A Management Algorithm for Mitomycin C Induced Cystitis
Abstract
Background/Objective: A post-bladder tumor resection dose of MMC can reduce non-invasive papillary (pTa) bladder cancer recurrences by up to 40%; this treatment is recommended in both the AUA and EUA non-muscle-invasive bladder cancer guidelines. A common complication of this treatment is eosinophilic cystitis. Symptoms range from mild urinary frequency and urgency to debilitating pain and dysuria. Currently, there is no established treatment algorithm for MMC-induced cystitis.
Methods: Members of the Urologic Surgery Quality Collaborative (USQC), a group composed of over 160 private and academic urologists, met to discuss the management of patients with cystitis following MMC therapy. They devised a treatment algorithm based on experiences of urologic oncologists and neurourologists to aid in the diagnosis and management of MMC-induced cystitis.
Results: The assessment begins with urinalysis and culture, followed by cystoscopy. For mild symptoms, behavioral therapy, including timed voids, fluid restriction and Kegel exercises are trialed. If symptoms have not resolved, treatment with an antihistamine, followed by a combination of anticholinergic and alpha-blocker medications. For persistent symptoms or severe symptoms at presentation, a course of prednisone plus antihistamine is prescribed. If symptoms are improving but have not resolved, this treatment is extended for a full 4 weeks prior to steroid taper. If symptoms do not improve, any visible bladder ulcerations are resected intraoperatively followed by an additional course of prednisone and antihistamine. Intravesical DMSO instillations and intra-ulcer steroid injection can be used as a final effort to treat this condition.
Conclusion: We present the first formal management algorithm with escalating treatment intensity tailored to patient symptoms.
INTRODUCTION
Mitomycin is an alkylating agent and inhibits DNA synthesis. In 1974, the Food and Drug Administration approved it for use in gastric and pancreatic cancer; today, Mitomycin C (MMC) is a current standard of care for suspected Ta bladder cancer [1]. According to American Urologic Association and European Association of Urology guidelines, following TURBT a single instillation of intravesical Mitomycin C (40 mg in 40 ml of saline) should be performed. For large volume, low grade Ta, an induction course of intravesical MMC is recommended [2, 3]. Post-TURBT intravesical MMC can reduce the rate of pTa recurrences by up to 40% [4]. In addition, a longer disease-free interval has been observed with the use of post-TURBT MMC [5, 6].
Despite the benefits of MMC, there are potential complications associated with administration of even a single dose. MMC administration has been found to be associated with a 5– 15% increased risk of complications compared to patients who did not receive MMC following TURBT [7]. Common complications following MMC include transient irritative bladder symptoms, allergic skin reactions, cystitis, pain, urinary retention, and urinary tract infections. The most common complication following a single dose of MMC is an aseptic cystitis that presents with dysuria, urinary urgency and frequency [7]. On occasion, MMC-induced cystitis can present with severe dysuria and pelvic pain, unresponsive to common management strategies such as alpha-blockers and anticholinergics. Biopsies of patients with MMC-associated cystitis typically demonstrate a predominantly eosinophil-rich inflammatory cellular infiltrate.
Eosinophils are the immune system’s primary response to parasitic infections; however, hyper-proliferation of eosinophils both circulating in the bloodstream and also accumulated in target tissues can be found in a variety of different types of disease processes including: asthma, allergic granulomatous angiitis (Churg-Strauss syndrome), primary hypereosinophilia syndrome, eosinophilic esophagitis, eosinophilic gastritis, and eosinophilic cystitis [8– 10]. Eosinophils also promote tissue remodeling, and associated fibrosis can lead to tissue rigidity with clinical complications that include esophageal strictures, pulmonary fibrosis, and endomyocardial fibrosis [11]. In Urology, intramural ureteral fibrosis has been shown to lead to development of hydronephrosis [12]. In general, the mainstay of treatment for eosinophil-rich inflammatory responses is corticosteroid therapy; eosinophilic conditions are generally very steroid responsive [13].
