Thermo Reversible Hydrogel Based Delivery of Mitomycin C (UGN-101) for Treatment of Upper Tract Urothelial Carcinoma (UTUC)
Abstract
Background:
There is an unmet need for an effective local treatment of upper tract urothelial carcinoma (UTUC). Drug delivery to the pyelocaliceal system and pursuant efficacy of intracavitary therapy is limited by urine production that washes the drug away, shortening dwell time and direct contact with the urothelium. Successful endoscopic management is often dictated by lesion size, grade, and focality. A thermo-reversible hydrogel formulation of Mitomycin C (UGN-101, formerly MitoGel) was developed and has demonstrated the safety and feasibility of increased time of the drug in the pyelocaliceal system resulting in chemoablation of tumors.
Objectives:
To examine the efficacy and safety of UGN-101 used for chemoablation of UTUC.
Methods:
There were 22 patients approved for compassionate use treatment at 14 institutions. Six-weekly instillations of UGN-101 were administered via ureteral catheter or percutaneous nephrostomy. Ureteroscopy was performed 2–6 weeks following treatment completion for response determination. Adverse events were recorded throughout treatment. Patients were followed up according to the standard of care of each center.
Results:
Median age of the cohort was 75 yrs., with 16 (73%) males. Eighteen patients had low-grade (LG) tumors, 2 high-grade (HG), and 2 had indeterminate grade. Median volume of UGN-101 instilled was 13.5cc with median Mitomycin dosage of 54 mg. Eight patients had complete response (36%) including 44% (8/18) of the patients with low grade tumors. Partial responses were observed in 23% and 28% of LG. Two patients had no response (9%) and 1 did not undergo ureteroscopy. Four patients could not complete 6 weeks due to adverse events (pyelonephritis, acute renal failure, pancytopenia, and unstable cardiac condition); 1 patient was diagnosed with a non-urothelial cancer during treatment; and 1 patient died prior to the third instillation due to suspected pulmonary embolus, determined to be unrelated to treatment with UGN-101. Out of the patients who had a complete response, 3 (37.5%) patients are recurrence free from 18–30 months. A total of 83 adverse events were recorded. Of these, 6 events related to UGN-101 were serious (requiring medical intervention), and 23 events related to UGN-101 were not serious.
Conclusions:
This compassionate use program of UGN-101 demonstrates proof of concept for chemoablation treatment of UTUC. A single arm Phase III multi-center registration trial to treat patients with low-grade low-volume renal pelvis tumors is open and enrolling patients (NCT02793128).
INTRODUCTION
Upper tract urothelial cancer (UTUC) is a rare malignancy and difficult to study in a randomized controlled setting [1]. Treatment guidelines are based mainly on retrospective series and expert opinion rather than strong (grade A) evidence [2]. Current practice is guided primarily by the clinical stage and histologic grade of the cancer. The European Association of Urology guidelines incorporate individual risk factors including location (ureter vs. renal pelvis) and extent of disease, tumor size, cytology, imaging, and previous history of urologic cancers [2]. Although these guidelines assist the clinical decision-making process of practicing urologists, when possible, the gold standard treatment for high-risk UTUC remains radical nephroureterectomy (NU) [3, 4]. There remains a large unmet need for an effective nephron-sparing treatment with comparable outcomes to NU.
Certain patients are not suitable candidates for NU and may require nephron-sparing therapies, including UTUC in a solitary kidney, low-grade and low-stage UTUC, patients with chronic kidney disease, and non-surgical candidates. Endoscopic or percutaneous treatment is a viable treatment option in patients with focal, low-grade, non-invasive disease. The recurrence rates are higher in patients managed endoscopically rather than with NU and range from 30–50% with 20–30% of patients eventually requiring NU [5–7]. At highly experienced centers, this minimally invasive approach has had comparable 5-year disease specific survival (DSS) to NU in patients with low-stage and low-grade disease, though careful patient selection is required for such cases [8].
Intracavitary, topical therapy (Bacillus Calmette-Guerin (BCG), interferon with BCG, thiotepa, Mitomycin C, and epirubicin) in aqueous formulation is infrequently given as an adjunct to endoscopic treatment for residual disease or for prophylaxis in non-metastatic stage Ta and T1 UTUC [2, 7]. Topical therapy can also be used for carcinoma in situ (CIS). Though numerous studies have examined endoscopic treatment with adjuvant intracavitary chemotherapy, there have not been studies examining the effectiveness of intracavitary therapy alone for tumor ablation [5]. There are no studies to date examining the efficacy of primary intracavitary therapy for UTUC like there have been for low-grade, non-muscle invasive urothelial carcinoma of the bladder. Distinct differences in anatomy hinder effective use of instillation therapy in the upper urinary tract. Furthermore, the continual production of urine and ureteral peristalsis are the two primary factors that limit the adequate delivery of intracavitary chemotherapy.
A temperature sensitive water-soluble gel formulation of Mitomycin C (UGN-101; formerly MitoGel; UroGen Pharma, Israel) has been formulated. UGN-101 has demonstrated increased drug delivery time (4 to 6 hours) and safety in the pelvicalyceal system of porcine and human bladders [9, 10]. During preparation, UGN-101 is cooled and becomes a liquid, which allows for instillation via an antegrade or retrograde approach. As UGN-101 warms to body temperature upon contact with the urothelium, the liquid becomes a hydrogel that conforms to the anatomy of each patient’s upper tract. The production of urine causes the gel’s slow dissolution. Immediate urine production has been detected upon UGN-101 administration to the upper tract of porcine with no evidence of renal insufficiency or obstruction [9].
