Patterns of Bladder Preservation Therapy Utilization for Muscle-Invasive Bladder Cancer
Abstract
Background: Trimodality bladder preservation therapy (BPT) in muscle invasive bladder cancer (MIBC) includes a maximal transurethral resection followed by concurrent chemoradiotherapy as an alternative to radical cystectomy (RC) in appropriately selected patients, or as a treatment option in non-cystectomy candidates. Several chemotherapy regimens can be used in BPT, but little is known about current practice patterns.
Objective: To describe utilization patterns of BPT and associated survival outcomes in MIBC.
Methods: Data were collected from the Retrospective International Study of Cancers of the Urothelial Tract (RISC), a database of 3,024 consecutive patients from 29 international academic centers from 2005 to 2013. Patients with clinical T2-T4aN0M0 urothelial cancer of the bladder were included.
Results: 265 patients received BPT. Compared with the 1,447 patients who received RC, BPT patients were older, had poorer performance status, and had more comorbidities (p < 0.01 for all). Median overall survival (OS) was similar for patients treated with curative radiation doses in BPT and patients treated with RC (41 vs 46 months, p = 0.33, respectively). 45% of BPT patients received concurrent chemotherapy with radiation. The most common regimens included cisplatin alone (23%), carboplatin alone (22%), gemcitabine alone (10%), paclitaxel alone (9%), and 5-FU+mitomycin (5%). There were no significant differences in survival among chemotherapy regimens. Only 10 patients (4% of BPT patients) underwent salvage cystectomy.
Conclusions: In clinical practice, BPT patients have similar survival to RC patients when treated with curative radiotherapy doses. Choice of concurrent chemotherapy regimen varied widely with no clear standard. Salvage cystectomy is rarely performed. Continued research is needed on the comparative effectiveness among BPT and RC, and among chemotherapy regimens in BPT.
INTRODUCTION
Bladder preservation therapy (BPT) utilizing a trimodality approach is a potential alternative to cystectomy for the treatment of MIBC in appropriately selected patients, as well as a treatment option in non-cystectomy candidates. This treatment combines a thorough and complete transurethral resection of the bladder tumor (TURBT), followed by radiotherapy and concurrent chemotherapy. This trimodal approach allows patients to maintain their native bladder, and avoids the potential morbidity and mortality associated with radical cystectomy (RC).
No large randomized trials exist that compare BPT to RC in muscle-invasive bladder cancer (MIBC). However, available data suggest that outcomes with BPT are similar to RC in carefully selected patients [1, 2]. Historically, patients were considered for BPT if they met certain criteria, including small tumor size (<5 cm), lack of carcinoma in situ, a visually complete TURBT, and absence of hydronephrosis [3, 4]. In practice, however, patients often undergo BPT due to patient and/or provider determination that cystectomy is not a preferred option. Current practice patterns and outcomes are less well studied in this patient population.
Chemotherapy, when given concurrently with radiotherapy in BPT, improves oncologic outcomes compared to radiation alone [5, 6]. Initial studies of BPT included cisplatin-based radiosensitizing chemotherapy [6–10]. Since then, several other chemotherapy regimens have been used including paclitaxel, gemcitabine, and 5-flourouracil (5-FU) with mitomycin C (MMC), among others, with varied strengths of published data supporting efficacy of each regimen [5, 11, 12]. In 2012, the BC2001 phase 3 randomized trial showed that the addition of 5-FU and MMC to radiation therapy for MIBC improves locoregional disease-free survival compared to radiation alone [5]. However, little is known about the comparative effectiveness of different chemotherapy regimens and actual patterns of chemotherapy administration in BPT [13, 14].
The current study utilizes a large, international clinical outcomes database to explore current patterns of care and associated outcomes in the use of BPT for MIBC. This database is uniquely able to make broad comparisons across treatment options in MIBC due to detailed clinical annotation including information on chemotherapy and radiation dose. We therefore present a comprehensive analysis of survival outcomes with different treatment modalities for BPT.
MATERIALS AND METHODS
Data source
Patients were identified from the Retrospective International Study of Cancers of the Urothelial Tract (RISC) database. RISC is a database of consecutive cases of urothelial cancer at 29 international academic medical centers from 2005 to 2013.
Patient population
Patients with clinical T2-T4aN0M0 urothelial cancer of the bladder were identified. Patients were designated as treated with BPT if they received radiation therapy to their primary tumor as initial therapy after diagnosis. Patients were designated as treated with RC if they received radical cystectomy or cystectomy not otherwise specified as initial therapy. Patients were excluded if they were treated with partial cystectomy or if initial treatment was unknown. Neoadjuvant chemotherapy (NAC) was not considered initial therapy, and patients who received NAC, either prior to RC or prior to BPT, were included in the analysis. Patients who received adjuvant chemotherapy were also included. Patients were excluded if they received chemotherapy as the sole treatment for MIBC.
Data items
Patient demographics, tumor characteristics, treatment information, and survival outcomes were collected. Demographic variables included age at diagnosis, sex, race, and treatment facility. Race was classified as white, black, or other. Comorbidities were estimated using the Charlson-Deyo comorbidity index (CCI) and categorized with a score of 0 (no comorbid conditions), 1, 2, or≥3 [15]. Performance status was recorded per the Eastern Cooperative Oncology Group (ECOG) scale.
