Id Proteins Contribute to Tumor Development and Metastatic Colonization in a Model of Bladder Carcinogenesis
Abstract
Background: Bladder cancer is one of the most common malignant genitourinary diseases worldwide. Despite advances in surgical technique, medical oncology and radiation therapy, cure of invasive tumors remains elusive for patients with late stage disease. Therefore, new therapeutic strategies are needed to improve the response rates with regard to recurrence, invasion and metastasis.
Objective: Inhibitor of DNA binding (Id) proteins have been proposed as therapeutic targets due to the key regulatory role they exert in multiple steps of cancer. We aimed to explore the role of Id proteins in bladder cancer development and the pattern of expression of Id proteins in bladder carcinomas.
Methods: We used a well-established chemically induced model of bladder carcinogenesis. Wild type and Id-deficient mice were given N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) in the drinking water and urinary bladder lesions were analyzed histopathologically and stained for Id1. We assessed the effects of Id1 inactivation in cultured bladder cancer cells and in a model of metastatic lung colonization. We also performed Id1 staining of human urothelial carcinoma samples and matched lymph node metastases.
Results: Id1 protein was overexpressed in the BBN-induced model of bladder cancer. Id1 deficiency resulted in the development of urinary bladder tumors with areas of extensive hemorrhage and decreased invasiveness when compared to wild type mice. Id1 inactivation led to decreased cell growth in vitro and lung colonization in vivo of human bladder cancer cells. Immunohistochemistry performed on human urothelial carcinoma samples showed Id1 positive staining in both primary tumors and lymph node metastases.
Conclusions: In summary, our studies reveal the physiological relevance of Id1 in bladder cancer progression and suggest that targeting Id1 may be important in the development of novel therapies for the treatment of bladder cancer.
INTRODUCTION
Bladder cancer is a significant health problem, with incidence continuing to rise. In terms of overall cancer frequency, it is ranked ninth, with more than 330,000 new cases diagnosed annually worldwide [1]. At presentation, about 70–80% of bladder tumors are non-muscle invasive papillary tumors with the majority of cancers amenable to initial transurethral resection and intravesical chemotherapy or immunotherapy. These tumors only rarely evolve into an invasive cancer, but they have a high rate of recurrence. The second main variant, which accounts for about 20% of urothelial carcinomas, presents as an invasive tumor. Tumors that show muscle invasion at the time of diagnosis have a much less favorable prognosis and often progress rapidly [2–7]. New therapeutic modalities are needed to decrease recurrence and progression in patients diagnosed with non-invasive tumors and to provide new treatments for patients diagnosed with invasive bladder cancer.
Id (inhibition of DNA binding) proteins belong to the helix-loop-helix (HLH) family of transcription factors. Id family members (called Id1, Id2, Id3 and Id4) control a wide range of developmental and cellular processes by inhibiting the activity of the basic helix-loop-helix (bHLH) family of transcription factors. Id proteins contain a HLH dimerization motif but lack a basic DNA binding domain and act as dominant negative regulators by binding to bHLH transcription factors and preventing the formation of DNA-binding transcriptionally active complexes [8, 9]. Since the identification of the first Id helix-loop-helix (HLH) protein in 1990, much work has demonstrated the regulatory roles of Id proteins in a variety of cellular processes including proliferation, differentiation, angiogenesis and metastasis [9–17]. The fact that Ids are expressed at low levels in post-natal tissues and have significant roles in tumorigenesis raises the possibility that targeting Id proteins might be a viable strategy for treating cancer [18]. The requirement of Id expression in endothelial cells to establish a tumor vasculature has been extensively studied [19–22]. The first study to assess the role of Id proteins for tumor vascularization showed that xenograft tumors failed to grow and/or metastasize in Id-deficient mice with extensive hemorrhage and necrosis [19]. The role of Id proteins in tumor endothelium was further investigated in several tumor mouse models. Studies using transgenic animals that express a HER/neu activated oncogene and develop mammary tumors (YD neu mice) have shown that Id deficiency did not prevent or delay tumor formation but did alter tumor phenotype. Tumors that developed in Id-deficient mice showed areas of necrosis and dramatically improved sensitivity to chemotherapeutic intervention [20]. Similar results were described in other tumor-prone animals like Pten+/- [21] or TRAMP (transgenic adenocarcinoma of the mouse prostate) [22] mice.
Although the importance of Id1 in tumor endothelial cells is well established, the expression and role of Id1 protein in some cancer cells was for a time controversial, owing to disparate observations depending on the cell line or tumor model used or the nature of the antibodies against the gene products [23–26]. To resolve this issue, our laboratory has developed and characterized a highly specific rabbit monoclonal antibody against Id1. Using this antibody, Perk et al. found that Id1 is expressed in tumor cells of metaplastic triple negative breast cancer and bladder cancer [13]. Importantly, Id protein reduction in both the vasculature and breast tumor cells leads to a profound reduction in metastatic propensity [10, 27, 28]. Other studies have revealed a role for Id1 in cancer cells of a variety of tumors including brain, salivary gland, colorectal and lung cancer [29–32]. A number of in vitro studies have pointed to a role for the Id proteins in bladder tumor cells. Overexpression of Id1 has been shown to enhance the invasiveness of bladder cancer cells, while Id1 downregulation results in increased chemosensitivity [26, 33]. A role for Id1 has also been proposed in the regulation of epithelial-to-mesenchymal transition in bladder cancer [34, 35].
To explore the role of Id1 in an in vivo setting, we have used a well-established chemically induced mouse model of urinary bladder carcinogenesis that relies on the administration of N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) [36, 37]. The use of this model can be of clinical relevance since BBN induced bladder tumors bear significant histopathological similarities to the human disease [38, 39].
MATERIALS AND METHODS
Mice
All animal studies were performed in accordance with MSKCC Institutional Animal Care and Use Committee guidelines via approved IACUC protocol # 06-10-025. The animal care programs and facilities of MSKCC are administered through the MSKCC Research Animal Resources Center, which provides full-time animal care. The Center’s programs are fully accredited by the American Association for Accreditation of Laboratory Animal Care and are in compliance with the NIH guidelines for the care and use of laboratory animals. All animals were closely monitored and promptly sacrificed if they exhibited any sign of morbidity or distress. Id-deficient mice have been described previously [19]. NCr nude mice were purchased from Taconic.
BBN treatment
For the induction of urinary bladder carcinomas, mice 8 weeks of age were exposed continuously to BBN (TCI, Japan) present in the drinking water at a concentration of 0.05% , during 20 weeks, as described previously [36]. Mice were sacrificed upon presentation of symptoms and urinary bladders were collected and paraffin embedded for histological analysis.
