Levodopa-Carbidopa Intestinal Gel Pharmacokinetics: Lower Variability than Oral Levodopa-Carbidopa
Abstract
In a double-blind, double-dummy, double-titration Phase 3 trial in advanced Parkinson’s disease (PD) patients, the efficacy and safety of Levodopa-carbidopa intestinal gel (LCIG) infusion were characterized relative to immediate-release oral levodopa-carbidopa (LC-oral) treatment. We present in this report the comparative pharmacokinetic profiles of LCIG and LC-oral from this pivotal study. The results presented in this report clearly demonstrate that LCIG results in lower variability and fluctuations in levodopa and carbidopa plasma concentrations compared to LC-oral. The superior pharmacokinetic profiles with LCIG were consistent with its improved efficacy compared to LC-oral as demonstrated in this study.
INTRODUCTION
Levodopa is considered to be the most effective treatment of Parkinson’s Disease (PD) [1, 2]. However, the high variability in levodopa plasma concentrations with oral levodopa-carbidopa (LC-oral) treatment often results in sub-optimal efficacy, especially as the disease progresses and the therapeutic window gets narrower [3]. One strategy to reduce levodopa exposure fluctuations is continuous delivery of levodopa and carbidopa to the jejunum to overcome the short elimination half-life of levodopa and by-pass the variable gastric emptying step prior to absorption [4, 5]. Levodopa-carbidopa intestinal gel (LCIG) was developed to overcome the limitations of oral levodopa carbidopa treatment. The LCIG system (Duodopa/Duopa®) consists of a suspension of levodopa and carbidopa monohydrate (4 : 1) in an aqueous gel (carboxymethyl cellulose) that is continuously delivered via a portable infusion pump to the proximal small intestine through a percutaneous endoscopic gastrostomy with jejunal extension (PEG-J). In a double-blind, double-dummy, double-titration Phase 3 trial in advanced PD patients (the LCIG Horizon study), LCIG has been shown to significantly reduce the “Off” time and increase the “On” time without troublesome dyskinesia compared to LC-oral treatment [6]. This work compared the pharmacokinetic profiles of both levodopa and carbidopa for LCIG and LC-oral treatment from this pivotal Phase 3 trial.
METHODS
In the LCIG Horizon Phase 3 trial [6], 71 patients with advanced PD were randomized to receivecontinuous LCIG infusion plus placebo LC-oral capsules (n = 37) or to receive LC-oral capsules (100/25 mg levodopa/carbidopa) plus continuous placebo gel infusion (n = 34) for 12 weeks.
Each day, study drugs (gel infusion and capsules) were administered over a 16-hour period and consisted of a morning dose (infusion or oral capsules) and a continuous infusion of the gel, and a regimen of oral capsules. Both groups were titrated to optimal effect. The schedule of LC-oral dosing during the double-blind period was to be based on the LC-oral dosing schedule established prior to double-blind randomization. Additional doses of open-label oral immediate-release levodopa-carbidopa tablets were used to treat acute changes in the subject’s Parkinson’s disease symptoms. Both groups were allowed to take LC-oral at night.
Out of the 71 advanced PD patients randomized, 20 patients (10 randomized to receive LCIG and 10 randomized to receive LC-oral) had intensive pharmacokinetic data on Weeks 4 and 12 of treatment; 41 of the enrolled subjects had less frequent pharmacokinetic samples collected on Week 6 of treatment. For the 20 subjects with intensive pharmacokinetic collection, planned pharmacokinetic sampling was as follows: at 12, 16, 17 and 18 hours post initiation of LCIG infusion on days 28 and 84 and prior to initiation of LCIG and after the start of infusion at the following time points: 5 minutes, end of morning dose, and 1, 1.33, 1.67, 2, 2.33, 2.67, 4, 4.33, 4.67, 8, 8.33 and 8.67 hours on days 29 and 85.
Levodopa and carbidopa plasma concentrations versus time profiles of the two formulations were compared for the subjects with intensive pharmacokinetic samples. Subjects with less frequent pharmacokinetic sampling had samples collected prior to initiation of intestinal gel infusion and after start of infusion at the following time points: 1, 2, 4 and 8 hours on study day 43.
Some subjects had additional blood samples collected at 12 and 16 hours post-infusion initiation on study day 42. For all of the subjects with pharmacokinetic data (N = 61), the inter- and intra-subject variability for levodopa and carbidopa plasma concentrations during the 2- to 16-hour interval relative to start of LCIG infusion (N = 33) or administration of the first morning LC-oral (N = 28) capsule was estimated using a linear mixed-effects model. The linear mixed-effects analysis was conducted on the logarithm of levodopa and carbidopa plasma concentrations with fixed effect (classification) for time and random effects for subject and occasion within subject.
