Dysexecutive symptoms and carer strain following acquired brain injury: Changes measured before and after holistic neuropsychological rehabilitation
Abstract
BACKGROUND: Following acquired brain injury (ABI), deficits in executive functioning (EF) are common. As a result many brain-injured patients encounter problems in every-day functioning, and their families experience significant strain. Previous research has documented the benefits of cognitive rehabilitation for executive dysfunction, and rehabilitation programmes designed to ameliorate functional problems associated with ABI.
OBJECTIVES: This study primarily aims to evaluate whether a neuropsychological rehabilitation programme reduces reported symptoms of everyday dysexecutive behaviour and carer strain.
METHODS: In this study 66 ABI outpatients attended comprehensive holistic neuropsychological rehabilitation programme. A repeated-measures design was employed to determine the effect of rehabilitation on EF and carer strain, as part of a service evaluation. Outcome measures comprised the dysexecutive questionnaire (DEX/DEX-I) and carer strain index (CSI), applied pre- and post-rehabilitation.
RESULTS: Results indicate rehabilitation benefited clients and carers in 5 of 6 DEX/DEX-I subscales, and 2 of 3 CSI subscales, (p < 0.05). An effect of aetiology on rehabilitation was found on the metacognitive scale of the DEX-I.
CONCLUSIONS: Therefore, this study supports a comprehensive holistic neuropsychological rehabilitation programme as effective in reducing reported symptoms of dysexecutive behaviour and carer strain following ABI.
1Introduction
Cognitive deficits are common following acquired brain injury (ABI), such as traumatic brain injury (TBI) and cerebrovascular accidents (CVA), (Cicerone et al., 2000). Executive function (EF) is an umbrella term for skills encompassing a range of higher-order capacities e.g. planning, organisation, initiation, error correction, monitoring or goal-oriented behaviour, (Lezak, 1982; Evans, 2003). Executive dysfunction is a frequent and disabling consequence of ABI, commonly impairing patients’ abilities to adapt to situations, develop and pursue goals and function independently in everyday life, (Burgess & Simons, 2005). Executive dysfunction has been extensively reported in TBI, (Bennet, Ong & Ponsford, 2005; Hart, Whyte, Kim & Vaccaro, 2005) and CVA patients (Leskela et al., 1999; Sachdevet al., 2004).
Many studies have documented significant strain on families of TBI clients, who generally provide long-term support, assistance and socialization, (Brooks, 1991; Perlesz, Kineslla & Crowne, 2000). Clinically significant anxiety and depression is evident in 25– 30% of relatives, and 60– 80% of carers report some degree of emotional distress (Kreutzer, Gervasio & Camplair, 1994, Ponsford, Olver, Ponsford & Nelms, 2003). Changes seen in individuals with an ABI in emotional control, personality, behaviour and cognitive difficulties,e.g. memory and EF problems, are commonly documented sources of carer strain, (Ponsford et al., 2003). Researchers have reported disruptions in family functioning, manifested by EF deficits after ABI, such as less effective coping, problem-solving, challenging behaviour and communication (Anderson, Parmenter & Mok, 2002). Furthermore, a study by Knight, Devereux & Godfrey (1998) showed levels of stress caused by high prevalence rates of emotional and behavioural change after ABI, was found to be predictive of the extent of carer strain.
Neuropsychological rehabilitation aims to alleviate problems associated with ABI. Research supports the efficacy of intensive holistic neuropsychological rehabilitation approaches, which place emphasis on the integration of emotional, social and cognitive features when planning and executing rehabilitation of ABI clients, (Parente & Stapleton, 1999; Salazar et al., 2000; Klonoff, Lamb & Henderson, 2001; Malec, 2001). Cicerone et al. (2005) concluded post-acute neuropsychological rehabilitation, integrating cognitive and interpersonal interventions, is recommended for moderate-severe TBI. However, Wilson, Gracey, Evans & Bateman (2009) state there is a general consensus that the major focus of neuropsychological rehabilitation is to treat cognitive deficits. Cognitive rehabilitation is a specialist facet of neuropsychological rehabilitation, aiming to reduce cognitive difficulties in attention, memory, perception and EF, using methods to assist restoration of lost functions and introduce compensatory strategies to reduce everyday problems. Chung, Pollock, Campbell, Durward & Hagen (2009), identified three categories of EF interventions: targeting specific components of EF, e.g. problem-solving techniques; compensation for impairment, e.g. goal-management training; use of external mechanisms, e.g. diaries.
The evidence base on the efficacy of cognitive rehabilitation for EF deficits is relatively small compared to other cognitive functions; however, several studies report success using group-based interventions. Ownsworth, McFarland & Young (2000) evaluated the effectiveness of a group support programme on self-awareness and psychosocial functioning in ABI patients. The intervention group showed significant improvement on the Self-Regulation Skills Interview compared to a control. Self-Monitoring Training (SMT) proved significant in reducing the frequency of delusional confabulations through the promotion of self-appraisal (Dayus & van den Broeak, 2000). However, generalizability of SMT techniques to other tasks and daily-life has not been supported. Compensatory strategies for EF problems following ABI have been more widely studied. Group-based Problem-Solving Training (PST) requires patients to break down problems in a slow, controlled, stepwise fashion, adopting a CBT approach (von Cramon, Matthes-von Cramon & Mai, 1991; von Cramon & Matthes-von Cramon, 1992). PST proved significant in improving performance on target assignments, and skills were translated to untrained tasks. However generalisation to everyday tasks was not established. Time Pressure Management (TPM) introduces a set of alternative cognitive strategies allowing ABI patients to compensate for their mental slowness in real-life tasks (Fasotti, Kovacs, Eling & Brouwer, 2000). A randomised control trial (RCT) comparing TPM to concentration training found TPM increased information gain which generalised to other measures of speed and memory function. Levine et al., (2000) executed an RCT assessing the effects of Goal Management Training (GMT), which encourages concepts of goal setting and prioritizing, listing main and sub-goals, and self-evaluation of performance. GMT was efficacious compared to motor skills training, through naturalistic observation and self-reported meal preparation performance. In addition, a group intervention combining PST and GMT was compared to an information booklet and traditional treatment (Miotto, Evans, Souza de Lucia & Scaff, 2009). Only the intervention showed significant improvement on target measures, the Multiple Errands Task and the DEX. A recent systematic review evaluating the effectiveness of cognitive rehabilitation for executive dysfunction following ABI concluded there was insufficient high quality evidence to reach a generalised conclusion (Chung, Pollock, Campbell, Durward & Hagen, 2013).
The National Service Framework for Long-term Conditions recommends the provision of support to ABI carers (Wade, 2005). Ponsford et al. (2003) demonstrated community-based rehabilitation benefited ABI families, as measured by the Family Assessment Device. However responsibility for their TBI relative predicted anxiety and depression in carers. Kreutzer et al. (2009) evaluated the impact of a systemic intervention on family members of ABI clients. Treatment included discussions of ABI sequalae, coping with loss and change, managing stress and intense emotions and taking care of one’s self. Results indicated a greater number of met needs and perceptions of fewer obstacles to receiving services; maintained at 3-month follow-up. However, despite the high level of need, the evidence-base evaluating the effects of specific interventions aimed at alleviating carer strain is extremely limited (Oddy & Herbert, 2003).
