Concordance in Biomarker Status Between Bladder Tumors at Time of Transurethral Resection and Subsequent Radical Cystectomy: Results of a 5-year Prospective Study
Abstract
Purpose:
To assess the concordance rate in alterations of molecular markers at the time of transurethral resection (TUR) and subsequent radical cystectomy (RC) among patients with high-grade urothelial carcinoma of the bladder (UCB).
Methods:
We prospectively performed immunohistochemical staining p53, p21, p27, Ki-67 and cyclin E1 on TUR and on RC specimens from 102 patients treated with RC and bilateral lymphadenectomy for high-grade UCB. We analyzed the concordance rate of individual markers and of the number of altered markers. Concordant and discordant findings were reported in the overall population and according to clinical stage.
Results:
Median patient age was 74 years (IQR 67–79) and mostly male (86%). Median time from TUR to RC was 1.5 months (IQR 1.0–2.4). Clinical stage at time of RC was cTa/Tis/T1 in 50% , cT2 in 47% , and cT4 in 1% of patients Nine (9%) patients received neoadjuvant chemotherapy. The concordance of biomarkers between TUR and RC specimens was 92.2% , 77.5% , 80.4% , 77.5% , and 83.3% for cyclin E1, p21, p27, p53 and Ki-67, respectively. The concordance between number of altered biomarkers was 51.0%.
Conclusions:
The rate of individual marker alterations at time of TUR closely approximates that found at RC specimens. However, the correlation of number of altered markers is lower. Molecular marker status at TUR could help predict the marker status at RC and may help guide multimodal therapeutic planning.
INTRODUCTION
Neoadjuvant chemotherapy provides a survival benefit for patients with muscle invasive bladder cancer [1, 2]. On the other hand, there are several studies that demonstrate that it is underutilized in treating UCB even among academic centers [3, 4]. There are multiple reasons which hinder the use of neoadjuvant chemotherapy. One concern is the assumption that patients can be cured by radical cystectomy (RC) alone and that the risks of chemotherapy outweigh its benefits in most patients. However, there is a significant risk of upstaging at the time of RC and current staging tools, such as computed tomography and magnetic resonance imaging, understage bladder cancer in 10–39% of cases [5–7].
Current risk-stratification systems, such as that of the American Joint Committee on Cancer, still rely on readily available clinical and histopathological data, yielding low accuracy in their predictions both pre- [8] and post-operatively [9, 10]. As a matter of fact, up to 51% of patients with non-invasive high-grade UBC actually harbor muscle invasive disease, and up to 16% already have involvement of the lymph nodes [8]. Furthermore, the predictive accuracy of pathological stage in predicting long-term outcome after RC is modest, and by constructing more comprehensive multivariable models including other clinical variables, the accuracy of the prediction is increased to 75–78% [9–11].
In the past years, there has been a growing interest in trying to improve staging and outcome prediction with the help of molecular markers [12]. These markers provide information of molecular alterations and could help in further stratification of heterogeneous cancers that have the same phenotype according to current staging methods. Among the different molecular markers, a panel of 5 cell-cycle molecules (cyclin E1, tumor protein p53 [Tp53], cyclin-dependent kinase inhibitor 1A [CDKN1A; formerly p21]; cyclin-dependent kinase inhibitor 1B [p27, Kip1]; and antigen identified by monoclonal antibody Ki-67 [MKI67; formerly Ki-67]) has been extensively characterized and validated in single- and multi-institutional studies[13–17, 18].
The use of this panel of markers recently has been shown in a prospective series to improve post-operative risk-stratification, increasing the accuracy of a model including standard pathologic features for prediction of cancer specific mortality from 80% to 83% [17]. However, to date there is little information [19, 20] on the correlation of marker alterations on TUR and RC specimens and no prospective evaluations. The aim of our study was to analyze the alteration of the aforementioned markers at time of TUR and RC in patients with high-grade UBC. A good correlation could potentially lead to earlier risk-stratification and improved decision-making regarding multi-modal therapeutic approaches.
MATERIALS AND METHODS
All procedures described in the present study were undertaken with the approval and oversight of the Institutional Review Board for the Protection of human subjects
Patient population
As previously published [17], immunohistochemical staining for cyclin E1, Tp53, CDKN1a, p27, and MKI67 on all UCB was performed at the hospitals of the University of Texas Southwestern Medical Center as part of a prospective study from January 2007. A similar protocol was initiated at New York Presbyterian Hospital in 2011. The IHC staining and scoring was done on each individual case in real time as compared with previously published TMAs where all cases are stained and scored together. As such the pathologist had to review and score the cases independent of the other cases and without knowledge of status of TUR (in cases of cystectomy) and without other prognostic information. Clinical and pathological variables for all patients were inserted in an Institutional Review Board-approved prospective database. Preoperative imaging included chest radiographs and computed tomography scans or magnetic resonance imaging of the abdomen and pelvis.
