Real-World Effectiveness of Chemotherapy in Elderly Patients With Metastatic Bladder Cancer in the United States
Abstract
Background:
Outcomes for patients with metastatic bladder cancer (mBC) are generally poor and progressively worse following first-line (1L) chemotherapy.
Objective:
To evaluate treatment patterns, survival outcomes, and characteristics of a large, real-world US population of elderly patients with advanced mBC receiving 1L and second-line (2L) treatment retrospectively.
Methods:
We identified patients with advanced mBC (aged ≥66 years)—newly diagnosed between January 1, 2004, and December 31, 2011—in the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program–Medicare linked database and assessed their palliative systemic chemotherapy treatments and survival outcomes.
Results:
Of 1703 eligible patients, 42% received 1L chemotherapy; 1L-treated patients tended to be younger and healthier than nontreated patients. Only 27% of 1L-treated patients received cisplatin-based chemotherapy, most commonly cisplatin-gemcitabine. Cisplatin-treated patients were younger and had fewer comorbidities than non-cisplatin–treated patients. Thirty-five percent of 1L-treated patients subsequently received 2L chemotherapy. Patients received a variety of 2L agents as combination chemotherapy (52%) or single-agent chemotherapy (39%). Median overall survival durations in 1L-treated and 2L-treated patients were 8.5 and 7.9 months, respectively.
Conclusions:
Results from this retrospective SEER-Medicare database analysis underscore the historical inadequacies of 1L and 2L treatments in elderly patients with advanced mBC. Few patients were treated with 1L chemotherapy, a minority of whom received 1L cisplatin-based chemotherapy, and even fewer received 2L chemotherapy. These findings highlight the disconnect between 1L treatment in clinical trials and treatment in the real-world setting and the lack of standard approaches to 2L treatment in the United States.
INTRODUCTION
In 2017, ≈79,000 patients in the United States (US) will receive a new diagnosis of bladder cancer (BC), and ≈16,800 will die [1]. Although clinically localized BC is potentially curable, metastatic BC (mBC) is generally associated with a poor prognosis. Approximately 11% of patients with BC have regional or distant metastases at initial presentation, with 5-year survival rates of ≈35% and 5%, respectively [2, 3].
A few decades of randomized trials established cisplatin-based combination chemotherapy as the standard first-line (1L) treatment for mBC [4–6]. However, a substantial disconnect exists between the results seen in patients enrolled in clinical trials and in those treated in the “real world.” Indeed, BC is largely a disease of the elderly (median age at diagnosis in the US of 65 years) [1, 7]. Elderly patients are generally underrepresented in clinical trials, or, if included, are more fit than the broader population, limiting the generalizability of such studies [8]. Although elderly patients, with age-associated physiological decline in renal function and/or other comorbidities, are commonly considered cisplatin ineligible, the extent to which elderly patients with BC treated in real-world settings receive non-cisplatin–based therapy has been under-researched [9–14]. Furthermore, before 2016, no treatments for patients with mBC that progresses following 1L chemotherapy were approved in the US.
We performed a retrospective observational cohort study to better understand the patterns of care of elderly patients with mBC in real-world settings and to explore the potential disconnect between the efficacy and effectiveness of systemic therapies for this disease.
MATERIALS AND METHODS
Data source
The Surveillance, Epidemiology, and End Results (SEER)–Medicare database is a linkage of 2 large, US population–based data sources: the SEER program of the National Cancer Institute and the Centers for Medicare and Medicaid Services, which provides detailed information on Medicare beneficiaries with cancer [15].
Patient population
We identified patients with BC including the upper and lower urinary tracts (International Classification of Diseases [ICD]-0-3 site codes C65.9, C66.9, C68.0, C67.x) (N = 214,444) and an initial diagnosis of stage IV transitional cell carcinoma (histology codes: 8120, 8122, and 8130) between January 1, 2004, and December 31, 2011 (n = 5801). Patients were included if they had no cancer during the year before or at any time after their mBC diagnosis (n = 4225), had a corresponding Medicare claim for BC (ICD Ninth Revision [ICD-9] codes 188.0–188.9, 189.1–189.3, 233.7, 236.7, 239.4, or V10.51) within 90 days before or after their SEER diagnosis (n = 3184), were 66 years or older at diagnosis (to ensure that all individuals had ≥1 full year of claims before BC diagnosis, n = 2840), had dates of birth and death that agreed in the SEER and Medicare databases (n = 2799), did not have their cancer reported solely from an autopsy or death certificate, and, during the year before their mBC diagnosis, had received no hospice care nor enrolled in a health maintenance organization but were covered by Medicare Parts A and B (n = 2043).
This analysis focused on patients whose chemotherapy was considered palliative rather than curative. Because a subset of patients in SEER with stage IV BC receiving chemotherapy might include patients with pathological evidence of nodal metastatic disease, who underwent potentially curative surgery and received chemotherapy in the perioperative setting, we excluded patients who had a cystectomy and perioperative chemotherapy after their initial stage IV diagnosis.
We defined neoadjuvant chemotherapy as presurgery chemotherapy claims that occurred ≤180 days before the surgery claim and ≤30 days before the diagnosis date (to account for delays in diagnosis recording). Drug claims made after the metastatic diagnosis date but >180 days before surgery were not considered neoadjuvant chemotherapy to avoid describing chemotherapy that may have been for other cancers. Adjuvant chemotherapy was defined as chemotherapy claims occurring within 120 days after the surgery claim. For patients who received neoadjuvant and adjuvant chemotherapy for 120 days, the latter was considered 1L chemotherapy (because it is usually given for 3 to 4 cycles in 21- or 28-day regimens). For patients who did not receive neoadjuvant chemotherapy but did receive adjuvant chemotherapy for 150 days, the latter was considered 1L chemotherapy. For patients with pT4a, pT4b, pN2, or pN3 tumors, chemotherapy that was initiated within 90 days of surgery and continued for ≤120 days was considered adjuvant with curative intent; these patients were excluded. Chemotherapy that did not meet this initiation time criteria or the criteria for number of cycles was considered 1L chemotherapy with palliative intent; these patients were included in the study.
Any patients whose records indicated a date of death prior to therapy were excluded as this may have been an error in their records.
