Phase I Study of Everolimus in Combination with Gemcitabine and Split-Dose Cisplatin in Advanced Urothelial Carcinoma
Abstract
Background:
Cisplatin-based combination chemotherapy is standard first-line treatment for patients with advanced urothelial carcinoma (UC). Molecular profiling studies reveal that the PI3K/AKT/mTOR pathway is altered in a significant percentage of UCs.
Objective:
We conducted a phase I trial to evaluate the feasibility of combining the mTOR inhibitor everolimus with gemcitabine and split-dose cisplatin (GC) in advanced UC in the first-line setting.
Methods:
Patients received gemcitabine 800 mg/m2 and cisplatin 35 mg/m2 on days 1 and 8 of 21-day cycles for a total of 6 cycles in combination with everolimus at increasing dose levels (DL1:5 mg QOD, DL2:5 mg daily, DL3:10 mg daily) following a standard 3+3 design. Responses were assessed every 2 cycles. Patients with at least stable disease (SD) continued everolimus until progression. Goals were to establish dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) for the combination.
Results:
12 patients were enrolled, 3 at DL1, 3 at DL2, and an additional 6 at DL1 *(DL1 following de-escalation). 3/3 patients at DL2 had DLTs during cycle 1. 2/8 evaluable patients at DL1/DL1 * had DLTs during cycle 1. DLTs were primarily hematologic. Further toxicities, also primarily hematologic, were observed during later treatment cycles, leading to 8 chemotherapy dose reductions overall. Partial responses were observed in 4/10 evaluable patients, and SD in 5/10. Median overall survival was 10.8 months (95% CI 6.9, not reached).
Conclusions:
The maximum tolerated dose was reached at the lowest dose level, 5 mg QOD, for everolimus in combination with gemcitabine and split-dose cisplatin in advanced UC. The regimen was limited by hematologic toxicity.
INTRODUCTION
Advanced urothelial carcinoma accounts for approximately 15,000 deaths in the United States annually [1]. Cisplatin-based combination chemotherapy regimens including gemcitabine and cisplatin (GC) and methotrexate, vinblastine, adriamycin and cisplatin (MVAC) were developed more than 15 years ago and remain the most effective treatments for the disease [2, 3]. Despite these therapies, prognosis remains poor with a median survival of about 15 months [3].
Significant efforts have been directed at the molecular profiling of urothelial carcinoma, with the goal of identifying actionable aberrations that can lead to novel therapies and improved outcomes [4]. In particular, the PI3K/AKT/mTOR pathway has been shown in multiple studies to be altered in up to 40% of urothelial carcinomas, either through PIK3CA mutation, PTEN deletion, AKT1 mutation, or TSC1/TSC2 mutation [5, 6]. This led to two phase II trials of single-agent everolimus (RAD001), an mTOR inhibitor, in patients who had progressed on prior chemotherapy [7, 8]. Both studies identified few partial responses and several cases with minor tumor regression, with one patient whose tumor harbored a TSC1 mutation displaying an exceptional response to the drug [9].
Preclinical data supports the use of everolimus in combination with chemotherapeutic agents [10–13], and multiple early phase studies in a variety of malignancies indicate that combining everolimus with chemotherapy may be feasible in patients [14–18]. Notably, a phase I study combining everolimus with gemcitabine and cisplatin in patients with a variety of solid tumors, primarily cholangiocarcinoma and gallbladder carcinoma, was recently reported [19]. This study identified the maximum tolerated dose (MTD) as everolimus 5 mg on Monday/Wednesday/Friday, gemcitabine 600 mg/m2 and cisplatin 12.5 mg/m2 on days 1 and 8 of 21-day cycles, doses that are significantly lower than standard dosing for urothelial cancer. All dose-limiting toxicities (DLTs) were hematologic.
We carried out a phase I clinical trial combining everolimus with standard gemcitabine and cisplatin (GC) exclusively in patients with advanced and metastatic urothelial carcinoma in the first-line setting. Given concern for toxicity with this regimen, we designed the study with split-dosing of cisplatin, considered an alternative to the full dosing regimen [20], and a lower initial dose of gemcitabine at 800 mg/m2 on days 1 and 8 of 21-day cycles. The primary objectives were to establish the dose-limiting toxicity and maximum tolerated dose of everolimus in combination with gemcitabine and split-dose cisplatin in patients with advanced urothelial cancer. Secondary objectives included evaluating the response rate as determined by response evaluation criteria in solid tumors (RECIST) version 1.0, time to disease progression and overall survival with this regimen.
