Exendin-4 Treatment Improves LPS-Induced Depressive-Like Behavior Without Affecting Pro-Inflammatory Cytokines
Abstract
Background: Exendin-4 is a peptide agonist of the glucagon-like peptide-1 (GLP-1) receptor, currently in clinical trials as a potential disease-modifying therapy for Parkinson’s disease. In light of this, it is important to understand potential modes of action of exendin-4 in the brain. Exendin-4 is neuroprotective and has been proposed to be directly anti-inflammatory, and that this is one way it reduces neurodegeneration. However, prior studies have focused on animal models involving both neurodegeneration and inflammation, therefore, it is also possible that the observed decreased inflammation is secondary to reduced neurodegeneration.
Objective: To investigate whether exendin-4 directly reduces inflammation in the brain following an insult that involves neuroinflammation but not neurodegeneration, namely systemic administration of lipopolysaccharide (LPS).
Methods: Rats were administered LPS systemically and were treated with either 0.5 μg/kg exendin-4 or saline vehicle injections over 5 days. Behavior was evaluated with forced swim test. We assayed TNF-α and IL-1β levels in cerebrospinal fluid and cytokine mRNA expression in striatal, hippocampal and cortical tissues using qPCR. We determined brain monoamines using high-performance liquid chromatography. Finally, we isolated primary brain microglia from rats and measured cytokine production after exendin-4 treatment and LPS stimulation.
Results: Exendin-4 treatment did not affect cytokine mRNA expression in brain, cytokine levels in cerebrospinal fluid or cytokine production from cultured microglia, although there was a trend towards increased striatal dopamine. Importantly, exendin-4 significantly prevented depressive-like behavior at 24 hours after LPS injection, indicating that the drug engaged a target in the brain. Depressive-like behavior was associated with altered dopamine turnover in the striatum.
Conclusion: We did not detect any anti-inflammatory effects of exendin-4. In previous studies exploring the effects of exendin-4 on brain insults involving neurodegeneration, observations of reduced inflammation might have been secondary to mitigation of neuronal death. Our results indicate that the effects of exendin-4 on behavior may be due to effects on dopamine synthesis or metabolism.
INTRODUCTION
A synthetic version (exendin-4, or Exenatide) of the endogenous glucagon-like peptide-1 (GLP-1) is currently used as medication for type II diabetes, regulating insulin secretion and glucose metabolism. GLP-1 receptors are widely expressed in the brain, with high levels in the hypothalamus and brainstem [1] and are known to regulate appetite and promote satiety [2]. The neurobiological effects of exendin-4 are still being characterized. Exendin-4 readily passes the blood-brain barrier [3] and increases neurogenesis in rodents, as well as modulates synaptic transmission in the subventricular zone and dentate gyrus of the hippocampus [4–6]. In addition, it is known that exendin-4 has neuroprotective properties, as it protects dopaminergic neurons in Parkinson’s disease (PD) rodent models [7, 8] and preserves synaptic plasticity in the hippocampus in animal models of Alzheimer’s disease (AD) [9–12]. Exendin-4 is also thought to exert anti-inflammatory effects in the central nervous system (CNS), such as suppress the production of the cytokines tumor necrosis factor-α (TNF-α), interleukin-1 β (IL-1β) and the inducible nitric oxide synthase (iNOS) [8, 13] although the precise underlying mechanisms are not well characterized [14]. Importantly, the reports of an anti-inflammatory effects of exendin-4 in the brain are derived from studies in animal models involving neurodegeneration, e.g. stroke, direct intracerebral injection of lipopolysaccharide (LPS) or systemic injections of the neurotoxin MPTP [13–17].
Currently, exendin-4 is being tested in clinical trials for both Alzheimer’s and PD [18, 19]. Results from an initial, single-blinded study in PD suggest that exendin-4 reduces rate of motor and cognitive deterioration following a one year treatment period [20] and that this effect is sustained for at least an additional year following cessation of treatment [21]. Therefore, exendin-4 was recently tested in a recently completed Phase II trial in PD, where the results are still pending [19]. Immune system activation has been suggested to contribute to both PD [22] and depression [23, 24]. Patients with clinical depression exhibit increased levels of cytokines in the blood and microglial activation [25, 26]. Similarly, patients with PD exhibit signs of inflammation in post-mortem brain tissue [27], and primarily non-motor symptoms of PD are closely related to the levels of inflammation in blood and cerebrospinal fluid [28]. In PD, it is possible that neuroinflammation, regardless of whether it is a primary trigger of the disease or a bystander effect of neurodegeneration, contributes to depressive symptoms [28].
In this study, we sought to investigate the potential anti-inflammatory effects of exendin-4 on the CNS independent of neurodegeneration. We utilized a peripheral challenge with endotoxin (lipopolysaccharide, LPS) that is known to establish transient inflammation in the CNS without causing acute neuronal death in the brain. LPS at the same or higher doses than the one given here can induce long-term neurodegeneration, as rat pups given 1-2 mg/kg body weight LPS show evidence of neurodegeneration around 3 months later [29]. Mice that are given 5 mg/kg LPS ip do not show any loss of dopaminergic neurons over the first four months after injection, whereas the tyrosine hydroxylase (TH)-positive cell loss became manifest at 7 months post-injection [30]. We hypothesized that in our model, exendin-4 treatment would reduce neuroinflammation as well as ameliorate the associated sickness behavior and depressive-like symptoms resulting from the peripheral LPS injection.
EXPERIMENTAL PROCEDURES
Experimental outline
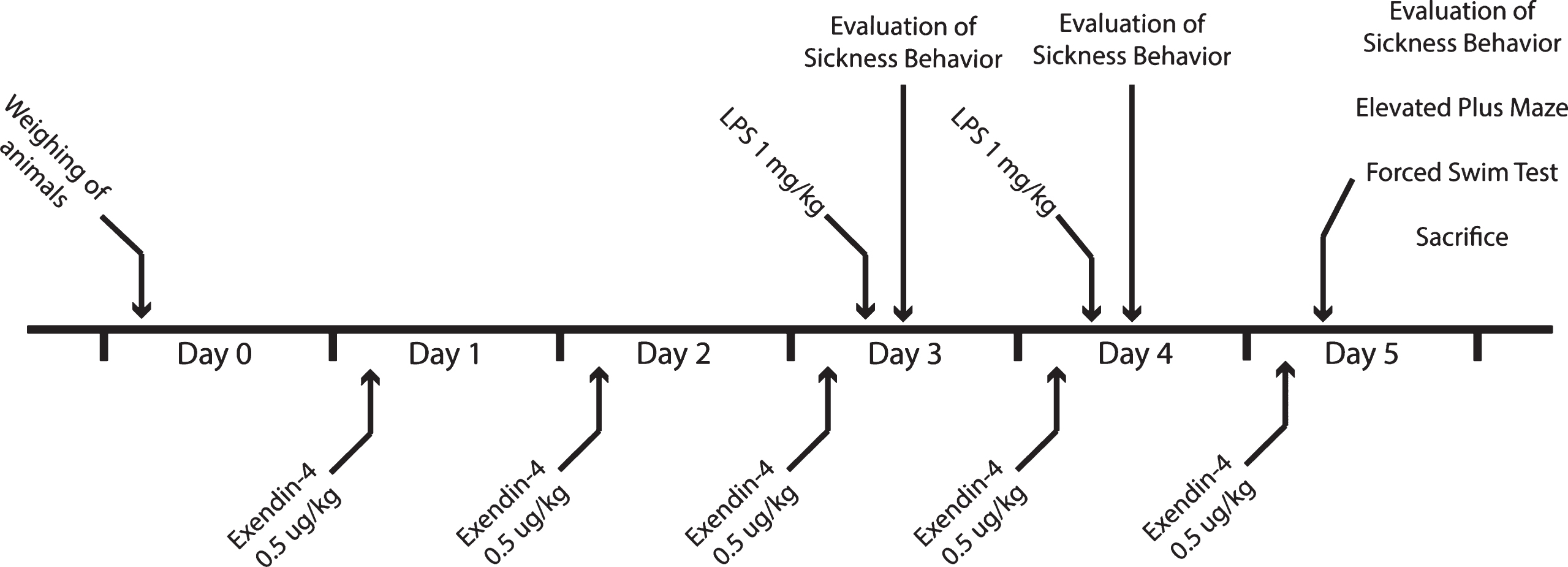