Cell culture
UM-UC-3 cells were obtained from the AmericanType Cell Culture (ATCC) and maintained in high-glucose Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Id1 knockdown in UM-UC-3 cells was achieved with the retroviral vector pRS-Puro-Id1sh previously validated [10]. Retroviral particles were produced via calcium phosphate transfection method in 293T-GP2 cells. Virus-containing supernatants were harvested between 48 and 72 h post-transfection and filtered using a 0.45 μm syringe filter. Target cells were infected a total of four times with viral supernatants containing 10 μg/mL polybrene (Sigma) and selected with 2 μg/ml puromycin (Sigma). For cell viability studies, 106 cells were plated in 10 cm dishes for various times and cell number was determined using a standard hemocytometer protocol with the addition of trypan blue to exclude dead cells.Experiments were performed in triplicate.
Protein analysis
For immunoblotting, cells were washed with PBS and lysed in RIPA buffer (50 nM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA) plus a cocktail of protease inhibitors (Thermo Scientist). After 10 min on ice, cellular debris was removed by centrifugation (14000 rpm, 10 min). Protein concentration was measured using the BioRad DC Protein Assay Kit. Samples corresponding to 20 μg of protein were resolved on 15% SDS-PAGE gels, wet-transferred to nitrocellulose (BioRad) and immunoblotted. Id1 protein was detected using rabbit monoclonal anti-Id1 clone 195–14 (Biocheck) 1:2000 dilution. The secondary antibody was HRP-linked anti-rabbit (N934 V, Amersham). Membranes were subsequently probed with anti-actin antibody (1:6000) (A2066, Sigma). Detection was performed by chemiluminescence using ECL-Prime (Amersham).
Lung colonization model
For the in vivo model of lung colonization, 2 × 106 cells were resuspended in 200 μl of PBS and injected into the lateral tail vein of 2 months old NCr nude mice. Metastatic burden was assessed by serial sectioning of formalin-fixed paraffin-embedded lung tissue whereby the entire lung was sectioned and seven sections from each mouse (4 mice from UM-UC-3 ctrl and 3 mice from UM-UC-3 sh-Id1) taken every 100 μm were H&E stained. Stained sections were digitized with a Mirax Scanner and areas of metastatic lesions and total lung were measured with ImageJ software.
Human urothelial carcinoma samples
All tumor samples and associated clinical data were collected retrospectively in accordance with Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board (protocol # 89-076). All patients provided written informed consent for the use of tissue in research or, when applicable, a waiver was obtained through the MSKCC Institutional Review Board. A total of 67 bladder carcinoma samples were stained for Id1. Six groups of samples were analyzed: pTa low grade (n = 10), pTis in situ (n = 10), pTa high grade (n = 10), pT1 (n = 9), pT2 (n = 9), pT3/T4 (n = 19). Id1 protein expression was analyzed in vessels as well as in tumor cells. The percentage of positive tumor cells was also determined. Id1 staining intensity in positive cases was scored as 1 (weak), 2 (moderate) or 3 (strong).
Immunohistochemistry staining
Immunohistochemical detection was done with a Discovery XT system (Ventana Medical Systems,Tucson, AZ). Slides were blocked with 10% normal goat serum and 2% bovine serum albumin for 30 minutes. Primary antibody incubation was done for 2 hours with anti- Id1 rabbit monoclonal 195–14 (diluted 1:500) followed by incubation with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA; 1:200 dilution) for 8 minutes. Endogenous biotin blocking kit, blocker D, streptavidin-horseradish peroxidase, and 3,3-diaminobenzidine detection kit were used according to the manufacturer’s instructions (Ventana Medical Systems). Immunostaining for CD31 was performed on a Leica Bondtrademark RX using the Bondtrademark Polymer Refine Detection Kit (Cat. No. DS9800). The sections were pre-treated using heat mediated antigen retrieval with EDTA (pH9, Epitope Retrieval Solution 2, Cat. No. AR9640) for 20mins. The sections were then incubated with anti-Mouse CD31 (PECAM-1) antibody (Dianova, Cat. No. DIA-310) diluted at 1:250 for 30mins. DAB was used as the chromogen. The sections were then counterstained with hematoxylin and mounted.
RESULTS
Id deficiency alters the phenotype of BBN-induced bladder carcinomas
Id1-deficient mice are viable, fertile, and do not display any gross physical or behavioral abnormalities. Evaluation of urinary bladder sections revealed no histological differences between the wild type and Id1-deficient mice and all components of the urinary bladder including vasculature are withinnormal limits (Supplementary Figure S1). To assess the role of Id proteins in a tumorigenic setting, we used an in vivo model of bladder cancer that consists of the administration of the carcinogen N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) (Fig. 1A). Eight week old wild type and Id-deficient (losing two copies Id1-/-Id3+/+ or three copies Id1-/- Id3+/- of Id genes) mice were administered 0.05% BBN in the drinking water for 20 weeks. After completion of BBN treatment, mice were maintained on tap water and monitored for development of signs of disease. Symptomatic mice were sacrificed and bladders were collected and processed for histopathologic analyses.
No differences in overall survival were observed among the different groups (Fig. 1B). However, we did observe dramatic differences in tumor phenotype. At the time of collecting the bladder for histopathological evaluation, we observed that tumors that developed in Id-deficient background were hemorrhagic in appearance when compared with tumors that developed in a wild type background (Fig. 2A, right). In particular, 52% of tumors that arose in Id-deficient mice showed areas of hemorrhagic tissue in contrast to only 8% of tumors in the wild type mice (Fig. 2A, left). No difference in the proportion of hemorrhagic lesions was observed between Id1-/-Id3+/+ mice (58% ) and Id1-/-Id3+/- mice (44% ). On histological examination, BBN-induced Id-deficient tumors showed extensive hemorrhage (Fig. 2B). It is possible that the accumulation of hemorrhagic material in the bladder of Id-deficient mice may cause obstruction of the bladder and ultimately morbidity in Id-deficient mice. Blood vessels were visualized by staining tumors for CD31 (Fig. 2C). Id-deficient tumors showed abnormal vascularization as evidenced by dilated and irregular vessels with anastomoses similar to what has been observed in other tumor settings [20–22]. The fact that there were no differences in survival but significant differences in tumor phenotype is reminiscence of the phenotype reported in the HER/neu mammary tumor mouse model where loss of Id did not affect the survival of the mice but rendered tumors to be hemorrhagic and dramatically more sensitive to chemotherapeutic intervention [20].
Bladder tumors were subjected to histopathological evaluation and classified according to tumor stage: dys, dysplasia; pTis, carcinoma in situ; pT1, invasive to lamina propria; pT2, invasive to muscular layer; pT3, invasive to serosa. The incidence of highly invasive tumors was reduced in Id-deficient mice when compared with the control group. 91.7% of tumors that developed in a wild type background were classified as pT3. In contrast, 52.4% of bladder tumors from Id-deficient mice were classified as pT3, while 9.5% were classified as pT2, 33.3% as pTis and 4.8% as dysplasia (Fig. 2D).
Together, these results suggest that Id proteins are involved in the establishment of a proper tumor vasculature and in the maintenance of tumorigenic properties of BBN-induced bladder carcinomas.
