Do Orthotopic Ileal Diversions Induce Immunological Changes in Retained Urethral Tissue?
Abstract
Background:
A second primary tumors of the urethra (urethral recurrence) after radical cystectomy has been reported to be more infrequent in patients with ileal orthotopic (neobladder) compared to incontinent diversions.
Objective:
To investigate whether an altered immunogenic environment of urethral tissue is induced by urethro-ileal anastomosis.
Methods:
Between 10/2008 and 12/2009 urethral biopsies of 19 patients (9 neobladder patients, 10 control patients without urethra-ileal anastomosis) were evaluated by conventional histopathological examination and immunohistochemistry for T- (CD3/CD5, CD4, CD8) and B-cell markers (CD20/22, CD79a, CD138). After semi-quantitative assessment, relative cell fractions (B vs. T cells) and subclasses (T4-helper vs. T8-killer cells vs. B cell clones, plasma cells) in neobladder vs. control patients were studied. Unpaired t-test was used for statistical analysis.
Results:
Of 19 included patients, 16 were eligible for analysis (7x neobladder, 9x controls). All neobladder patients had undergone cystectomy for UBC. Comparing relative fractions of cells positive for T- and B-cell markers in NB and CO patients, no statistical differences were observed. In 4/7 neobladder patients relative fraction of CD79a positive B-cells was higher than relative fraction of CD3/CD5 positive T-cells (B/T-ratio 1.3). B cells were predominantly CD138 positive plasma cells (5/7 NB patients). Relative B-cell fraction was lower than T-cell fraction in 7/9 control patients (B/T-ratio 0.6). Neither CD 138 positive plasma cells nor CD22 positive B cell clones were predominant. T-helper and CD8 positive T-killer cells were equally distributed in both neobladder (CD4/CD8-ratio: 2.1) and control patients (CD4/CD8-ratio: 1.9).
Conclusions:
Comparing neobadder and control patients the distribution of B- and T-cells was statistically not different. However, a trend towards an increased presence of B-cells in urethral tissues of NB patients that could become relevant in a larger study might be suggestive for an immunological response induced by connecting urethral and ileal tissue.
INTRODUCTION
In the recent literature urethral tumor recurrences/second urethral primaries following radical cystectomy for muscle invasive bladder cancer have been reported with a frequency of 3–6% [1–4]. Interestingly, several retrospective analyses found a lower frequency of second primary tumors of the urethra in patients with orthotopic ileal neobladder compared to patients with non-orthotopic diversions [1, 3, 5–8]. The most simple explanation for these findings might be that patients specifically selected for orthotopic neobladders present with favorable disease characteristics (tumor stage, tumor grade). This hypothesis is supported, for example, by the findings of Huguet et al. who described that favorable disease characteristics were responsible for a lower rate of second primary tumors of the urethra in neobladder patiens [2]. However, in other analyses, including a recent retrospective analysis of Boorjian et al. the presence of orthotopic urinary diversion was an independent prognostic factor for second primary tumors of the urethra after correction for multiple disease and patient characteristics. These authors generally refer to the hypothesis of Freeman et al., who proposed an anti-tumorigenic “ileal factor” responsible for the lower rate of second primary tumors of the urethra in neobladder patients compared to patients with non-orthotopic diversion and retained urethra [9].
Urothelial carcinoma is known to be responsive to immune based treatments especially intravesical BCG (bacillus Calmette-Guerin) instillation therapy for non muscle invasive bladder cancer. While the mechanism of BCG-triggered immune response has not been fully elucidated yet, a T-cell mediated inflammation recruiting granulocytes (polymorphonuclear neutrophils) has been suggested [10, 11].
In this context, we investigated whether an immune response of urethral tissue potentially induced by the contact of the urethra with ileal tissue might be present as a morphological substrate of an “ileal factor”. To test this hypothesis, we compared lymphocyte infiltrates in urethral biopsies of patients with orthotopic diversion to patients with an intact lower urinary tract. If a difference between both groups indicating an immune response in neobladder patients would have been observed further investigation focusing on potential antineoplastic effects of this response would be justified.
MATERIAL AND METHODS
Sample collection
Between 11/2008 and 11/2009, urethral biopsies of patients who had previously undergone radical cystectomy and orthotopic ileal diversion (neobladder patients) and of patients without urinary diversion (control patients) were taken and analyzed prospectively. In neobladder patients, urethral biopsy was performed during the regular follow up for urothelial bladder cancer. Cystocopy and biopsy in neobladder patients was performed specifically for this study. In control patients, urethral biopsy was performed simultaneously during endoscopic treatment of benign prostate hyperplasia and/or non-muscle invasive urothelial bladder cancer. Urethral biopsy was taken specifically for this study.