MATERIALS AND METHODS
The study was reviewed and approved by the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board and conducted within institutional and national guidelines on human experimentation. Patients at MSKCC with histologically confirmed advanced urothelial cancer were eligible for enrollment. Patients may not have received prior systemic chemotherapy for metastatic disease, though they may have received prior neoadjuvant or adjuvant systemic chemotherapy if it was completed at least 1 year prior to the diagnosis of metastatic disease. Eligible patients were required to have a Karnofsky Performance Status (KPS) of at least 70% , expected survival of at least 3 months, and adequate organ function based on screening laboratory values. Measurable disease was encouraged but not required. Exclusion criteria included any anticancer therapy or major surgery within 4 weeks of start of study, uncontrolled brain or leptomeningeal metastases, chronic systemic corticosteroids equivalent to prednisone 20 mg daily or higher, evidence of another malignancy (excluding non-melanoma skin cancer), and any severe or uncontrolled medical condition.
The goals of the study were to define the safety and establish the MTD of everolimus in combination with gemcitabine and split-dose cisplatin in advanced urothelial cancer. Secondary objectives were to evaluate the response rate, progression-free and overall survival observed with this regimen.
Treatment
Everolimus was administered orally daily or every other day depending on the dose level indicated below, and started on day 1 of 21-day cycles. Cisplatin was administered intravenously at 35 mg/m2 on days 1 and 8 of 21-day cycles. Gemcitabine was administered intravenously at 800 mg/m2 on days 1 and 8 of 21-day cycles. Supportive medications and intravenous fluids were administered per institutional guidelines for these drugs. Colony stimulating factors were not administered routinely, but were allowed for thetreatment of neutropenic fever or prophylactically in the case of observed neutropenia after chemotherapy. Patients received a maximum of 6 cycles of gemcitabine and cisplatin, and were eligible to continue everolimus at the same dose until disease progression or study discontinuation. Dose reductions were allowed for gemcitabine (level -1 = 650 mg/m2, level -2 = 500 mg/m2) and cisplatin (level -1 = 30 mg/m2) in case of toxicity. Patients requiring dose reductions below these levels were removed from study. Dose reductions for everolimus were not allowed.
Dose escalation and dose-limiting toxicities (DLTs)
Standard 3+3 design [21] was planned, with the goal of evaluating 3 everolimus dose levels (DLs): DL1: everolimus 5 mg every other day, DL2: everolimus 5 mg daily, DL3: everolimus 10 mg daily (Table 1). Toxicities were graded according to the National Cancer Institute (NCI) common terminology criteria for adverse events (CTCAE) version 3.0. DLTs during the first cycle included any event requiring a gemcitabine or cisplatin dose reduction, febrile neutropenia, grade 4 neutropenia lasting at least 7 days, any grade 4 hematologic toxicity, non-hematologic grade 3 or 4 treatment-related toxicity, and any non-hematologic toxicity requiring treatment delay of greater than 7 days.
Three patients were to be enrolled at DL1. If no DLTs were observed during cycle 1, 3 patients would be enrolled at the next dose level. If 1 DLT was observed during cycle 1, up to 3 additional patients would be enrolled at the same dose level. If 2 or more DLTs were observed at a given dose level, toxicity was exceeded at that dose level, and 3 additional patients would be enrolled at the lower dose level (de-escalation), or the trial ended if toxicity was exceeded at DL1. The protocol allowed for enrollment of additional patients at a dose level in case of inevaluable patients or concern for additional toxicity. The maximum tolerated dose (MTD) was defined as one dose level below the dose that induces DLTs in more than 1/6 patients.
Evaluation and follow-up
At screening, patients underwent a medical history and physical examination, laboratory testing including complete blood count, comprehensive metabolic panel, lipids, and hepatitis serologies, a 12-lead electrocardiogram, and disease assessment with baseline imaging (CT or MRI). Physical examination, blood count and metabolic panels were repeated prior to every chemotherapy treatment. Response assessments were performed every 2 cycles by imaging. For those patients continuing on everolimus alone, imaging studies were performed at 3 month intervals. Responses were assessed using RECIST version 1.0.