All procedures were performed in accordance with national laws and were approved by the Regional Animal Ethics Committee in Malmö/Lund, Sweden. In these experiments, we used male Wistar rats (Harland, Sweden) weighing around 200 g, housed in groups of three. We allowed the animals to acclimatize for at least five days before treatment with exendin-4 (Sigma, Germany) or vehicle (0.1 % BSA in saline), and with LPS (Sigma, Germany) or saline. The treatment groups were as follows: Vehicle + Saline (Control), Vehicle + LPS (LPS), Exendin-4 + Saline (Ex4) and Exendin-4 + LPS (LPS + Ex4) (n = 12 animals/group). We gave exendin-4 (0.5 μg/mL/kg) to the rats or vehicle i.p. once daily for five days (Fig. 1). This dose was selected based on a previous study [8]. On day three and four, the animals also received LPS (1 mg/mL/kg) or saline injections (i.p.) one hour after the exendin-4/vehicle injection. The dose of LPS was selected based on previous LPS experiments inducing brain inflammation and depressive-like behavior [31]. To assess LPS-induced sickness behavior, we scored stillness in the home cage, one hour and 24 hours after the LPS injection. On day five, after the last exendin-4/vehicle injection and after the last session of stillness assessment, we analyzed the animals for depressive-like behavior (despair) with forced swim test (FST). After the behavioral tests, we terminally anesthetized the animals with sodium pentobarbital (60 mg/kg, i.p.). Cerebrospinal fluid (CSF) was collected with transcutaneous cisterna magna puncture and blood by cardiac puncture. Brain tissue were collected from prefrontal cortex, dorsal striatum and hippocampus. The CSF and brain tissue were snap-frozen and stored at – 80°C. We kept the blood at room temperature for 30 min and then on ice for 1 h prior to serum collection. Serum was collected following centrifugation (10 min; 1300 g; 4°C) and stored at – 80°C. Glucose levels in the serum were assessed using HemoCue Glucose 201 apparatus (HemoCue A, Sweden).
Fig.1
Schematic illustration of the study design.
Sickness behavior and weight loss
To confirm that LPS induced sickness behavior, and subsequently that this had ceased at the time-point when we evaluated depressive- and anxious-like behaviors, we assessed activity of the rats one hour after each LPS injection and 24 hours after the last LPS injection [32]. The rats were individually placed into a novel cage and assessed blindly every five seconds over 5 consecutive minutes (in total 60 periods). Each period a rat sat motionless, not performing any grooming or rearing, was counted as a period of stillness. Due to sickness, rats lose weight following the first LPS injection. The LPS treated rats that did not lose any significant weight, and did not exhibit increased stillness compared to the vehicle/saline treated group, were excluded from further analysis (n = 3 [LPS]). Twenty-four hours after the last LPS injection, rats are expected to have recovered from sickness behavior and to have regained normal locomotor activity. One rat did not recover from LPS (n = 1 [LPS + Ex4]), and two rats from the control group showed health complications due to the i.p. injections and showed abnormal levels of stillness; they were excluded from further analysis (n = 1 [Control], n = 1 [LPS]).
Forced swim test
One hour after elevated plus maze, we analyzed the rats for depressive-like behavior (despair) in FST. The animals were placed in a Plexiglas cylinder (height: 70 cm, diameter: 30 cm) filled with water over the 30 cm level at a temperature of 23±1°C and videotaped for seven minutes. The last four minutes were blindly analyzed for every 5 s period. The modified version of the Porsolt swim test employed here has been described previously [33]. The predominant behavior during each period was recorded as swimming, climbing, or floating. Rats were classified as immobile when they remained floating motionless in the water, making only movements necessary to keep their head above water. Swimming was defined as horizontal movements throughout the cylinder and climbing was defined as vertical movements against the wall.
Analysis of monoamines
We analyzed the levels of serotonin, dopamine, and their respective metabolites; 5-hydroxyindoleacetic acid (5-HIAA), and 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), in the prefrontal cortex and striatum using high-performance liquid chromatography (HLPC) with electrochemical detection as previously described [34]. Brain tissue was homogenized using an ultrasonic tissue homogenizer (Biologics, Gainesville, VA) in an antioxidant solution (0.4 N perchlorate, 1.34 mM ethylenediaminetetraacetic acid and 0.53 mM sodium metabisulfite). The suspension was spun down (14,000 g, 20 min, 4°C) and supernatants collected. Samples were separated on a Microsorb MV C-18 column (5 μm, 4.6×250 mm2, Varian, Walnut Creek, CA). Unknown samples were quantified against a six-point standard curve with a minimum r2 of 0.97. Quality control samples were interspersed with each run to ensure HPLC calibration. The analysis failed to measure monoamines in two samples of striatum and one sample of prefrontal cortex (total n in each group for measurements indicated in Table 1).
Table 1
The levels (ng/mg protein; mean±SD) of dopamine (DA), 3,4-dihydroxyphenyl-acetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) in the prefrontal cortex (PFC) and striatum of the experimental groups. *indicates one measurement less due to method failure in some samples
| Brain | Analyte | Vehicle | LPS | Exendin4 | LPS + Exendin4 | Two-way ANOVA, |
| region | (n = 11) | (n = 7) | (n = 12) | (n = 11) | effect of Exendin-4 | |
| *(n = 10) | and LPS | |||||
| PFC | DA | 0.86±0.33 | 0.79±0.31 | 0.78±0.23 | 0.94±0.14 | unaltered |
| DOPAC | 0.17±0.14 | 0.17±0.07 | 0.13±0.07 | 0.21±0.05 | unaltered | |
| HVA | 0.21±0.29 | 0.33±0.16 | 0.18±0.25 | 0.37±0.21 | unaltered | |
| 5-HT | 6.88±1.00 | 7.19±1.63 | 7.48±1.66 | 7.32±1.19 | unaltered | |
| 5-HIAA | 6.70±0.82 | 6.88±1.38 | 6.11±1.57 | 7.03±2.43 | unaltered | |
| DOPAC/DA ratio | 0.19±0.10 | 0.23±0.10 | 0.16±0.05 | 0.22±0.05 | Significant increasing effect of LPS, p = 0.026 | |
| HVA/DA ratio | 0.22±0.31 | 0.39±0.22 | 0.18±0.25 | 0.39±0.21 | Significant increasing effect of LPS, p = 0.03 | |
| Striatum | DA | 154.02±22.32 | 160.12±20.24 | 164.28±33.40 | 184.22±28.17* | Trend towards increasing effect of Exendin-4, p = 0.059 |
| DOPAC | 14.50±2.84 | 16.32±2.28 | 15.09±3.38 | 16.21±1.99* | unaltered | |
| HVA | 10.89±1.85 | 12.42±2.56 | 11.06±2.83 | 14.04±3.66* | Significant increasing effect of LPS, p = 0.017 | |
| 5-HT | 7.95±1.23 | 7.10±1.35 | 6.77±1.53 | 7.00±3.30* | unaltered | |
| 5-HIAA | 13.35±1.27 | 14.20±3.89 | 11.64±2.80 | 14.69±5.52* | Significant increasing effect of LPS, p = 0.047 | |
| DOPAC/DA ratio | 0.095±0.02 | 0.1028±0.01 | 0.0923±0.01 | 0.0892±0.01* | Trend towards decreasing effect of Exendin-4, p = 0.1 | |
| HVA/DA ratio | 0.071±0.00 | 0.077±0.01 | 0.067±0.01 | 0.076*±0.01 | Significant increasing effect of LPS, p = 0.031 |
Cytokine protein level assay
We quantified the CSF levels of IL-1β and TNF-α, serum levels of IL-1β, and supernatant (cell media) levels of IL-1β and TNF-α from cell cultures by electrochemiluminescence in a SECTOR SI6000 imager (Meso-Scale Discovery, USA) following the manufacturer’s instructions. CSF from six animals were not used because of insufficient amounts. Discovery Workbench software (Meso-Scale Discovery) was used to compute the results. Samples were analyzed in duplicates and the mean was used for statistical analysis. All reported values were above detection limit. Detection limits: IL-1β [CSF] = 8.72 pg/mL; TNF-α [serum] = 1.27 pg/mL; IL-1β [serum] = 9.11 pg/mL; IL-1β [cell culture] = 24.5 pg/mL and TNF-α [cell culture] = 8.16 pg/mL.