This new formulation of mitomycin C is a promising treatment for those who have no other therapeutic options. Thus, UGN-101 has been granted Fast Tract and Orphan Designation Status by the Food and Drug Administration (FDA).
This retrospective cohort study aims to examine the feasibility, efficacy, and safety of UGN-101 administration for UTUC on a compassionate use basis. UGN-101 is available for patients who suffer from a life-threatening or debilitating disease and have been treated unsuccessfully with approved existing medications, or for whom, in the opinion of their treating physician, other treatment options have been exhausted.
MATERIALS AND METHODS
Regulatory and Institutional Review Board (IRB) approval
Compassionate use approval was obtained on an individual patient basis from the respective regulatory authorities and IRBs. In the United States, UGN-101 was granted Orphan Designation Status by the FDA (09/08/2014) prior the study’s inception.
UGN-101 chemistry and formulation
UGN-101 consists of RTGel, a poloxamer-based hydrogel combined with hydroxy propyl methyl cellulose (HPMC) and polyethylene glycol (PEG), which is formulated with mitomycin C prior to instillation.
Patient selection
Retrospective chart review was performed at 14 institutions in 5 countries (Austria, Netherlands, Israel, Switzerland, and the United States). A total of 22 patients were approved for treatment. Tumor grade was designated as either low-grade (LG) or high-grade (HG), and patients without tumor biopsies were designated indeterminate grade (I/D).
UGN-101 administration
The treatment regimen included 6-weekly instillations of UGN-101 instilled via percutaneous nephrostomy tube (2) or ureteral catheter and performed without anesthesia in most patients. Two patients received twice-weekly instillations. Two patients received maintenance treatment following a complete response (CR), consisting of 3 or 4 additional treatments of UGN-101. The volume of UGN-101 ranged from 5–20 cc and the concentration of Mitomycin was between 2–6 mg/cc. Target volume was determined by contrast assessment of the renal pelvis on an individual patient basis by the investigator. Three patients received doses of 2 mg/cc, 3 patients (two of them diagnosed with high-grade tumor) received doses of 6 mg/cc, and 18 received doses of 4 mg/cc.
Efficacy and safety monitoring of UGN-101
Adverse events were recorded throughout treatment and throughout patient follow-up. Ureteroscopy was performed 2–6 weeks following treatment completion for response determination with cytology and biopsies if indicated and feasible. A complete response (CR) was defined as either tumor necrosis or no evidence of neoplasm on cytology or biopsy. Partial response (PR) was defined as decrease in tumor size with evidence of persistent cancer by cytology or biopsy after completion of treatment. No response (NR) was defined as an increase or no change in tumor size after treatment.
RESULTS
Patient demographics
The median age of the cohort was 75 years (range 57 to 92 years), with 16 males (73%). Eighteen patients had LG tumor (81.8%), 2 had HG tumor (9.1%), and 2 were classified as indeterminate grade (9.1%). Ten patients had a solitary kidney (45.5%), 17 had un-resectable able tumor due to location and/or tumor volume (77.3%), 3 additional patients were treated as chemoablation and adjuvant and 2 were treated as adjuvant. The ten patients with a solitary kidney were treated in order to avoid dialysis. Two patients were high-risk for general anesthesia (9%) and therefore were not considered candidates for nephroureterectomy (NXU). In the remaining 9 patients, the tumor(s) could not be ablated via ureteroscopy and therefore chemoablation was chosen as an alternative to NXU (7) or the patient refused NXU (2). Out of the patients who had complete response, 3 (37.5%) patients are recurrence free >12 months (18; 28 and 30 months), 1 recurred at 4 months, 1 at 8 months and 2 at 9 months. One patient who was CR is lost to follow up (Supplemental Table 1). One patient who was determined to be PR on PDE on visual inspection turned to CR after 3 monthly maintenance treatments and 11 months following was found to have a recurrence. See Table 1 for a summary of patient demographics and UGN-101 administration. Treatment and outcome is described for each patient in Supplemental Table 1.
Table 1
Patient demographics
| Patient Demographics | |||
| Median age: | 75 years (range 57 – 92) | ||
| Males (%) | 15 (68.2%) | ||
| Histologic grade: | 18 LG | 2 HG | 2 N/D* |
| Solitary kidney: | 10 (45.5%) | ||
| Non-resectable: | 17 (77.3%) | ||
| High-risk anesthesia: | 3 (13.6%) |
*HG - High grade; LG - Low grade; N/D = not determined /indeterminate grade.
UGN-101 administration
Of the 22 patients, 16 (72.7%) patients completed 6 weeks of UGN-101 treatment and 15 were evaluated by endoscopy (Fig. 1). Two of the patients with complete response received maintenance treatment with 3–5 additional instillations of UGN-101. Figure 2 demonstrates the correct retrograde catheter placement for UGN-101 instillation. Fluoroscopy was used to ensure proper delivery of chemotherapy to the upper tract and aid in determining adequate volume of UGN-101 delivery. Time-lapse fluoroscopy in a porcine model (Fig. 3) demonstrates retention time of greater than 6 hours in the upper tract and the gradual dissolution of UGN-101 over time.
Fig.1
Response to treatment. URS – ureteroscopy.
