Health-Related Quality of Life in Children with Duchenne Muscular Dystrophy: A Review
Abstract
In pediatric chronic illness, improving health-related quality of life (HRQOL) has become one of the most important goals of disease management. Duchenne muscular dystrophy (DMD) is a debilitating, progressive and chronic neuromuscular disorder affecting boys. The purpose of this review is to provide an overview of published research on HRQOL in the pediatric DMD population, describe the instruments used and summarize the study findings. The databases searched were Medline, Embase and PsycInfo. The literature search yielded 167 articles, of which 19 were included in this review. The studies were published between 2005 and 2013 across nine countries. Thirteen different generic and disease-specific measures were used, the most common being the Pediatric Quality of Life 4.0 Generic Core module.
HRQOL in boys with DMD is worse than that of healthy peers and children with other chronic illnesses, especially in the physical domains. Boys who are at a more severe stage of the disease reported worse physical HRQOL but not necessarily psychosocial HRQOL than boys at a less severe stage. Traditional clinical outcome measures correlated well only with physical HRQOL. Parents’ proxy-reports of their sons’ HRQOL and the boys’ self-reports had poor concordance. More research is needed to assess trends in HRQOL over time and to elucidate factors that affect HRQOL.
ABBREVIATIONS
CHQ | child health questionnaire |
DMD | Duchenne muscular dystrophy |
FDA | Food and Drug Administration |
HRQOL | health-related quality of life |
ICC | intraclass correlation coefficient |
LSI-A | Life Satisfaction Index-Adolescent |
NM | Neuromuscular |
PODCI | Pediatric Outcomes Data Collection Instrument |
SOLE | strips of life with emoticons |
INTRODUCTION
Duchenne muscular dystrophy (DMD) is the most common and severe form of neuromuscular illness in children, affecting between 1 in 3, 500 and 1 in 6, 000 newborn boys [1, 2]. It is characterized by progressive muscle loss that begins in proximal, lower extremity muscles. Muscle degeneration eventually leads to loss of ambulation, occurring between 10 to 14 years of age [3]. The respiratory, cardiac and bone health of boys with DMD are compromised. There is also a higher prevalence of cognitive disability in this population [4]. Respiratory or cardiac failure eventually leads to premature death, typically in the second to third decade of life [5, 6].
Medical interventions have extended the quantity of life of DMD patients, however, their quality of life (QOL) remains greatly jeopardized by the condition [7]. The World Health Organization Quality of Life group has defined quality of life as ‘individuals’perception of their position in life, in the context of culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns [8]. Health-related quality of life (HRQOL) narrows the scope of QOL and focuses specifically on the impact of illness and treatments on a person’s life [9–11]. Although the concept of HRQOL has been operationalized rather inconsistently across the various measures developed, there are two elements of the concept that are widely agreed to be core: that it includes both subjective and objective dimensions of the impact a health condition and/or its management has on an individual and that it is multidimensional, encompassing multiple aspects of a person’s life. Thus, most instruments are made up of multiple domains, including emotional, social and physical health [12].
The Food and Drug Administration (FDA) has mandated that all clinical trials include patient reported outcomes such as HRQOL [13]. A number of promising molecular therapies for DMD are currently undergoing various stages of clinical trials [14]. A greater understanding of HRQOL would enable researchers and clinicians to meaningfully interpret forthcoming trial results and better incorporate HRQOL measures into future studies. Furthermore, understanding the state of HRQOL in the DMD population is important in and of itself due to its chronic nature and the prolonged life expectancy that has become possible.
While research on HRQOL in DMD is still scarce, it has gained momentum in the past few years. The aim of this article is to provide a thorough review of existing literature on HRQOL in the pediatric DMD population. Key themes, such as comparison of HRQOL with healthy children and other disease groups will be addressed and the limitations of existing literature will be discussed.
METHODS
A literature search was conducted in between October 2014 and April 2015 in the electronic databases Medline, Embase and PsycINFO using the following key words: 1) Duchenne Muscular Dystrophy; 2) quality of life OR health related quality of life OR health-related quality of life OR health status; 3) pediatri * OR paediatri OR teenage * OR adolescent OR child OR child * 4) 1 AND 2 AND 3. Medical Subject Heading (MeSH) terms were used whenever possible. Through the initial search criteria, 167 articles were identified that were published from 1986 to 2015. The abstracts were examined and, if suitable, full-length articles were retrieved (Figure 1). Articles were included in this review if they met the inclusion criteria. The reference lists of relevant studies were used to identify potential studies not discovered upon initial search.
Inclusion criteria:
1) Child or parent-reported QOL or HRQOL was the main outcome of the study; 2) study population was specific to or included children with Duchenne Muscular Dystrophy (≤18); and 3) was published in the English language.
RESULTS
Nineteen studies were identified that fit the inclusion criteria. The studies were published between 2005 and 2013 and were conducted in the following countries: United States, the Netherlands, Germany, China, Brazil, France, Australia, Italy, and Canada. There are regional differences in the selection of HRQOL measures: almost all studies carried out in North America used either the PedsQL4.0 Generic Core or the CHQ-50 while almost none of the European countries used these two measures even though they are available in the languages of these countries. Each of the studies done in Europe used a different HRQOL measure.
Thirteen measures of HRQOL were used. Ten were generic measures and most consisted minimally of physical, psychological and social domains. Four studies used disease specific questionnaires: the English [15] and Chinese [16] versions of the PedsQL 3.0 Neuromuscular module, the PedsQL 3.0 DMD module [17] and the Strips of Life with Emoticons [18]. The development of the PedsQL 3.0 Neuromuscular module was described in detail by Iannacone [19]. It underwent extensive review with input from clinicians and families of children with spinal muscular atrophy and DMD. The Neuromuscular module and DMD module were developed simultaneously. A summary of all measures used can be found in Table 1.