Immunohistochemical data on final pathology specimens was available for 234 patients who underwent RC and bilateral lymph node dissection for high grade and/or muscle invasive bladder cancer. Of these, 102 patients (41 from University of Texas and 61 from New York) had immunohistochemical data available also for TUR specimens. Compared to patients without marker status at TUR, patients with immunohistochemical information at TUR had significantly higher rates of non-muscle invasive disease (54% vs. 33% ; p = 0.0005), while there were no significant differences regarding clinical grade, concomitant CIS at TUR, months from TUR to RC and neoadjuvant chemotherapy rates. Overall, 9 patients (9%) underwent neoadjuvant chemotherapy based on physician/patient preference. No patient received prior pelvic radiotherapy.
Both TUR and RC pathological specimens were processed and examined by expert pathologists dedicated to genito-urinary cancers, following previously described protocols [14, 21]. Staging and grading of the specimens was conducted using the AJCC 2002 TNM classification and the 1998 WHO/ISUP classification, respectively [22].
Immunohistochemistry
Immunohistochemical staining for cyclin E1, Tp53, CDKN1a, p27, and MKI67 was performed on serial sections from the same paraffin-embedded tumor block. The staining protocols for all antibodies were described in detail in previous studies [14, 17]. Briefly, immunostaining was performed on the Dako Autostainer (Carpinteria, CA, USA). Optimum primary antibody dilutions were predetermined using known positive control tissues. Multiple known positive control sections were included in each run. Tumor sections with the primary antibodies substituted with rabbit immunoglobulin fraction (normal) and/or immunoglobulin G1 monoclonal were used as negative controls.
Staining was performed both on tissue from TUR and RC. Internal controls of non-malignant urothelium were obtained from specimens of patients undergoing RC for non-neoplastic causes. We used bright field microscopy imaging coupled with advanced color detection software (Automated Cellular Imaging System, Clarient, CA) to detect, classify, and count stained cellular objects based on predetermined color morphology. We obtained the mean, maximum, range, and standard deviation of staining intensity and percentage of positive nuclei/area measurements by using 10 random hot spots within each specimen. The mean of the triplicate cores was calculated for data analysis. All markers were placed in 1 of 2 categories, altered or normal.
Immunohistochemical scoring
As shown and validated in previous studies [13–17, 18], nuclear Tp53 immunoreactivity was considered altered when samples demonstrated at least 10% nuclear reactivity. On the other hand, CDKN1a findings were considered altered when samples demonstrated less than 10% staining. Nuclear p27 and cyclin E1 were considered altered when samples demonstrated less than 30% nuclear reactivity. Finally, MKI67 immunoreactivity was considered altered when samples showed >10% nuclear reactivity. Based on the number of altered biomarkers, an established prognostic score as has been previously reported was used. An altered expression of none to two biomarkers was considered favorable, whereas an alteration of at least three biomarkers represented an unfavorable prognostic score.
Statistical analyses
First, we evaluated the concordance of marker alterations between TUR and RC specimens. In detail, we tested several levels of concordance: the overall concordance was divided in negative and positive concordance, that is patients who had respectively negative or positive markers in both TUR and RC specimens. Furthermore, among patients who did not exhibit concordance, we explored how many patients underwent alteration of a marker from TUR to RC and how many underwent a normalization of a marker. This scrutiny was carried out for each individual marker, for the total number of altered makers, and for a prognostic score based on the number of altered numbers (≤2 vs >2). Figures 1 and 2 demonstrate positive and negative concordance as well as changes in marker status from TUR to RC.
Second, Fisher’s exact test and the Mann-Whitney U test were used to evaluate associations between marker concordance rates and clinic-pathological parameters.
All statistical tests were performed with the use of STATA version 12 (Stata Corp, College Station, TX, USA). All tests were two-sided with a significance level at 0.05.
RESULTS
Demographics
Clinical and pathological characteristic of patients with immunohistochemical data for both TUR and RC specimens are shown in Table 1. The median age at RC was 74 years (IQR 67–79 years), with the majority of patients (86%) being male. The median time from TUR to RC was 1.5 months (IQR 1.0–2.4 months). The majority of patients (n = 51) had non-muscle invasive disease at clinical staging, while 48 of them had invasion of the muscle layer, and only 1 had tumor spreading into extravesical tissues. Clinical stage was unknown for 2 patients. Almost all patients (n = 99) had high-grade disease at TUR, only 3 patients had low grade cancer but were subsequently found to have high grade disease at cystectomy. Sixteen patients presented with concomitant CIS at TUR. Neoadjuvant chemotherapy was administered in 9 patients.
