Is Exam under Anesthesia Still Necessary for the Staging of Bladder Cancer in the Era of Modern Imaging?
Abstract
Background:
The ability to accurately determine tumor stage in bladder cancer is critical because it impacts the management paradigm and overall prognosis. There is often discrepancy between clinical and pathologic staging. Historically, exam under anesthesia (EUA) has been recommended to assist in the staging of bladder cancer.
Objective:
In this era of modern imaging technology, we sought to determine if EUA still contributes meaningfully to the local staging of bladder cancer.
Methods:
We retrospectively reviewed the charts of 1898 patients from 1994–2013 in our radical cystectomy database at MD Anderson Cancer Center. There were 414 patients that had complete information including EUA and whose surgery was performed by one of two surgeons and included in the final analysis. Univariate and multiple logistic regression models were generated to determine the ability of EUA, imaging, and other patient characteristics to predict pathological fat extension at the time of cystectomy.
Results:
38% of patients had≥pT3 disease at the time of cystectomy. 30.9% of patients had findings on EUA suggestive of T3 disease and 28.7% had radiologic findings suggestive of T3 disease. In a model including age, BMI, ethnicity, year of operation, and neoadjuvant chemotherapy among other factors, the only factors predictive of pT3 disease were EUA and imaging (p = 0.002). The combination of EUA and imaging improved the accuracy of clinical staging compared to either modality alone.
Conclusions:
Despite modern advances in imaging, EUA contributes meaningfully to accurate determination of local bladder cancer stage.
INTRODUCTION
With over 70,000 new cases per year, bladder cancer represents the fifth most commonly diagnosed cancer in the American population [1]. While many bladder cancers are superficial at diagnosis, about 10–15% will progress to muscle-invasive disease and approximately one-third of patients are found to have muscle-invasive disease at the initial diagnosis [2, 3]. For patients with muscle-invasive bladder cancer, accurate determination of clinical stage can help predict survival and likelihood of success with curative treatment options [4].
Current practice for the initial staging evaluation of bladder cancer includes: transurethral resection of bladder tumors (TURBT), bimanual exam under anesthesia (EUA), abdominal and pelvic imaging (typically CT or MRI), baseline laboratory studies, and chest imaging. The EUA is performed to determine local tumor extent outside the bladder and imaging is performed to assess regional lymph nodes for tumor involvement. However, imaging may add helpful information about local staging of the primary tumor as well. The EUA is performed after complete tumor resection by placing one hand on the anterior abdominal wall and a finger of the other hand in the rectal vault with the patient in the dorsal lithotomy position. In a female patient, two fingers of the other hand are placed in the vagina. This allows direct palpation of the bladder between the two hands to manually determine if the tumor is palpable on the outer surface of the bladder and whether it is a mobile or fixed mass, distinguishing clinical stage T3 and T4 disease respectively.
Given the complexity and potential morbidity associated with radical cystectomy, patients with muscle-invasive bladder cancer are often referred to higher volume academic centers for radical cystectomy. Ploeg et al highlight that the EUA is an integral component of the clinical staging of bladder cancer. Unfortunately, this is less often performed in non-teaching hospitals with a rate of < 29.9% in their population [5]. We have observed a similarly low rate of EUA performance at the time of initial TURBT in those patients referred to our academic center. There seems to be a trend in the clinical staging of bladder cancer to rely more on the radiographic findings alone as imaging technology has improved. In this study, we investigate the relative contribution of EUA and imaging in predicting extravesical spread of bladder cancer in the cystectomy specimen.
