusions, opinions and statements expressed herein are those of the authors and not necessarily those of Cancer Care Ontario or CIHI.
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by CIHI. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources and CIHI. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Dr. Booth had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: 10.3233/BLC-180184.
REFERENCES
[1] | Heit JA , Silverstein MD , Mohr DN , Petterson TM , O’Fallon WM , Melton LJ . Risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med. (2000) ;160: (6):809. 10.1001/archinte.160.6.809. |
[2] | Dyer J , Wyke S , Lynch C . Hospital episode Statistics data analysis of postoperative venous thromboembolus in patients undergoing urological surgery: A review of 126,891 cases. Ann R Coll Surg Engl. (2013) ;95: (1):65–69. 10.1308/003588413X13511609956219. |
[3] | Clément C , Rossi P , Aissi K , et al. Incidence, risk profile and morphological pattern of lower extremity venous thromboembolism after urological cancer surgery. J Urol. (2011) ;186: (6):2293–7. 10.1016/j.juro.2011.07.074. |
[4] | Alberts BD , Woldu SL , Weinberg AC , Danzig MR , Korets R , Badani KK . Venous thromboembolism after major urologic oncology surgery: A focus on the incidence and timing of thromboembolic events after 27,455 operations. Urology. (2014) ;84: (4):799–806. 10.1016/j.urology.2014.05.055. |
[5] | Schmid M , Abraham Chiang H , Sood A , et al. Causes of hospital readmissions after urologic cancer surgery. Urol Oncol Semin Orig Investig. (2016) ;34: (236):236.e1–236.e11. 10.1016/j.urolonc.2015.11.019. |
[6] | James AC , Holt SK , Wright JL , Porter MP , Gore JL . Burden and timing of venothrombolic events in patients younger than 65 years undergoing radical cystectomy for bladder cancer. Urol Oncol. (2014) ;32: (6):815–9. 10.1016/j.urolonc.2014.02.016. |
[7] | Sun AJ , Djaladat H , Schuckman A , Miranda G , Cai J , Daneshmand S . Venous thromboembolism following radical cystectomy: Significant predictors, comparison of different anticoagulants and timing of events. J Urol. (2015) ;193: (2):565–9. 10.1016/j.juro.2014.08.085. |
[8] | Sandhu R , Pan C-X , Wun T , et al. The incidence of venous thromboembolism and its effect on survival among patients with primary bladder cancer. Cancer. (2010) ;166: (4):NA–NA 10.1002/cncr.25004. |
[9] | Tully CM , Apolo AB , Zabor EC , et al. The high incidence of vascular thromboembolic events in patients with metastatic or unresectable urothelial cancer treated with platinum chemotherapy agents. Cancer. (2016) ;122: (5):712–21. 10.1002/cncr.29801. |
[10] | Murray KM , Parker W , Stephany H , et al. Venous thromboembolism after radical cystectomy: Experience with screening ultrasonography. Arab J Urol. (2016) ;14: (1):37–43. 10.1016/j.aju.2015.11.002. |
[11] | Moore RA , Adel N , Riedel E , et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J Clin Oncol. (2011) ;29: (25):3466–73. 10.1200/JCO.2011.35.5669. |
[12] | Zareba P , Patterson L , Pandya R , et al. Thromboembolic events in patients with urothelial carcinoma undergoing neoadjuvant chemotherapy and radical cystectomy. Urol Oncol (2014) ;32: (7):1–6. 10.1016/j.urolonc.2014.03.025. |
[13] | Doiron RC , Booth CM , Wei X , Siemens DR . Risk factors and timing of venous thromboembolism after radical cystectomy in routine clinical practice: A population-based study. BJU Int. (2016) ;118: (5):714–22. 10.1111/bju.13443. |
[14] | Vandlac AA , Cowan NG , Chen Y , et al. Timing, incidence and risk factors for venous thromboembolism in patients undergoing radical cystectomy for malignancy: A case for extended duration pharmacological prophylaxis. J Urol (2014) ;191: (4):943–7. 10.1016/j.juro.2013.10.096. |
[15] | Pariser JJ , Pearce SM , Anderson BB , et al. Extended duration enoxaparin decreases the rate of venous thromboembolic events after radical cystectomy compared to inpatient only subcutaneous heparin. J Urol. (2017) ;197: (2):302–7. 10.1016/j.juro.2016.08.090. |
[16] | Duivenvoorden WCM , Daneshmand S , Canter D , et al. Incidence, characteristics and implications of thromboembolic events in patients with muscle invasive urothelial carcinoma of the bladder undergoing neoadjuvant chemotherapy. J Urol (2016) ;196: (6):1627–33. 10.1016/j.juro.2016.06.017. |
[17] | Gopalakrishna ASM , Longo TA , et al. High rates of venous thromboembolic events in patients undergoing systemic therapy for urothelial carcinoma: A systematic review and meta-analysis. Urol Oncol Semin Orig Investig. (2016) ;34: :1–8. 10.1016/j.urolonc.2016.05.009. |
[18] | Clarke EA , Marrett LD , Kreiger N . Cancer registration in Ontario: A computer approach. IARC Sci Publ. (1991) ;(95):246–57. http://www.ncbi.nlm.nih.gov/pubmed/1894327. |
[19] | Booth CM , Li G , Zhang-Salomons J , Mackillop WJ . The impact of socioeconomic status on stage of cancer at diagnosis and survival: A population-based study in Ontario, Canada. Cancer. (2010) ;116: (17):4160–7. 10.1002/cncr.25427. |
