Neuroimaging of Freezing of Gait
Abstract
Functional brain imaging techniques appear ideally suited to explore the pathophysiology of freezing of gait (FOG). In the last two decades, techniques based on magnetic resonance or nuclear medicine imaging have found a number of structural changes and functional disconnections between subcortical and cortical regions of the locomotor network in patients with FOG. FOG seems to be related in part to disruptions in the “executive-attention” network along with regional tissue loss including the premotor area, inferior frontal gyrus, precentral gyrus, the parietal and occipital areas involved in visuospatial functions of the right hemisphere. Several subcortical structures have been also involved in the etiology of FOG, principally the caudate nucleus and the locomotor centers in the brainstem. Maladaptive neural compensation may present transiently in the presence of acute conflicting motor, cognitive or emotional stimulus processing, thus causing acute network overload and resulting in episodic impairment of stepping.
In this review we will summarize the state of the art of neuroimaging research for FOG. We will also discuss the limitations of current approaches and delineate the next steps of neuroimaging research to unravel the pathophysiology of this mysterious motor phenomenon.
INTRODUCTION
Functional brain imaging techniques appear ideally suited to explore the pathophysiology of freezing of gait (FOG) in patients with Parkinson’s disease (PD) and other neurological disorders. In contrast with other techniques [e.g. electroencephalography (EEG), magnetoencephalography (MEG) or functional near-infrared spectrography (fNIRS)], magnetic resonance imaging (MRI) techniques, positron emission tomography (PET) or single photon emission computed tomography (SPECT) offer sufficient to excellent spatial and temporal resolution for brain imaging, though spatial resolution is more limited for SPECT. Both MRI and nuclear medicine techniques enable the study of neurovascular coupling (i.e., changes in blood flow during neuronal activity) during a specific task, however radioisotope imaging allows also the study of neurotransmiter and pathobiological alterations associated with FOG and can be also performed in patients who underwent deep brain stimulation (DBS). On the other hand, nuclear medicine techniques are more invasive and might be less informative in terms of anatomical and microstructuralchanges.
The principal limitation of all these tools is that they require that patient’s head to remain immobile, thus precluding actual gait execution. In order to overcome these limits, different research strategies are currently used (Table 1).
In this review, we will summarize the state of the art of neuroimaging research for FOG, particularly focusing on nuclear medicine techniques (PET and SPECT) and both structural and functional MRI techniques. We will also discuss the limitations of current approaches and delineate the next steps of neuroimaging research to unravel the pathophysiology of this mysterious phenomenon.
PET and SPECT
Study of the resting condition: Perfusion & glucose metabolic studies
Both PET and SPECT can assess cerebral glucose metabolism or regional cerebral blood flow (rCBF), and can quantify brain activity with good spatial resolution because synaptic activity is accompanied by regional changes in glucose utilization and capillary perfusion.
One of the earliest SPECT studies using 133Xenon did not find any particular perfusion alteration in PD patients with FOG [1]. However, subsequent PET and SPECT studies have consistently shown evidence of cortical changes in parietal and frontal regions of FOG patients [2–4].
Patients with FOG have shown hypometabolism in the bilateral orbitofrontal (Brodmann areas 10 and 11) and parietal cortices as well as in the basal ganglia when compared to patients without FOG or healthy subjects [2, 4, 5]. The frontal lobe changes may explain why FOG patients are consistently performing poorly when executive functions are tested (e.g. [6]).
Other studies reported hypoperfusion within the right anterior cingulate cortex (ACC) and primary visual cortex, thus suggesting that FOG results from neuronal circuitry dysfunction of the parietal-lateral premotor circuits in the right hemisphere [7]. This hypothesis is further supported by the studies linking right hemisphere pathology with FOG episodes triggered by spatial constrain [8]. In keeping with these notions, PD patients with onset on the left body side (worse right brain pathology) have an increased risk for FOG [9] and have more problems in negotiating obstacles like narrow doorways. In addition, the posterior cortex is also involved in limb-independent antiphase movement [10] and execution of complex sequential movement task [11].
Interestingly, a SPECT study on patients with FOG due to vascular parkinsonism (VP) found significant reduction in the perfusion of the right parietal cortex and bilateral ACC [12].
Other data showed hypometabolism in the basal ganglia and midbrain in pure akinesia with gait freezing (PAGF) compared with controls [13]. Progressive supranuclear palsy (PSP) patients may present a similar topographic distribution of glucose hypometabolism with additional areas including the frontal cortex. Similar results were shown in an earlier SPECT study showing normal frontal lobe perfusion in patients with primary progressive freezing of gait (PPFG), whereas frontal lobe hypoperfusion was only present in the PSP patients [1]. Neuropathological studies have demonstrated the common presence of tau protein deposition in PPFG, PAFG and PSP patients [14] where imaging studies suggest a continuum between shared involvement of subcortical structures and perhaps later (frontal) cortical involvement.
The aforementioned perfusion and glucose metabolic studies compared FOG patients with patients without FOG or healthy controls, thus exploring diagnostic correlations rather than investigating specific pathophysiological mechanisms of FOG. However, SPECT or PET studies using specific neurotransmitter or pathobiological ligands may provide more specific cluesto the etiopathogenesis of FOG (see below).
Dopamine
The dopaminergic system has been extensively investigated in patients with different forms of FOG. The severity of FOG does not seem to correlate with the loss of dopaminergic terminals [15], although a more recent study found that, compared to non-freezers, freezers had a lower striatal dopaminergic activity, but also a longer disease duration [16].
A dopaminergic PET study showed evidence of more prominent (predominantly right) caudate nucleus denervation in patients with FOG [3]. This observation is in keeping with dopaminergic PET studies in patients receiving fetal graft implants, which have linked the improvement of FOG with the restoration of dopaminergic transmission in the caudate nucleus [17, 18]. The biological plausibility of caudate in the FOG pathophysiology is supported by its connection with prefrontal regions, which are involved in the initiation of behavioral responses to the environment [19]. In addition, dopamine deficit in the right caudate has been found to correlate with difficulty in bimanual hand movements [20].
We have shown that about 40% of patients with PPFG have a normal dopaminergic system, as measured by means of dopamine transporter (DAT) SPECT [21]. It has been speculated that PPFG patients with abnormal dopaminergic imaging might slowly evolve towards pure akinesia with FOG (PAFG) or PSP [21, 22], whereas those with normal DAT imaging might evolve towards a cortico-basal syndrome or motor neuron diseases. On the other hand, FOG has been also described in patients with amyotrophic lateral sclerosis and abnormal dopaminergic imaging [23].
These data provide support for the hypothesis that patients with FOG undergo a progressive extension of the degenerative process to non-dopaminergic structures affecting locomotion (see below).
Acetylcholine and β-amyloid
Advancing PD is associated with prominent axial motor complications, such as FOG, with diminishing responsiveness to dopaminergic medications [24].
A PET study using a cholinergic marker has linked the deficiency of thalamic activity (possibly reflecting loss of cholinergic projections from the pedunculopontine nucleus – PPN) with the propensity to falls in PD patients [25]. Although this study did not specifically evaluate the occurrence and severity of FOG, donepezil [26] or galantamine [27] have been found to improve gait and the perfusion in bilateral frontal cortex in PD patients without dementia.