Treatment and line-of-therapy definitions
The 1L and second-line (2L) chemotherapeutic agents of interest were carboplatin, cisplatin, cyclophosphamide (2L only), gemcitabine, docetaxel, doxorubicin, 5-fluorouracil, ifosfamide, methotrexate, nab-paclitaxel, paclitaxel, pemetrexed, and vinblastine. For patients with no claims for these agents, we searched for other evidence of chemotherapy care (eg, J9999 [not otherwise classified, antineoplastic drugs]).
1L chemotherapy was defined by Medicare claims for chemotherapy drugs received at any time after the mBC diagnosis date or ≥120 days after surgery if surgery occurred after diagnosis. Any drugs added within 30 days of the first drug were considered part of the 1L chemotherapy regimen. 2L chemotherapy was defined as a change or switch in therapy (including initiation of any drugs not part of the previous regimen), except for a cisplatin-to-carboplatin switch or resumption of the same therapy after ending the 1L regimen with a <180-day gap. For a cisplatin-to-carboplatin switch, patients were categorized based on the initial regimen (ie, cisplatin-based regimen if cisplatin was used first before switching to carboplatin) to avoid double counting.
Time to treatment initiation was defined as the duration between mBC diagnosis date and start of 1L chemotherapy only in patients treated with chemotherapy. Duration of treatment was defined as the period between the start and end dates of each line of treatment. The median duration of treatment and 95% CI were calculated by the Kaplan-Meier method, and patients whose end date of treatment was ≤1 month before the end of nondeath follow-up were censored at the last claim. Time between 1L and 2L chemotherapy was defined as the duration between the end of 1L and the start of 2L chemotherapy only in patients who received 2L chemotherapy.
Baseline comorbidities
Patients were considered to have selected clinically relevant comorbid conditions if they had ≥1 inpatient claim or ≥2 outpatient/physician claims ≥30 days apart between 12 months and 1 month before diagnosis. Comorbidities relevant to mBC [16] were selected from the Adult Comorbidity Evaluation–27 list and identified using ICD-9 clinical modification codes (see footnotes in Table 1). The modified Charlson Comorbidity Index (CCI), unlike the original CCI, does not include solid tumors [17–19].
Table 1
Demographic and Clinical Characteristics of Patients With Metastatic Bladder Cancer in the SEER-Medicare Database
| All Patients (N = 1703) | No 1L Chemo-therapy (n = 987) | Any 1L Chemo-therapy (n = 717) | Any 2L Chemo-therapy (n = 254) | 1L Cisplatin Based (n = 192) | 1L Non-Cisplatin Based (n = 525) | 1L Single Agent (n = 156) | 1L Combo Agent (n = 501) | 2L Single Agent, Taxane (n = 60) | 2L Single Agent, Non-Taxane (n = 38) | 2L Combo, Taxane Based (n = 60) | 2L Combo, Non-Taxane based (n = 73) | |
| Age at metastatic diagnosis, years | ||||||||||||
| Mean ± SD | 78.2 ± 6.9 | 79.7 ± 7.0 | 76.1 ± 6.2 | 75.3 ± 5.6 | 73.7 ± 5.3 | 76.9 ± 6.3 | 78.4 ± 6.3 | 75.2 ± 5.8 | 75.3 ± 5.2 | 76.9 ± 6.0 | 74.0 ± 5.8 | 75.5 ± 5.6 |
| Median (Q1, Q3) | 78.0 | 80.0 | 76.0 | 75.0 | 73.0 | 77.0 | 79.0 | 75.0 | 76.0 | 76.5 | 73.0 | 75.0 |
| (73.0, 83.0) | (75.0, 84.0) | (71.0, 80.0) | (71.0, 79.0) | (69.0, 77.0) | (72.0, 82.0) | (73.0, 83.0) | (71.0, 79.0) | (71.0, 78.5) | (73.0, 82.0) | (68.5, 78.5) | (71.0, 79.0) | |
| Sex, n (%) | ||||||||||||
| Male | 1100 (64.6) | 601 (61.0) | 499 (69.6) | 186 (73.2) | 134 (69.8) | 365 (69.5) | 110 (70.5) | 343 (68.5) | 42 (70.0) | 30 (79.0) | 45 (75.0) | 53 (72.6) |
| Race, n (%) | ||||||||||||
| White | 1518 (89.1) | 874 (88.6) | 644 (89.8) | 236 (92.9) | 169 (88.0) | 475 (90.5) | 142 (91.0) | 451 (90.0) | 57 (95.0) | 36 (94.7) | 57 (95.0) | 66 (90.4) |
| Marital status, n (%) | ||||||||||||
| Married | 880 (51.7) | 450 (45.6) | 430 (60.0) | 170 (66.9) | 118 (61.5) | 312 (59.4) | 81 (51.9) | 315 (62.9) | 45 (75.0) | 22 (57.9) | 37 (61.7) | 48 (65.8) |
| Residence location, n (%) | ||||||||||||
| Metro/urban | 1533 (90.0) | 891 (90.4) | 642 (89.5) | 228 (89.8) | 172 (89.6) | 470 (89.5) | 143 (91.1) | 446 (89.0) | 58 (97.0) | 36 (95.0) | 49 (82.0) | 64 (88.0) |
| Region, n (%) | ||||||||||||
| West | 693 (40.7) | 404 (41.0) | 289 (40.3) | 103 (40.5) | 87 (45.3) | 202 (38.5) | 59 (37.8) | 200 (39.9) | 17 (28.3) | 18 (47.4) | 26 (43.3) | 32 (43.8) |
| Northeast | 411 (24.1) | 226 (22.9) | 185 (25.8) | 70 (27.6) | 43 (22.4) | 142 (27.0) | 45 (28.9) | 126 (25.2) | 25 (41.7) | 11 (29.0) | 11 (18.3) | 19 (26.0) |
| Midwest | 282 (16.6) | 156 (15.8) | 126 (17.6) | 51 (20.1) | 29 (15.1) | 97 (18.5) | 29 (18.6) | 92 (18.4) | *** | ** | 12 (20.0) | *** |