The neobladder patients were taken to OR and underwent cysto/biopsy specifically for this study. The controls were in the OR for other reasons, but the urethral biopsy was taken specifically for this study.
Cold-cup biopsies were taken proximally of the external sphincter muscle using an endoscopic forceps. Active urinary Infection was excluded by microbiological examination in all of the participants.
Informed consent was obtained from all participants before biopsies were taken. The study was approved by the local review board and was in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Histopathological assessment and immunohistochemistry
After routine fixation in 4% buffered formalin and processing to paraffin blocks, serial sections were cut at two levels for hematoxylin/eosin (HE) and immunohistochemical sections. Based on HE staining, biopsy specimens were examined for benign and malignant urethral abnormalities as well as chronic and acute inflammation. Biopsy specimens showing pathological alterations other than inflammatory reactions were excluded from further analysis. Inflammatory reaction was graded using a 4-point Likert scale (0 – no inflammation, 1 – mild, 2 – moderate, 3 – severe inflammation).
For immunohistochemistry, inflammatory cells were detected using antibodies against CD20 (Thermo Scientific), CD22 (ITK) and CD79 (DAKO) to detect B-cells, CD138 (Klinipath) to detect plasmacells, CD 3 (Thermo Scientific) and CD 5 (Novocastra) to detect T-cells, and CD4 (Thermo Scientific) and CD8 (DAKO) to detect T-helper and T-killer cells, respectively. Antibodies were visualized by appropriate biotin-labelled secondary antibodies and avidin peroxidase, (Poly-HRP-GAM/R/R IgG – Immunologic). All antibodies that we used have been validated for routine histopathological use. Formalin fixed lymphnode tissue served as positive controls for all B- and T-cell markers. After immunostaining, semiquantitative analysis of lymphocyte infiltration was performed. First, we evaluated the relative fractions of CD79a positive B and CD3/5 positive T cells (CD79a positive + CD3/5 positive = 100% ). Subsequently, we quantified the relative fractions B cell subclasses (CD 20/22: B cell clones, CD138: plasma cells) and T cell subclasses (CD4: T4-helper cells, CD8: T8-killer cells).
Statistical analysis
Statistical analysis was performed using Graph Pad Prism Version 5.01 (La Jolla, CA). Descriptive statistics with no baseline assumption were used. Calculated median and mean values were amended by inter-quartile ranges (IQR) and the standard error of mean (SEM). Unpaired t-test was used to compare observations in neobladder and control patients. A two-sided p-value of <0.05 was considered statisticalsignificant.
RESULTS
Patients characteristics
Between 11/2008 and 11/2009, 19 patients were included in the study. Of these, 7 neobladder patients and 9 controls were eligible for analysis. Three patients (neobladder: 1×IHC not available for review, 1×only intestinal epithelial in biopsy, control: 1×presence of urethral Cis in biopsy) were excluded from analysis.
All of the 7 neobladder patients had undergone radical cystectomy for urothelial bladder cancer and had no clinical signs of local or distant recurrence at the time urethral biopsy was performed. Information on the cancer history of the individual neobladder patients is provided in Table 1.
Urethral biopsy in the control patients was performed during endoscopic treatment of primary or recurrent non-muscle invasive urothelial bladder cancer in 5 patients and of benign prostatic hyperplasia in 4 patients. In none of the NMIBC patients, spread of urothelial cancer to the urethra was evident. Four of the control patients had a history of BCG treatment, in none of these patients BCG has been applied within a time frame of two years prior to study inclusion. Two of these 4 patients had additional treatment with mitomycin. In both patients, this treatment was finished more than 6 months before study inclusion.
Further baseline data of included patients are detailed in Table 2.
Immunohistochemical staging of B and T cells
In 4 of 7 neobladder patients CD79a positive B-cells were more frequently observed than CD3/CD5 positive T-cells resulting in a B/T ratio of 1.3 In control patients, CD3/CD5 positive T-cells were more frequently observed (1/9 control patients; B/T ratio 0.6). Comparing B/T ratio of neobladder patients to control patients, the difference was statistically not significant (p = 0.166) (Fig. 1).
Further investigation showed that in 5 of 7 neobladder patients CD138 positive plasma cells were the predominant B-cell subclass. In control patients, neither CD138 positive plasma cells nor CD22 positive B-cell clones were predominant (Fig. 2).
