Benefit of Adjuvant Chemotherapy and Pelvic Lymph Node Dissection in pT3 and Node Positive Bladder Cancer Patients Treated with Radical Cystectomy
Abstract
Background: Benefits of adjuvant chemotherapy (AC) and extent of pelvic lymph node dissection (PLND) in radical cystectomy (RC) are debated. Results from randomized trials are still expected.
Objective: To analyze the effects of AC and PLND in two academic centers with opposite policies regarding their use.
Methods: 581 bladder cancer patients who underwent RC without neoadjuvant chemotherapy, from Toronto (University Health Network), Canada, and Turku University Hospital, Finland were included. Disease specific survival (DSS) and failure patterns were assessed.
Results: Centers differed in PLND rate (93% and 36% in Toronto and Turku respectively, p < 0.001), PLND extent (≥10 removed nodes, 58% vs. 8%, p < 0.001) and AC rate (21% vs. 2%, p < 0.001). Survival between centers among pT≤1 or pT4 patients was similar. pT3 patients in Toronto had an improved 10 year DSS (43% vs. 22%, p = 0.025). Distant failures were less common after AC (HR 0.56, 95% CI 0.33–0.98, p < 0.042). In node positive (N+) patients, mortality was significantly higher in Turku (HR 2.19, 95% CI 1.44–3.34, p < 0.001) and lower in patients receiving AC (HR 0.60, 95% CI 0.37–0.99, p = 0.044). 41% DSS at 10 years was observed in N+ Toronto patients. Limitations included the non-randomized retrospective design and absence of propensity score analysis.
Conclusion: Combining AC and PLND to RC is associated with improved survival in pT3 and N+ patients. PLND did not affect survival independently but helps in selecting patients for AC. Our data adds to the growing body of evidence supporting the usefulness of AC in addition to PLND in high risk patients operated by cystectomy.
INTRODUCTION
Radical cystectomy (RC) offers excellent local control for muscle-invasive bladder cancer (BC), but only half of patients with extravesical extension and 15% to 35% patient with nodal metastasis (N+) are long term survivors [1–4]. This speaks to the important risk of micro-metastases in these high risk patients, suggesting a multimodality approach might be of benefit.
There is obviously a need to improve outcomes in pT3 and N+ patients operated by RC [5].
Neoadjuvant cisplatin-based chemotherapy, which results in ∼5% absolute survival benefit, has been utilized infrequently [6, 7]. In recent years, there have been reports of increased use of neoadjuvant chemotherapy especially in academic centers and by fellowship-trained uro-oncologists [8]. Postoperative adjuvant chemotherapy (AC) is offered more often than neoadjuvant, although the evidence supporting its benefit is less robust [9]. A meta-analysis reported that ∼500 patients in 6 trials were randomized to RC with or without adjuvant cisplatin-based chemotherapy [9]. A 25% relative risk reduction favoring chemotherapy was noted, but the power of the meta-analysis was limited.
Because outcomes in pT3-pT4 or N+ patients are suboptimal, the EORTC 30994 aimed to compare immediate versus deferred cisplatin-based combination chemotherapy after RC in these patients [5]. Results have been recently published. The trial was unfortunately closed after recruitment of 284 of the planned 660 patients. Although it did not show a significant improvement in overall survival, with immediate versus deferred chemotherapy after RC and bilateral lymphadenectomy, immediate treatment significantly prolonged progression-free survival compared with deferred treatment (HR 0.54, 95% CI 0.4–0.73, p < 0.0001), with 5-year progression-free survival of 47.6% (95% CI 38.8–55.9) in the immediate treatment group and 31.8% (24.2–39.6) in the deferred treatment group [5]. In a recent large retrospective study, Galsky et al. also demonstrated that AC was associated with improved outcome when compared to observation after RC [10].
There is obvious room for improvement. These data nevertheless suggest that AC might be of benefit in this high risk population.
The role of pelvic node dissection (PLND) remains debated and no universally accepted definition for extended PLND (ePLND) exists. When ePLND is performed, more nodal metastases are detected, but the magnitude of its effect on survival is unclear [11, 12].
The results of the SWOG–S1011 trial and the German multicenter study LEA22, evaluating the benefit of a standard versus an extended pelvic lymphadenectomy, performed at time of RC, are eagerly expected [13].
In the present study, we aimed to analyze the effects of AC and PLND on survival and type of failure patterns for patients undergoing RC, with a special emphasis on high risk pT3-4 and N+ patients, using data from two academic centers. As these 2 centers have different approaches with regards to AC and PLND, the setting offered an opportunity to explore the effect of these treatment modalities.
PATIENTS AND METHODS
An Institutional Research Ethics Boards approved a RC database including consecutive RC patients from University Health Network, Toronto, Canada (study period 1991–2008), and Turku University Hospital, Turku, Finland (1986–2007), were analyzed to ensure a long follow-up period. All patients undergoing RC during the study period were included into the study. Patients with non-urothelial BC (n = 53) or neoadjuvant chemotherapy (n = 31) were excluded.
In both centers, staging included CT of the abdomen and pelvis and chest x-ray. There was a substantial difference in the policies for PLND in the two study centers. In Turku University Hospital, formal PLND was not performed before 1995 but macroscopically suspicious, i.e. grossly abnormal nodes were removed. Limited PLND (obturator nodes) was performed beginning in 1995. In Toronto, most patients underwent ePLND (cranial boundary of dissection either the aortic bifurcation or mid common iliac vessels). PLND was not performed only in unusual cases (24/330) when it was not feasible or safe (e.g. due to prior lymphadenectomy or radiation for causes other than BC) and ePLND was performed in 204 cases. The number of removed nodes defined the extent of PLND. Tumor grade was reported according to the WHO 1973 or WHO/ISUP 2004 classification and stage according to the 2002 TNM-classification [14–16]. All pathology reports were reviewed by a single person (first author) and stages were uniformly defined (e.g. all pT4a patients had prostatic stromal tumor invasion).