Staging was defined by the American Joint Commission on Cancer TNM clinical stage at the time of diagnosis. Treatment information was heavily annotated in the database and included type, timing, and dose of chemotherapy. Total radiation dose received was also collected, although intent of treatment (palliative vs curative) and intended dose was not available. Total radiation doses of at least 59 Gray (Gy) were considered to be of curative intent based on recently reportedly dose ranges, which was a pre-specified cutoff based on expert opinion prior to data analysis [4]. Overall survival (OS) was defined as the time between the date of diagnosis and the date of death from any cause. Metastasis-free survival was defined as the time between the date of diagnosis of MIBC and the date of metastatic disease or death from any cause. The database did not include data on localized recurrence after BPT.
Statistical analysis
Fisher’s exact tests were used to compare categorical variables between groups and two group t-tests were used for continuous variables. The Kaplan-Meier method and Log rank tests were used to estimate and compare OS. Cox proportional hazards modeling was used to compare survival between different treatment groups with adjustment for potential confounders. Covariates were considered for inclusion in modeling based on a priori determination of clinical significance and availability in the dataset, and covariates were included in final modeling based on stepwise selection. All statistical tests were 2-sided and a p value of <0.05 was considered statistically significant. All analyses were performed using SAS statistical software (version 9.4; SAS Institute, Cary, NC). Since all patient information was deidentified in the RISC database, this study was approved for exempt status by the University of North Carolina Institutional Review Board.
RESULTS
Overall, 265 patients received BPT compared with 1,447 patients that received RC. A flow chart to identify the study population is shown in Fig. 1. The median age in the total population was 68. Seventy-eight percent of patients were male and 91% were white. The median age of patients who received BPT was 9 years older than patients who received RC; BPT patients also had higher comorbidity scores and higher ECOG performance status scores (p < 0.01 for all) (Table 1). There was no significant difference in sex, race, or T stage between the groups. Twenty-seven percent of patients received NAC prior to RC and 33% received NAC prior to BPT (p = 0.04). The regimen of gemcitabine and cisplatin was the most common NAC regimen in both groups (given to 66% and 50% of BPT and RC patients who underwent NAC, respectively). Eighty-two percent of RC patients had pathologic lymph node stage available and 28% had positive lymph nodes.
Survival data was available on 94% of RC patients and 92% of BPT patients. Median OS in the RC group was 46 months compared with 27 months in the BPT group (Log rank p < 0.01) (Fig. 2a). Median follow-up for the BPT and RC patients was 23.4 months and 24.0 months, respectively, with 132 deaths in the BPT group and 611 deaths in the RC group. The unadjusted hazard ratio (HR) for death for patients treated with BPT compared to those treated with RC was 1.50 (95% CI 1.23–1.82). This difference lost significance when adjusted for age, T stage, and CCI (HR 1.21, 95% CI 0.97–1.50). Median metastasis-free survival was not significantly different between treatment groups (22 months in the RC group compared with 19.0 months in the BPT group, Log rank p = 0.53). Data on metastases was available for 96% and 89% of RC and BPT patients, respectively, with 811 patients in the RC group and 107 patients in the BPT developing metastases.
When the analysis was restricted to only include BPT patients who received at least 59 Gy of radiation (n = 162), the median OS improved to 41 months in the BPT group compared with 46 months in the RC group (Log rank p = 0.33)(Fig. 2b). The unadjusted HR for death was 1.13 (95% CI 0.88–1.46) for BPT patients compared with RC, and the HR for death remained non-significant when adjusted for age, T stage, and CCI (HR 0.89, 95% CI 0.67–1.17). Of all patients that received BPT, 10 (3.8%) eventually underwent salvage cystectomy. Of those, 4 were upstaged at the time of surgery, and 4 had received curative doses of radiation prior to cystectomy.
Of the 265 patients that were treated with BPT (any dose), 109 patients received concurrent chemotherapy (45% of the 244 patients with data on concurrent chemotherapy use). Of the patients who received curative dose radiation, 57% received concurrent chemotherapy. Patients who received concurrent chemotherapy were younger (p = 0.03) with lower T stage (p < 0.01), but more comorbidities (p < 0.01) compared to those patients treated with radiation alone (Table 2). Of the 79 patients who received NAC prior to BPT, 27 (34%) subsequently received concurrent chemotherapy with their radiation treatment. Patients treated in Europe were much less likely to receive concurrent chemotherapy with radiation after NAC than patients from other countries (14% vs 80%, respectively, p < 0.01). Use of NAC prior to BPT was also more common in Europe compared to other countries, although this difference was not statistically significant (38% vs 27%, respectively, p = 0.09). Ten BPT patients (3.7%) and 30 RC patients (2.1%) received adjuvant chemotherapy.
Cisplatin alone was the most commonly administered chemotherapy regimen given concurrently with radiation (23% of patients). Other common regimens included carboplatin alone (22%), gemcitabine alone (10%), paclitaxel alone (9%), and combination chemotherapy as outlined in Fig. 3. All of the patients that were treated with 5-FU+mitomycin were diagnosed in 2011 or later. There was no significant difference in survival between patients who received concurrent chemotherapy compared to those who received radiation alone (unadjusted HR 0.96, 95% CI 0.64–1.40, p = 0.82). This remained insignificant when adjusted for age, comorbidity score, and T stage with a HR of 0.89 (95% CI 0.59–1.34, p = 0.58).