Concerning subdivision of T cell classes, CD4 positive T-helper and CD8 positive T-killer cells were equally distributed in urethral biopsies of both neobladder (CD4/CD8 ratio: 2.1) and control patients (CD4/CD8 ratio: 1.9) (p = 0.417). CD4 positive T-helper cells were the predominant T-cell class in both neobladder and control patients (Fig. 3)
DISCUSSION
A lower incidence of second primary tumors of the urethra in patients who had undergone orthotopic urinary diversion was first reported in 1996 by Freeman and coworkers [9]. In their retrospective analysis only 5/174 patients (2.9% ) with an orthotopic Kock pouch presented with second primary tumors of the urethra compared to 29/262 patients (11.1% ) with cutaneous urinary diversion. This difference has been confirmed by further retrospective analysis [1–3, 6, 8, 12]. In search of an explanation of these findings, most authors refer to Freeman’s hypothesis of some “ileal factor” prevailing cancer progression in urethral tissue. Apart from physiological, genetic and biochemical features of ileal tissues, immunogenicity has been suggested a main contributing factor in this hypothesis [9]. This hypothesis is supported by histological findings of a chronic inflammation of the ileal wall after urinary diversions and/or cystoplasties both in the treatment of benign diseases and bladder cancer have been performed [13, 14]. However, further systematic investigation on this topic has never been performed.
In this context, our pilot study was set up in order to obtain a first signal, whether a lower recurrence rate in neobladder patients might be related to immunogenic changes of urethral tissue. According to this hypothesis, we expected to observe a distinct change in distribution of immune cells in urethral tissue as it has been described, for example, in the bladder wall after BCG treatment [15–19]. However, differences in the distribution of B- and T-cells comparing neobladder and control patients were statistically not significant. Though in neobladder patients CD138-positive lymphocytes were more frequently while predominantly CD79a-positive T-lymphocytes were observed in urethral tissue of control patients. If despite the limited validity this indeed points toward an immunologic response induced in urethral tissue by contact to ileal tissue potentially bearing a “antineoplastic effect”, this would be a rather unexpected finding as anticancer immunity in general has traditionally been attributed to the activity of different types of T and not B-cells [20, 21].
The role of B cell in general tumor immunology seems to be ambiguous. While regulatory B cells have been reported to favor tumor progression by inhibiting T cell mediated cytotoxicity, antigen presenting B cells (APBCs) are a prerequisite for antigen dependent cytotoxicity counteracting tumor growth and progression [21]. Data on the impact of plasma cell infiltration of malignant cancers is contradictory. For example, in breast cancer patients, plasma cell infiltration was correlated with a poorer outcome [22]. In contrast, in colorectal cancer, plasma cell infiltration has been reported to be a favorable prognostic factor it in [23]. However, the question by which mechanism tumorigenicity is promoted in either the one or the other direction by plasma cells has not been answered yet.
Specifically in bladder cancer patients, current and recent investigations on immunological interactions has been focusing on BCG induced immune response in the treatment of patients suffering from non muscle invasive bladder cancer. Although details of the complex BCG-dependent mechanisms are still awaiting final elucidation, evidence is condensing that BCG response is mainly dependent on T cell mediated immunity [24]. As morphological substrate of this assumption, several groups described a boosted frequency of T cells in the bladder mucosa upon intravesical treatment with BCG [15–19]. T cells infiltrating the bladder wall could mainly be classified as CD4 positive T helper cells [17, 19]. In our study, the presence of T cells was lower in neobladder compared to control patients. In addition, a difference of the CD4/CD8 ratio was not found comparing neobladder and control patients. Thus, our data suggest that a similar T cell dependent immune reaction as seen in BCG treatment, is unlikely to be a candidate for potential immunological mechanism protective against second primary tumors of the urethra.
Concerning the impact of lymphocyte infiltration on recurrence and progession in urothelial cancer data are inconsistent and focusing on T- and NK (natural killer)-cells. While some groups described peritumoral lymphocyte infiltration as a poor prognostic factor for recurrence-free and/or progression-free survival, in other studies peritumoral lymphocyte infiltration seems to be a beneficial prognosticator [25–28]. However, the role of B-cells for tumorigenicity in bladder cancer is yet unclear.
