The Profile of Long-term Parkinson’s Disease Survivors with 20 Years of Disease Duration and Beyond
Abstract
Background:
Parkinson’s disease (PD) patients with 20 years or more survival (PD-20) are not well characterized.
Objective:
To evaluate PD-20 patient characteristics and identify areas for improvement of their health care.
Methods:
The international, multicenter National Parkinson’s Foundation Quality Improvement Initiative (NPF-QII) study database was queried to identify PD-20 subjects. Demographic and clinical data were analyzed.
Results:
We identified 187 PD-20 subjects (55% men) representing 4% (187/4,619) of all NPF-QII participants. Subjects were mean age 69.5 years; mean age at PD onset was 44.0 years. The majority (75%) had 20-25 years of PD duration, the longest duration being 49 years. They were median Hoehn and Yahr stage 3, and 75% had motor fluctuations. Half (54%) reported exercising. The majority (89%) were living at home and required a caregiver (88%). They were mildly cognitively impaired for age (Montreal Cognitive Assessment estimate 22.6±3.7), with most deficits in verbal fluency and delayed recall. Quality of life (Parkinson’s Disease Quality of Life Questionnaire index 36±15%) was mild to moderately impaired, with most impairment in mobility and activities of daily living. Caregiver strain measured by the Multidimensional Caregiver Strain Index (27±16%), recorded highest subscores in social constraint. PD-20 subjects aged <70 years versus ≥70 only differed significantly by worse cognition (P < 0.0001).
Conclusions:
PD-20 subjects reflect an elite group of PD survivors with early-onset disease and relatively mild cognitive disability despite long disease duration. Interventions for caregivers, mobility, and activities of daily living are areas that could improve caregiver burden and patient quality of life.
INTRODUCTION
Parkinson’s disease (PD) patients have shortened survival when compared with age-matched controls [1–4]. Several risk factors negatively impact PDsurvival including demographic factors, such as older age at onset [1]; PD factors, such as psychosis, dementia, and motor severity [4, 5]; and non-PD factors, such as pneumonia and cachexia [3]. Very few PD patients survive beyond 20 years, particularly those who develop PD in their seventh or eighth decades. Natural history studies are limited but suggest dementia and nursing home placement are almost inevitable in those who reach the 20-year milestone [6]. Consequently, PD patients with long-term survival of 20 years or greater have not been well characterized. Hence, the aim of this study was to describe PD-20 patients derived from a large community-dwelling cohort. Characterization of these patients may reveal favorable factors for survival in PD. It may also help improve health care delivery in thispopulation.
METHODS
Study design
The international, multicenter National Parkinson Foundation’s Quality Improvement Initiative (NPF-QII) study database prospectively records annual data on PD subjects from 20 NPF Centers of Excellence in the United States, Canada, Netherlands, and Israel [7]. At the time of the study, NPF-QII was in its third year of enrollment, having registered 4,619 PD participants. All enrolled subjects provide written informed consent.
The NPF-QII dataset was queried in a cross-sectional manner to identify and characterize PD-20 subjects. Demographic and clinical data extracted included gender, race, age at onset, age at diagnosis, disease duration, presence of tremor, motor fluctuations, Hoehn and Yahr stage, co-morbidities, living situation, presence or absence of a regular care partner, exercise, hospital and emergency room visits within the past year, medications, and other treatments (physical therapy, occupational therapy, speech therapy). Data instruments included Timed Up and Go test [8], Parkinson’s Disease Quality of Life Questionnaire (PDQ-39) [9], Multiple Caregiver Strain Index (MCSI) [10], and Montreal Cognitive Assessment (MoCA) estimate [11] scores. The PDQ-39 is a validated, health-related, quality of life (QoL) scale that is completed by the subject and measures health status and impact of PD on daily life. There are 39 items subdivided into eight domains: mobility, activities of daily living (ADLs), emotional well-being, stigma, social support, cognition, communication, and bodily discomfort. The sum score is expressed as a percentage (PDQ index, %), where 0% is no disability and 100% is maximum disability [9]. MCSI is an 18-item tool completed by the caregiver, measuring 6 domains of subjective response to stressors. Subscores include physical strain, social constraints, financial strain, time constraints, interpersonal strain, and elder demanding/manipulative. Similar to the PDQ-39, the MCSI is expressed as an index percentage [10]. The scale has not been validated in PD, but expert review recommended this was appropriate for the PD population [7]. The MoCA estimate, a weighted cognitive score formed from a combination of immediate and delayed 5-word recall and semantic fluency (animals) scores to optimally predict MoCA [11] results, was concurrently collected at one center (University of Pennsylvania). Multiple medication use was defined as 2 or more medications. A subgroup analysis comparing PD-20 patients aged <70 years versus ≥70 years was also performed to assess whether current age may be a factor in the recorded outcomes. We arbitrarily chose age 70, where patients younger than age 70 would have had PD onset beforeage 50.