Many studies do not control for varying aetiologies of clients during analysis. However, recent research has shown aetiology can influence outcomes of rehabilitation (Fish, Manly, Emslie, Evans & Wilson, 2008). TBI and CVA are the largest subtypes of ABI, however they are associated with differing patterns of pathology, which result in TBI patients typically reporting complaints of memory, attention and executive problems, whilst CVA patients’ deficits differ according to lesion location (Levine et al., 2000). In addition, demographics are divergent; CVA primarily affects clients over 65 years of age, whereas TBI incidence is highest between 15– 24 years (Royal College of Physicians, 2003). Fish et al. (2008) studied the differential effects of a paging system comparing TBI and CVA clients, and found TBI had greater maintenance of pager-related benefits associated with increased EF, whilst CVA performance returned to baseline. Comparisons of demographics showed the CVA group was older, shorter post-injury interval and poorer EF than TBI. Therefore, when selecting an intervention on an individual basis, aetiology should be considered as a potential moderator of other factors known to be important.
In summary, literature confirms common complaints associated with EF impairments and carer strain following ABI. Research supports neuropsychological rehabilitation alleviating ABI problems in general, and a variety of targeted stand-alone interventions ameliorating EF deficits. However the effectiveness of neuropsychological rehabilitation for alleviating EF deficits specifically is insubstantial. Furthermore literature on the benefits of interventions aimed at reducing carer strain is inadequate, and particularly the effects of neuropsychological rehabilitation remain undetermined. Many studies employ standardized outcome measures or evaluate performance on targeted tasks, and often generalization to real-life is poor or undetermined. Self-report questionnaires provide information about a variety of everyday behaviours, and their application as outcome measures has become common. However these questionnaires possess poor construct validity; hence measuring change over time using total scores may mislead conclusions on the effectiveness of rehabilitation. Literature has proved aetiology to be a moderator of the effectiveness of specific interventions following ABI. However this is often overlooked during evaluation of neuropsychological rehabilitation, hence studies are frequently excluded from meta-analyses and the effect of aetiology remains undetermined.
This study primarily aims to evaluate whether a comprehensive, holistic neuropsychological rehabilitation programme reduces reported symptoms of everyday dysexecutive behaviour and carer strain. DEX, DEX-I and CSI will be applied pre- and post-rehabilitation to provide subjective reports of real-life problems. Additionally, Rasch-based subscales will be employed to ensure changes over time are recognised. A secondary aim is to assess whether aetiology interacts with the effects of rehabilitation on DEX, DEX-I and CSI performance. It is hypothesized that clients will show reduced reported symptoms on all questionnaires following neuropsychological rehabilitation. In addition traumatic clients are expected to show increased improvement over time, compared to the non-traumatic group.
2Methods
2.1Participants
Data were available for 66 people who underwent intensive outpatient neuropsychological rehabilitation at the Oliver Zangwill Centre for Neuropsychological Rehabilitation (OZC), UK. See Fig. 1 for points of routine assessment at OZC. Information about 407 patients, referred between 1996 and 2011, was available, however many data sets were incomplete as clients had not returned questionnaires, or had attended a preliminary assessment but not the programme. Reasons for incomplete data sets were not available; hence only complete data sets were used for analysis to ensure included clients had attended the full rehabilitation programme. To compensate for late returns of questionnaires and increase the number of included data sets, analysis was extended to include preliminary assessment and three-month follow-up data. The 66 completed data sets were located from existing databases and individual electronic and paper client files. Each client’s performance on DEX, DEX-I and CSI, pre- and post-rehabilitation was collated and entered into a single database for analysis.
Admission criteria included: aged over 16 years; non-progressive ABI; one year post-injury; multiple interacting difficulties; adequate physical recovery. The sample consisted of 41 males and 25 females, demographic data of the sample are demonstrated in Table 1. The aetiology of brain damage comprised 50 traumatic injuries (closed head injuries [n = 46]; open head injuries [n = 4]), and 16 non-traumatic injuries (cerebrovascular accidents [n = 9]; aneurysms [n = 3]; anoxia [n = 1]; encephalitis [n = 2]; hypoxaemia [n = 1]).
2.2Design
This study was conducted as part of a service evaluation, undertaking analysis of routine data, collected at OZC. Ethical approval was obtained from the University of Nottingham. Analysis was conducted on existing data and all clients included in analysis had received comparable rehabilitation. A repeated-measures design was employed, each patient’s performance was analysed, at two time points: pre- and post-rehabilitation. Scores on the three questionnaires were further broken down into subscales.
2.3Measures
The effects of neuropsychological rehabilitation were evaluated through performance on subjective self-report measures: DEX, DEX-I and CSI, (Wilson et al., 1996; Teasdale et al., 2009).
The use of standardised questionnaires have become widespread allow patients and carers to communicate everyday problems, providing opportunities to identify personally relevant goals for rehabilitation, (Hart & Evans, 2006; Lewis, Babbage & Leathem, 2011). The Dysexecutive Questionnaire was developed as an informant (DEX-I) and self-rating scale (DEX), sampling everyday problems commonly associated with executive dysfunction, (Wilson, Alderman, Burgess, Emslie & Evans, 1996). Initial research suggested DEX/DEX-I covered four executive domains: emotional, motivational, behavioural and cognitive.The Modified Carer Strain Index (CSI) aimed to explore subjective perceptions of the care-taking relationship, and emotional health of carers, (Teasdale et al., 2009). CSI, like DEX/DEX-I, is commonly used as an outcome measure for rehabilitation programmes, as a concurrent indicator of success. However, recent research has proposed DEX, DEX-I and CSI do not measure one psychological construct each, but a series of related psychological constructs. Hence these questionnaires should be analysed as separate subscales to ensure change scores are not misleading during future research establishing the efficacy of rehabilitation, (Simblett & Bateman, 2010). Recent Rasch analyses, using ABI samples, have provided construct validity for DEX, DEX-I and CSI, and proposed subscales for each questionnaire, (Badham, 2010; Greening, 2011; Simblett et al., 2010).
DEX and DEX-I are parallel, standardised scales measuring behavioural aspects of EF. The dysexecutive questionnaire requires participants (DEX) or relatives/carers (DEX-I) to rate 20 items, such as ‘seems lethargic, or unenthusiastic about things’, on a 5-point Likert scale. Total scores range from 0– 80; a high score demonstrates increased dysexecutive behaviour. Responses were categorised into three revised subscales: executive/cognitive functions, behavioural/emotional self-regulatory functions and metacognitive processes, (Badham, 2010; Simblett et al., 2010). Bennett, Ong & Ponsford (2005) presented evidence supporting DEX as a sensitive measure of executive dysfunction following ABI.
CSI is a standardised scale of carer strain, requiring carers to rate 16 items, such as ‘helping takes up a lot of time’, on an 11-point Likert scale. Total scores range from 0– 160; a high score demonstrates increased carer strain. Responses were categorised into three revised subscales: time/practical strain, personal/emotional strain and personal/role strain, (Greening, 2010). Teasdale et al. (2009) proved the CSI to have good internal reliability.