Quantification of cytokine mRNA expression
Brain tissue from striatum, prefrontal cortex, and hippocampus was homogenized with Trizol reagent followed by RNA isolation with RNeasy mini kit according to the manufacturer’s instructions with the addition of a DNA digestion step (DNase) (Qiagen, Germany). Total RNA was transcribed to cDNA using Super-Script III (Invitrogen, Sweden).
In prefrontal cortex and hippocampus, the expression of IL-1β and TNF-α was analyzed with RT qPCR on a C1000 thermal cycler with CFX 96 real-time system (Bio-Rad, Sweden) using Maxima SYBR Green qPCR Mix (Fermentas, Sweden) with β-actin and Hprt1 as the control genes. Primer sequences for IL-1β and TNF-α have been described previously, [35], as well as primers for the control genes, [36]. The PCR program had an initial hot start for 5 min at 95°C followed by 40 cycles with a denaturing step of 15 s, a 30 s annealing step at 55°C and an extension step of 30 s at 72°C. For the melt curve, samples were initially heated to 95°C for 1 min and then cooled for 1 min at 55°C followed by 10 s increments of 0.5°C with the temperature increasing from 55°C to 95°C. All samples were run in triplicates and data were analyzed using the Pfaffl method. For striatum, the PCR reactions were carried out with TaqMan Universal MasterMix (ABI, Carlsbad, CA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control (Rn99999916_s1, ABI, Carlsbad, CA). Analysis of relative gene expression was carried out using the 2-ΔΔCT method.
Adult primary microglia cultures
Cell culture
A rat (weighing around 200 g) was anesthetized with pentobarbital and perfused (transcardiac perfusion) with HBSS w/o (Life Technologies, Sweden). Cerebellum and hindbrain were removed and the remainder was cut into small pieces followed by centrifugation (300 g; 2 min; RT) in HBSS w/o. The pellet was enzymatically homogenized with a pre-heated enzyme mix (37°C) containing papain (Neural Tissue Dissociation Kit, Miltenyi Biotec, Fisher Scientific, Sweden). After 5 min incubation at 37°C, the tissue was dissociated mechanically by pipetting. This was followed by addition of DNase (Neural Tissue Dissociation Kit, Miltenyi Biotec, Fisher Scientific, Sweden). The tissue was further dissociated with fire-polished Pasteur pipettes of decreasing diameter and incubated in a water bath for a maximum time of 30 min with enzymes. The suspension was filtered through a 70 μm cell strainer to obtain a single cell suspension, next centrifuged (300 g; 8 min; RT) in regular HBSS. To eliminate debris, the pellet was re-suspended in 8 mL 20% isotonic Percoll (Sigma Aldrich, Stockholm Sweden) with 2 mL HBSS on top. Following centrifugation (400 g; 25 min; 22°C) the pellet was washed and re-suspended in sorting buffer containing HBSS, EDTA (Sigma Aldrich, Sweden), HEPES and 5% FBS (Sigma Aldrich, Sweden). Parenchymal microglia were isolated by fluorescence activated cell sorting (FACS) as described below. The acquired cells were finally re-suspended in culture media (DMEM + high glucose + HEPES (Sigma Aldrich, Sweden), 10 % FBS) and distributed into a Poly-L-Lysine (PLL) (Sigma Aldrich, Sweden) coated 96-well plate (75 000 cells/well). Cells were treated with 2 μM (8.34 μg/mL) and 12 μM (50.21 μg/mL) of exendin-4. After 1 hour of incubation with exendin-4, 2 μg/mL LPS (Sigma Aldrich, Sweden) were added. After 24 hours of incubation, the supernatant was centrifuged (400 g; 4 min; 4°C), aliquoted and stored in – 80°C. The experiment was repeated three times.
Fluorescence-activated cell sorting
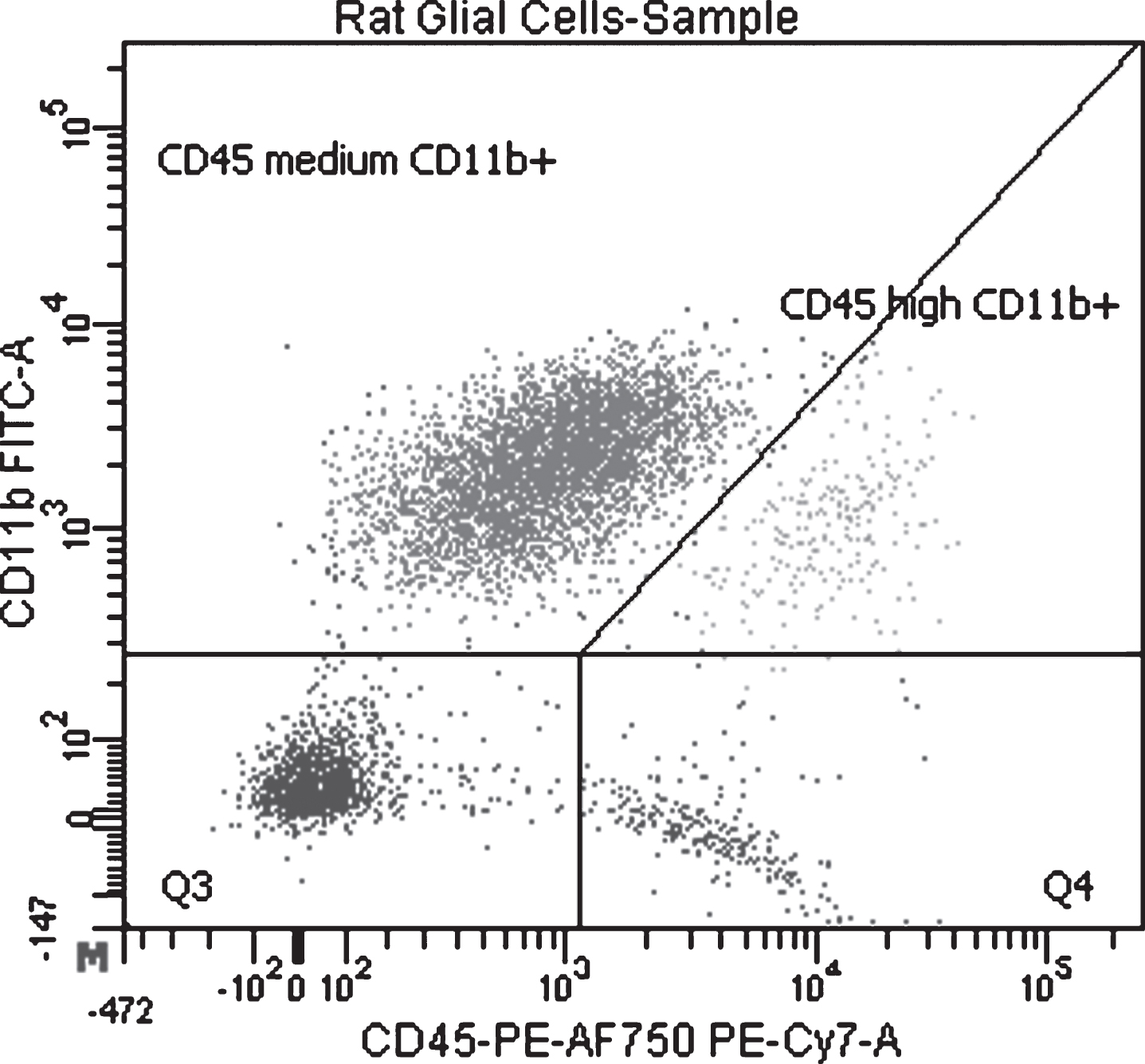
Antibodies targeting CD11b/c (OX-42, FITC conjugated, BD #554861) and CD45 (OX-1, R-Phycoerythrin and Alexa Fluor 750 conjugated, AbD Serotec #MCA43P750) were added to the single cell suspensions (after Percoll gradient centrifugation) which were then incubated for 15 minutes at + 4°C. Centrifugation (300 g; 5 min; 4°C) was performed to remove excess antibodies and the pellet was resuspended in sorting buffer. Parenchymal microglia were isolated by FACS (BD FACS Aria III) and based on their characteristic expression of CD11b/c and CD45 (CD11b/c + CD45low, Fig. 2) [37].