DISCUSSION
Several current guidelines recommend RC as the gold standard treatment option for MIBC, despite emerging evidence of similar outcomes with trimodality BPT [16, 17]. Our study delves deeper into the comparison of BPT and RC in a varied clinical practice database, and also explores patterns of care in chemotherapy use in BPT. We found that in clinical practice, BPT is used in a minority of cases as primary treatment for MIBC and those treated with curative doses of radiation (≥59 Gy) appear to have similar survival outcomes to patients treated with RC. Chemotherapy is used concurrently with radiation in a minority of BPT cases, with wide variation in regimen choice and no clear standard. Salvage cystectomy is rare.
Our data indicate clear differences between patients with MIBC treated with primary BPT and those treated with RC. Patients who receive BPT are older, have poorer performance status, and have more comorbidities than patients who receive RC. It is likely these differences in large part drive the decision for treatment with BPT due to lack of ideal candidacy for RC. Accordingly, median OS is significantly shorter by 19 months for patients who receive BPT compared with those who receive RC. Prior published findings similarly show that patients who are not cystectomy candidates have worse OS when treated with BPT than cystectomy candidates treated with BPT [10]. We suspect that baseline patient fitness explains much of the survival disparity between BPT and RC in our study. This is also evident from the survival curves in our analysis demonstrating a clear and prompt separation of the survival curves, indicating the cause of death is likely related to baseline patient or tumor characteristics, and less likely related to efficacy of chosen treatmentmodality.
However, when RC patients are compared to the subset of BPT patients who received curative (> = 59 Gy) doses of radiation during BPT, the survival difference is much less pronounced with an absolute difference in median OS of only 5 months and a non-significant HR for death (HR 1.13, 95% CI 0.88–1.46). In addition, in multivariable analysis which adjusts for patient factors including age and comorbidities, the hazard ratio for death in RT patients is actually 0.89, lower than that for radical cystectomy patients, though not statistically significant. This finding may suggest that outside of a clinical trial setting and in patients who are fit enough to complete RC or curative doses of RT, survival outcomes are similar between these two treatment regimens.
The survival outcomes in our analysis are generally in agreement with prior reports. Previous clinical trials of radical cystectomy for MIBC report a median OS of between 38 to 77 months, depending on use of NAC [18, 19]. The median OS in our RC group was 46 months which falls well within the historical range. Furthermore, the median OS in our BPT group who received curative doses of radiation therapy was 41 months, similar to the range seen in recent trials of chemoradiotherapy in BPT [5]. The group that received curative dosing of radiation was also more likely to receive concurrent chemotherapy. We therefore demonstrate that dose of radiation received and receipt of concurrent chemotherapy are critical factors for survival analyses in BPT, and any future comparisons of BPT and RC need to include radiation dose as a consideration. Since a minority of patients received concurrent chemotherapy with BPT, our data are also in agreement with prior studies examining outcomes with radiation alone in MIBC[20–23].
There is level 1 evidence supporting the use of concurrent chemotherapy along with radiation for definitive treatment of MIBC [5]. Despite this, we show that a minority of patients undergoing BPT receive concurrent chemotherapy, and the types of chemotherapy used vary widely in clinical practice. The low rates of chemotherapy utilization may be related to the same baseline patient characteristics that informed the initial treatment choice against RC. Surprisingly, patients in our study with more comorbidities were more likely to receive concurrent chemotherapy, suggesting that comorbidity score may not be driving the choice of chemotherapy use. It is possible that patients with fewer comorbidities that undergo BPT instead of RC have other baseline characteristics that may exclude chemotherapy use, such as poor performance status.
Although cisplatin-based therapy has been utilized in this setting for many years, we also demonstrated that many other regimens are frequently used. The addition 5-FU and MMC to radiotherapy in the BC2001 trial demonstrated a statistically significant improvement in locoregional disease-free survival, but this regimen was used in only a small proportion of the patients in our study [5]. This may be related to the timeframe over which our data was collected, or patient/provider dislike of infusional chemotherapy. The most recently treated patients in our analysis were diagnosed in 2013, and the BC2001 trial was published in 2012. Therefore, the use of the 5-FU and MMC regimen may have increased in the last several years, which could not be captured in our analysis. Nevertheless, there remains an unclear picture of which chemotherapy regimen is most effective for use in BPT and further studies are needed on the comparative effectiveness of chemotherapyin BPT.
The use of NAC in our study produced interesting results that again highlight the variability in practice patterns around the world. More patients undergoing BPT received NAC compared with patients undergoing RC, despite the fact that NAC has shown no definitive benefit when added to concurrent chemoradiotherapy in BPT [4, 24, 25]. Presumably the frequent use of NAC is based on the randomized BA06 30894 trial comparing NAC followed by definitive therapy with either cystectomy or radiation with definitive therapy alone. This trial showed an overall survival benefit of 6% at 10 years, although when analysis was restricted to only those who received definitive radiotherapy, the survival benefit of NAC was not statistically significant (HR 0.90, 95% CI 0.62–1.02), perhaps related to the higher risk baseline tumor and patient characteristics in the radiotherapy patients. It is clear from our dataset that many providers are continuing to use NAC, likely based on the potential benefit seen in this trial. There is also substantial regional variation in use of NAC, and in use of concurrent chemotherapy during XRT after NAC. Some prior studies have selected patients for BPT based on response to NAC, and it may be that there is regional variation in the use of this method [24, 25]. In addition, if patients with a complete response to NAC are preferentially referred for BPT, this could select for patients with less aggressive tumors and could alter the survival comparison between BPT and RC.
