Efficacy of Rotigotine at Different Stages of Parkinson’s Disease Symptom Severity and Disability: A Post Hoc Analysis According to Baseline Hoehn and Yahr Stage
Abstract
Background: The efficacy of rotigotine has been demonstrated in studies of patients with early (i.e. not receiving levodopa) and advanced (i.e. not adequately controlled on levodopa; average 2.5 h/day in ‘off’ state) Parkinson’s disease (PD).
Objective: To further investigate the efficacy of rotigotine transdermal patch across different stages of PD symptom severity and functional disability, according to baseline Hoehn and Yahr (HY) staging.
Methods: Post hoc analysis of six placebo-controlled studies of rotigotine in patients with early PD (SP506, SP512, SP513; rotigotine ≤8 mg/24 h) or advanced-PD (CLEOPATRA-PD, PREFER, SP921; rotigotine ≤16 mg/24 h). Data were pooled and analyzed according to baseline HY stage (1, 2, 3 or 4) for change from baseline to end of maintenance in Unified Parkinson’s Disease Rating Scale (UPDRS) II (activities of daily living), UPDRS III (motor) and UPDRS II+III; statistical tests are exploratory.
Results: Data were available for 2057 patients (HY 1 : 262; HY 2 : 1230; HY 3 : 524; HY 4 : 41). Patients at higher HY stages were older, had a longer time since PD diagnosis and higher baseline UPDRS II+III scores vs patients at lower HY stages. Rotigotine improved UPDRS II+III versus placebo for each individual HY stage (p < 0.05 for each HY stage), with treatment differences increasing with increasing HY stages. Similar results were observed for UPDRS II and UPDRS III.
Conclusions: This post hoc analysis suggests that rotigotine may be efficacious across a broad range of progressive stages of PD symptom severity and functional disability (HY stages 1–4).
INTRODUCTION
The management of Parkinson’s disease (PD), including decisions on the optimal timing and type of treatment, are guided by important factors such as the stage of the disease and the type and severity of symptoms [1]. While treatment initiation is often delayed until symptoms of PD begin to limit the patient’s ability to function [2– 4], evidence suggests that the early period after diagnosis may be critical for treatment initiation [5, 6]. Moreover, adding to the complexity of treatment decision in PD, due to the progressive nature of the disease, there is a common assumption that the efficacy of PD medication decreases as the severity of the symptoms of the disease progresses. However, no studies have assessed the effect of PD medication over progressive stages of the disease. The Hoehn and Yahr (HY) staging scale provides an estimate of clinical function in PD, focusing on motor symptom severity and relative level of disability [7].
Although levodopa is considered the most effective drug for the treatment of symptoms of PD, its long-term use is associated with the development of motor complications [8]. Dopamine receptor agonists (DAs) are commonly used as first-line monotherapy for symptomatic control in the early stages of PD to delay the initiation of levodopa therapy and as adjunctive therapy to levodopa as the severity of the disease progresses [1, 2, 9]. Nevertheless, there still is a degree of clinical uncertainty when to initiate levodopa.
Rotigotine is a non-ergoline DA with activity across D1 through D5 receptors as well as select adrenergic and serotonergic sites [10]. Continuous, steady transdermal delivery of rotigotine maintains stable plasma levels over 24 hours with a single daily application [11]. Several large, placebo-controlled studies have demonstrated the efficacy of rotigotine as monotherapy in patients with early PD (improvements in activities of daily living and motor symptoms) [12– 15], and as adjunctive therapy in patients with advanced PD not adequately controlled with levodopa (reductions in ‘off’ time) [16– 18]. In addition, a placebo-controlled study in patients with PD and early morning motor dysfunction demonstrated that rotigotine provided significant benefits in control of both motor function and nocturnal sleep disturbances [19]. However, rotigotine’s effects across the progressive stages of PD symptom severity and functional disability have not previously been reported.
The objective of this post hoc analysis of six double-blind placebo-controlled studies of rotigotine in early- and advanced-stage PD was to investigate benefits of rotigotine based on symptom severity and disability according to baseline HY staging.
METHODS
Studies included in analyses
Studies of rotigotine transdermal patch in patients with early- (defined as not receiving levodopa) and advanced-stage PD (defined as not adequately controlled on levodopa, and with an average 2.5 h/day spent in ‘off’ state) were included in this post hoc analysis only if they met the following criteria: (1) randomized, double-blind, placebo-controlled study (UCB Pharma sponsored); (2) ≥7 weeks maintenance phase duration; (3) Unified Parkinson’s Disease Rating Scale (UPDRS) II+III total score as efficacy variable; and (4) patients having HY stage assessments at baseline/screening (specifically performed during ‘on’ state for advanced-stage PD studies).