2.4Procedure
Clients who met the criteria for admission attended a 24-week rehabilitation programme. Intensive phase lasted 12-weeks, 4 full days a week; re-integration phase lasted 12-weeks, 2/3 full days a week. The OZC programme, established in 1996, was modelled on Ben-Yishay and Prigatano’s holistic approach (Ben-Yishay & Prigatano, 1990). Ben-Yishay and Prigatano (1990) describe a holistic approach to brain injury rehabilitation as consisting of well-integrated interventions that exceed in scope and kind, the highly specific and circumscribed interventions, usually termed cognitive rehabilitation. A holistic approach considers cognitive, emotional and social consequences interactively, and incorporates engagement, awareness and acceptance into its programme, alongside attention & goal management (A&GM), mood and psychological support. The OZC A&GM group employed PST and GMT techniques. Groups met for one hour, twice a week during the intensive phase; sessions incorporated education, practical tasks, facilitated discussion and homework. The programme also aimed to help carers develop an understanding of the consequences of ABI; individual family consultation was integrated if appropriate and a relatives peer group ran every6-weeks. Therapy team included clinical psychologists, occupational therapists (OT), speech and language therapists (SALT) and a physiotherapist. Full details of the programme can be found in Wilson et al. (2009).
Routine assessment of clients occurred at multiple time points, see Fig. 1. DEX, DEX-I and CSI were administered by therapists, or completed independently by clients for return to OZC. Pre-rehabilitation data were provided from ‘preliminary assessment’ or ‘detailed assessment’; post-rehabilitation data were supplied using ‘discharge’ or ‘three-month follow-up’. The time between pre-rehabilitation data and programme entry varied, but would usually be no more than 3 months.
2.5Statistical analysis
Client performance of individual items on each questionnaire was collated for analysis. Scores of items proposed to measure the same psychological construct were summed to form total scores of Rasch-based subscales in each questionnaire. The skew of the sample was determined to assess normality of distribution, followed by the employment of parametric analyses. A series of repeated-measures t-tests established the effect of rehabilitation, comparing pre-and post-rehabilitation scores of each subscale of DEX, DEX-I and CSI. A series of 2×2 mixed-model ANOVAs were employed to establish the interaction between one repeated-measures independent variable with two levels (Effect of rehabilitation: Pre-rehabilitation and Post-rehabilitation) and one between-group independent variable with two groups (Aetiology: traumatic and non-traumatic), on each subscale. Post-hoc analyses using multiple t-tests were performed to determine the nature of interactions between independent variables. Statistical analyses were performed using IBM SPSS Statistics (version 19) using an alpha level set at 0.05 for all analyses. Family-wise errors were considered using the Bonferroni correction.
3Results
Normality of the sample was assessed by calculating the skew of the distribution of performance on the 9 subscales, at both time points. The skew proved distribution was normal for 16 measures, 2 measures demonstrated slightly positively skewed distribution, hence the vast majority of measures had normal distribution, and parametric analyses were employed.
3.1Effectiveness of neuropsychological rehabilitation
3.1.1Dysexecutive questionnaire
Results obtained by clients’ performance on subscales of DEX and DEX-I, pre- and post-rehabilitation are displayed in Table 2. Scores on all post-rehabilitation DEX subscales showed a significantly lower number of reported symptoms of dysexecutive behaviour than scores on their corresponding pre-rehabilitation subscales, hence showing an effect of rehabilitation. Scores on the post-rehabilitation DEX-I behavioural/emotional and executive function subscales also showed a significantly lower number of reported symptoms of dysexecutive behaviour, compared to pre-rehabilitation performance. No significant effect of rehabilitation was established on the metacognitive subscale of DEX-I.
3.1.2Carer strain index
Results obtained by clients’ performance on subscales of CSI, pre- and post-rehabilitation are displayed in Table 3. Scores on the post-rehabilitation CSI time/practical and personal/emotional subscales showed a significantly lower number of reported symptoms of carer strain, compared to pre-rehabilitation performance, hence showing an effect of rehabilitation. No significant effect of rehabilitation was established on the personal/role subscale of CSI.
Therefore seven of the nine subscale analyses demonstrate a benefit of rehabilitation, when using the traditional criterion variable of 0.05. Multiple testing can lead to family-wise error; applying the Bonferroni correction to this set of 9 analyses suggests p values should be interpreted with an alpha level of 0.006. Subsequent re-interpretation of p values supports the significance of the effectiveness of rehabilitation on aforementioned subscales.
3.2Effect of aetiology on neuropsychological rehabilitation
Clients were classified according to their aetiological group (traumatic, non-traumatic). Clients’ performance on all subscales, pre- and post- rehabilitation are displayed in Table 4.
Results demonstrate a significant interaction between the effect of rehabilitation and aetiology on the metacognitive subscale of DEX-I. Carers of traumatic clients reported less symptoms of dysexecutive behaviour post- than pre-rehabilitation, whereas carers of non-traumatic clients reported increased symptoms post-rehabilitation; this interaction is demonstrated in Fig. 2. No significant interaction was found between the effect of rehabilitation and aetiology on any subscales on DEX or CSI, or on the behavioural/emotional or executive function subscales on DEX-I.
In order to establish the nature of the interaction between aetiology and effect of rehabilitation on the metacognitive subscale of DEX-I, post-hoc simple effects analyses were performed. A set of two repeated-measures t-tests established the difference between pre- and post-rehabilitation data sets in both traumatic and non-traumatic groups. A set of two independent-sample t-tests were used to establish the difference between traumatic and non-traumatic groups at both pre- and post-rehabilitation time points. Results are displayed in Table 5.
Results show an effect of rehabilitation on performance in the traumatic group, as clients reported significantly fewer dysexecutive behaviours at post- than pre-rehabilitation. A significant effect of aetiology was found on pre-rehabilitation performance, where traumatic clients reported more symptoms than non-traumatic clients. No significant effect of rehabilitation was found in the non-traumatic group, or of aetiology on post-rehabilitation performance. Applying the Bonferroni correction to this set of 4 analyses suggests p values should be interpreted with an alpha level of. 0125. Subsequent re-interpretation of p values supports the significance of aforementioned findings.
4Discussion
Primarily this study aimed to evaluate the efficacy of comprehensive, holistic neuropsychological rehabilitation in reducing perceived symptoms of dysexecutive behaviour in clients and carer strain. Analysis comparing pre- and post-rehabilitation performance on DEX showed a significant reduction in reported symptoms of dysexecutive behaviour on all three subscales. Therefore rehabilitation alleviated client-perceived symptoms of executive cognition problems, e.g. planning, and distractibility; behavioural and emotional problems, such as insight and apathy; and metacognitive problems, e.g. aggression and impulsivity. Analysis comparing pre- and post-rehabilitation performance on DEX-I found a significant reduction in reported symptoms on the behavioural/emotional and executive function subscales. However a significant reduction in metacognitive complaints was not established. Therefore rehabilitation alleviated carer-perceived symptoms of executive cognition problems and behavioural and emotional problems, however metacognitive symptoms such as impulsivity and euphoria were not reduced.