Final pathological stage showed significant upstaging, with 32% of patients with pT3 disease and 5% with pT4. Non-muscle invasive disease was seen in 44% of the patients and pT2 in 17% . Concomitant CIS at final pathology was seen in 50% of individuals, 27% had evidence of lymphovascular invasion, while 13% had lymph node metastases.
Marker concordance
Table 2.1 shows concordance rates of markers between TUR and RC specimens. Overall, the concordance rate for each of the five individual markers varied between 77.5% to 92.2% % , with the lowest concordance for CDKN1a and Tp53, and the highest concordance for cyclin E1. Concordance between the number of altered markers was seen in 51.0% of patients and the prognostic marker score (≤2 vs >2) in 75.5% .
With regards to the markers whose status was normal at TUR, cyclin E1 was found to be altered at RC only in 3.9% of cases, followed by Tp53 (7.8%), MKI67 (9.8%), p27 (12.7%), while CDKN1a showed the highest rate of alteration (16.7%). On the other end, concerning markers that were found altered at TUR, cyclin E1 was the markers who showed the lowest rate of normalization at final pathology (3.9%), followed by CDKN1a(5.9%), MKI67 and p27 (both 6.9%) and Tp53(14.7%).
Table 2.2 shows markers concordance rates according to clinical stage. Significant differences were not recorded for concordance of each marker even when patients were stratified according to non-muscle invasive (cTa/Tis/T1) versus muscle invasive disease (cT2/T3/T4) at TUR (all p > 0.2). Furthermore, we did not record significant differences regarding the concordance of number of altered markers and prognostic marker score (all p > 0.4).
DISCUSSION
Marker concordance between diagnostic biopsy and final pathological specimen is of importance when planning neoadjuvant therapies. Studies have shown good concordance of HER-2/neu alterations between biopsy and surgical specimens in breast cancer patients, allowing clinicians to rely on biopsy results to plan neoadjuvant chemotherapy [25, 26]. As with breast cancer, a good concordance of markers between TUR and RC specimens could help extrapolate data from biopsy setting to RC setting, thereby allowing a risk-stratified approach to multimodal neoadjuvant therapies. Indeed, despite level-1 evidence of the efficacy of neoadjuvant chemotherapy in UCB, its use is not widespread, possibly due to the concerns of over-treatment and side effects [27]. Markers assessment at TUR could potentially overcome these problems by selecting only the patients at higher-risk of adverse outcomes such as upstaging and micrometastatic disease.
Our study compared biomarker alterations in UCB specimens at TUR and RC in a large prospectively-collected series. Furthermore, we tested a panel of five markers that has gone through a stringent process of validation [12, 13, 15, 16] and that has shown good performance in predicting oncological outcomes [14, 17]. In addition, the aforementioned markers panel was shown to improve the detection of patients that will harbor more invasive disease at RC [18]. Our results showed a good rate of concordance between individual marker findings at immunohistochemical analysis of TUR and RC specimens of patients with high-grade UBC. The concordance rate of individual markers alterations ranged from 77.5% to 92.2% . These rates are similar to those reported in a previous retrospective study, where concordance rates of alteration of Tp53, pRB, CDKN1a and p27 at the time of TUR and RC were reported in 22 patients [20]. However, when analyzing the number of total altered markers the concordance rate dropped to 51.0% but was 75.5% for marker score. Interestingly, when comparing concordance rates between patients who had clinically non muscle-invasive disease (cTa/cTis/cT1) to those who had a clinical stage T2 or higher at TUR, we did not record any statistically significant difference.
While the study’s finding that individual marker alterations were mostly accurate is reassuring, the finding that the number of alterations was less accurate is disappointing. Several studies have demonstrated that the number of alterations has a greater impact on predicting outcomes than any single marker [14, 17]. This may be due to the limitations of staining, differences between observations on different days of resection, changes that relate to the TUR such as necrosis from cautery, or variability within tumors such that some populations of cells exhibit more abnormalities than other areas.
For most individual markers, the concordance was high and this is an important fact since other tumors that are multifocal by nature can have variability between the tumor sampled and the most aggressive tumor in the organ. This is seen in multifocal tumors such as prostate cancer, for example, where it is not uncommon that the biopsy shows a Gleason score of lower than that found at radical prostatectomy, with rates of upgrading of 28% [28, 29].
Our prospective study has several limitations. First of all, immunohistochemistry can be influenced by many factors, such as choice of antibody, antibody dilution, tissue sampling, etc. However, pathological assessment was carried out following highly standardized protocols and advanced software-based autoscoring systems, which have been shown to improve staining reproducibility. Furthermore, it is important to stress that in our series marker alterations were assessed prospectively. This means that TUR and RC specimens were processed in the context of normal pathology routine, unlike previous studies were samples were retrospectively collected and validated in an experimental setting. Another limitation is that the use of neoadjuvant chemotherapy was not standardized. Hence, it might have affected the alterations of markers at RC. However, results in the population of patient that did not undergo pre-operative chemotherapy reflected the overall results. Finally, the extent of TUR could have had an impact on the alterations of markers.