MATERIALS AND METHODS
We retrospectively reviewed the IRB-approved MD Anderson Cancer Center cystectomy database and identified patients that had undergone radical cystectomy for bladder cancer at our institution between 1994 and 2013. Given the potential for variability in the performance and reporting of the EUA, we limited our analysis to patients with complete clinical information whose EUA, operative report dictation, and radical cystectomy had all been performed by the same attending surgeon. We limited our analysis to 414 patients of 1898 patients in the MD Anderson radical cystectomy database in order to reduce interobserver variability. We analyzed the 414 patients who underwent cystectomy under the care of two urologists who routinely perform their own EUA and document it in their operative reports. For these patients, we collected demographic, clinical, and pathologic information. Surgeon operative reports were reviewed to obtain EUA results and radiology reports were reviewed to determine if CT or MRI was suggestive of clinical fat invasion (stage cT3).
Imaging findings that were suggestive of cT3 disease included significant bladder wall thickening, fat stranding, obliteration of normal fat planes surrounding the bladder or invasive into contiguous pelvic structures. Additionally, the presence of hydronephrosis on abdominal imaging was also suggestive of at least cT3 disease, particularly for posterior tumors. A vast majority of patients underwent CT scan compared to MRI (395 patients versus 19 patients). The mean time between imaging and TURBT was 34.6 days, the median was 15 days, and our analysis utilized the imaging and TURBT information closest to the time of cystectomy. A positive EUA was defined as a palpable, mobile or fixed 3D mass. For those patients that underwent neoadjuvant chemotherapy and had multiple EUAs performed, the EUA, after chemotherapy and prior to cystectomy, was used in our analysis.
We constructed univariate and multiple logistic regressions to identify factors predictive of pathological fat invasion (stage pT3) at the time of radical cystectomy. To determine the relative contribution of EUA, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value of EUA and imaging modalities alone and then the combination of EUA and imaging together in their ability to predict pathological fat invasion. We also calculated the under-staging and over-staging percentages of each modality alone and then the combination of both modalities. In using a combination of EUA and imaging, we created two different predictive models: first including those patients who had agreement of the EUA and imaging findings and second using those patients where either EUA or imaging was suggestive of T3 disease. The either positive EUA or positive imaging model more closely represents clinical practice, whereby if either is positive on staging evaluation suggesting cT3 disease, it would potentially change the management paradigm. A 2-sided p-value of 0.05 was used to determine significance for all hypothesis testing.
Given the possibility that EUA may be easier to perform in women (due to the absence of the prostate) and in patients with lower BMI (due to less visceraladiposity), we constructed regression models that included these and other potential modifiers of effect as interaction terms.
RESULTS
Of the 1898 patients in the radical cystectomy database, we identified 414 patients that met inclusion criteria. Descriptive characteristics for these patients are listed in Table 1. In this cohort, the median age was 68 years, 80% were men, and almost 90% were white. The median BMI was 27.3 kg/m2. In our cohort, 146 of the 414 patients (35.6%) had received neoadjuvant chemotherapy.
On EUA, 128 (30.9%) patients had findings suggestive of tumor extension at least into the perivesical fat. On imaging, 119 (28.7%) patients had radiologic findings suggestive of perivesical tumor extension. At final pathological analysis of cystectomy specimens, 119 (28.7%) patients had pathologic T3 disease and 40 (9.7%) patients had pathologic T4 disease. Test characteristics for EUA and imaging to accurately predict extravesical tumor extension on the final cystectomy specimen are shown in Table 2. In assessing for potential interactions, we found that BMI, year of surgery, and gender did not moderate the effect of EUA or imaging findings.
Further statistics regarding specificity, sensitivity, positive predictive value, and negative predictive value for EUA and Imaging are shown in Table 3. Table 4 demonstrates the staging accuracy of using EUA or Imaging alone and also in combination, in which either EUA or imaging are suggestive of T3 disease and also when EUA and imaging findings are in agreement.
On univariate analysis (Table 2), the only factors predictive of pathological fat invasion were EUA (OR 2.12, p = 0.001) and imaging (OR 2.20, p < 0.001). Age, race, gender, BMI, year of surgery, imaging type (CT versus MRI), and receipt of neoadjuvant chemotherapy were not found to be predictive. On multivariate analysis (Table 2), both positive EUA and positive imaging retained significance for predicting pathological fat extension at cystectomy (p = 0.002 for each). Figure 1 demonstrates the added benefit of EUA in clinical staging of bladder cancer. In this receiver operating curve (ROC) regression model, there was a statistically significant improvement in predicting T3 disease with the inclusion of EUA in pre-operative staging (p = 0.004).