| South | 317 (18.6) | 200 (20.3) | 117 (16.3) | 30 (11.8) | 33 (17.2) | 84 (16.0) | 23 (14.7) | 83 (16.6) | ** | ** | 11 (18.3) | ** |
| Stage IV, n (%) | ||||||||||||
| T4b N0 M0 | 112 (6.6) | 77 (7.8) | 35 (4.9) | ** | ** | 29 (5.5) | ** | 24 (4.8) | ** | ** | ** | ** |
| Any T N1-3 M0 | 484 (28.4) | 263 (26.7) | 221 (30.8) | 64 (25.2) | 77 (40.1) | 144 (27.4) | 52 (33.3) | 151 (30.1) | ** | ** | 16 (26.7) | 23 (31.5) |
| Any T N1 M0 | 283 (16.6) | 155 (15.7) | 128 (1.9) | 41 (16.1) | 44 (22.9) | 84 (16.0) | 36 (23.1) | 81 (16.2) | ** | ** | 12 (20.0) | 15 (20.5) |
| Any T N2 M0 | 189 (11.1) | 102 (10.3) | 87 (12.1) | 21 (8.3) | 31 (16.1) | 56 (10.7) | 15 (9.6) | 67 (13.4) | ** | ** | ** | ** |
| Any T N3 M0 | 12 (0.7) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Any T Any N M1 | 1058 (62.1) | 615 (62.4) | 443 (61.8) | 170 (66.9) | 106 (55.2) | 337 (64.2) | 94 (60.3) | 313 (62.5) | 50 (83.3) | 25 (65.8) | 38 (63.3) | 46 (63.0) |
| Tumor grade III-IV, n (%) | 1415 (83.1) | 807 (81.8) | 608 (84.8) | 212 (83.5) | 167 (86.9) | 441 (84.0) | 137 (87.8) | 423 (84.4) | 48 (80.0) | 34 (89.5) | 46 (76.7) | 63 (86.3) |
| Poor PS, n (%) | 875 (51.4) | 533 (54.1) | 342 (47.7) | 117 (46.1) | 80 (41.7) | 262 (49.9) | 82 (52.6) | 233 (46.5) | 33 (55.0) | 16 (42.1) | 23 (38.3) | 32 (43.8) |
| Charlson Comorbidity Index (modified, no tumors) | ||||||||||||
| Mean ± SD | 0.83 ± 1.35 | 1.00 ± 1.51 | 0.60 ± 1.06 | 0.53 ± 0.95 | 0.41 ± 0.75 | 0.67 ± 1.15 | 0.69 ± 1.09 | 0.54 ± 1.00 | 0.48 ± 0.81 | 0.42 ± 0.95 | 0.47 ± 0.87 | 0.66 ± 1.13 |
| 0, n (%) | 1002 (58.8) | 538 (54.6) | 464 (64.7) | 172 (67.7) | 136 (70.8) | 328 (62.5) | 90 (57.7) | 337 (67.3) | 40 (66.7) | 29 (76.3) | 45 (75.0) | 44 (60.3) |
| 1, n (%) | 359 (21.1) | 204 (20.7) | 155 (21.6) | 49 (19.3) | 41 (21.3) | 114 (21.7) | 44 (28.2) | 103 (20.6) | 14 (23.3) | ** | ** | 20 (27.4) |
| 2, n (%) | 156 (9.2) | 101 (10.2) | 55 (7.7) | 19 (7.5) | ** | 45 (8.6) | *** | 35 (6.9) | ** | ** | ** | ** |
| ≥3, n (%) | 186 (10.9) | 143 (14.5) | 43 (6.0) | 14 (5.5) | ** | 38 (7.2) | ** | 26 (5.2) | ** | ** | ** | ** |
| Comorbid conditionsa, n (%) | ||||||||||||
| Cardiovascular disease | 1053 (61.8) | 634 (64.3) | 419 (58.4) | 142 (55.9) | 105 (54.7) | 314 (59.8) | 91 (58.3) | 292 (58.3) | 39 (65.0) | 18 (47.4) | 31 (51.6) | 43 (58.9) |
| Diabetes mellitus | 341 (20.0) | 215 (21.8) | 126 (17.6) | 41 (16.1) | 30 (15.6) | 96 (18.3) | 32 (20.5) | 86 (17.2) | ** | ** | ** | 16 (21.9) |
| Gastrointestinal disease | 137 (8.0) | 98 (9.9) | 39 (5.4) | 15 (5.9) | ** | 32 (6.1) | 11 (7.1) | 24 (4.8) | ** | ** | ** | ** |
| Hearing loss | 19 (1.12) | 12 (1.22) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| NYHA class III heart failure | 126 (7.4) | 88 (8.9) | 38 (5.3) | ** | ** | 35 (6.7) | ** | 21 (4.2) | ** | ** | ** | ** |
| Neurological disease | 74 (4.3) | 52 (5.3) | 22 (3.1) | ** | ** | 18 (3.4) | ** | ** | ** | ** | ** | ** |
| Peripheral neuropathy | 62 (3.6) | 42 (4.5) | 20 (2.8) | ** | ** | 15 (2.9) | ** | 16 (3.2) | ** | ** | ** | ** |
| Psychiatric disease | 74 (4.3) | 51 (5.2) | 23 (3.2) | ** | ** | 15 (2.9) | ** | 18 (3.6) | ** | ** | ** | ** |
| Renal function impairment | 136 (8.0) | 103 (10.4) | 33 (4.6) | 15 (5.9) | ** | 28 (5.3) | ** | 21 (4.2) | ** | ** | ** | ** |
| Respiratory disease | 207 (12.2) | 143 (14.5) | 64 (8.9) | 21 (8.3) | 14 (7.3) | 50 (9.5) | 15 (9.6) | 40 (8.0) | ** | ** | ** | ** |
| Rheumatologic disease | 34 (2.0) | 21 (2.1) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
Abbreviations: 1L, first line; 2L, second line; combo, combination; NYHA, New York Heart Association; PS, performance status; Q1, first quartile; Q3, third quartile; SEER, Surveillance, Epidemiology, and End Results. **Includes <11 patients. ***Number of cases <15 censored to eliminate the potential for re-identification of person with cancer. aInternational Classification of Diseases, Ninth Revision, Clinical Modification codes: chronic pulmonary disease (490–496, 500–505, 506.4); diabetes (250–250.3, 250.7); diabetes with complications (250.4–250.6); congestive heart failure (428–428.9); cerebrovascular disease (430–438); peripheral vascular disease (443.9, 441.1–441.9, 785.4, V43.4, Procedure 38.48); acute myocardial infarction (410–410.9); old myocardial infarction (410–410.9); rheumatologic disease (710.0, 710.1, 710.4, 714.0–714.2, 714.81, 725); moderate/severe renal disease (582.0–582.9, 583.0–583.7, 585, 586, 588.0–588.9); dementia (290.0–290.9); ulcer disease (531.0–534.9, 531.4–531.7, 532.4–532.7, 533.4–533.7, 534.4–534.7); paralysis (344.1, 342.0–342.9); mild liver disease (571.2, 571.5, 571.6, 571.4–571.49); moderate/severe liver disease (572.2–572.8, 456.0–456.21); AIDS (042–044.9).