[20] | Deyo R , Cherkin D , Ciol M . Adapting a clinical comorbidity index for use with ICD-9-CM Administrative Databases. J Clin Epidemiol. (1992) ;45: :613–9. 10.1016/0895-4356(92)90133-8. |
[21] | Siemens DR , Mackillop WJ , Peng Y , et al. Processes of care and the impact of surgical volumes on cancer-specific survival: A population-based study in bladder cancer. Urology. (2014) ;84: (5):1049–57. 10.1016/j.urology.2014.06.070. |
[22] | von Elm E , Altman DG , Egger M , et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. (2007) ;335: (7624):806–8. 10.1136/bmj.39335.541782.AD. |
[23] | Czaykowski PM , Moore MJ , Tannock IF . High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. (1998) ;160: (6 Pt 1):2021–4. http://www.ncbi.nlm.nih.gov/pubmed/9817314. |
[24] | Botten J , Sephton M , Tillett T , et al. Thromboembolic events with cisplatin-based neoadjuvant chemotherapy for transitional cell carcinoma of urinary bladder. In: Journal of Clinical Oncology. (2013) ;31. http://meeting.ascopubs.org/cgi/content/abstract/31/6_suppl/277?sid=73caa98f–4cae-967a-82d42efb9909%5Cnhttp://ovidsovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed11&NEWS=N&AN=71085156. |
[25] | Patterson L , Pandya R , Margel D , et al. Incidence, characteristics and implications of thromboemboli events in patients with muscle invasive bladder cancer undergoing cisplatin based neo-adjuvant chemotherapy. J Urol. (2013) ;189: (4):590–1. |
[26] | Gould MK , Garcia DA , Wren SM , et al. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2012) ;141: (2 SUPPL.):227–77. 10.1378/chest.11-2297. |
[27] | Forrest JB , Clemens JQ , Finamore P , et al. AUA Best Practice Statement for the prevention of deep vein thrombosis in patients undergoing urologic surgery. J Urol. (2009) ;181: (3):1170–7. 10.1016/j.juro.2008.12.027. |
[28] | Streiff B , Holmstrom B , Ashrani A , et al. Cancer-associated venous thromboembolic disease, version 1. 2015. J Natl Compr Canc Netw. (2015) ;13: (9):1079. |
[29] | Farge-Bancel D , Bounameaux H , Brenner B , et al. Implementing thrombosis guidelines in cancer patients: A review. Rambam Maimonides Med J. (2014) ;5: (4):e0041. doi: 10.5041/RMMJ.10175 . |
[30] | Tikkinen KAO , Craigie S , Agarwal A , et al. Procedure-specific risks of thrombosis and bleeding in urological cancer surgery: Systematic review and meta-analysis. Eur Urol. (2018) ;73: (2):242–51. doi: 10.1016/j.eururo.2017.03.008 . |
[31] | Klaassen Z , Arora K , Goldberg H , et al. Extended venous thromboembolism prophylaxis after radical cystectomy: A call for adherence to current guidelines. J Urol. (2018) ;199: (4):906–14. doi: 10.1016/j.juro.2017.08.130 . |
[32] | European Association of Urology. Guidelines on Thromboprophylaxis in Urological Cancer Surgery. (2017) . |
[33] | National Comprehensive Cancer Network. Cancer-Associated Venous Thromboembolic Disease, Version 1.2018. (2018) . |
[34] | Bergqvist D , Agnelli G , Cohen AT , et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. (2002) ;346: (13):975–80. doi: 10.1056/NEJMoa012385 . |
[35] | Rasmussen MS , Jorgensen LN , Wille-Jørgensen P , et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: A multicenter randomized open-label study. J Thromb Haemost. (2006) ;4: (11):2384–90. doi: 10.1111/j.1538-7836.2006.02153.x . |
[36] | Agnelli G , Gussoni G , Bianchini C , et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. (2009) ;10: (10):943–9. doi: 10.1016/S1470-2045(09)70232-3 . |
[37] | Pridgeon S , Allchorne P , Turner B , Peters J , Green J . Venous thromboembolism (VTE) prophylaxis and urological pelvic cancer surgery: A UK national audit. BJU Int. (2015) ;115: (2):223–9. doi: 10.1111/bju.12693 . |
[38] | Sterious S , Simhan J , Uzzo RG , et al. Familiarity and self-reported compliance with American urological association best practice recommendations for use of thromboembolic prophylaxis among american urological association members. J Urol. (2013) ;190: (3):992–8. doi: 10.1016/j.juro.2013.03.076 . |
[39] | Cohen AT , Davidson BL , Gallus AS , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: Randomised placebo controlled trial. BMJ. (2006) ;332: (7537):325–9. doi: 10.1136/bmj.38733.466748.7C . |
[40] | Khorana AA , Kuderer NM , Culakova E , Lyman GH , Francis CW . Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. (2008) ;111: (10):4902–7. doi: 10.1182/blood-2007-10-116327 . |
[41] | Rajkumar SV , Jacobus S , Callander NS , et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. (2010) ;11: (1):29–37. doi: 10.1016/S1470-2045(09)70284-0 . |
[42] | Lyman GH , Bohlke K , Khorana AA , et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. (2015) ;33: (6):654–6. doi: 10.1200/JCO.2014.59.7351 . |
[43] | Srikanthan A , Tran B , Beausoleil M , et al. Large retroperitoneal lymphadenopathy as a predictor of venous thromboembolism in patients with disseminated germ cell tumors treated with chemotherapy. J Clin Oncol. (2015) ;33: (6):582–7. doi: 10.1200/JCO.2014.58.6537 . |