Six studies (SP506, SP512, SP513, CLEOPATRA-PD, PREFER and SP921) were identified and included in the current analyses. Detailed methodologies of the 6 studies have been published previously [12– 18]; the main study characteristics, including key inclusion criteria, are summarized in Table 1. Eligible patients were to be HY stage ≤3 at baseline in the 3 early PD studies and HY stage 2– 4 in both the ‘on’ and ‘off’ states in the 3 advanced-PD studies.
All 6 studies were conducted in accordance with the local laws of the countries, Good Clinical Practice and the Declaration of Helsinki. The study protocols and their amendments were approved by national, regional or investigational site ethics committees or institutional review boards. Written informed consent was obtained from all patients prior to participation.
Post hoc analyses according to baseline HY staging
The HY is a descriptive five-point staging scale, based on the concept that severity of overall dysfunction in PD relates to evolution from unilateral to bilateral motor dysfunction and progressive impairment of gait and balance [7]. The five HY stages are: HY stage 1: unilateral involvement only, usually with minimal/no functional disability; HY stage 2: bilateral or midline involvement without impairment of balance; HY stage 3: bilateral disease: mild to moderate disability with impaired postural reflexes; physically independent; HY stage 4: severely disabling disease; still able to walk/stand unassisted; HY stage 5: confined to wheelchair unless aided [7]. The scale is widely utilized and has high correlations with standardized scales of motor impairment, disability and quality of life; the Movement Disorder Society Task Force supports the use of the HY scale to categorize patients and to capture important aspects of PD progression [7].
Data from the six studies were pooled, and patients were analyzed according to HY stage at baseline (1, 2, 3 or 4). Of note, no patients at HY stage 5 were included in the rotigotine studies assessed in this post hoc analysis. For the 3 advanced-PD studies, data are reported based on the HY stage in the ‘on’ state. For each HY stage subgroup, change from baseline to end of maintenance (EoM) in the UPDRS II+III total score and UPDRS II (activities of daily living) and III (motor) subscores were analyzed. In addition, percentage change of the mean UPDRS score was analyzed to evaluate the relative magnitude of improvement with rotigotine across different HY stages.
Statistical analyses
Demographic and baseline characteristics along with efficacy assessments are reported for the full analysis set (patients who had a baseline and at least one post-baseline UPDRS II+III total score assessment). Efficacy assessments were performed using a last observation carried forward approach. Rotigotine vs placebo treatment differences for the change from baseline to EoM in UPDRS scores were assessed using an analysis of covariance model with treatment and study as factors and baseline UPDRS scores as a covariate. Analyses were performed in an exploratory manner only and p values <0.05 do not infer statistical significance.
RESULTS
Demographics and baseline characteristics
Data were available for 2057 patients. Compared with patients at a lower HY stage, patients at higher HY stages were generally older, had a longer time since PD diagnosis, and higher baseline UPDRS II+III total scores (Table 2).
Levodopa and rotigotine exposure
Proportion of patients receiving concomitant levodopa increased with HY stage, from 2% (5/262) at stage 1 to 100% (41/41) at stage 4 (this relates to the study designs; levodopa was not permitted in the early PD studies but was part of the inclusion criteria in the advanced PD studies). Baseline mean dose of concomitant levodopa also increased with HY stage (Table 2). Mean±SD dose of rotigotine at EoM was 5.9±2.3 mg/24 h for HY stage 1 group (n = 184), 7.1±3.7 mg/24 h for HY stage 2 (n = 849), 7.5±3.9 mg/24 h for HY stage 3 (n = 368), and 7.7±3.9 mg/24 h for HY stage 4 (n = 25) (mean dose was calculated from all studies, whether optimal or fixed-dose design).
Change from baseline to EoM in UPDRS scores
Rotigotine improved mean UPDRS II+III total score vs placebo for each individual HY stage (p < 0.05 for each HY stage), with treatment differences increasing with increasing HY stage (Fig. 1A). The percentage change of the mean UPDRS II+III total score indicated a relative improvement with rotigotine between ∼18 and ∼25% across the different HY stages (Fig. 1B). Similar results were observed for UPDRS II subscore (p < 0.01 for each HY stage) (Fig. 2A and B) and UPDRS III subscore (p < 0.01 for HY stage 1, 2, and 3; p = 0.053 for HY stage 4) (Fig. 2C and D).
DISCUSSION
The results of this post hoc analysis reporting data from over 2000 patients suggest that rotigotine transdermal patch may improve activities of daily living and motor symptoms across the different stages of PD symptom severity and disability, according to baseline HY stage.