The protocol for the study was approved by the institutional review board at each site and written informed consent was obtained from each subject before any study-related procedures were performed.
RESULTS
On the pharmacokinetic sampling days for the 20 subjects with intensive pharmacokinetic sampling on Week 4 and Week 12, the mean daily study drug levodopa doses ranged from 1004 to 1118 mg for subjects receiving LCIG and from 1211 to 1417 mg for subjects receiving LC-oral. The mean daily study drug carbidopa doses ranged from 251 to 280 mg for subjects receiving LCIG and from 306 to 354 mg for subjects receiving LC-oral.
For all 61 patients for which pharmacokinetic data were available (intensive or sparse), the mean daily study drug levodopa doses ranged from 1004 to 1284 mg for subjects receiving LCIG and from 1211 to 1417 mg for subjects receiving LC-oral. The mean daily study drug carbidopa doses ranged from 251 to 321 mg for subjects receiving LCIG and from 303 to 354 mg for subjects receiving LC-oral.
The pharmacokinetic profiles of the subjects with intensive pharmacokinetic sampling are presented in Fig. 1. Results from the linear mixed-effects analysis of levodopa and carbidopa plasma concentrations for all subjects with pharmacokinetic data are presented in Table 1. The intra-subject variability in levodopa plasma concentrations with LCIG treatment was approximately 1/3rd the intra-subject variability observed with LC-oral treatment, indicating lower fluctuations in levodopa plasma concentrations within a subject with LCIG treatment.
Fig.1
Levodopa and Carbidopa Plasma Concentrations for Subjects with Advanced Parkinson’s Disease Receiving LCIG or LC-Oral. Each color represents a different subject for each treatment. LCIG = levodopa-carbidopa intestinal gel. LC-Oral = oral levidopa-carbidopa.
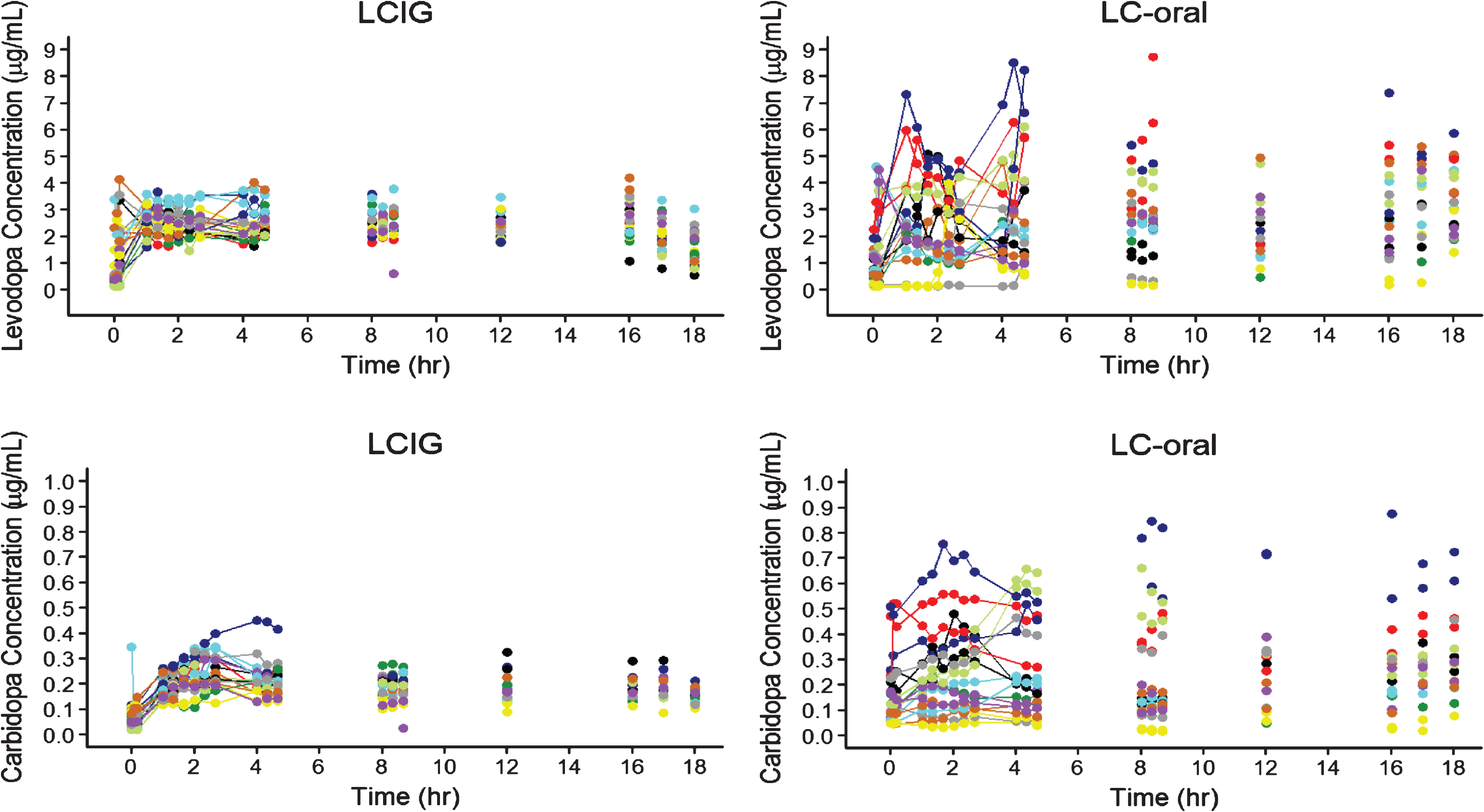
Table 1
Inter-Subject and Intra-Subject Coefficients of Variations (% CV) for Levodopa and Carbidopa Plasma Concentrations During Hour 2 to 16 Following Initiation of LCIG Infusion or Administration of the First Morning LC-oral Capsule
| N | Inter-Subject CV (%) | Intra-Subject CV (%) | ||||||||
| Analyte | LCIG | LC-Oral | LCIG | LC-oral | LCIG | LC-oral | ||||
| Levodopa | 33 | 28 | 35 | 93 | 21 | 67 | ||||
| Carbidopa | 33 | 28 | 31 | 70 | 25 | 39 | ||||
CV = coefficient of variation; N = number of subjects. LCIG = levodopa-carbidopa intestinal gel. LC-Oral = oral levidopa-carbidopa.
DISCUSSION
The pharmacokinetic profiles and the linear mixed-effects analysis of levodopa and carbidopa plasma concentrations presented in this report clearly demonstrate that LCIG results in lower variability and fluctuations in levodopa and carbidopa plasma concentrations compared to LC-oral (Fig. 1 and Table 1). These results are consistent with previous work characterizing the pharmacokinetics for LCIG [4, 5, 7, 8]. The improved pharmacokinetic profile for levodopa with LCIG as compared to LC-oral is consistent with the observed lower mean (±SE) “Off” times of 1.91±0.57 hours and higher mean (±SE) “On” times of 1.86±0.65 hours without troublesome dyskinesia observed in this study [6]. Furthermore, this improved efficacy for LCIG compared to LC-oral was observed with lower average daily levodopa delivered (1004 to 1284 mg for LCIG and 1211 to 1417 mg for LC-oral), which provides further evidence for the importance of the improved pharmacokinetic profile.