Statistical analysis
The study was designed for 3–6 patients to be enrolled at each everolimus dose level following traditional 3+3 design. The method of dose escalation between cohorts and dose limiting toxicities are described above. The design allowed for additional patients to be enrolled at a dose level if a patient was considered inevaluable for DLTs. Event-free survival (EFS) was calculated from treatment start to coming off study due to disease progression, toxicity, or patient’s withdrawal of consent. Overall survival (OS) was calculated from treatment start to date of death or last contact.
RESULTS
Patient characteristics and treatment
The study enrolled 12 patients between September 2010 and November 2013, 8 of them male and 4 female. Median age was 64. 7 patients had visceral metastases and 5 patients had lymph node-only disease. Table 2 summarizes patient characteristics.
Three patients were initially enrolled at DL1 and did not experience DLTs during the first cycle, leading to escalation to DL2. All 3 patients at DL2 experienced DLTs during cycle 1 (pancytopenia, hypersensitivity to everolimus and anemia requiring transfusion). In concordance with the 3+3 design of the study, de-escalation to DL1 occurred and 3 patients were enrolled at that dose level (called DL1 * after de-escalation). One of these patients experienced a DLT during cycle 1 (neutropenia) and another was considered inevaluable for DLTs due to disease progression prior to completing cycle 1. Given the inevaluable patient and concern for underrepresentation of DLTs, 3 additional patients were enrolled at DL1 *, for a total of 9 patients at the lowest everolimus dose level. Of these, 1 patient experienced a DLT (diarrhea) during cycle 1. DLTs for patients at each dose level are summarized in Table 3. In total, 2/8 (25%) of evaluable patients at the lowest dose level experienced DLTs during cycle 1. Using a 33% threshold for unacceptable toxicity during cycle 1 [22], the MTD was reached at the lowest dose level of everolimus 5 mg every other day. Notably one additional patient at DL1 experienced a DLT during a later cycle (pancytopenia leading to a gemcitabine dose reduction during cycle 5).
Toxicities
As noted above, 5 of 11 evaluable patients experienced DLTs during cycle 1, with one additional patient experiencing a DLT at a later point in treatment (Table 3). 4 of those DLTs were hematologic, including neutropenia, leukopenia, lymphopenia, anemia, and thrombocytopenia. Additional DLTs included diarrhea and a hypersensitivity reaction to everolimus resulting in grade 3 rash. Other grade 3 and 4 events included hypomagnesemia, hypophosphatemia, urinary tract infections, hyponatremia, hypokalemia and fatigue. Grade 3 and 4 adverse events possibly attributable to study treatment, as well as all grade 3 and 4 events occurring in at least 10% of patients, are listed in Table 4. Likewise, grade 1 and 2 adverse events are listed in Supplementary Table 1. Cumulative incidence by cycle of grade 3 and 4 hematologic toxicities is represented in Supplementary Figure 1A, while cumulative incidence of mucositis/stomatitis is represented in Supplementary Figure 1B.
Tumor response
Ten of 12 patients were considered evaluable for tumor response (Table 5). One of these patients had clinical progression while on therapy and was counted as having progressive disease (PD) despite having no imaging assessed by RECIST (patient 7). The two remaining patients were considered inevaluable for response due to coming off protocol prior to the first imaging assessment (patient 6, withdrew consent), and discontinuing everolimus during cycle 1 due to hypersensitivity reaction (patient 5).
Of the 10 evaluable patients, 4 had a partial response (PR), 5 had stable disease (SD), and 1 had progressive disease (PD) as their best response to therapy. Two patients (patients 3 and 8) completed 6 cycles of GC and had maintained PRs on everolimus monotherapy for a total of 19 and 9 cycles, respectively, until disease progression.
The MSKCC risk score, which includes KPS <80% and the presence of bone or visceral metastases as independent risk factors, was previously shown to accurately predict disease response and survival [23] and has been independently validated. Of the 10 patientsevaluable for response, 3/4 patients with a risk score of 0 had a PR (75%), 1/5 patients with a risk score of 1 had a PR (20%), and 0/1 patient with a risk score of 2 had a PR.