Eosinophilic cystitis can present with severe urinary frequency, urgency, dysuria and pelvic pain refractory to initial therapy. On cystoscopy, the urothelium can be ulcerated, inflamed, necrotic and nodular. Cross-sectional imaging of the bladder can show asymmetrical thickening with the appearance of a locally advanced bladder cancer. Historically, refractory bouts of eosinophilic cystitis have been treated with cystectomy in desperation [14].
In the context of this background, we aimed to create a step-wise algorithm for the management of MMC-induced eosinophilic cystitis based on expert experience and opinion with escalating treatment intensity according to patient symptom severity. Specifically, our goal was to develop a treatment algorithm for patients with aseptic cystitis refractory to behavioral therapies. This algorithm is meant to lend guidance to the practitioner managing patients presenting with post-MMC urinary symptoms, including more severe symptoms of aseptic, eosinophilic cystitis. Up to now, this algorithm has not been evaluated in a clinical trial setting but has brought the resolution of even the most severe symptoms of post-MMC cystitis.
METHODS
The Urologic Surgery Quality Collaborative set out to develop a treatment algorithm for MMC-induced cystitis based on expert opinion. The Urologic Surgery Quality Collaborative comprises more than 160 urologists from both academic centers and private practices. The goal of the Urologic Surgery Quality Collaborative is to bring together urologists of different backgrounds to improve the quality of care delivered to patients. The USQC has representatives from the states of Michigan, Indiana, Ohio, Virginia and Tennessee [15].
The algorithm was developed at a USQC meeting convened to discuss the treatment of non-muscle invasive bladder cancer in May 2013. A team of urologic oncologists and voiding dysfunction experts met in a focus session to develop the algorithm based on their experience, and assessment of clinical outcomes following treatment and treatment of other eosinophilic-associated disease processes. The team then presented the algorithm at the USQC general meeting to all participating members. Input was obtained from the members, and the final algorithm was approved by the USQC membership.