The finding that baseline UPDRS II+III scores increased with HY stage is consistent with previous studies, demonstrating that progression in HY stage correlates with motor decline and impairment of activities of daily living [20– 22]. In the current analyses, mean absolute UPDRS II+III treatment differences in favor of rotigotine increased with HY stage, with the largest absolute change with rotigotine in the mean UPDRS II+III total score observed in the HY stage 4 group (∼15 points vs ∼7– 11 points in the HY stage 1, 2 and 3 groups). A similar pattern of improvement with rotigotine was observed in UPDRS II and III subscores. These data suggest that the efficacy of rotigotine may increase with increasing baseline disease severity. This may be because patients at more severe HY stages had higher baseline UPDRS scores and thus more scope for improvement. In addition, although the UPDRS is commonly used to assess signs of PD at all disease stages, the scale’s utility to detect treatment effects may be limited by a ‘floor effect’ in the early stages where impairment is subtle [23, 24]. Despite this, an improvement with rotigotine was observed in the HY stage 1 group (∼3 UPDRS II+III points treatment difference), and the percentage change of the mean UPDRS II+III total score indicated a relative improvement of ∼18 to ∼25% with rotigotine across the different HY stages. These data suggest that rotigotine may also provide benefits in the activities of daily living and motor symptoms very early in the disease course, and is consistent with the concept of earlier initiation of symptomatic treatment in PD [3, 5, 6]. In line with this, in a post hoc analysis of two open-label studies of rotigotine in early-stage PD (HY stage 1-2), a 6-month earlier vs postponed initiation of rotigotine resulted in a slower return to baseline of the mean UPDRS II+III total score [25]. However, to fully evaluate any potential benefit of early initiation of treatment with rotigotine on the activities of daily living and motor symptoms, there is a need for prospective studies specifically designed to assess the timing of rotigotine initiation on the outcome of UPDRS scores.
In the current analysis, patients in the more advanced HY stages were more likely to be receiving levodopa from study onset (due to study designs) and at higher doses. Despite this, the addition of rotigotine provided an extra benefit on top of that achieved with levodopa. Thus, in those patients with more severe axial symptoms and disability (i.e. HY stage 3 or 4) and already receiving levodopa, rotigotine may further improve activities of daily living and motor symptoms. Overall, our data suggest that the efficacy of rotigotine may be observed across the different stages of PD symptom severity and functional disability, with benefits more numerically pronounced as the underlying severity increases. Further prospectively designed, adequately powered studies are required to confirm theseobservations.
The current post hoc analyses have a number of limitations. Firstly, due to their exploratory nature, interpretation of the data and conclusions should be made with caution. Secondly, as patients were required to fulfill strict eligibility criteria, enrolled patients may not be fully representative of the wider PD population. In addition, as older patients are generally prone to gait and postural disturbances [26, 27], the observed increasing age at baseline with HY stage represents a potential confounding factor in our analysis. However, increased age at more advanced HY stages [28], as well as other baseline patient characteristics reported here (i.e., longer disease duration and higher UPDRS scores), have been previously described [20– 22, 29, 30]. Finally, there were considerable variations in study design between the included studies: 1) the analyses included studies with different treatment durations (maintenance phase varied from 7– 33 weeks); 2) the analyses combined studies with fixed and optimal dosing designs. However, pooling of all available double-blind, placebo-controlled studies of rotigotine (despite their variable durations and dosing schemes) allowed for a large sample size. This is also the first comprehensive assessment of the effectiveness of a DA across progressive stages of PD symptom severity and functional disability.
CONCLUSION
This post hoc analysis suggests that rotigotine transdermal patch may be efficacious in patients with PD across the stages of the disease from mild symptoms and minimal functional disability (HY stage 1) to increasing symptom severity and disability (HY stage 4).