Id1 protein expression in BBN-induced bladder carcinomas
Since Id1 protein has been reported to be upregulated in human bladder tumors [13, 26, 35], we wanted to test the levels and expression pattern of Id1 protein in our experimental model of induced bladder carcinogenesis. Similar to the findings in humans [13], Id1 protein was expressed at low levels in normal bladder tissue (Fig. 3A, Supplementary Figure S2). In contrast, high levels of Id1 were found in BBN-induced bladder tumors by western blot analysis and immunohistochemistry (Fig. 3A, Supplementary Figure S2).
Among the Id family members, Id1 and Id3 are more extensively overexpressed in various tumor types. Id1 and Id3 are known to be closely related evolutionarily and have overlapping biochemical functions [10, 19]. It has also been shown that they can compensate for each other and in some cases, loss of Id1 results in an increase in Id3 protein levels. We analyzed protein levels of Id3 in these samples and we did not find an upregulation of Id3 in response to loss of Id1. We also did not find that Id3 is overexpressed in the BBN-induced bladder carcinomas, suggesting that Id1 may play a pivotal role in these types of tumors (Fig. 3A). This is consistent with the fact that we did not observe differences in tumor phenotype between BBN-induced bladder carcinomas from Id1-/-Id3+/+ and Id1-/-Id3+/- mice.
To determine the pattern of Id1 expression we performed immunohistochemistry for Id1 in these tumors. We found that Id1 is expressed both in tumor vessels (Fig. 3B, red arrow) and in the tumor cells (Fig. 3B, black arrow), suggesting that Id1 could play a role in both endothelial and uroepithelial cells in bladder carcinomas.
Functional requirement for Id1 during metastatic lung colonization
The data obtained in the mouse model of bladder carcinogenesis prompted us to study the effects of Id1 inactivation in human bladder cancer. The role of Id1 in the human bladder cancer cell line UM-UC-3 (derived from a urinary bladder transitional cell carcinoma from a male patient [40, 41]) was assessed by knockdown of Id1 expression using short hairpin RNAs. UM-UC-3 cells were transduced with retroviral particles encoding small hairpin RNA against Id1 (shId1) or with control vector. After puromycin selection, Id1 protein expression was analyzed by western blot. We detected a reduction in Id1 protein levels in UM-UC-3-shId1 cells (Fig. 4A) that led to inhibition of cell growth (Fig. 4B-D).
To test the in vivo relevance of these findings we used an experimental model of lung metastasis. Female NCr nude homozygous mice were injected via tail vein with UM-UC-3 ctrl and sh-Id1 cells. Ten weeks after injection, mice were euthanized and lungs were collected and paraffin embedded. Paraffin blocks were serially sectioned and stained with hematoxylin and eosin for histologic examination. The analysis of these sections revealed that Id1 inactivation dramatically reduces metastasis in the lung (Fig. 4E). Mice injected with UM-UC-3 ctrl cells developed lung metastases positive for Id1 (Fig. 4F). Interestingly, the small metastatic lesions found in the UM-UC-3 sh-Id1 group stained positive for Id1, indicating that metastases were derived from a subpopulation of cells escaping the Id1 knockdown (Fig. 4G).
Together, these results indicate that downregulation of Id1 decreases cell growth in vitro and lung colonization potential in vivo of bladder cancer cells.
Immunohistochemical analysis of Id1 in human bladder tumors
Id1 levels were evaluated in tumor specimens from patients with bladder cancer and adjacent normal-appearing tissues by immunohistochemistry (Fig. 5, Table 1). Staining intensity was graded as 1 (weak), 2 (moderate) or 3 (strong). Tumor vessels were positive for Id1 staining in all samples analyzed, in agreement with the established role for Id1 in tumor endothelial cells. Normal-appearing urothelium adjacent to neoplastic urothelium showed weak to moderate Id1 staining (average Id1 intensity = 1.7, n = 31), whereas urothelial carcinoma tumor cells showed moderate to high Id1 levels (average Id1 intensity = 2.6, n = 63).
Within the bladder carcinoma samples, pTa low grade and pTis (in situ) were positive for Id1 in 10/10 samples analyzed with an average percentage of Id1 positive tumor cells of 84% and an average staining intensity of 2.9. 10/10 of pTa high grade samples showed Id1 expression in an average of 83% tumor cells with an average staining intensity of 2.7. In the case of pT1, 9/9 were positive, average percentage was 49% and intensity 2.6. For pT2 samples, 9/9 were positive, average percentage was 50% and intensity 2.3. For pT3/pT4, 15/19 samples were positive, average percentage of 47% and intensity 2.3.
We have also evaluated levels of Id1 protein in lymph node metastasis (Fig. 5F). 8/9 of these lymph node metastatic lesions showed Id1 positive staining with an average percentage of 58% and staining intensity of 2.8.
The Kruskal–Wallis test was used to compare groups followed by Dunn’s multiple comparisons test. Kruskal-Wallis test revealed a significant difference in Id-1 staining intensity between the various groups (**** p < 0.0001). Post hoc Dunn’s test showed significant differences between tumors (**** p < 0.0001), pTa low grade (**** p < 0.0001), pTis (**** p < 0.0001), pTa high grade (**** p < 0.01), pT1 (**** p < 0.01) and lymph node metastases (**** p < 0.01) when compared with normal tissue.
DISCUSSION
Id1 is a known marker of tumor vasculature for a wide variety of cancers, where it plays a role in tumor angiogenesis [19]. Id1 could also play a role in tumor cells where it contributes to the neoplastic phenotype by inhibiting differentiation and stimulating proliferation and invasiveness [8–10, 27]. Our knowledge of the role of Id proteins in bladder cancer cells comes from in vitro studies that implicate Id proteins in tumorigenic processes like invasion, chemorresistance and conversion between epithelial and mesenchymal states [26, 27, 33–35]. Knockdown of Id1 increases the sensitivity of bladder cancer cells to the chemotherapeutic agent epirubicin, suggesting that inactivation of Id1 may be a potential means to improve the efficiency of chemotherapeutic drugs [33].
We have used a chemically induced model of bladder cancer to ascertain the role of Id proteins in a physiologically relevant setting. Cross-species comparison analysis has shown that this model of bladder cancer accurately represent the clinical situation found in human bladder cancers [42] and has been extensively used to characterize the tumorigenic process for urinary bladder cancer and to assess the efficacy of potential chemopreventive agents [43–47]. We found that Id1 is overexpressed in tumors arising in this mouse model of bladder cancer. Id1 expression was detected in both bladder epithelium and endothelium of these bladder carcinomas induced by BBN. Interestingly, Id1 deficiency results in hemorrhagic tumors and reduced frequency of invasive tumors, suggesting a functional involvement for Id1 in both the establishment of a proper tumor vasculature and the maintenance of tumorigenic properties of BBN-induced bladder carcinomas. The change in biology of the lesions upon Id loss could be of relevance since similar changes profoundly sensitizes the tumors to chemotherapy in a model of breast cancer [20].