We recently reported on extra-nigral PET changes in patients with PD and FOG assessing cholinergic denervation and cortical β-amyloid deposition by means of the Pittsburgh compound B (PIB) [16]. FOG was not only more common in patients with diminished neocortical cholinergic innervation but also independently in patients with increased neocortical β-amyloid deposition. Within the group of freezers, 90% had at least one of the two extra-nigral pathological conditions studied. Furthermore, there was evidence of a dose-response association between absence, presence of single or dual presence of these extranigral pathologies: frequency of FOG was lowest with absence of both pathological conditions (4.8%), intermediate in subjects with single extra-nigral pathological condition (14.3%), and highest with combined neocortical cholinopathy and amyloidopathy (41.7%) (Fig. 1) [16].
These data indicate that extranigral pathological conditions in the presence of more significant nigrostriatal denervation may contribute to FOG in PD. These data support also the concept that the emergence of dopamine less-responsive axial motor problems, such as FOG, may reflect the transition from a predominantly hypodopaminergic disorder to a multisystem neurodegenerative disorder involving non-dopaminergic locomotor network structures and pathologies [16]. Moreover, the regionally localized cortical and striatocortical changes may reflect the cognitive and attentional dependency of gait in PD [28].
Repeated studies following prolonged walking
A dynamic PET study investigated the dynamic changes in DAT availability (inversely related to dopamine release) in association with walking exercise in 6 normal subjects and 7 age-matched unmedicated PD patients; it was found that putaminal dopamine release was greater in normal subjects, whereas in PD patients gait execution depended on dopamine release in the caudate nucleus and orbitofrontal cortex [29].
SPECT using technetium-99m-hexamethyl-propyleneamine oxime (99mTc-HMPAO) in normal subjects have revealed that the supplementary motor area (SMA), medial primary sensorimotor area, the striatum, the cerebellar vermis and the visual cortex are activated during walking [30]. A study employing this approach in PD patients has reported a relative under-activation in the left medial frontal area, right precuneus and left cerebellar hemisphere and a relative over-activity in the left temporal cortex, right insula, left cingulate cortex and cerebellar vermis [31]. A similar study using 99mTc-ethyl cysteinate dimer revealed under-activation in the SMA, thalamus and basal ganglia, and over-activation of the premotor cortex in patients with VP and FOG during walking on a treadmill, compared with VP patients without gait difficulties [32]. SMA is also an important player in the execution of complex movement [11], its lesions have been linked to the appearance of stuttering and FOG [33] and an increase of its metabolism has been associated with improvement of walking in PD [34, 35].
Repeated studies following a given intervention
A SPECT study using 99mTc-HMPAO found that visual cues during gait on a treadmill induced activations in the premotor, parietal cortices and cerebellar hemispheres in PD patients with FOG compared with controls, thus suggesting the recruitment of cerebral areas involved in visuomotor control to overcome FOG [36].
Similar approaches have been used to explore the effect of medications (levodopa in normal pressure hydrocephalus [37], tandospirone in PSP [38], donepezil in PAFG [26], rasagiline in PPFG [39], selegiline in PD [4]) or other experimental procedures (e.g. motor cortex stimulation [34]). These studies point to enhancement of frontal metabolism in relationship to improved mobility. Lyoo et al. [40] showed that improvement in the total motor score following DBS of suthalamic nucleus (STN) was correlated with increased metabolism activity in the prefrontal cortex, SMA and ACC, whereas FOG improvement was associated with metabolic activity in parietal, occipital and temporal sensory association cortices.
Study while the patient executes a surrogate of walking
Few nuclear imaging studies using this paradigm have been performed. A study evaluating the effect of unilateral PPN area stimulation during self-paced alternating lower limb movements showed a bilateral increase in rCBF in the thalamus, putamen, cerebellum and midbrain locomotor regions, which correlated with rCBF changes in the sensorimotor cortex and SMA [35].
MAGNETIC RESONANCE IMAGING
MRI has witnessed the greatest expansion of techniques and experimental approaches in the last decade. The following paragraphs will summarize the knowledge gathered so far in terms of both brain morphology and function.
Structural imaging studies
Table 2 summarizes recent findings from studies of structural brain imaging. More specifically, these papers were selected if they depicted the contribution of various brain structures to FOG using voxel-based morphometry (VBM) or diffused tensor imaging (DTI).
Gray matter
Five studies investigated the involvement of gray matter (GM) atrophy in the pathogenesis of FOG [41–45]. Global GM, white matter (WM), and cerebral spinal fluid volume were similar in PD patients and controls, and when comparing freezers and non-freezers [42, 45]. These whole brain comparisons indicate that GM differences between freezers and non-freezers are attributable to site-specific tissue alterations and not general brain GM atrophy.
The extent and distribution of GM atrophy in 71 subjects were studied by Kostic et al. using VBM [43]. PD patients with and without FOG, and healthy controls were administered a neuropsychological and behavioral evaluation, including the Addenbrooke’s Cognitive Examination–Revised, the Frontal Assessment Battery (FAB), Executive Interview (EXIT-25) and the Hamilton Depression and Anxiety Rating Scales. Particular attention was given to executive functions. They reported increased GM atrophy in the left inferior frontal gyrus, precentral gyrus, and inferior parietal gyrus (regions involved in visuomotor functioning) among freezers relative to both non-freezers and healthy controls. In the freezers, the FOG-Questionnaire (FOG-Q) total score was associated with GM volumes of the left superior, middle, and inferior frontal gyri and right superior frontal, middle cingulate, and posterior cingulate gyri, independent of the motor score of the Unified PD Rating Scale (UPDRS) and FAB scores. They suggested that a specific pattern of brain network damage bilaterally, in particular, involving frontal and parietal cortices, contributes to the presence of FOG in PD. Furthermore and in keeping with SPECT/PET literature, there was an association between FOG severity and frontal executive deficits, emphasizing the role of cognitive function, mostly executive function, in FOG emergence [43].
Tessitore et al. [42] studied 36 subjects of which 24 were patients with PD. They used the motor UPDRS and the FOG-Q in addition to a variety of cognitive tests including the Mini-Mental State Examination, the spatial (Corsi Block-Tapping) and verbal span, Rey Auditory Verbal Learning Test, the constructional apraxia test, the Raven’s Colored Progressive Matrices test to evaluate abstract nonverbal reasoning, FAB, phonological verbal fluency, the Stroop test, Ten-Point Clock Test, and cancellation attentional matrices. The authors reported reduced GM volumes in the left cuneus, precuneus, lingual gyrus, and posterior cingulated cortex in the freezers compared to the non-freezers [42]. In the neurocognitive battery, the non-freezers scored significantly better on four of the cognitive tests compared to the freezers. FOG severity was correlated with GM loss in the left cuneus. The authors suggested that cognitive impairment, namely executive and visuospatial dysfunction and structural GM loss in posterior cortical regions may be associated with the development of FOG.
The group by Herman et al. [45] studied 30 PD patients with FOG and 76 PD patients without FOG. The patients with FOG had significantly more GM atrophy in the inferior parietal lobe and angular gyrus compared to non-freezers, however, this was only evident in a well-matched case-control study of freezers compared with non-freezers; 22 subjects in each group (Fig. 2). In the entire cohort, FOG severity was related to bilateral caudate volumes. These few studies demonstrate that FOG is associated with GM atrophy in posterior cortical regions and brain areas suggestive of executive-visuospatial dysfunction.