All 12 patients were used for EFS and OS analysis. Median EFS was 3.9 month (95% CI 3.7 –not reached) (Fig. 1). Two patients who had partial responses to therapy and came off trial to undergo consolidative surgery were censored for EFS at the date when they were taken off-study (patients 1 and 10). Median OS was 10.8 months (95% CI 6.9 –not reached) (Fig. 1). Eleven of twelve patients have died.
DISCUSSION
The poor prognosis associated with advanced and metastatic urothelial carcinoma calls for increased pre-clinical and clinical investigation of novel therapies in this disease [4]. Targeting the PI3K/AKT/mTOR pathway in combination with standard first-line cisplatin-based therapy presents a rational approach, given evidence of alteration of the pathway in urothelial carcinoma [5, 6], and evidence of some single-agent activity for the mTOR inhibitor everolimus in chemotherapy-refractory disease [7, 8]. Herein, we report the results of a phase I trial investigating the safety of combining everolimus with gemcitabine and split-dose cisplatin in advanced urothelial carcinoma. We established the MTD at everolimus 5 mg every other day, gemcitabine 800 mg/m2 on days 1 and 8, and cisplatin 35 mg/m2 on days 1 and 8 of 21-day cycles, with 2/8 evaluable patients exhibiting DLTs during cycle 1 at that dose level. Overall, the regimen was difficult to tolerate, with a total of 8 dose reductions of gemcitabine and cisplatin in a cohort of 12 patients and non-negligible numbers of hematologic toxicities occurring during later cycles of therapy.
Our study findings are in agreement with others, finding that a significant number of patients developed DLTs that were hematologic, likely due to the combined myelosuppressive effects of everolimus combined with gemcitabine. A recent study tested the combination of everolimus with gemcitabine and cisplatin in a variety of solid tumors primarily consisting of cholangiocarcinoma and gallbladder cancer, and established the MTD at everolimus 5 mg on Monday/Wednesday/Friday, gemcitabine 600 mg/m2 on days 1 and 8, and cisplatin 12.5 mg/m2 on days 1 and 8 of 21-day cycles [19]. These doses are well below standard therapy for urothelial carcinoma, and the MTD fell at dose level -2, suggesting that the regimen had worse tolerability than expected. All DLTs identified in that study were hematologic, namely thrombocytopenia and neutropenia.
This regimen resulted in 4/10 partial responses and an overall survival of 10.8 months (95% CI 6.9 –not reached). Given the small numbers of patients and wide confidence interval, this is roughly in line with the phase I/II study of gemcitabine and split-dose cisplatin alone, which reported a response rate of 66% and median overall survival of 16 months [20]. In addition, we find a higher number of partial responses in patients with lower MSKCC risk scores, consistent with known worse outcomes associated with higher risk scores [23]. Of note, gemcitabine and split-dose cisplatin has not been shown to be equivalent to standard GC in a Phase III trial, thus it is possible that patients on the study may have received suboptimal doses of cisplatin. We also aimed to perform studies correlating alterations in the PI3K/AKT/mTOR pathway with response to therapy, but did not have sufficient tissue to proceed.
In conclusion, we established the MTD for everolimus, gemcitabine and split-dose cisplatin in advanced urothelial carcinoma. The regimen, utilizing lower doses of gemcitabine than is typical of urothelial cancer regimens, was difficult to tolerate due to predominantly hematologic toxicities. A different approach will likely have to be employed targeting the PI3K/AKT/mTOR pathway in advanced and metastatic urothelial cancer. Based on reports of exceptional response to everolimus in specific molecular contexts [9, 24, 25], we believe the agent will be most appropriate as monotherapy or in combination for patients with specific molecular alterations. Targeted trials of everolimus in specific molecular contexts are currently ongoing (e.g. NCT02201212, NCT02352844).
CONFLICT OF INTEREST
Dean F. Bajorin received research funding and minor consulting fees from Novartis.