Statistical analysis
Standard descriptive statistics were used for mean and standard deviation values. For comparison ofPD-20 subjects <70 versus ≥70 years old, Studentt and chi-square tests were conducted. The statistical significance was set at P < 0.0001.
RESULTS
There were 187 PD-20 subjects (55% men), representing 4% (187/4,619) of all PD subjects in the NPF-QII study (Table 1). PD-20 subjects were mean age 69.5 years old (±8.3 years), with a mean age of onset of 44.0 years (±9.0 years). All had PD onset before age 66. The majority (75%) had 20–25years of PD duration, and the longest duration was 49 years (Fig. 1). They were median Hoehn and Yahr stage 3, with rest tremor reported by 55% , and motor fluctuations in 75% . Almost all (98%) were taking levodopa, with dopamine agonists (40%) and amantadine (37%) the next most commonly used medications for motor symptoms. Antidepressants (40%), cognitive enhancers (16%), and antipsychotics (14%) were the most commonly used medications for non-motor symptoms. Eighty-four (45%) had undergone deep brain stimulation (DBS). Just over half (54%) reported some form of exercise, although the frequency and intensity was not known. The use of therapists occurred in a minority of patients. A large proportion (41%) had either visited the emergency room or had been admitted to hospital in the last year. Encouragingly, the vast majority (89%) were still living at home, although most required a caregiver (88%). They were on average mildly cognitively impaired for age (MoCA estimate 22.6±3.7), with most impairment in verbal fluency and delayed recall. Patient self-reported QoL was mild to moderately impaired (PDQ-39 index 36±15%), with highest subscores in mobility and ADLs. Caregiver strain was also mild to moderately impaired (27±16%), with highest subscores in social constraint.
Comparison of PD-20 patients aged < 70 years old versus ≥70, revealed that they differed significantly by worse cognition (measured by delayed word recall and MOCA estimate) (P < 0.0001). Those younger than 70 were more likely to have undergone DBS (64 [66%] versus 20 [22%]; P < 0.0001). They were otherwise similar in all other domains, including medication use, mobility scores, likelihood of requiring a relative or paid caregiver, caregiver strain, and perceived QoL (Table 1).
DISCUSSION
PD subjects enrolled in the NPF-QII study that survived 20 years or longer had a more robust health status than anticipated.; they were not inevitably demented or residing in nursing homes [6]. The most apparent explanation is the PD-20 population had earlier onset disease, on average in the fifth decade compared with the seventh decade for PD cases overall. Early-onset PD is known to predict a milder disease course with longer survival and less disability compared to later-onset PD [5, 12, 13]. In addition, early-onset PD is more likely to include patients with autosomal recessive genetic variants [14]. These hereditary forms are commonly pure motor phenotypes with long disease duration [15]. It is likely that a proportion of our PD-20 patients had genetic variants, although this information was not captured in the NPF-QII study. The proportion of male subjects was lower (55% vs 45% female; ratio 1.2:1.0) than the reported overall PD ratio of 1.5-2.5 times higher in males [16]. Higher male mortality has been reported in the normal elderly as well as other chronic diseases. Female gender may be a protective factor for longer disease duration, possibly relating to hormonal or other gender-specific factors [16–18]. There were less tremor-predominant PD patients than anticipated, as the presence of tremor is reported to confer a more benign course with slower disease progression [19–21]. Tremor in our cohort may have been masked by PD drugs, or an artifact of the study recording protocol (examiner classification vs. strict classification).