On the other hand, two studies reported reduced GM volumes in freezers compared to non-freezers in subcortical areas. A combined fMRI and VBM study [41] studied 24 patients with PD and 21 healthy matched controls. They found grey matter atrophy in a small portion of the mesencephalic locomotor region (MLR) in freezers. The gait-related hyperactivity of the MLR correlated with FOG severity and disease duration, but not with the degree of atrophy. Sunwoo and colleagues [44] evaluated the association between subcortical cholinergic structures and FOG in 46 patients with PD. A comprehensive neuropsychological assessment was performed along with volumetric analysis of sub-cortical brain structures. PD patients with FOG had lower cognitive performance in the frontal executive and visual-related functions compared to non-freezers. The analysis of sub-cortical structures revealed that thalamic volumes were significantly reduced in the freezers compared to non-freezers, adjusting for disease related symptoms and total intracranial volume. Thalamic volume was correlated with visual recognition memory. The authors suggested that thalamic volume and related visual recognition may be a major contributor to FOG development in non-demented PD patients [44].
While site specific GM atrophy appears to be related to FOG, inconsistencies among the findings and the lack of full agreement with regard to the brain areas (cortical and subcortical) involved does not allow one to draw definitive conclusions. The disparate results suggest that freezing may reflect other functional features associated with this paroxysmal gait disturbance like cognition and mood, rather than simply anatomical and structural changes. It is also possible these divergent imaging findings may reflect different sub-groups of FOG.
White matter
In a relatively small sample, Schweder et al. [46] used DTI to characterize the PPN connectivity in PD patients with FOG. In healthy controls and non-freezers, the PPN showed good connectivity with the cerebellum, unlike in patients with FOG, who showed an absence of cerebellar connectivity and increased visibility of the decussation of corticopontine fibers. These results suggest that cortico-pontine projections are increased in freezers, highlighting the importance of corticopontine-cerebellar pathways in the pathophysiology of this phenomenon [46]. In line with these findings, Fling and colleagues [47] found reduced structural connectivity between the right PPN and the other locomotor regions in patients with FOG. However, in a study designed to assess differences in PD motor subtypes, Herman et al. [48] found similar white matter hyperintensities (WMHs) in both phenotypes: the PIGD and the TD subtypes. In contrast with the above-mentioned papers, while performing additional analysis on this cohort (unpublished data), PD patients with and without FOG, did not differ in WMHs scoring nor in analysis of DTI.
Resting state and task-related fMRI studies (Table 2)
Task-related fMRI (T-fMRI) measures blood oxygenation level dependent (BOLD) signal changes between task-stimulated states and control states. In the last years, several T-fMRI studies, using virtual reality and motor imagery paradigms, have investigated the neural correlates of FOG in PD patients. Shine and colleagues have explored the neural mechanisms associated with FOG in two studies using a virtual reality gait task that has been shown to correlate with actual episodes of FOG. In the first study [49], they demonstrated, during motor arrests, an increased BOLD response within frontoparietal and insular cortices and a concomitant decreased response in bilateral sensorimotor areas. Motor arrests were also associated with decreased BOLD response in the basal ganglia, thalamus and MLR. These functional changes were correlated with the clinical severity of FOG. In the second study [50], patients with and without FOG were compared under different cognitive load during the fMRI data acquisition. Patients with FOG were unable to properly recruit specific cortical and subcortical regions within the cognitive control network during the performance of simultaneous motor and cognitive functions. Taken together, these findings support the hypothesis that the pathophysiology of FOG implicates a dysfunction across coordinated cognitive and motor neural networks. A motor imagery paradigm has been used by Snijders and colleagues [41] in patients with and without FOG and healthy controls. Interestingly, they found an increased activation of the MLR and a decreased response in frontal and posterior parietal regions in patients with FOG during motor imagery of gait. The authors suggest that FOG might emerge when an altered cortical control of gait is combined with a limited ability of the MLR to react to this cortical functional change. More recently, Vercruysse et al. [51] investigated the neural mechanisms of upper limb motor blocks and their relation with FOG during bilateral finger movements. During successful upper limb movement, patients with FOG showed decreased activation in cortical frontal areas and increased subcortical activity compared with patients without FOG and controls. Upper limb motor blocks were instead associated with increased cortical brain activity while subcortical activity was decreased. These findings indicate that the neural drive for rhythmic movement generation and, more specifically, the balance between subcortical and cortical activation, is altered in patients with FOG.
However, it is not clear from these previous studies whether observed BOLD response differences in each region associated with FOG are related to more complex changes in the functional brain connectivity. Resting-state (RS) fMRI constitutes a novel paradigm that examines brain connectivity between functionally linked cortical regions with minimal bias towards a specific motor, visual and cognitive function [52]. Spatially distributed networks of temporal synchronization can be detected that can characterize RS networks (RSNs). The most commonly reported RSNs are the default mode network, the executive-attention network, the sensorimotor network and the visual and auditory networks. However, only a few studies have so far investigated RS functional connectivity in patients with FOG. Tessitore et al. [53] revealed that patients with FOG exhibit a significantly reduced functional connectivity only within the executive-attention and visual networks which was correlated with the FOG clinical severity. These data may suggest that a cognitive networks dysfunction may be relevant to the pathogenesis of FOG. Recently, Fling and colleagues [54], using a multimodal imaging seed-based approach, have explored the functional and structural integrity of the locomotor neural network in patients with and without FOG. They demonstrated that patients with FOG showed a greater functional connectivity between the SMA and MLR and cerebellar (CLR) locomotor regions compared to both patients without FOG and controls. This functional reorganization within the locomotor network in patients with FOG was positively correlated with both clinical and self-reported ratings of freezing severity.
Although functional brain connectivity has generally been investigated by means of RS-fMRI, it can also be explored by measuring the co-ordination between neuronal networks during the performance of behavioral tasks. A task-based functional connectivity analysis has been performed in a recent study [28] where patients with PD performed a virtual reality gait task. During task performance, patients with FOG demonstrate an impairment of functional connectivity between the basal ganglia network and the bilateral cognitive control network. These results support the hypothesis that FOG is associated with paroxysmal episodes of functional decoupling between complementary yet competing neuronal networks.
FUTURE DEVELOPMENTS
The studies discussed this review have certainly contributed to a better understanding of FOG pathophysiology but their findings are limited by a number of drawbacks intrinsic to the given technique (Table 1) or study design. As for the latter, since FOG is very common in the advanced stages of the disease, patient groups with and without FOG should be accurately matched for disease severity and other confounders (e.g. cognition). For instance, while many studies have confirmed the hypometabolism of frontal lobe in FOG, these patients have a high falling risk and a reduced orbitofrontal rCBF has been related to fear of falling [55]. In addition, categorizing patients as controls may be very difficult because FOG is episodic and presents a very variable expression; thus, patients should be studied using reliable biomarkers, preferably with continuous or semi-quantitative metrics to allow correlation analysis (e.g. rating scales or instrument variables) and FOG motor assessments. Besides these general considerations, future studies should address specific needs in this field.
Need for multimodal neuroimaging studies to reconcile presence of local injury markers and episodic nature of FOG
Although there is growing consensus for widespread neural changes in higher-order cortical motor control areas in conjunction with brainstem and cerebellar locomotor areas underlying FOG, less is known how to explain the episodic nature of this motor phenomenon and whether the primary etiology of this is cortical, subcortical or a pure network-wide disruption. Future studies should employ multimodal neuroimaging techniques to reconcile the presence of local injury markers and episodic nature of FOG. In fact, markers of local nervous system injury, such as cortical atrophy, β-amyloid proteinopathy or cholinopathy cannot explain the episodic nature of FOG but may identify a weak link within a neural circuit where freezing behavior in PD may occur because of impaired communication between complimentary yet competing neural networks [28]. Functional MRI techniques are geared towards detecting pathological or compensatory alterations in functional network organizations that may occur even before alterations in local injury markers become evident.