Fig.2
Separation of rat microglial cell used in the experiment where LPS and exendin-4 were added to cell cultures. Gating of the cell sorting with CD11b/c+CD45low/medium (parenchymal microglia) in Q1, CD11b/c+CD45high in Q2 (macrophages, perivascular microglia), CD11b/c-CD45low in Q3 and CD11b/c-CD45high in Q4.
Cell viability
After a 6-hour incubation, the percentage of surviving cells was measured with a high content screening platform (CellomicsTM, ArrayScan VTI HCS Reader, Thermo Scientific). The cells nuclei were stained with H342 (Sigma Aldrich, Sweden) (1.25 μg/mL) and propidium iodide (PI) (Sigma Aldrich, Sweden) (0.5%). The percentages of PI-positive cells were measured.
Statistical analysis
The statistical analysis was performed using SPSS Statistics 21 (IBM, US). When applicable, the effects of the drug treatments (LPS and exendin-4) on animal behavior and on levels of analytes were analyzed with two-way ANOVA. In case of interaction effects; these were further characterized with simple effects analysis. Cell culture data was analyzed with one-way ANOVA. The CSF cytokine levels were analyzed with Student’s t-test. Spearman’s rho was used for correlation analysis. Alpha-level of significance was set at p = 0.05.
RESULTS
Effects on Inflammation
Effects on inflammation in the CNS and periphery
The LPS-treated rats displayed an ongoing inflammation in the CSF and brain tissue at 24 hours after the last LPS injection. The protein levels of TNF-α and IL-1β were significantly increased in the CSF of LPS treated rats Fig. 3(A)and (B). At this time-point, there were no differences in the serum levels of IL-1β between the groups (two-way ANOVA, NS).
Fig.3
(A-B) The levels of cytokines in CSF (pg/mL). The LPS-treated rats exhibited higher levels IL-1β (A) and TNF-α (B), but exendin-4 treatment had no effect on cytokine levels (Student’s t-tests, NS). Non-LPS treated rats were below detection limit. CSF from six animals were not used because of insufficient amounts (n = 1 [Control], n = 2 [LPS], n = 3 [LPS+Ex4]). (C) The mRNA expression of IL-1β in the striatum. The IL-1β expression was higher in LPS treated rats but was not affected by exendin-4 treatment (two-way ANOVA, LPS effect, F(1,38) = 44.04, p < 0.001). Bars represent the mean±SEM, ***p < 0.001.
![(A-B) The levels of cytokines in CSF (pg/mL). The LPS-treated rats exhibited higher levels IL-1β (A) and TNF-α (B), but exendin-4 treatment had no effect on cytokine levels (Student’s t-tests, NS). Non-LPS treated rats were below detection limit. CSF from six animals were not used because of insufficient amounts (n = 1 [Control], n = 2 [LPS], n = 3 [LPS+Ex4]). (C) The mRNA expression of IL-1β in the striatum. The IL-1β expression was higher in LPS treated rats but was not affected by exendin-4 treatment (two-way ANOVA, LPS effect, F(1,38) = 44.04, p < 0.001). Bars represent the mean±SEM, ***p < 0.001.](https://content.iospress.com:443/media/jpd/2017/7-2/jpd-7-2-jpd171068/jpd-7-jpd171068-g003.jpg)
LPS treatment increased the expression of IL-1β mRNA in all three brain regions analyzed, striatum (F(1,38) = 44.04, p < 0.001, two-way ANOVA), Fig. 3(C), prefrontal cortex (F(1,17) = 10.04, p < 0.01), and hippocampus (F(1,27) = 24.20, p < 0.001). In contrast, there was no effect of LPS on TNF-α mRNA levels in any of the three regions (NS).
Exendin-4 did not have an effect on cytokine levels in the CSF, serum or mRNA expression in any of the three brain regions investigated (two-way ANOVA, NS).
Finally, there were no significant differences in glucose levels in the serum between groups related to exendin-4 treatment (two-way ANOVA, NS). The glucose levels were similar in the four different groups.
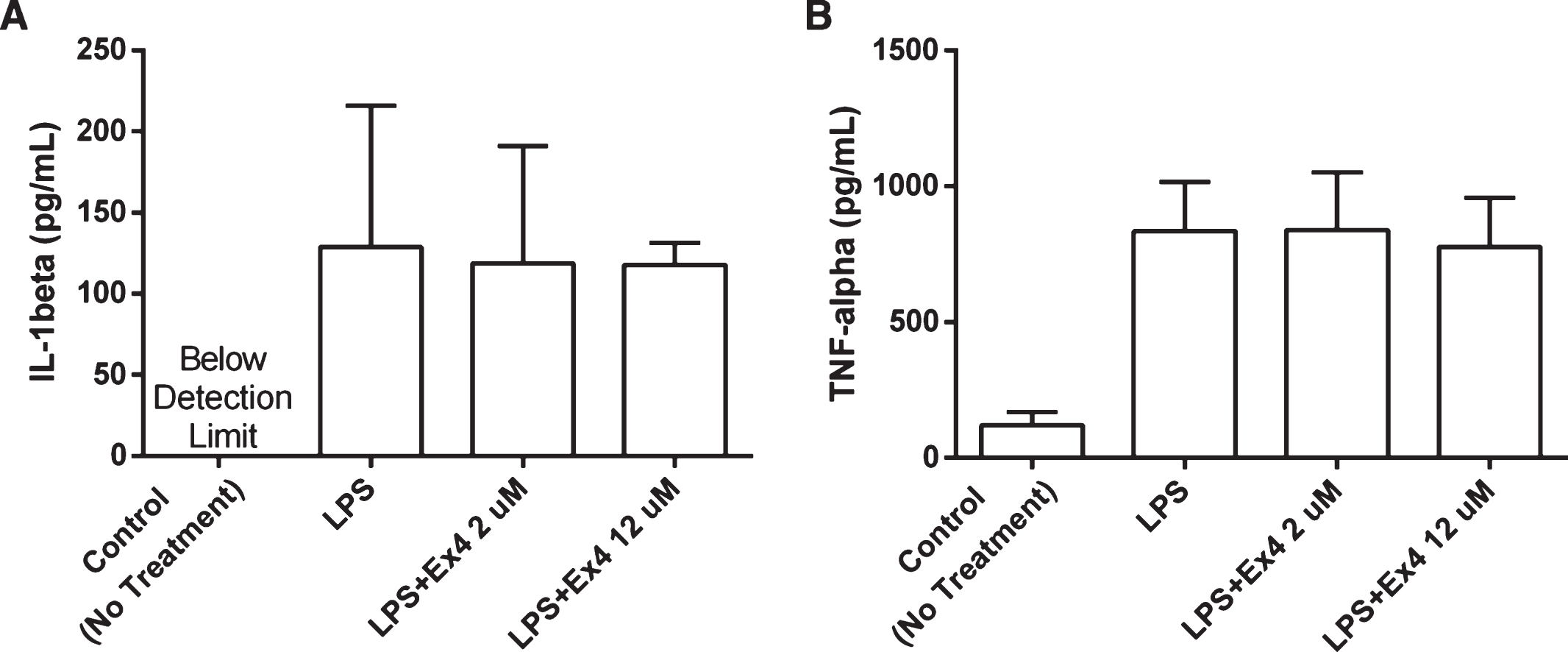
Cytokine levels from LPS-treated adult primary microglia cell cultures
Incubation with exendin-4 (2 μM and 12 μM) did not affect cytokine production from rat primary microglial cells cultured in LPS. No significant difference in IL-1β or TNF-α levels was observed following 24-hour incubation (Student’s t-tests, NS), Fig. 4(A) and (B). There were no significant differences in cell death (% PI positive cells) between groups after 6-hour incubation confirming that differences in cell viability/numbers did not influence the results (NS).