Surprisingly, a high proportion of PD-20 subjects were still living at home (89%). This percentage was lower than that reported in a study of NPF-QII patients with 10 years PD survival [22], probably reflective of disease progression. The need for caregivers in this population (88%) is important to highlight and has implications for increased costs of care. Another important finding was caregiver burden, which also translates to higher costs [23, 24]. Likewise, almost half of the PD-20 subjects visited an emergency room or a hospital in the past year, which would also increase cost of care [23]. Of interest is that half of the patients reported exercising, although the type, frequency, and intensity were not recorded. This warrants further study, particularly given the postulated neuroprotective effect of exercise in dementia and Parkinson’s disease. Almost half had undergone DBS; whether this could be a factor relating to better survival is unknown, and will need to be examined in future prospective case-control natural history studies.
Remarkably, there was little difference betweenPD-20 subjects aged <70 years old versus those ≥70. The older subjects had worse function in a single cognitive domain (delayed verbal recall) likely explained by the age difference [12, 13]. It is noteworthy that this did not lead to a measurable difference in QoL, caregiver burden, or increased use of cognitive enhancement or antipsychotic medications. We anticipated worse mobility in the older PD-20 group, but their motor performance is likely an integral link to their survival. Also, the absence of a difference in mobility could explain why the QoL and caregiver burden was similar for the two subgroups, as impaired gait impacts QoL [25]. Those younger than age 70 had significantly higher rates of DBS treatment, which is not surprising given the risks of DBS surgery increase in those above age 70. However, the age at which DBS was performed was not recorded.
There has been a paucity of data on long-term PD survivors to date [5, 6]. The Sydney Multicenter Study followed 136 community-dwelling PD patients over a 20-year period. Of the 36 survivors, 83% were demented and almost all were nursing home residents according to Hely et al. [6]. This has helped to shape a pessimistic view amongst neurologists that patients surviving 20 years and beyond are destined to have a poor prognosis. However, our findings highlight that this is not universal. While ours is a cross-sectional study, and therefore cannot be directly compared with the Hely study, it does demonstrate that certain PD patients may survive for two decades or more and have a reasonably acceptable QoL, still residing at home with retained cognition. The important lesson for neurologists is that these patients are likely to be encountered in PD clinics after 20 years of PD duration and functioning reasonably well.
The major limitation of our study is its cross-sectional nature. There is survival bias, as the original denominator of the PD-20 patients is unknown. There is sampling bias for PD patients attending NPF centers and enrollment bias for PD subjects listed in the NPF-QII study. Thus, PD-20 subjects with severe dementia or residing in a nursing home may not be represented in the NPF-QII. The study strengths are utilization of a uniform dataset and identification of the PD-20 subset from multiple geographic real-world clinics.
Despite the positive picture that emerged in ourPD-20 subjects, the mild to moderate impairment in QoL, and the mild to moderate caregiver burden will be important to address. Early prevention of motor symptom progression, dementia, and psychosis has been suggested as an important direction for increasing life expectancy in PD [5], although there are no existing preventive medications or strategies. The prospective nature of the NPF-QII clinical study enhances its ability to provide this type of information.
In conclusion, subjects surviving 20 years or longer with PD largely represent an elite subset of patients with early-onset disease, which is associated with a more benign course. They have relatively mild cognitive disability and continue to reside at home, despite long disease duration. Support for PD-20 caregivers could relieve caregiver burden, and targeting patient mobility and ADLs could improve PD-20 QoL. Further prospective research will be necessary to identify modifiable factors that confer long-term PD survival.