CONFLICTS OF INTEREST
Nir Giladi serves as a member of the Editorial Board for the Journal of Parkinson’s Disease, and as a consultant to Teva-Lundbeck, Intec Pharma, NeuroDerm, Armon Neuromedical Ltd\Dexel, Mon4t, Pharma Two B, and Lysosomal Therapeutics Inc., provided expert testimony to GSK, served on advisory boards to Teva-Lundbeck, Lysosomal Therapeutics, Dexel, Abbvie, NeuroDerm, and Sionara, received honoraria from Teva-Lundbeck, Novartis, UCB Pharma, Movement Disorder Society, Genzyme and Shire, received payments for lectures at Teva-Lundbeck, Novartis, UCB Pharma, Abbvie, Shire and Genzyme, received research support from the Michael J Fox Foundation, the National Parkinson Foundation, the European Union 7th Framework Program, the Israel Science Foundation, Teva NNE program, Lysosomal Therapeutics, and Abbvie, and owns stocks in Lysosomal Therapeutics Inc. Anthony P Nicholas has been a consultant for Cynapsus, US WorldMeds, Lundbeck and ACADIA Pharmaceuticals, and has served as a local site Principal Investigator for clinical trials sponsored by Adamas, Cynapsus and the Michael J Fox Foundation. Werner Poewe has received personal fees from AbbVie, Allergan, AstraZeneca, BIAL, Boehringer Ingelheim, Boston Scientific, GlaxoSmithKline, Ipsen, Lundbeck, Medtronic, MSD, Merck Serono, Merz Pharmaceuticals, Novartis, Orion Pharma, TEVA Pharmaceuticals, UCB Pharma, and Zambon (consultancy and lecture fees in relation to clinical drug development programs for Parkinson’s disease), and royalties from Cambridge University Press, Oxford University Press, Thieme, and Wiley-Blackwell; Mahnaz Asgharnejad, Lars Bauer, Elisabeth Dohin, and Franz Woltering are salaried employees of UCB Pharma; Mahnaz Asgharnejad and Lars Bauer receive stock options from their employment.
ACKNOWLEDGMENTS
This post hoc analysis was supported by UCB Pharma, Monheim am Rhein, Germany. The analysis and manuscript development was a full partnership between the sponsor (UCB Pharma) and academic experts in the field of PD, who were investigators/principal investigators for the original double-blind studies included. The final data reported in this manuscript are the outcome of scientific discussions between all authors. Specifically, the original analysis was initially designed by the study sponsor, and subsequently modified upon review by the academic authors. All authors contributed significantly to the design/specifics of the analyses included in the manuscript, reviewed the data, were involved in the interpretation of the results, and reviewed and critiqued the manuscript. The sponsor statistician author was involved in running the data analysis. The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to the studies included in these analyses. The