Strengths and flaws
Without any doubt, the main flaw of this trial is the limited patient number which permits further statistical analysis. However, as to our knowledge the question of immunological changes in the urethra of neobladder patients has not been addressed, it seemed reasonable to perform a hypotheses-generating pilot study. These hypotheses may help to perform further targeted analysis of potential relevant immunological changes and to calculate appropriate patient numbers for a reasonable clinical investigative trial. Another flaw may be the selection of the control group. For example, patients having an incontinent urinary diversion might have been a more appropriate control. However, by choosing patients with an unaltered lower urinary tract, we were able to focus on ‘the ileal factor’ itself without considering additional confounder as the lack of urinary flow in patients with incontinent urinary diversions.
CONCLUSIONS
To our knowledge, this is the first report dealing with the impact of the urethra-ileal anastomosis on immunological features of the urethral tissues. In this pilot study, we did not observe any differences comparing B- and T-cell fractions when comparing patients who had undergone radical cystectomy including orthotopic ileal urinary diversion to patients with an intact lower urinary tract. However, the presence of preferably B cells being classified predominantly as plasma cells in urothelial tissues of patients with orthotopic diversion may nevertheless point towards an immunogenic effect induced by contact of ileal to urethral tissue. To clarify this finding and the hypothesis that this immunogenic response might be “tumor-protective”, further investigations (including a larger patient cohort preferably including patients who had undergone non-orthotopic diversion and in which the urethra is still in place) are required.
CONFLICTS OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
This work was supported by the European Urological Scholarship Program of the European Association of Urology (EUSP/CV-06-2013) for G.N.
REFERENCES
1 | Boorjian SA, Kim SP, Weight CJ, Cheville JC, Thapa P, Frank I(2011) Risk factors and outcomes of urethral recurrence following radical cystectomyEur Urol60: 612661272 |
2 | Huguet J, Monllau V, Sabate S, Rodriguez-Faba O, Algaba F, Palou J(2008) Diagnosis, risk factors, and outcome of urethral recurrences following radical cystectomy for bladder cancer in 729 male patientsEur Urol53: 4785792discussion 92–3 |
3 | Stein JP, Clark P, Miranda G, Cai J, Groshen S, Skinner DG(2005) Urethral tumor recurrence following cystectomy and urinary diversion: Clinical and pathological characteristics in 768 male patientsJ Urol173: 411631168 |
4 | Perlis N, Turker P, Bostrom PJ, Kuk C, Mirtti T, Kulkarni G(2013) Upper urinary tract and urethral recurrences following radical cystectomy: Review of risk factors and outcomes between centres with different follow-up protocolsWorld J Urol31: 1161167 |
5 | Nieder AM, Sved PD, Gomez P, Kim SS, Manoharan M, Soloway MS(2004) Urethral recurrence after cystoprostatectomy: Implications for urinary diversion and monitoringUrology64: 5950954 |
6 | Yossepowitch O, Dalbagni G, Golijanin D, Donat SM, Bochner BH, Herr HW(2003) Orthotopic urinary diversion after cystectomy for bladder cancer: Implications for cancer control and patterns of disease recurrenceJ Urol169: 1177181 |
7 | Varol C, Thalmann GN, Burkhard FC, Studer UE(2004) Treatment of urethral recurrence following radical cystectomy and ileal bladder substitutionJ Urol172: 3937942 |
8 | Hassan JM, Cookson MS, Smith JAJr, Chang SS(2004) Urethral recurrence in patients following orthotopic urinary diversionJ Urol172: 4 Pt 113381341 |
9 | Freeman JA, Tarter TA, Esrig D, Stein JP, Elmajian DA, Chen SC(1996) Urethral recurrence in patients with orthotopic ileal neobladdersJ Urol156: 516151619 |