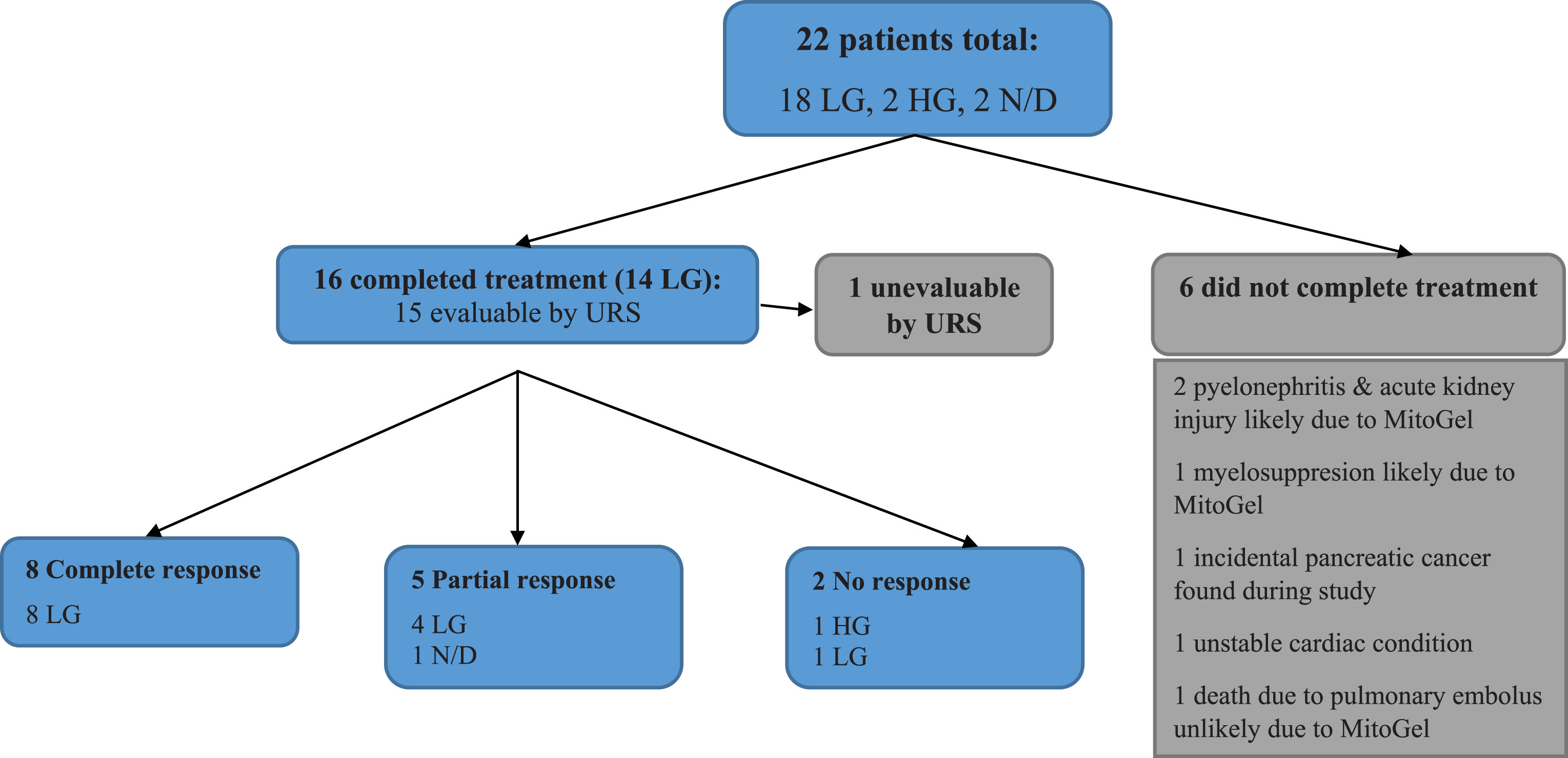
Fig.2
Intraoperative fluoroscopy helps facilitate correct placement of retrograde catheter for instillation of MitoGel into the upper tract.
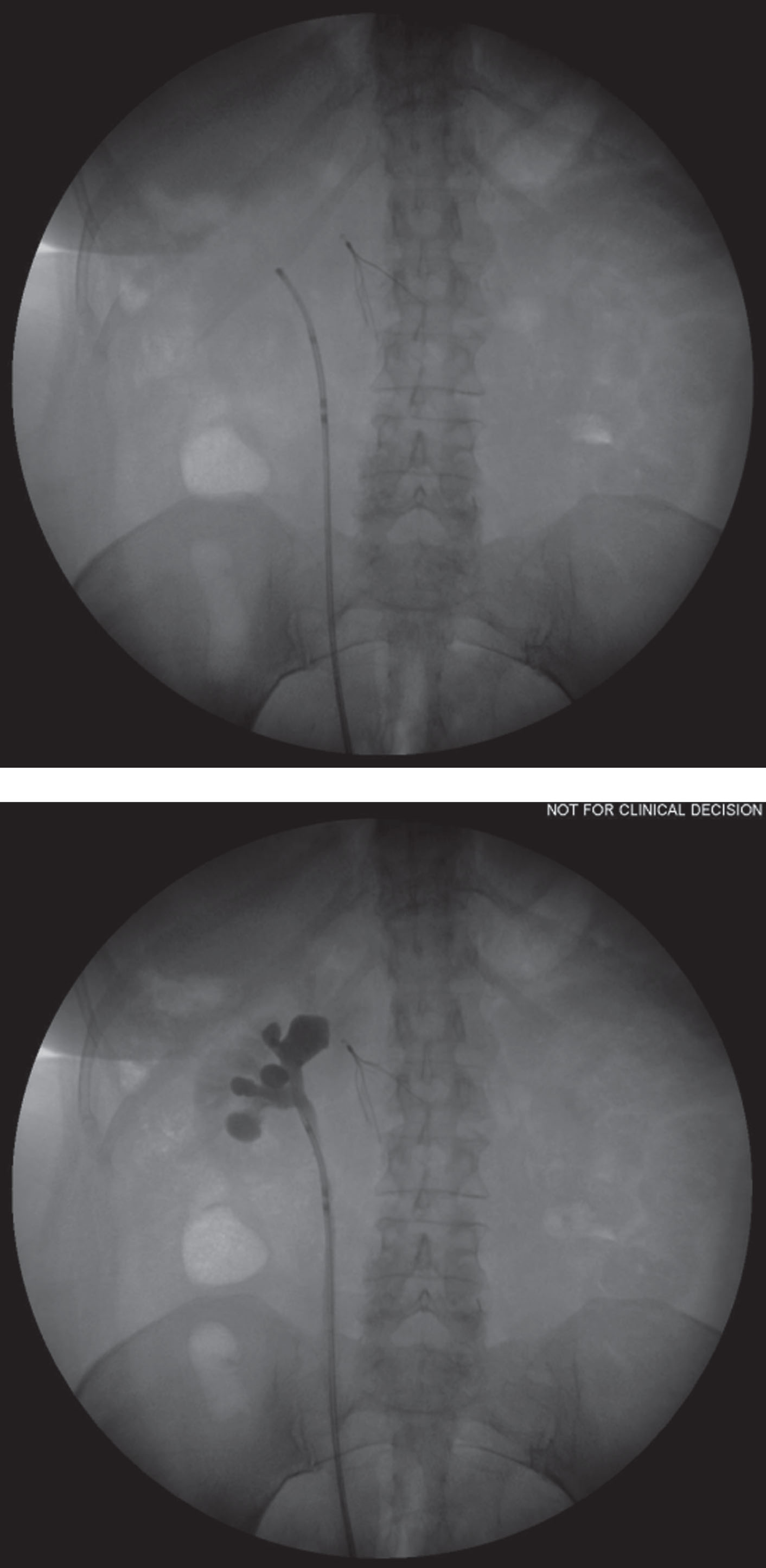
Fig.3
Time lapse fluoroscopy in a swine model demonstrates the prolonged indwelling time of intracavitary chemotherapy of >6 hours.
Response to UGN-101 intracavitary chemotherapy
Eight patients had a CR (36%) including 44% of patients with low-grade disease; 5 had PR (23% and 28%, respectively); and 2 patients had NR (9.1%) to UGN-101. One patient did not undergo URS and therefore is not evaluable. All 13 patients who had a CR or PR had LG tumor on initial biopsy. Of the 2 patients with no response