CONFLICT OF INTEREST
Dr. Hassan has nothing to disclose.
Dr. Wu has nothing to disclose.
Dr. Schmidt is employed by National Parkinson Foundation; receives royalties from a patent for knee replacement administered by the New York Society for the Relief of the Ruptured and Crippled; and has stocks and financial instruments.
Dr. Simuni is a consultant for Novartis, Ibsen, General Electric, UCB Pharma, Teva, and Boehringer Ingelheim and received grant support from the NIH (grants U01 NS050324-01A1, U01 NS050095-03, and U10 NS053377-02), Michael J. Fox Foundation for Parkinson’s Research, Teva, and Takeda.
Dr. Giladi serves as Associate Editor for Journal of Neural Transmission, as a member of the Editorial Board for Current Treatment Options in Neurology, and Journal of Parkinson’s Disease; serves as consultant for Teva-Lundbeck, Intec Pharma, NeuroDerm, Armon Neuromedical Ltd., and Pharma Two B; has received payment for lectures from Teva-Lundbeck, Novartis and UCB Pharma; and receives research support from Michael J. Fox Foundation for Parkinson’s Research (Grant: AJ -6201, BDPD- 9555, PPMI- 108/2310), the National Parkinson Foundation (PD in non-AJ- 1395), the European Union 7th Framework Programme (V-TIME- 278169 and CuPiD- 288586), and the Israel Science Foundation.
Dr. Miyasaki has received consultancy fees from for Novartis (end date December 31, 2013), speaking honorarium from Merz Pharma and Teva Innovations Canada, and a research grant from Allergan.
Dr. Bloem has served as an editorial board member of Movement Disorders; currently serves as an editorial board member of Physiotherapy Canada and Associate Editor for the Journal of Parkinson’s disease; received honoraria from serving on the scientific advisory board for Danone, Glaxo-Smith-Kline, UCB; received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, the Prinses Beatrix Foundation, the Stichting Parkinson Fonds and the Parkinson Vereniging, Netherlands Organization for Scientific Research, Prinses Beatrix Foundation, Stichting Parkinson Fonds, Michael J Fox Foundation, Parkinson Vereniging, and National Parkinson Foundation; and consultant for Danone, Glaxo-Smith-Kline, UCB; and received speaking fees fromAbbvie.
Dr. Malaty has received research support funding from the National Parkinson Foundation, Michael J. Fox Foundation, Tourette Syndrome Association; NIH, Abbott, Acadia, Allergan, EMD-Sorono, IPSEN, Merz, and TEVA, and honoraria from PRIME CME.
Dr. Okun has received consultant fees for the National Parkinson Foundation, research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, Quantia, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
FUNDING SOURCES FOR STUDY
Supported by the National Parkinson Foundation.
ACKNOWLEDGMENTS
This study was funded by the National Parkinson Foundation. The National Parkinson Centers of Excellence included in the study were: University of Florida, Markham Stouffville Hospital, Struthers Parkinson’s Center, Oregon Health & Science University, Northwestern University, Pennsylvania Hospital, Baylor College of Medicine, Beth Israel Deaconess Medical Center, The University of Kansas Medical Center, Johns Hopkins Medical Institute, The Parkinson’s Institute, Medical College of Georgia, University of South Florida, Vanderbilt University Medical Center, Mt. Sinai Hospital, Muhammad Ali Parkinson Center, Tel Aviv Sourasky Medical Center, and Nijmegen Parkinson Center.