In Toronto, the option of AC was discussed with all medically fit patients with pT3-4 disease or positive nodes, and with high risk pT2 cases (pT2b with lymphovascular invasion (LVI)). AC was not offered in Turku during the study period. In contrast, surveillance with salvage chemotherapy at the time of recurrence was the practice of choice in Turku.
After surgery, patients were followed every 3 months for the first year and then semi-annually. In addition to history, physical examination, and blood chemistry, imaging by CT scans was undertaken as indicated by symptoms. Details of recurrence were recorded according to available imaging and pathological evidence. Any recurrent disease in the pelvis, caudal to the aortic bifurcation, was defined as a local recurrence. In the event of metastatic progression, patient in University of Turku were recommended cisplatin-based salvage chemotherapy.
The primary endpoint was Disease Specific Survival (DSS). Survival was calculated from date of surgery to death or last follow-up. Survival data were obtained from patient charts and hospital registries and then confirmed from cancer registries and governmental death registries. Any death with recurrent or metastatic BC was defined as cancer specific mortality. Secondary endpoints were overall survival (OS), local and distant recurrences. Time to recurrence was defined as time from surgery to detection of recurrence, with either imaging or biopsy. The Pearson chi-square test was used to analyze categorical data. Numerical data were analyzed by the Student’s t-test. The Kaplan-Meier method and log-rank test were used to analyze survival. Cox proportional hazards models were used to estimate the effect of clinicopathological factors, and center (model #1), or PLND and AC (model #2) on survival.
RESULTS
Study cohorts
The study included 581 patients. The patient cohorts according to pT-categories are presented in Table 1. Patients in Toronto were older in all categories. There was no significant difference in tumor grade. 93% of patients in Toronto underwent PLND with a median of 12 nodes removed (mean = 14, range = 1–62). There were no differences in the Toronto cohort in node dissection rate or extent among different tumor stages. In Turku, only 36% of RC had PLND (mean and median 7, range 1–18). PLND was more commonly done in higher stage disease (p = 0.015) but the number of removed nodes was unaffected by stage. In Toronto, positive nodes were detected in 7–43% of patients depending on the stage and this was significantly higher than in Turku (N+ rate 0–28%). Positive surgical margins status was uncommon in both institutions (Toronto 4.3%, Turku 3.5). Average ASA-score was 2.4 in Toronto cohort, and 2.3 in Turku cohort, respectively.
In Toronto, the rate of AC was 21% and it was more commonly given in higher pT-categories ranging from 3 to 39%. Furthermore, AC was administered to 44% and 15% for node-positive and negative patients, respectively. Only five patients (2%) of the Turku cohort received AC. In Toronto 92% of chemotherapy patients and all five in Turku received cisplatin based combination chemotherapy (MVAC, CMV or cisplatin-gemcitabine).
Survival
A total of 193 patients (33%) died of BC during a median FU of 5.7 years (8.5 years in Turku, and 3.9 years in Toronto, respectively). A Kaplan-Meier analysis for DSS and OS for different pT-categories in the two study centers is presented in Fig. 1.
Figure 2 presents Kaplan-Meier curves for pT2 and pT3 patients. Survival was worse in the Turku cohort but it was statistically significantly worse only among pT3 patients (p = 0.001). The effect of nodal stage on DSS is presented in Fig. 3. The survival was better in Toronto both for node negative and positive patients (both p < 0.05). For those patients who did not have any nodes removed (Nx), the survival was identical to node negative patients in the Turku cohort (n = 47). In Toronto, Nx patients had survival rates similar to node positive patients (5y DSS 45%, and 41% for Nx, and N+, respectively).
Cox regressions analyses were used to explore factors associated with DSS among all pT2 and pT3 patients (Table 2). In univariate analysis for DSS, high grade, advanced pT-stage, positive margin status, and positive nodal status were significantly associated with worse outcome. In univariate analysis pT3-category, positive nodal status, and Turku as study center were significantly associated with poorer survival. Two multivariable models were used due to the dependent association of center and AC, as well as center and PLND, due to the different treatment policies at the two centers. In model #1, all clinicopathological variables, which were significant in univariate analysis, remained significant; also study center (Turku vs. Toronto, HR 2.19, 95% CI 1.44–3.34, p < 0.001) was significantly associated with worse DSS. In model #2, patients receiving AC had improved survival (HR for BC mortality 0.60, 95% CI 0.37–0.99, p = 0.044), but the extent of nodal dissection did not impact survival. Different cut-offs were studied for number of nodes removed both in univariate and multivariate analysis and 10 nodes had the best, albeit not significant, discrimination.
Failure patterns
Among pT2 and pT3 patients BC recurrence was detected in 123 (44%) patients (58% in Turku, and 36 in Toronto, respectively). Of these, 31 (25%) had an isolated local recurrence, 72 (59%) had distant metastasis, and 20 (16%) had concurrent local and distant metastases. The risk of local recurrence was similar in both cohorts (p = 0.38), but distant metastasis were seen significantly more often in Turku cohort (p = 0.004) (Fig. 4). Analyses for risk factors for distant failures are presented in Table 3. Only female gender and nodal metastases were significantly associated with local failure and study center, AC or PLND did not affect the risk (data not shown).
Distant failure risk was affected by pT-category, nodal metastases, study center (Turku vs. Toronto, HR 2.24, 95% CI 1.41–3.57, p = 0.001), and use of AC (HR 0.56, 95% CI 0.33–0.98, p = 0.042) but not the extent of PLND (Table 3).
DISCUSSION
Our present study suggests that the outcome of muscle-invasive BC patients at high risk of harbouring micrometastatic disease in regional and distant sites (pT3 and N+ disease), is improved when adjuvant chemotherapy is administered. Although PLND does not independently provide a survival advantage, it may help to select patients for AC, likely indirectly contributing to the advantage provided by AC.