Claims-based proxy for performance status
The SEER-Medicare database does not include measures of performance status, such as the Eastern Cooperative Oncology Group performance score. Instead, we used Medicare claims from 12 months before the mBC diagnosis to identify several indicators of poor performance status, including the use of oxygen and related respiratory therapy supplies and home health agency services [20].
Survival analysis
The Kaplan-Meier method was used for survival analyses. Median survival and milestone survival (12 and 24 months) were calculated with 95% CIs around the estimates. Patients who had not died by the end of study follow-up (December 31, 2013) were censored. Overall survival (OS) was calculated either from the time of diagnosis or the start of 1L or 2L chemotherapy until death from any cause.
Statistical analysis
Descriptive analyses included means and standard deviations or 95% CIs and/or medians and interquartile ranges for continuous variables and sample sizes and proportions for categorical variables.
RESULTS
Patient characteristics
Of the patients with mBC identified, 1703 met all inclusion and exclusion criteria. The median age of the 1703 patients with mBC was 78 years (66–101 years), and the cohort was 65% male. Fifty-one percent of patients had poor proxy performance status, and the mean modified CCI score was 0.83. The most common comorbidities were cardiovascular disease (62%) and diabetes (20%). Table 1 provides these and additional baseline characteristics.
Treatment patterns and characteristics: 1L chemotherapy in patients with mBC in the SEER-Medicare database
Of the 1703 patients with mBC, 42% received any 1L systemic chemotherapy (Table 1). Treated patients were younger, more likely to be men, and more likely to be married than were nontreated patients. Treated patients were also more likely to be healthier than nontreated patients were per CCI score and proxy performance status (Table 1).
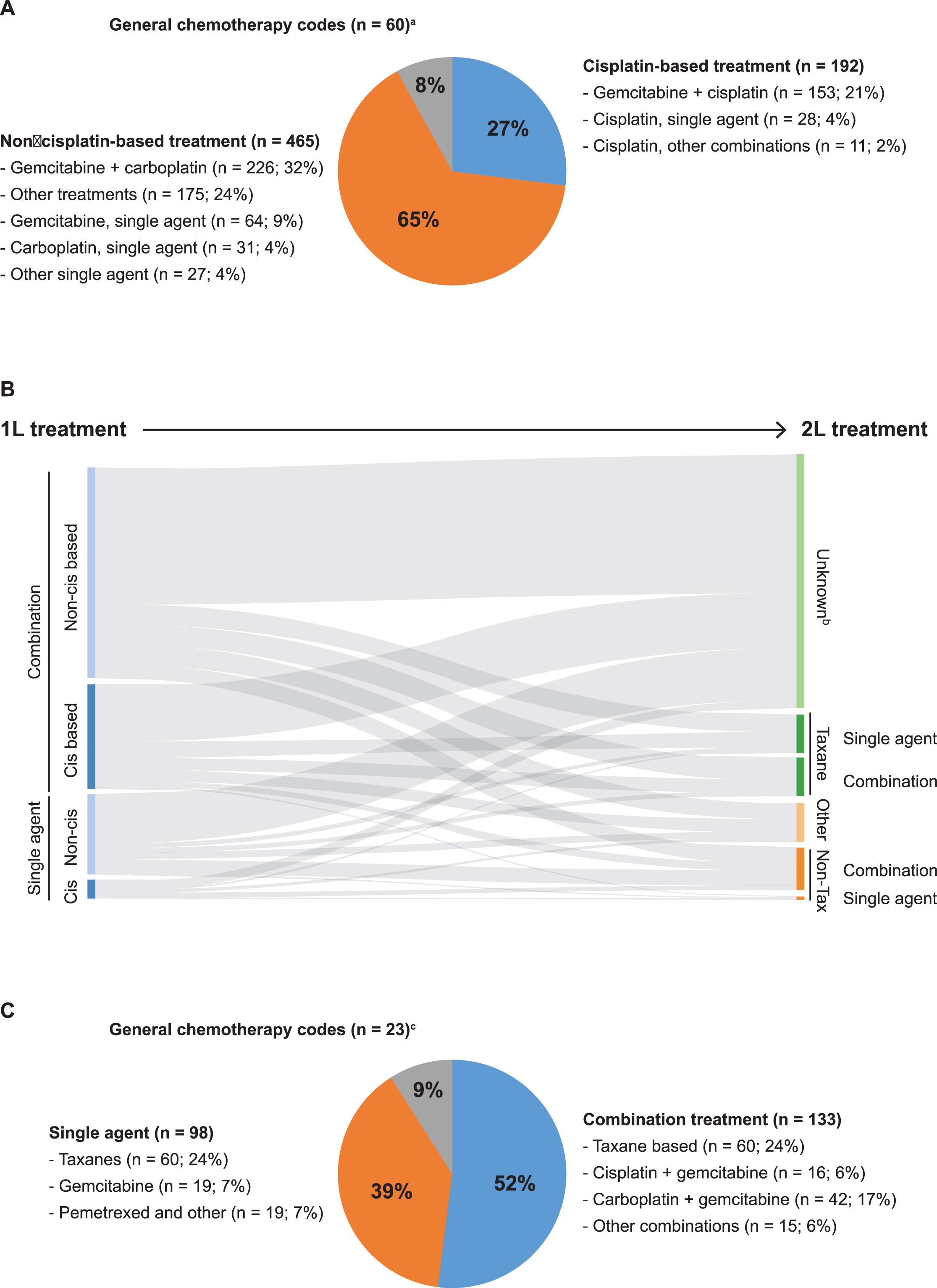
Of those receiving 1L chemotherapy, only 27% had 1L cisplatin-based regimens (Fig. 1A)—most commonly cisplatin-gemcitabine. In patients not receiving 1L cisplatin, the most common treatment regimen was gemcitabine-carboplatin (43%), then single-agent gemcitabine (12%) (Fig. 1A). Baseline characteristics of patients receiving cisplatin- or non-cisplatin–based chemotherapy and single-agent or combination chemotherapy are shown in Table 1. Patients receiving 1L cisplatin were younger, had fewer comorbidities, and had a higher likelihood of metastatic disease limited to lymph nodes (any T, N1-3, M0) versus patients receiving non-cisplatin–based chemotherapy. For 484 patients with metastases limited to lymph nodes (any T, N1-3, M0), 1L cisplatin-based regimens were also the most common 1L treatments (Supplementary Table 1).