Salvage cystectomy is incorporated in all major treatment paradigms for patients with MIBC who do not respond or recur with MIBC after BPT [4, 26]. However, our study demonstrates that salvage cystectomy after primary BPT outside of a clinical trial setting is rare, occurring in only 4% of patients. Historically, rates reported in the literature of BPT have been much higher, with approximately 30% of patients undergoing salvage cystectomy [4]. The low rates of salvage cystectomy in our study could have several explanations. The most likely reason is that the patients undergoing BPT were not candidates for cystectomy, either at initial diagnosis or at completion of chemoradiotherapy. Alternatively, BPT patients may have rejected cystectomy as a treatment option or be subject to varying practice patterns at our included institutions. Unfortunately, the RISC database does not include the reason for choosing the primary therapy to distinguish between these possibilities. In addition, follow-up is limited in some patients, so the data may be underreporting the rate of salvage cystectomy. Nevertheless, we believe this is important information when counseling patients about real-world rates of salvage cystectomy, and important feedback for providers about the current status of post-BPT care.
This analysis has several important limitations to consider. First, we made comparisons across treatment options and were unable to fully account for all potential confounders or selection bias in treatment choice, including initial surgical candidacy. Since this is a retrospective database from 29 academic medical centers, it is not truly population-based and there is variability in completeness of reporting and practice patterns at individual centers. There was substantial missing data when analyzing ECOG performance status, making its inclusion in multivariable modeling impossible due to loss of power. Second, although serum creatinine was collected at multiple timepoints in the database, there was no timepoint that had consistent data to use as a covariate. Third, we were unable to differentiate between split course vs continuous courses or variations in fractionation of radiation, we did not distinguish extent of the radiation field used, which can vary geographically, and we did not have information on completeness of the TURBTs. Finally, although the primary intent of the study was to look at differences in survival outcomes in different treatment groups, the wide variation in treatment patterns and limited follow-up caused many of the analyses to be underpowered to compare survival. Although we found no survival differences between chemotherapy regimens in BPT, we suspect that this is due to the small numbers of patients who received each treatment. Analysis of metastasis-free survival was similarly limited due to lack of consistent reporting of involved disease sites of metastatic recurrence.
Despite our increased knowledge of patterns of care of BPT, a number of questions remain. Our analysis includes a broad array of academic medical centers, but the patterns of BPT use in the global community setting are unknown and may be even more varied. In addition, the comparative effectiveness of curative intent BPT and RC remains unanswered, as does the optimal chemotherapy to administer with BPT. These outstanding questions should guide future prospective research in thisarea.
CONFLICTS OF INTEREST
M.D.G. reports previous grants from Bristol-Myers Squibb, Novartis AG, and CElgene Corporation and advisory/consulting fees from BioMotiv and Merck & Company; he is a cofounder of Dual Therapeutics. J.B. reports lectures fees from Pierre Fabre; he serves without compensation on the advisory boards of Pierre Fabre, Genentech, and Merck & Company Inc., and he is an uncompensated lecturer for Merck & Company Inc. Y.N.W. reports grant funding from Pfizer for an unrelated project and institutional clinical trial support from Bayer, Astellas Pharma US Inc, Jansen Pharmaceuticals, and Millennium Pharmaceuticals. L.C.H. has acted as a compensated member of the advisory boards of Genentech, Dendreon, Pfizer, and Medivation/Astellas for work performed outside this study. E.Y.Y. reports grant funding from ImClone (an Eli Lilly company) for a sponsored clinical trial. S.K.P. receives honoraria from Medivation and consulting fees from Astellas Pharma UA for work performed outside the current study. R.C.C. has received a grant from Accuray Inc and personal fees from Astellas/Medivation for work performed outside the current study. M.E.N. reports consulting feeds from the American College of Physicians High Value Care Task Force. M.I.M. reports research support from Pfizer, BIND Therapeutics, Dendreon, Exelixis, Johnson & Johnson, Astellas Pharma, Mirati Therapeutics, Merck and Cerulean Pharma, Inc, outside the submitted work. The authors have no other conflicts of interest to report.
ACKNOWLEDGMENTS
T.L.R. is supported by the NIH Grant No. 5K12CA120780-09. A.Z.W. is supported by R01CA178748 and U54-CA151652 from the National Institutes of Health/National Cancer Institute. M.E.N. is supported in part by the American Cancer Society (grant MRSG-13-154-01-CPPB) and the Urology Care Foundation/Astellas. A.B.S. was supported in part by the National Center for Research Resources, the National Center for Advancing Translational Sciences, the National Institutes of Health (grant KL2TR000084), and the Patient-Center Outcomes Research Institute.