Our main finding was that patients with pT3 and N+ disease had better outcome in the Toronto cohort, and AC chemotherapy was the main reason for this improved survival. Our results support the results of adjuvant chemotherapy meta-analysis [6, 7], and are in line with those observed in the EORTC 30994 trial, closed prematurely because of poor accrual [5]. In our study, AC resulted in a significant 40% relative risk reduction for BC related death. The AC meta-analysis reported a 25% relative risk and 9% absolute risk reduction [6, 7]. All AC trials have been greatly affected by small sample size. Although the number of patients receiving chemotherapy in this study was only 78, it is surprisingly more than in any of the individual randomized trials included in the meta-analysis, except the EORTC 30994 trial [5–7]. Our results are also in agreement with the results reported recently by Svatek and coworkers [16]. In this multicenter study with similar AC rate, chemotherapy improved survival especially for patients in highest risk quintile (≥pT3 and node positive disease) [17]. There was a trend towards improved survival in pT2 patients from Toronto, but it was far from statistical significance (p = 0.26).
Interestingly, a post hoc analysis of the p53-MVAC trial revealed an association between LVI and shorter time to recurrence in patients with pT1–T2 N0 disease, as this was the focus of that trial [18]. The analysis did not show however, a statistically significant benefit of adjuvant chemotherapy in patients with LVI and pT1-2 N0 on survival, although a possible benefit could not be excluded [17]. These results support that the most significant benefit of ePLND and adjuvant chemotherapy is likely to be observed in groups such as pT3 or N+ disease rather than pT2, even with LVI.
The delivery of AC may be challenging for patients with BC. Even with an active policy of delivering AC to fit patients, only 44% of node positive and 31% of pT3 and pT4 patients received chemotherapy in the Toronto series, rates comparable to other large series [19]. Reported reasons for the low rate of AC includes an elderly patient population, renal insufficiency, postoperative complications, and patient preferences [18, 19]. Currently neoadjuvant chemotherapy, supported with level I evidence, is increasingly embraced by guidelines and experts. Obviously our study cannot comment on the differences of neoadjuvant vs. adjuvant approaches but stresses the importance of peri-operative chemotherapy especially in high risk groups.
Regarding the effect of PLND on survival after RC, awaiting the results of the SWOG and LEA22 randomized trials, retrospective evidence only is available to suggest the importance of extended PLND in detection of metastases and its impact on outcome. Herr and coworkers reported a study analyzing the material from the landmark neoadjuvant trial by Grossmann and coworkers [10]; ePLND (defined as >10 removed nodes) was associated with reduced local recurrence rates and improved survival [10]. Similar results were also reported by Leissner et al. [21]. A group from Bern compared the outcome of their series with ePLND (cranial dissection up to mid-common iliac) to results from the Cleveland clinic (limited PLND) and to USC (“super-extended” dissection to origin of inferior mesenteric artery) [21]. The results suggest that the mid-common iliac level is appropriate for PLND as the Bern results were superior to Cleveland but equal to USC results.
In this study combining Cleveland and USC, the 5-year recurrence-free survival for pT2 N0-2 cases was 63% for limited and 71% for ePLND, very much in line with our 68% DSS observed in the Toronto cohort at 10 years. In pT3 N0-2 disease, the respective figures were 19% and 49%, much in line with the 22% and 43% 10 DSS observed in the present study in Toronto and Turku, respectively [22].
Unfortunately the rate of perioperative chemotherapy was not reported in the Bern/Cleveland study.
In light of our results, we feel that extended PLND is an important part of RC. Although we could not detect a survival advantage for patients with an extended PLND, PLND is an important staging procedure and helps to select patients for AC.
The analysis of failure pattern revealed that local recurrence was not different in the two study centers and was not affected either by AC or PLND. In contrast, distant failure rates was significantly higher in Turku and among patients receiving AC, but not PLND, highlighting the importance of chemotherapy in eradicating distant micrometastatic deposits.
The major limitations of the current study are related to possible biases associated with its retrospective and non-randomized design. We also did not perform a propensity score analysis. We tried to minimize biases in data collection. Only consecutive cases with same exclusion criteria were analyzed. Database design and data collection was done by a single uro-oncologist (first author). A central pathology review was not performed however, as we focused mainly on pT-stage, it is unlikely that such a review would have a substantial effect on our results. There may have been changes in in operative techniques, perioperative care and pathological sampling, for example, during the study period. In controlled prospective trials, data are often less than optimal. In the neoadjuvant trial, Herr et al. reported that 9% of patients had no PLND done and only 50% had more than 10 nodes removed [12]. In our study, although not randomized, the selection for AC and node dissection was made according to institutional practice policies and therefore many selection biases were avoided.
CONCLUSION
Our study suggests that patients with pT3 and node positive BC benefit from a multimodal approach including RC, ePLND and AC for high risk patients. Efforts should continue to be made to improve outcome in this high risk population operated by RC.
ACKNOWLEDGEMENTS (INCLUDING SOURCES OF SUPPORT)
Mrs. Sally Hannah helped with establishing the database. This work was supported by the Uro-Oncology Fellowship Program at the University of Toronto.