The PedsQL 4.0 Generic Core, PedsQL 3.0 Neuromuscular, PedsQL 3.0 DMD modules as well as the PARS-III have been validated in pediatric DMD populations; none of the other measures has been validated in this population. The PedsQL 4.0 Generic core module and the PedsQL 3.0 Neuromuscular module were tested in a group of 44 boys with DMD and their parents [15]. Both modules were found to be feasible and have adequate construct validity for child and parent reports. The internal consistency reliability on the PedsQL Neuromuscular module exceeded the conventional minimum alpha coefficient of 0.7 for all scales in child and parent reports. The internal consistency reliability on the PedsQL Generic Core module exceeded the minimum alpha coefficient for all scales on the parent report. The test-retest reliability of the PedsQL Neuromuscular module is supported for child and parent reports [15]. The PedsQL 3.0 DMD module was tested in a group of 203 families of boys with DMD. All scales on child and parent reported questionnaires exceeded the minimum alpha coefficient needed for group comparisons [17]. Construct validity was also found to be adequate. The PARS-III was tested in a group of 282 males with DMD [20]. Internal consistency reliability was found to be good for the total score and most scale scores. Construct and convergent validities were also found to be adequate.
Six of the studies used parent reports only, seven used child report only, and six used both child reports and parent reports. Two studies had the same cohort of patients [21, 22], one used parent reports only, one used both child and parent reports. Seventeen of the studies were cross-sectional and two were longitudinal, conducted over the span of nine months and one year [23, 24]. The sample sizes of the studies ranged from 25 to 287. It should be noted, however, that some of studies’ patient populations included adults with DMD or children with other types of neuromuscular disorders [18, 25–27].
Although almost all of the studies of HRQOL in children with DMD are descriptive, some comparative analyses were completed. Four recurring themes were identified: 1) comparison of HRQOL between boys with DMD to either healthy peers or those with another chronic illness; 2) comparison among sub-groups within the pediatric DMD sample; 3) investigation of relationship between clinical measures of function and HRQOL; and 4) comparison of children’s and parents’ perception of children’s HRQOL. Each of the four themes will be discussed in detail below. A summary of major findings of all nineteen studies reviewed can be found in Table 2.
Comparison to healthy peers or those with another chronic illness
Ten studies compared HRQOL in boys with DMD to a healthy cohort or children with other chronic illnesses, such as diabetes, cerebral palsy and epilepsy. Four of these studies recruited healthy controls along with their DMD sample [18, 24, 28, 29], and six studies used established normative data from the literature as basis of their comparison to healthy children [15, 17, 22, 30–32].
Elsenbruch et al. [31] divided their sample into child and adolescent groups. In the children’s group, all domain scores as well as the total HRQOL score were significantly lower than the scores of age-matched children with other chronic illnesses. In the adolescent group, only the social inclusion domain score was significantly lower in boys with DMD than the normative data. Orcesi et al. [18] developed a new HRQOL instrument targeting young children with neuromuscular illnesses, the Strips of Life with Emoticons (SOLE), which only has an overall score and no domains scores. Compared to healthy boys, boys with DMD had significantly lower scores on the SOLE.
Eight studies found that boys with DMD had significantly worse physical HRQOL than healthy boys or children with other types of chronic illnesses [15, 17, 22, 24, 28–30, 32]. Five studies reported that boys with DMD had significantly lower psychosocial score than healthy controls [15, 22, 28–30]. The difference in physical HRQOL score were consistently larger than difference in psychosocial score, and these deficiencies were observed in both children’s and parent’s reports.
Comparison among sub-groups within the pediatric DMD sample
In DMD studies, age is often used as a proxy for disease progression given that generally, older children are at a more severe stage of the illness. Patients with DMD may also be classified by ambulation status (ambulant or non-ambulant) or ventilation status (requires ventilation or not).
Three studies used the PedsQL 4.0 Generic Core scale and reported that younger boys had significantly higher physical HRQOL scores than older boys [17, 21, 29]. In contrast, another study reported that boys younger than 10 years of age did not report significantly higher physical score than those who were older [28]. Two studies reported boys using wheelchairs had significantly lower physical domains scores than boys who were still ambulant [15, 30]. Kohler et al. [33], found that boys with DMD who required ventilation did not report significantly lower physical or mental HRQOL scores than those not requiring ventilation. However, this study used the Short Form-36, which was designed to assess HRQOL in adults rather than in children.
There is less consistency across studies assess psychosocial HRQOL. Four studies reported that there were no significant differences between older and younger boys in psychosocial HRQOL scores, [22, 28–30]. Similarly, Davis et al. reported no significant differences in psychosocial HRQOL scores between children using wheelchairs and those who were not [15]. In contrast, Hendriksen et al. found the psychosocial adjustment score to be positively associated with age in a survey of parents of boys with DMD [20]. As well, Uzark et al. found that boys in the oldest age group reported significantly higher psychosocial scores than boys in younger age groups; this difference was not observed in parent report [17]. Mah et al. found that, based on parent reports, children who require ventilation support had significantly lower physical and psychosocial HRQOL scores than children not requiring ventilation [26].
In a longitudinal study, Simon et al. followed a group of boys with DMD over nine months and found that life satisfaction score improved over time for all age groups [23]. One major limitation of the study was that the questionnaire used, the LSI-A, was designed for adolescents, but the majority of the study sample was under 12 years of age. There was no psychometric evaluation to justify this deviation.
On the PedsQL 3.0 Neuromuscular module, boys who were using wheelchairs full-time and their parents reported lower about My Neuromuscular Disease domain score than part-time wheelchair users and those not using wheelchairs [15]. There were no significant differences in the other domains or the total score. On the PedsQL 3.0 DMD module, boys receiving steroids had significantly higher scores on the Daily Activities domain than boys not receiving steroids by parent-reports; and higher Worry score (less worry) by self-reports. Parents of children in the youngest age group reported them to have significantly higher scores in Daily Activities, Treatment Barriers and Worry domains than the two olderage groups.