authors also acknowledge Karolina Rzeniewicz, PhD (Evidence Scientific Solutions, London, UK) for writing assistance, which was funded by UCB Pharma, Brussels, Belgium, and Cédric Laloyaux (Strategic Publication Lead Neurology, UCB Pharma, Brussels, Belgium) for publication coordination. The authors also acknowledge Marc Derycke, MSc (UCB Pharma, Monheim am Rhein, Germany) for his contributions to the specifications and programming of the post hoc analyses.
REFERENCES
[1] | Ferreira JJ , Katzenschlager R , Bloem BR , Bonuccelli U , Burn D , Deuschl G , Dietrichs E , Fabbrini G , Friedman A , Kanovsky P , Kosti V , Nieuwboer A , Odin P , Poewe W , Rascol O , Sampaio C , Schupbach M , Tolosa E , Trenkwalder C , Schapira A , Berardelli A & Oertel WH ((2013) ) Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol, 20: , 5–15. |
[2] | Connolly BS & Lang AE ((2014) ) Pharmacological treatment of Parkinson disease: A review. JAMA, 311: , 1670–1683. |
[3] | Löhle M , Ramberg C-J , Reichmann H & Schapira AV ((2014) ) Early versus delayed initiation of pharmacotherapy in Parkinson’s disease. Drugs, 74: , 645–657. |
[4] | Miyasaki JM , Martin W , Suchowersky O , Weiner WJ & Lang AE ((2002) ) Practice parameter: Initiation of treatment for Parkinson’s disease: An evidence-based review: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology, 58: , 11–17. |
[5] | Schapira AH & Obeso J ((2006) ) Timing of treatment initiation in Parkinson’s disease: A need for reappraisal? Ann Neurol, 59: , 559–562. |
[6] | Schrag A , Dodel R , Spottke A , Bornschein B , Siebert U & Quinn NP ((2007) ) Rate of clinical progression in Parkinson’s disease. A prospective study. Mov Disord, 22: , 938–945. |
[7] | Goetz CG , Poewe W , Rascol O , Sampaio C , Stebbins GT , Counsell C , Giladi N , Holloway RG , Moore CG , Wenning GK , Yahr MD & Seidl L ((2004) ) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov Disord, 19: , 1020–1028. |
[8] | Jankovic J & Aguilar LG ((2008) ) Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat, 4: , 743–757. |
[9] | Oertel WH , Bloem BR , Bonuccelli U , Burn D , Deuschl G , Dietrichs E , Fabbrini G , Ferreira JJ , Friedman A , Kanovsky P , Kostic V , Nieuwboer A , Odin P , Poewe W , Rascol O , Sampaio C , Schupbach M , Tolosa E & Trenkwalder C ((2011) ) Early (uncomplicated) Parkinson’s disease In European Handbook of Neurological Management. Blackwell Publishing Ltd., pp. 217–236. |
[10] | Scheller D , Ullmer C , Berkels R , Gwarek M & Lubbert H ((2009) ) The receptor profile of rotigotine: A new agent for the treatment of Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol, 379: , 73–86. |