We further investigated the functional role of Id1 in urinary bladder cancer by knocking-down Id1 in human bladder cancer cells. Loss of Id1 results in decreased cellular proliferation rates in vitro and dramatically decreased lung colonization capacity in an experimental mouse model of metastasis. These results are in alignment with previous studies [10] showing that Id proteins mediate tumor re-initiation during breast cancer lung metastasis.
We analyzed Id1 staining in human urothelial carcinoma samples and found that Id1 was expressed in both vessels and tumor cells making it an extremely attractive target for therapeutic intervention. Staining in tumor cells was nuclear in agreement with a previous study [13]. Other studies have shown cytoplasmic Id1 staining, which can be explained based on the use of a different antibody in these assays which can have a lower degree of specificity [26, 35]. In agreement with a previous study, benign urothelium adjacent to neoplastic urothelium showed weak to moderate Id1 staining, which may suggest an onset of molecular changes in these tissues adjacent to tumors [13]. This suggests a role for Id1 in the transition from normal to malignant urothelium and could be an early marker of the disease. Similar findings have been described in premalignant lesions in the pancreas [48] and recently in colon cancer, where elevated levels of Id1 occur in colorectal adenomas and in dysplasia implying that upregulation of Id1 occurs as a relatively early event during tumorigenesis [49, 50]. It is likely that other genetic and epigenetic alterations are also required for the development of late stage disease. We have also analyzed Id1 staining in lymph node metastases and found high levels of Id1 expression. These results agree with and complement previous studies on Id1 protein in other types of tumors like breast cancer, where positive staining for Id1 was found both in primary tumors and metastases [10]. Interestingly, higher levels of Id1 staining were found at early stages and in metastatic lesions. A similar pattern of expression is found in melanomas, where Id1 protein is expressed in early primary melanomas and in metastatic melanoma cell lines and lesions [51–53]. These data suggest that Id1 is required but may not be sufficient for tumor initiating functions, both in the context of primary tumor formation and during metastatic colonization of the lymph node microenvironment.
Collectively, our results in an in vivo model provide new insights into the role of Id1 in bladder carcinogenesis and the potential of using Id1 as a therapeutic target for bladder cancer treatment.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
We thank members of the Benezra laboratory; L. Barrett, S. Pavlovic and P. Wojnarowicz for critical reading of this manuscript and Courtney Coker for assistance with animal husbandry. We also thank Smitha Pillai and Maria S. Jiao of the MSKCCComparative Pathology Core Facility for pathology analysis and immunohistochemistry. This facility is supported by NIH/NCI grant P30 CA008748. This work was also supported by funds from the Pin Down Bladder Cancer, and the Michael and Zena Wiener for Therapeutics Program in Bladder Cancer to BHB and Cycle for Survival to HAA. MG-C was funded by fellowships from the Human Frontier Science Program (HFSP) and the Spanish Ministry of Education and Science (MEC).
Appendices
The supplementary table and figure are available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-150023.
REFERENCES
1 | Ploeg M, Aben KK, Kiemeney LA(2009) The present and future burden of urinary bladder cancer in the worldWorld J Urol27: 3289293 |
2 | Friedrich MG, Pichlmeier U, Schwaibold H, Conrad S, Huland H(2007) Long-term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short-term intravesical chemotherapy and short-term therapy with Bacillus Calmette-Guerin (BCG) in patients with non-muscle-invasive bladder carcinomaEur Urol52: 411231129 |
3 | Chung PW, Bristow RG, Milosevic MF, Yi QL, Jewett MA, Warde PR(2007) Long-term outcome of radiation-based conservation therapy for invasive bladder cancerUrol Oncol25: 4303309 |
4 | Herr HW, Donat SM, Bajorin DF(2001) Post-chemotherapy surgery in patients with unresectable or regionally metastatic bladder cancerJ Urol165: 3811814 |
5 | Xu N, Zhang XC, Xiong JP, Fang WJ, Yu LF, Qian J(2007) A phase II trial of gemcitabine plus carboplatin in advanced transitional cell carcinoma of the urotheliumBMC Cancer7: 98 |
6 | Bamias A, Moulopoulos LA, Koutras A, Aravantinos G, Fountzilas G, Pectasides D(2006) The combination of gemcitabine and carboplatin as first-line treatment in patients with advanced urothelial carcinomaA Phase II study of the Hellenic Cooperative Oncology GrouCancer106: 2297303 |
7 | Ahmad I, Sansom OJ, Leung HY(2012) Exploring molecular genetics of bladder cancer: Lessons learned from mouse modelsDis Model Mech5: 3323332 |
8 | Lasorella A, Benezra R, Iavarone A(2014) The ID proteins: Master regulators of cancer stem cells and tumour aggressivenessNat Rev Cancer14: 27791 |
9 | Perk J, Iavarone A, Benezra R(2005) Id family of helix-loop-helix proteins in cancerNat Rev Cancer5: 8603614 |
10 | Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K(2007) ID genes mediate tumor reinitiation during breast cancer lung metastasisProc Natl Acad Sci U S A104: 491950619511 |
11 | Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD(2005) Genes that mediate breast cancer metastasis to lungNature436: 7050518524 |
12 | Romero-Lanman EE, Pavlovic S, Amlani B, Chin Y, Benezra R(2012) Id1maintains embryonic stem cell self-renewalbyup-regulation of Nanog and repression of BrachyuryexpressionStem Cells Dev21: 3384393 |
13 | Perk J, Gil-Bazo I, Chin Y, de Candia P, Chen JJ, Zhao Y(2006) Reassessment of id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-id1 antibodyCancer Res66: 221087010877 |
14 | Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H(1990) The protein Id: A negative regulator of helix-loop-helix DNA binding proteinsCell61: 14959 |
15 | Fong S, Itahana Y, Sumida T, Singh J, Coppe JP, Liu Y(2003) Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasisProc Natl Acad Sci U S A100: 231354313548 |
16 | Fong S, Debs RJ, Desprez PY(2004) Id genes and proteins as promising targets in cancer therapyTrends Mol Med10: 8387392 |
17 | Nair R, Teo WS, Mittal V, Swarbrick A(2014) ID proteins regulate diverse aspects of cancer progression and provide novel therapeutic opportunitiesMol Ther22: 814071415 |
18 | Benezra R(1551) The Id proteins: Targets for inhibiting tumor cells and their blood supplyBiochim Biophys Acta2F39F47 |