to UGN-101, 1 had a tumor classified as indeterminate, and 1 as HG. Figure 1 and Table 2 outline the treatment outcomes by histologic grade. The 8 patients who achieved an initial CR were followed for durability of response with initial data demonstrating at least 12 months and up to 30 months without recurrence in 3 patients.
Table 2
UTUC response to MitoGel table
| Type enrolled | LG | HG | N/D |
| N | 18 | 2 | 2 |
| Completed treatment | 14 | 1 | 1 |
| Evaluated by URS | 13 | 1 | 1 |
| CR/PR/NR* | 8/4/1 | 0/0/1 | 0/1/0 |
*HG - High grade; LG - Low grade; CR – complete response; PR – partial response; NR – no response. N/D = not determined/indeterminate grade.
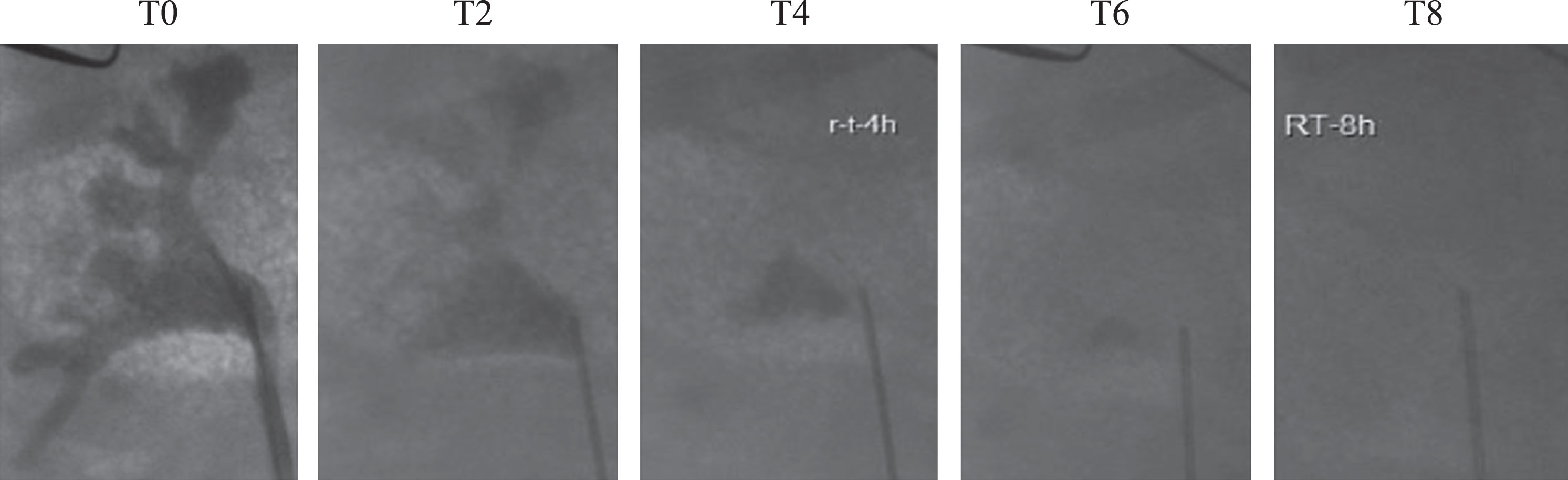