CONCLUSIONS
Our study is the first one to address the concordance rate of marker panels at TUR and at RC in patients with high-grade UCB, in a prospective fashion. Our results indicate low rates of concordance when analyzing the number of altered markers, but high concordance rates when analyzing individual markers, independent of stage or neoadjuvant chemotherapy. Alterations of individual markers in TUR specimens closely reflect those found at RC and could be used for pre-operative risk stratification and neoadjuvant treatment planning.
REFERENCES
[1] | Grossman HB , Natale RB , Tangen CM , et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med (2003) ;349: , 859–866. |
[2] | International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al.: International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer:Long-term results of the BA06 30894 trial. Journal of Clinical Oncology (2011) ;29: :2171–7. |
[3] | David KA , Milowsky MI , Ritchey J , et al. Low incidence of perioperative chemotherapy for stage III bladder cancer to A report from the National Cancer Data Base. J Urol (2007) ;178: , 451–454. |
[4] | Raj GV , Karavadia S , Schlomer B , et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer (2011) ;117: , 276–282. |
[5] | Shariat SF , Palapattu GS , Karakiewicz PI , et al. Discrepancy between Clinical and Pathologic Stage: Impact on Prognosis after Radical Cystectomy. Eur Urol (2007) ;51: , 137–151. |
[6] | Green DA , Rink M , Hansen J , et al. Accurate preoperative prediction of non-organ-confined bladder urothelial carcinoma at cystectomy. BJU Int (2013) Mar;111: (3):404–11. doi: 10.1111/j.1464-410X.2012.11370.x. Epub 2012 Jul 13. PMID: 22805163. |
[7] | Lawrentschuk N , Lee ST , Scott AM . Current role of PET, CT, MR for invasive bladder cancer. Curr Urol Rep (2013) ;14: , 84–89. |
[8] | Fritsche H-M , Burger M , Svatek RS , et al. Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: Results from an international cohort. Eur Urol (2010) ;57: , 300–309. |
[9] | International Bladder Cancer Nomogram Consortium, Bochner BH , Kattan MW , et al. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. Journal of Clinical Oncology (2006) ;24: , 3967–72. |
[10] | Karakiewicz PI , Shariat SF , Palapattu GS , et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol (2006) ;176: , 1354–1361. discussion 1361–2. |
[11] | Shariat SF , Karakiewicz PI , Palapattu GS , et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res (2006) ;12: , 6663–6676. |