19 | Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R(1999) Id1 and Id3 are required forneurogenesis, angiogenesis and vascularization of tumourxenograftsNature401: 6754670677 |
20 | de Candia P, Solit DB, Giri D, Brogi E, Siegel PM, Olshen AB(2003) Angiogenesis impairment in Id-deficient mice cooperates with an Hsp90 inhibitor to completely suppress HER2/neu-dependent breast tumorsProc Natl Acad SciU S A100: 211233712342 |
21 | Ruzinova MB, Schoer RA, Gerald W, Egan JE, Pandolfi PP, Rafii S(2003) Effect of angiogenesis inhibition by Id loss and thecontribution of bone-marrow-derived endothelial cells inspontaneous murine tumorsCancer Cell4: 4277289 |
22 | Li H, Gerald WL, Benezra R(2004) Utilization of bone marrow-derived endothelial cell precursors in spontaneous prostate tumors varies with tumor gradeCancer Res64: 1761376143 |
23 | Schoppmann SF, Schindl M, Bayer G, Aumayr K, Dienes J, Horvat R(2003) Overexpression of Id-1 is associated with poor clinical outcome in node negative breast cancerInt J Cancer104: 6677682 |
24 | Coppe JP, Itahana Y, Moore DH, Bennington JL, Desprez PY(2004) Id-1 and Id-2 proteins as molecular markers for human prostate cancer progressionClin Cancer Res10: 620442051 |
25 | Ouyang XS, Wang X, Lee DT, Tsao SW, Wong YC(2002) Over expression ofID-1 in prostate cancerJ Urol167: 625982602 |
26 | Ding Y, Wang G, Ling MT, Wong YC, Li X, Na Y(2006) Significanceof Id-1 up-regulation and its association with EGFR in bladdercancer cell invasionInt J Oncol28: 4847854 |
27 | Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L(2013) TGF-beta-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transitionCell Rep5: 512281242 |
28 | Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V(2008) Endothelial progenitor cells control the angiogenic switch in mouse lung metastasisScience319: 5860195198 |
29 | Soroceanu L, Murase R, Limbad C, Singer E, Allison J, Adrados I(2013) Id-1 is a key transcriptional regulator of glioblastoma aggressiveness and a novel therapeutic targetCancer Res73: 515591569 |
30 | Sumida T, Murase R, Onishi-Ishikawa A, McAllister SD, Hamakawa H, Desprez PY(2013) Targeting Id1 reduces proliferation and invasion in aggressive human salivary gland cancer cellsBMC Cancer13: 141 |
31 | O’Brien CA, Kreso A, Ryan P, Hermans KG, Gibson L, Wang Y(2012) ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21Cancer Cell21: 6777792 |
32 | Pillai S, Rizwani W, Li X, Rawal B, Nair S, Schell MJ(2011) ID1 facilitates the growth and metastasis of non-small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signalingMol Cell Biol31: 1430523067 |
33 | Hu H, Han HY, Wang YL, Zhang XP, Chua CW, Wong YC(2009) The role of Id-1 in chemosensitivity and epirubicin-induced apoptosis in bladder cancer cellsOncol Rep21: 410531059 |
34 | Cubillo E, Diaz-Lopez A, Cuevas EP, Moreno-Bueno G, Peinado H, Montes A(2013) E47 and Id1 interplay in epithelial-mesenchymal transitionPLoS One8: 3e59948 |
35 | Hu H, Wang YL, Wang GW, Wong YC, Wang XF, Wang Y(2013) A novel role of Id-1 in regulation of epithelial-to-mesenchymal transition in bladder cancerUrol Oncol31: 712421253 |
36 | Yamamoto S, Tatematsu M, Yamamoto M, Fukami H, Fukushima S(1998) Clonal analysis of urothelial carcinomas inC3H/HeN< –>BALB/c chimeric mice treated with N-butyl-N-(4-hydroxybutyl)nitrosamineCarcinogenesis19: 5855860 |
37 | Ozaki K, Sukata T, Yamamoto S, Uwagawa S, Seki T, Kawasaki H(1998) High susceptibility of p53 (+/-) knockout mice in N-butyl-N-(4-hydroxybutyl)nitrosamine urinary bladder carcinogenesis and lack of frequent mutation in residual alleleCancer Res58: 1738063811 |
38 | Cohen SM(2002) Comparative pathology of proliferative lesions of the urinary bladderToxicol Pathol30: 6663671 |
39 | Cohen SM(1998) Urinary bladder carcinogenesisToxicol Pathol26: 1121127 |
40 | Bellet D, Lazar V, Bieche I, Paradis V, Giovangrandi Y, Paterlini P(1997) Malignant transformation of nontrophoblastic cells is associated with the expression of chorionic gonadotropin beta genes normally transcribed in trophoblastic cellsCancer Res57: 3516523 |
41 | Grossman HB, Wedemeyer G, Ren L, Wilson GN, Cox B(1986) Improved growth of human urothelial carcinoma cell culturesJ Urol136: 4953959 |
42 | Lu Y, Liu P, Wen W, Grubbs CJ, Townsend RR, Malone JP(2010) Cross-species comparison of orthologous gene expression in human bladder cancer and carcinogen- induced rodent modelsAm J Transl Res3: 1827 |
43 | Ding Y, Paonessa JD, Randall KL, Argoti D, Chen L, Vouros P(2010) Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissuesCarcinogenesis31: 1119992003 |
44 | Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE(2000) Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 ratsCancer Res60: 2055995602 |
45 | Lubet RA, Huebner K, Fong LY, Altieri DC, Steele VE, Kopelovich L(2005) 4- Hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers in mice: Characterization of FHIT and survivin expression and chemopreventive effects of indomethacinCarcinogenesis26: 3571578 |
46 | Steele VE, Rao CV, Zhang Y, Patlolla J, Boring D, Kopelovich L(2009) Chemopreventive efficacy of naproxen and nitric oxide-naproxen in rodent models of colon, urinary bladder, and mammary cancersCancer Prev Res (Phila)2: 11951956 |
47 | Yao R, Lemon WJ, Wang Y, Grubbs CJ, Lubet RA, You M(2004) Altered gene expression profile in mouse bladder cancers induced by hydroxybutyl(butyl)nitrosamineNeoplasia6: 5569577 |
48 | Maruyama H, Kleeff J, Wildi S, Friess H, Buchler MW, Israel MA(1999) Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitisAm J Pathol155: 3815822 |
49 | Zhang N, Subbaramaiah K, Yantiss RK, Zhou XK, Chin Y, Benezra R(2015) Id1 Deficiency Protects against Tumor Formation in ApcMin/+Mice but Not in a Mouse Model of Colitis-Associated Colon CancerCancer Prev Res (Phila) |
50 | Zhang N, Yantiss RK, Nam HS, Chin Y, Zhou XK, Scherl EJ(2014) ID1 is a functional marker for intestinal stem and progenitor cells required for normal response to injuryStem Cell Reports3: 5716724 |
51 | Polsky D, Young AZ, Busam KJ, Alani RM(2001) The transcriptional repressor of p16/Ink4a, Id1, is up-regulated in early melanomasCancer Res61: 1660086011 |
52 | Zigler M, Villares GJ, Dobroff AS, Wang H, Huang L, Braeuer RR(2011) Expression of Id-1 is regulated by MCAM/MUC A missing link in melanoma progressionCancer Res71: 1034943504 |
53 | Ryu B, Kim DS, DeLuca AM, Healey MA, Dunlap S, Fackler MJ(2007) Id1 expression is transcriptionally regulated in radial growth phase melanomasInt J Cancer121: 817051709 |
Figures and Tables
Fig.1
BBN-induced model of bladder carcinogenesis. A) Schematic diagram of the BBN administration protocol. B) Kaplan-Meier survival curves of mice treated with BBN.