Results generally mirror research evaluating similar interventions, such as PST and GMT, in alleviating dysexecutive behaviour e.g. planning and dissociation problems (von Cramon, et al., 1991; von Cramon et al., 1992; Levine et al., 2000). Specifically, this study complements Miotto et al. (2009), in supporting the effectiveness of the OZC A&GM group as a stand-alone intervention, in reducing EF impairments. Furthermore, this study adds to past research by assessing the effects of a whole rehabilitation package on perceived EF, rather than a sole targeted-intervention. It also assesses multiple constructs within EF individually, allowing the evaluation of rehabilitation efficacy on meaningful components of dysexecutive behaviour. Results propose carers did not perceive an improvement in client metacognition following rehabilitation. Questions on the metacognitive subscale measure symptoms relating to interaction with others, e.g. ‘no concern for social rules’, whilst other DEX-I subscales appear to focus on non-social aspects, e.g. planning or perseveration. Lack of improvement, despite a benefit in other EF skills, could be a consequence of the programme targeting cognitive and behavioural/emotional facets of EF more effectively than metacognitive problems. The A&GM group employed PST and GMT techniques to alleviate EF impairments, therefore it could be argued therapy was not focussed at alleviating metacognitive symptoms. Alternatively, carer-perceived reports of metacognition function was relatively unimpaired at baseline, therefore analysis measuring post- performance relative to pre-rehabilitation would not show a significant benefit of rehabilitation. However, clients did perceive their metacognition to be improved on DEX; this discrepancy may reflect a tendency for clients to under-report metacognitive attributes, such as aggression (Willner, Joner, Tams & Green, 2002).
Analysis comparing pre- and post-rehabilitation performance on CSI showed significant reduction in reported symptoms of carer strain on the time/practical and personal/emotional subscales. However no significant reduction in personal/role symptoms was established. Therefore rehabilitation alleviated carer-perceived symptoms of strain, such as changes to personal plans and disrupted routines, as well as personal upset, tiredness and feeling overwhelmed. However, carers did not perceive an improvement in personal/role features, such as financial strain, disturbed sleep and responsibility. Despite the limited literature evaluating the efficacy of rehabilitation on carer strain, this study complements research reporting reduction in emotional and practical features (Kreutzer et al., 2009; Ponsford et al., 2003). Furthermore results support Schonberger et al. (2010), demonstrating the emotional status of the carer as improved following rehabilitation. A possible explanation for the ineffectiveness of rehabilitation on personal/role elements of strain, could be attributed to the programme focussing on educating carers on ABI consequences, adapting to living with ABI and peer support. These could be seen to focus on the time/practical and personal/emotional symptoms of strain, rather than personal/role. However personal/role strains such as, adjustment to work and disturbed sleep would logically be expected to improve as symptoms from the other scales improve, such as upsetting behaviour and changes to personal plans. This inconsistency could be due to the 24-week duration of the programme, and the relatively long-term adaptations associated with personal/role aspects have yet to occur. Benefits of rehabilitation may appear after a more extended period post-rehabilitation.
The secondary objective of the study was to investigate whether aetiology of ABI interacts with the effects of rehabilitation on performance on the subscales of DEX, DEX-I and CSI. Whilst results found no interactive effect on any subscales of DEX or CSI, the metacognitive subscale of DEX-I showed a significant effect of aetiology on rehabilitation. Furthermore, traumatic scores were significantly reduced from pre- to post-rehabilitation, whilst non-traumatic scores increased non-significantly. These results indicate rehabilitation reduced carer-perceived metacognitive problems for traumatic clients, however rehabilitation had no effect for non-traumatic clients. Analysis also found a significant difference between aetiological groups at pre-rehabilitation; traumatic clients reported more dysexecutive behaviour than non-traumatic. No significant difference was present at post-rehabilitation. Non-traumatic clients had relatively high baseline metacognitive function therefore analysis measuring discharge performance relative to baseline would not show a benefit of rehabilitation. These results are particularly interesting as the primary analysis of DEX-I showed no effect of rehabilitation on the metacognitive scale. In light of the secondary analysis, the difference in direction of effect between aetiological groups, could have masked an effect of rehabilitation during primary analysis. Hence, despite a lack of interaction between aetiology and rehabilitation on most subscales, the findings on the metacognitive subscale demonstrates the importance of considering aetiology of ABI whilst interpreting mixed group results. Although literature evaluating the effect of aetiology on the efficacy of rehabilitation is limited; this study partially supports Fish et al. (2008), which established an increased improvement following rehabilitation in TBI, compared to CVA patients. Fish et al. (2008) proposed traumatic clients maintained the benefits of rehabilitation due to increased EF skills, compared to non-traumatic clients. An increased benefit of rehabilitation could also be explained by the younger average age of the traumatic group compared to non-traumatic. This, coupled with increased EF skills, could be indicative of enhanced ability to implement compensatory strategies, motivation and familial support, and therefore improved responsiveness to rehabilitation. However, as no interaction was found between aetiology and rehabilitation on any other subscales, the degree of aetiology as a moderator remainsundetermined.
This study is unique in evaluating the efficacy of a holistic neuropsychological rehabilitation programme on EF and carer strain specifically. The employment of recently developed Rasch-based subscales further enhances previous literature evaluating EF and carer strain, by allowing the investigation of individual psychological constructs within the umbrella terms ‘EF’ and ‘carer strain’. As part of a clinical service evaluation there were constraints to the study design. However, service evaluations are essential to establish service performance against target aims. The data analysed in this study was collected over 15 years, therefore the centre will have experienced staff turnover and minor updates to the programme during this time period; however key staff have remained, as have the underlying principles of the programme. A limitation of the study was large amount of missing data, leading to the sole use of complete data sets; clients who failed to return post-rehabilitation data might have had specific characteristics, such as increased or decreased benefit from rehabilitation, hence biasing the included group for analysis. A recommendation for future research could include enhanced monitoring of post-rehabilitation data return. A lack of a prospectively designed control condition meant randomisation, allocation concealment and blinding were not employed. However, clients’ average time since injury was 3 years, therefore spontaneous recovery had occurred and clients’ disabilities were chronic, hence any improvement was due to engagement in the rehabilitation programme. Furthermore if a control group was assigned in future research, any interaction with the rehabilitation team could serve as an intervention, as therapeutic milieu played a large part in the programme. Perhaps future research could employ an RCT-style design, using a randomized, waiting-list normal-treatment control, with blinding of scorers. Alternatively multiple single-case experimental designs of patients with similar profiles could provide a scope of generalizability without masking individual differences. This study employed a pre- and post-rehabilitation design using a large sample to increase power, however maintenance of effects was not determined. Analysis of 6 or 12-month follow-up data would provide a useful insight into long-term effects of rehabilitation, and uncover whether some benefits appear post-discharge.
Baseline characteristics of the CVA sample showed lower average age at injury, compared to the general stroke population, hence the sample was atypical and the generalizability of aetiological differences established in this study is questionable. Client severity of EF and carer strain was not classified due to lack of cut-off scores on employed measures; this could be considered in future research to increase translation of results across studies. However, baseline performance on subscales was established, therefore future research or practice can determine the level of severity, and hence the degree of generalizability.
The use of standardised measures to evaluate rehabilitation is challenging; the group effect is crucial but masks the uniqueness of individual goals. Despite their frequent use as standardised outcome measures, DEX, DEX-I and CSI do not use interval scaling for scoring; hence the measure of difference between pre- and post-rehabilitation scores is not psychometrically valid. However, this study used Rasch-based subscales, hence construct validity of subscales is established. The inclusion of qualitative outcome measures would provide a platform for clients and carers to suggest improvements and offer insight.