More patients received 1L combination chemo-therapy (70%) than 1L single-agent chemotherapy (22%) (Table 1). Compared with patients who got single-agent chemotherapy, patients who received combination chemotherapy were younger and had fewer comorbidities and a better proxy performance status (Table 1). In the 717 patients treated with 1L chemotherapy, the median time to initiation of 1L chemotherapy was 2.4 months, and the median duration of 1L chemotherapy was 2.1 months (Supplementary Table 2).
Fig.1
Treatment patterns in patients with metastatic bladder cancer. (A) 1L systemic chemotherapy regimens used. aIncludes 60 patients who had evidence of systematic chemotherapy, but the agent was not specified. (B) Flow from 1L to 2L treatment. bPatients with “Unknown” 2L treatments may include those who died before 2L treatment, who refused treatment or had no need for 2L treatment, or who were still being treated with 1L treatment. (C) 2L systemic chemotherapy regimens used. cIncludes 23 patients who had evidence of systematic chemotherapy, but the agent was not specified. 1L, first line; 2L, second line; Cis, cisplatin; Tax, taxane.
Treatment patterns and characteristics: 2L chemotherapy in patients with mBC in the SEER-Medicare database
The median age of the 254 patients treated with 2L chemotherapy was 75 years, the mean CCI score was 0.53, and 46% of these patients had poor proxy performance status (Table 1); cardiovascular disease and diabetes were the most common comorbidities.
Baseline characteristics of the patients treated with 2L chemotherapy were also assessed per taxane treatment status (Table 1). Patients who received 2L single-agent taxanes and non-taxanes were similar in age, race, and location of residence (Table 1). Compared with patients who received a non-taxane combination, patients treated with combination taxanes were slightly younger and were more likely to be male and white and to have a lower CCI score. The combination taxane subgroup also had comparatively fewer cases of poor proxy performance status (Table 1).
Patients who received 1L chemotherapy received a wide variety of 2L agents. Regardless of 1L chemo-therapy, most patients did not receive 2L chemotherapy (Fig. 1B). The most common 2L chemotherapy regimens were single agent (24%) or combination taxane-based agents (24%), and more patients received combination chemotherapy (52%) than single-agent chemotherapy (39%) (Fig. 1C). Characteristics of patients treated with 2L non-taxane single agents and non-taxane combination agents are detailed in Table 1.
The median time to switching to 2L chemotherapy (ie, duration between the start of 1L chemotherapy and that of 2L chemotherapy) in the 254 patients treated with 2L chemotherapy was 6.5 months (Supplementary Table 2). The median time between the end of 1L and the start of 2L chemotherapy was 2.1 months (Supplementary Table 2). The median duration of 2L chemotherapy was 1.8 months (Supplementary Table 2). Approximately one-third of patients who received any 2L chemotherapy had received prior cisplatin-based 1L chemotherapy (Supplementary Table 2).
Median and milestone overall survival
Survival results are shown in Fig. 2A-E. In all patients with mBC (treated and nontreated, n = 1703), the median OS (mOS) from time of diagnosis was 6.4 months (95% CI, 5.9 months to 6.9 months). The probabilities of surviving 12 and 24 months were 29.2% (95% CI, 27.0% to 31.3%) and 13.1% (95% CI, 11.6% to 14.8%), respectively. The mOS from diagnosis was 12 months in patients treated with 1L chemotherapy and <4 months in patients not treated with 1L chemotherapy (Fig. 2A).
Fig.2
Kaplan-Meier plots of overall survival in patients with metastatic bladder cancer. (A) Any 1L treatment and no 1L treatment. Survival was measured from the index date. (B) 1L cisplatin-based and non-cisplatin–based treatments. Survival was measured from the start of 1L treatment. (C) 1L treatment with combination agents and with single agents. Survival was measured from the start of 1L treatment. (D) 2L single-agent treatment: non-taxane and taxane based. Survival was measured from the start of 2L treatment. (E) 2L combination treatment: non-taxane and taxane based. Survival was measured from the start of 2L treatment. 1L, first line; 2L, second line. **Includes <11 patients.
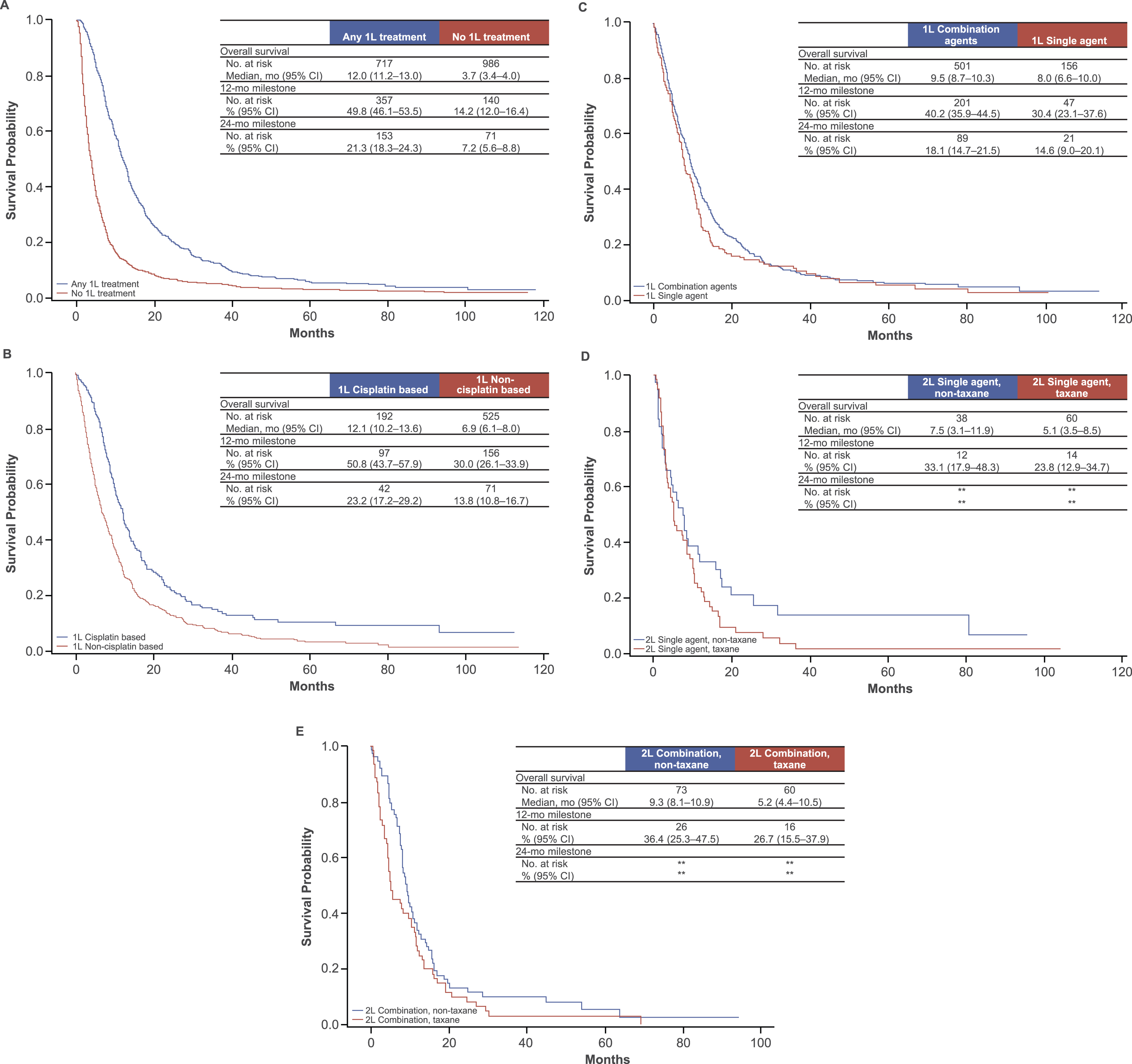