Overall, physical HRQOL in children who are at a more severe stage of the disease is worse than children who are at a less severe stage. Less consistent is the relationship between disease severity andpsychosocial HRQOL. Some studies found older children reported better psychosocial HRQOL despite having more severe disease progressions [17, 20], while others reported no significant differences between older and younger children [22, 28–30]. It is worth noting that no conclusions can be drawn regarding a possible causal relationship between disease severity and HRQOL from cross-sectionalstudies.
Correlation with clinical measures
Traditional outcomes used in clinical trials of DMD involve quantitative measures of strength and mobility. With the increasing recognition of the importance of patient-reported outcomes such as HRQOL, some studies have examined the relationship between clinical measures and HRQOL.
The self-reported domains of the KIDSCREEN-52 [17, 20] and the DISABKIDS [31] did not correlate well with clinical measures. The only significant correlations were between the physical domain of the KIDSCREEN-52 and the Vignos scale, a measure of upper body strength; and between emotional domain of the DISABKIDS and the Vignos scale. McDonald et al. [29] found that only the physical domains of the parent-reported PedsQL 4.0 Generic Core and the Pediatric Outcomes Data Collection Instrument (PODCI) significantly correlated with functional measures. Similarly, in two separate studies, Bray and colleagues [21, 22] found that only the physical domains of the CHQ-50 parent report and the physical domain of the self-reported PedsQL 4.0 Generic Core module correlated with the Vignos scale. The 6-minute walk test is a recently adopted outcome measure in clinical trials of DMD (NCT01462292). Henricson et al. [24] found that decline in 6-minute walk test over one year correlated significantly with decline in parent-reported PODCIscore.
Vuillerot et al. [27] examined self-reported well-being of a group of 43 adolescents with various neuromuscular disorders, 19 of whom were DMD patients. They concluded that HRQOL did not correlate with motor function.
Overall, it appears that while the physical domains of some HRQOL measures correlated significantly with commonly used clinical measures, neither the psychosocial domains nor the overall HRQOL scores correlated with clinical end points.
Comparison and agreement between child report and parent report
Parents generally rated their child’s HRQOL lower than children themselves did [15–17, 21, 34], although no statistical comparisons were conducted in these studies. This difference tended to be greater in the psychosocial domains than the physical domain.
Six studies included both child self-reports and parent proxy-reports, and of these, five studies examined concordance between child and parent reports. Parent and child concordance is determined by intraclass correlation coefficient (ICC). For the purposes of this review, an ICC of ≤0.40 is considered poor, of 0.41 to 0.75 is considered moderate, of >0.75 is considered excellent [17].
In studies that used the PedsQL 4.0 Generic Core module [15, 17, 21], only the school domain had moderate concordance, while other domains had poor concordance. Davis et al. [15] found that all subdomains of the English PedsQL 3.0 Neuromuscular have poor parent-child concordance. Hu et al. [16] tested the Chinese version of the same questionnaire and found that for 50 child-parent pairs, the ICC of the overall score and subdomains were moderate. Lim et al. [34] found that by both classical test analysis, which examines scale-level agreement, and Rasch analysis, which examines item-level agreement, there was better concordance between children and parents on the physical scale than psychosocial scale.
Overall, parents tend to rate their child’s HRQOL as worse than children themselves do, and the concordance between parent and child is in the poor to moderate range with the majority of domains and total scores in the poor range. The more observable aspects of a child’s life, such as school function tended to yield higher concordance.
DISCUSSION AND LIMITATIONS OF CURRENT STUDIES
There is heterogeneity across studies in the definition and constructs HRQOL. Some measures focus only on individuals’ feelings about their well-being (PARS-III, LSI-A), while other instruments are closer to measures of health status in that the questions evaluate the extent of a problem has occurred (CHQ and PedsQL questionnaires). Furthermore, it is difficult to compare results across measures that have different domains, particularly if they do not have summary score(s). Fortunately, the most commonly used questionnaires are CHQ and PedsQL, both of which have physical and psychosocial summary scores, making it easier to compare the findings.
Many of the studies used convenience sampling and drew participants from a single clinic. Given the severity of the disease, it is reasonable to assume that almost all boys with DMD are managed at a tertiary-care clinic, thus patients recruited through such clinics are likely to be representative of the DMD population. However, most studies did not report a response rate and it is unclear whether the respondents differ from those who chose not to participate.
Most of the studies had relatively small sample sizes, with 10 of the 18 studies having a sample size of 50 participants or less. When subgroup comparisons were made, the sample sizes became even smaller. Thus the lack of any significant differences across groups could be due to insufficient statistical power. Additionally, of all HRQOL measures, only the PedsQL modules and the PARS-III have been validated specifically in a pediatric DMD population [15, 20]. The reliability and validity of other measures in this population are unknown. Some studies have employed measures designed for adults [33] or adolescents [23] to children, without any psychometric evaluation of their appropriateness in pediatric populations.
In four of the studies [18, 25–27], boys with DMD were part of a sample that included children with other types of neuromuscular disorders, making it hard to elucidate specifically the HRQOL of boys with DMD. Some studies excluded boys younger than 8 years old [15, 31], or boys who have lost ambulation [24, 29]. Finally, not all of the studies assessed HRQOL from both parent and child perspectives, and few studies used disease-specific measures [15–18].
CONCLUSIONS
Based on existing literature, the HRQOL of boys with DMD appears to be significantly poorer than that of their healthy peers, particularly in the physical domain. Within the DMD sample, boys who are at a more severe stage of the disease consistently reported poorer physical HRQOL than boys who are at a less severe stage of the disease; such a difference was not as consistent for psychosocial HRQOL. Similarly, while the physical domains of HRQOL instruments correlated well with clinical measures of functioning, psychosocial domains did not. Finally, the parent and child concordance for most measures of HRQOL was poor.