Discrepancy between client and carer ratings is common, and often dependent on relationship type, (Wilner et al., 2002). A professional-rater DEX would be a useful tool to highlight familial biases, however client and carer DEX provides a voice for their subjective perceptions; crucial in planning and evaluating rehabilitation. Examining the relationship between responses on outcome measures would provide interesting scope for future research. Furthermore, the relationship between metacognitive and personal/role subscales could aid understanding of interactions between EF and carer strain. A recent study by Raskin et al. (2010), showed the effectiveness of rehabilitation was only observed by individually defined goals. The association between goal attainment scores with performance on questionnaires could determine the ecological validity of the measure. OZC is a unique centre in Europe, and may not be reflective of the majority of rehabilitation programmes in the UK. Therefore comparisons of the efficacy of the programme with other types would be recommended for future research to determine generalizability of findings.
In conclusion, consistent with the hypotheses, neuropsychological rehabilitation generally appears to be effective in reducing client and carer reports of perceived dysexecutive behaviours. Initially, client-perceived metacognitive behaviours appeared unimproved. However it was subsequently revealed that traumatic clients did improve, although non-traumatic had higher baseline performance and therefore showed little change from rehabilitation. Therefore, this research implicates the importance of considering aetiology and baseline characteristics in practice. Neuropsychological rehabilitation also generally appears to be effective in reducing symptoms of carer strain, consistent with the predictions. Personal/role aspects of carer strain did not significantly improve, it is suggested that follow-up assessment would reveal benefits in this area. This study demonstrates the importance of executing service evaluation to assure efficacy of rehabilitation. Despite difficulties associated with using routine outcome data, evaluation conveys the impact of a service that aims to improve the everyday life of ABI clients. Another important implication of this study is that even after the spontaneous recovery period, rehabilitation can benefit chronic ABI clients who suffer a variety of long-term disabilities, and their families. Finally, these results should be interpreted with respect to the limitations of the study, and in consideration of the distinctive style of the neuropsychological rehabilitation programmeevaluated.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgments
Andrew Bateman is supported by National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East of England at Cambridgeshire and Peterborough NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We are grateful to the clients and staff at the Oliver Zangwill Centre and Cambridgeshire Community Services NHS Trust who provided extra support in the collection and release of data for this research.
References
1 | Allen K. , Linn R. T. , Gutierrez H. , & Willer B. S. ((1994) ). Family burden following traumatic brain injury. Rehabilitation Psychology, 39: (1), 29. |
2 | Anderson M. I. , Parmenter T. R. , & Mok M. The relationship between neurobehavioural problems of severe traumatic brain injury (TBI), family functioning and the psychological well-being of the spouse/caregiver: Path model analysis. Brain Injury, 16: (9), 743–757. |
3 | Badham R. ((2010) ). Evaluation of carer responses to the independent rater version of the Dysexecutive (DEX) questionnaire using Rasch analysis. Unpublished manuscript. |
4 | Ben-Yishay Y. , & Prigatano G. ((1990) ). Post-acute neuropsychological rehabilitation: A holistic perspective. In Christensen A. L. , and Uzzell B. P. , eds., Critical Issues in Neuropsychology, International Handbook of Neuropsychological Rehabilitation. New York: Kluwer Academic/Plenum Publishers. |
5 | Bennett P. C. , Ong B. , & Ponsford J. . Measuring executive dysfunction in an acute rehabilitation setting: Using the dysexecutive questionnaire (DEX. Journal of the International Neuropsychological Society ((2005) ) 11: , 376–385. |
6 | Brooks N. ((1991) ). The head-injured family. Journal of Clinical and Experimental Neuropsychology, 13: , 155–188. |
7 | Burgess P. W. , & Simons J. S. ((2005) ). Theories of frontal lobe executive function: Clinical applications. In Halligan P. W. , & Wade D. T. , (Eds.), Effectiveness of Rehabilitation for Cognitive Deficits. Oxford: Oxford University Press. |
8 | Cicerone K. D. , Dahlberg C. , Kalmar K. , Langenbahn D. M. , Malec J. F. , & Bergquist T. F. , et al ((2000) ). Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Archives of Physical Medicine and Rehabilitation, 81: , 1596–1615. |
9 | Cicerone K. D. , Dahlberg C. , Malec J. F. , Langenbahn D. M. , Felicetti T. , & Kneipp S. , et al ((2005) ). Evidence-based cognitive rehabilitation: Updated review of the literature from 1988 through 2002. Archives of Physical Medicine and Rehabilitation, 86: , 1681–1692. |
10 | Cicerone K. D. , Langenbahn D. M. , Braden C. , Malec J. F. , Kalmar J. , & Fraas M. , et al ((2011) ). Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation, 92: , 519–530. |
11 | Chung C. S. Y. , Pollock A. , Campbell T. , Durward B. R. , & Hagen S. ((2009) ). Cognitive rehabilitation for executive dysfunction in patients with stroke or other adult non-progressive acquired brain damage. The Cochrane Library. |
12 | Chung C. , Pollock A. , Campbell T. , Durward B. , & Hagen S. ((2013) ). Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult nonprogressive acquired brain damage. Stroke, 44: (7), e77–e78. |
13 | Dancey C. P. , & Rediy J. ((2004) ). Statistics Without Maths for Psychology. London: Pearson Education Ltd.. |
14 | Dayus B. , & van den Broeak M. D. ((2000) ). Treatment of stable delusional confabulations using self-monitoring training. Neuropsychological Rehabilitation, 10: (4), 415–427. |
15 | Evans J. ((2003) ). Rehabilitation of executive deficits. In Wilson B. A. (Eds.), Neuropsychological Rehabilitation. Abingdon: Swets and Zeitlinger. |
16 | Fasotti L. , Kovacs F. , Eling P. A. T. M. , & Brouwer W. H. ((2000) ). Time pressure management as a compensatory strategy training after closed head injury. Neuropsychological Rehabilitation, 10: (1), 47–65. |
17 | Fish J. , Manly T. , Emslie H. , Evans J. J. , & Wilson B. A. ((2008) ). Compensatory strategies for acquired disorders of memory and planning: Differential effects of a paging system for patients with brain injury of traumatic versus cerebrovascular aetiology. Journal of Neurology, Neurosurgery and Psychiatry, 79: , 930–935. |
18 | Gracey F. , Palmer S. , Rous B. , Psalia K. , Kendra S. , & O’Dell J. , et al ((2008) ). Feeling part of things”: Personal construction of the self after brain injury. Neuropsychological Rehabilitation, 18: (5), 627–650. |
19 | Greening K. ((2011) ). Evaluation of the Modified Carer Strain Index using Rasch analysis and factor analysis in a sample of carer of adults with acquired brain injury. Unpublished manuscript. |
20 | Hart T. , Whyte J. , Kim J. , & Vaccaro M. ((2005) ). Executive function and self-awareness of ‘real world’ behaviour and attention deficits following traumatic brain injury. Journal of Head Trauma Rehabilitation, 20: , 333–347. |
21 | Hart T. , & Evans J. ((2006) ). Self-regulation and goal theories in brain injury rehabilitation. Journal of Head Trauma Rehabilitation 142–155. |
22 | Intercollegiate Stroke Working Party. ((2004) ). National Clinical Guidelines for Stroke. (2nd Ed). London: RCP. |
23 | Jackson D. , Turner-Stokes L. , Murray J. , Leese M. , & Mcpherson K. M. ((2009) ). Acquired brain injury and dementia: A Comparison of carer experiences. Brain Injury, 23: (5), 433–444 . |
24 | Klonoff P. S. , Lamb D. G. , & Henderson S. W. ((2001) ). Outcomes from milieu-based neurorehabilitation at up to 11 years post-discharge. Brain Injury, 15: (5), 413–428. |