In patients treated with any 1L chemotherapy (n = 717), the mOS from the start of 1L chemotherapy was 8.5 months (95% CI, 7.7 months to 9.3 months), and the 12- and 24-month survival probabilities were 35.6% (95% CI, 32.1% to 39.1%) and 16.3% (95% CI, 13.6% to 19.0%), respectively. Patients treated with 1L cisplatin-based chemotherapy had a longer mOS (12.1 months) than patients treated with non-cisplatin–based 1L chemotherapy (6.9 months) (Fig. 2B). The mOS from the start of 1L therapy in patients who had received 1L combination chemotherapy was 9.5 months—slightly longer than in patients treated with 1L single-agent chemotherapy (8.0 months) (Fig. 2C).
In patients who may have been possibly considered cisplatin ineligible because they either were not treated with 1L systemic chemotherapy or received a non-cisplatin–based 1L chemotherapy regimen (n = 1511), the mOS from time of diagnosis was 5.5 months (95% CI, 5.1% to 5.9%), and the 12- and 24-month survival probabilities were 25% and 11%, respectively (data not shown). For patients with metastases limited to lymph nodes, mOS was 21.3 months (95% CI, 17.6 months to 29.3 months) with cisplatin-based treatment and 16.1 months (95% CI, 13.6 months to 18.5 months) with non-cisplatin–based treatment (Supplementary Figure 1).
In patients treated with any 2L chemotherapy (n = 254), the mOS from start of 2L chemotherapy was 7.9 months (95% CI, 6.0% to 8.8%), and the 12- and 24-month survival probabilities were 31.3% (95% CI, 25.5% to 37.0%) and 13.3% (95% CI, 9.0% to 17.6%), respectively. For patients treated with a single 2L agent, mOS was 5.1 months with a taxane and 7.5 months with a non-taxane agent (Fig. 2D); for patients who received 2L combination chemotherapy, mOS was 5.2 months for those treated with taxane-based combinations and 9.3 months for those treated with non-taxane–based combinations (Fig. 2E).
In patients who received 1L chemotherapy but not 2L chemotherapy for unknown reasons, the mOS from end of 1L chemotherapy was 2.9 months (95% CI, 2.6% to 3.4%).
DISCUSSION
This retrospective analysis evaluated 1L and 2L treatment patterns and survival outcomes in a large, real-world US population of elderly patients with mBC. This study found that >50% of these patients received no 1L systemic therapy. This result is striking but agrees with previous observational findings that ≥50% of patients with mBC do not receive chemotherapy [12, 21]. Furthermore, although patients treated with 1L cisplatin-based chemotherapy had survival times similar to those in prospective clinical trials [4, 5], the survival outcomes were suboptimal, and durable disease control was uncommon.
Many patients with mBC are elderly and have age-associated comorbidities, negatively affecting the risk:benefit ratio of cisplatin-based chemotherapy [22–24], but there have been a paucity of studies specifically focused on chemotherapy for mBC in elderly patients (eg, [9, 14]). In this real-world study, we found survival outcomes with 1L cisplatin similar to those in clinical trials [4, 5] that generally enrolled younger patients. However, patients receiving any 1L treatment (vs none), 1L cisplatin (vs non-cisplatin treatment) and 1L combination chemotherapy (vs single agent)—as well as those receiving any 2L treatment (vs none), and 2L combination chemotherapy (vs single agent)—still tended to have a younger median age than their cognate groups. These findings echo prior data suggesting a trend toward non-cisplatin 1L treatment with increasing age [11]. Here, only 27% of patients who received 1L chemotherapy received a cisplatin-based therapy, and these patients tended to be younger, have fewer comorbidities, and have metastatic disease limited to lymph nodes compared with their non–cisplatin-treated counterparts. A consensus definition of cisplatin ineligibility has been developed to facilitate the development of novel therapeutic approaches in this setting and adopted as the eligibility criteria for recently completed and ongoing registration trials [22]. However, the extent to which such criteria reflect the reasons for administration of non-cisplatin–based chemotherapy in the real world has not been explored. Although not all variables that define cisplatin ineligibility were available from the SEER-Medicare database, our analysis indicates that patients who received non-cisplatin 1L chemotherapy had higher CCI scores and poorer proxy performance status than patients who received cisplatin. Our results confirm a disconnect in treatment efficacy of standard-of-care 1L cisplatin and its effectiveness in real-world settings. These data reinforce the need for safe and effective regimens that can be used more broadly in patients with mBC.