The PedsQL Inventory has both generic and disease-specific measures, available in multiple languages, with the English versions having been validated in the pediatric DMD populations [15, 17]. Their brevity is advantageous in the clinical and research settings. Many past and current clinical trials in DMD have used PedsQL instruments as outcomes [35]. However, the internal consistency reliability of PedsQL instruments is low to moderate in some domains, and none has reached the level of 0.9 ideal for use at the individual patient level [36]. Furthermore, their responsiveness to important clinical changes has not been established, but this is the case for all other HRQOL measures in the DMD population. Although further psychometric testing, as well as establishing values such as minimally clinically important differences should be carried out, the PedsQL inventory at present, appear to be the most comprehensive and validated measures for clinical and research use in this population. Other multidimensional instruments such as DISABKIDS are available in multiple languages, including English, and are also candidates for clinical use. However, psychometric testing in the DMD population should be performed.
Most of the existing studies have significant limitations that restrict generalizability of the data and comparison of results. Further, the methodological variability in this emerging literature makes it difficult to establish conclusive ideas about sensitivity to change and minimally clinically important differences that will be important for interpreting patient reported outcomes in clinical trials. Longitudinal studies, in large and diverse DMD patient populations, with examination of multiple aspects of HRQOL and disease characteristics, are needed to evaluate HRQOL over time, and to potential determinants of HRQOL.
COMPETING INTEREST
The authors declare that they have no competing interests.
AUTHOR’S CONTRIBUTIONS
This review was adapted from the Master’s thesis of YW, who contributed to the design of the study, extracted the relevant articles and drafted the manuscript. CC and KS were involved in the design of the study, reviewed relevant articles and contributed to the manuscript at all stages.
ACKNOWLEDGEMENT
We thank Gracia Mabaya and Rhiannon Hicks for reviewing the manuscript and providing valuable feedback. YW was supported by the Children’s Health Research Institute’s Quality of Life Graduate Student Award.
REFERENCES
1 | Brooke M.H., Fenichel G.M., Griggs R.C., Mendell J.R., Moxley R., Florence J., King W.M., Pandya S., Robison J., Schierbecker J.(1989) Duchenne muscular dystrophy: Patterns of clinical progression and effects ofsupportive therapyNeurology39: 4475481 |
2 | Bushby K., Finkel R., Birnkrant D.J., Case L.E., Clemens P.R., Cripe L., Kaul A., Kinnett K., McDonald C., Pandya S., Poysky J., Shapiro F., Tomezsko J., Constantin C.(2010) DMD Care Considerations WorkingGrouDiagnosis and management of Duchenne muscular dystrophy, part Diagnosis, and pharmacological andpsychosocial managementLancet Neurol9: 17793 |
3 | Bushby K., Connor E.(2011) Clinical outcome measures for trials in Duchenne muscular dystrophy: Report fromInternational Working Group meetingsClin Investig.1: 912171235 |
4 | Snow W.M., Anderson J.E., Jakobson L.S.(2013) Neuropsychological and neurobehavioral functioning in Duchennemuscular dystrophy: A reviewNeurosci Biobehav Rev37: 5743752 |
5 | Kohler M., Clarenbach C.F., Bahler C., Brack T., Russi E.W., Bloch K.E.(2009) Disability and survival inDuchenne muscular dystrophyJ Neurol Neurosurg Psychiatry.80: 3320325 |
6 | Moxley R.T., Pandya S., Ciafaloni E., Fox D.J., Campbell K.(2010) Change in natural history of duchenne musculardystrophy with long-term corticosteroid treatment: Implications for managementJ Child Neurol25: 911161129 |
7 | Biggar W.D.(2006) Duchenne Muscular DystrophyPediatr Rev27: 38388 |
8 | The World Health Organization quality of life assessment (WHOQOL): Position paper from the World HealthOrganization(1995) Soc Sci Med41: 1014031409 |
9 | De Civita M., Regier D., Alamgir A.H., Anis A.H., Fitzgerald M.J., Marra C.A.(2005) Evaluating health-relatedquality-of-life studies in paediatric populations: Some conceptual, methodological and developmentalconsiderations and recent applicationsPharmacoEconomics23: 7659685 |
10 | Guyatt G.H., Feeny D.H., Patrick D.L.(1993) Measuringhealth-related quality of lifeAnn Intern Med118: 8622629 |
11 | Spieth L.E., Harris C.V.(1996) Assessment of health-related quality of life in children and adolescents: Anintegrative reviewJ Pediatr Psychol21: 2175193 |
12 | Davis E., Waters E., Mackinnon A., Reddihough D., Graham H.K., Mehmet-Radji O., Boyd R.(2006) Paediatricquality of life instruments: A review of the impact of the conceptual framework on outcomesDev Med ChildNeurol48: 4311318 |
13 | U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual Life Outcomes. 2006; 4: 79. |
14 | Liew W.K.M., Kang P.B.(2013) Recent developments in the treatment of Duchenne muscular dystrophy and spinalmuscular atrophyTher Adv Neurol Disord6: 3147160 |
15 | Davis S., Hynan L.S., Limbers C.a., Andersen C.M., Greene M.C., Varni J.W., Iannaccone S.T.(2010) The PedsQLin pediatric patients with Duchenne muscular dystrophy: Feasibility, reliability, and validity of the Pediatricquality of life inventory neuromuscular module and generic core scalesJ Clin Neuromuscul Dis11: 397109 |
16 | Hu J., Jiang L., Hong S., Cheng L., Kong M., Ye Y.(2013) Reliability and validity of the Chinese version of thePediatric Quality Of Life InventoryTM (PedsQLTM) 3.0 neuromuscular module in children with Duchenne musculardystrophyHealth Qual Life Outcomes11: 14747 |
17 | Uzark K., King E., Cripe L., Spicer R., Sage J., Kinnett K., Wong B., Pratt J., Varni J.W.(1559) Health-related quality of life in children and adolescents with Duchenne muscular dystrophyPediatrics130: 6e1559e1566 |
18 | Orcesi S., Ariaudo G., Mercuri E., Beghi E., Rezzani C., Balottin U.SOLE NMDs Study Group(2014) A new self-report quality of life questionnaire for children with neuromuscular disorders: Presentation of the instrument, rationale for its development, and some preliminary results.J Child Neurol29: 2167181 |
19 | Iannaccone S.T., Hynan L.S., Morton A., Buchanan R., Limbers C.A., Varni J.W.(2009) The pedsQL(TM) inpediatric patients with spinal muscular atrophy: Feasibility, reliability, and validity of the pediatric qualityof life inventory(TM) generic core scales and neuromuscular moduleNeuromuscul Disord NMD19: 12805812 |