REFERENCES
[1] | Mak RH , Hunt D , Shipley WU , Efstathiou JA , Tester WJ , Hagan MP , Kaufman DS , Heney NM , Zietman AL . Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of radiation therapy oncology group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol (2014) ;32: (34):3801–9. |
[2] | Huddart R , Birtle A , Lewis R , Bahl A , Falconer A , Maynard L , Hall E . Results of the SPARE Feasibility Study; Selective Bladder Preservation Against Radical Excision in Muscle Invasive T2/T3 Transitional Cell Carcinoma of the Bladder. International Journal of Radiation Oncology Biology Physics (2012) ;84: (3):S119–S20. |
[3] | Rodel C , Grabenbauer GG , Kuhn R , Papadopoulos T , Dunst J , Meyer M , Schrott KM , Sauer R . Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J Clin Oncol (2002) ;20: (14):3061–71. |
[4] | Ploussard G , Daneshmand S , Efstathiou JA , Herr HW , James ND , Rodel CM , Shariat SF , Shipley WU , Sternberg CN , Thalmann GN , Kassouf W . Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: A systematic review. Eur Urol (2014) ;66: (1):120–37. |
[5] | James ND , Hussain SA , Hall E , Jenkins P , Tremlett J , Rawlings C , Crundwell M , Sizer B , Sreenivasan T , Hendron C , Lewis R , Waters R , Huddart RA . Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med (2012) ;366: (16):1477–88. |
[6] | Sauer R , Dunst J , Altendorf-Hofmann A , Fischer H , Bornhof C , Schrott KM . Radiotherapy with and without cisplatin in bladder cancer. Int J Radiat Oncol Biol Phys (1990) ;19: (3):687–91. |
[7] | Chen WC , Liaw CC , Chuang CK , Chen MF , Chen CS , Lin PY , Chang PL , Chu SH , Wu CT , Hong JH . Concurrent cisplatin, 5-fluorouracil, leucovorin, and radiotherapy for invasive bladder cancer. Int J Radiat Oncol Biol Phys (2003) ;56: (3):726–33. |
[8] | Coppin CM , Gospodarowicz MK , James K , Tannock IF , Zee B , Carson J , Pater J , Sullivan LD . Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol (1996) ;14: (11):2901–7. |
[9] | Gogna NK , Matthews JH , Turner SL , Mameghan H , Duchesne GM , Spry N , Berry MP , Keller J , Tripcony L . Efficacy and tolerability of concurrent weekly low dose cisplatin during radiation treatment of localised muscle invasive bladder transitional cell carcinoma: A report of two sequential Phase II studies from the Trans Tasman Radiation Oncology Group. Radiother Oncol (2006) ;81: (1):9–17. |
[10] | Hussain MH , Glass TR , Forman J , Sakr W , Smith DC , Al-Sarraf M , Jones J , Balcerzak SP , Crawford ED , Grossman HB . Combination cisplatin, 5-fluorouracil and radiation therapy for locally advanced unresectable or medically unfit bladder cancer cases: A Southwest Oncology Group Study. J Urol (2001) ;165: (1):56–60; discussion -1. |
[11] | Dunst J , Weigel C , Heynemann H , Becker A . Preliminary results of simultaneous radiochemotherapy with paclitaxel for urinary bladder cancer. Strahlenther Onkol (1999) ;175: (Suppl 3):7–10. |
[12] | Oh KS , Soto DE , Smith DC , Montie JE , Lee CT , Sandler HM . Combined-modality therapy with gemcitabine and radiation therapy as a bladder preservation strategy: Long-term results of a phase I trial. Int J Radiat Oncol Biol Phys (2009) ;74: (2):511–7. |
[13] | Cahn DB , Ristau BT , Ghiraldi EM , Churilla TM , Geynisman DM , Horwitz EM , Uzzo RG , Smaldone MC . Bladder Preservation Therapy: A Review of the Literature and Future Directions. Urology (2016) ;96: :54–61. |
[14] | Rose TL , Milowsky MI . Improving Systemic Chemotherapy for Bladder Cancer. Curr Oncol Rep (2016) ;18: (5):27. |
[15] | Charlson ME , Pompei P , Ales KL , MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis (1987) ;40: (5):373–83. |
[16] | Bellmunt J , Orsola A , Leow JJ , Wiegel T , De Santis M , Horwich A . Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2014) ;Supp 3: :iii40–8. |
[17] | Milowsky MI , Rumble RB , Lee CT . Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement Summary. J Oncol Pract (2016) ;12: (6):588–90. |
[18] | Grossman HB , Natale RB , Tangen CM , Speights VO , Vogelzang NJ , Trump DL , deVere White RW , Sarosdy MF , Wood DP Jr , Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med (2003) ;349: (9):859–66. |
[19] | Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: A randomised controlled trial. International collaboration of trialists. Lancet (1999) ;354: (9178):533–40. |
[20] | Pollack A , Zagars GZ . Radiotherapy for stage T3b transitional cell carcinoma of the bladder. Semin Urol Oncol (1996) ;14: (2):86–95. |
[21] | De Neve W , Lybeert ML , Goor C , Crommelin MA , Ribot JG . Radiotherapy for T2 and T3 carcinoma of the bladder: The influence of overall treatment time. Radiother Oncol (1995) ;36: (3):183–8. |
[22] | Mameghan H , Fisher R , Mameghan J , Brook S . Analysis of failure following definitive radiotherapy for invasive transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys (1995) ;31: (2):247–54. |
[23] | Jenkins BJ , Caulfield MJ , Fowler CG , Badenoch DF , Tiptaft RC , Paris AM , Hope-Stone HF , Oliver RT , Blandy JP . Reappraisal of the role of radical radiotherapy and salvage cystectomy in the treatment of invasive (T2/T3) bladder cancer. Br J Urol (1988) ;62: (4):343–6. |
[24] | Shipley WU , Winter KA , Kaufman DS , Lee WR , Heney NM , Tester WR , Donnelly BJ , Venner PM , Perez CA , Murray KJ , Doggett RS , True LD . Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol (1998) ;16: (11):3576–83. |
[25] | Tester W , Caplan R , Heaney J , Venner P , Whittington R , Byhardt R , True L , Shipley W . Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: Results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol (1996) ;14: (1):119–26. |
[26] | Rodel C , Weiss C , Sauer R . Trimodality treatment and selective organ preservation for bladder cancer. J Clin Oncol (2006) ;24: (35):5536–44. |
Figures and Tables
Fig.1
Flow chart of patients included in primary analysis.