DISCUSSION
For bladder cancer, precise determination of tumor stage is a key requirement for determining the most appropriate treatment strategy. The ability to accurately stage bladder cancer clearly impacts the paradigm for management of this disease. It is well known that both prognosis and survival are directly correlated to stage [4, 6]. Despite progress in our understanding of bladder cancer and the advances in the imaging technology available to visualize the disease, there is still a notable discrepancy between clinical staging and pathologic staging and high rates of under-staging reported in the literature [6, 7]. Ficarra et al noted that 50% of patients with clinical stage≤T2 were found to have pathologic stage≥T3 at cystectomy [7]. Shariat et al reported a 36% rate of upstaging from cT2 at TURBT to pT3 disease at cystectomy [6]. Studies show that 42% – 54% of bladder cancer patients are under-staged and 20% – 27% are over-staged. The overall preoperative staging of locally advanced bladder cancer may be inaccurate in 23–50% of patients [8, 9].
Classically, exam under anesthesia (EUA) has been used to aid in the clinical staging of bladder cancer, with a palpable mass indicating more advanced disease with higher rates of lymph node positive disease and poorer overall survival [10–13]. Wijkstrom et al found that those with a palpable bladder mass had survival rates of 53% and 45% at 5 and 10 years respectively, compared to 80% and 70% without palpable masses at 5 and 10 years respectively [12]. These early studies underscored the importance of EUA as part of the clinical staging evaluation for the primary tumor. Despite this, many consider clinical staging with EUA to be highly subjective and therefore of prohibitively low sensitivity [7]. Mehrsai and colleagues found EUA to have a sensitivity of 46% , specificity of 82% , and a positive predictive value of 70% for extravesical disease [14]. These numbers are similar to the test characteristics we determined for EUA in our much larger group of patients.
Many have evaluated the use of advanced imaging (CT, MRI) to aid in clinical staging of disease instead of the EUA [16, 17]. However, imaging modalities have been deemed notoriously inaccurate in regards to staging the primary tumor and its depth of invasion in older studies [16]. Paik et al evaluated the use of CT scans to determine clinical staging and depth of tumor invasion; the overall accuracy of CT was 54.9% with a 39% rate of under-staging and 6.1% rate of over-staging [17]. CT scan is severely limited in its ability to detect microscopic invasion of perivesical fat and micrometastatic disease in lymph nodes [18]. There are well-described limitations of CT to determine the T stage of bladder cancer, especially in regards to variability of radiologic interpretation [17, 19]. Despite the advances in modern imaging techniques and the increasing ability to stage bladder cancer non-invasively, we have found that the performance of a thorough EUA still independently improves our ability to determine local tumor stage.
In the current series we demonstrate a supplemental benefit of combining EUA and imaging to determine the most accurate staging information. The specificity, positive predictive value, and negative predictive value are all improved with the combination of the EUA and imaging, rather than each modality alone (Table 3). We also note a decrease in under-staging and over-staging with the combination of EUA and imaging compared to each modality alone (Table 4). There is a clear benefit of including EUA as part of the staging evaluation in bladder cancer. Grossman and colleagues demonstrated a survival benefit with neoadjuvant chemotherapy in patients with locally advanced bladder cancer, especially those whose pathologic tumor stage was downgraded at time of cystectomy. Those patients receiving neoadjuvant chemotherapy had median survival of 77 months compared to 46 months for cystectomy alone [21]. Accurate determination of clinical stage may help select patients most likely to benefit from neoadjuvant chemotherapy. Culp et al found that patients with muscle-invasive bladder cancer lacking certain high-risk features (lymphovascular invasion (LVI), hydronephrosis, 3-D mass on EUA, cT4a, micropapillary histology, or neuroendocrine tumor type) showed greater than 80% 5-year disease specific survival without neoadjuvant chemotherapy [22].