[12] | Rink M , Cha EK , Green D , et al. Biomolecular predictors of urothelial cancer behavior and treatment outcomes. Curr Urol Rep (2012) ;13: , 122–135. |
[13] | Shariat SF , Ashfaq R , Sagalowsky AI , et al. Correlation of cyclin D1 and E1 expression with bladder cancer presence, invasion, progression, and metastasis. Hum Pathol (2006) ;37: , 1568–1576. |
[14] | Shariat SF , Karakiewicz PI , Ashfaq R , et al. Multiple biomarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer (2008) ;112: , 315–125. |
[15] | Shariat SF , Tokunaga H , Zhou J , et al. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol (2004) ;22: , 1014–1024. |
[16] | Shariat SF , Chromecki TF , Cha EK , et al. Risk Stratification of Organ Confined Bladder Cancer After Radical Cystectomy Using Cell Cycle Related Biomarkers. J Urol (2012) ;187: , 457–462. |
[17] | Lotan Y , Bagrodia A , Passoni N , et al. Prospective Evaluation of a Molecular Marker Panel for Prediction of Recurrence and Cancer-specific Survival After Radical Cystectomy. Eur Urol (2013) ;64: (3), 465–471. |
[18] | Shariat SF , Passoni N , Bagrodia A , et al. Prospective Evaluation of a preoperative biomarker panel for prediction of upstaging at radical cystectomy. BJU Int(2013) ; in press. |
[19] | Tokunaga H , Shariat SF , Green AE , et al. Correlation of immunohistochemical molecular staging of bladder biopsies and radical cystectomy specimens. Int J Radiat Oncol Biol Phys (2001) ;51: , 16–22. |
[20] | Shariat SF , Zlotta AR , Ashfaq R , et al. Cooperative effect of cell-cycle regulators expression on bladder cancer development and biologic aggressiveness. Mod Pathol (2007) ;20: , 445–459. |
[21] | Margulis V , Shariat SF , Ashfaq R , et al. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin Cancer Res (2006) ;12: , 7369–7373. |
[22] | Epstein JI , Amin MB , Reuter VR , et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee (1998) ;22: , 1435–1448. |
[23] | Shariat SF , Karakiewicz PI , Palapattu GS , et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: A contemporary series from the Bladder Cancer Research Consortium. J Urol (2006) ;176: , 2414–2422. discussion 2422. |
[24] | Margulis V , Lotan Y , Karakiewicz PI , et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. JNCI Journal of the National Cancer Institute (2009) ;101: , 114–119. |