[11] | Elshoff JP , Braun M , Andreas JO , Middle M & Cawello W ((2012) ) Steady-state plasma concentration profile of transdermal rotigotine: An integrated analysis of three, open-label, randomized, phase I multiple dose studies. Clin Ther, 34: , 966–978. |
[12] | Parkinson Study Group ((2003) ) A controlled trial of rotigotine monotherapy in early Parkinson’s disease. Arch Neurol, 60: , 1721–1728. |
[13] | Jankovic J , Watts RL , Martin W , Boroojerdi B & Group SPRTSCS ((2007) ) Transdermal rotigotine: Double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol, 64: , 676–682. |
[14] | Watts RL , Jankovic J , Waters C , Rajput A , Boroojerdi B & Rao J ((2007) ) Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology, 68: , 272–276. |
[15] | Giladi N , Boroojerdi B , Korczyn AD , Burn DJ , Clarke CE & Schapira AH ((2007) ) Rotigotine transdermal patch in early Parkinson’s disease: A randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord, 22: , 2398–2404. |
[16] | LeWitt PA , Lyons KE & Pahwa R ((2007) ) Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology, 68: , 1262–1267. |
[17] | Nicholas AP , Borgohain R , Chana P , Surmann E , Thompson EL , Bauer L , Whitesides J , Elmer LW & S.P. Study Investigators ((2014) ) A randomized study of rotigotine dose response on ‘off’ time in advanced Parkinson’s disease. J Parkinsons Dis, 4: , 361–373. |
[18] | Poewe WH , Rascol O , Quinn N , Tolosa E , Oertel WH , Martignoni E , Rupp M , Boroojerdi B & Investigators SP ((2007) ) Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: A double-blind, double-dummy, randomised controlled trial. Lancet Neurol, 6: , 513–520. |
[19] | Trenkwalder C , Kies B , Rudzinska M , Fine J , Nikl J , Honczarenko K , Dioszeghy P , Hill D , Anderson T , Myllyla V , Kassubek J , Steiger M , Zucconi M , Tolosa E , Poewe W , Surmann E , Whitesides J , Boroojerdi B , Chaudhuri KR & Recover Study G ((2011) ) Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: A double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord, 26: , 90–99. |
[20] | Alves G , Wentzel-Larsen T , Aarsland D & Larsen JP ((2005) ) Progression of motor impairment and disability in Parkinson disease: A population-based study. Neurology, 65: , 1436–1441. |
[21] | Zhao YJ , Wee HL , Chan YH , Seah SH , Au WL , Lau PN , Pica EC , Li SC , Luo N & Tan LC ((2010) ) Progression of Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Mov Disord, 25: , 710–716. |
[22] | Marinus J , Visser M , Stiggelbout AM , Rabey JM , Martinez-Martin P , Bonuccelli U , Kraus PH & van Hilten JJ ((2004) ) A short scale for the assessment of motor impairments and disabilities in Parkinson’s disease: The SPES/SCOPA. J Neurol Neurosurg Psychiatry, 75: , 388–395. |
[23] | Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease ((2003) ) The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord, 18: , 738–750. |