RESULTS
When a patient presents with new onset urinary symptoms (eg. dysuria, urgency and/or frequency) after treatment with MMC, a urinalysis and culture should be performed to rule out urinary tract infection. If urinalysis is negative, a cystoscopy should be performed to evaluate the patient’s bladder and baseline extent of involvement with eosinophilic cystitis changes prior to treatment. If there is concern for bladder perforation a cystogram should be considered. If urinalysis and cystoscopy are both negative, then behavioral therapy should be utilized as first-line therapy for mild symptoms.
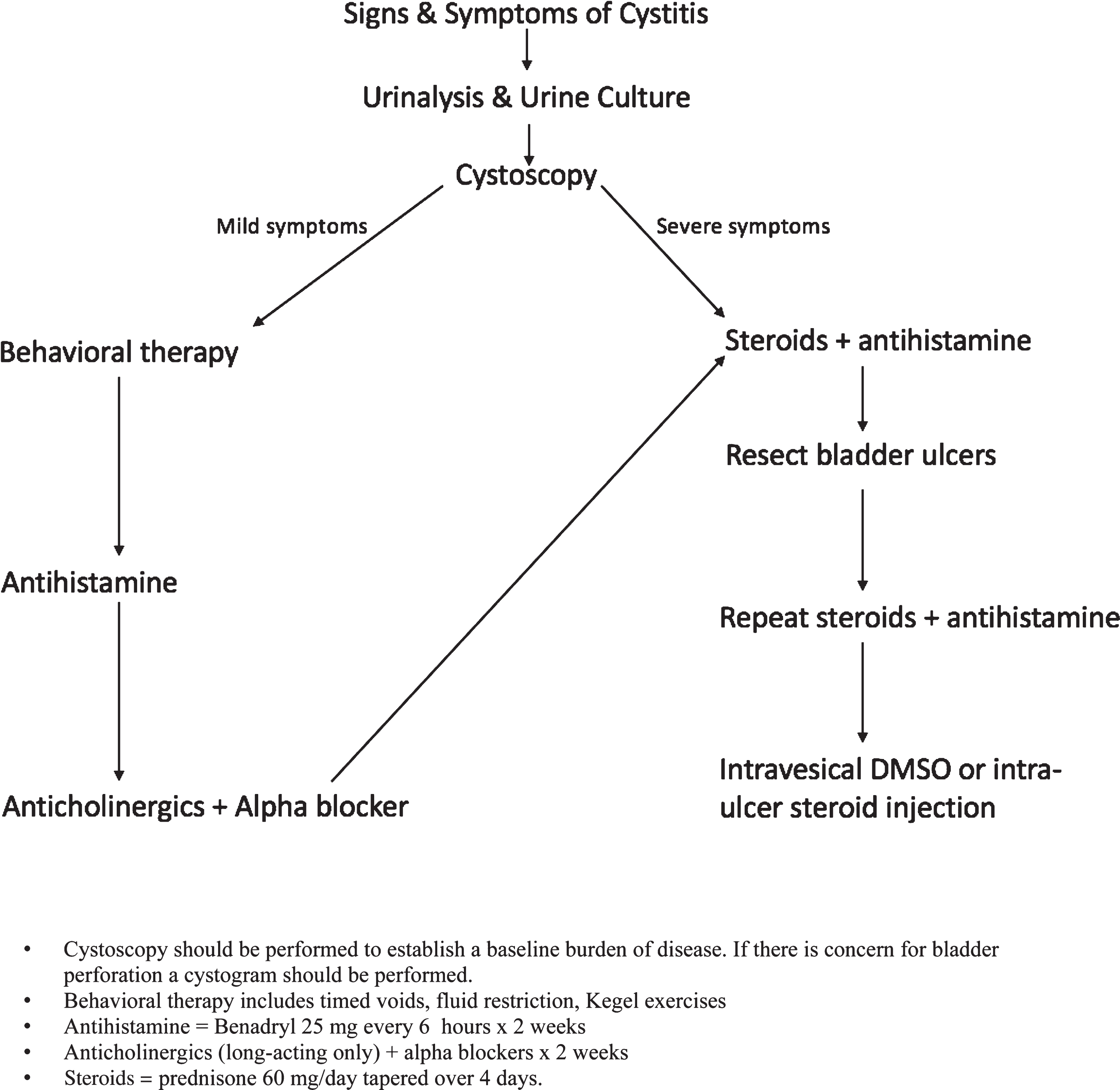
Behavior therapy includes timed voids, fluid restriction and Kegel exercises. In a systematic fashion, if this fails, an antihistamine (ie: Benadryl 25 mg every 6 hours) is trialed for 1 week. Next, long-acting anticholinergics and alpha-blockers should be prescribed for 1 week. If symptoms persist, prednisone 60 mg daily is prescribed for 2 weeks with an additional 4 day taper, reducing the dose to 40 mg the first day of the taper, then by 10 mg per day. Simultaneously, an antihistamine is prescribed and taken for two weeks. Patients presenting with severe symptoms should enter the treatment algorithm at this point after assuring the patient does not have a urinary tract infection or bladder perforation. If symptoms are improving but have not resolved after an initial two week steroid and antihistamine course, this treatment is extended for a full 4 weeks prior to steroid taper. If symptoms do not improve or still persist, any visible bladder ulcerations are resected intraoperatively followed by an additional 2 week course of prednisone and antihistamine. Intravesical DMSO instillations followed by intra-ulcer steroid injection can be used as a final effort to treat this condition (Fig. 1).
Fig.1
Mitomycin-C treatment algorithm demonstrating the escalation of therapy for management of symptoms. Depending on the severity of the patient’s symptoms steps in the algorithm may be skipped.