20 | Hendriksen J.G.M., Poysky J.T., Schrans D.G.M., Schouten E.G.W., Aldenkamp A.P., Vles J.S.H.(2009) Psychosocial adjustment in males with Duchenne muscular dystrophy: Psychometric properties and clinical utilityof a parent-report questionnaireJ Pediatr Psychol34: 16978 |
21 | Bray P., Bundy A.C., Ryan M.M., North K.N., Everett A.(2010) Health-related quality of life in boys withDuchenne muscular dystrophy: Agreement between parents and their sonsJ Child Neurol25: 1011881194 |
22 | Bray P., Bundy A.C., Ryan M.M., North K.N., Burns J.(2011) Health status of boys with Duchenne musculardystrophy: A parent’s perspectiveJ Paediatr Child Health47: 8557562 |
23 | Simon V.A., Resende M.B.D., Simon M.A.V.P., Zanoteli E., Reed U.C.(2011) Duchenne muscular dystrophy:Quality of life among 95 patients evaluated using the life satisfaction index for adolescentsArq Neuropsiquiatr69: 11922 |
24 | Henricson E., Abresch R., Han J.J., Nicorici A., Goude Keller E., de Bie E., McDonald C.M.(2013) The 6-minutewalk test and person-reported outcomes in boys with duchenne muscular dystrophy and typically developingcontrols: Longitudinal comparisons and clinically-meaningful changes over one yearPLoS Curr5 |
25 | Grootenhuis M.A., de Boone J., van der Kooi A.J.(2007) Living with muscular dystrophy: Health related quality oflife consequences for children and adultsHealth Qual Life Outcomes5: 3131 |
26 | Mah J.K., Thannhauser J.E., Kolski H., Dewey D.(2008) Parental stress and quality of life in children withneuromuscular diseasePediatr Neurol39: 2102107 |
27 | Vuillerot C., Hodgkinson I., Bissery A., Schott-Pethelaz A.-M., Iwaz J., Ecochard R., D’Anjou M.-C., Commare M.-C., Berard C.(2010) Self-perception of quality of life by adolescents with neuromuscular diseasesJ Adolesc Health Off Publ Soc Adolesc Med46: 17076 |
28 | Bendixen R.M., Senesac C., Lott D.J., Vandenborne K.(2012) Participation and quality of life in children withDuchenne muscular dystrophy using the International Classification of Functioning, Disability, and HealthHealth Qual Life Outcomes10: 43 |
29 | McDonald C.M., McDonald D.a., Bagley A., Sienko Thomas S., Buckon C.E., Henricson E., Nicorici a., Sussman M.D.(2010) Relationship between clinical outcome measures and parent proxy reports of health-related qualityof life in ambulatory children with Duchenne muscular dystrophyJ Child Neurol25: 911301144 |
30 | Baiardini I., Minetti C., Bonifacino S., Porcu A., Klersy C., Petralia P., Balestracci S., Tarchino F., Parodi S., Canonica G.W., Braido F.(2011) Quality of life in Duchenne muscular dystrophy: The subjective impact onchildren and parentsJ Child Neurol26: 6707713 |
31 | Elsenbruch S., Schmid J., Lutz S., Geers B., Schara U.(2013) Self-reported quality of life and depressive symptomsin children, adolescents, and adults with duchenne muscular dystrophy: A cross-sectional survey studyNeuropediatrics44: 5257264 |
32 | Opstal S.L.S.H., Jansen M., van Alfen N., de Groot I.J.M.Health-related quality of life and itsrelation to disease severity in boys with duchenne muscular dystrophy:: Satisfied boys, worrying parents–Acase-control studyJ Child Neurol2013 |
33 | Kohler M., Clarenbach C.F., Böni L., Brack T., Russi E.W., Bloch K.E.(2005) Quality of life, physicaldisability, and respiratory impairment in Duchenne muscular dystrophyAm J Respir Crit Care Med172: 810321036 |
34 | Lim Y., Velozo C., Bendixen R.M.(2014) The level of agreement between child self-reports and parent proxy-reportsof health-related quality of life in boys with Duchenne muscular dystrophyQual Life Res Int J Qual Life AspTreat Care Rehabil |
35 | Bushby K., Finkel R., Wong B., Barohn R., Campbell C., Comi G.P., Connolly A.M., Day J.W., Flanigan K.M., Goemans N., Jones K.J., Mercuri E., Quinlivan R., Renfroe J.B., Russman B., Ryan M.M., Tulinius M., Voit T., Moore S.A., Lee Sweeney H., Abresch R.T., Coleman K.L., Eagle M., Florence J., Gappmaier E., Glanzman A.M., Henricson E., Barth J., Elfring G.L., Reha A., Spiegel R.J., O’donnell M.W., Peltz S.W., Mcdonald C.M.(2014) PTC124-GD-007-DMDSTUDY GROUPAtaluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve50: 4477487 |
36 | Varni J.W., Burwinkle T.M., Lane M.M.(2005) Health-related quality of life measurement in pediatric clinicalpractice: An appraisal and precept for future research and applicationHealth Qual Life Outcomes3: 3434 |
37 | Reid D.T., Renwick R.M.(1994) Preliminary validation of a new instrument to measure life satisfaction inadolescents with neuromuscular disordersJ Rehabil Res17: 2 |
38 | Landgraf J.M., Maunsell E., Speechley K.N., Bullinger M., Campbell S., Abetz L., Ware J.E.(1998) Canadian-French, German and UK versions of the Child Health Questionnaire: Methodology and preliminary itemscaling resultsQual Life Res7: 5433445 |
39 | Ravens-Sieberer U., Gosch A., Rajmil L., Erhart M., Bruil J., Duer W., Auquier P., Power M., Abel T., Czemy L., Mazur J., Czimbalmos A., Tountas Y., Hagquist C., Kilroe J.KIDSCREEN Group E(2005) KIDSCREEN-52 quality-of-life measure for children and adolescentsExpert Rev Pharmacoecon Outcomes Res5: 3353364 |
40 | Simeoni M.-C., Schmidt S., Muehlan H., Debensason M.DISABKIDS Group(2007) Field testing of a Europeanquality of life instrument for childrenand adolescents with chronic conditions: The 37-item DISABKIDS Chronic Generic ModuleQual Life Res Int J Qual Life Asp Treat Care Rehabil16: 5881893 |
41 | Vogels T., Verrips G.H.W., Verloove-Vanhorick S.P., Fekkes M., Kamphuis R.P., Koopman H.M., Theunissen N.C.M., Wit J.M.(1998) Measuring health-related quality of life in children: The development of theTACQOL parent formQual Life Res7: 5457465 |
42 | Simeoni M.C., Auquier P., Antoniotti S., Sapin C., San Marco J.L.(2000) Validation of a French health-relatedquality of life instrument for adolescents: The VSP-AQual Life Res Int J Qual Life Asp Treat Care Rehabil9: 4393403 |
43 | Daltroy L.H., Liang M.H., Fossel A.H., Goldberg M.J.(1998) The POSNA pediatric musculoskeletal functionalhealth questionnaire: Report on reliability, validity, and sensitivity to change. Pediatric Outcomes InstrumentDevelopment Group. Pediatric Orthopaedic Society of North AmericaJ Pediatr Ortho18: 5561571 |
Figures and Tables
Fig.1
Process of selecting studies included in this reviewProcess of selecting studies included in this review.