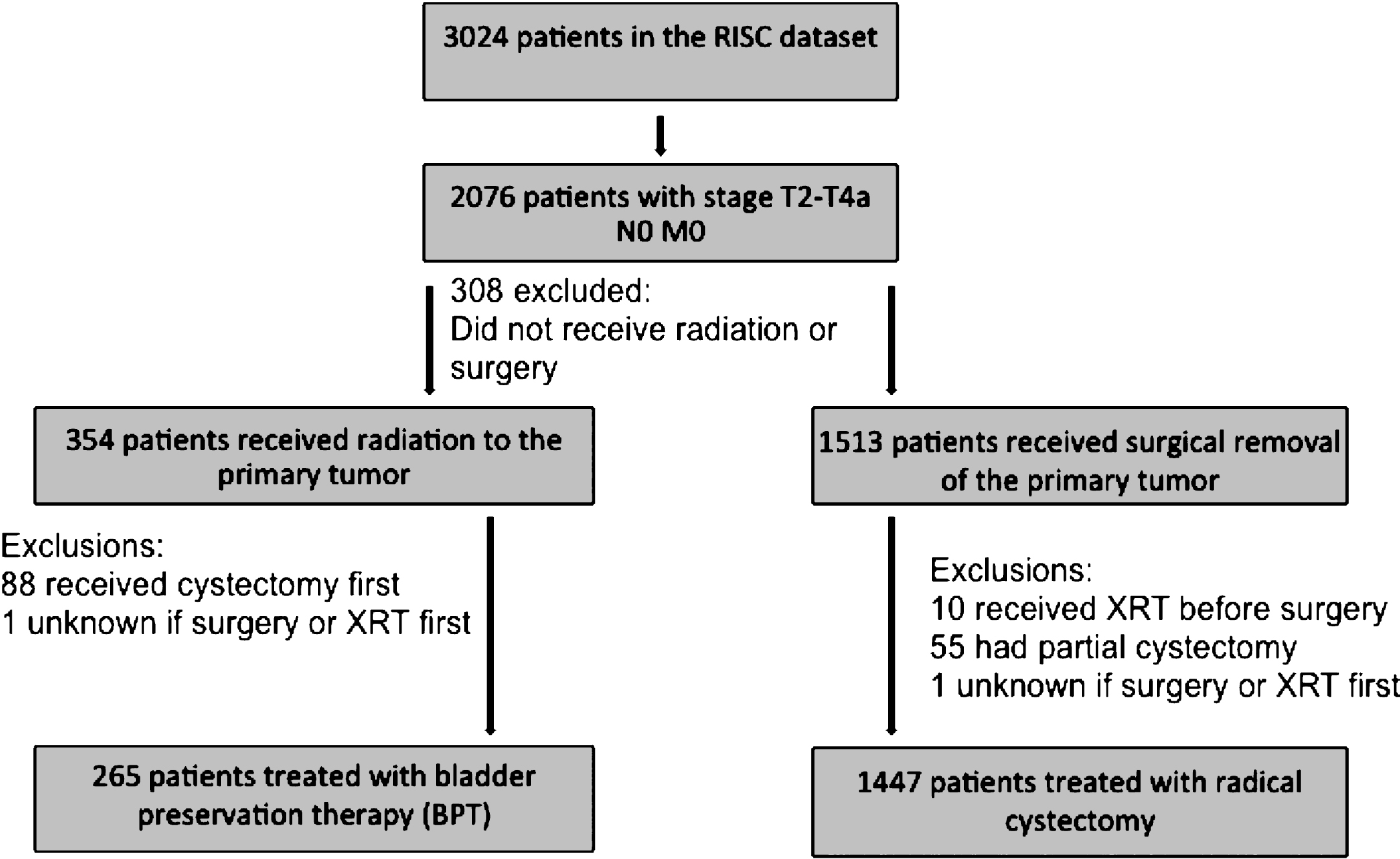
Fig.2
Probability of survival with BPT vs radical cystectomy in (a) all patients and (b) patients who received at least 59 Gy radiation.