Fig.4
(A-B) The levels of cytokines in supernatants from adult primary microglia cell cultures. The cell cultures were treated with LPS (2 μg/mL) and exendin-4 (2 μM and 12 μM) and incubated for 24 hours (n = 3). No significant difference in the levels of IL-1β and TNF-α could be observed between the groups, (one-way ANOVA, NS). The IL-1β levels in the control group were below detection range. Bars represent the mean±SEM.
Effects on behavior
LPS-induced sickness behavior and general motor activity
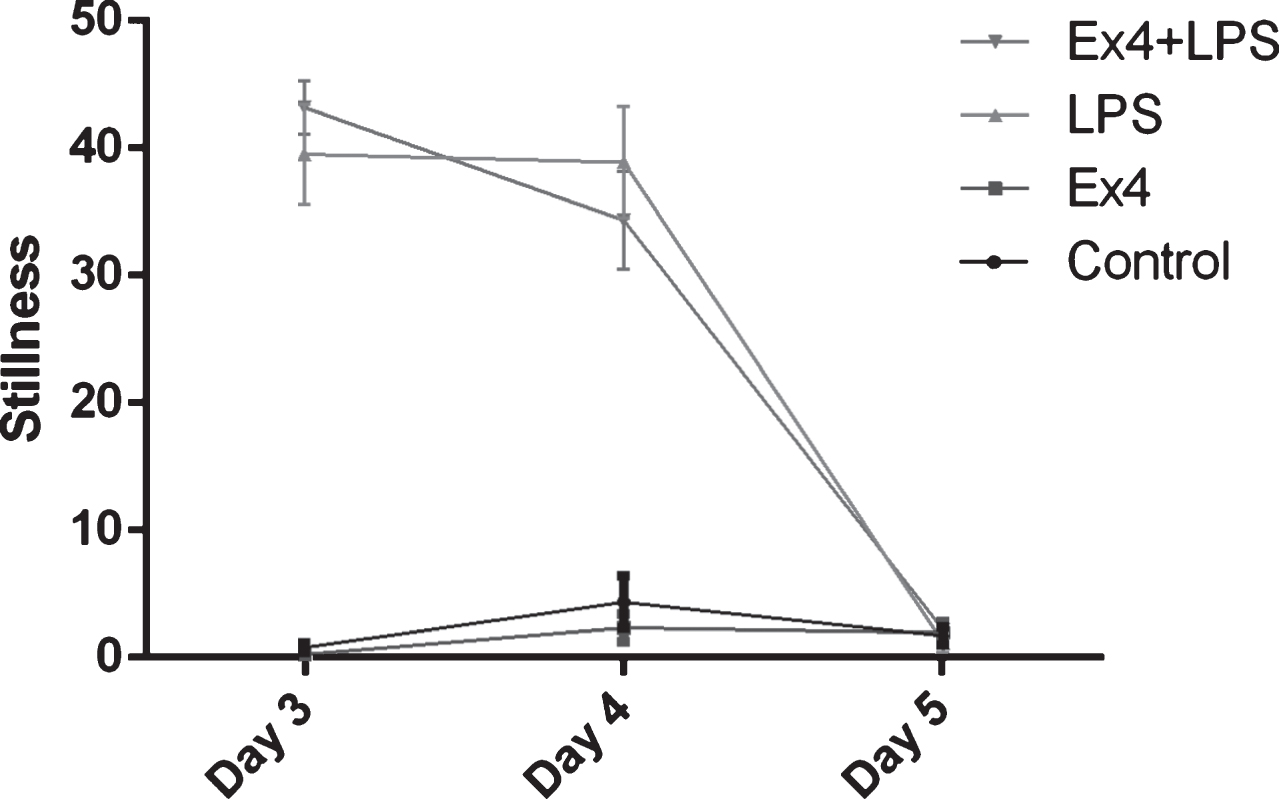
Rats displayed sickness behavior one hour after first LPS injection, as confirmed by significantly increased stillness in the LPS treated rats (F(1,38) = 473.87, p < 0.001, two-way ANOVA), Fig. 5. One day after the injection, the LPS treated rats lost 28.6 grams in weight (mean value) compared to 6.4 grams of weight gain in saline treated rats. Exendin-4 treatments did not affect weight (two-way ANOVA, NS). The rats also displayed sickness behavior after the second LPS injection (F(1,38) = 129.72, p < 0.001). At 24 hours after the second LPS injection there was no significant difference in stillness between groups. Thus, acute sickness behavior had ceased as expected 24 hours after the last LPS injection. Exendin-4 did not have any effect on sickness behavior (NS).
Fig.5
The number of periods a rat sat motionless (stillness). One hour after first LPS injection all LPS treated rats showed increased stillness, two-way ANOVA, F(1,38) = 473.87, p < 0.001 (day 3). Again, one hour after the second LPS injection, all LPS-treated rats showed increased stillness, two-way ANOVA, F(1,38) = 129.72, p < 0.001 (day 4). At 24 hours after the second LPS injection, all LPS-treated rats recovered and no significant difference in stillness was seen between groups, two-way ANOVA, NS (day 5). Bars represent the mean±SEM.
Depressive-like behavior
LPS had the strongest effect on immobility (behavioral despair) out of the three measures in the FST. There was a significant interaction between the LPS and exendin-4 treatments (F(1,38) = 5.33, p < 0.05, two-way ANOVA). LPS treated animals showed increased immobility (14.25±10.85 events, mean±SD) compared to vehicle treated rats (3.09±3.89, p < 0.01), whereas this effect was not present in the exendin-4 treated rats (6.33±6.34), Fig. 6(A).
Fig.6
The forced swim test. (A) Periods of 5 secs the rats spent immobile. There was a significant LPS×Exendin-4 interaction (two-way ANOVA, F(1,38) = 5.33, p < 0.05). LPS significantly increased the time spent immobile (induced behavioral despair) in animals without exendin-4 treatment (simple effect analysis, p < 0.01) but this effect was not present in the exendin-4 treated rats (NS). (B) The number of counts the rats spent climbing (vertical movements). There was no effect of either LPS or exendin-4 on climbing behavior (two-way ANOVA, NS). (C) The number of periods the rats spent swimming (horizontal movements). LPS treatment decreased the counted periods of swimming, (two-way ANOVA, F(1,38) = 10.03, p = 0.003). Bars represent the mean±SEM, *p < 0.05, **p < 0.01.