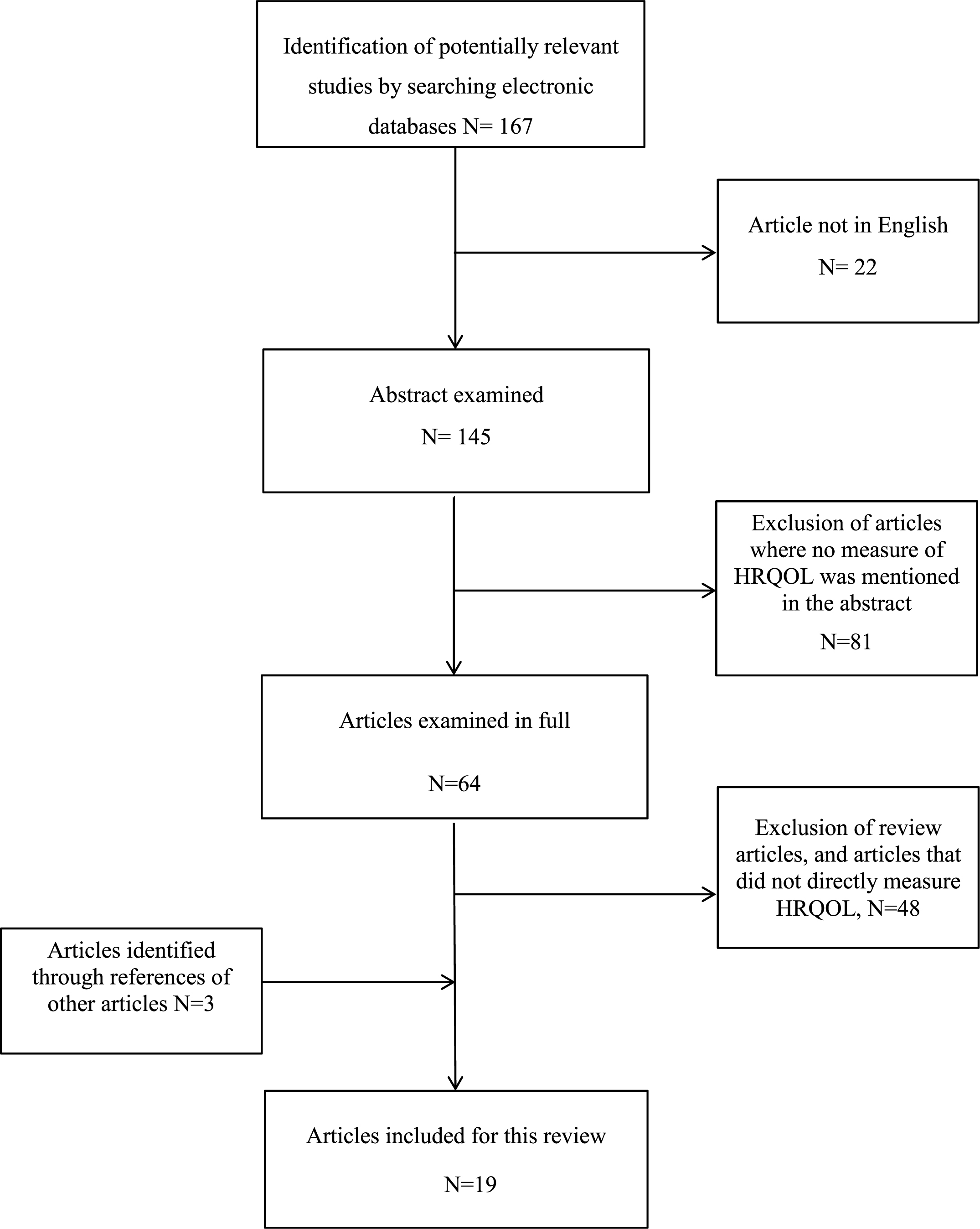
Table 1
Summary of health-related quality of life instruments used by studies reviewed
| Instrument | Dimensions or domains | Scoring | Psychometric properties * | |
| Pediatric Quality of Life | Physical, emotional, social and schoolfunctions | 23 items | English version valid | |
| 4.0 Generic Core | and reliable in DMD | |||
| (PedsQL 4.0 Generic) | Scores on four domains | population [15] | ||
| English and Chinese | Physical and Psychosocial | α= 0.45–0.89 | ||
| summary scores | ||||
| Parent and child reports | ||||
| available | Has total score | |||
| Life Satisfaction Index for | General wellbeing, interpersonalrelationship, | 45 items | Validated on | |
| Adolescents (LSI-A) | personal development, personal satisfaction, | adolescents with DMD | ||
| recreation | Scores on five domains | |||
| English and Dutch | α= 0.52–0.88 [37] | |||
| No total score | ||||
| Child report available | ||||
| Personal Adjustment and | Peer relations, dependency, hostility, | 28 items | Valid and reliable in US | |
| roles Skills Scale (PARS- | productivity, anxiety/depression, and | and Dutch DMD | ||
| III) | withdrawal | Scores on six domains | populations | |
| Dutch | Has total score | α= 0.75–0.91[20] | ||
| Parent report only | ||||
| PedsQL Neuromuscular | About My Neuromuscular Disease, | 25 items | Both English [15] and | |
| Module | communication, About our family resources | Chinese[16] versions | ||
| 3 domain scores | valid and reliable in | |||
| English and Chinese | children with DMD. | |||
| Has total score | ||||
| Parent and child reports | α= 0.71-0.89 | |||
| available | ||||
| PedsQL DMD module | Daily Activities, Treatment, Worry, | 4 domainscores | Valid and reliable in | |
| communication | children with DMD | |||
| English version | No total score | [17]. | ||
| Parent and child reports | α= 0.66-0.86 | |||
| available | ||||
| Child Health | Physical Functioning, Role/Social Limitations– | 50 items; Scores on 15 | Valid and reliable in | |
| Questionnaire Parent | Physical, | domains | UK, US, German and | |
| Form 50 | General Health Perceptions, Bodily | Canadian English and | ||
| (CHQ-50) | Pain/Discomfort, Family Activities, Role/Social | 2summary scores: physical | French populations [38] | |
| Limitations - Emotional, Role/Social | and psychosocial | |||
| English | Limitations - Behavioral, Parent Impact Time, | |||
| Parent Impact Emotion, Self-Esteem, Mental | No total score | |||
| Parent report only | Health, Behavior, Family Cohesion, Change in | |||
| health | ||||