In addition to the unmet needs identified in the 1L treatment setting, our analysis showed that only 35% of patients who received 1L chemotherapy subsequently received 2L chemotherapy. Patients received a wide range of 2L chemotherapy regimens, including an expectedly high proportion of combination regimens, underscoring the lack of consensus during the study period around best choice of treatment. These findings suggest that the identification and development of novel, tolerable 2L therapeutic approaches should be a priority. For instance, such approaches might need to be introduced earlier in treatment to achieve a greater impact on population-based outcomes, given that most patients with mBC are currently unable to proceed to 2L chemotherapy. Additionally, further studies identifying appropriate sequencing of therapies, run-in, or maintenance regimens may help to improve outcomes in a setting with such stark drop-off between 1L and 2Ltreatments.
Our analysis had several strengths and potential weaknesses. The SEER-Medicare database provided a large representative sample of US adults with mBC who received care across geographic regions and in multiple patient care settings. However, this database is limited to Medicare recipients and thus is not generalizable to a nonelderly population. Although SEER provides very detailed information on cancer diagnoses and treatment billing, the claims from Medicare may be prone to errors or misclassification due to coding practices. In addition, if a healthcare encounter is not required, comorbidities may be underestimated (both in occurrence and severity) when quantified solely by claims.
The observational study design and limited information on patient characteristics (eg, Eastern Cooperative Oncology Group performance status), laboratory values, and lifestyle behaviors (eg, smoking) that may serve as the basis of treatment choice preclude any conclusions regarding different treatment outcomes because treatment effectiveness may be confounded by baseline characteristics; thus, no formal comparative analyses of effectiveness adjusted for baseline characteristics were conducted.
This analysis detailed the treatment regimens used and regimen sequencing; however, some of these details were accompanied by small patient counts for certain treatment subgroups, less stable survival estimates, and wide CIs. Additionally, this analysis was limited to patients with an initial diagnosis of metastatic disease (rather than previously diagnosed localized disease). Neither disease progression nor the reason for treatment switch are captured in the SEER-Medicare database; therefore, some patients in the group whom we identified as having received 2L treatment may have been misclassified. Further, patients with “unknown” 2L treatment status may have died before 2L treatment, refused treatment, had no need for 2L treatment, or were still being treated with 1L treatment, so it is not possible to fully know all reasons why patients with 1L treatment did not receive 2L treatment. Therefore, treatment improvements need to be focused on 1L-treated patients with true disease progression who are eligible for 2L treatment.
After our study period (up to 2013), an important shift in the landscape of treatment for mBC occurred. Several large phase 1/2 studies and a phase 3 study showed that a subset of patients with platinum-resistant mBC achieved durable disease control with programmed death-ligand 1/programmed death-1 inhibitors (atezolizumab, nivolumab, pembrolizumab, avelumab, durvalumab), which led to their approval by the US Food and Drug Administration [25–33]. In addition, phase 2 studies have demonstrated the safety and activity of these therapies as 1L treatment for cisplatin-ineligible patients [25, 34]. The impact of such therapies on treatment patterns and outcomes in patients with BC in real-world settings requires further study to determine whether the gap between efficacy and effectiveness will begin to narrow.
CONFLICT OF INTEREST
MG reports a consulting/advisory role with Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, EMD-Serono, Pfizer, and Astellas and research funding from Merck and Bristol-Myers Squibb; SP reports a consulting/advisory role with Genentech, Pfizer, Novartis, Exelixis, Ipsen, and Astellas; S-WL, SO, JS, CD, and CS report employment with Genentech/Roche, including stock; MZ reports a consulting/advisory role with Genentech; CD also reports travel sponsored by Genentech/Roche; CS also reports employment with Genentech/Roche, including stock, by a family member; GS reports a consulting/advisory role with Pfizer, Genentech, Novartis, Argos, Merck, Sanofi, Agensys, AstraZeneca, UpToDate, Biotheranostics, Exelixis, Bristol-Myers Squibb, Janssen, Amgen, Eisai, and National Comprehensive Care Network and research funding from Boehringer-Ingelheim, Bayer, Onyx-Amgen, Pfizer, Merck, and Celgene.
FUNDING
This study was sponsored by F. Hoffmann-La Roche, Ltd. Medical writing assistance for this manuscript was provided by Eric Berlin, MD, of Health Interactions, Inc (San Francisco, CA), and was funded by F. Hoffmann-La Roche.
ACKNOWLEDGMENTS
The authors thank James Chuo, of Genentech, for assistance with figures; Irmarie Reyes-Rivera and Shivani Mhatre, of Genentech, for their contributions to the study; and Andy Surinach and Jingbo Yi, of Genesis, for assistance with data analysis. This study was sponsored by F. Hoffmann-La Roche, Ltd. Medical writing assistance for this manuscript was provided by Eric Berlin, MD, of Health Interactions, and funded by F. Hoffmann-La Roche, Ltd. This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
SUPPLEMENTARY MATERIAL
[1] The supplementary material (Supplementary Tables 1-2 and Supplementary Figure 1) is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-170149.