REFERENCES
[1] | Stein J , Lieskovsky G , Cote R , Groshen S , Feng AC , Boyd S , Skinner E , Bochner B , Thangathurai D , Mikhail M , Raghavan D , Skinner DG . Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol (2001) ;19: :666–75. |
[2] | Madersbacher S , Hochreiter W , Burkhard F , Thalmann GN , Danuser H , Markwalder R , Studer UE . Radical cystectomy for bladder cancer today–a homogeneous series without neoadjuvant therapy. J Clin Oncol (2003) ;21: :690–6. |
[3] | Hautmann R , Gschwend J , de Petriconi R , Kron M , Volkmer BG . Cystectomy for transitional cell carcinoma of the bladder: Results of a surgery only series in the neobladder era. J Urol (2006) ;176: :486–92. |
[4] | Shariat S , Karakiewicz P , Palapattu G , Lotan Y , Rogers CG , Amiel GE , Vazina A , Gupta A , Bastian PJ , Sagalowsky AI , Schoenberg MP , Lerner SP . Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: A contemporary series from the Bladder Cancer Research Consortium. J Urol (2006) ;176: :2414–22. |
[5] | Sternberg CN , Skoneczna I , Kerst JM , Albers P , Fossa SD , Agerbaek M , Dumez H , de Santis M , Théodore C , Leahy MG , Chester JD , Verbaeys A , Daugaard G , Wood L , Witjes JA , de Wit R , Geoffrois L , Sengelov L , Thalmann G , Charpentier D , Rolland F , Mignot L , Sundar S , Symonds P , Graham J , Joly F , Marreaud S , Collette L , Sylvester R . Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): An intergroup, open-label, randomised phase 3 trial. Lancet Oncol (2015) ;16: (1), 76–86. |
[6] | Collaboration ABCO. Neoadjuvant chemotherapy for invasive bladder cancer. Cochrane Database Syst Rev (2005) ;CD005246. |
[7] | Collaboration ABCAM-a. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol (2005) ;48: :202–5. |
[8] | Bochner BH , Feifer A , Sperling D , Mashni JW , Bajorin DF , Bakul Shah J , Kamat AM , Steinberg GD , Stadler WM , Grubb RL , Kibel AS , Schoenberg M , Black PC , Zlotta A , Kassouf W , Lerner SP , Lotan Y , Collaborators of the BCAN Quality of Care Initiative Consortium Multi-institutional quality care initiative (QCI) to improve the care of patients with invasive bladder cancer. J Clin Oncol (2014) ;32: (suppl 4; abstr 298). |
[9] | Collaboration ABCAM-a. Adjuvant chemotherapy for invasive bladder cancer (individual patient data). Cochrane Database Syst Rev (2006) ;CD006018. |
[10] | Galsky MD , Stensland K , Moshier EL , Sfakianos J , McBride RB , Tsao C-K , Casey MF , Hall SJ , Boffetta P , Oh WE , Wisnivesky JP . Comparative effectiveness of adjuvant chemotherapy (AC) versus observation in patients with ≥pT3 and/or pN+ bladder cancer. J Clin Oncol (2015) ;33: :(suppl 7; abstr 292). |
[11] | Leissner J , Ghoneim MA , Abol-Enein H , Thüoff JW , Franzaring L , Fisch M , Schulza H , Managadze G , Allhoff EP , el-Baz MA , Kastendieck H , Buhtz P , Kropf S , Hohenfellner R , Wolf HK . Extended radical lymphadenectomy in patients with urothelial bladder cancer: Results of a prospective multicenter study. J Urol (2004) ;171: :139–44. |
[12] | Herr HW , Faulkner JR , Grossman HB , Natale RB , deVere White R , Sarosdy MF , Crawford ED . Surgical factors influence bladder cancer outcomes: A cooperative group report. J Clin Oncol (2004) ;22: :2781–9. |
[13] | ClinicalTrials. gov: Identifiers: NCT01224665, NCT01215071, http://clinicaltrials.gov/ct2/home. |
[14] | Mostofi F , Sobin L , Torloni H Histological typing of urinary bladder tumors, in Organization WH (ed): International classification of tumors (ed 1st Ed): Geneva, Switzerland. (1973) ;34. |
[15] | Eble J , Sauter G , Epstein J , Sesterhenn IA . The world health organization classification of tumors of the urinary system and male genital system, Lyon, France: IARC Press, (2004) ;354. |
[16] | Sobin D . WC: TNM classification of Malignant Tumors, in Sobin D, CH W (eds): TNM classification of malignant tumors, New York, NY: Wiley-Liss. (2002) ;199–202. |
[17] | Svatek RS , Shariat SF , Lasky RE , Skinner EC , Novara G , Lerner SP , Fradet Y , Bastian PJ , Kassouf W , Karakiewicz PI , Fritsche HM , Müller SC , Izawa JI , Ficarra V , Sagalowsky AI , Schoenberg MP , Siefker-Radtke AO , Millikan RE , Dinney CP . The effectiveness of off-protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder. Clin Cancer Res (2010) ;16: :44461–7. |
[18] | von Rundstedt FC , Mata DA , Groshen S , Stein JP , Skinner DG , Stadler WM , Cote RJ , Kryvenko ON , Godoy G , Lerner SP . Significance of lymphovascular invasion in organ-confined, node-negative urothelial cancer of the bladder: Data from the prospective p53-MVAC trial. BJU Int (2015) ;116: (1), 44–9. |
[19] | Raj GV , Karavadia S , Schlomer B , Arriaga Y , Lotan Y , Sagalowsky A , Frenkel E . Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer (2011) ;117: :276–82. |
[20] | Donat SM , Shabsigh A , Savage C , Cronin AM , Bochner BH , Dalbagni G , Herr HW , Milowsky MI . Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: A high-volume tertiary cancer center experience. Eur Urol (2009) ;55: :177–85. |
[21] | Leissner J , Hohenfellner R , Thüroff JW , Wolf HK . Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int (2000) ;85: :817–23. |
[22] | Dhar NB , Klein EA , Reuther AM , Thalmann GN , Madersbacher S , Studer UE . Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol (2008) ;179: :873–8. |
Figures and Tables
Fig.1
Kaplan-Meier analysis for disease specific survival for the University of Toronto (A) and the University of Turku (B).