REFERENCES
1 | Morgante L, Salemi G, Meneghini F, Di Rosa AE, Epifanio A, Grigoletto F, Ragonese P, Patti F, Reggio A, Di Perri R, Savettieri G (2000) Parkinson disease survival: A population-based study Arch Neurol 57: 507 512 |
2 | Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD (2001) Parkinsonism in Ontario: Increased mortality compared with controls in a large cohort study Neurology 57: 2278 2282 |
3 | D’Amelio M, Ragonese P, Morgante L, Reggio A, Callari G, Salemi G, Savettieri G (2006) Long-term survival of Parkinson’s disease: A population-based study J Neurol 253: 33 37 |
4 | Posada IJ, Benito-Leon J, Louis ED, Trincado R, Villarejo A, Medrano MJ, Bermejo-Pareja F (2011) Mortality from Parkinson’s disease: A population-based prospective study (NEDICES) Mov Disord 26: 2522 2529 |
5 | Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G (2010) What predicts mortality in Parkinson disease?: A prospective population-based long-term study Neurology 75: 1270 1276 |
6 | Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years Mov Disord 23: 837 844 |
7 | Okun MS, Siderowf A, Nutt JG, O’Conner GT, Bloem BR, Olmstead EM, Guttman M, Simuni T, Cheng E, Cohen EV, Parashos S, Marsh L, Malaty IA, Giladi N, Schmidt P, Oberdorf J (2010) Piloting the NPF data-drivenquality improvement initiative Parkinsonism Relat Disord 16: 517 521 |
8 | Morris S, Morris ME, Iansek R (2001) Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease Phys Ther 81: 810 818 |
9 | Peto V, Jenkinson C, Fitzpatrick R (1998) PDQ-39: A review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures J Neurol 245: Suppl 1 S10 S14 |
10 | Stull D (1996) The multidimensional caregiver strain index (MCSI): Its measurement and structure J Clin Geropsychol 2: 175 196 |
11 | Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, Porter RJ, Watts R, Anderson TJ (2010) The MoCA: Well-suited screen for cognitive impairment in Parkinson disease Neurology 75: 1717 1725 |
12 | Diederich NJ, Moore CG, Leurgans SE, Chmura TA, Goetz CG (2003) Parkinson disease with old-age onset: A comparative study with subjects with middle-age onset Arch Neurol 60: 529 533 |
13 | Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD (1989) Effect of age at onset on progression and mortality in Parkinson’s disease Neurology 39: 1187 1190 |
14 | Simon-Sanchez J, Kilarski LL, Nalls MA, Martinez M, Schulte C, Holmans P, Gasser T, Hardy J, Singleton AB, Wood NW, Brice A, Heutink P, Williams N, Morris HR (2012) Cooperative genome-wide analysis shows increased homozygosity in early onset Parkinson’s disease. e PLoS One 7: 28787 |
15 | Bonifati V (2012) Autosomal recessive parkinsonism Parkinsonism Relat Disord 18: Suppl 1 S4 S6 |
16 | Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW (2007) Gender differences in Parkinson’s disease J Neurol Neurosurg Psychiatry 78: 819 824 |
17 | Chillag-Talmor O, Giladi N, Linn S, Gurevich T, El-Ad B, Silverman B, Friedman N, Peretz C (2013) Estimation of Parkinson’s disease survival in Israeli men and women, using health maintenance organization pharmacy data in a unique approach J Neurol 260: 62 70 |
18 | Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA (2012) Predictors of survival in patients with Parkinson disease Arch Neurol 69: 601 607 |
19 | Hershey LA, Feldman BJ, Kim KY, Commichau C, Lichter DG (1991) Tremor at onset. Predictor of cognitive and motor outcome in Parkinson’s disease? Arch Neurol 48: 1049 1051 |
20 | Marras C, Rochon P, Lang AE (2002) Predicting motor decline and disability in Parkinson disease: A systematic review Arch Neurol 59: 1724 1728 |
21 | Eggers C, Pedrosa DJ, Kahraman D, Maier F, Lewis CJ, Fink GR, Schmidt M, Timmermann L (2012) Parkinson subtypes progress differently in clinical course and imaging pattern PLoS One 7: e46813 |
22 | Hassan A, Wu SS, Schmidt P, Malaty IA, Dai YF, Miyasaki JM, Okun MS (2012) What are the issues facing Parkinson’s disease patients at ten years of disease and beyond? Data from the NPF-QII study Parkinsonism Relat Disord 18: Suppl 3 S10 S14 |
23 | Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD (2003) Burden of parkinsonism: A population-based study Mov Disord 18: 313 319 |
24 | Dowding CH, Shenton CL, Salek SS (2006) A review of the health-related quality of life and economic impact of Parkinson’s disease Drugs Aging 23: 693 721 |
25 | Duncan GW, Khoo TK, Yarnall AJ, O’Brien JT, Coleman SY, Brooks DJ, Barker RA, Burn DJ (2014) Health-related quality of life in early Parkinson’s disease: The impact of nonmotor symptoms Mov Disord 29: 195 202 |
Figures and Tables
Fig.1
Disease duration of PD-20 subjects (N = 187).