25 | Knight R. G. , Devereux R. , & Godfrey H. P. D. ((1998) ). Caring for a family member with traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 90: (6), 939–946. |
26 | Kreutzer J. S. , Gervasio A. H. , & Camplair P. S. ((1994) ). Primary caregivers’ psychological status and family functioning after traumatic brain injury. Brain Injury, 8: (3), 197–210. |
27 | Kreutzer J. S. , Rapport L. J. , Marwitz J. H. , Harrison-Felix C. , Hart T. , Glenn M. , & Hammond F. ((2009) ). Caregivers’ well-being after traumatic brain injury: A multicenter prospective investigation. Archives of Physical Medicine and Rehabilitation, 90: (6), 939–946. |
28 | Leskela M. , Hietanen M. , Kalska H. , Yliskoski R. , Pohjasvaara T. , & Mantyla R. , et al ((1999) ). Executive functions and speed of mental processing in elderly patients with frontal or nonfrontal ischemic stroke. European Journal of Neurology, 6: , 653–661. |
29 | Levine B. , Robertson I. H. , Clare L. , Carter G. , Hong J. , & Wilson B. A. , et al ((2000) ). Rehabilitation of executive functioning: An experimental-clinical validation of goal management training. Journal of the International Neuropsychological Society, 6: , 299–312. |
30 | Lewis M. W. , Babbage D. R. , & Leathem J. M. ((2011) ). Assessing executive performance during cognitive rehabilitation. Neuropsychological Rehabilitation, 21: (2), 145–163. |
31 | Lezak M. D. ((1982) ). The problem of assessing executive functions. International Journal of Psychology, 17: , 281–297. |
32 | Mai N. ((1992) ). Discussion: Evaluation in constructing neuropsychological treatments. In von Steinbuchel N. , von Cramon D. Y. , & Poppel E. , (Eds.), Neuropsychological Rehabilitation. Berlin: Springer-Verlag. |
33 | Malec J. F. ((2001) ). Impact of comprehensive day treatment on the societal participantion for persons with acquired brain injury. Archives of Physical Medicine and Rehabilitation, 82: , 885–895. |
34 | Miotto E. C. , Evans J. J. , Souza de Lucia M. C. , & Scaff M. ((2009) ). Rehabilitation of executive dysfunction: A controlled trial of an attention and problem solving treatment grou. Neuropsychological Rehabilitation, 19: (4), 517–540. |
35 | Oddy M. , & Herbert C. ((2003) ). Intervention with families following brain injury: Evidence-based practice. Neuropsychological Rehabilitation, 13: (1/2), 259–273. |
36 | Ownsworth T. L. , McFarland K. , & Young R. McD. ((2000) ). Self-awareness and psychosocial functioning following acquired brain injury: An evaluation of a group support programme. Neuropsychological Rehabilitation, 10: (5), 465–484. |
37 | Parente R. , & Stapleton M. ((1999) ). Development of a cognitive strategies group for vocational training after traumatic brain injury. NeuroRehabilitation, 13: , 13–20. |
38 | Perlesz A. , Kinsella G. , & Crowne S. ((2000) ). Psychological distress and family satisfaction following traumatic brain injury: Individuals and their primary, secondary and tertiary carers. Journal of Head Trauma Rehabilitation, 15: , 909–929. |
39 | Ponsford J. , Olver J. , Ponsford M. , & Nelms R. ((2003) ). Long-term adjustment of families following traumatic brain injury where comprehensive rehabilitation has been provided. Brian Injury, 17: (6), 453–468. |
40 | Raskin S. M. C. , Bouwens S. F. M. , Dijcks B. , Winkens I. , Bakx W. G. M. , & van Heugten C. M. ((2010) ). Effectiveness of a low intensity outpatient cognitive rehabilitation programme for patients in the chronic phase after acquired barin injury. Neuropsychological Rehabilitation, 20: (5), 760–777. |
41 | Royal College of Physicians. ((2003) ). British Society of Rehabilitation Medicine. In Turner-Stokes L. , (Ed). Rehabilitation Following Acquired Brain Injury. London: RCP, BSRM. |
42 | Robinson B. C. ((1983) ). Validation of a caregiver strain index. Journal of Gerontology, 38: (3), 344–348. |
43 | Sachdev P. S. , Braodaty H. , Valenzuela M. J. , Lorentz L. , Looi J. C. , & Wen W. , et al ((2004) ). The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology, 62: , 912–919. |
44 | Salazar A. M. , Warden D. L. , Schwab K. , Spector J. , Braverman S. , & Walter J. , et al ((2000) ). Cognitive rehabilitation for traumatic brain injury. Journal of the American Medical Association, 283: , 3075–3081. |
45 | Salmond G. H. , Chatfeld D. A. , Menon D. , Pickard J. D. , & Sahakian B. J. ((2005) ). Cognitive sequalae of head injury: Involvement of basal forebrain an associated structures. Brain, 128: , 189–200. |
46 | Schonberger M. , Ponsford J. , Olver J. , & Ponsford M. ((2010) ). A longitudinal study of family functioning after TBI and relatives emotional status. Neuropsychological Rehabilitation, 20: (6), 813–829. |
47 | Simblett S. K. , & Bateman A. ((2010) ). Dimensions of the dysexecutive questionnaire (DEX) Examined using Rasch analysis. Neuropsychological Rehabilitation, i First 1–25. |
48 | Spikman J. M. , Boelen D. H. E. , Lamberts K. F. , Brouwer W. H. , & Fasotti L. ((2010) ). Effects of a multifaceted treatment program for executive dysfunction after acquired brain injury on indications of executive functioning in daily life. Journal of International Neuropsychological Society, 16: , 118–129. |
49 | Sunderland A. , Harris J. E. , & Gleave J. ((1984) ). Memory failures in everyday life following severe head injury. Journal of the Clinical Neuropsychology, 6: , 127–142. |
50 | Tate R. L. ((2010) ). A compendium of tests, scales and questionnaires: The practitioner’s guide to measuring outcomes after acquired brain impairment. Hove: Psychology Press. |
51 | Teasdale T. W. , Emslie H. , Quirk K. , Evans J. , Fish J. , & Wilson B. A. ((2009) ). Alleviation of carer strain during the use of the Neuropage device by people with acquired brain injury. Journal of Neurology, Neurosurgery and Psychiatry, 80: , 781–783. |
52 | von Cramon D. M. , Matthes-von Cramon G. , & Mai N. ((1991) ). Problem-solving deficits in brain-injured patients: A therapeutic approach. Neuropsychological Rehabilitation, 1: (1), 45–64. |
53 | von Cramon D. M. , & Matthes-von Cramon G. ((1992) ). Reflections on the treatment of brain-injured suffering from problem-solving disorders. Neuropsychological Rehabilitation, 2: (3), 207–229. |
54 | Wade D. T. ((2005) ). Applying the WHO ICF framework to the rehabilitation of cognitive deficits rehabilitation. In Halligan P. W. , and Wade D. T. , (Eds.), Effectiveness of Rehabilitation for Cognitive Deficits. Oxford: Oxford University Press. |
55 | Willner P. , Joner J. , Tams R. , & Green G. ((2002) ). A randomized controlled trial of the efficacy of a cognitive-behavioural anger management group for clients with learning disabilitites. Jounral of Applied Research in Intellectual Disabilities, 15: (3), 224–235. |
56 | Wilson B. A. , Alderman N. , Burgess P. W. , Emslie H. , & Evans J. J. ((1996) ). BADS. Behoural Assessment of the Dysexecutive Syndrome. Bury St Edmunds, UK: Thames Valley Test Company. |
57 | Wilson B. A. , Evans J. J. , Emslie H. , & Malinek V. ((1997) ). Evaluation of NeuroPage: A new memory aid. Journal of Neurology, Neurosurgery and Psychiatry, 63: , 113–115. |
58 | Wilson B. A. , Emslie H. C. , Quirk K. , & Evans J. J. ((2001) ). Reducing everyday memory and planning problems by means of a paging system: A randomised control crossover study. Journal of Neurology, Neurosurgery and Psychiatry, 70: , 477–482. |
59 | Wilson B. A. ((2002) ). Towards a comprehensive model of cognitive rehabilitation, 12: (2), 97–110. |
60 | Wilson B. A. ((2003) ). Neuropsychological Rehabilitation: Theory and Practice. Hove: Psychology Press Ltd. |
61 | Wilson B. A. , Gracey F. , Evans J. J. , & Bateman A. ((2009) ). Neuropsychological Rehabilitation: Theory, Models, Therapy and Outcome. Cambridge: Cambridge University Press. |
62 | Wood R. L. , McCrea J. D. , Wood L. M. , & Merriman R. N. . Clinical and cost effectiveness of post-acute neurobehavioural rehabilitation. Brain Injury ((1999) ) 13: (2), 69–88 . |
Figures and Tables
Fig.1
Points of routine assessment at OZC.