REFERENCES
[1] | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Bladder cancer. V5.2017. 2017; cited 2017 Sep 7]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. |
[2] | National Cancer Institute. SEER Cancer Stat Fact Sheets: Bladder cancer. 2017 [updated 2017; cited 2017 Sep 7]. Available from: https://seer.cancer.gov/statfacts/html/urinb.html. |
[3] | National Cancer Institute. SEER Cancer Statistics Review, 1975-2012. [2015; cited 2017 Sep 7]. Available from: https://seer.cancer.gov/csr/19752012/. |
[4] | Loehrer PJSr , Einhorn LH , Elson PJ , Crawford ED , Kuebler P , Tannock I , et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. (1992) ;10: (7):1066–73. |
[5] | von der Maase H , Sengelov L , Roberts JT , Ricci S , Dogliotti L , Oliver T , et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. (2005) ;23: (21):4602–8. |
[6] | Bellmunt J , Fougeray R , Rosenberg JE , von der Maase H , Schutz FA , Salhi Y , et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol. (2013) ;24: (6):1466–72. |
[7] | American Cancer Society. Key statistics for bladder cancer. 2017 [updated 2017 Jan 5; cited 2017 Sep 7]. Available from: .http://www.cancer.org/cancer/bladdercancer/detailedguide/bladder-cancer-key-statistics. |
[8] | Stensland KD , Galsky MD . Current approaches to the management of bladder cancer in older patients. Am Soc Clin Oncol Educ Book. (2014) :e250–6. |
[9] | Galsky MD , Krege S , Lin CC , Hahn N , Ecke TH , Moshier E , et al. Cisplatin-based combination chemotherapy in septuagenarians with metastatic urothelial cancer. Urol Oncol. (2014) ;32: (1):e15–30. e21 |
[10] | Guancial EA , Roussel B , Bergsma DP , Bylund KC , Sahasrabudhe D , Messing E , et al. Bladder cancer in the elderly patient: Challenges and solutions. Clin Interv Aging. (2015) ;10: :939–49. |
[11] | Sonpavde G , Watson D , Tourtellott M , Cowey CL , Hellerstedt B , Hutson TE , et al. Administration of cisplatin-based chemotherapy for advanced urothelial carcinoma in the community. Clin Genitourin Cancer. (2012) ;10: (1):1–5. |
[12] | Sonpavde G , Galsky MD , Latini D , Chen GJ . Cisplatin-ineligible and chemotherapy-ineligible patients should be the focus of new drug development in patients with advanced bladder cancer. Clin Genitourin Cancer. (2014) ;12: (2):71–3. |
[13] | Sonpavde G , Bellmunt J , Rosenberg JE , Regazzi AM , Bajorin DF , Choueiri TK , et al. Patient eligibility and trial design for the salvage therapy of advanced urothelial carcinoma. Clin Genitourin Cancer. (2014) ;12: (6):395–8. |
[14] | Bamias A , Efstathiou E , Moulopoulos LA , Gika D , Hamilos G , Zorzou MP , et al. The outcome of elderly patients with advanced urothelial carcinoma after platinum-based combination chemotherapy. Ann Oncol. (2005) ;16: (2):307–13. |
[15] | National Cancer Institute. SEER-Medicare: Brief description of the SEER-Medicare database. 2017 [updated 2017 Feb 24; cited 2017 Sep 7]. Available from: https://healthcaredelivery.cancer.gov/seermedicare/overview/. |
[16] | Megwalu II , Vlahiotis A , Radwan M , Piccirillo JF , Kibel AS . Prognostic impact of comorbidity in patients with bladder cancer. Eur Urol. (2008) ;53: (3):581–9. |
[17] | Deyo RA , Cherkin DC , Ciol MA . Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. (1992) ;45: (6):613–9. |
[18] | Klabunde CN , Potosky AL , Legler JM , Warren JL . Development of a comorbidity index using physician claims data. J Clin Epidemiol. (2000) ;53: (12):1258–67. |
[19] | National Cancer Institute. SEER-Medicare: Calculation of comorbidity weights. [2017; cited 2017 Sep 7]. Available from: https://healthcaredelivery.cancer.gov/seermedicare/considerations/calculation.html. |
[20] | Davidoff AJ , Tang M , Seal B , Edelman MJ . Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. (2010) ;28: (13):2191–7. |
[21] | Porter MP , Kerrigan MC , Donato BM , Ramsey SD . Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol. (2011) ;29: (3):252–8. |
[22] | Galsky MD , Hahn NM , Rosenberg J , Sonpavde G , Hutson T , Oh WK , et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. (2011) ;29: (17):2432–8. |
[23] | Sonpavde G , Galsky MD , Vogelzang NJ . First-line systemic therapy trials for advanced transitional-cell carcinoma of the urothelium: Should we stop separating cisplatin-eligible and -ineligible patients? J Clin Oncol. (2010) ;28: (25):443–4. |
[24] | Houede N , Locker G , Lucas C , Parra HS , Basso U , Spaeth D , et al. Epicure: A European epidemiological study of patients with an advanced or metastatic Urothelial Carcinoma (UC) having progressed to a platinum-based chemotherapy. BMC Cancer. (2016) ;16: (1):752. |
[25] | Balar AV , Galsky MD , Rosenberg JE , Powles T , Petrylak DP , Bellmunt J , et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. (2017) 389: (10064):67–76. |
[26] | Bellmunt J , de Wit R , Vaughn DJ , Fradet Y , Lee JL , Fong L , et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. (1015) ;376: (11):26. |
[27] | Rosenberg JE , Hoffman-Censits J , Powles T , van der Heijden MS , Balar AV , Necchi A , et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. (2016) ;387: (10031):1909–20. |
[28] | Sharma P , Retz M , Siefker-Radtke A , Baron A , Necchi A , Bedke J , et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. (2017) ;18: (3):312–22. |
[29] | Bellmunt J , Sonpavde G , de Wit R , Choueiri TK , Siefker-Radtke AO , Plimack ER , Lewis NM , Brown H , Mai Y , Gause CK , Kaufman DR , Bajorin DF . KEYNOTE-045 Randomized phase 3 trial of pembrolizumab (MK-3475) versus paclitaxel, docetaxel, or vinflunine for previously treated metastatic urothelial cancer. J Clin Oncol. (2015) ;33: (suppl; abstr TPS4571).. |
[30] | Apolo AB , Ellerton JA , Infante JR , Agrawal M , Gordon MS , Aljumaily R , Britten CD , Dirix LY , Lee KW , Taylor MH , Schöffski P , Wang D , Ravaud A , Gelb A , Xiong J , Rosen G , Patel MR . Updated efficacy and safety of avelumab in metastatic urothelial carcinoma (mUC): Pooled analysis from 2 cohorts of the phase 1b Javelin solid tumor study. J Clin Oncol. (2017) ; 35: (suppl; abstr 4528). |
[31] | Massard C , Gordon MS , Sharma S , Rafii S , Wainberg ZA , Luke J , et al. Safety and efficacy of durvalumab (MEDI 4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. (2016) ;34: (26):3119–25. |
[32] | Bavencio (avelumab) [package insert]. New York, NY: EMD Serono, Inc and Pfizer Inc. 2017. |
[33] | Imfinzi (durvalumab) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP. 2017. |
[34] | Bajorin DF , Plimack ER , Siefker-Radtke AO , Choueiri TK , de Wit R , Sonpavde G , Gipson A , Brown H , Mai Y , Pang L , Perini RF , Bellmunt J . KEYNOTE-052 Phase 2 study of pembrolizumab (MK-3475) as first-line therapy for patients (pts) with unresectable or metastatic urothelial cancer ineligible for cisplatin-based therapy. J Clin Oncol. (2015) ;33: (suppl; abstr TPS4572). |