This algorithm has brought the resolution of even severe symptoms and bladder changes associated with post-MMC eosinophilic cystitis. The images in Fig. 2 depict a patient who initially presented following TURBT for a solitary, 1 cm Ta bladder tumor after his first perioperative mitomycin C instillation. Following this he developed urinary frequency, urgency, gross hematuria, severe dysuria and pelvic pain. Cross-sectional imaging was performed and demonstrated findings concerning for a recurrent bladder cancer. Cystoscopy demonstrated multiple areas of ulceration, necrosis and nodular urothelium (Fig. 3). The patient was taken to the operating room and TURBT was performed. The pathology demonstrated a dense eosinophil-rich acute inflammatory infiltrate, consistent with mitomycin C eosinophilic cystitis (Fig. 4).
Fig.2
CT scan of MMC cystitis: pre-algorithm treatment image demonstrates bladder wall thickening suggestive of bladder cancer recurrence; however, this was biopsy proven MMC cystitis. The post-treatment image on the bottom was following steroids and antihistamine treatment per algorithm.
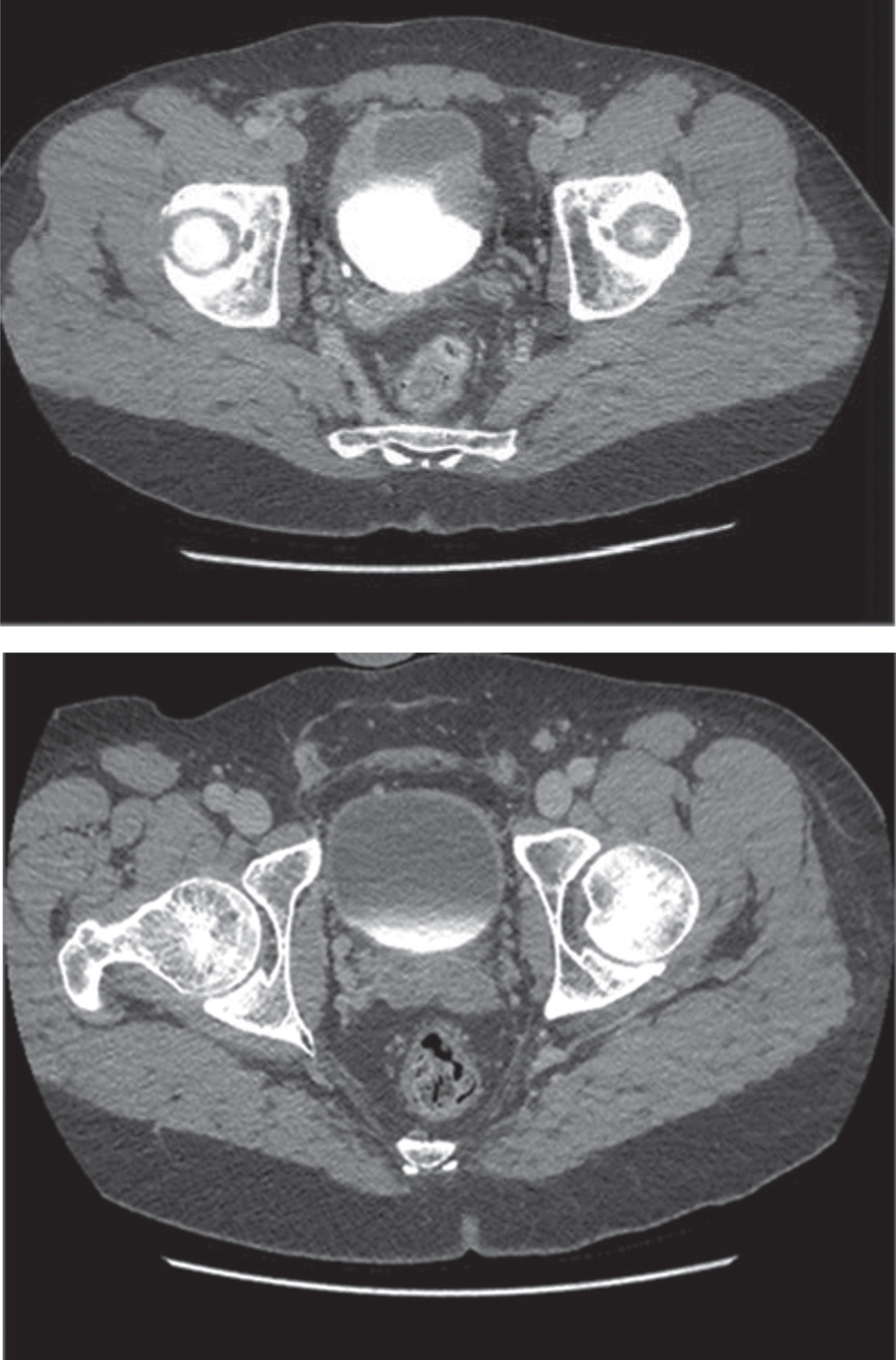
Fig.3
Cystoscopic images: Mitomycin-C induced cystitis pre- and post-treatment (top and bottom respectively) utilizing the algorithm.