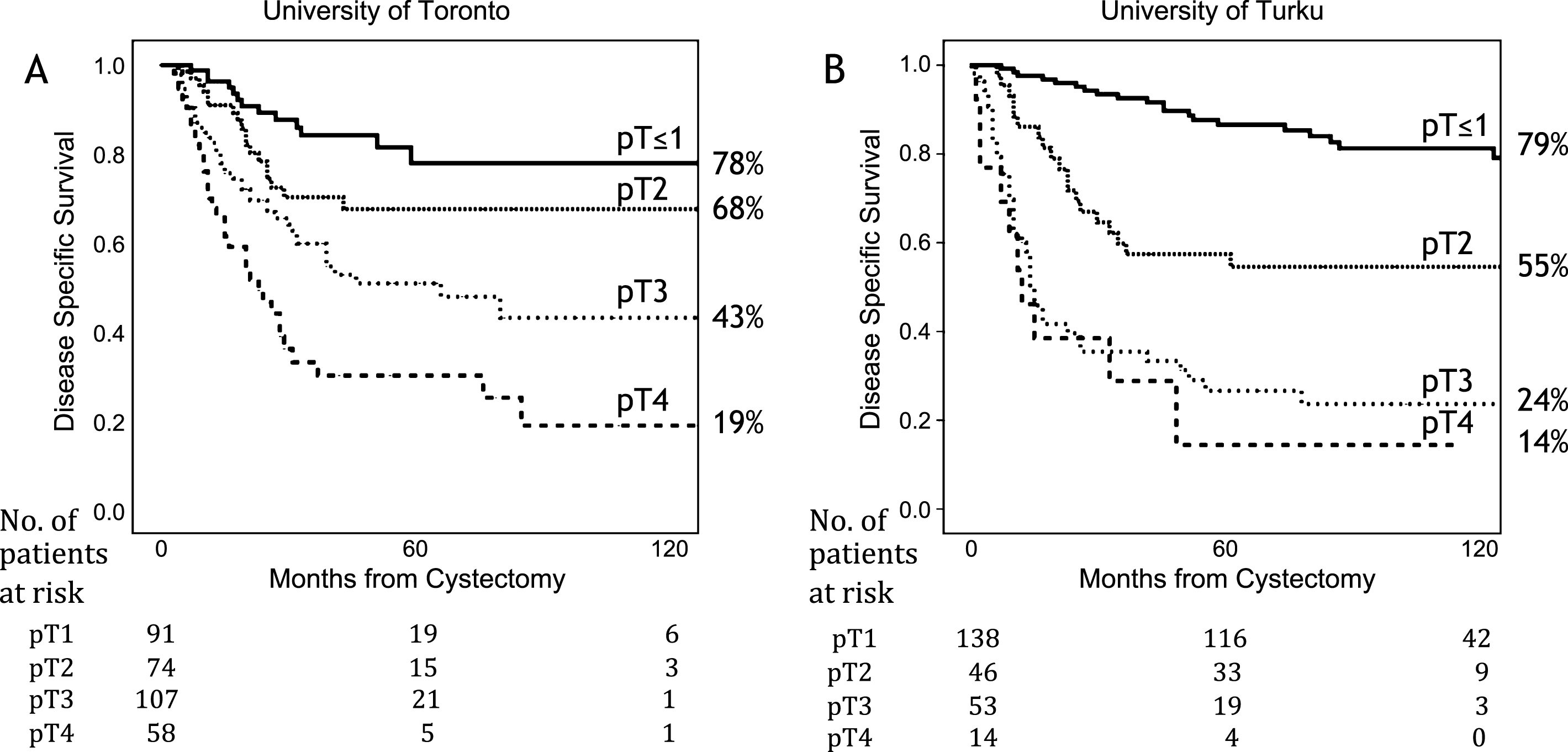
Fig.2
Kaplan-Meier analysis comparing disease specific survival between the University of Toronto and the University of Turku cohorts among pT2 patients (A) and pT3 patients (B).
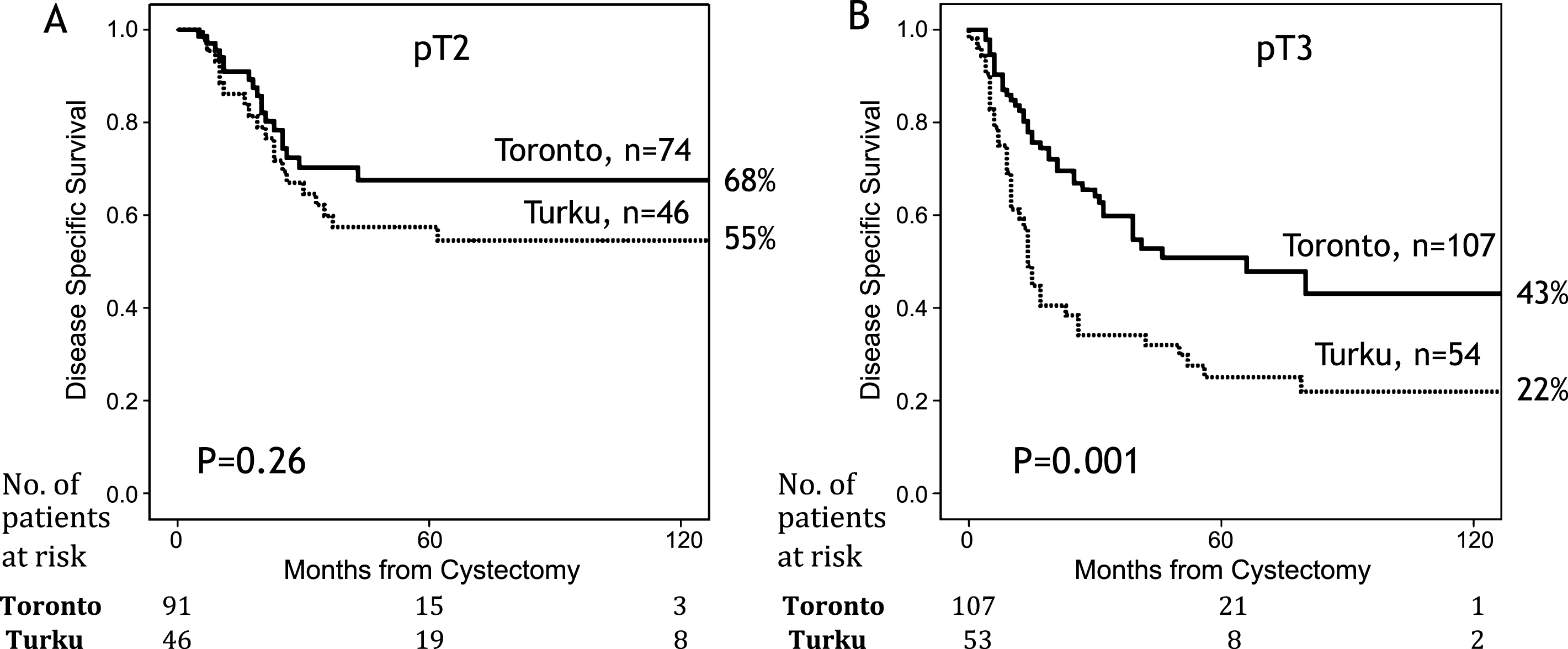
Fig.3
Kaplan-Meier analysis for disease specific survival for University of Toronto (A) and University of Turku (B) for different nodal status.