The lower intra-subject variability of levodopa and carbidopa concentrations with LCIG administration compared to LC-oral administration is a result of the continuous infusion and bypassing the impact of intra-subject variability in gastric emptying rate on absorption rate with LCIG administration [9, 10]. Levodopa oral controlled release formulations as well as combination with additional COMT inhibitors have been explored as alternative strategies to reduce the large fluctuations in levodopa plasma concentrations; however, these strategies have been shown not to reduce the levodopa fluctuations to any major extent [11, 12]. Several new levodopa controlled release formulations are developed or under development [13]; however, the predicted low colonic absorption of levodopa is thought to be a major barrier to developing effective oral controlled release levodopa formulations [14]. Overall, the clinical advantage of controlled release oral preparations is considered to be marginal [15–17] while the clinical impact of LCIG vs LC-oral appears to be more substantial based on the measured daily time of troublesome PD symptoms and quality of lifemeasures [6].
The objective of constant LCIG infusion is to achieve continuous dopaminergic stimulation with an optimized dose that results in stable plasma levels within the patient’s individualized therapeutic window. However, even with stable plasma levodopa concentrations achieved with LCIG infusion, there are a number of factors that may influence the pharmacodynamic response, such as transport across the blood-brain barrier, enzymatic conversion of levodopa to dopamine, the storage capacity for dopamine in the dopaminergic nerve terminals, dopamine release at the effect site, and changes in pre- and post-synaptic dopamine receptor sensitivity. Therefore, residual variability in the response can always be expected, even after achieving an optimal pharmacokinetic profile.
Conflict of Interest
For each author, there is no financial/personal interest or belief that could affect his objectivity.
Funding Source Declaration
This study was funded by AbbVie. AbbVie contributed to the study design, research, and interpretation of data, writing, reviewing, and approving the publication.
Financial Disclosures
All authors are current or former AbbVie employees and may hold AbbVie stocks or options.
Author Agreement/Declaration
All authors have seen and approved the final version of the brief report being submitted. The article is the authors’ original work, hasn’t received prior publication and isn’t under consideration for publication elsewhere.