ACKNOWLEDGMENTS
This study was funded by the Novartis Pharmaceuticals Corporation and conducted with support from NIH grant P30-CA008748 to MSKCC. Wassim Abida is the recipient of a Prostate Cancer Foundation Young Investigator Award and of a Conquer Cancer Foundation of ASCO Young Investigator Award.
Appendices
The supplementary table and figure are available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-150038.
REFERENCES
[1] | Siegel R , Naishadham D , Jemal A . Cancer statistics, 2013, CA Cancer J Clin (2013) ;63: (1):11–30. |
[2] | Sternberg CN , et al. Randomized phase III trial of high-doseintensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol (2001) ;19: (10):2638–2646. |
[3] | von der Maase H , et al.Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol (2005) ;23: (21):4602–4608. |
[4] | Nadal R , Bellmunt J . New treatments for bladder cancer: When will we make progress? Curr Treat Options Oncol (2014) ;15: (1):99–114. |
[5] | Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature (2014) ;507: (7492):315–322. |
[6] | Iyer G , et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol (2013) ;31: (25):3133–3140. |
[7] | Milowsky MI , et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int (2013) ;112: (4):462–470. |
[8] | Seront E , et al. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: Clinical activity, molecular response, and biomarkers. Ann Oncol (2012) ;23: (10):2663–2670. |
[9] | Iyer G , et al. Genome sequencing identifies a basis for everolimus sensitivity. Science (2012) ;338: (6104):221. |
[10] | Beuvink I , et al (2005) ; The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell (6104) ;120: (6):747–759. |
[11] | O’Reilly T , et al. Evaluation of the mTOR inhibitor, everolimus, in combination with cytotoxic antitumor agents using human tumor models in vitro and in vivo. Anticancer Drugs (2011) ;22: (1):58–78. |
[12] | Pinto-Leite R , et al. Everolimus combined with cisplatin has a potential role in treatment of urothelial bladder cancer. Biomed Pharmacother (2013) ;67: (2):116–121. |
[13] | Pinto-Leite R , et al. Everolimus enhances gemcitabine-induced cytotoxicity in bladder-cancer cell lines. J Toxicol Environ Health A (2012) ;75: (13–15):788–799. |
[14] | Besse B , et al. A phase Ib dose-escalation study of everolimus combined with cisplatin and etoposide as first-line therapy in patients with extensive-stage small-cell lung cancer. Ann Oncol (2014) ;25: (2):505–511. |
[15] | Chiang CT , et al. Combinations of mTORC1 inhibitor RAD001 with gemcitabine and paclitaxel for treating non-Hodgkin lymphoma. Cancer Lett (2010) ;298: (2):195–203. |
[16] | Fury MG , et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol (2012) ;69: (3):591–598. |
[17] | Fury MG , et al. A phase 1 study of everolimus plus docetaxel plus cisplatin as induction chemotherapy for patients with locally and/or regionally advanced head and neck cancer. Cancer (2013) ;119: (10):1823–1831. |
[18] | Joka M , et al. Combination of antiangiogenic therapy using the mTOR-inhibitor everolimus and low-dose chemotherapy for locally advanced and/or metastatic pancreatic cancer: A dose-finding study. Anticancer Drugs (2014) ;25: (9):1095–1101. |
[19] | Costello BA , et al. Phase I trial of everolimus, gemcitabine and cisplatin in patients with solid tumors. Invest New Drugs (2014) ;32: (4):710–716. |
[20] | Hussain SA , et al. A phase I/II study of gemcitabine and fractionated cisplatin in an outpatient setting using a 21-day schedule in patients with advanced and metastatic bladder cancer. Br J Cancer (2004) ;91: (5):844–849. |
[21] | Le Tourneau C , Lee JJ , Siu LL . Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst (2009) ;101: (10):708–720. |
[22] | Ivy SP , et al. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: A report from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin Cancer Res (2010) ;16: (6):1726–1736. |
[23] | Bajorin DF , et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol (1999) ;17: (10):3173–3181. |
[24] | Ali SM , et al. Exceptional Response on Addition of Everolimus to Taxane in Urothelial Carcinoma Bearing an NF2 Mutation. Eur Urol (2015) ;67: (6):1195–1196. |
[25] | Wagle N , et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov (2014) ;4: (5):546–553. |
Figures and Tables
Fig.1
Kaplan-Meier curves for event-free survival (EFS) and overall survival (OS). Dotted lines represent 95% confidence intervals.