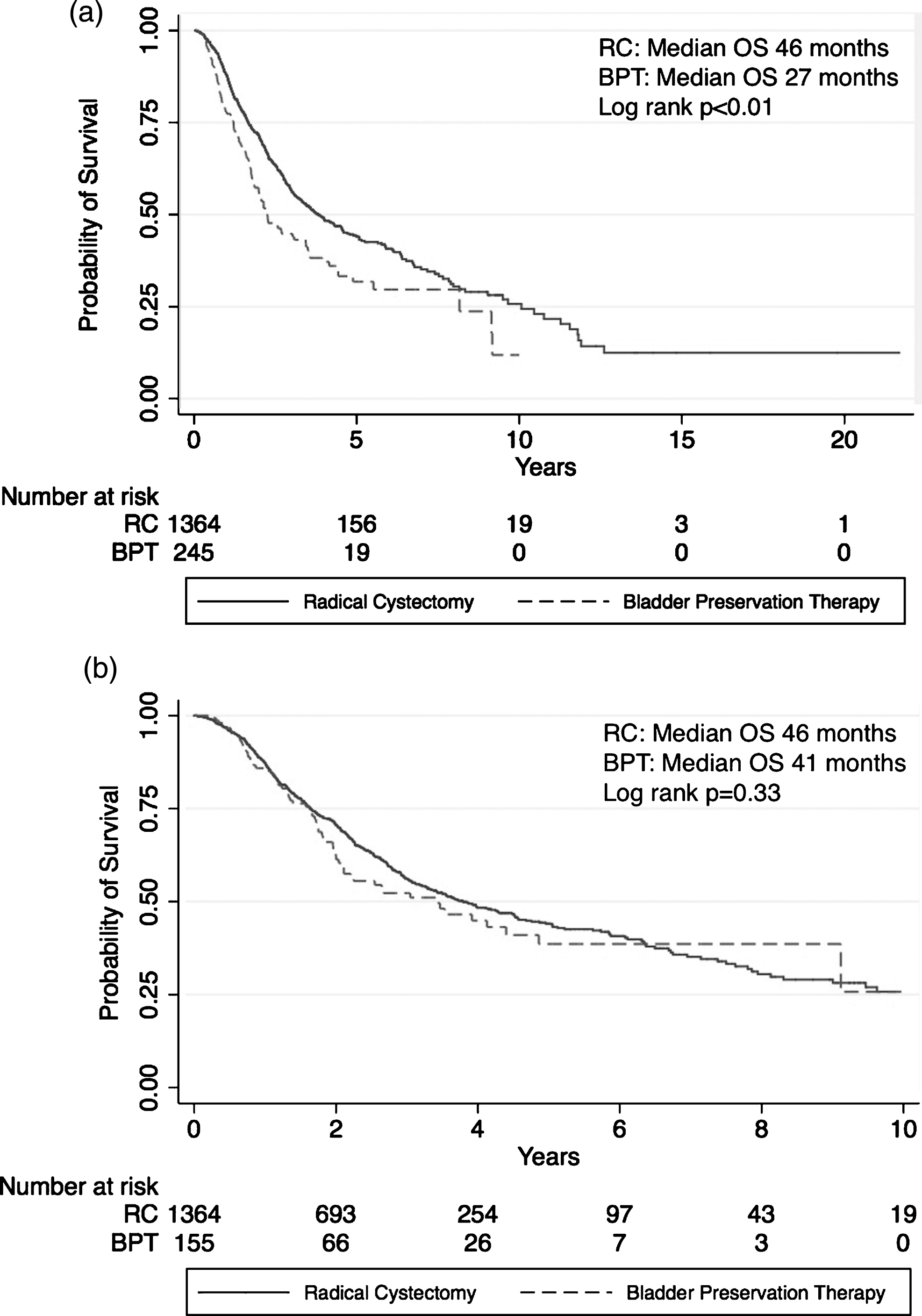
Fig.3
Frequency of concurrent chemotherapy regimens used in patients undergoing bladder preservation therapy (n = 109).
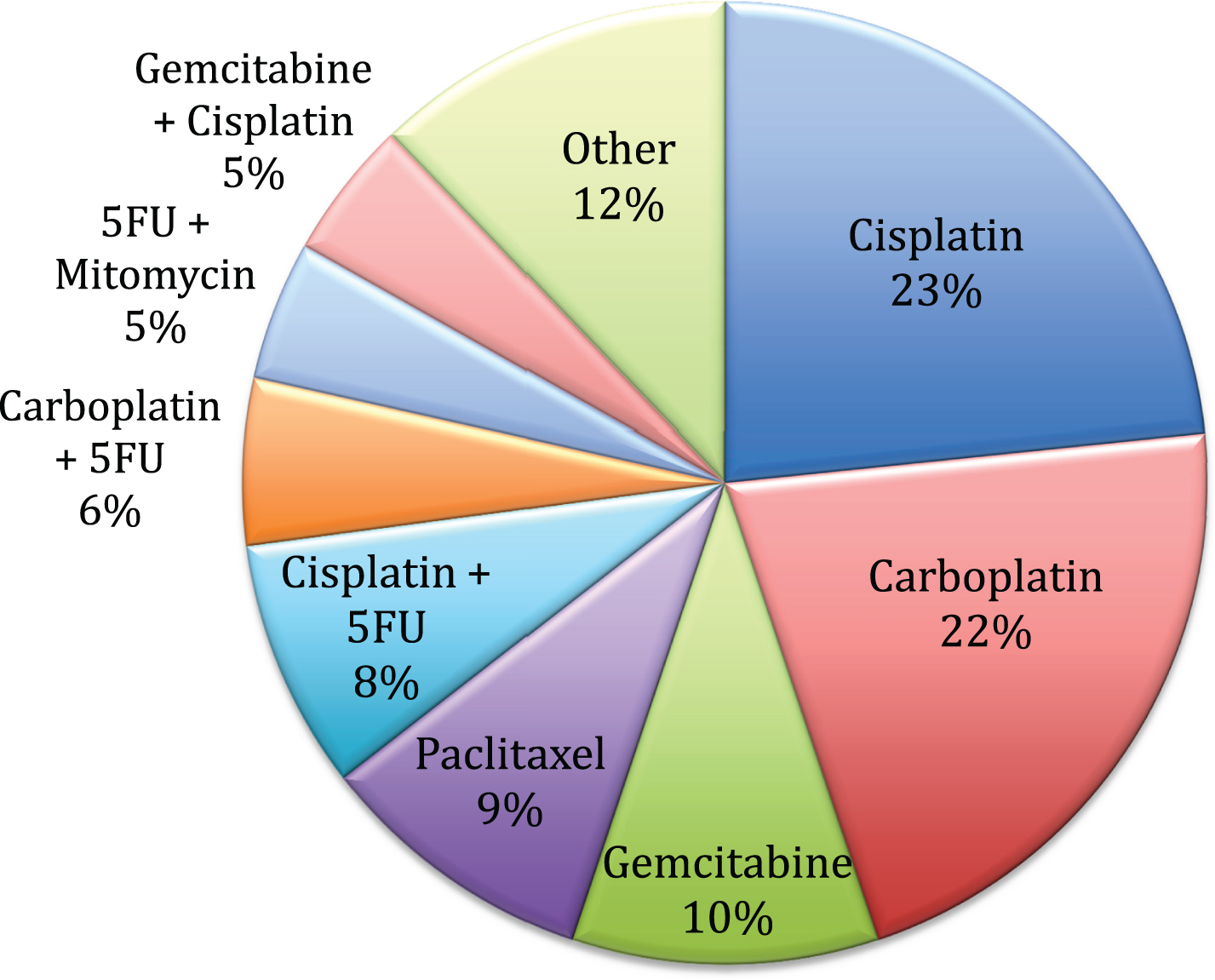
Table 1
Baseline factors of patients who received BPT compared to RC
| BPT | RC | p value | |
| (n = 265) | (n = 1447) | ||
| Age, median (range) | 75 (33–96) | 66 (32–100) | <0.01* |
| Sex | |||
| Male | 211 (79.6%) | 1123 (78.1%) | 0.63 |
| Female | 54 (20.4%) | 315 (21.9%) | |
| Race | |||
| White | 227 (92.3%) | 1309 (91.4%) | 0.77 |
| Black | 9 (3.7%) | 50 (3.5%) | |
| Other | 10 (4.1%) | 74 (5.2%) | |
| ECOG PS | |||
| 0-1 | 79 (29.8%) | 402 (27.8%) | <0.01* |
| 2 | 16 (6.0%) | 9 (0.6%) | |
| 3-4 | 6 (2.3%) | 4 (0.3%) | |
| Unknown | 164 (61.9%) | 1032 (71.3%) | |
| Charlson Comorbidity Index | |||
| 0 | 86 (33.6%) | 512 (37.7%) | <0.01* |
| 1 | 39 (15.2%) | 83 (6.1%) | |
| 2 | 59 (23.1%) | 403 (29.7%) | |
| ≥3 | 72 (28.1%) | 360 (26.5%) | |
| Treating Site Location | |||
| Asia | 8 (3.0%) | 34 (2.3%) | <0.01* |
| Europe | 163 (61.5%) | 588 (40.6%) | |
| United States | 94 (35.5%) | 825 (57.0%) | |
| Clinical T stage | |||
| T2 | 161 (64.9%) | 797 (70.4%) | 0.07 |
| T3 | 59 (23.8%) | 254 (22.4%) | |
| T4 | 28 (11.3%) | 81 (7.2%) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status. *p value significant at level of p < 0.05.