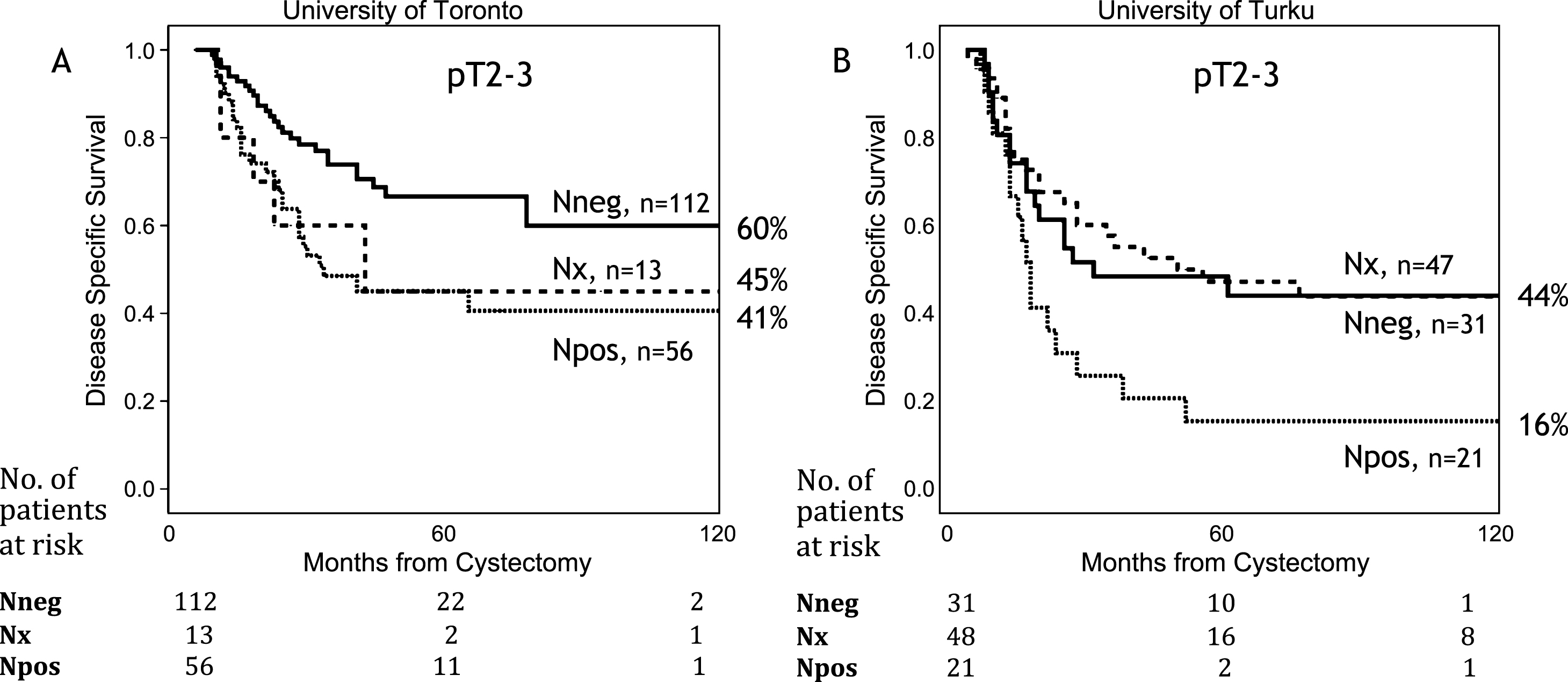
Fig.4
Kaplan-Meier analysis for local recurrence free survival (A) and distance free recurrence survival (B) for University of Toronto and University of Turku.