10 | Redelman-Sidi G, Glickman MS, Bochner BH(2014) The mechanism of action of BCG therapy for bladder cancer-a current perspectiveNat Rev Urol11: 3153162 |
11 | Brincks EL, Risk MC, Griffith TS(2013) PMN and anti-tumor immunity–the case of bladder cancer immunotherapySemin Cancer Biol23: 3183189 |
12 | Taylor JM, Spiess PE, Kassouf W, Munsell MF, Kamat AM, Dinney CP(2010) Management of urethral recurrence after orthotopic urinary diversionBJU Int106: 15661 |
13 | Dellis AE, Demonakou M, Papatsoris AG, Chrisofos M, Bamias A, Deliveliotis C(2008) Insight into long-term histological, proliferative and apoptotic modifications in ileal orthotopic neobladder and conduit mucosaTumori94: 5701705 |
14 | Byard RW, Ahmed S, Phillips GE, Dewan PA(1997) Further observations on histological changes at the ureteroileal junction in ileal conduitsPediatr Surg Int12: 5-6397400 |
15 | Elsasser J, Janssen MW, Becker F, Suttmann H, Schmitt K, Sester U(2013) Antigen-specific CD4 T cells are induced after intravesical BCG-instillation therapy in patients with bladder cancer and show similar cytokine profiles as in active tuberculosisPLoS One8: 9e69892 |
16 | Honda S, Sakamoto Y, Fujime M, Kitagawa R(1997) Immunohistochemical study of tumor-infiltrating lymphocytes before and after intravesical bacillus Calmette-Guerin treatment for superficial bladder cancerInt J Urol4: 16873 |
17 | Peuchmaur M, Benoit G, Vieillefond A, Chevalier A, Lemaigre G, Martin ED(1989) Analysis of mucosal bladder leucocyte subpopulations in patients treated with intravesical Bacillus Calmette-GuerinUrol Res17: 5299303 |
18 | Patard JJ, Muscatelli-Groux B, Saint F, Popov Z, Maille P, Abbou C(1996) Evaluation of local immune response after intravesical bacille Calmette-Guerin treatment for superficial bladder cancerBr J Urol78: 5709714 |
19 | Saint F, Patard JJ, Groux Muscatelli B, Lefrere Belda MA, Gil Diez de Medina S, Abbou CC(2001) Evaluation of cellular tumour rejection mechanisms in the peritumoral bladder wall after bacillus Calmette-Guerin treatmentBJU Int88: 6602610 |
20 | Darcy PK, Neeson P, Yong CS, Kershaw MH(2014) Manipulating immune cells for adoptive immunotherapy of cancerCurr Opin Immunol 27C4652 |
21 | Fremd C, Schuetz F, Sohn C, Beckhove P, Domschke C(2013) B cell-regulated immune responses in tumor models and cancer patientsOncoimmunology2: 7e25443 |
22 | Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC(2012) The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancerBr J Cancer107: 5864873 |
23 | Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG(2012) The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancerBr J Cancer106: 1220102015 |
24 | Kawai K, Miyazaki J, Joraku A, Nishiyama H, Akaza H(2013) Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: Current understanding and perspectives on engineered BCG vaccineCancer Sci104: 12227 |
25 | Krpina K, Babarovic E, Dordevic G, Fuckar Z, Jonjic N(2012) The association between the recurrence of solitary non-muscle invasive bladder cancer and tumor infiltrating lymphocytesCroat Med J53: 6598604 |
26 | Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R(1992) Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancerEur J Cancer29A: 16975 |
27 | Morita T, Tokue A, Minato N(1990) Analysis of natural killer activity and natural killer cell subsets in patients with bladder cancerCancer Immunol Immunother32: 3191194 |
28 | Ikemoto S, Kishimoto T, Wada S, Nishio S, Maekawa M(1990) Clinical studies on cell-mediated immunity in patients with urinary bladder carcinoma: Blastogenic response, interleukin-2 production and interferon-gamma production of lymphocytesBr J Urol65: 4333338 |
Figures and Tables
Fig.1
Comparison of B/T cell ratio in urethral biopsies of neobladder and control patients.