In this respect, the greater degree of communication between cortical motor control areas and subcortical locomotor centers may reflect a failed attempt to compensate for presumed cortical or striatocortical dysfunction. More specifically, findings of greater communication between the cortex and subcortical locomotor centers in PD subjects with FOG may indicate a failed attempt to compensate for the loss of connectivity between the cortex and key subcortical locomotor centers [54]. Therefore, maladaptive neural compensation in injured brain regions may present transiently in the presence of acute conflicting motor, cognitive or emotional stimulus processing causing transient neural network overload resulting in FOG. The recent technical development of integrated and simultaneous PET-MRI imaging may offer new tools to identify neural substrate and network changes in FOG.
Investigation of mechanisms of compensation and decompensation in neural motor control networks underling gait functions
FOG appears to be more common in patients with more severe nigrostriatal degeneration. Degraded striatal dopaminergic function in PD likely places additional burden on other brain systems mediating attentional functions, such as forebrain cholinergic neurons, to increase monitoring of previously automatic motor tasks [56]. In this respect, neural compensation may occur when a relatively isolated impairment of a single neurotransmission system in PD (i.e., nigrostriatal denervation) may not lead to the development of significant impairments because of adaptive plasticity in other brain systems that are still relatively intact [57]. Support for this model comes from a recent animal dual forebrain dopaminergic and cholinergic lesioning study where the significant presence of dual dopaminergic and cholinergic lesions was associated with motor freezing behavior when the rats had to pass through a narrow doorway put over a walking beam [58]. The results support the hypothesis that after dual cholinergic–dopaminergic lesions, attentional resources can no longer be recruited to compensate for diminished striatal control of complex movement, thereby “unmasking” loss of lower-order, automatic control of gait by the basal ganglia forcing (failing) attempts for higher-order cortical top-down control of gait. Pharmacological evidence for the cholinergic compensation hypothesis of FOG is shown by clinical observation that increasing exposure to anti-muscarinic drugs is associated with FOG in PD [59].
Other neurotransmitters should be investigated and new tracers have been developed in the recent past, e.g. 11C-yohimbine for alpha2-adrenergic receptors [60] or 11C-DASB for the serotonin transporter.
There is now abundant evidence to demonstrate that stimulation of the output structures of the basal ganglia leads to GABAergic inhibition of the brainstem and thalamic structures that control gait, which ultimately manifests as akinesia [61]. Therefore, there is a need to test the hypothesis that FOG manifests via a common final pathway, namely overwhelming of GABAergic inhibition of subcortical structures controlling gait as proposed by Lewis & Shine [62].
The cerebellar locomotor region, which may regulate speed and give rhythmical impulses to the brainstem and spinal cord [63], remains a largely unexplored area of research in parkinsonian gait and FOG. Cerebellar processing of proprioceptive information is also important to regulate ongoing movements and to maintain stable standing posture [64]. Therefore, the cerebellum may prove to be an important site to investigate neural mechanisms of compensation in parkinsonian gait. Another important target of research is the STN. Hill and colleagues compared effects of dorsal versus ventral STN regions on gait functions in PD using H2 15O PET in a within-subject design [65]. These authors found differential correlations with gait velocity and premotor cortex rCBF changes with ventral STN DBS whereas dorsal STN DBS produced similar changes in the anterior cerebellum. These findings suggest that effects of STN DBS on gait may be mediated by different circuits depending on the site of STN region stimulation through basal ganglia-thalamo-cortical versus cerebellar-thalamo-cortical circuits [66]. These findings illustrate also complementary roles of basal ganglia and cerebellum in motor control.
Need to investigate specific subtypes of FOG
The pathophysiology of freezing involves context-dependent dysfunction across multiple levels of the locomotor system, including cortical, subcortical and brainstem regions [67, 68]. Neuroimaging studies of network functions provide support for a proposed model where a cortico-striatal loop of motor control involves functions of volition, cognition and attention. In contrast, a subcortical-brainstem system seems to be required for the automatic regulation and modulation of muscle tone and rhythmic limb movements [64, 69]. Furthermore, the manifestation of FOG may be related to specific triggers that may result in acute conflicting motor, cognitive or emotional stimulus processing, including cognition, motor or anxiety stimulus processing [28, 70]. Some FOG types may be L-DOPA responsive and others not. These divergent neural pathways and mechanistic triggers indicate the presence of different subtypes of FOG, which may have different neural mechanisms and implications for treatments. A network hypothesis of FOG may postulate that the presence of the weakest link within a network may be the primary site of triggering FOG. However, further research is needed whether there is a final common neural pathway for FOG or not.
Need to correlate in vivo dynamic imaging findings to neurophysiology of FOG
There is a need to correlate in vivo dynamic imaging changes with neurophysiology of FOG as well as with quantitative gait and postural changes relevant to FOG, such as dual task cost, rhythmicity of gait and coupling of gait and postural functions using quantitative real-life or virtual reality mobility assessments. Such studies may help to elucidate dynamic brain network underlying key motor changes during FOG episodes in vivo. EEG and MEG have excellent temporalresolution and will make it possible to investigate changes in brain activity immediately preceding FOG or freezing of limb movements [71]. In addition, fNIRS may allow real-life or virtual-reality mobility assessment during FoG provocation maneuvers [72].
Such approaches might overcome the main limitations of PET, SPECT and MRI studies: the lack of ecological validity with respect to balance and mobility demands, as patients are scanned while lying down and while we still need to validate the imaging mental imagery paradigm of motor surrogateof gait.
CONCLUSIONS
In the last decade several studies have shed light on the cerebral areas and networks involved in FOG pathophysiology. Recent work implicates compromised structural integrity and transient functional disconnection between subcortical and cortical regions of the locomotor network in patients who experience FOG. Overall, there is reasonable agreement regarding the brain structures associated with FOG. FOG seems to be related in part to disruptions in the executive-attention network along with regional tissue loss including the premotor area, inferior frontal gyrus, precentral gyrus, and the parietal lobe e.g., the precuneus, cuneus and angular gyrus, areas involved in visuospatial functions of the right hemisphere. Several subcortical structures have been also involved in the etiology FOG, principally the caudate nucleus and the locomotor centers in the brainstem. Maladaptive neural compensation in injured brain regions may present transiently in the presence of acute conflicting motor, cognitive or emotional stimulus processing causing acute network overload resulting in FOG. FOG may represent an advancing multisystem stage of PD where extra-nigral pathologies contribute to more widespread neural injury that may parallel the diminished striatal control of complex movement, thereby unmasking loss of lower-order, automatic control of gait by the basal ganglia. Multimodal neuroimaging approaches that combine structure and function, including network connectivity, will be important to unravel critical mechanisms underlying FOG in PD.
ACKNOWLEDGMENTS
NIB acknowledges funding from the NIH, Department of Veterans Affairs and the Michael J. Fox Foundation.