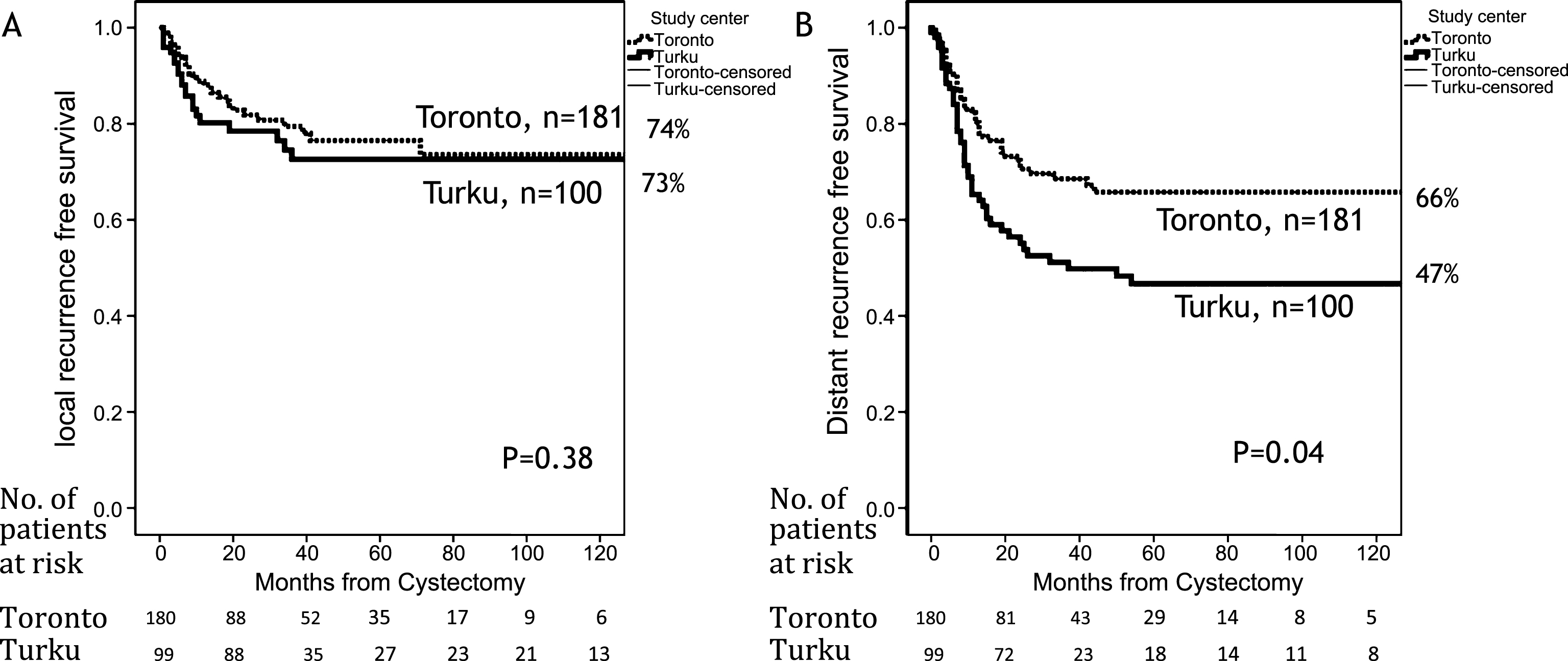
Table 1
Basic clinicopathological variables
| Variable | pT≤1 | pT2 | pT3 | pT4 | ||||||||||||||||
| Turku n = 138 | Toronto n = 91 | Turku n = 46 | Toronto n = 74 | Turku n = 53 | Toronto n = 107 | Turku n = 14 | Toronto n = 58 | |||||||||||||
| N | % | N | % | p-value | n | % | n | % | p-value | n | % | n | % | p-value | n | % | N | % | p-value | |
| Gender | ||||||||||||||||||||
| male | 117 | 85 | 71 | 78 | 0.19 | 37 | 80 | 58 | 78 | 0.79 | 36 | 68 | 82 | 77 | 0.24 | 11 | 79 | 45 | 78 | 0.94 |
| Age | ||||||||||||||||||||
| median±SD | 65±8 | 68±12 | 0.016 | 62±11 | 67±12 | 0.015 | 68±11 | 65±8 | 0.022 | 64±10 | 71±9 | 0.005 | ||||||||
| Grade | ||||||||||||||||||||
| G1-2/Low grade | 27 | 20 | 16 | 18 | 4 | 9 | 8 | 11 | 2 | 4 | 4 | 4 | 2 | 14 | 6 | 10 | ||||
| G3/High grade | 111 | 80 | 75 | 82 | 0.71 | 42 | 91 | 66 | 89 | 0.71 | 51 | 96 | 103 | 96 | 0.99 | 12 | 86 | 12 | 86 | 0.67 |
| N-status | ||||||||||||||||||||
| N0 | 46 | 33 | 80 | 88 | 15 | 33 | 53 | 72 | 16 | 30 | 59 | 55 | 5 | 36 | 27 | 47 | ||||
| N1 | 0 | 0 | 6 | 7 | 6 | 13 | 17 | 23 | 15 | 28 | 39 | 36 | 1 | 7 | 15 | 43 | ||||
| Nx | 92 | 67 | 5 | 6 | <0.001 | 25 | 54 | 4 | 5 | <0.001 | 22 | 42 | 9 | 8 | <0.001 | 8 | 57 | 6 | 10 | <0.001 |
| No of nodes Median±SD1 | 8±4 | 15±9 | <0.001 | 8±4 | 13±7 | 0.003 | 9±5 | 14±10 | 0.006 | 12±5 | 12±9 | 1.0 | ||||||||
| 0 | 92 | 67 | 5 | 6 | 25 | 54 | 4 | 5 | 22 | 42 | 9 | 8 | 8 | 57 | 6 | 10 | ||||
| 1–9 | 30 | 22 | 24 | 26 | 14 | 34 | 24 | 32 | 17 | 32 | 32 | 30 | 2 | 14 | 22 | 38 | ||||
| ≥10 | 16 | 12 | 62 | 68 | 7 | 15 | 46 | 62 | 14 | 26 | 66 | 62 | 4 | 29 | 30 | 52 | ||||
| Adj. chemotherapy | ||||||||||||||||||||
| No | 138 | 100 | 88 | 97 | 45 | 98 | 59 | 80 | 49 | 93 | 65 | 61 | 13 | 93 | 46 | 79 | ||||
| Yes | 0 | 0 | 3 | 3 | 0.032 | 0 | 0 | 15 | 20 | 0.005 | 4 | 8 | 42 | 39 | <0.001 | 1 | 7 | 12 | 21 | <0.001 |
1median number of removed lymph nodes, patients without node dissection excluded.