| KIDSCREEN-52 | Physical, psychological, moods/emotions, self- | 52items | Valid and reliable in | |
| perception, autonomy, parent relations/home | healthypopulation [39]. | |||
| Dutch version | life, financial resources, social support/peers, | Scores on ten domains | ||
| school environment, social acceptance | ||||
| Child report only | No total score | |||
| DISABKIDS | Independence, emotion, social inclusion, social | 37items; Scores on six | Valid and reliable in | |
| exclusion, physical limitation, treatment | domains and totalscore | children and adolescents | ||
| German version | with various chronic | |||
| illnesses [40]. | ||||
| Child report only | ||||
| TACQoL | Motor functioning, physical symptoms, social | 56 items;Scores on seven | Valid and reliable in | |
| functioning, cognitive functioning, positive | domains | children with various | ||
| Dutch | emotions, negative emotions, autonomy | chronic illnesses [41] | ||
| No total score | ||||
| Child report only | ||||
| Vecu Sante percu par | Vitality, leisure, relationship withparents, | 36 items | Valid and reliable in | |
| l’adolescent | relationship with friends, relationship with | group of healthy and ill | ||
| (VSPA) | teachers, body image, school performance, | Scores 9domains | adolescents [42]. | |
| physical and psychological wellbeing | ||||
| French | No total score | |||
| Child report only | ||||
| Paediatric Outcome Data | Upper extremity, transfer/basicmobility, | Scores on five domains | Valid and reliable in a | |
| Collection Instrument | sports/physical function, pain, happiness, and | range of children with | ||
| (PODCI) | global functioning | Global functioning score is | functional limitations | |
| mean of all domains | [43] | |||
| English | excluding happiness | |||
| Child and parent reports | ||||
| available | ||||
| Strips of Life with | 33 individual items that assess how a childat | Total score of all 33 items | Not validated | |
| Emoticons (SOLE) | different times in a typical day | |||
| No domain scores | ||||
| Italian | ||||
| Child report only | ||||
| Short Form 36 | Vitality, physical functioning, bodily pain, | 36items; Scores on eight | Not validated in the | |
| general health perceptions, physical role | domains | pediatricpopulation | ||
| English | functioning, emotional role functioning, social | |||
| role functioning, mental health | No total score |
*Internal consistency reliability (Cronbach’salpha) of instruments that have been tested in the pediatric DMDpopulation are reported; validity and reliability instruments thathave not been tested in other populations are referenced.