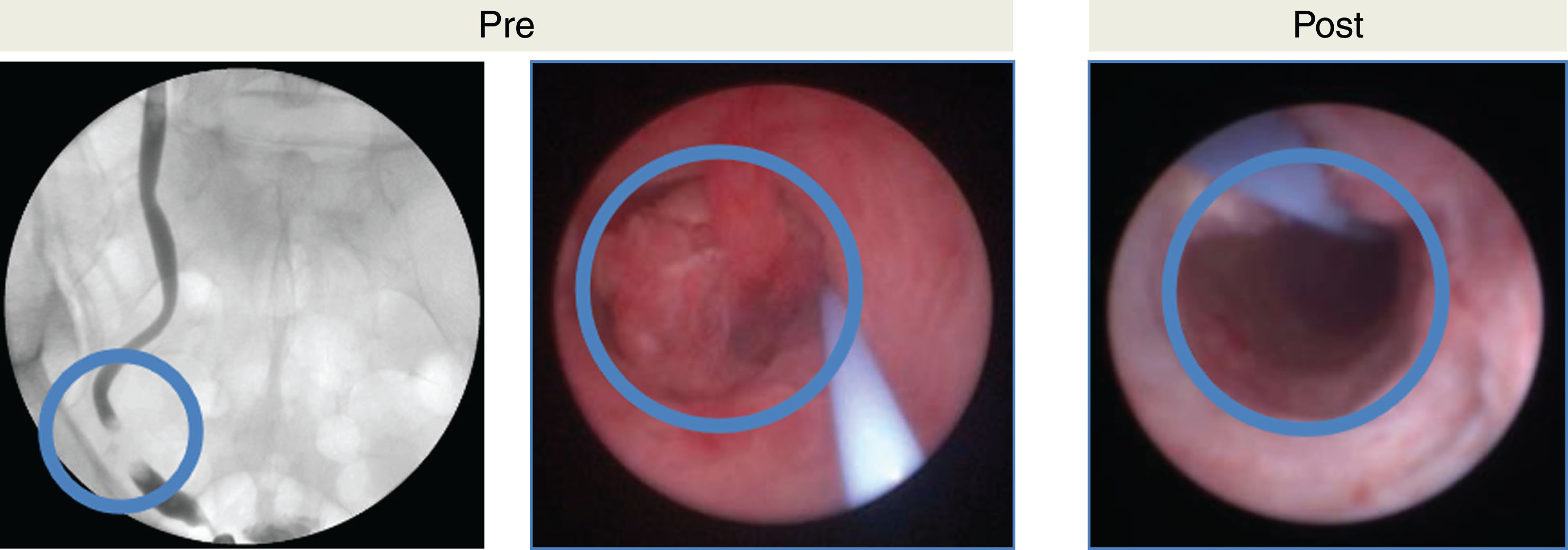
Figures 4 and 5 demonstrate efficacy of UGN-101 for tumors in different anatomic locations of the upper tract. Figure 4 shows a small, LG tumor of the renal pelvis. After 6 weeks of UGN-101, biopsy of the residual tumor bed showed tumor necrosis and no viable cancer. Figure 5 demonstrates a non-obstructive distal ureteral tumor that after 6 weeks of UGN-101 treatment, no viable tumor was evident on ureteroscopy or biopsy and the patient remains free of recurrence at 21 months.
Fig.4
Top image displays small renal pelvis tumor captured on ureteroscopy. Following several weeks of intracavitary MitoGel demonstrated chemoablation with necrotic tumor and no evidence of viable cancer.