Of the factors we reviewed, only EUA and imaging were found to be predictive of final pathologic stage. Ploeg et al found that bimanual exam (EUA) was less accurate in males compared to females (58.1% vs 64.9%) and more likely to under-stage cancers in men; however, this finding did not hold true for our population [5]. In our series, neither BMI nor gender moderated the ability of EUA to predict pathologic stage.
The current study provides results that are salient to clinical practice and management of bladder cancer. The goal of this study was to demonstrate the importance of utilizing the information from a thorough EUA in the clinical staging of bladder cancer, and that it should not be ignored as part of the preoperative staging evaluation of muscle invasive bladder cancer. We understand that there are certain limitations and like any staging tool, EUA is not perfect. However, when included in clinical staging, we did find an additive benefit. Despite this we must recognize the limitations of our study: it is a retrospective single-institution study limited to the exams of two bladder cancer focused urologists. Despite EUA being a subjective evaluation, we believe we were able to control for this by using only EUAs that were done by one of two experienced surgeons who routinely perform and document EUAs as part of every evaluation, thereby reducing inter-observer variability in our staging. Additionally, we cannot reliably distinguish between clinical staging error and pathologic downstaging in the group of patients who had clinical T3 disease and received neoadjuvant chemotherapy and did not have pT3 disease on final pathology. Future research may focus on the utility of repeat EUA in the NAC population.
While recognizing that the presence LVI, prostatic stromal invasion, variant histology are all risk factors for more advanced disease, the focus of our analysis was to characterize the impact of EUA on clinical staging of bladder cancer. In our cohort there were no patients with prostate stromal invasion. In our practice we utilize high risk pathologic characteristics, such as LVI, in order to risk stratify our patients to determine who may benefit most from neoadjuvant chemotherapy. Future studies would include these pathologic characteristics in the analysis to assess their importance in predicting extra-vesical disease both independent and in combination with EUA. While we do not address the presence of hydronephrosis on imaging independently in our analysis, it is indicative of cT3 disease and is one of the imaging factors characterizing cT3 disease. Another limitation of our study is that multiple radiologists reviewed the imaging findings over a period from 1994–2013 and the time point at which imaging was obtained was not standardized to a particular time point relative to TURBT or cystectomy for all of our patients. The quality of imaging has changed over this time period as well. Future studies may benefit from a single experienced genitourinary radiologist interpreting all imaging independently and standardizing the time point at which the imaging was obtained relative to TURBT and cystectomy. In this study we only focus on local staging of the primary tumor. It may be of added benefit in the future to determine our ability to predict lymph node positive disease since that clearly has an impact on the prognosis of bladder cancer.
CONCLUSION
The clinical staging of bladder cancer is critical because of its impact on management. Treatment options and outcomes are directly linked to clinical staging. It is crucial for urologists to use the appropriate staging tools to provide the best care for patients with bladder cancer. EUA is a valuable staging tool that is readily available to all physicians and one that should not be omitted as part of the staging evaluation of bladder cancer.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DISCLOSURES
None.