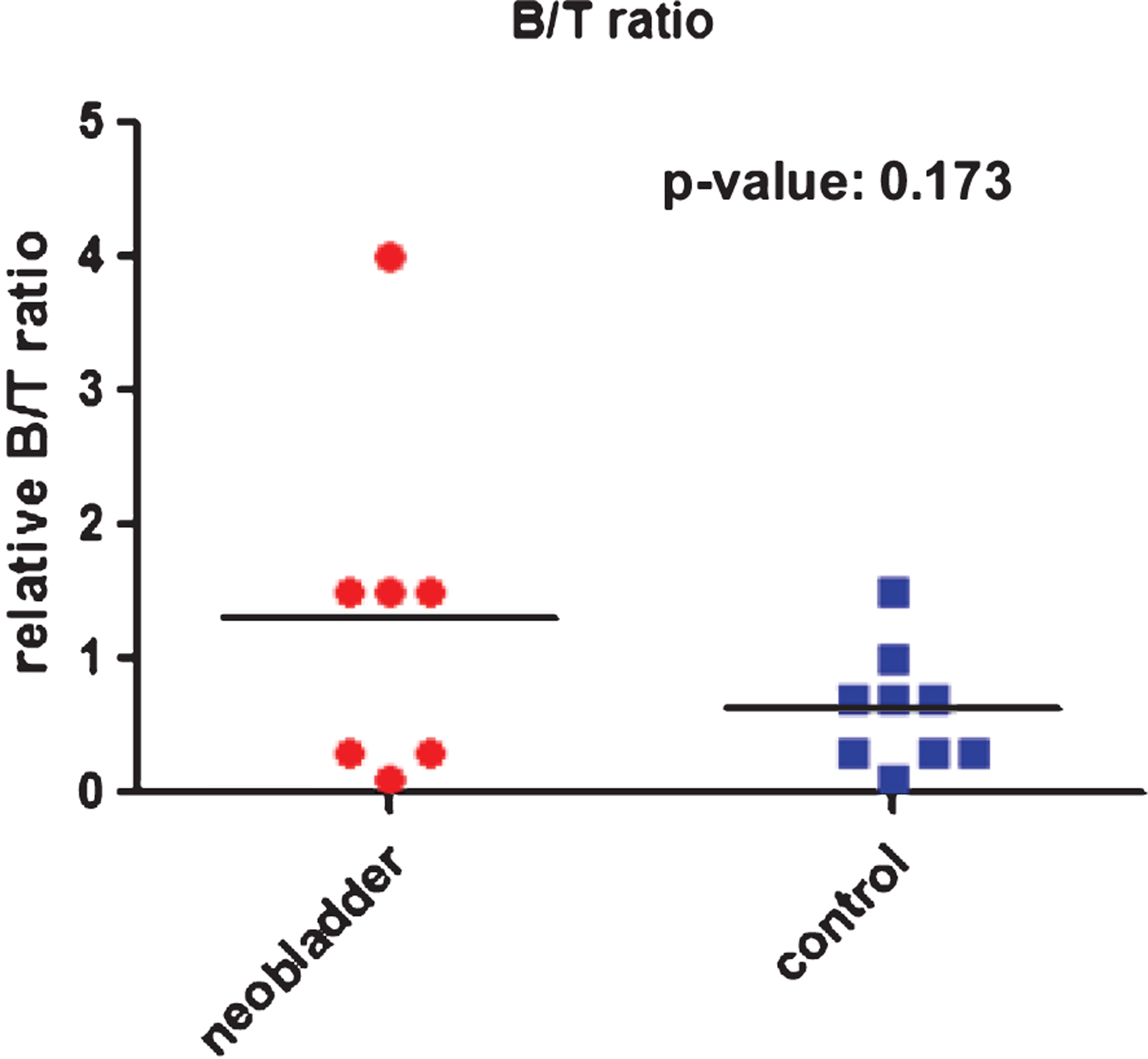
Fig.2
Distribution of B cells in urethral biopsies of neobladder and control patients. A. Relative fractions of CD79a positive lymphocytes. B. relative fractions of CD20/22 positive B cell clones. C. Relative fractions of CD138 positive plasma cells. (IQR – inter-quartile range, SEM – standard error of mean, unpaired t-test was used to compare cell fractions in neobladder and control patients).
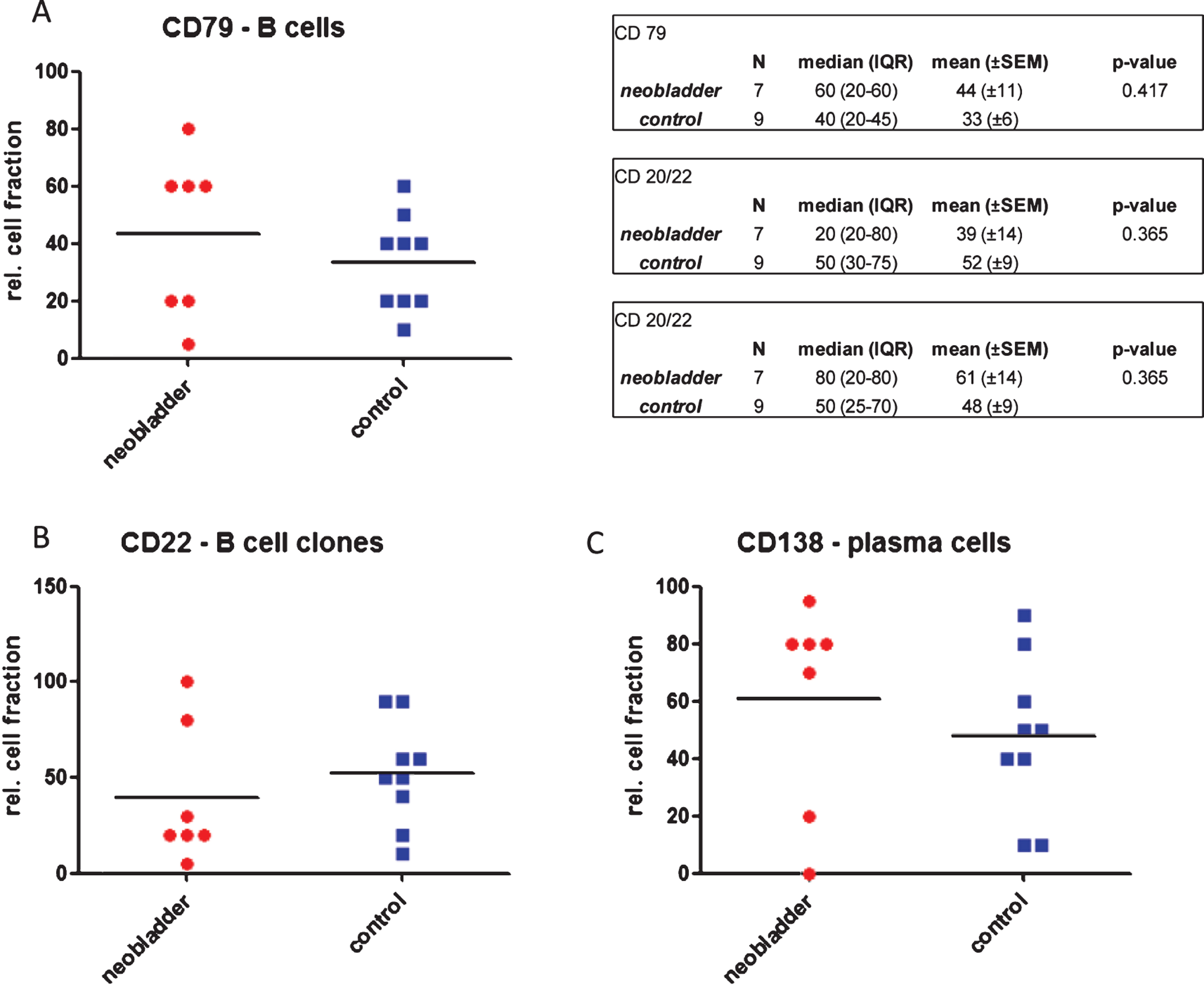
Fig.3
Distribution of T cells in urethral biopsies of neobladder and control patients. A. Relative fractions of CD3/5 positive lymphocytes. B. relative fractions of CD4 positive T-helper cells. C. Relative fractions of CD8 positive T-killer cells. (IQR – inter-quartile range, SEM – standard error of mean, unpaired t-test was used to compare cell fractions in neobladder and control patients).