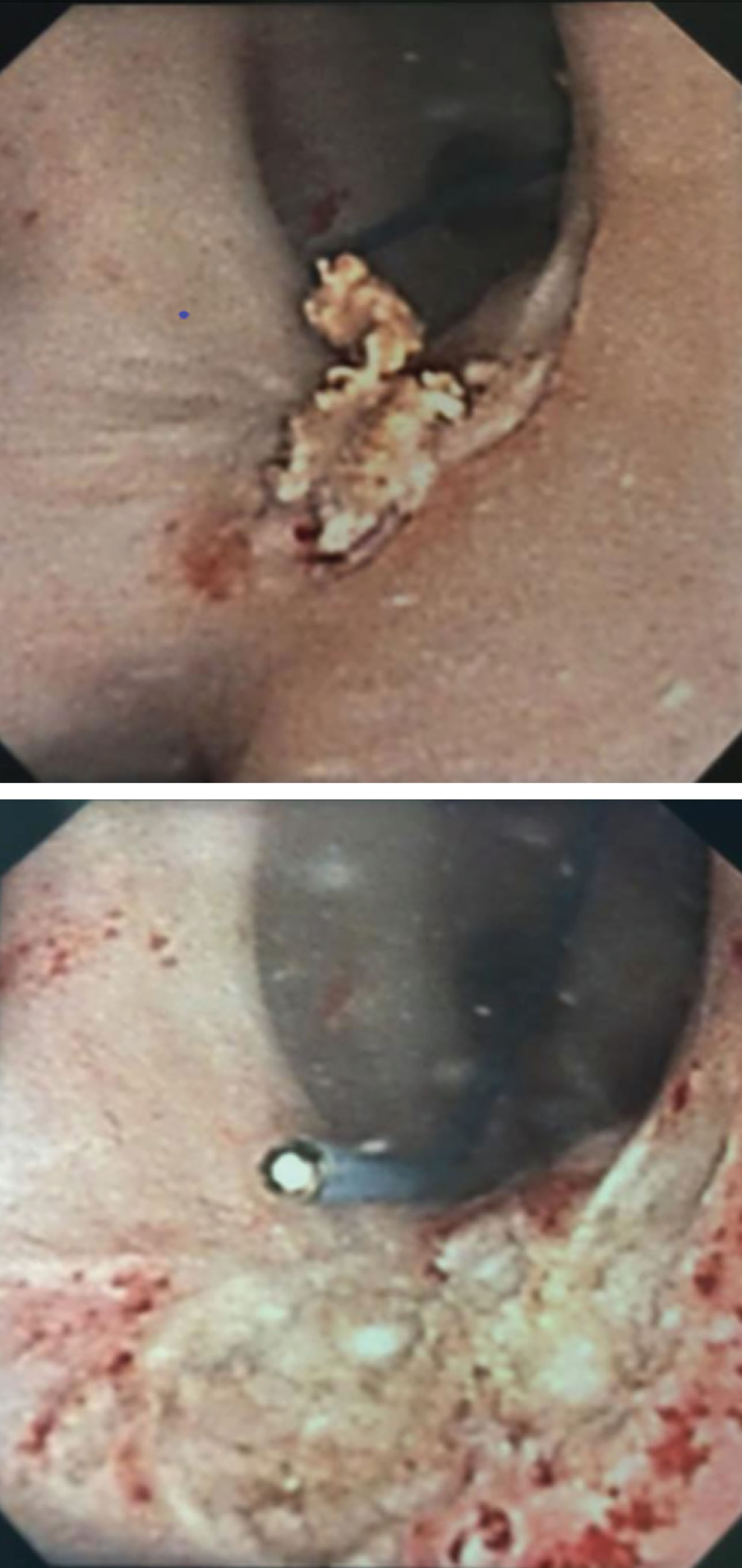
Fig.5
Chemoablative affects of MitoGel demonstrated in ureteroscopy. Left image demonstrates filling defect in lower ureter, corresponding to middle image captured prior to MitoGel treatment demonstrating large tumor protruding into lumen of ureter. Right image demonstrates chemoablative affects of tumor status-post 6 weeks of MitoGel treatment with no tumor evident on ureteroscopy.
Early treatment cessation
Six patients did not complete treatment. Of these, treatment was discontinued in three patients due to adverse events: one patient with high grade disease based on risk benefit analysis suggesting that the patient would not benefit from continued treatment in the trial related to unstable cardiac condition; one patient developed myelosuppression due to simultaneous dosing of bladder and upper tract and was withdrawn from the program by the treating center; one patient developed narrowing of the ureter, which caused difficulty with instillation and the patient was withdrawn from the program.
One patient was diagnosed with a second, non-urothelial cancer (pancreatic adenocarcinoma) after the first instillation of UGN-101, resulting in the cessation of treatment. One patient with a history of pulmonary embolus (PE) died prior to the third instillation due to suspected PE, determined to be unrelated to UGN-101 treatment. One patient was lost to follow-up.
Adverse events
A total of 83 adverse events were recorded during the compassionate use program. There were 29 events attributed to UGN-101, and of these, 6 were considered serious (requiring intervention). Table 3 describes the adverse events and Table 4 lists the type and frequency of events. Serious and UGN-101-related adverse events included hydronephrosis, severe upper tract inflammation, acute kidney injury exacerbating chronic kidney disease, pancytopenia, and hyperkalemia. These events affected 4 patients (22.2%), resulting in 1 patient discontinuing UGN-101 therapy. Serious adverse events determined not related to UGN-101 were death from pulmonary embolus, radical cystectomy secondary to primary bladder urothelial carcinoma, acute pyelonephritis, second primary metastatic pancreatic carcinoma, severe arrhythmia, and recurrent cardiac asthma. Four patients discontinued UGN-101 therapy due to treatment-related toxicities.
Table 3
Adverse events
| Adverse events | MitoGel-related | Not related |
| Serious | 6 | 9 |
| Not serious | 23 | 44 |
Table 4
Adverse events detailed with type of event and frequency noted in parenthesis if greater than one
| MitoGel-related | Not related | |||
| Serious | Not serious | Serious | Not serious | |
| General: | Chills, Fatigue (2), Recurrent fever, Weakness, | Death | Discomfort, Dizziness, Drowsiness, Fatigue (2), Fevers and chills, | |
| Genitourinary: | Hydronephrosis, Severe inflammation of upper tract | Bladder inflammation Dysuria (3) Frequency Narrowing at previous tumor site Nephrostomy tube obstruction | Acute pyelonephritis (3), Radical cystectomy | Asymptomatic bacteriuria (2), Asymptomatic rupture of fornix with extravasation, Bladder spasms, Gross hematuria, Macroscopic hematuria (3), Urethral stricture, Urinary retention Acute kidney injury |
| Renal: | Acute kidney injury on chronic kidney disease (2) | |||
| Rheumatologic: | Allergic (2) Mild pancytopenia | Pubic pruritus | ||
| Hematologic/ oncologic: | Hyperkalemia, Pancytopenia | Metastatic carcinoma of the head of pancreas | ||
| Gastrointestinal: | Nausea (4), Metallic taste | Dry mouth, Icteric sclera, Vomiting (2) Flank fullness (3), Flank pain (5), Lower back pain, Lower limb numbness, Pressure at instillation site (3), Soreness | ||
| Musculoskeletal: | Cool flank sensation Dull headache Flank pain during instillation | |||
| Cardiology | Severe arrhythmia Recurrent cardiac asthma | |||
| Pulmonary | Upper respiratory infection | |||
| Endocrine: | Fasting hyperglycemia | |||
| Psychologic | Depression | |||
| Total events: | 6 | 23 | 8 | 40 |
| Patients effected: | 4 (18.2%) | 10 (45.5%) | 6 (27.3%) | 15 (68.2%) |
DISCUSSION
This cohort study describing compassionate use of UGN-101 demonstrates proof of concept for the primary chemoablation of low-grade upper urinary tract urothelial cancer [9]. Sixteen of 22 patients (72.7%) completed 6 weekly treatments of UGN-101 in the upper tract with 15 (68.1% of all patients) of these patients evaluated by ureteroscopy for treatment response. Additionally, two patients with CR and one with PR tolerated maintenance treatment of 3–5 and 12, respectively, additional instillations of UGN-101.