Table 2
Univariate and multivariate Cox proportional hazards regression analysis for disease specific survival in pT2 and pT3 cohorts
| Univariate | Multivariate, model #1 | Multivariate, model #2 | ||||||||
| Variable | n | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Gender | ||||||||||
| Male | 214 | ref | ref | ref | ||||||
| Female | 67 | 1.31 | 0.87–1.96 | 0.20 | 1.51 | 0.98–2.32 | 0.06 | 1.42 | 0.93–2.18 | 0.11 |
| Age | ||||||||||
| <60 | 77 | ref | ref | ref | ||||||
| 60–69 | 75 | 1.14 | 0.70–1.83 | 0.61 | 1.20 | 0.73–1.97 | 0.47 | 1.10 | 0.67–1.81 | 0.70 |
| ≥70 | 129 | 1.15 | 0.74–1.79 | 0.52 | 1.32 | 0.84–2.08 | 0.22 | 1.08 | 0.68–1.72 | 0.74 |
| Grade | ||||||||||
| G1-2/low grade | 18 | ref | ref | ref | ||||||
| G3/high grade | 253 | 2.48 | 0.92–6.74 | 0.074 | 2.02 | 0.73–5.65 | 0.18 | 2.33 | 0.84–6.45 | 0.10 |
| p-Tcategory | ||||||||||
| pT2 | 120 | ref | ref | ref | ||||||
| pT3 | 161 | 2.00 | 1.36–2.96 | <0.001 | 1.85 | 1.24–2.77 | 0.003 | 1.95 | 1.29–2.94 | 0.001 |
| Nodal status N0 | 143 | ref | ref | ref | ||||||
| Npos | 77 | 2.12 | 1.40–3.22 | <0.001 | 1.98 | 1.28–3.06 | 0.002 | 2.16 | 1.37–3.39 | 0.001 |
| Nx | 61 | 1.52 | 0.94–2.44 | 0.085 | 0.95 | 0.55–1.63 | 0.85 | 1.56 | 0.92–2.66 | 0.10 |
| Study Center Toronto | 181 | ref | ref | |||||||
| Turku | 100 | 1.69 | 1.18–2.44 | 0.005 | 2.19 | 1.44–3.34 | <0.001 | |||
| Adj.chemotherapy No | 219 | ref | ref | |||||||
| Yes | 62 | 0.88 | 0.57–1.36 | 0.57 | 0.60 | 0.37–0.99 | 0.044 | |||
| Removed nodes | ||||||||||
| 0 | 61 | ref | ref | |||||||
| 1–9 | 86 | 1.09 | 0.67–1.76 | 0.73 | 1.23 | 0.74–2.06 | 0.43 | |||
| ≤ | 134 | 0.78 | 0.49–1.24 | 0.29 | 0.78 | 0.47–1.29 | 0.33 | |||
In multivariate model #1 in addtion to clinicopathological variables (gender, age, grade, pT-category, and nodal status) study center is added to the model; In model #2 Adjuvant chemotherapy and node dissesction groups, but not center are included.
Table 3
Univariate and multivariate Cox proportional hazards regression analysis for distant recurrence in pT2 and pT3 cohorts
| Univariate | Multivariate, model #1 | Multivariate, model #2 | ||||||||
| Variable | n | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Gender | ||||||||||
| Male | 214 | ref | ref | ref | ||||||
| Female | 67 | 1.01 | 0.62–1.64 | 0.98 | 1.20 | 0.72–2.00 | 0.48 | 1.17 | 0.70–1.93 | 0.56 |
| Age | ||||||||||
| <60 | 77 | ref | ref | ref | ||||||
| 60–69 | 75 | 0.84 | 0.49–1.43 | 0.51 | 0.89 | 0.51–1.55 | 0.89 | 0.82 | 0.47–1.43 | 0.48 |
| ≥70 | 129 | 0.88 | 0.55–1.43 | 0.61 | 1.02 | 0.62–1.67 | 0.95 | 0.80 | 0.48–1.33 | 0.39 |
| Grade | ||||||||||
| G1-2/low grade | 18 | ref | ref | Ref | ||||||
| G3/high grade | 253 | 2.60 | 0.82–8.21 | 0.10 | 2.19 | 0.67–7.13 | 0.19 | 2.52 | 0.78–8.18 | 0.12 |
| p-Tcategory | ||||||||||
| pT2 | 120 | ref | ref | ref | ||||||
| pT3 | 161 | 1.97 | 1.27–3.04 | 0.002 | 1.70 | 1.08–2.67 | 0.23 | 1.80 | 1.14–2.85 | 0.012 |
| Nodal status | ||||||||||
| N0 | 143 | ref | ref | ref | ||||||
| Npos | 77 | 2.87 | 1.80–4.56 | <0.001 | 2.53 | 1.56–4.09 | <0.001 | 2.91 | 1.76–4.83 | <0.001 |
| Nx | 61 | 1.55 | 0.89–2.70 | 0.12 | 1.02 | 0.54–1.91 | 0.96 | 1.62 | 0.88–2.99 | 0.12 |
| Study Center | ||||||||||
| Toronto | 181 | ref | ref | |||||||
| Turku | 100 | 1.79 | 1.19–2.70 | 0.005 | 2.24 | 1.41–3.57 | 0.001 | |||
| Adj.chemotherapy | ||||||||||
| No | 219 | ref | ref | |||||||
| Yes | 62 | 1.01 | 0.63–1.62 | 0.98 | 0.56 | 0.33–0.98 | 0.042 | |||
| Removed nodes | ||||||||||
| 0 | 61 | ref | ref | |||||||
| 1–9 | 86 | 1.21 | 0.69–2.12 | 0.51 | 1.18 | 0.65–2.12 | 0.59 | |||
| ≤ | 134 | 0.88 | 0.52–1.50 | 0.64 | 0.81 | 0.46–1.44 | 0.47 | |||
In multivariate model #1 in addtion to clinicopathological variables (gender, age, grade, pT-category, and nodal status) study center is added to the model; In model #2 Adjuvant chemotherapy and node dissesction groups, but not center are included.