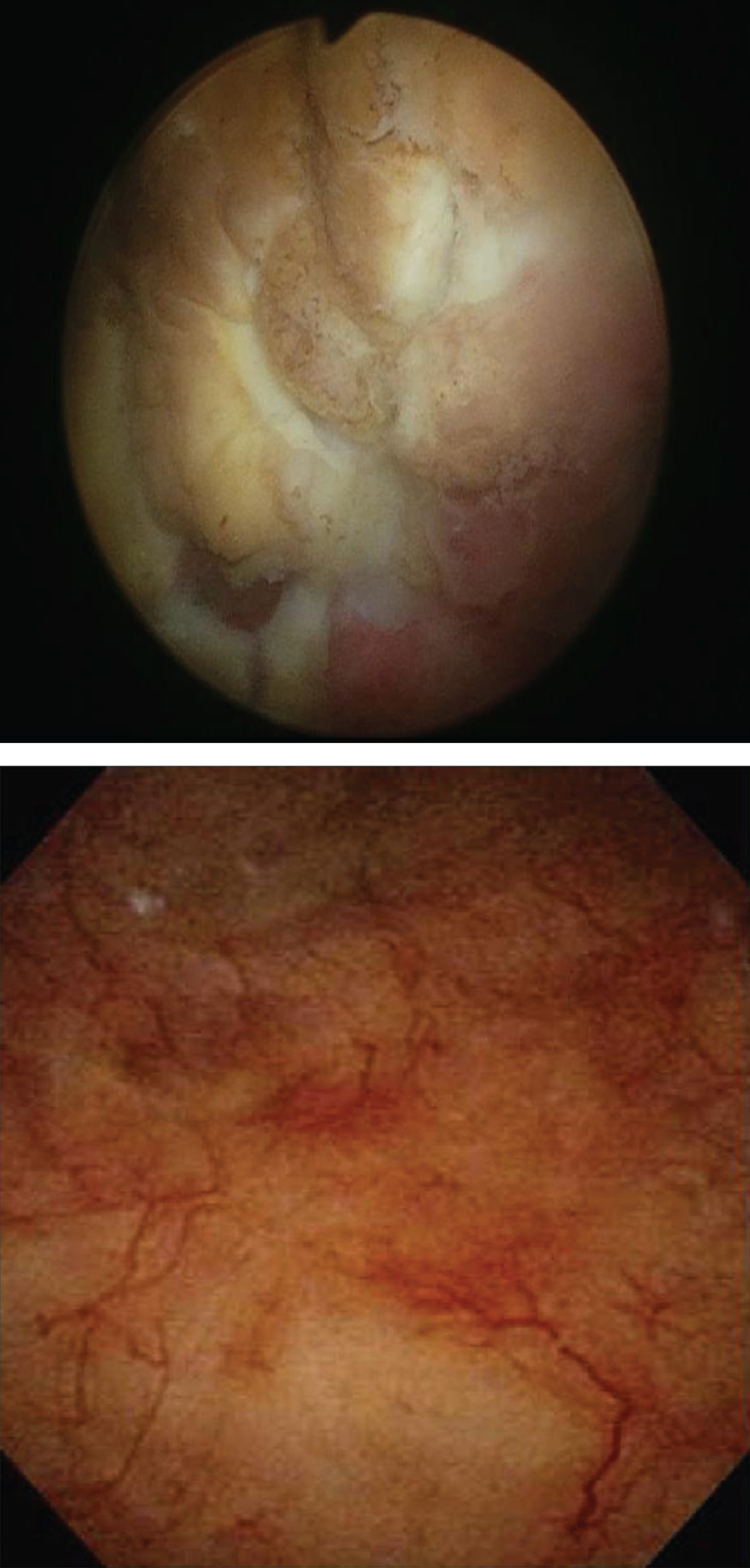
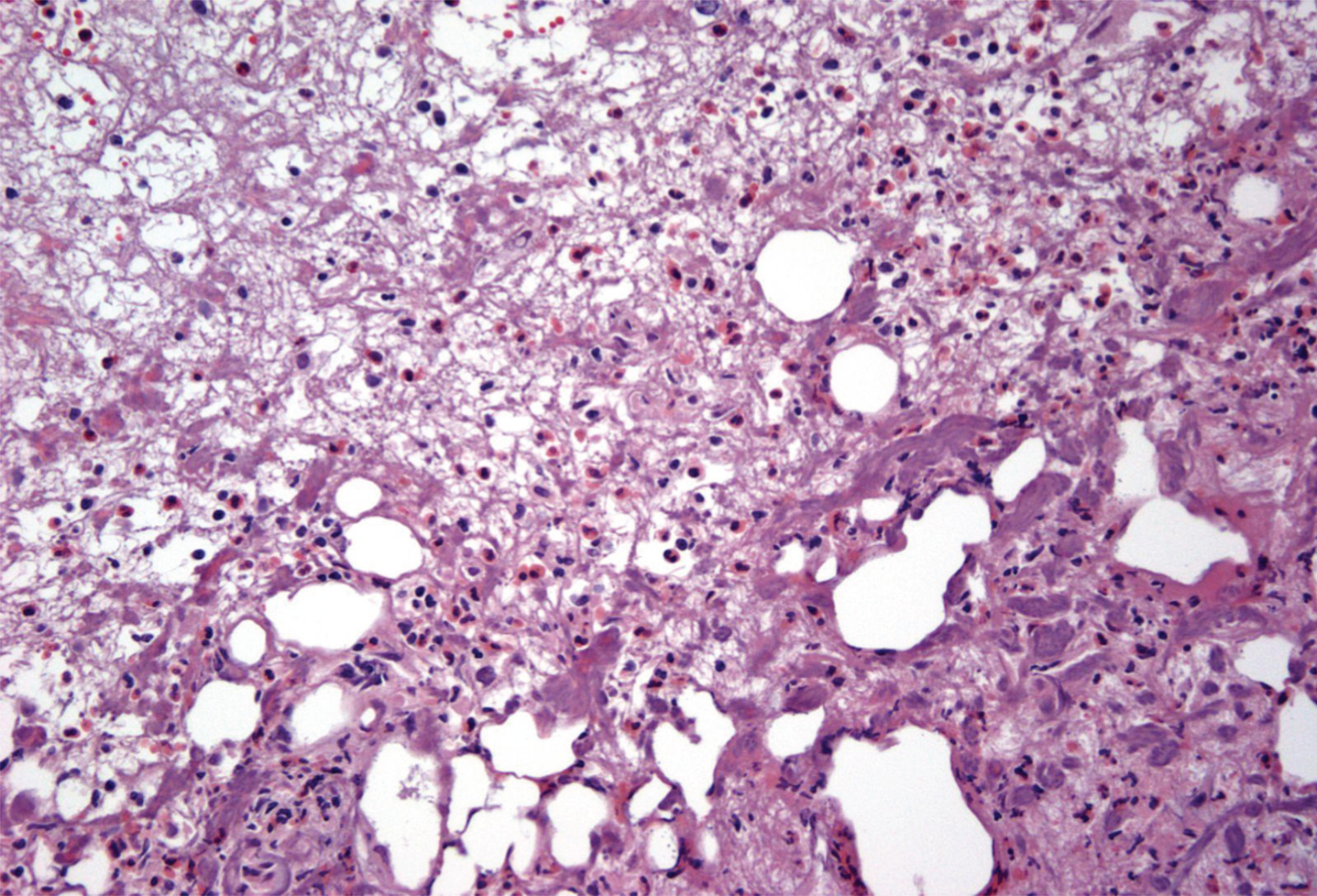
Fig.4
Bladder biopsy of Mitomycin-C cystitis. Biopsy demonstrates numerous eosinophils consistent with MMC induced cystitis.