REFERENCES
1 | Fabre N, Brefel C, Sabatini U, Celsis P, Montastruc JL, Chollet F, Rascol O (1998) Normal frontal perfusion in patients with frozen gait Mov Disord 13: 677 683 |
2 | Matsui H, Udaka F, Miyoshi T, Hara N, Tamaura A, Oda M, Kubori T, Nishinaka K, Kameyama M (2005) Three-dimensional stereotactic surface projection study of freezing of gait and brain perfusion image in Parkinson’s disease Mov Disord 20: 1272 1277 |
3 | Bartels AL, de Jong BM, Giladi N, Schaafsma JD, Maguire RP, Veenma L, Pruim J, Balash Y, Youdim MB, Leenders KL (2006) Striatal dopa and glucose metabolism in PD patients with freezing of gait Mov Disord 21: 1326 1332 |
4 | Imamura K, Okayasu N, Nagatsu T (2012) Cerebral blood flow and freezing of gait in Parkinson’s disease Acta Neurol Scand 126: 210 218 |
5 | Hayashi H, Odano I, Nishihara M, Higuchi S, Sakai K, Ishikawa A (1989) Clinical evaluation of Parkinson’s disease using 123I-IMP SPECT Kaku Igaku 26: 1405 1415 |
6 | Ricciardi L, Bloem BR, Snijders AH, Daniele A, Quaranta D, Bentivoglio AR, Fasano A (2014) Freezing of gait in Parkinson’s disease: The paradoxical interplay between gait and cognition Parkinsonism Relat Disord 20: 824 829 |
7 | Bartels AL, Leenders KL (2008) Brain imaging in patients with freezing of gait Mov Disord 23: Suppl 2 S461 S467 |
8 | Cohen RG, Horak FB, Nutt JG (2012) Peering through the FoG: Visual manipulations shed light on freezing of gait Mov Disord 27: 470 472 |
9 | Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, Tanner CParkinson Study G (2001) Freezing of gait in PD: Prospective assessment in the DATATOP cohort Neurology 56: 1712 1721 |
10 | de Jong BM, Leenders KL, Paans AM (2002) Right parieto-premotor activation related to limb-independent antiphase movement Cereb Cortex 12: 1213 1217 |
11 | Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y (1997) Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements J Neurosci 17: 9667 9674 |
12 | Terashi H, Ishimura Y, Utsumi H (2012) Regional cerebral blood flow patterns in patients with freezing of gait due to lacunar infarction: SPECT study using three-dimensional stereotactic surface projections Int J Neurosci 122: 423 430 |
13 | Park HK, Kim JS, Im KC, Oh SJ, Kim MJ, Lee JH, Chung SJ, Lee MC (2009) Functional brain imaging in pure akinesia with gait freezing: [18F] FDG PET and [18F] FP-CIT PET analyses Mov Disord 24: 237 245 |
14 | Williams DR, Lees AJ (2009) Progressive supranuclear palsy: Clinicopathological concepts and diagnostic challenges Lancet Neurol 8: 270 279 |
15 | Lorberboym M, Treves TA, Melamed E, Lampl Y, Hellmann M, Djaldetti R (2006) [123I]-FP/CIT SPECT imaging for distinguishing drug-induced parkinsonism from Parkinson’s disease Mov Disord 21: 510 514 |
16 | Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Constantine GM, Scott PJ, Albin RL, Muller ML (2014) Extra-nigral pathological conditions are common in Parkinson’s disease with freezing of gait: An in vivo positron emission tomography study Mov Disord 29: 1118 1124 |
17 | Iacono RP, Tang ZS, Mazziotta JC, Grafton S, Hoehn M (1992) Bilateral fetal grafts for Parkinson’s disease: 22 months’ results Stereotact Funct Neurosurg 58: 84 87 |
18 | Lee CC, Lin SZ, Wang Y, Lin JJ, Liu JY, Chen GJ, Chiang YH, Liu JC, Zhou FC (2003) First human ventral mesencephalon and striatum cografting in a Parkinson patient Acta Neurochir Suppl 87: 159 162 |
19 | Rolls ET, Thorpe SJ, Maddison SP (1983) Responses of striatal neurons in the behaving monkey. 1. Head of the caudate nucleus Behav Brain Res 7: 179 210 |
20 | de la Fuente-Fernandez R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ (2000) Nigrostriatal dopamine system and motor lateralization Behav Brain Res 112: 63 68 |
21 | Fasano A, Baldari S, Di Giuda D, Paratore R, Piano C, Bentivoglio AR, Girlanda P, Morgante F (2012) Nigro-striatal involvement in primary progressive freezing gait: Insights into a heterogeneous pathogenesis Parkinsonism Relat Disord 18: 578 584 |
22 | Compta Y, Valldeoriola F, Tolosa E, Rey MJ, Marti MJ, Valls-Sole J (2007) Long lasting pure freezing of gait preceding progressive supranuclear palsy: A clinicopathological study Mov Disord 22: 1954 1958 |
23 | Park HK, Lim YM, Kim JS, Lee MC, Kim SM, Kim BJ, Kim KK (2011) Nigrostriatal dysfunction in patients with amyotrophic lateral sclerosis and parkinsonism J Neurol Sci 301: 12 13 |
24 | Vu TC, Nutt JG, Holford NH (2012) Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment Br J Clin Pharmacol 74: 267 283 |
25 | Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL (2009) History of falls in Parkinson disease is associated with reduced cholinergic activity Neurology 73: 1670 1676 |
26 | Yener GG, Kaya GC, Ozturk V, Akdal G (2005) Improvement in Tc-99m HMPAO brain SPECT findings during donepezil therapy in a patient with pure akinesia Ann Nucl Med 19: 607 609 |
27 | Litvinenko IV, Khalimov RR, Trufanov AG, Krasakov IV, Khaimov DA (2012) New approach to gait disorders therapy in late stages of Parkinson’s disease Adv Gerontol 25: 267 274 |
28 | Shine JM, Matar E, Ward PB, Frank MJ, Moustafa AA, Pearson M, Naismith SL, Lewis SJ (2013) Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia Brain 136: 3671 3681 |
29 | Ouchi Y, Kanno T, Okada H, Yoshikawa E, Futatsubashi M, Nobezawa S, Torizuka T, Tanaka K (2001) Changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson’s disease Brain 124: 784 792 |
30 | Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, Kimura J, Shibasaki H (1997) Brain functional activity during gait in normal subjects: A SPECT study Neurosci Lett 228: 183 186 |
31 | Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H (1999) Mechanisms underlying gait disturbance in Parkinson’s disease: A single photon emission computed tomography study Brain 122: Pt 7 1271 1282 |
32 | Iseki K, Hanakawa T, Hashikawa K, Tomimoto H, Nankaku M, Yamauchi H, Hallett M, Fukuyama H (2010) Gait disturbance associated with white matter changes: A gait analysis and blood flow study Neuroimage 49: 1659 1666 |
33 | Chung SJ, Im JH, Lee JH, Lee MC (2004) Stuttering and gait disturbance after supplementary motor area seizure Mov Disord 19: 1106 1109 |
34 | Fasano A, Piano C, De Simone C, Cioni B, Di Giuda D, Zinno M, Daniele A, Meglio M, Giordano A, Bentivoglio AR (2008) High frequency extradural motor cortex stimulation transiently improves axial symptoms in a patient with Parkinson’s disease Mov Disord 23: 1916 1919 |
35 | Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, Cilia R, Houle S, Poon YY, Lang AE, Strafella AP (2009) Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: A [(15)O] H2O PET study Hum Brain Mapp 30: 3901 3909 |
36 | Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H (1999) Enhanced lateral premotor activity during paradoxical gait in Parkinson’s disease Ann Neurol 45: 329 336 |
37 | Aggarwal S, Childers MK, Jimenez D (1997) Use of carbidopa-levodopa in a patient with hydrocephalus and frozen movement Brain Inj 11: 831 836 |
38 | Watanabe H, Arahata Y, Tadokoro M, Kato T, Sobue G (2000) Effects of tandospirone citrate on frozen gait in patients with early stage of progressive supranuclear palsy, investigated by walk-induced activation single photon emission computed tomography method Rinsho Shinkeigaku 40: 1130 1132 |