[24] | Visser M , Marinus J , Stiggelbout AM & van Hilten JJ ((2006) ) Responsiveness of impairments and disabilities in Parkinson’s disease. Parkinsonism Relat Disord, 12: , 314–318. |
[25] | Timmermann L , Asgharnejad M , Boroojerdi B , Dohin E , Woltering F & Elmer LW ((2015) ) Impact of 6-month earlier versus postponed initiation of rotigotine on long-term outcome: analysis of patients with early Parkinson’s disease with mild symptom severity. Expert Opin Pharmacother, 16: , 1423–1433. |
[26] | Giladi N , Horak FB & Hausdorff JM ((2013) ) Classification of gait disturbances: Distinguishing between continuous and episodic changes. Mov Disord, 28: , 1469–1473. |
[27] | Salzman B ((2010) ) Gait and balance disorders in older adults. Am Fam Physician, 82: , 61–68. |
[28] | Wagner ML , Fedak MN , Sage JI & Mark MH ((1996) ) Complications of disease and therapy: A comparison of younger and older patients with Parkinson’s disease. Ann Clin Lab Sci, 26: , 389–395. |
[29] | Hoehn MM & Yahr MD ((1967) ) Parkinsonism: Onset, progression and mortality. Neurology, 17: , 427–442. |
[30] | Kang GA , Bronstein JM , Masterman DL , Redelings M , Crum JA & Ritz B ((2005) ) Clinical characteristics in early Parkinson’s disease in a central California population-based study. Mov Disord, 20: , 1133–1142. |
Figures and Tables
Fig.1
Mean change (A) and percentage change of the mean (B) in UPDRS II+III total score from baseline to EoM. aAnalysis of covariance (ANCOVA) model adjusted for baseline UPDRS II+III total score and study. All statistical tests are exploratory in nature and p values <0.05 do not infer statistical significance. Abbreviations: CI, confidence interval; EoM, end of maintenance; LS, least squares; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
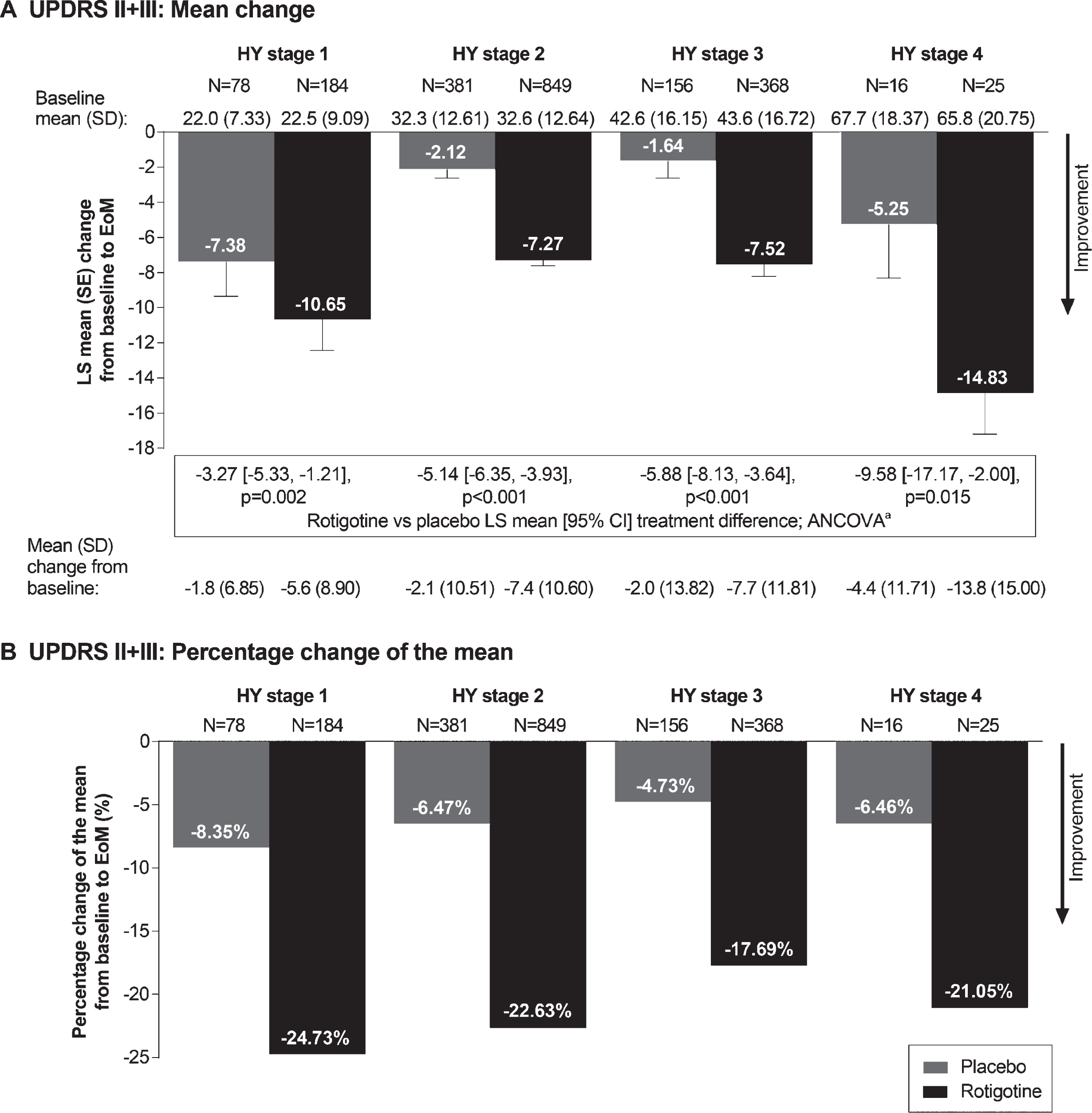
Fig.2
Mean change and percentage change of the mean in UPDRS II (A and B) and UPDRS III (C and D) subscores from baseline to EoM. aAnalysis of covariance (ANCOVA) model adjusted for baseline UPDRS II subscore or UPDRS III and study. All statistical tests are exploratory in nature and p values <0.05 do not infer statistical significance. Abbreviations: CI, confidence interval; EoM, end of maintenance; LS, least squares; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
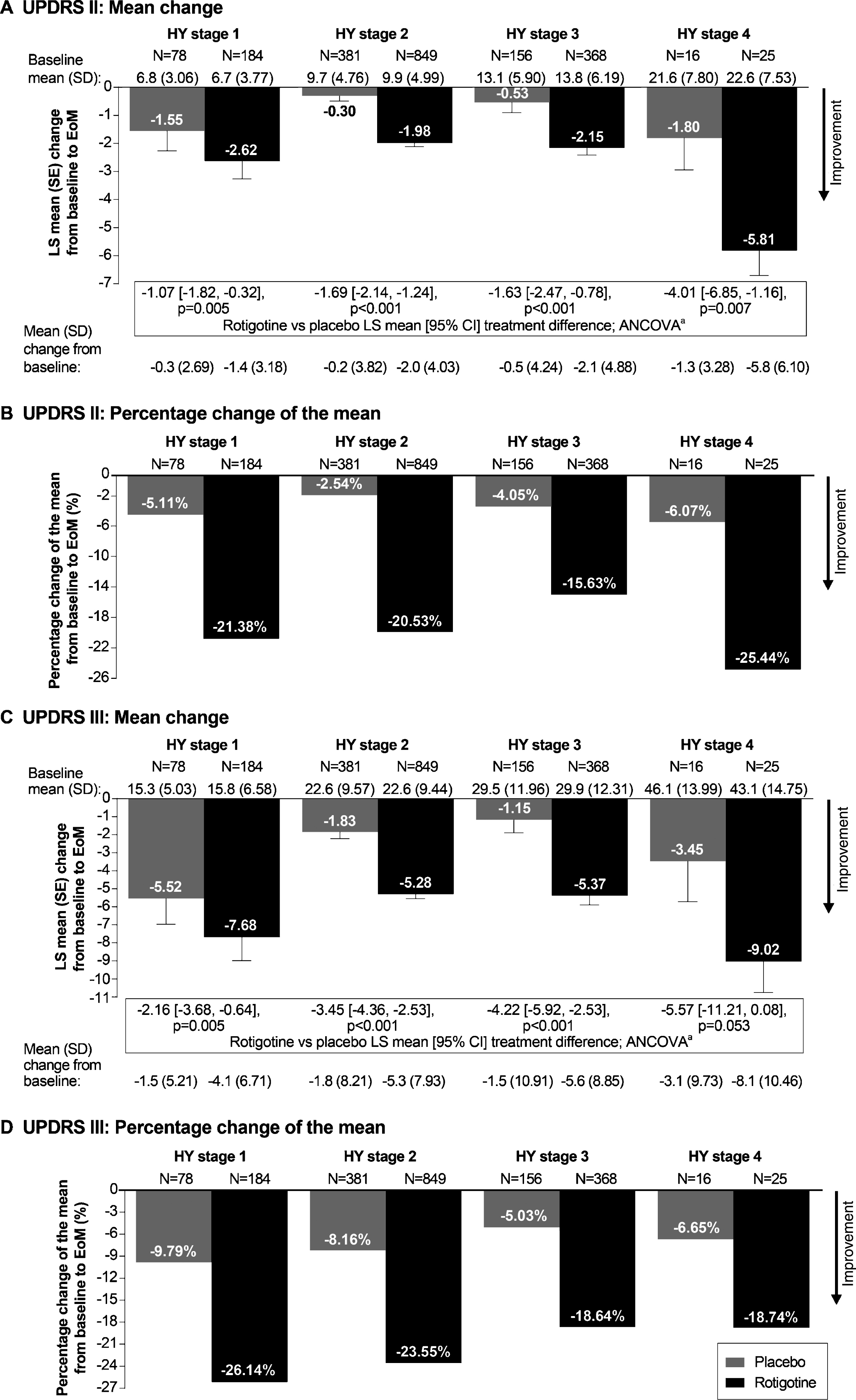
Table 1
The main characteristics of the six double-blind, randomized, placebo-controlled studies included in this post hoc analysis
| Early-stage PDa | Advanced-stage PDb | ||||||
| SP506 (Phase IIb) [12] | SP512 (Phase III) | SP513 (Phase III) [15]c | CLEOPATRA-PD | PREFER (Phase III) | SP921 (Phase III) | ||
| [13, 14] | (Phase III) [18]d | [16] | (NCT00522379) [17] | ||||
| (NCT00244387) | |||||||