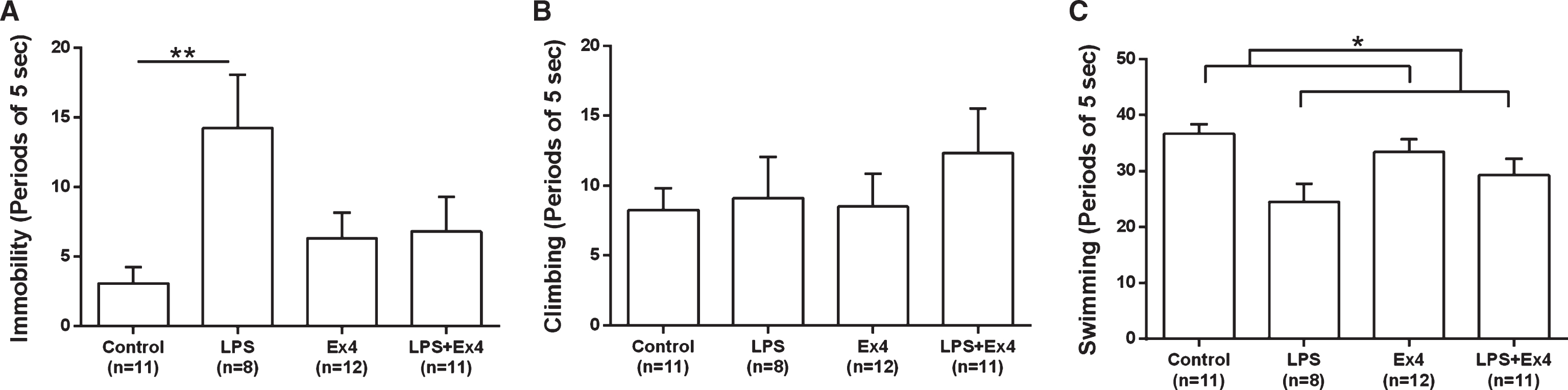
There were no significant differences in climbing between the experimental groups (Fig. 6(B)), however LPS-treated animals (LPS and LPS + Ex4) spent significantly less time swimming (horizontal movements), (F(1,38) = 10.03, p < 0.01, two-way ANOVA), Fig. 6(C). These results demonstrate that exendin-4 has anti-depressant-like properties, preventing despair in this model of inflammation-induced depression.
Effects on monoamines
Exendin-4 treatment did not significantly alter the levels of the monoamines or their metabolites in the striatum or pre-frontal cortex (two-way ANOVAs, NS). However, there was a trend towards an increasing effect of exendin-4 on the striatal dopamine levels (two-way ANOVA, F(1,37) = 5.46, p = 0.059) and towards a lower DOPAC/DA ratio in striatum (two-way ANOVA, p = 0.10). The levels of the dopamine, serotonin and their metabolites in striatum and pre-frontal cortex of the rats in the four experimental groups are shown in Table 1.
As expected, LPS had an effect on the monoamine metabolism (two-way ANOVAs). The HVA/DA and the DOPAC/DA ratios were both increased in the pre-frontal cortex of LPS treated animals; in the striatum the HVA/DA ratio was increased (Table 1).
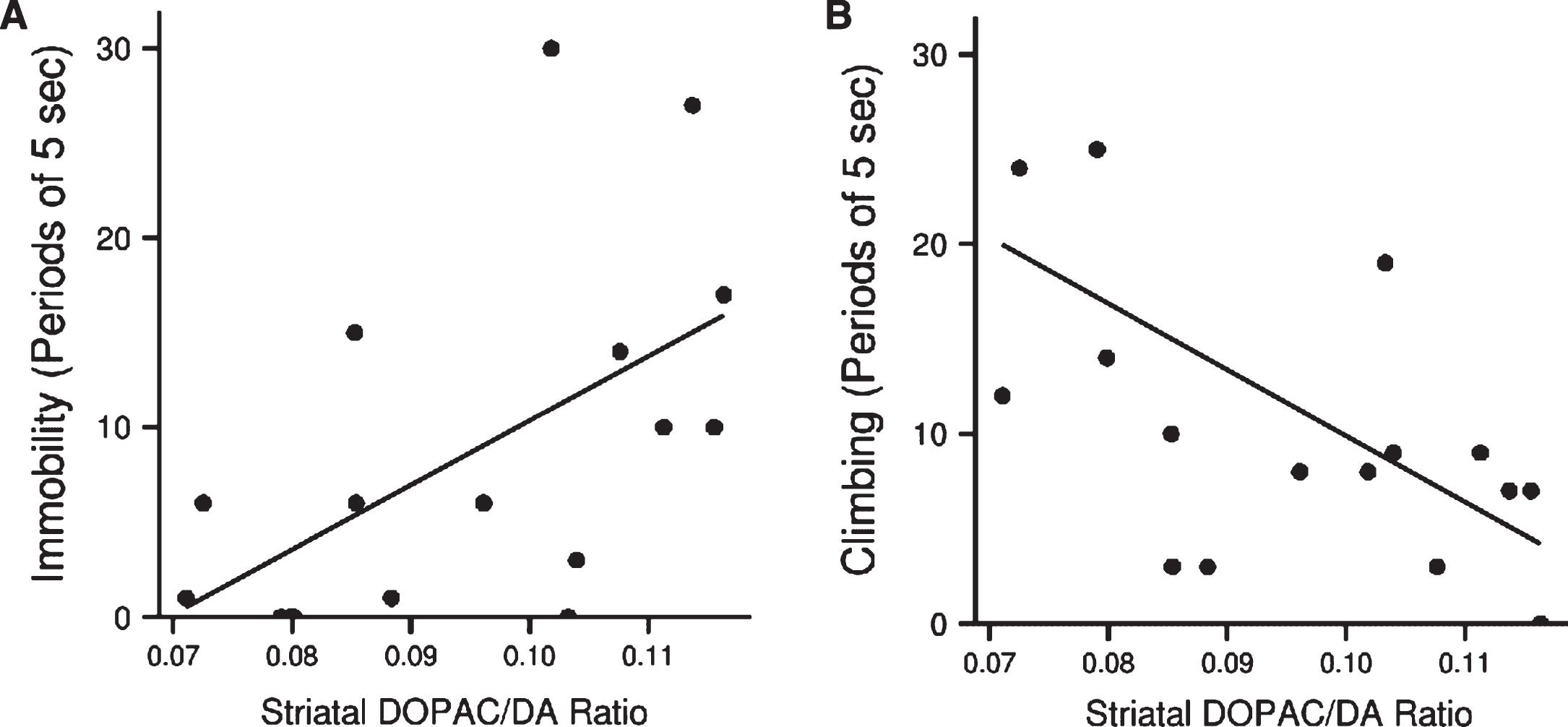
The DOPAC/DA ratio in the striatum of the LPS-treated animals (with and without exendin-4) was significantly associated with both floating (Spearman’s rho = 0.6, p < 0.05, n = 17) and climbing (rho = –0.7, p < 0.01, n = 17), Fig. 7(A) and (B). Hence, lower dopamine turnover resulted in less depressive-like behavior.
Fig.7
(A) Association between dopamine turnover in striatum of LPS treated animals and immobility (Spearman’s rho = 0.6, p < 0.05, n = 17) and (B) climbing (rho = – 0.7, p < 0.01, n = 17) in the forced swim test.
DISCUSSION
We did not detect any anti-inflammatory effect of exendin-4 in the periphery or CNS of LPS-treated rats. In addition, exendin-4 was not anti-inflammatory of on cultured primary LPS-stimulated microglia cells from adult rats. Similarly, exendin-4 did not affect sickness behavior in rats, a behavior that is thought to mainly result from the actions of pro-inflammatory cytokines [38]. By contrast, we found that exendin-4 prevents LPS-induced depressive-like symptoms, known to occur at about 24 hours after a peripheral immune challenge. However, the effect was not mediated through modulation of neuroinflammation as we had hypothesized. Instead we found that an increased dopamine turnover in striatum was significantly associated with increased immobility and less climbing in the FST, e.g. with more pronounced depressive-like symptoms. Indeed, the treatment with exendin-4 had a near-significant increasing effect on the dopamine levels in the striatum of the animals.