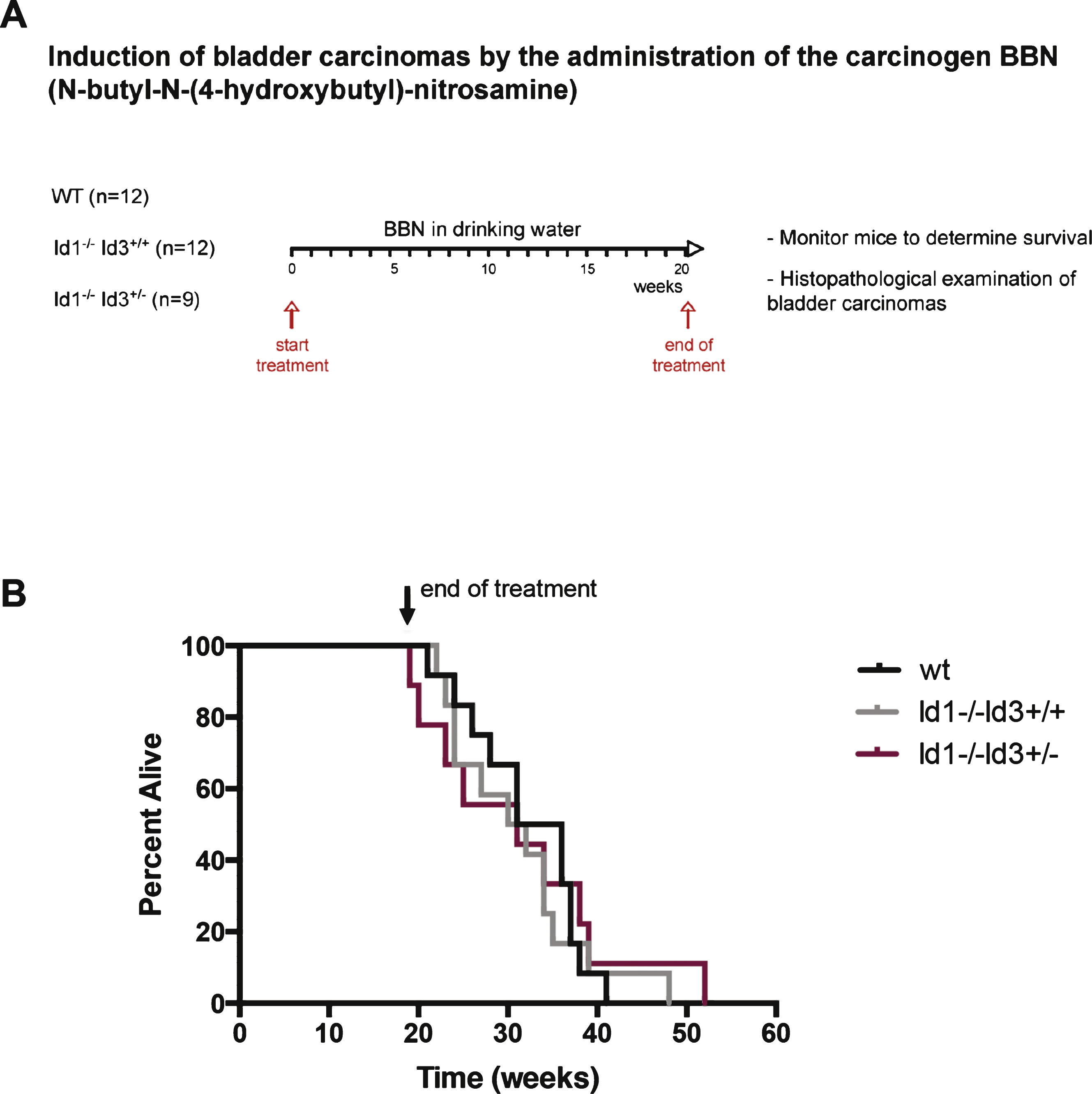
Fig.2
Bladder tumors that develop in Id-deficient background show hemorrhagic areas and decreased frequency of highly invasive tumors. A) Percentage of hemorrhagic tumors found in wild type or Id-deficient background (left) and representative images of BBN-induced tumors (right). *p < 0.05 (χ 2 test). B) Wild type: Section of the bladder showing high grade urothelial carcinoma deeply invasive into perivesical tissue (pT3) in the form of solid sheets and nests of invasive tumor. Id-deficient: section of bladder wall showing abundant intravesical hemorrhage at different stages of organization. This finding was not present in the bladders of the wild type mice. The urothelial lesion in this bladder was only urothelial carcinoma in situ (CIS) without evidence of invasion. C) CD31 immunostaining highlights the endothelial lining of the vessels. The vessels in the wild type mouse are small and scattered through the bladder wall without dilation or endothelial proliferation. In contrast, in the Id-deficient mice, there is extensive vascular dilation and endothelial proliferation resulting in anastomosing and gaping vessels. D) Bladder tumors were classified according to tumor stage (dys: dysplasia; pTis: carcinoma in situ; pT2: invasive to muscular layer; pT3: invasive to serosa). *p < 0.05 (incidence of pT3 tumors, χ 2 test). Picture displays a high grade and high stage urothelial carcinoma in a wild type mouse. The tumor is deeply invasive to the level of perivesical tissue (pT3). In comparison, the urothelial lesion that developed in this Id-deficient mouse is non-invasive urothelial carcinoma (in situ), characterized by nuclear pleomorphism and disorder, without any evidence of invasion.