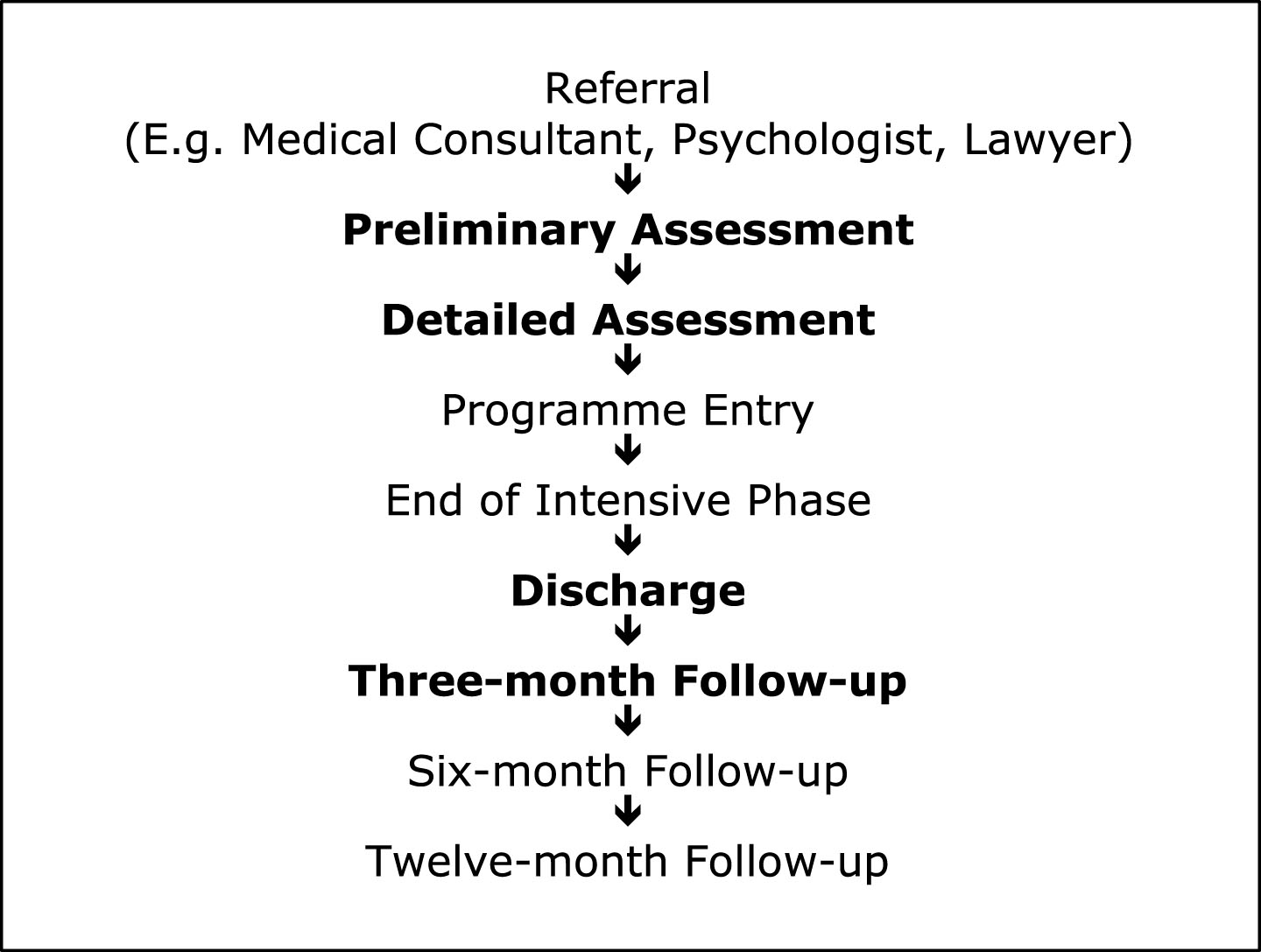
Fig.2
Interaction between aetiology and rehabilitation on the metacognitive subscale of DEX-I.