[25] | D’Alfonso T , Liu Y-F , Monni S , et al. Accurately assessing her-2/neu status in needle core biopsies of breast cancer patients in the era of neoadjuvant therapy: Emerging questions and considerations addressed. Am J Surg Pathol (2010) ;34: , 575–581. |
[26] | Lee AHS , Key HP , Bell JA , et al. Concordance of HER2 status assessed on needle core biopsy and surgical specimens of invasive carcinoma of the breast. Histopathology (2012) ;60: , 880–884. |
[27] | Apolo AB , Grossman HB , Bajorin D , et al. Practical use of perioperative chemotherapy for muscle-invasive bladder cancer: Summary of session at the Society of Urologic Oncology annual meeting. Urol Oncol (2012) ;30: , 772–780. |
[28] | Chun FK-H , Briganti A , Shariat SF , et al. Significant upgrading affects a third of men diagnosed with prostate cancer: Predictive nomogram and internal validation. BJU Int (2006) ;98: , 329–334. |
[29] | Epstein JI , Feng Z , Trock BJ , et al. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: Incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol (2012) ;61: , 1019–1024. |
Figures and Tables
Fig.1
In this patient, there was marker number and overall score concordance between transurethral resection (TUR) and radical cystectomy (RC) specimens - a total of 2 markers were altered in both specimens - favorable prognosis. This concordance in number of markers and score, when resolved to the level of individual markers, was achieved by a combination of individual marker concordance (Ki67 showed positive concordance, and p21 & Cyclin E showed negative concordance between the TUR and RC specimens, as well as non-concordance (normalization of p53 and alteration of p27, from TUR to RC). The images in this panel (Panel 1) show positive concordance of Ki-67 for TUR to RC (top), negative concordance for Cyclin E (middle), and normalization of P53 (bottom) between the TUR to RC specimens (see main paper for definition of alteration for each marker).
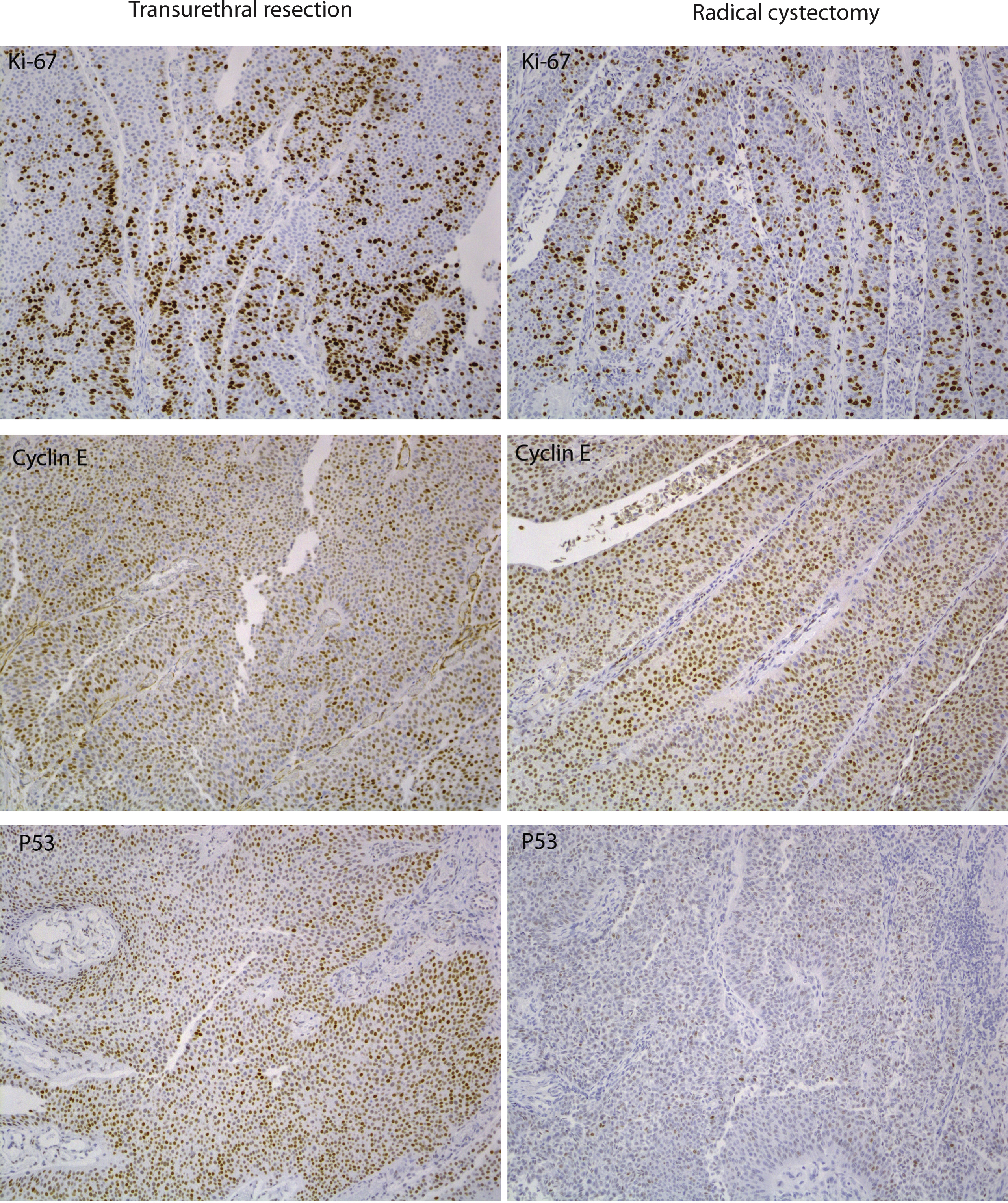
Fig.2
In this patient, there is marker number and overall score non-concordance between TUR and RC specimens. A total of 2 markers, P53 and Ki-67, were altered in the TUR specimen (favorable prognosis), but a total of 3 markers –p21, p27 and Ki67 - were altered in RC (i.e. p21 & p27 underwent alteration and p53 underwent normalization from TUR to RC), with an unfavorable prognosis. The images in this panel (Panel 2) show, from top to bottom: H&E images (topmost), positive concordance for Ki-67 marker, negative concordance for Cyclin E, alteration for p21 and normalization of P53 (bottom) between the TUR to RC specimens (see main paper for definition of alteration for each marker).