39 | Coria F, Cozar-Santiago Mdel P (2008) Rasagiline improves freezing in a patient with primary progressive freezing gait Mov Disord 23: 449 451 |
40 | Lyoo CH, Aalto S, Rinne JO, Lee KO, Oh SH, Chang JW, Lee MS (2007) Different cerebral cortical areas influence the effect of subthalamic nucleus stimulation on parkinsonian motor deficits and freezing of gait Mov Disord 22: 2176 2182 |
41 | Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I (2011) Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait Brain 134: 59 72 |
42 | Tessitore A, Amboni M, Cirillo G, Corbo D, Picillo M, Russo A, Vitale C, Santangelo G, Erro R, Cirillo M, Esposito F, Barone P, Tedeschi G (2012) Regional gray matter atrophy in patients with Parkinson disease and freezing of gait AJNR Am J Neuroradiol 33: 1804 1809 |
43 | Kostic VS, Agosta F, Pievani M, Stefanova E, Jecmenica-Lukic M, Scarale A, Spica V, Filippi M (2012) Pattern of brain tissue loss associated with freezing of gait in Parkinson disease Neurology 78: 409 416 |
44 | Sunwoo MK, Cho KH, Hong JY, Lee JE, Sohn YH, Lee PH (2013) Thalamic volume and related visual recognition are associated with freezing of gait in non-demented patients with Parkinson’s disease Parkinsonism Relat Disord 19: 1106 1109 |
45 | Herman T, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff JM (2014) Gray matter atrophy and freezing of gait in Parkinson’s disease: Is the evidence black-on-white? Mov Disord 29: 134 139 |
46 | Schweder PM, Hansen PC, Green AL, Quaghebeur G, Stein J, Aziz TZ (2010) Connectivity of the pedunculopontine nucleus in parkinsonian freezing of gait Neuroreport 21: 914 916 |
47 | Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB (2013) Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait Brain 136: 2405 2418 |
48 | Herman T, Rosenberg-Katz K, Jacob Y, Auriel E, Gurevich T, Giladi N, Hausdorff JM (2013) White matter hyperintensities in Parkinson’s disease: Do they explain the disparity between the postural instability gait difficulty and tremor dominant subtypes? PLoS One 8: e55193 |
49 | Shine JM, Matar E, Ward PB, Bolitho SJ, Gilat M, Pearson M, Naismith SL, Lewis SJ (2013) Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease Brain 136: 1204 1215 |
50 | Shine JM, Matar E, Ward PB, Bolitho SJ, Pearson M, Naismith SL, Lewis SJ (2013) Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load PLoS One 8: e52602 |
51 | Vercruysse S, Spildooren J, Heremans E, Wenderoth N, Swinnen SP, Vandenberghe W, Nieuwboer A (2014) The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait Cereb Cortex 24: 3154 3166 |
52 | Friston KJ (2011) Functional and effective connectivity: A review Brain Connect 1: 13 36 |
53 | Tessitore A, Amboni M, Esposito F, Russo A, Picillo M, Marcuccio L, Pellecchia MT, Vitale C, Cirillo M, Tedeschi G, Barone P (2012) Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait Parkinsonism Relat Disord 18: 781 787 |
54 | Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB (2014) Functional reorganization of the locomotor network in Parkinson patients with freezing of gait PLoS One 9: e100291 |
55 | Kent JM, Coplan JD, Mawlawi O, Martinez JM, Browne ST, Slifstein M, Martinez D, Abi-Dargham A, Laruelle M, Gorman JM (2005) Prediction of panic response to a respiratory stimulant by reduced orbitofrontal cerebral blood flow in panic disorder Am J Psychiatry 162: 1379 1381 |
56 | Sarter M, Albin RL, Kucinski A, Lustig C (2014) Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function Exp Neurol 257C: 120 129 |
57 | Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJ, Albin RL, Muller ML (2013) Gait speed in Parkinson disease correlates with cholinergic degeneration Neurology 81: 1611 1616 |
58 | Kucinski A, Paolone G, Bradshaw M, Albin RL, Sarter M (2013) Modeling fall propensity in Parkinson’s disease: Deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation J Neurosci 33: 16522 16539 |
59 | Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, Meissner WG, Schelosky L, Tison F, Rascol O (2014) Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease JAMA Neurol 71: 884 890 |
60 | Jakobsen S, Pedersen K, Smith DF, Jensen SB, Munk OL, Cumming P (2006) Detection of alpha2-adrenergic receptors in brain of living pig with 11C-yohimbine J Nucl Med 47: 2008 2015 |
61 | Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T (2003) Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: A new concept for understanding motor disorders in basal ganglia dysfunction Neuroscience 119: 293 308 |
62 | Lewis SJ, Shine JM (2014) The Next Step: A Common Neural Mechanism for Freezing of Gait Neuroscientist |
63 | Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T (2004) Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging Neuroimage 22: 1722 1731 |
64 | Takakusaki K (2008) Forebrain control of locomotor behaviors Brain Res Rev 57: 192 198 |
65 | Hill KK, Campbell MC, McNeely ME, Karimi M, Ushe M, Tabbal SD, Hershey T, Flores HP, Hartlein JM, Lugar HM, Revilla FJ, Videen TO, Earhart GM, Perlmutter JS (2012) Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease Exp Neurol 241C: 105 112 |
66 | Doya K (2000) Complementary roles of basal ganglia and cerebellum in learning and motor control Curr Opin Neurobiol 10: 732 739 |
67 | Naismith SL, Lewis SJ (2010) A novel paradigm for modelling freezing of gait in Parkinson’s disease J Clin Neurosci 17: 984 987 |
68 | Shine JM, Naismith SL, Lewis SJ (2011) The pathophysiological mechanisms underlying freezing of gait in Parkinson’s Disease J Clin Neurosci 18: 1154 1157 |
69 | Zwergal A, la Fougere C, Lorenzl S, Rominger A, Xiong G, Deutschenbaur L, Schoberl F, Linn J, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K (2013) Functional disturbance of the locomotor network in progressive supranuclear palsy Neurology 80: 634 641 |
70 | Lieberman A (2006) Are freezing of gait (FOG) and panic related? J Neurol Sci 248: 219 222 |
71 | Handojoseno AM, Shine JM, Nguyen TN, Tran Y, Lewis SJ, Nguyen HT (2012) The detection of Freezing of Gait in Parkinson’s disease patients using EEG signals based on Wavelet decomposition Conf Proc IEEE Eng Med Biol Soc 2012: 69 72 |
72 | Mirelman A, Maidan I, Bernad-Elazari H, Nieuwhof F, Reelick M, Giladi N, Hausdorff JM (2014) Increased frontal brain activation during walking while dual tasking: An fNIRS study in healthy young adults J Neuroeng Rehabi 11: 85 |
73 | Maillet A, Thobois S, Fraix V, Redoute J, Le Bars D, Lavenne F, Derost P, Durif F, Bloem BR, Krack P, Pollak P, Debu B (2015) Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson’s disease: A kinesthetic imagery approach Hum Brain Mapp 36: 959 980 |
74 | Schweder PM, Joint C, Hansen PC, Green AL, Quaghebeur G, Aziz TZ (2010) Chronic pedunculopontine nucleus stimulation restores functional connectivity Neuroreport 21: 1065 1068 |
Figures and Tables
Fig.1
Proportion of freezers in subgroups of patients with absence, single or combined presence of cortical amyloidopathy and/or cholinopathy (reproduced with permission from [16]).