REFERENCES
1 | American Cancer Society(2012) Cancer Facts and Figures Atlanta: American Cancer Society2012 |
2 | Chang SS, Hassan JM, Cookson MS, Wells N, Smith JAJr(2003) Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stageJournal of Urology170: 410851087 |
3 | Malkowicz SB, van Poppel H, Mickisch G(2007) Muscle-invasive urothelial carcinoma of the bladderUrology69: 1 Suppl316 |
4 | Stein JP, Lieskovsky G, Cote R(2001) Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patientsJ Clin Oncol19: 3666675 |
5 | Ploeg M, Kiemeney LA, Smits GA(2012) Discrepancy between clinical staging through bimanual palpation and pathological staging after cystectomyUrol Oncol30: 3247251 |
6 | Shariat SF, Palapattu GS, Karakiewicz PI(2007) Discrepancy between clinical and pathologic stage: Impact on prognosis after radical cystectomyEur Urol51: 1137151 |
7 | Ficarra V, Dalpiaz O, Alrabi N, Novara G, Galfano A, Artibani W(2005) Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinomaBJU Int95: 6786790 |
8 | Levy D, Grossman HB(1996) Staging and prognosis of T3b bladder cancerSemin Urol Oncol14: 25661 |
9 | MacVicar AD(2000) Bladder Cancer StagingBJU Int86: Suppl 1111122 |
10 | Jewett HJ, Strong GH(1946) Infiltrating carcinoma of the bladder; relation of depth of penetration of the bladder wall to incidence of local extension and metastasesJournal of Urology55: 366372 |
11 | Jewett H(1973) Cancer of the bladder; diagnosis and stagingCancer32: 510721074 |
12 | Wijkström H, Norming U, Lagerkvist M, Nilsson B, Näslund I, Wiklund P(1998) Evaluation of clinical staging before cystectomy in transitional cell bladder carcinoma: A long-term follow-up of 276 consecutive patientsBJU81: 5686691 |
13 | Fosså SD, Ous S, Berner A(1991) Clinical significance of the “palpable mass” in patients with muscle-infiltrating bladder cancer undergoing cystectomy after pre-operative radiotherapyBJU67: 15460 |
14 | Mehrsai A, Mansoori D, Taheri Mahmoudi M, Sina A, Seraji A, Pourmand GH(2004) A comparison between clinical and pathologic staging in patients with bladder cancerUrol J1: 28589 |
15 | McLaughlin S, Shephard J, Wallen E, Maygarden S, Carson CC, Pruthi RS(2007) Comparison of the clinical and pathologic staging in patients undergoing radical cystectomy for bladder cancerInt Braz J Urol33: 12532 |
16 | Kundra V, Silverman PMImaging in oncology from the University of Texas M. D. Anderson Cancer Center(2003) Imaging in the diagnosis, staging, and follow-up of cancer of the urinary bladderAJR Am J Roentgenol180: 410451054 |
17 | Paik ML, Scolieri MJ, Brown SL, Spirnak JP, Resnick MI(2000) Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomyJ Urol163: 616931696 |
18 | Setty BN, Holalkere NS, Sahani DV, Uppot RN, Harisinghani M, Blake MA(2007) State-of-the- art cross-sectional imaging in bladder cancerCurr Probl Diagn Radiol36: 28396 |
19 | Tritschler S, Mosier C, Tilki D, Buchner A, Stief C, Graser A(2012) Interobserver variability limits exact preoperative staging by computed tomography in bladder cancerUrology79: 613171321 |
20 | Tekes A, Kamel I, Imam K(2005) Dynamic MRI of bladder cancer: Evaluation of staging accuracyAJR Am J Roentgenol184: 1121127 |
21 | Grossman HB, Natele RB, Tangen CM(2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancerNew England Journal of Medicine348: 9859866 |
22 | Culp SH, Dickstein RJ, Grossman HB(2014) Refining patient selection for neoadjuvant chemotherapy before radical cystectomyJournal of Urology191: 14047 |
Figures and Tables
Fig.1
ROC curves of models to predict pathological fat invasion at radical cystectomy. Blue line depicts the ROC curve for the regression model which dose not include EUA and the red line depicts the ROC curve for the model which includes EUA. AUC improves significantly from 0.648 to 0.676 with inclusion of EUA in the model (p = 0.004).