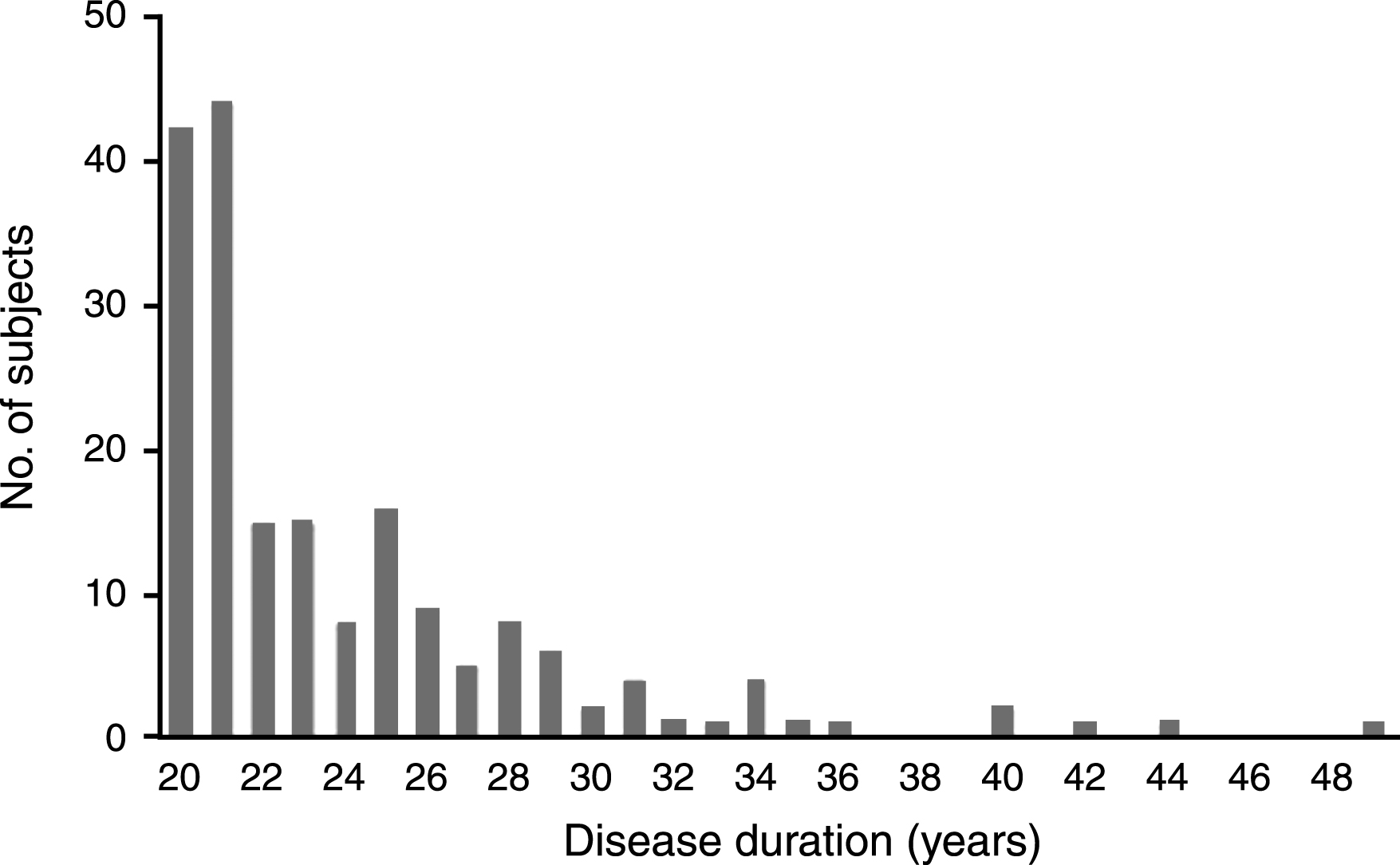
Table 1
Comparison of demographic and clinical characteristics for PD-20 patients with those aged <70 years versus those ≥70 years
| Overall PD-20 | Age | P Value | ||
| (N = 187) | <70 yrs (N = 97) | ≥70 (N = 90) | ||
| Demographic variables | ||||
| Male, N (%) | 103 (55%) | 54 (56%) | 49 (54%) | 0.866 |
| Age of onset, mean±SD | 44.0±9.0 | 38.8±6.5 | 49.8±7.8 | <0.0001 * |
| Current age, mean±SD | 69.5±8.3 | 63.2±5.0 | 76.3±5.1 | <0.0001* |
| Social variables | ||||
| Living situation, N (%) | ||||
| At home | 166 (89%) | 91 (94%) | 75 (83%) | 0.062 |
| Skilled care | 19 (10%) | 5 (5%) | 14 (16%) | |
| Other | 2 (1%) | 1 (1%) | 1 (1%) | |
| Regular care partner, N(%) | ||||
| No | 22 (12%) | 15 (16%) | 7 (8%) | 0.021 |
| Spouse/partner | 128 (68%) | 68 (70%) | 60 (67%) | |
| Other relative | 20 (11%) | 8 (8%) | 12 (13%) | |
| Paid caregiver | 14 (8%) | 3 (3%) | 11 (12%) | |
| Other | 3 (2%) | 3 (3%) | ||
| Clinical variables | ||||
| Certainty of idiopathic PD | 174 (94%) | 95 (98%) | 79 (90%) | 0.019 |
| diagnosis ≥90% | ||||
| Rest tremor present | 101 (55%) | 48 (50%) | 53 (60%) | 0.192 |
| Motor fluctuations | 140 (75%) | 73 (75%) | 67 (74%) | 0.898 |
| Standardized TUG (sec) | 0.6±1.0 | 0.4±1.1 | 0.9±0.9 | 0.001 |
| Immediate word recall | 3.9±1.1 | 4.2±0.9 | 3.6±1.2 | 0.0004 |
| Delayed word recall | 2.5±1.4 | 2.9±1.3 | 2.0±1.4 | <0.0001 * |
| Verbal fluency | 15.2±6.2 | 16.4±6.5 | 13.9±5.7 | 0.006 |