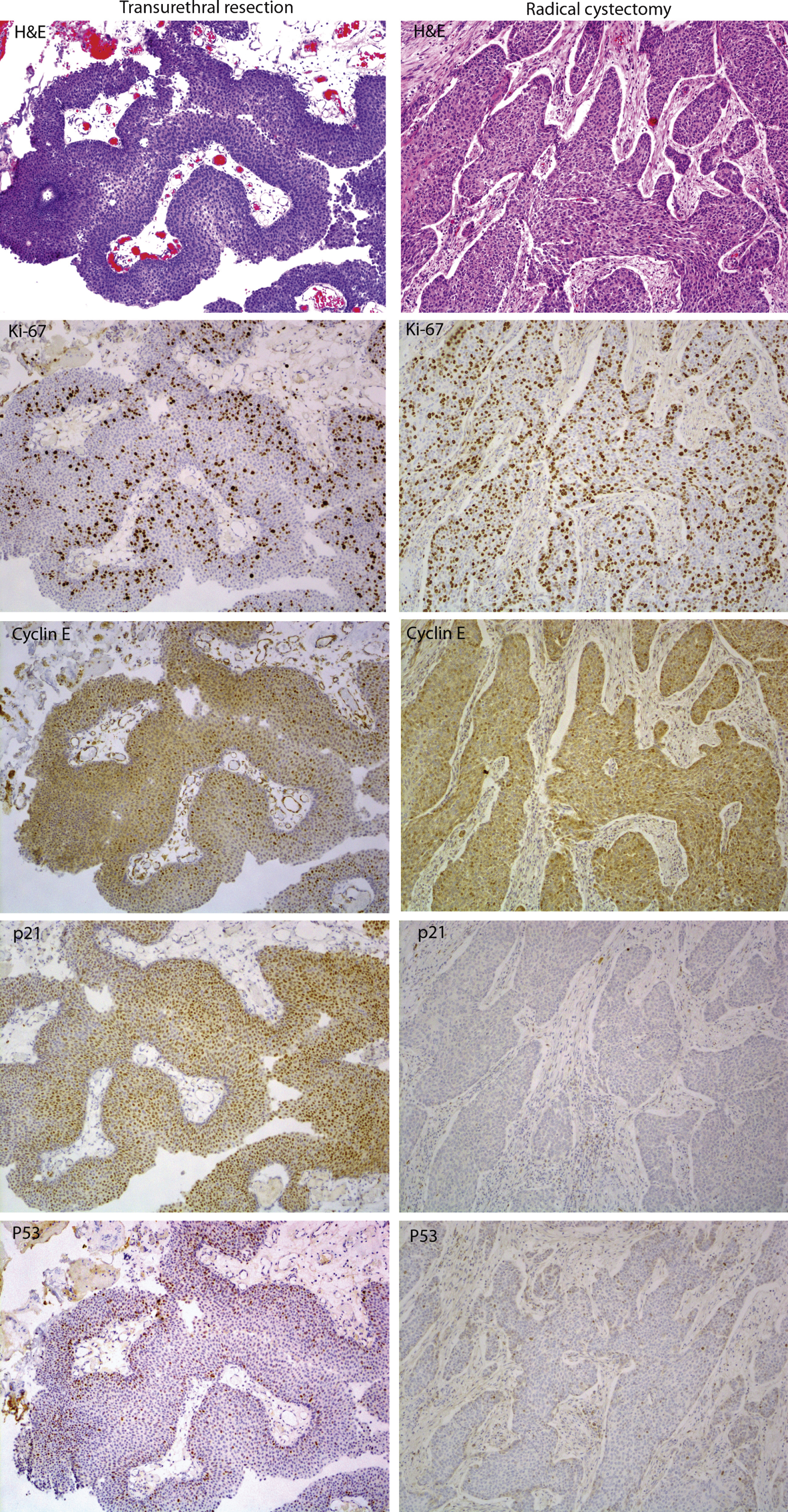
Table 1
Population characteristics, overall and according to Institution
| Overall population (N = 102) | UTSW (N = 41) | NYPH (N = 61) | |
| Age (median, IQR) | 74 (67, 79) | 72 (60, 77) | 76 (69, 80) |
| Gender (n, %) | |||
| Female | 14 (14%) | 7 (17%) | 7 (11%) |
| Male | 88 (86%) | 34 (83%) | 54 (89%) |
| Clinical stage (n, %) | |||
| cTa | 16 (16%) | 11 (27%) | 5 (8%) |
| cTis | 6 (6%) | 4 (10%) | 2 (3%) |
| cT1 | 29 (28%) | 11 (27%) | 18 (30%) |
| cT2 | 48 (47%) | 12 (29%) | 36 (59%) |
| cT4 | 1 (1%) | 1 (2%) | 0 (0%) |
| Unknown | 2 (2%) | 2 (5%) | 0 (0%) |
| Grade at TUR (n, %) | |||
| Low | 3 (3%) | 3 (7%) | 0 (0%) |
| High | 99 (97%) | 38 (93%) | 61 (100%) |
| Concomitant CIS at TUR (n, %) | |||
| No | 86 (84%) | 33 (80%) | 53 (87%) |
| Yes | 16 (16%) | 8 (20%) | 8 (13%) |
| Number of altered markers at TUR (median, IQR) | 2 (2, 3) | 2 (2, 3) | 2 (1, 3) |
| Number of altered markers at TUR (n, %) | |||
| 0 | 6 (6%) | 4 (10%) | 2 (3%) |
| 1 | 19 (19%) | 5 (12%) | 14 (23%) |
| 2 | 49 (48%) | 21 (51%) | 28 (46%) |
| 3 | 25 (25%) | 10 (24%) | 15 (25%) |
| 4 | 2 (2%) | 1 (2%) | 1 (2%) |
| 5 | 1 (1%) | 0 (0%) | 1 (2%) |
| Neoadjuvant chemotherapy (n, %) | |||
| No | 93 (91%) | 35 (85%) | 58 (95%) |
| Yes | 9 (9%) | 6 (15%) | 3 (5%) |
| Months from TUR to RC (median, IQR) | 1.5 (1.0, 2.4) | 1.7 (1.3, 2.6) | 1.3 (0.8, 2.2) |
| Pathological stage (n, %) | |||
| pTa | 15 (15%) | 7 (17%) | 8 (13%) |
| pTis | 15 (15%) | 5 (12%) | 10 (16%) |
| pT1 | 14 (14%) | 5 (12%) | 9 (15%) |
| pT2 | 20 (20%) | 5 (12%) | 15 (25%) |
| pT3 | 33 (32%) | 19 (46%) | 14 (23%) |
| pT4 | 5 (5%) | 0 (0%) | 5 (8%) |
| Pathological grade (n, %) | |||
| High | 102 (100%) | 41 (100%) | 61 (100%) |
| Pathological concomitant CIS (n, %) | |||
| No | 51 (50%) | 22 (54%) | 29 (48%) |
| Yes | 51 (50%) | 19 (46%) | 32 (52%) |
| Number of altered markers at RC (median, IQR) | 2 (2, 3) | 2 (1, 3) | 2 (2, 3) |
| Number of altered markers at RC (n, %) | |||
| 0 | 4 (4%) | 3 (7%) | 1 (2%) |
| 1 | 21 (21%) | 8 (20%) | 13 (21%) |
| 2 | 42 (41%) | 17 (41%) | 25 (41%) |
| 3 | 28 (27%) | 13 (32%) | 15 (25%) |
| 4 | 6 (6%) | 0 (0%) | 6 (10%) |
| 5 | 1 (1%) | 0 (0%) | 1 (2%) |
| LVI (n, %) | |||
| No | 74 (73%) | 32 (78%) | 42 (69%) |
| Yes | 28 (27%) | 9 (22%) | 19 (31%) |
| LNI (n, %) | |||
| No | 89 (87%) | 35 (85%) | 54 (89%) |
| Yes | 13 (13%) | 6 (15%) | 7 (11%) |