The work in this brief report has been submitted as an abstract for a poster presentation to the 2016 World Parkinson’s Congress: Abstract #674
REFERENCES
[1] | Olanow CW , Watts RL , & Koller WC ((2001) ) An algorithm (decision tree) for the management of Parkinson’s disease (2001): Treatment guidelines. Neurology, 56: , S1–S88. |
[2] | LeWitt PA , Nelson MV , Berchou RC , Galloway MP , Kesaree N , Kareti D , & Schlick P ((1989) ) Controlled-release carbidopa/levodopa (Sinemet 50/200 CR4): Clinical and pharmacokinetic studies. Neurology 39: , 45–53; discussion 59. |
[3] | Nyholm D , Lennernas H , Gomes-Trolin C , & Aquilonius SM ((2002) ) Levodopa pharmacokinetics and motor performance during activities of daily living in patients with Parkinson’s disease on individual drug combinations. Clin Neuropharmacol, 25: , 89–96. |
[4] | Nyholm D , Askmark H , Gomes-Trolin C , Knutson T , Lennernas H , Nystrom C , & Aquilonius SM ((2003) ) Optimizing levodopa pharmacokinetics: Intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol, 26: , 156–163. |
[5] | Nyholm D , Odin P , Johansson A , Chatamra K , Locke C , Dutta S , & Othman AA ((2013) ) Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J 15: , 316–323. |
[6] | Olanow CW , Kieburtz K , Odin P , Espay AJ , Standaert DG , Fernandez HH , Vanagunas A , Othman AA , Widnell KL , Robieson WZ , Pritchett Y , Chatamra K , Benesh J , Lenz RA , Antonini A , & Group LHS ((2014) ) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol, 13: , 141–149. |
[7] | Othman AA , & Dutta S ((2014) ) Population pharmacokinetics of levodopa in subjects with advanced Parkinson’s disease: Levodopa-carbidopa intestinal gel infusion vs. oral tablets. Br J Clin Pharmacol, 78: , 94–105. |
[8] | Othman AA , Chatamra K , Mohamed ME , Dutta S , Benesh J , Yanagawa M , & Nagai M ((2015) ) Jejunal Infusion of levodopa-carbidopa intestinal gel versus oral administration of levodopa-carbidopa tablets in japanese subjects with advanced Parkinson’s disease: Pharmacokinetics and pilot efficacy and safety. Clin Pharmacokinet, 54: , 975–984. |
[9] | Kurlan R , Nutt JG , Woodward WR , Rothfield K , Lichter D , Miller C , Carter JH , & Shoulson I ((1988) ) Duodenal and gastric delivery of levodopa in parkinsonism. Ann Neurol, 23: , 589–595. |
[10] | Bredberg E , Nilsson D , Johansson K , Aquilonius SM , Johnels B , Nystrom C , & Paalzow L ((1993) ) Intraduodenal infusion of a water-based levodopa dispersion for optimisation of the therapeutic effect in severe Parkinson’s disease. Eur J Clin Pharmacol, 45: , 117–122. |
[11] | Sagar KA , & Smyth MR ((2000) ) Bioavailability studies of oral dosage forms containing levodopa and carbidopa using column-switching chromatography followed by electrochemical detection. Analyst, 125: , 439–445. |
[12] | LeWitt PA , Jennings D , Lyons KE , Pahwa R , Rabinowicz AL , Wang J , Guarnieri M , Hubble JP , & Murck H ((2009) ) Pharmacokinetic-pharmacodynamic crossover comparison of two levodopa extension strategies. Mov Disord, 24: , 1319–1324. |
[13] | Kianirad Y , & Simuni T ((2016) ) Novel Approaches to Optimization of Levodopa Therapy for Parkinson’s Disease. Curr Neurol Neurosci Rep, 16: , 34. |
[14] | Fagerholm U , Lindahl A , & Lennernas H ((1997) ) Regional intestinal permeability in rats of compounds with different physicochemical properties and transport mechanisms. J Pharm Pharmacol, 49: , 687–690. |
[15] | Nutt JG , & Woodward WR ((1986) ) Levodopa pharmacokinetics and pharmacodynamics in fluctuating parkinsonian patients. Neurology, 36: , 739–744. |
[16] | Poewe WH , Lees AJ , & Stern GM ((1986) ) Treatment of motor fluctuations in Parkinson’s disease with an oral sustained-release preparation of L-dopa: Clinical and pharmacokinetic observations. Clin Neuropharmacol, 9: , 430–439. |
[17] | Cedarbaum JM , Kutt H , & McDowell FH ((1989) ) A pharmacokinetic and pharmacodynamic comparison of Sinemet CR (50/200) and standard Sinemet (25/100). Neurology 39: , 38–44; discussion 59. |