![Proportion of freezers in subgroups of patients with absence, single or combined presence of cortical amyloidopathy and/or cholinopathy (reproduced with permission from [16]).](https://content.iospress.com:443/media/jpd/2015/5-2/jpd-5-2-jpd150536/jpd-5-2-jpd150536-g001.jpg)
Fig.2
Areas of gray matter atrophy derived from a voxel-based morphometric direct comparison between PD patients with FOG (n = 22) and PD patients without FOG (n = 22). The GM maps were analyzed using analysis of variance (ANOVA) as implemented in SPM5. The model was adjusted for age and disease duration. The results are superimposed in representative sagittal and axial sections of a customized gray matter template, at a threshold of p < 0.005, uncorrected cluster size >50. *indicates p < 0.05, cluster level corrected. Note that if we applied FWE correction on the contrasts maps, the group differences were no longer significant. Significantly lower values of GM in the freezers compared to the non-freezers were observed in the precuneus, frontal gyrus/supplementary motor area, cerebellum declive and middle temporal gyrus (p < 0.005, uncorrected). In addition, in the left IPL and the right angular gyrus, significant differences were found when correcting for multiple comparisons (p < 0.015, cluster level corrected). IFG = inferior frontal gyrus; SMA = supplementary motor area; IPL = inferior parietal lobe; MTG = middle temporal gyrus.
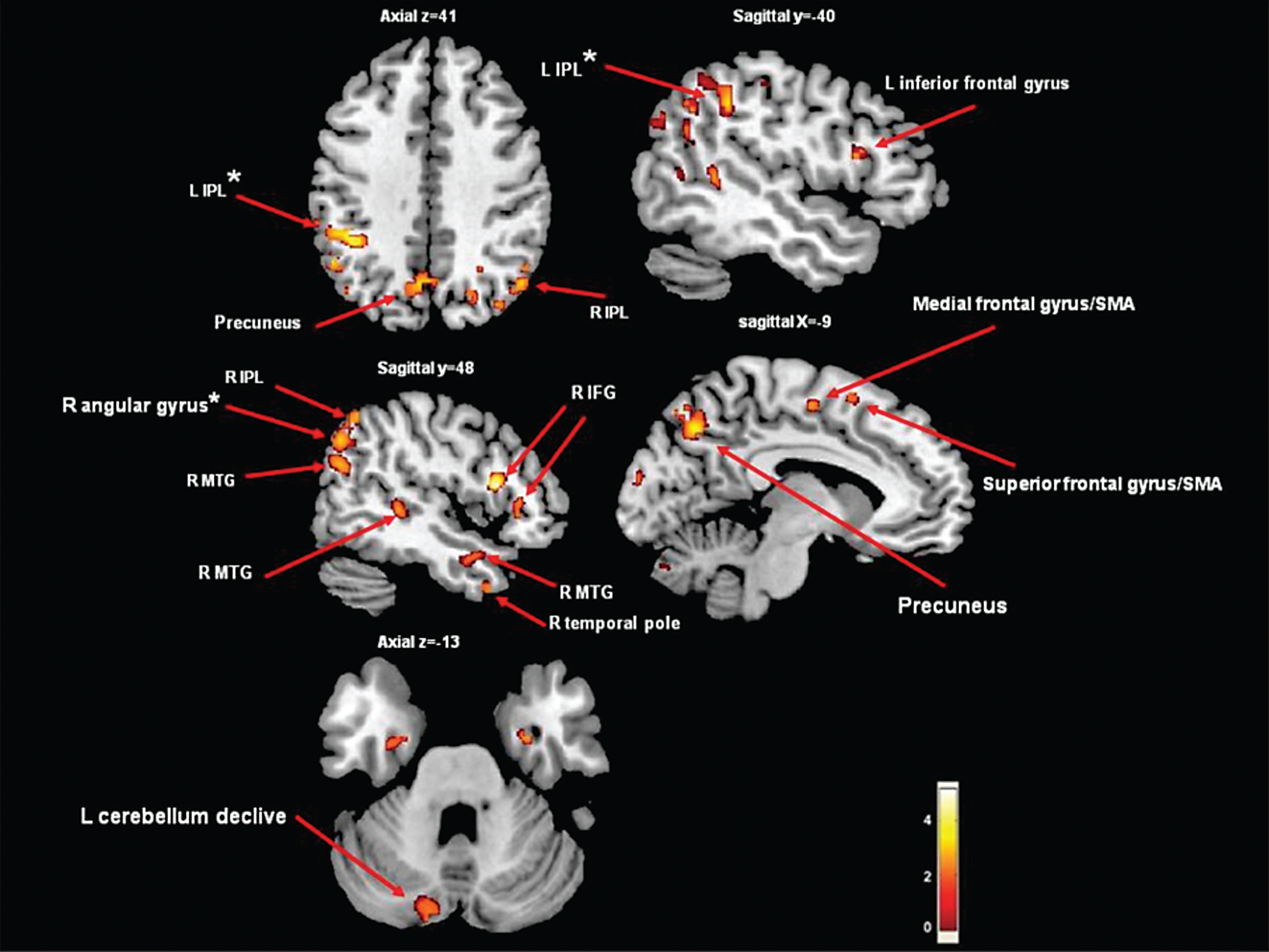
Table 1
Neuroimaging protocols currently used to study FOG
| Neuroimaging protocols | Techniques | Advantages | Disadvantages |
| Study of the resting condition | DTI, PET (FDG), RS-fMRI, SPECT (rCBF), SPECT/PET tracers (e.g., neurotransmitters), VBM | Widely employed in studies comparing patients with or without FOG. Its usefulness relies on the robustness of the result and on the use of specific tracers (e.g. neurotransmitters). | Inability to definitively find a causative (rather than associative) correlation between the observed abnormalities and FOG. Limited experience with tracers exploring neurotransmitters other than dopamine. Findings may reflect compensatory mechanisms |
| Repeated studies following prolonged walking | SPECT using radionuclides with long half-life of decay (e.g. 99mTc-HMPAO or 99mTc-ECD), PET displacement studies (e.g., 11C-CFT for DAT) | Since the half-life duration of its radiotracers is long, SPECT enables the description of the cerebral structures involved in gait maintenance, meaning that the radiotracer is fixed in the active brain regions during walking prior to image acquisition. | Slight variation in head position between studies may jeopardize the comparison. Treadmill walking might not be the right gait surrogate (e.g. visual input does not change, there is no turning) The hemodynamic changes induced by motion may affect binding and blood flow. |
| Repeated studies following a given intervention | PET using radionuclides with short half-life of decay (H2 15O), SPECT (rCBF) | Used to explore the effect of external factors (e.g. environment, visual cues), therapeutic intervention (e.g. medications, DBS) or other experimental procedures | Slight variation in head position between studies may jeopardize the comparison. Intervention may produce a ancillary effect unrelated to gait changes (e.g. DBS may activate the neural pathways passing near the electrode, with remote antidromic and/or orthodromic effects) |
| Study while the patient executes a surrogate of walking | PET using radionuclides with short half-life of decay (H2 15O), RS-fMRI, T-fMRI | During these studies, the patients are asked to step or cycling while lying down. | Repetitive foot or lower limb movement performed while lying supine lack several important features of gait control, the most important being the need for balance control FOG pts have a normal rhythmic leg movements when seated or lying |
| Study during motor imagery (i.e., the mental simulation of a given action, without actual execution) or virtual reality | PET using radionuclides with short half-life of decay (H215O) T-fMRI | It allows investigation of the internal dynamics of movement planning and preparation of gait, while avoiding sensory and motor compounds related to motor execution. | Lying supine lack several important features of gait control, the most important being the need for balance control. Only a single PET study has been performed so far [73]. |
Abbreviation: 11C-CFT: 11C-2-β-carbomethoxy-3β-(4-fluorophenyl) tropane; 99mTc-ECD: 99mTc-ethyl cysteinate dimer; 99mTc-HMPAO: technetium-99m-hexamethyl-propyleneamine oxime; DAT: dopamine transporter; DBS: deep brain stimulation; DTI: diffused tensor imaging; FDG: fluorodeoxyglucose; FOG: freezing of gait; PET: positron emission tomography; rCBF: regional cerebral blood flow; RS-fMRI: Resting-state functional magnetic resonance imaging; SPECT: single photon emission computed tomography; T-fMRI: Task-related functional magnetic resonance imaging; VBM: voxel-based morphometry.