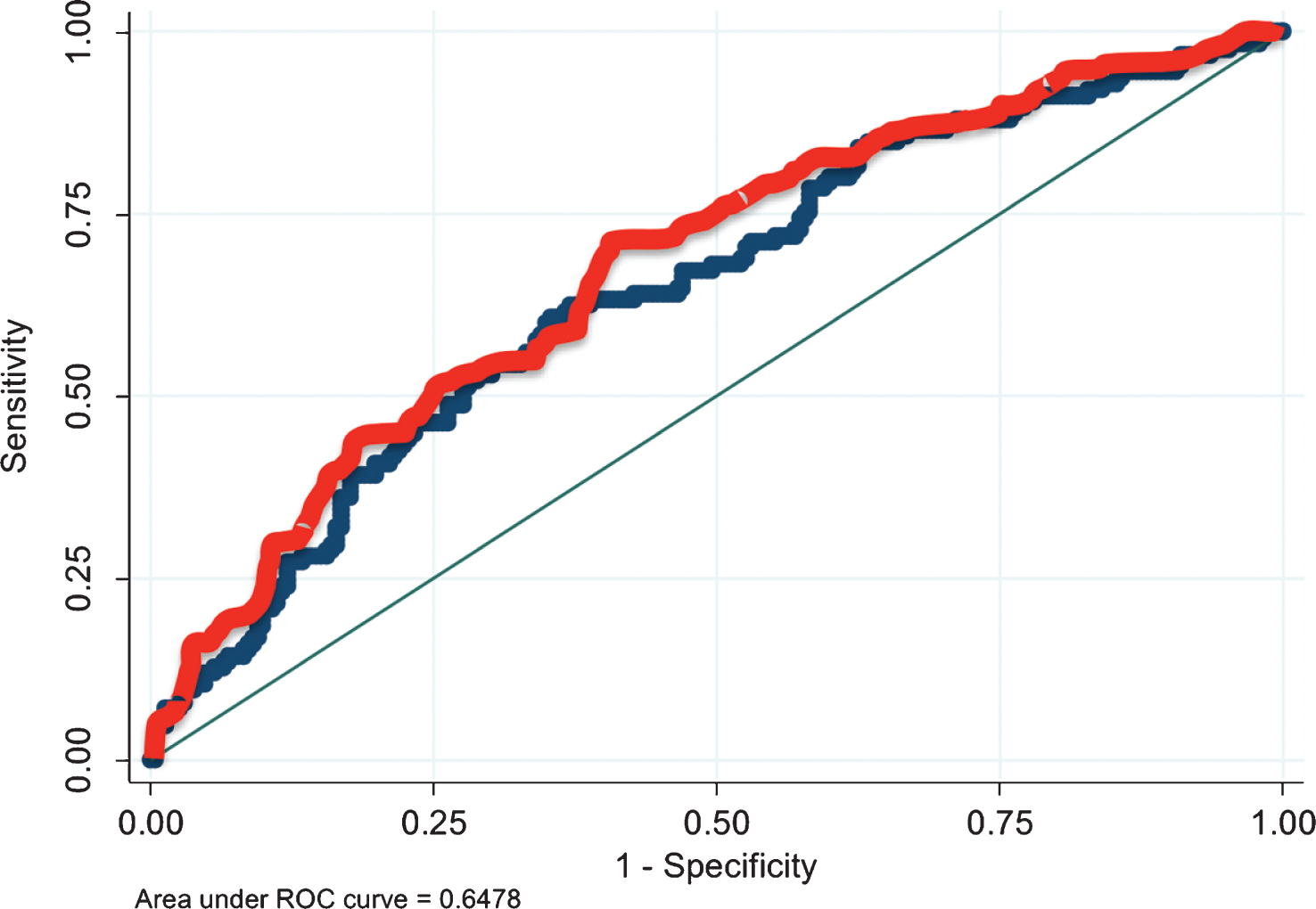
Table 1
Patient characteristics
| N | 414 |
| Age in years, median [IQR] | 68 [62–76] |
| Gender, n (%) | |
| Male | 332 (80.2%) |
| Female | 82 (19.8%) |
| Race, n (%) | |
| White | 369 (89.1%) |
| Black | 19 (4.6%) |
| Latino | 23 (5.6%) |
| Asian | 3 (.7%) |
| BMI, median [IQR] | 27.3 [24.3–31.5] |
| Clinical T Stage, n (%) | |
| cTa/Tis/T1 | 94 (22.7%) |
| cT2 | 123 (29.7%) |
| ≥cT3 | 197 (47.6%) |
| Pathologic T Stage, n (%) | |
| p0 | 58 (14.0%) |
| pTa/Tis/T1 | 126 (30.4%) |
| pT2 | 71 (17.1%) |
| pT3 | 119 (28.7%) |
| pT4 | 40 (9.7%) |
| Neoadjuvant chemotherapy, n (%) | 146 (35.3%) |
| EUA suggestive of extravesical disease, n (%) | 128 (30.9%) |
| Imaging suggestive of extravesical disease, n (%) | 119 (28.7%) |
Table 2
Univariate and Multivariate analysis of factors predicting pathological fat invasion at radical cystectomy
|
Univariate Odds Ratio [95% CI] | p-value |
Multivariate Odds Ratio [95% CI] | p-value | |
| Age | 1.01 [1.00–1.04] | 0.119 | 1.02 [0.99–1.04] | 0.19 |
| Race | ||||
| White | 1.00 [Referent] | – | Referent | |
| Black | 1.32 [0.52–3.34] | 0.56 | 1.15 [0.37–3.59] | 0.81 |
| Hispanic/Latino | 0.79 [0.32–1.98] | 0.62 | 0.91 [0.34–2.42] | 0.85 |
| Asian | 0.91 [0.82–10.1] | 0.94 | 1.55 [0.12–20.5] | 0.74 |
| Gender | ||||
| Male | 1.00 [Referent] | – | Referent | |
| Female | 1.56 [0.95–2.55] | 0.08 | 1.49 [0.85–2.61] | 0.17 |
| BMI | 0.76 [0.55–1.06] | 0.11 | 0.85 [0.59–1.22] | 0.37 |