Two of the 8 patients with CR, who initially had multifocal LG tumor, were found to have a 1 mm and 2 mm recurrence at 4 and 8-month follow-up. Although this tumor was resected successfully endoscopically, this highlights the importance for continual follow-up in patients treated with intracavitary chemotherapy.
This cohort study included 2 patients with HG tumor, with 1 patient completing UGN-101 instillation and follow-up ureteroscopy. This patient had NR to UGN-101 despite undergoing two rounds of UGN-101 instillation (6-weekly instillations each round) with increased concentration of mitomycin C from 2 to 4 mg/mL during the second round. Patients with HG disease are at high risk for invasive disease and are much less likely to respond to intracavitary chemotherapy when used as primary therapy. The benefits and risks must be weighed in light of each patient’s medical co-morbidities.
Though the majority of patients tolerated UGN-101 administration with grade 1 or 2 adverse events, serious adverse events were increased compared with previous studies using mitomycin C in the adjuvant setting. Adverse events highlighted in Tables 3 and 4 demonstrate the need for careful monitoring when utilizing UGN-101 in patients. The patients in this cohort had multiple medical co-morbidities and this may have placed patients at a higher risk for adverse events, both related and unrelated to UGN-101 therapy.
Aqueous mitomycin C has been used in the adjuvant setting in UTUC, occasionally after ureteroscopic resection, and has been studied in multiple case series. The small numbers of patients limited these studies significance and extraction of adverse event data may be confounded by ureteroscopic resection [11–16]. One study by Martinez-Pinerio et al. described one death (3%) out of 33 patients treated due to systemic absorption of Mitomycin [14]. Other studies report an incidence of 3% acute kidney injury (1 event in 35 patients) and 5–23% incidence of upper urinary tract adverse events (strictures, inflammation) [12, 14, 15]. Cornu et al. found a 3% (1 of 35 patients) incidence of acute kidney injury [16]. It is difficult to assess adverse events related to mitomycin C versus the incidental effects of ureteroscopy plus multiple manipulations, and thus it is difficult to make precise adverse event attribution in these studies.
Although aqueous mitomycin C has demonstrated relative safety for use in the upper tract, this compassionate use cohort demonstrates a different adverse effect profile likely related to the increase dwell time of chemotherapy in the upper tract. Local effects of UGN-101 include hydronephrosis found in one patient perhaps secondary to local edema induced by inflammation of the urothelium. Another patient had bladder inflammation that resolved without intervention during the fifth instillation of UGN-101. Strictures have not been found in this cohort in contrast to those reported in previous studies. This may be related to the absence of ureteroscopic resection and more long-term follow-up is needed to monitor for stricture disease after UGN-101.
Although very rare, mitomycin C has been found to cause renal insufficiency (3% incidence found by Cornu et al.) [16]. Two patients in our cohort (11.1%) were found to have exacerbation of chronic renal disease with one patient suffering from hyperkalemia. The mechanism of acute kidney injury by mitomycin C is unknown, though UGN-101’s additional hydrogel properties may cause transient post-renal obstruction, although no evidence of this was seen in pre-clinical studies [9].
Systemic effects of mitomycin C are well known and most notably include pancytopenia. In one patient (5.6%), pancytopenia was mild and discovered on a routine complete blood count. Another patient (5.6%) had pancytopenia requiring a temporary hold of UGN-101 instillations. This patient was treated in the same setting with transurethral resection of bladder tumor and immediate peri-operative intravesical mitomycin C for co-morbid bladder urothelial carcinoma concomitant with the first UGN-101 upper urinary tract treatment. The increased exposure of mitomycin C in both the upper tract and bladder urothelium was likely responsible for the increased absorption of mitomycin C and resulting pancytopenia.
There are some important limitations of this study. The primary purpose of this cohort study was to examine the feasibility of UGN-101 instillation in the upper tract. The number of patients who completed therapy together with the short-term follow-up of these patients limit conclusions about the efficacy and safety of UGN-101. Furthermore, it is difficult to elucidate adverse events directly attributable to UGN-101. With the increased contact time achieved with UGN-101, there may be increased risk of systemic absorption of mitomycin C. UGN-101 concentration and volume have not been standardized and were left up to each investigator’s discretion. Volume of UGN-101 was estimated through contrast-enhanced imaging intraoperatively and may have underestimated the volume needed to fill the upper tract for adequate drug delivery. Depending on the tumor location, this may additionally limit chemotherapy contact time with the tumor.