Exendin-4 has previously been suggested to exert anti-inflammatory effects in Type II diabetes [39] and experimental PD models. Specifically, exendin-4 has been shown to inhibit MPTP-induced microglial activation and suppress the increase of TNF-α and IL-1β mRNA in the substantia nigra [13]. Exendin-4 has also been shown to reduce depressive-like behavior in a model of PD, where mechanisms were not detailed [40]. In a brain injury model, exendin-4 was protective, but the effects on neuroinflammation were less clear. There was no effect on the levels of pro-inflammatory cytokines, although markers of wound-healing reparative microglia (M2 phenotype) were upregulated [41]. No previous study has specifically defined the anti-inflammatory actions of exendin-4 in the CNS in a paradigm that lacks neurodegeneration. Thus, prior studies have focused on e.g. stroke models or toxin-induced PD models.
Here, we explored for the first time if exendin-4 could alleviate inflammation-induced depressive-like behavior in rats treated with peripheral LPS injections. The LPS injections induced the expected acute transient inflammatory response, including sickness behavior. At 24 hours post injection, there was an increased IL-1β mRNA expression in the prefrontal cortex, striatum and hippocampus; this was the time-point when the animals also exhibited depressive-like behavior in the FST [31, 38, 42]. We could not, however, detect any effects of the exendin-4 treatment on the cytokine levels, either in serum, CSF or mRNA expression in the brain. It should be noted that our study has only tested one dosage regimen of exendin-4, and different effects might be observed in more chronic treatment models. However, also when we cultured primary microglia cells and treated them with LPS to induce cytokine production, there were no effects of exendin-4 on IL-1β or TNF-α production even though we used similar experimental conditions as previous macrophage cell cultures [43]. These in-vitro results indicate that exendin-4 does not have a direct anti-inflammatory effect on rodent microglia cells. Altogether, our results do not indicate that the effects of exendin-4 on behavioral despair were due to a reduction in neuroinflammation. In fact, all LPS-treated animals (LPS and LPS + exendin-4) displayed similar weight loss and showed the same amount of sickness behavior. Instead, our results indicated that the anti-depressive effects of exendin-4 might be associated with changes in dopamine production or metabolism in the striatum.
Several prior studies have shown that exendin-4 can affect striatal dopamine neurotransmission, mostly in toxin-induced PD models [8]. Exendin-4 has also been shown to increase the release of dopamine [44]. Interestingly, activation of the GLP-1 receptor by exendin-4 increases the synthesis of intracellular cAMP [45]; and elevated levels of cAMP increases the expression and activity of tyrosine hydroxylase [46], the rate limiting enzyme in the synthesis of dopamine, which could be the underlying mechanism of action [8]. Peripheral LPS injections in rodents are known to increase the turnover (metabolite/monoamine ratio) of serotonin and dopamine in multiple structures in the brain [47, 48]. In agreement with this, we found that, at least 24 hours after last LPS injection, LPS-treated animals (LPS and LPS + exendin-4) had increased levels of HVA in striatum, suggestive of increased dopamine metabolism. Dopamine activity in the dorsal striatum has been linked to goal-directed behavior [49] and incentive motivation [50] in rodents. Furthermore, depressive-like behavior has been associated with changes in striatal dopamine neurochemistry in various PD models [51, 52]. In our study, the rats receiving exendin-4 treatment exhibited a trend towards higher striatal dopamine and lower DOPAC/DA ratio, suggestive of a reduced dopamine turnover. The DOPAC/DA ratio in striatum correlated with immobility, a sign of behavioral despair, in LPS treated rats. In other words, rats with a high ratio exhibited more immobility. This indicates that exendin-4 might exert anti-depressive effects at least in part by affecting the dopamine system. Although our results suggest that the effects of exendin-4 on behavioral despair might be coupled to changes in striatal dopamine signaling, additional mechanisms that we did not explore might also be involved. For example, a recent study examined the effects of exendin-4 on behavioral despair under baseline conditions (no lesion or challenge), and found that the treatment affected serotonergic signaling in the amygdala [53].
In conclusion, we have shown that exendin-4 prevents LPS-induced behavioral despair by attenuating immobility in the FST. This behavioral effect occurred independently of inflammatory changes in the CNS or periphery, but was associated with alterations in dopamine metabolism. In light of ongoing trials with exendin-4 in PD, further studies detailing the effects of exendin-4 on the changes in striatal dopamine metabolism are highly warranted.
ACKNOWLEDGMENTS AND SOURCES OF SUPPORT
We thank Anna Hammarberg, who kindly operated and provided the FACS data necessary for our analysis.
This work was supported The Swedish Research Council (LB, PB), The Strong Research Environment Multipark (Multidisciplinary research in Parkinson’s disease at Lund University) (LB, PB), The Swedish Parkinson Foundation (PB); in part by the Udall Center of Excellence in Parkinson’s Disease Research at Michigan State University, P50NS58830 (JWL) and by Van Andel Institute (LB, PB). None of the supporters had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
[1] | Goke R , Larsen PJ , Mikkelsen JD , & Sheikh SP ((1995) ) Distribution of GLP-1 binding sites in the rat brain: Evidence that exendin-4 is a ligand of brain GL-1 binding sites. Eur J Neurosci, 7: , 2294–2300. |
[2] | Alvarez E , Martinez MD , Roncero I , Chowen JA , Garcia-Cuartero B , Gispert JD , Sanz C , Vazquez P , Maldonado A , de Caceres J , Desco M , Pozo MA , & Blazquez E ((2005) ) The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem, 92: , 798–806. |
[3] | Kastin AJ , & Akerstrom V ((2003) ) Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord, 27: , 313–318. |
[4] | Bertilsson G , Patrone C , Zachrisson O , Andersson A , Dannaeus K , Heidrich J , Kortesmaa J , Mercer A , Nielsen E , Ronnholm H , & Wikstrom L ((2008) ) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res, 86: , 326–338. |
[5] | Isacson R , Nielsen E , Dannaeus K , Bertilsson G , Patrone C , Zachrisson O , & Wikstrom L ((2011) ) The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol, 650: , 249–255. |
[6] | Kobayashi K , Iwai T , Sasaki-Hamada S , Kamanaka G , & Oka J ((2013) ) Exendin (5-39), an antagonist of GLP-1 receptor, modulates synaptic transmission via glutamate uptake in the dentate gyrus. Brain Res, 1505: , 1–10. |
[7] | Li Y , Perry T , Kindy MS , Harvey BK , Tweedie D , Holloway HW , Powers K , Shen H , Egan JM , Sambamurti K , Brossi A , Lahiri DK , Mattson MP , Hoffer BJ , Wang Y , & Greig NH ((2009) ) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad SciU S A, 106: , 1285–1290. |
[8] | Harkavyi A , Abuirmeileh A , Lever R , Kingsbury AE , Biggs CS , & Whitton PS ((2008) ) Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation, 5: , 19. |
[9] | Gault VA , & Holscher C ((2008) ) GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol, 587: , 112–117. |
[10] | Abbas T , Faivre E , & Holscher C ((2009) ) Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res, 205: , 265–271. |
[11] | Wang XH , Li L , Holscher C , Pan YF , Chen XR , & Qi JS ((2010) ) Val8-glucagon-like peptide-1 protects against Abeta1-40-induced impairment of hippocampal late-phase long-term potentiation and spatial learning in rats. Neuroscience, 170: , 1239–1248. |