Table 2
Baseline factors of BPT patients who received concurrent chemotherapy compared to radiation alone
| Concurrent | Radiation | p value | |
| chemotherapy | alone | ||
| and radiation | (n = 135) | ||
| (n = 109) | |||
| Age, median (range) | 73 (33–93) | 76 (47–92) | 0.03* |
| Sex | |||
| Male | 87 (79.9%) | 108 (80.0%) | 1.0 |
| Female | 22 (20.2%) | 27 (20.0%) | |
| Race | |||
| White | 99 (91.7%) | 112 (94.1%) | 0.49 |
| Black | 4 (3.7%) | 5 (4.2%) | |
| Other | 5 (4.6%) | 2 (1.7%) | |
| ECOG PS | |||
| 0-1 | 23 (21.1%) | 55 (40.7%) | <0.01* |
| 2 | 6 (5.5%) | 8 (5.9%) | |
| 3-4 | 0 | 6 (4.4%) | |
| Unknown | 80 (73.4%) | 66 (48.9%) | |
| Charlson Comorbidity Index | |||
| 0 | 17 (16.7%) | 60 (45.1%) | <0.01* |
| 1 | 16 (15.7%) | 22 (16.5%) | |
| 2 | 30 (29.4%) | 24 (18.1%) | |
| > = 3 | 39 (38.2%) | 27 (20.3%) | |
| Treating Site Location | |||
| Asia | 5 (4.6%) | 3 (2.2) | <0.01* |
| Europe | 38 (34.9%) | 113 (83.7%) | |
| United States | 66 (60.6%) | 19 (14.1%) | |
| Clinical T stage | |||
| T2 | 82 (77.4%) | 68 (53.1%) | <0.01* |
| T3 | 13 (12.2%) | 44 (34.4%) | |
| T4 | 11 (10.4%) | 16 (12.5%) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status. *p value significant at level of p < 0.05.