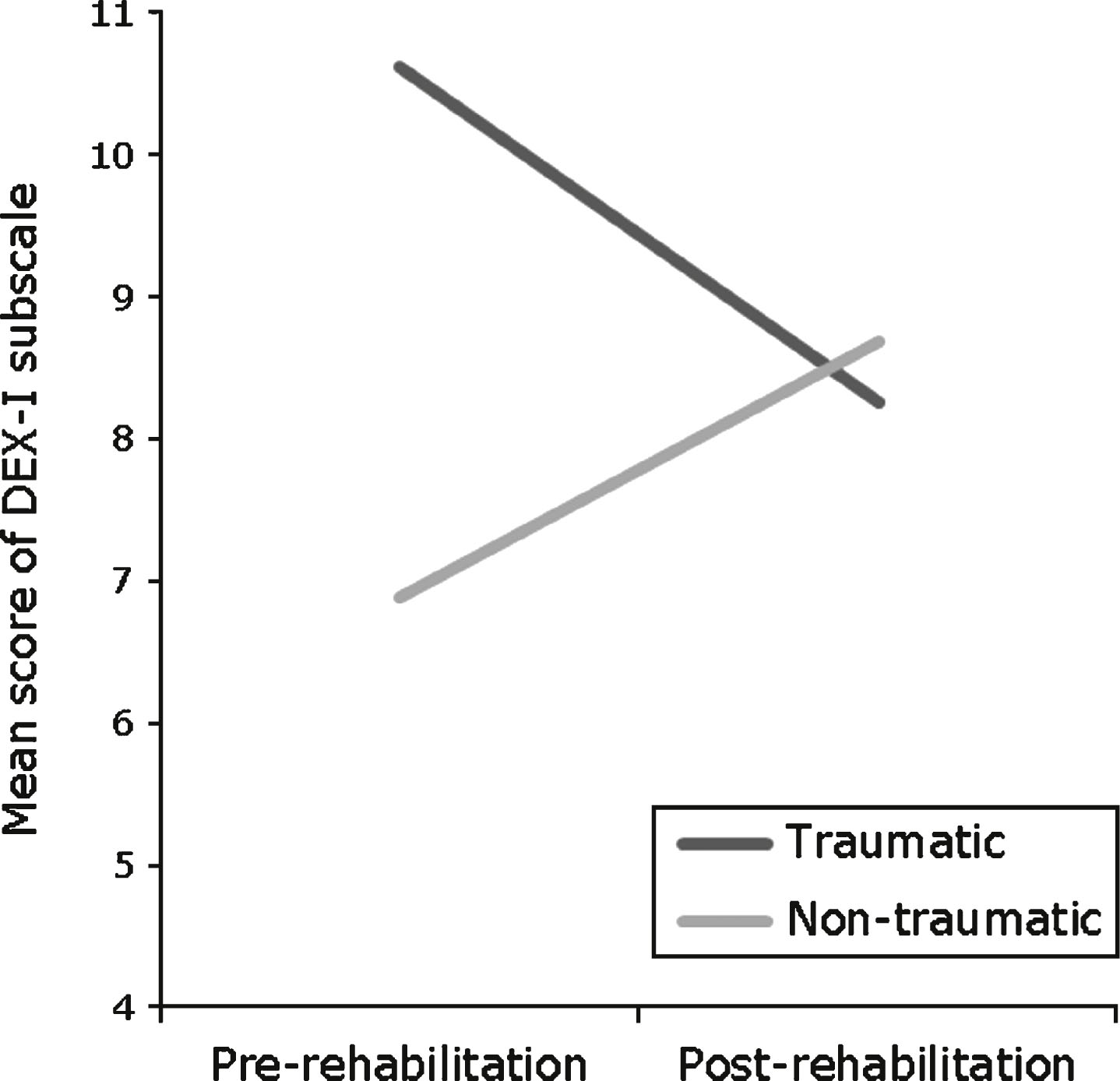
Table 1
Demographic characteristics of the sample
| Traumatic | Non-traumatic | |||
| Mean (SD) | Range | Mean (SD) | Range | |
| Age at Injury (years) | 31.60 (11.75) | 11– 55 | 42.24 (11.70) | 12– 56 |
| Age at Assessment (years) | 35.02 (11.72) | 18– 61 | 45.08 (9.44) | 31– 58 |
| Time Since Injury (years) | 2.89 (2.17) | 0– 13 | 2.84 (4.57) | 1– 20 |
Table 2
Performance on DEX/DEX-I subscales
| Subscales | Pre-rehabilitation Mean (SD) | Post-rehabilitation Mean (SD) | t (65) | p | |
| DEX | Behavioural/Emotional | 12.58 (6.40) | 8.38 (7.16) | 4.63 | 0.00* |
| Metacognitive | 8.06 (4.69) | 5.23 (3.73) | 5.74 | 0.00* | |
| Executive Function | 8.73 (3.48) | 6.74 (3.40) | 4.14 | 0.00* | |
| DEX-I | Behavioural/Emotional | 13.52 (7.19) | 10.24 (6.23) | 4.52 | 0.00* |
| Metacognitive | 9.71 (5.13) | 8.36 (6.46) | 1.87 | 0.66 | |
| Executive Function | 12.59 (3.78) | 9.86 (3.90) | 5.91 | 0.00* |
Repeated-measures t-tests.*p < 0.05.
Table 3
Performance on CSI subscales
| Subscale | Pre-rehabilitation Mean (SD) | Post-rehabilitation Mean (SD) | t (65) | p |
| Time/Practical | 20.41 (12.04) | 14.88 (10.54) | 3.85 | 0.00* |
| Personal/Emotional | 35.61 (15.56) | 28.50 (18.42) | 3.82 | 0.00* |
| Personal/Role | 24.56 (15.09) | 21.23 (16.09) | 1.90 | 0.63 |
Repeated-measures t-tests.*p < 0.05.
Table 4
Aetiologically grouped performance on all subscales
| Traumatic (n = 50) | Non-traumatic (n = 16) | |||||||
| Questionnaire | Subscales | Pre-rehab Mean (SD) | Post-rehab Mean (SD) | Pre-rehab Mean (SD) | Post-rehab Mean (SD) | F (1,64) | MSE | p |
| DEX | Behavioural/Emotional | 13.38 (6.36) | 8.90 (7.78) | 10.06 (6.03) | 6.75 (4.60) | 0.30 | 27.42 | 0.59 |
| Metacognitive | 8.74 (4.73) | 5.66 (3.97) | 5.94 (3.96) | 3.88 (2.50) | 7.78 | 8.07 | 0.38 | |
| Executive Function | 9.28 (3.46) | 6.84 (3.54) | 7.00 (3.01) | 6.44 (2.99) | 2.90 | 7.38 | 0.09 | |
| DEX-I | Behavioural/Emotional | 14.16 (7.44) | 10.54 (6.40) | 11.50 (6.13) | 9.31 (5.76) | 0.71 | 17.41 | 0.40 |
| Metacognitive | 10.62 (5.05) | 8.26 (4.80) | 6.88 (4.40) | 8.69 (10.27) | 6.67 | 15.83 | 0.01* | |
| Executive Function | 12.80 (3.52) | 9.86 (4.14) | 11.94 (4.58) | 9.88 (3.18) | 0.66 | 7.06 | 0.42 | |
| CSI | Time/Practical | 19.98 (12.46) | 14.60 (10.54) | 21.75 (10.89) | 15.75 (10.82) | 0.03 | 69.03 | 0.86 |
| Personal/Emotional | 35.94 (15.18) | 28.12 (17.61) | 34.56 (17.16) | 29.69 (21.35) | 0.46 | 115.27 | 0.50 | |
| Personal/Role | 23.54 (16.08) | 20.96 (16.32) | 27.75 (11.26) | 22.06 (15.86) | 0.57 | 102.76 | 0.45 | |
2×2 mixed-model ANOVAs: One between-group independent variable with two groups (Aetiology: traumatic and non-traumatic); one repeated-measures independent variable with two levels (Effect of rehabilitation: Pre-rehabilitation and Post-rehabilitation).*p < 0.05.
Table 5
Post-hoc analyses determining the nature of interaction between aetiology and rehabilitation on performance on DEX-I Metacognitive scale
| Interactions | t | df | p |
| Traumatic: Rehabilitation (pre-; post-)a | 4.01 | 49 | 0.00* |
| Non-traumatic: Rehabilitation (pre-; post-)a | – 0.82 | 15 | 0.43 |
| Pre-rehabilitation: Aetiology (traumatic; non-traumatic)b | 2.66 | 64 | 0.01* |
| Post-rehabilitation: Aetiology (traumatic; non-traumatic)b | 0.23 | 64 | 0.82 |
aRepeated-measures t-test; bIndependent-samples t-test. *p < 0.05.