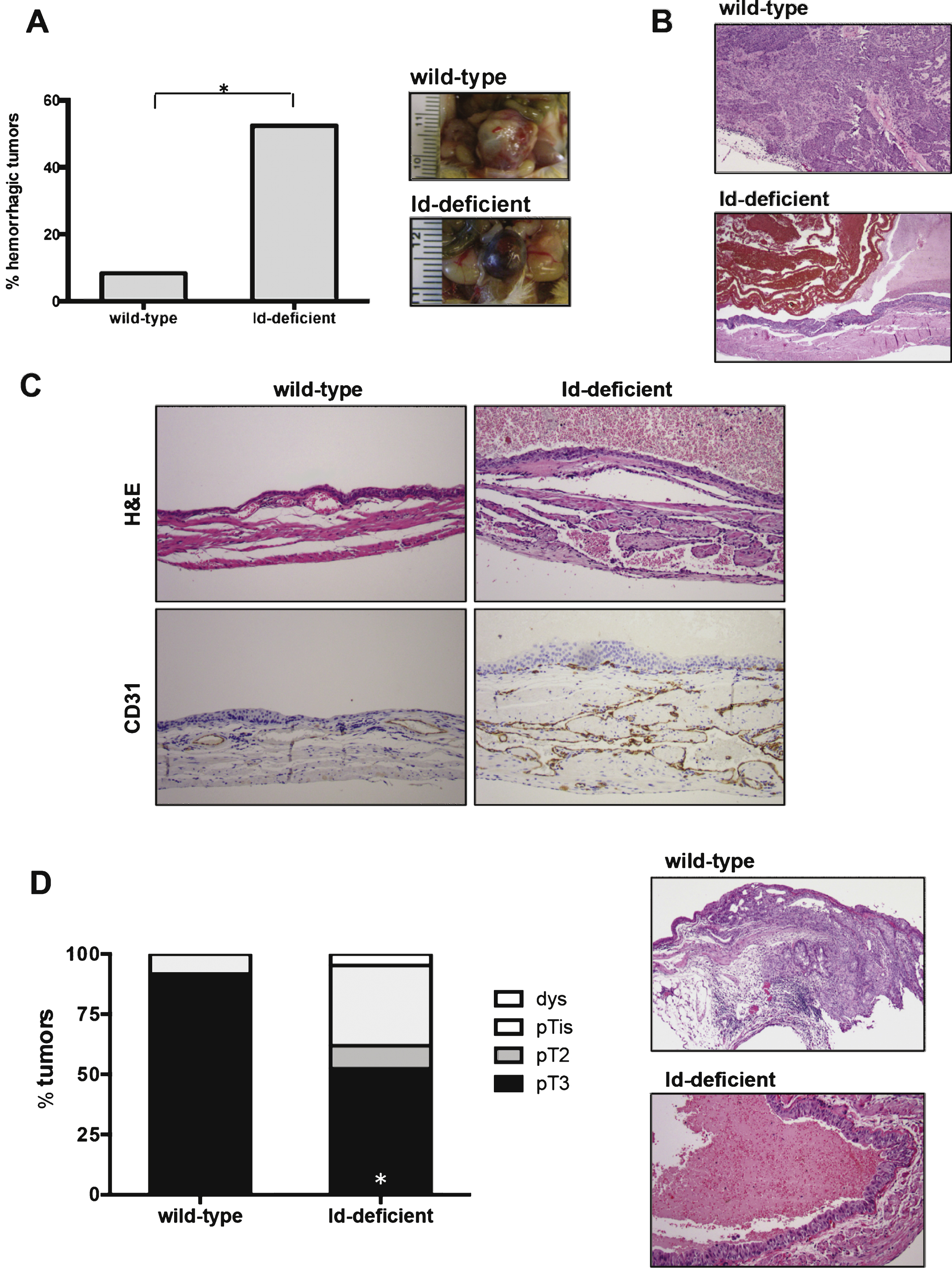
Fig.3
Id1 protein is expressed in BBN-induced bladder tumors. A) Western blot analysis of Id1 and Id3 protein expression in normal bladder (N) and bladder tumors (T). B) Immunohistochemistry showing Id1 staining in tumor cells (black arrow) and vessels (red arrow) of BBN-induced bladder tumors. Id1-/- BBN-induced bladder tumor is shown as a negative control. Scale bars = 20 μm.
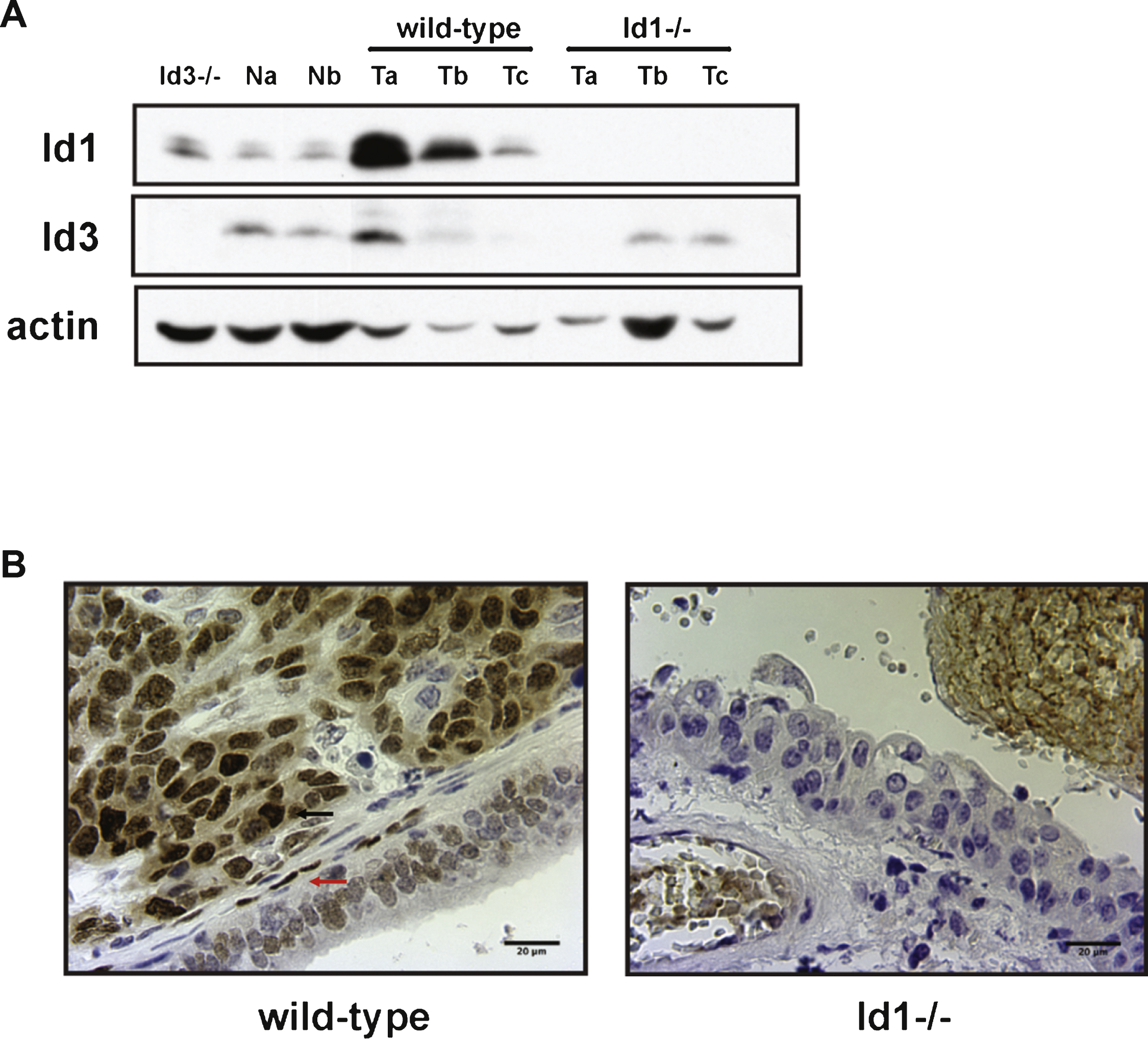
Fig.4
Effects of Id1 knockdown in vitro and in an in vivo model of lung colonization. A) UM-UC-3 cells were transduced with ctrl or sh-Id1 retrovirus and Id1 protein expression was analyzed by western blot. B) Growth curves of UM-UC-3 ctrl and sh-Id1 cells. Cells were plated at 106 cells per plate in triplicates in 10 cm plates for various times, and cell numbers were estimated by using Trypan Blue. Unpaired t-test (**** p < 0.05, **** p < 0.01). Representative images of UM-UC-3 ctrl (C) and sh-Id1 cells (D) after 72 h in culture. E) Quantification of metastasis area in lungs from mice injected with UM-UC-3 ctrl and sh-Id1 cells. Metastatic burden was assessed by serial sectioning of formalin-fixed paraffin-embedded lung tissue whereby the entire lung was sectioned and sections 100 μm apart were digitally scanned and the percentage of lung metastasis area to total lung area was determined. Mann-Whitney U test (**** p < 0.0001). Immunohistochemical staining for Id1 of lungs from UM-UC-3 ctrl (F) and sh-Id1 (G) injected mice. Original magnification: x20.