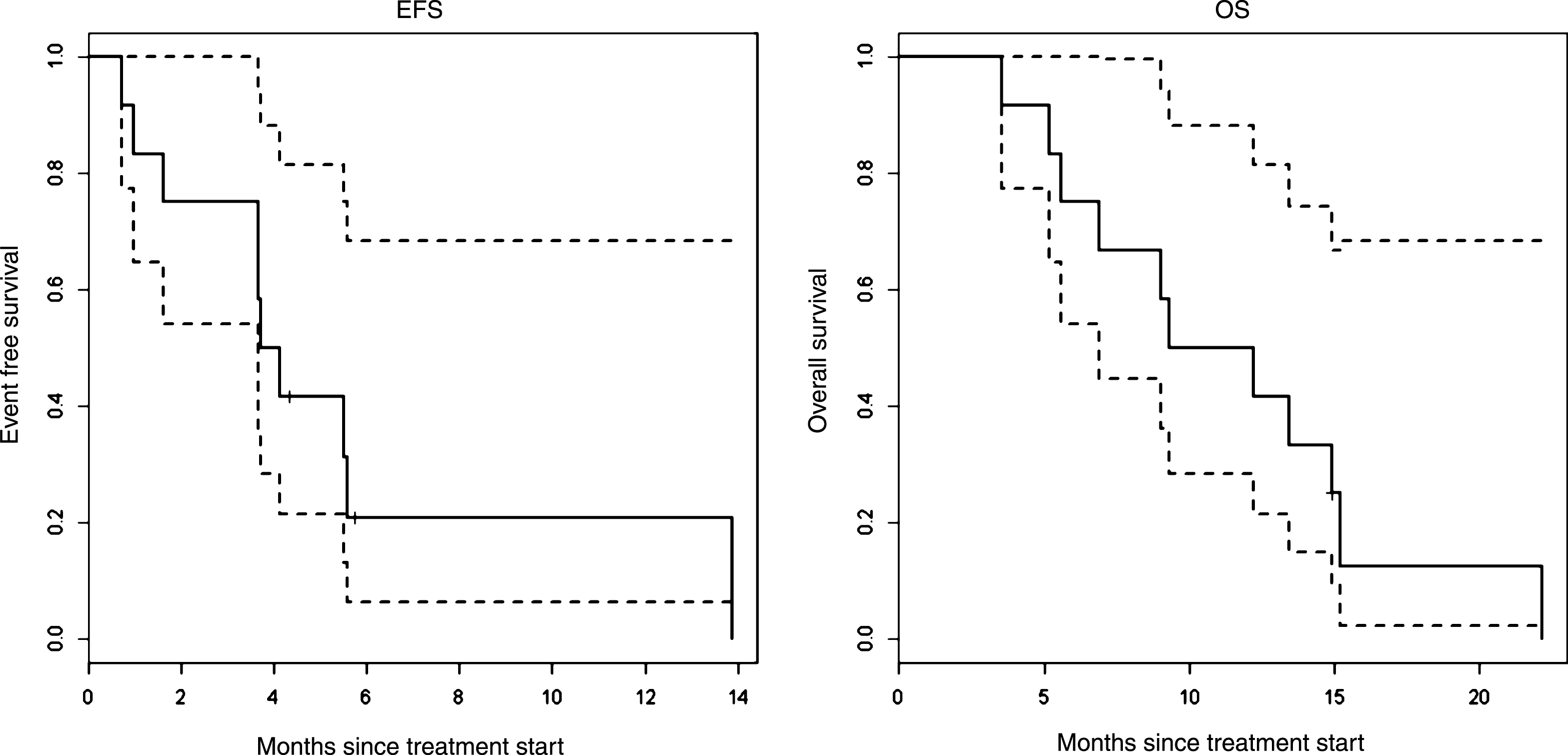
Table 1
Drug doses by dose level (21-day cycles)
| Dose level | Everolimus | Gemcitabine | Cisplatin |
| DL1 | 5 mg every other day | 800 mg/m2 days 1 and 8 | 35 mg/m2 days 1 and 8 |
| DL2 | 5 mg daily | 800 mg/m2 days 1 and 8 | 35 mg/m2 days 1 and 8 |
| DL3 | 10 mg daily | 800 mg/m2 days 1 and 8 | 35 mg/m2 days 1 and 8 |
Table 2
Patient characteristics (N = 12)
| Summary | |
| Age, median (range) | 64 (47–73) |
| Gender | |
| Male | 8 (66%) |
| Female | 4 (33%) |
| KPS, median (range) | 80% (70–90) |
| Primary site | |
| Bladder | 7 |
| Renal pelvis | 4 |
| Ureter | 1 |
| Visceral metastasis (any one site below) | 7 |
| Lung | 3 |
| Liver | 1 |
| Bone | 4 |
| Other soft tissue | 2 |
| Prior curative surgery | 3 |
| MSKCC risk group [23] | |
| 0 | 5 |
| 1 | 6 |
| 2 | 1 |
| Number of disease sites, median (range) | 2 (1–4) |
Table 3
Treatment and DLT summary
| Patient | Dose level | DLT in | DLT in | Cycles | Dose | Reason for |
| Cycle 1 | Later Cycle | completed | Reductions | end treatment | ||
| 1 | DL1 | None | Pancytopenia | 5.5 | 1 | Consolidation surgery |
| 2 | DL1 | None | None | 4 | 0 | Unrelated fall |
| 3 | DL1 | None | None | 19 | 0 | Progression |
| 4 | DL2 | Pancytopenia | N/A | 5 | 2 | Toxicity |
| 5 | DL2 | Hypersensitivity (Grade 3 rash) | N/A | 6 | 2 | Completed GC |
| 6 | DL2 | Anemia | N/A | 0.5 | 0 | Withdrew consent |
| 7 | DL1 * | Inevaluable | N/A | 0.5 | 0 | Progression |
| 8 | DL1 * | Neutropenia | N/A | 9 | 1 | Progression |