| Rotigotine dose | Fixed dose: | Optimal dose: | Optimal dose: | Optimal dose: | Fixed dose: | Fixed dose: | |
| 2, 4, 6 or 8 mg/24 h | 2– 6 mg/24 h | 2– 8 mg/24 h | 4– 16 mg/24 h | ≤8 or ≤12 mg/24 h | 2, 4, 6 or 8 mg/24 h | ||
| Rotigotine maintenance phase | 7 weeks | 24 weeks | 33 weeks | 16 weeks | 24 weeks | 12 weeks | |
| Key inclusion criteria | •Aged ≥30 years | •Aged ≥30 years | |||||
| •Idiopathic PD of ≤5 yearse | •Idiopathic PD of >3 years | ||||||
| •HY stage ≤3 | •HY stage 2– 4 (in both the ‘on’ and ‘off’ state) | ||||||
| •Levodopa not permitted | •Inadequately controlled on stable dose of levodopa | ||||||
| Number randomized to rotigotine or placebo | •Rotigotine | •Rotigotine, n = 181 | •Rotigotine, n = 215 | •Rotigotine, n = 204 | •Rotigotine | •Rotigotine | |
| 2 mg/24 h, n = 67 | •Placebo, n = 96 | •Placebo, n = 118 | •Placebo, n = 101 | ≤8 mg/24 h, n = 120 | 2 mg/24 h, n = 101 | ||
| 4 mg/24 h, n = 63 | ≤12 mg/24 h, n = 111 | 4 mg/24 h, n = 107 | |||||
| 6 mg/24 h, n = 65 | •Placebo, n = 120 | 6 mg/24 h, n = 104 | |||||
| 8 mg/24 h, n = 70 | 8 mg/24 h, n = 94 | ||||||
| •Placebo, n = 64 | •Placebo, n = 108 | ||||||
aDefined as not receiving levodopa. bDefined as not adequately controlled on levodopa and with an average 2.5 h/day spent in ‘off’ state. cSP513 also included active comparator arm (ropinirole), which was not included in current analyses. dCLEOPATRA-PD also included active comparator arm (pramipexole), which was not included in current analyses. eDuration not specified in SP506. Abbreviations: PD, Parkinson’s disease.
Table 2
Demographic and baseline characteristics by HY stage at baseline (pooled data)
| HY stage 1 | HY stage 2 | HY stage | HY stage | |
| N = 262 | N = 1230 | N = 524 | N = 41 | |
| SP506 | 109 | 172 | 35 | 0 |
| SP512 | 66 | 155 | 52 | 0 |
| SP513 | 82 | 202 | 46 | 0 |
| CLEOPATRA-PD | 1a | 174 | 119 | 7 |
| PREFER | 4a | 205 | 115 | 17 |
| SP921 | 0 | 322 | 157 | 17 |
| Age, mean±SD, years | 59.0±10.5 | 62.8±9.6 | 66.0±10.2 | 71.4±8.9 |
| Male, n (%) | 164 (62.6) | 825 (67.1) | 316 (60.3) | 24 (58.5) |
| White, n (%) | 250 (95.4) | 1037 (84.3) | 437 (83.4) | 33 (80.5) |
| Time since PD diagnosis, mean±SD, years | 1.3±1.67 | 4.9±4.25 | 6.6±5.20 | 10.3±5.24 |
| UPDRS II+III total score, mean±SD | 22.4±8.59 | 32.5±12.62 | 43.3±16.55 | 66.5±19.64 |
| Concomitant levodopa at baseline, n (%) | 5 (2)a | 701 (57) | 392 (75) | 41 (100) |
| Levodopa dose at baseline, mean±SD, mg/day | 630.0±426.61 | 696.1±423.57b | 740.5±416.32c | 823.2±469.99 |
aOne patient from CLEOPATRA-PD and four patients from PREFER (protocol deviations: HY stage 1). bData available for 700/701 patients. cData available for 391/392 patients. Abbreviations: HY, Hoehn and Yahr; PD, Parkinson’s disease.