| MoCA estimate | 22.6±3.7 | 23.7±3.3 | 21.3±3.7 | <0.0001 * |
| Comorbidities | 1.9±1.3 | 1.9±1.4 | 2.0±1.2 | 0.643 |
| Deep brain stimulation, N (%) | 84 (45%) | 64 (66%) | 20 (22%) | <0.0001 * |
| Hospital/ER visit, N (%) | ||||
| Missing | 51 (27%) | 25 (26%) | 26 (29%) | 0.310 |
| None | 80 (43%) | 44 (45%) | 36 (40%) | |
| Hospital only | 17 (9%) | 12 (12%) | 5 (6%) | |
| ER only | 16 (9%) | 7 (7%) | 9 (10%) | |
| Hospital and ER | 23 (12%) | 9 (9%) | 14 (16%) | |
| Medications, N (%) | ||||
| Any form of levodopa | 182 (98%) | 93 (97%) | 89 (99%) | 0.344 |
| Dopamine agonist | 74 (40%) | 41 (43%) | 33 (37%) | 0.400 |
| MAO-B inhibitor | 32 (17%) | 13 (14%) | 19 (21%) | 0.182 |
| COMT inhibitor | 56 (30%) | 32 (33%) | 24 (27%) | 0.322 |
| Amantadine | 68 (37%) | 42 (44%) | 26 (29%) | 0.031 |
| Antidepressant medications | 75 (40%) | 48 (50%) | 27 (30%) | 0.006 |
| Cognitive enhancers | 29 (16%) | 11 (12%) | 18 (20%) | 0.115 |
| Stimulants | 4 (2%) | 3 (3%) | 1 (1%) | 0.344 |
| Antipsychotic medications | 25 (14%) | 9 (9%) | 16 (18%) | 0.082 |
| Anticholinergic medications | 12 (7%) | 9 (10%) | 3 (3%) | 0.090 |
| Other treatments, N (%) | ||||
| Physical therapy | 87 (47%) | 42 (43%) | 45 (50%) | 0.359 |
| Occupational therapy | 37 (20%) | 11 (11%) | 26 (29%) | 0.003 |
| Speech therapy | 40 (21%) | 22 (23%) | 18 (20%) | 0.655 |
| Exercise | 100 (54%) | 47 (49%) | 53 (59%) | 0.153 |