| 9 | DL1 * | None | None | 5.5 | 0 | Progression |
| 10 | DL1 * | None | None | 6 | 0 | Consolidation surgery |
| 11 | DL1 * | Diarrhea | N/A | 2.5 | 2 | Toxicity |
| 12 | DL1 * | None | None | 6 | 0 | Progression |
Table 4
Grade 3 and 4 adverse events possibly attributable to protocoltherapy, or occurring in at least 10% of patients (Total N = 12)
| Adverse event (CTCAE v 3.0) | N (%) |
| Anemia | 8 (67%) |
| Leukopenia | 5 (42%) |
| Neutropenia | 5 (42%) |
| Lymphopenia | 5 (42%) |
| Thrombocytopenia | 3 (25%) |
| Hypomagnesemia | 2 (17%) |
| Urinary tract infection | 3 (25%) |
| Pain | 2 (17%) |
| Hypophosphatemia | 2 (17%) |
| Diarrhea | 1 (8%) |
| Hypokalemia | 1 (8%) |
| Muscle weakness | 1 (8%) |
| Rash/desquamation | 1 (8%) |
| INR | 1 (8%) |
| Hyponatremia | 1 (8%) |
| Hypermagnesemia | 1 (8%) |
| DVT | 1 (8%) |
| Dehydration | 1 (8%) |
| Fatigue | 1 (8%) |
| Urinary frequency | 1 (8%) |
Table 5
Best tumor responses by RECIST v. 1.0. PR: partial response. SD: Stable disease. PD: Progressive disease
| Patient | Dose level | MSKCC risk | Tumor |
| score | response | ||
| 1 | DL1 | 0 | PR |
| 2 | DL1 | 1 | SD |
| 3 | DL1 | 0 | PR |
| 4 | DL2 | 1 | SD |
| 5 | DL2 | 0 | Inevaluable (stopped |
| everolimus in cycle 1) | |||
| 6 | DL2 | 1 | Inevaluable |
| (withdrew in cycle 1) | |||
| 7 | DL1 * | 2 | PD (clinical) |
| 8 | DL1 * | 1 | PR |
| 9 | DL1 * | 1 | SD |
| 10 | DL1 * | 0 | PR |
| 11 | DL1 * | 0 | SD |
| 12 | DL1 * | 1 | SD |