| Year of surgery | 0.99 [0.99–1.00] | 0.57 | 1.00 [0.99–1.00] | 0.53 |
| Imaging type (CT v. MRI) | 0.47 [0.15–1.44] | 0.19 | 0.38 [0.11–1.31] | 0.13 |
| Neoadjuvant Chemo | 0.96 [0.63–1.47] | 0.86 | 0.77 [0.46–1.28] | 0.31 |
| EUA suggestive of T3 | 2.12 [1.38–3.25] | 0.001 | 2.22 [1.34–3.69] | 0.002 |
| Imaging suggestive of T3 | 2.20 [1.42–3.41] | <0.001 | 2.18 [1.33–3.58] | 0.002 |
Table 3
Statistical analysis of the accuracy of EUA and imaging in predicting final pathological stage
| EUA alone | |
| Sensitivity | 43.40% |
| Specificity | 75.30% |
| Positive Predictive Value | 52.30% |
| Negative Predictive Value | 68.10% |
| Imaging alone | |
| Sensitivity | 40.10% |
| Specificity | 78.80% |
| Positive Predictive Value | 54.60% |
| Negative Predictive Value | 68.10% |
| EUA + Imaging | |
| Sensitivity | 35.30% |
| Specificity | 89.20% |
| Positive Predictive Value | 61.20% |
| Negative Predictive Value | 74.10% |
| EUA or Imaging (either/or) | |
| Sensitivity | 65.40% |
| Specificity | 61.60% |
| Positive Predictive Value | 51.50% |
| Negative Predictive Value | 74.10% |
Table 4
Under-staging and Over-staging
| EUA alone | Imaging alone | EUA + Imaging | EUA or Imaging (either/or) | |
| Correctly Staged | 261/414 (63.0%) | 266/414 (64.3%) | 187/261 (71.6%) | 261/414 (63.0%) |
| Understaged | 90/414 (21.7%) | 94/414 (22.7%) | 55/261 (21.1%) | 55/414 (13.3%) |
| Overstaged | 63/414 (15.2%) | 54/414 (13.0%) | 19/261 (7.3%) | 98/414 (23.7%) |