Table 2
Summary of studies reviewed
| Citation | Study design; DMD sample characteristics [N; mean age, (age range)]; Recruitment method | HRQOL measure used | Major findings | |
| [15] | Cross-sectional; | PedsQL 4.0 Generic Core | All HRQOL scores significantly | |
| and PedsQL 3.0 | poorer than normative sample. | |||
| [44; 12.9 years; (8–18)] | Neuromuscular modules | |||
| Poorer physical HRQOL in both modules | ||||
| Neuromuscular clinics in | Child and parent report | among non-ambulatory boys. | ||
| the United States | ||||
| [16] | Cross-sectional; | Chinese version of PedsQL | The Chinese translation of the | |
| 4.0 Generic Core and | Neuromuscular module was feasible, | |||
| [56; 7.5 years; (2–13)] | Neuromuscular 3.0 module | reliable and valid | ||
| Tertiary hospitals in urban China | Child and parent report | Moderate agreement between | ||
| parent and child | ||||
| [17] | Cross-sectional; | PedsQL 4.0 Generic Core | All HRQOL scores significantly | |
| and DMD module | lower than healthy children. | |||
| [203; 10.4 years; (5–17)] | ||||
| Child and parent report | Self-reported psychosocial scores | |||
| Neuromuscular clinics in the | significantly higher for older than | |||
| United States (Michigan) | younger boys. | |||
| Psychosocial score not related to | ||||
| use of mobility aids. | ||||
| [18] | Cross-sectional; | Strips Of Life with | Poorer HRQOL than | |
| Emoticons (SOLE) | healthy controls. | |||
| [43; 8.6 years; (range 5–13)] | questionnaire | HRQOL not related to degree | ||
| of functional disability. | ||||
| Six tertiary centres in Italy | Child report | |||
| [20] | Cross-sectional | Personal Adjustment and | Adjustment score did not differ significantly | |
| Role Skills Scale (PARS-III) | from boys with other chronic conditions. | |||
| [287; 10.9 years; (5–18)] | Adjustment score increased with age. | |||
| Dutch and American Parent | ||||
| Project Muscular Dystrophy | Parent report | |||
| organizations | ||||
| [23] | Longitudinal | Life Satisfaction Index | HRQOL in most domains | |
| for Adolescents | improved over time. | |||
| [95; unknown; (5–17)] | ||||
| Child report | No significant difference | |||
| Single neuromuscular centre | between age groups. | |||
| in Brazil | ||||
| [24] | Longitudinal | PedsQL 4.0 Generic | Decline in PODCI score but not | |
| Core PODCI | PedsQL were significantly correlated | |||
| [24; 7.9 years; (4–12) | with decline in 6 minute walk test. | |||
| Parent-report | ||||
| Neuromuscular clinics in the | ||||
| United States (California) | ||||
| 25] | Cross-sectional | TACQoL children for | Only the ‘motor functioning’ domain | |
| under 16 year olds | was poorer than healthy peers | |||
| [36; 12.6 years; (8–17)] | TACQoL adult for 16 and older | |||
| Dutch Neuromuscular centres | ||||
| Child report | ||||
| [26] | Cross-sectional | PedsQL 4.0 General Core | Children who required ventilation had | |
| significantly lower overall HRQOL than | ||||
| [24 (out of 109 NM patients; | Parent-report | children not on ventilation | ||
| 10.5 years; (2–18)] | ||||
| Single Neuromuscular centre | ||||
| in Canada | ||||
| [27] | Cross-sectional | Vecu Sante Percu par | HRQOL scores not significantly | |
| L’adolescent (self-perceived | different than nondisabled group. | |||
| [19 (out of 43 NM patients); | perceived health states in | |||
| 13.8 years; (10–17)] | adolescents) | HRQOL scores did not correlate | ||
| Self-report | with physical impairment | |||
| Single neuromuscular centre | ||||
| in France | ||||
| [21] * | Cross-sectional | PedsQL 4.0 Generic Core | Self-reported scores significantly correlated | |
| with physical domain and Vignos scale | ||||
| [35; 12.5 years; (9–17)] | Child and parent reports | |||
| Parent-child concordance range from poor | ||||
| Neurogenetics clinics and | to modest for different domains. | |||
| community newsletters | ||||
| in Australia | ||||
| [22] * | Cross-sectional | Child Health | Parents reported significantly lower | |
| Questionnaire 50-Parent | HRQOL score than normative sample and | |||
| [34; 9.9 years; (5–18)] | Form | Charcot-Marie-Tooth disease sample. | ||
| Three urban paediatric hospitals | Parent-report | Parents experienced greatest stress during | ||
| in Australia | disease transition points. | |||
| [28] | Cross-sectional | PedsQL 4.0 Generic Core | All HRQOL scores poorer than healthy | |
| sample except for emotional domain. | ||||
| [50; 8.0 years; (5–17)] | Child and parent report | |||
| Participation level is not | ||||
| Neuromuscular clinic in the | correlated to HRQOL | |||
| United States (Florida) | ||||
| Older boys had significantly lower | ||||
| participation level, but not lower | ||||
| HRQOL scores than younger boys | ||||
| [29] | Cross-sectional | PedsQL 4.0 Generic Core | HRQOL in both measures are | |
| Module | poorer than controls | |||
| [52; 8.4 years; (4–17)] | ||||
| PODCI | The physical function domain of PedsQL | |||
| Neuromuscular clinics in the | and of PODCI correlated with age and | |||
| United States | Parent report | clinical measures of strength | ||
| [30] | Cross-sectional | Child Health | HRQOL scores significantly poorer | |
| Questionnaire 50- Parent | than healthy sample | |||
| [27, 11.4 years; (unknown)] | Form | |||
| Use of wheelchairs and ventilation were | ||||
| Neuromuscular clinics in Italy | Parent report | significantly associated with lower physical | ||
| HRQOL. | ||||
| [31] | Cross-sectional | DISABKIDS chronic | In children, all HRQOL scores poorer than | |
| generic module for | children with other chronic illnesses. In | |||
| [50; 15.4 years; (8–23)] | children and adolescents; | adolescents, only social inclusion domain | ||
| Short Form-36 for young | was poorer. | |||
| adults | ||||
| Single paediatric neurology | No correlation between total HRQOL score | |||
| clinic in Germany | Child report | and Vignos function score. | ||
| [32] | Cross-sectional | KIDSCREEN-52 | Apart from physical domain, HRQOL | |
| in not significantly different from that | ||||
| [40; 11.5 years; (8–20)]; | Child report | of healthy boys. | ||
| Dutch Duchenne Parent | Significant correlations between physical | |||
| Database | domain and some functional scales | |||
| Parent scores were significantly lower | ||||
| than child score in three domains. | ||||
| [33] | Cross-sectional | Short Form-36 | Physical and mental HRQOL not found to | |
| be correlated with physical impairment or | ||||
| [N = 35; 17 years; (8–33)] | Child report | FVC. | ||
| Swiss facility for NM patients | ||||
| [34] | Cross-sectional | PedsQL 4.0 Generic Core | Parents reported significantly lower | |
| DMD sample N = 63 | physical and psychosocial HRQOL than | |||
| parent-child pairs | Child and parentreport | boys themselves. | ||
| Mean age 10.3 (range 5-16) | ||||
| The agreement between children and | ||||
| Florida, United States | parents in physical domain was better | |||
| than psychosocial domains. |
*Participants of two studies by Bray andcolleagues were from the same cohort of families.