Table 2
Structural and functional MRI studies in PD patients with FOG
| Reference | Subjects (age) | Neuroimaging technique | Brain measures | Main findings |
| Schweder et al. [74] | 2 PD+FOG, 8 PD-FOG 17 HC * | DTI around PPN in DBS patients | WM connectivity | In PD+FOG, reduced WM connectivity in pontine-cerebellar projections, (i.e., absence of PPN connectivity to any part of the cerebellum) compared to HC and PD-FOG. Increased WM connectivity in cortico-pontine projections |
| Snijders et al. [41] | 12 PD+FOG (59±9) 12 PD-FOG (63±7) 21 HC (57±9) ** | VBM | Gray matter atrophy | In PD+FOG, reduced GM volume in the MLR. |
| Kostic et al. [43] | 17 PD+FOG (64±8) 20 PD-FOG (63±5) 34 HC (64±7) ** | VBM | Gray matter atrophy | In PD+FOG, compared to both PD no-FOG and HC, reduced GM volume in left inferior frontal gyrus, precentral gyrus and inferior parietal gyrus (un- corrected). FOG severity correlated with: frontal executive deficits, bilateral frontal and parietal cortices GM volumes |
| Tessitore et al. [42] | 12 PD+FOG (67±5) 12 PD-FOG (66±6) 12 HC (66±6) ** | VBM | Gray matter atrophy | In PD+FOG, reduced GM volume in left precuneus, cuneus, lingual gyrus and posterior cingulated gyrus, compared to both PD no-FOG. FOG severity correlated with posterior cortical GM loss |
| Fling et al. [47] | 14 PD+FOG (67±5) 12 PD-FOG (65±7) 15 HC (67±8) ** | DTI | WM connectivity | In PD+FOG, reduced WM connectivity from the PPN to the cerebellar locomotor regions, thalamus, and multiple regions of the frontal and prefrontal cortex. These structural differences were observed only in the right hemisphere of the freezers |
| Sunwoo et al. [44] | 16 PD+FOG (67±5) 30 PD-FOG (69±4) ** | VBM Region of-interest-based volumetric analysis | Sub-cortical gray matter volumes | The normalized substantia innominata volume did not differ significantly between freezers and non-freezers. The automatic analysis of subcortical structures revealed that the thalamic volumes were significantly reduced in PD patients with FOG compared to those without |
| Herman et al. [45] | 30 PD+FOG (65±9) 76 PD-FOG (65±10) *** | VBM | Gray matter atrophy | In the entire cohort (n = 106), no differences in GM atrophy between freezers and non-freezers. In the matched smaller groups, PD+FOG had reduced GM volume in the left IPL and angular gyrus |
| Snijders et al. [41] | 12 PD+FOG (59±9) 12 PD-FOG (63±7) 21 HC (57±9) | fMRI | Task-related | In FOG+ patients compared to FOG- and HC: increased activation in MLR; decreased activation in SMA, frontal and posterior parietal lobes |
| Tessitore et al. [53] | 16 PD+FOG (67±6) 15 PD-FOG (66±6) ** | fMRI | Resting-state | In FOG+ patients compared to FOG- and HC: reduced functional connectivity in both executive (fronto-parietal) and visual (occipito-temporal) networks. FOG severity was significantly correlated with decreased connectivity within the two resting state networks |
| Shine et al. 2013 [49] | 18 PD+FOG (67±8) | fMRI | Task-related | In FOG+ patients: motor arrests were associated with an increased BOLD response within fronto-parietal and insular cortices and a concomitant decreased response in bilateral sensorimotor areas and subcortical areas (caudate, thalamus and GPi) Changes were inversely correlated with FOG severity |
| Shine et al. 2013 [50] | 14 PD+FOG (63±7) 15 PD-FOG (63±8) ** | fMRI | Task-related | In FOG+ patients compared to FOG-: decreased BOLD response in the bilateral anterior insula, ventral striatum, pre-SMA and left STN during the performance of simultaneous motor and cognitive tasks |
| Shine et al. 2013 [28] | 10 PD+FOG (67±6) 10 PD-FOG (66±6) ** | fMRI | Task-based functional connectivity study | In FOG+ patients compared to FOG-: functional decoupling between the basal ganglia network (left and right caudate, rostral cingulate) and the cognitive control network (left and right PPC, DLPFC, VLPFC). This decoupling was also associated with paroxysmal motor arrests |
| Vercruysse et al. 2013 [51] | 16 PD+FOG (66±7) 16 PD-FOG (67±5) 16 HC (67±6) ** | fMRI | Task-related | In FOG+ patients compared to FOG- and HC: during successful movement decreased activation in right DLPFC, left PMd and left M1, and bilateral increased activation in dorsal putamen, pallidum and STN. During upper limb motor blocks, FOG+ showed increased activation in right M1, PMd, SMA and left DLPFC and decreased activation in bilateral pallidum and putamen |
| Fling et al. 2014 [54] | 15 PD (65±6): 8 PD+FOG 7 PD-FOG 14 HC (67±8) ** | fMRI | Resting-state | In FOG+ patients compared to FOG- and HC: increased functional connectivity between SMA and bilateral MLR and between SMA and left CLR. Connectivity in these regions was positively correlated with FOG severity |
Abbreviations: *: No age and matching details; **: age- and gender-matched; ***: age- and gender-matched, plus disease-related group matching (n = 22 each); CLR: cerebellar locomotor region; DBS: deep brain stimulation; DLPFC: dorsolateral prefrontal cortex; DTI: Diffused Tensor Imaging; FLAIR: fluid attenuated inversion recovery; fMRI: functional magnetic resonance imaging; FOG: freezing of gait; GM: gray matter; GPi: globus pallidus internus; HC: healthy control; IPL: inferior parietal lobe; M1: primary motor cortex; MLR: mesencephalic locomotor region; PD: Parkinson’s disease; p-PIGD: predominant postural instability gait difficulty; p-TD: predominant tremor dominant; PMd: dorsal premotor cortex; PPC: posterior parietal cortex; PPN: pedunculopontine nucleus; SMA: supplementary motor area; STN: subthalamic nucleus; VBM: voxel-based morphometry; VLPFC: ventrolateral prefrontal cortex; WMh: white matter hyperintencities.