UGN-101’s novel formulation overcomes the two main obstacles for effective intracavitary chemotherapy by increasing contact time and enhancing the delivery of mitomycin C chemotherapy to the upper tract. Just as mitomycin C is used effectively in non-muscle invasive bladder cancer as adjuvant treatment [17, 18], UGN-101 may provide urologists with an additional tool for patients with LG UTUC. A single arm Phase III multi-center registration trial to treat patients with low-grade low-volume renal pelvis tumors is open and accruing patients (NCT02793128).
CONFLICTS OF INTEREST
| Author | COI |
| Agmon-Gerstein, Yael | |
| Chamie, Karim | UroGen:Consultant |
| Altor Biosciences: Scientific Advisory Board | |
| AstraZenica: Scientific Advisory Board | |
| Hakim, Gil | Urogen Employee |
| Jeshurun-Gutshtat Michal | Urogen Employee |
| Klein, Ifat | Urogen Employee |
| Kleinmanm, Nir | Urogen:Advisory Board |
| Kopelen, Helen | No conflict |
| Lin, Jeffry | None |
| Malchi, Nadav | None |
| Matin, Surena F. | Consultant: Urogen, QED Theraputics, Taris Bio |
| Mayer, Gil | None |
| Nativ, Ofer | Urogen-Reserch support |
| Pantuck, Allan | Allogene, Kynan Pharma, National Insititutes of Health, Pfizer, UpToDate, Urogen |
| Schoenberg, Mark | Urogen Pharma |
| Shvero, Asaf | None |
| Smith, Angela | Grant funding: AHRQ, PCORI |
| Consulting: Photocure | |
| Wirth, Gregory | None |
| Witjes, Alfred J. | Roche, MSD, Tocagen, BMS, Nucleix, Cephied, Taris, Spectrum, BioCanCell, MEL (Synergo) |
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-180182.
REFERENCES
[1] | Siegel R , Naishadham D , Jemal A . Cancer statistics. CA Cancer J Clin. (2012) ;62: :10–29. |
[2] | Roupret M , et al.. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2015 Update. Eur Uro. (2015) ;68: :868–79. |
[3] | Margulis V , et al.. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. (2009) ;115: :1124–33. |
[4] | Gadzinski AJ , Roberts WW , Faerbert GJ , Wolf JS . Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol. (2010) ;183: :2148–53. |
[5] | Cutress ML , et al.. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): A systematic review. BJUI. (2012) ;110: :614–28. |
[6] | Leow JJ , et al.. A contemporary review of management and prognostic factors of upper tract urothelial carcinoma. Cancer Treatment Reviews. (2015) ;41: :310–9. |
[7] | Audenet F , Traxer O , Bensalah K , Roupret M . Upper urinary tract instillations in the treatment of urothelial carcinomas: A review of technical constraints and outcomes. World J Urol. (2013) ;31: :45–52. |
[8] | Yakoubi R , et al.. Radical nephroureterectomy versus endoscopic procedures for the treatment of localized upper tract urothelial carcinoma: A meta-analysis and systematic review of current evidence from comparative studies. Eur J Surg Oncol. (2014) ;14: (20):1629–34. |
[9] | Donin N , et al.. Preclinical trial of serial MitoGel instillations into the pelvicalyceal system of the Yorkshire Swine. J Urol. (2016) ;195: :e289. |
[10] | Meiron M , et al.. MitoGel: Optimizing drug delivery to the upper urinary tract – a preclinical evaluation. J Urol. (2014) ;191: :e914. |
[11] | Smith AD , Orihuela E , Crowley AR . Percutaneous management of renal pelvic tumors: A treatment option in selected cases. J Urol. (1987) ;137: :852–6. |
[12] | Keeley FX , Bagley DH . Adjuvant mitomycin C following endoscopic treatment of upper tract transitional cell carcinoma. J Urol. (1997) ;158: :2074–7. |
[13] | Eastham JA , Huffman JL . Technique of mitomycin C instillation in the treatment of upper urinary tract urothelial tumors. J Urol. (1993) ;150: :324–5. |
[14] | Martinez-Pineiro JA , Matres MJG , Martinez-Pineiro L . Endourological treatment of upper tract urothelial carcinomas: Analysis of a series of 59 tumors. J Urol. (1996) ;156: :377–85. |
[15] | Cutress ML , Stewart GD , Wells-Cole S , Phipps S , Thomas BG , Tolley DA . Long-term endoscopic management of upper tract urothelial carcinoma (UTUC): 20-year single centre experience. BJU Int. (2012) ;110: :1608–17. |
[16] | Cornu JN , Roupret M , Carpentier X , Geavlete B , Diez de Medina SG , Cussenot O , Traxer O . Oncologic control obtained after exclusive flexible ureteroscopic management of upper urinary tract urothelial carcinoma. World J Urol. (2010) ;28: :151–6. |
[17] | Chang SS , Boorjian SA , Chou R , Clark PE , Daneshmand S , Konety BR , Pruthi R , Quale DZ , Ritch CR , Seigne JD , Skinner EC , Smith ND , McKiernan JM . Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. (2016) ;196: :1021–9. |
[18] | Babjuk M , Bohle A , Burger M , Comperat E , Kaasinen E , Palou J , Roupret M , van Rhijn BWG , Shariat S , Sylvester R , Zigeuner R . EAJU guidelines on non-muscle-invasive bladder cancer: Update 2016. Eur Urol. (2017) ;71: :447–61. |