This patient presenting with severe symptoms entered the treatment algorithm with a 2 week course of prednisone and antihistamine. His symptoms improved, but did not fully resolve. He was again taken to the OR for resection of residual ulcers, and re-treated with prednisone and antihistamine. His symptoms completely resolved. Repeat cystoscopy demonstrated complete resolution of bladder ulceration (Fig. 3). A repeat CT scan demonstrated resolution of the asymmetric bladder wall thickening which had previously been concerning for bladder cancer recurrence (Fig. 2).
DISCUSSION
Mitomycin C cystitis can occur with just a single dose in mitomycin C naïve patients. Symptoms can range from mild irritative voiding symtpoms which are self-limited to severe, debilitating dysuria and pain. It can be refractory to behavioral therapies, and treatment with anticholinergics and alpha blockers. There is no published accepted standard treatment protocol for management of mitomycin C-induced eosinophilic cystitis. Our algorithm has been used successfully to treat this condition in several patients.
Mitomycin C is recommended as a form of intravesical chemotherapy as either a single perioperative dose or as part of induction therapy for patients with low risk Ta bladder cancer [2, 3]. The use of mitomycin C is not without complications, including reports of eosinophilic cystitis, other forms of inflammation and lower urinary tract symptoms [7, 16, 17]. One analysis of cystectomy patients who received perioperative intravesical chemotherapy including mitomycin, demonstrated widespread perivesical fat necrosis out of proportion to typical necrosis seen after TURBT indicating that mitomycin C causes an inflammatory response [18]. Patients who have symptomatic eosinophilic cystitis are often refractory to first line behavioral therapy and treatment with anticholinergics. Our treatment algorithm is applicable to patients who present with mild or severe symptoms and is in concordance with treatment approaches to other types of eosinophil-rich conditions.
Patients who present with more severe symptoms should move directly to the initial course of combined steroid and antihistamine therapy once infection and perforation are ruled out. Side-effects and toxicity from steroid treatment is not expected because of the short course(s) administered. We do advise close monitoring of blood glucose levels in diabetic patients as steroids worsen hyperglycemic control and monitoring blood pressure in hypertensive patients throughout this treatment.
Our report must be considered in the context of several limitations. First, this algorithm has been developed based on expert opinion and literature review alone rather than experimental data. This has not been evaluated in a formal trial, so there is no direct comparison between a treated and an untreated control group. Further work to validate this algorithm could be considered.
Despite these limitations, to our knowledge this is the first formal treatment algorithm offered for the symptomatic relief of patients presenting with post-mitomycin C urinary symptoms. The algorithm includes commonly used medications in combination to treat a specific set of urinary symptoms associated with intravesical mitomycin C in a step-wise fashion. This algorithm can alleviate profoundly disabling and distressing voiding symptoms and pain. Recognizing a patient presenting with symptoms of mitomycin C induced eosinophilic cystitis and initiating this algorithm early on can prevent prolonged patient symptoms and the possible evolution of more permanent bladder fibrosis. For providers, this algorithm provides guidelines for managing a particularly challenging patient population.