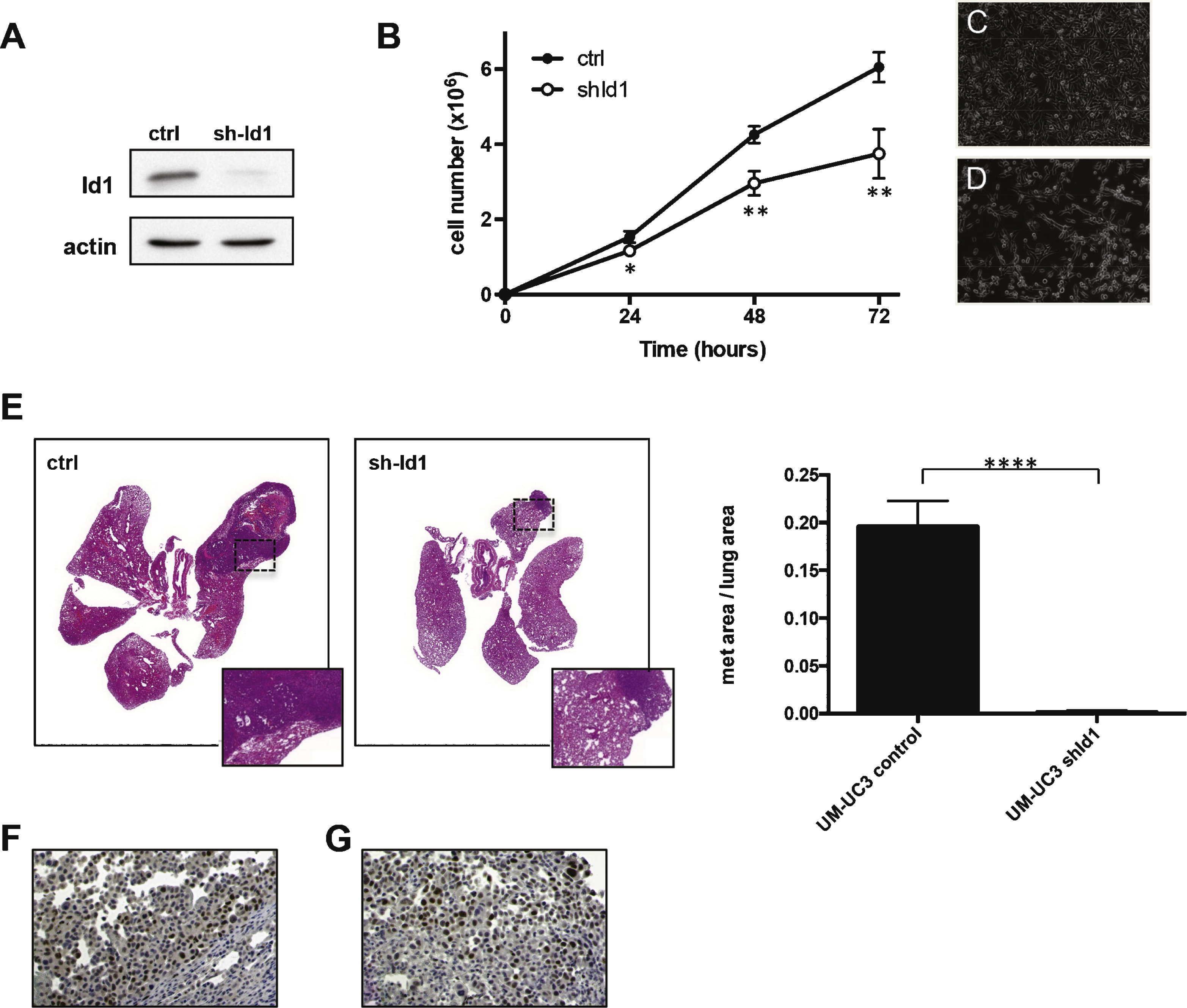
Fig.5
Id1 expression by immunohistochemistry in human samples. Positive expression in low grade papillary urothelial carcinoma (A); high grade papillary urothelial carcinoma (B); and urothelial carcinoma in situ (C). One sample of invasive urothelial carcinoma without Id1 expression (D, note Id1 expression in endothelial cells of tumor associated vasculature tumor [arrow heads] and Id1 protein expression in overlying normal urothelium [inset]). Id1 expression in invasive urothelial carcinoma (E) and matched lymph node metastasis (F).
![Id1 expression by immunohistochemistry in human samples. Positive expression in low grade papillary urothelial carcinoma (A); high grade papillary urothelial carcinoma (B); and urothelial carcinoma in situ (C). One sample of invasive urothelial carcinoma without Id1 expression (D, note Id1 expression in endothelial cells of tumor associated vasculature tumor [arrow heads] and Id1 protein expression in overlying normal urothelium [inset]). Id1 expression in invasive urothelial carcinoma (E) and matched lymph node metastasis (F).](https://content.iospress.com:443/media/blc/2015/1-2/blc-1-2-blc150023/blc-1-2-blc150023-g005.jpg)
Table 1
Id1 staining in human bladder carcinoma samples
| Id1 staining intensity | |
| normal-appearing (n = 31) | 1.7 |
| tumors (n = 63) | 2.6**** |
| pTa low grade (n = 10) | 2.9**** |
| pTis (n = 10) | 2.9**** |
| pTa high grade (n = 10) | 2.7**** |
| pT1 (n = 9) | 2.6**** |
| pT2 (n = 9) | 2.3 |
| pT3/pT4 (n = 15) | 2.3 |
| lymph node mets (n = 8) | 2.8**** |
Id1 staining intensity was scored as 1 (weak), 2 (moderate) or 3 (strong). Kruskal-Wallis test revealed significant differences in Id1 staining intensity between the various groups (**** p < 0.0001). Subsequent Dunn’s test showed significant differences between tumors (**** p < 0.0001), pTa low grade (**** p < 0.0001), pTis (**** p < 0.0001), pTa high grade (**** p < 0.01), pT1 (**** p < 0.01) and lymph node metastases (**** p < 0.01) when compared with normal tissue.