[12] | Holscher C ((2012) ) Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs, 26: , 871–882. |
[13] | Kim S , Moon M , & Park S ((2009) ) Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol, 202: , 431–439. |
[14] | Athauda D , & Foltynie T ((2016) ) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: Mechanisms of action. Drug Discov Today, 21: , 802–818. |
[15] | Cao L , Li D , Feng P , Li L , Xue GF , Li G , & Holscher C ((2016) ) A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson’s disease by reducing chronic inflammation in the brain. Neuroreport, 27: , 384–391. |
[16] | Huang HJ , Chen YH , Liang KC , Jheng YS , Jhao JJ , Su MT , & Lee-Chen GJ , Hsieh-Li HM ((2012) ) Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS One, 7: , e39656. |
[17] | Darsalia V , Mansouri S , Ortsater H , Olverling A , Nozadze N , Kappe C , Iverfeldt K , Tracy LM , Grankvist N , Sjoholm A , & Patrone C ((2012) ) Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond), 122: , 473–483. |
[18] | McIntyre RS , Powell AM , Kaidanovich-Beilin O , Soczynska JK , Alsuwaidan M , Woldeyohannes HO , Kim AS , & Gallaugher LA ((2013) ) The neuroprotective effects of GLP-1: Possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res, 237: , 164–171. |
[19] | Athauda D , & Foltynie T ((2015) ) The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol, 11: , 25–40. |
[20] | Aviles-Olmos I , Dickson J , Kefalopoulou Z , Djamshidian A , Ell P , Soderlund T , Whitton P , Wyse R , Isaacs T , Lees A , Limousin P , & Foltynie T ((2013) ) Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest, 123: , 2730–2736. |
[21] | Aviles-Olmos I , Dickson J , Kefalopoulou Z , Djamshidian A , Kahan J , Fmedsci PE , Whitton P , Wyse R , Isaacs T , Lees A , Limousin P , & Foltynie T ((2014) ) Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis, 4: , 337–344. |
[22] | McGeer PL , & McGeer EG ((2008) ) Glial reactions in Parkinson’s disease. Mov Disord, 23: , 474–483. |
[23] | Smith RS ((1991) ) The macrophage theory of depression. Med Hypotheses, 35: , 298–306. |
[24] | Miller AH , Maletic V , & Raison CL ((2009) ) Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry, 65: , 732–741. |
[25] | Dowlati Y , Herrmann N , Swardfager W , Liu H , Sham L , Reim EK , & Lanctot KL ((2010) ) A meta-analysis of cytokines in major depression. Biol Psychiatry, 67: , 446–457. |
[26] | Steiner J , Bielau H , Brisch R , Danos P , Ullrich O , Mawrin C , Bernstein HG , & Bogerts B ((2008) ) Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res, 42: , 151–157. |
[27] | Wang Q , Liu Y , & Zhou J ((2015) ) Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener, 4: , 19. |
[28] | Lindqvist D , Hall S , Surova Y , Nielsen HM , Janelidze S , Brundin L , & Hansson O ((2013) ) Cerebrospinal fluid inflammatory markers in Parkinson’s disease–associations with depression, fatigue, and cognitive impairment. Brain Behav Immun, 33: , 183–189. |
[29] | Cai Z , Fan LW , Kaizaki A , Tien LT , Ma T , Pang Y , Lin S , Lin RC , & Simpson KL ((2013) ) Neonatal systemic exposure to lipopolysaccharide enhances susceptibility of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Dev Neurosci, 35: , 155–171. |
[30] | Qin L , Wu X , Block ML , Liu Y , Breese GR , Hong JS , Knapp DJ , & Crews FT ((2007) ) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia, 55: , 453–462. |
[31] | Bay-Richter C , Janelidze S , Hallberg L , & Brundin L ((2011) ) Changes in behaviour and cytokine expression upon a peripheral immune challenge. Behav Brain Res, 222: , 193–199 . |
[32] | Swiergiel AH , & Dunn AJ ((2007) ) Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav, 86: , 651–659. |
[33] | Cryan JF , Markou A , & Lucki I ((2002) ) Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci, 23: , 238–245. |
[34] | McNamara RK , Sullivan J , Richtand NM , Jandacek R , Rider T , Tso P , Campbell N , & Lipton J ((2008) ) Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: Relationship with ventral striatum dopamine concentrations. Synapse, 62: , 725–735 . |
[35] | Tonelli LH , Holmes A , & Postolache TT ((2008) ) Intranasal immune challenge induces sex-dependent depressive-like beavihor and cytokine expression in the brain. Neuropsychopharmacology, 33: , 1038–1048. |
[36] | Bonefeld BE , Elfving B , & Wegener G ((2008) ) Reference genes for normalization: A study of rat brain tissue. Synapse, 62: , 302–309. |
[37] | Ford AL , Goodsall AL , Hickey WF , & Sedgwick JD ((1995) ) Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+T cells compared. J Immunol, 154: , 4309–4321. |
[38] | Dantzer R , O’Connor JC , Freund GG , Johnson RW , & Kelley KW ((2008) ) From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci, 9: , 46–56. |
[39] | Cechin SR , Perez-Alvarez I , Fenjves E , Molano RD , Pileggi A , Berggren PO , Ricordi C , & Pastori RL ((2012) ) Anti-inflammatory properties of exenatide in human pancreatic islets. Cell Transplant, 21: , 633–648. |
[40] | Rampersaud N , Harkavyi A , Giordano G , Lever R , Whitton J , & Whitton P ((2012) ) Exendin-4 reverts behavioural and neurochemical dysfunction in a pre-motor rodent model of Parkinson’s disease with noradrenergic deficit. Br J Pharmacol, 167: , 1467–1479. |
[41] | Darsalia V , Hua S , Larsson M , Mallard C , Nathanson D , Nystrom T , Sjoholm A , Johansson ME , & Patrone C ((2014) ) Exendin-4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial M2 polarization. PLoS One, 9: , e103114. |
[42] | Yirmiya R ((1996) ) Endotoxin produces a depressive-like episode in rats. Brain Res, 711: , 163–174. |
[43] | Chang SY , Kim DB , Ryu GR , Ko SH , Jeong IK , Ahn YB , Jo YH , & Kim MJ ((2013) ) Exendin-4 inhibits iNOS expression at the protein level in LPS-stimulated Raw264.7 macrophage by the activation of cAMP/PKA pathway. J Cell Biochem, 114: , 844–853. |
[44] | Abuirmeileh A , Harkavyi A , Rampersaud N , Lever R , Tadross JA , Bloom SR , & Whitton PS ((2012) ) Exendin-4 treatment enhances L-DOPA evoked release of striatal dopamine and decreases dyskinetic movements in the 6-hydoxydopamine lesioned rat. J Pharm Pharmacol, 64: , 637–643. |
[45] | Drucker DJ , Philippe J , Mojsov S , Chick WL , & Habener JF ((1987) ) Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A, 84: , 3434–3438. |
[46] | Kim KS , Park DH , Wessel TC , Song B , Wagner JA , & Joh TH ((1993) ) A dual role for the cAMP-dependent protein kinase in tyrosine hydroxylase gene expression. Proc Natl Acad Sci U S A, 90: , 3471–3475. |
[47] | Lavicky J , & Dunn AJ ((1995) ) Endotoxin administration stimulates cerebral catecholamine release in freely moving rats as assessed by microdialysis. J Neurosci Res, 40: , 407–413. |
[48] | Dunn AJ ((1992) ) Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: Comparison with interleukin-1. J Pharmacol Exp Ther, 261: , 964–969. |
[49] | Robinson S , Sotak BN , During MJ , & Palmiter RD ((2006) ) Local dopamine production in the dorsal striatum restores goal-directed beavihor in dopamine-deficient mice. Behav Neurosci, 120: , 196–200. |
[50] | Tomer R , Goldstein RZ , Wang GJ , Wong C , & Volkow ND ((2008) ) Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol, 77: , 98–101. |
[51] | Tadaiesky MT , Dombrowski PA , Figueiredo CP , Cargnin-Ferreira E , Da Cunha C , & Takahashi RN ((2008) ) Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience, 156: , 830–840 . |
[52] | Santiago RM , Barbieiro J , Lima MM , Dombrowski PA , Andreatini R , & Vital MA ((2010) ) Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry, 34: , 1104–1114. |
[53] | Anderberg RH , Richard JE , Hansson C , Nissbrandt H , Bergquist F , & Skibicka KP ((2016) ) GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology, 65: , 54–66. |