In summary, collaborative efforts based on expert opinion have led to the first algorithm for management of MMC induced cystitis. This algorithm serves as a guideline for escalation of therapy, and has been successfully implemented within the USQC collaborative.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
We would like to acknowledge the members of the Urologic Surgery Quality Collaborative (USQC). No funding was received for this project.
REFERENCES
[1] | Doll DC , Weiss RB , Issell BF . Mitomycin: Ten years after approval for marketing. J Clin Oncol (1985) ;3: (2):276–86. |
[2] | Babjuk M , Burger M , Zigeuner R , Shariat SF , van Rhijn BW , Comperat E , et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol (2013) ;64: (4):639–53. |
[3] | Hall MC , Chang SS , Dalbagni G , Pruthi RS , Seigne JD , Skinner EC , et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol (2007) ;178: (6):2314–30. |
[4] | Sylvester RJ , Oosterlinck W , van der Meijden AP . A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J Urol (2004) ;171: (6 Pt 1):2186–90, quiz 435. |
[5] | Solsona E , Iborra I , Ricos JV , Monros JL , Casanova J , Dumont R . Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: Short and long-term followup. J Urol (1999) ;161: (4):1120–3. |
[6] | Tolley DA , Hargreave TB , Smith PH , Williams JL , Grigor KM , Parmar MK , et al. Effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: Interim report from the Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party). Br Med J (Clin Res Ed) (1988) ;296: (6639):1759–61. |
[7] | Filson CP , Montgomery JS , Dailey SM , Crossley HS , Lentz H , Tallman CT , et al. Complications associated with single-dose, perioperative mitomycin-C for patients undergoing bladder tumor resection. Urol Oncol (2014) ;32: (1):40 e1–8. |
[8] | Akuthota P , Weller PF . Eosinophils and disease pathogenesis. Semin Hematol (2012) ;49: (2):113–9. |
[9] | Simon D , Wardlaw A , Rothenberg ME . Organ-specific eosinophilic disorders of the skin, lung, and gastrointestinal tract. J Allergy Clin Immunol (2010) ;126: (1):3–13; quiz 4-5. |
[10] | Kita H . Eosinophils: Multifaceted biological properties and roles in health and disease. Immunol Rev (2011) ;242: (1):161–77. |
[11] | Aceves SS . Remodeling and fibrosis in chronic eosinophil inflammation. Dig Dis (2014) ;32: (1-2):15–21. |
[12] | Itano NM , Malek RS . Eosinophilic cystitis in adults. J Urol (2001) ;165: (3):805–7. |
[13] | Landolina NA , Levi-Schaffer F . Eosinophils as a pharmacological target for the treatment of allergic diseases. Curr Opin Pharmacol (2014) ;17: , 71–80. |
[14] | Sidh SM , Smith SP , Silber SB , Young JD Jr . Eosinophilic cystitis: Advanced disease requiring surgical intervention. Urology (1980) ;15: (1):23–6. |
[15] | Miller DC , Murtagh DS , Suh RS , Knapp PM , Dunn RL , Montie JE . Establishment of a urological surgery quality collaborative. J Urol (2010) ;184: (6):2485–90. |
[16] | Cliff AM , Romaniuk CS , Parr NJ . Perivesical inflammation after early mitomycin C instillation. BJU Int (2000) ;85: (4):556–7. |
[17] | Clark T , Chang SS , Cookson MS . Eosinophilic cystitis presenting as a recurrent symptomatic bladder mass following intravesical mitomycin C therapy. J Urol (2002) ;167: (4):1795. |
[18] | Doherty AP , Trendell-Smith N , Stirling R , Rogers H , Bellringer J . Perivesical fat necrosis after adjuvant intravesical chemotherapy. BJU Int (1999) ;83: (4):420–3. |