| Total removed lymph nodes (median, IQR) | 19.5 (14.0, 29.0) | 20.0 (16.0, 30.0) | 19.0 (13.0, 28.0) |
| Positive lymph nodes (median, IQR) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Soft tissue/Ureteral margins (n, %) | |||
| Negative | 86 (84%) | 32 (78%) | 54 (89%) |
| Positive | 16 (16%) | 9 (22%) | 7 (11%) |
UTSW: University of Texas South-Western, NYPH: New York Presbyterian Hospital, TUR: trans-urethral resection, RC: radical cystectomy, CIS: carcinoma in-situ, LVI: lymphovascular invasion, LNI: lymph node invasion.
Table 2.1
General concordance rates
| Concordance | Negative | Positive | Alteration | Normalization | |
| rate | concordance | concordance | at RC | at RC | |
| % (n) | % (n) | % (n) | % (n) | % (n) | |
| Cyclin E1 | 92.2 (94) | 88.2 (90) | 3.9 (4) | 3.9 (4) | 3.9 (4) |
| CDKN1A | 77.5 (79) | 62.7 (64) | 14.7 (15) | 16.7 (17) | 5.9 (6) |
| p27 | 80.4 (82) | 53.9 (55) | 26.5 (27) | 12.7 (13) | 6.9 (7) |
| Tp53 | 77.5 (79) | 27.5 (28) | 50.0 (51) | 7.8 (8) | 14.7 (15) |
| MKI67 | 83.3 (85) | 15.7 (16) | 67.6 (69) | 9.8 (10) | 6.9 (7) |
| No. altered markers | 51.0 (52) | – | – | – | – |
| Score concordance (≤2 vs. >2 altered markers) | 75.5 (77) | – | – | – | – |
Table 2.2
General concordance rates according to clinical stage (non-muscle invasive vs. muscle invasive)
| NON-MUSCLE INVASIVE (cTa/Tis/T1) | MUSCLE INVASIVE (cT2/T3/T4) | ||||||||||
| (N = 51) | (N = 49) | ||||||||||
| Overall | Neg. | Pos. | Alt. | Norm. | Overall | Neg. | Pos. | Alt. | Norm. | p-value | |
| Conc. | Conc. | Conc. | at RC | at RC | Conc. | Conc. | Conc. | at RC | at RC | ||
| % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | ||
| Cyclin E1 | 88.2 (45) | 84.3 (43) | 3.9 (2) | 3.9 (2) | 7.8 (4) | 95.9 (47) | 91.8 (45) | 4.1 (2) | 4.1 (2) | – | 0.3 |
| CDKN1A | 76.5 (39) | 62.7 (32) | 13.7 (7) | 15.7 (8) | 7.8 (4) | 79.6 (39) | 63.3 (31) | 16.3 (8) | 16.3 (8) | 4.1 (2) | 0.8 |
| p27 | 76.5 (39) | 51.0 (26) | 25.5 (13) | 13.7 (7) | 9.8 (5) | 87.8 (43) | 59.2 (29) | 28.6 (14) | 8.2 (4) | 4.1 (2) | 0.2 |
| Tp53 | 76.5 (39) | 39.2 (20) | 37.3 (19) | 5.9 (3) | 17.6 (9) | 77.6 (38) | 14.3 (7) | 63.3 (31) | 10.2 (5) | 12.2 (6) | 1 |
| MKI67 | 80.4 (41) | 13.7 (7) | 66.7 (34) | 7.8 (4) | 11.8 (6) | 85.7 (42) | 18.4 (9) | 67.3 (33) | 12.2 (6) | 2.0 (1) | 0.6 |
| No. altered markers | 56.9 (29) | – | – | – | – | 46.9 (23) | – | – | – | – | 0.4 |
| Score concordance | 78.4 (40) | – | – | – | – | 75.5 (37) | – | – | – | – | 0.8 |