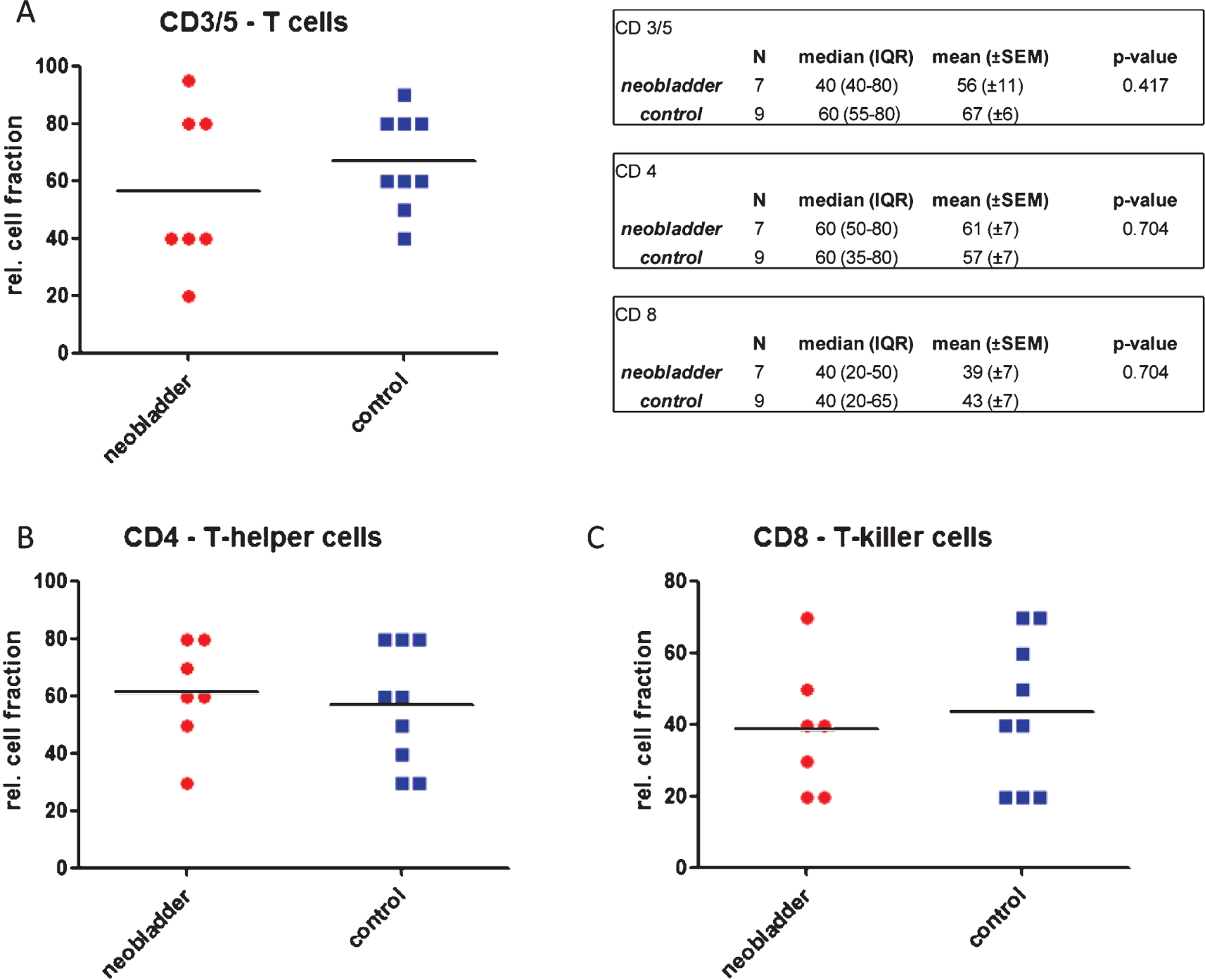
Table 1
Clinical details on neobladder and control patients (NC – neoadjuvant chemotherapy)
| Gender | Intervention | Year of cystectomy | Histopathology | Prior intravesical treatment | |
| patient 1 | male | cystoprostatectomy | 2004 | TCC, ypT0 pN0 (after NC) | – |
| patient 2 | male | cystoprostatectomy | 1995 | TCC, pT1 G3 pN0 | – |
| patient 3 | male | cystoprostatectomy | 1999 | TCC, pT3a pN0 | – |
| patient 4 | male | cystoprostatectomy | 1991 | TCC, pT3b pN0 | – |
| patient 5 | male | prostate-sparing cystectomy | 2005 | TCC, pTa G2 pN0 | gemcitabine, mitomycin, apaziquone |
| patient 6 | female | uterus-sparing cystectomy | 2008 | TCC, ypT3b pN2 (after NC) | – |
| patient 7 | male | cystoprostatectomy | 2005 | TCC, pT1 G3 pN0 | – |
| control 1 | male | laserresection of prostate | 2009 | BPH | – |
| control 2 | female | TUR-B | 2009 | pTa G2a | mitomycine, epirubicine, BCG |
| control 3 | male | TUR-B | 2009 | pT1a G3 | – |
| control 4 | male | TUR-P | 2009 | BPH | – |
| control 5 | male | TUR-P | 2009 | BPH | – |
| control 6 | male | TUR-P | 2009 | BPH | – |
| control 7 | male | TUR-B | 2009 | Cis | BCG |
| control 8 | male | TUR-B | 2009 | Cis | BCG |
| control 9 | male | TUR-B | 2009 | pTa G2b pN0 | mitomycine, BCG |
Table 2
Baseline data of patients included in the study (IQR – inter-quartile range, HE – hematoxylin/eosin staining)
|
Hautmann neobladder (n = 7) |
controls (n = 9) | |
| median age (IQR) | 66 (59–74) | 69 (61–80) |
| Degree of chronic inflammation, | ||
| based on HE | ||
| grade 0 – none | 0 | 1 |
| grade 1 – mild | 4 | 7 |
| grade 2 – moderate | 0 | 0 |
| grade 3 – severe | 3 | 1 |