| Social worker/counseling | 25 (13%) | 12 (12%) | 13 (15%) | 0.655 |
| No mental health therapy/referral | 16 (9%) | 12 (12%) | 4 (4%) | 0.053 |
| PDQ-39, mean±SD | ||||
| PDQ39 mobility | 22.1±11.7 | 19.9±11.7 | 24.4±11.4 | 0.008 |
| PDQ39 ADL | 12.1±6.7 | 10.7±6.4 | 13.7±6.6 | 0.003 |
| PDQ39 emotion | 7.3±5.3 | 7.3±5.4 | 7.3±5.2 | 0.978 |
| PDQ39 stigma | 3.8±3.4 | 3.8±3.4 | 3.7±3.6 | 0.774 |
| PDQ39 social support | 2.2±2.3 | 2.3±2.4 | 2.0±2.1 | 0.292 |
| PDQ39 cognition | 5.6±3.4 | 5.3±3.5 | 6.0±3.3 | 0.151 |
| PDQ39 communication | 4.8±3.0 | 4.9±3.0 | 4.7±3.0 | 0.583 |
| PDQ39 pain | 4.4±2.9 | 4.5±2.9 | 4.3±3.0 | 0.745 |
| PDQ39 summary index (%) | 35.8±16.3 | 34.3±16.8 | 37.4±15.7 | 0.204 |
| MCSI, mean±SD | ||||
| Physical strain | 3.5±2.9 | 3.5±2.9 | 3.5±2.9 | 0.937 |
| Social constraints | 6.3±3.7 | 6.0±4.1 | 6.7±3.4 | 0.334 |
| Financial strain | 1.2±1.4 | 1.1±1.3 | 1.3±1.5 | 0.575 |
| Time constraints | 3.4±2.2 | 3.4±2.2 | 3.5±2.1 | 0.844 |
| Interpersonal strain | 3.4±3.0 | 3.2±3.1 | 3.5±2.9 | 0.679 |
| Elder demanding/manipulative | 1.2±1.6 | 0.9±1.4 | 1.6±1.8 | 0.023 |
| MCSI index (%) | 26.5±16.0 | 25.1±16.2 | 27.8±15.8 | 0.349 |
ADL, activities of daily living; COMT, catechol-O-methyl transferase; ER, emergency room; MAO, monoamine oxidase; MCSI, Multiple Caregiver Strain Index; MoCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; PDQ, Parkinson’s Disease Quality of Life Questionnaire; TUG, timed up and go. * P < 0.0001 significant.



