Cholinergic Deep Brain Stimulation for Memory and Cognitive Disorders
Abstract
Memory and cognitive impairment as sequelae of neurodegeneration in Alzheimer’s disease and age-related dementia are major health issues with increasing social and economic burden. Deep brain stimulation (DBS) has emerged as a potential treatment to slow or halt progression of the disease state. The selection of stimulation target is critical, and structures that have been targeted for memory and cognitive enhancement include the Papez circuit, structures projecting to the frontal lobe such as the ventral internal capsule, and the cholinergic forebrain. Recent human clinical and animal model results imply that DBS of the nucleus basalis of Meynert can induce a therapeutic modulation of neuronal activity. Benefits include enhanced activity across the cortical mantle, and potential for amelioration of neuropathological mechanisms associated with Alzheimer’s disease. The choice of stimulation parameters is also critical. High-frequency, continuous stimulation is used for movement disorders as a way of inhibiting their output; however, no overexcitation has been hypothesized in Alzheimer’s disease and lower stimulation frequency or intermittent patterns of stimulation (periods of stimulation interleaved with periods of no stimulation) are likely to be more effective for stimulation of the cholinergic forebrain. Efficacy and long-term tolerance in human patients remain open questions, though the cumulative experience gained by DBS for movement disorders provides assurance for the safety of the procedure.
INTRODUCTION
In recent years, functional neuromodulation has offered an immense therapeutic opportunity through deep brain stimulation (DBS) for varied neurological and psychiatric disorders. DBS offers the potential to stimulate or suppress the function of specific spatial regions close to the activated lead. Electrode leads are implanted in the target neuronal structure deep within the brain, electrical pulses are applied to modulate neural responses near the lead, and corollary discharges alter neuronal activity to alter aberrant neural function. DBS has become an accepted form of minimally invasive procedure in restorative neurosurgery for Parkinson’s disease [1–3]. It has also found application for other movement disorders including essential tremors [4–6], dystonias [7–9], and Gilles de la Tourette syndrome [10–12], though its success rate has been more limited. More recently, DBS has been explored as a treatment for neuropsychological conditions including obsessive compulsive disorder (OCD) [13–16] and treatment-resistant depression [17–19]. The memory and cognitive deficits associated with cognitive dysfunction as sequela of neurogenerative disorders like dementia and Alzheimer’s disease (AD) represent the newest frontier for DBS [20–23]. In this article, we first review mechanisms of DBS action that provide the foundation for understanding the therapeutic potential of the procedure in cognitive disorders. We then examine the relevant pathology of AD that could benefit from stimulation. We discuss the brain structures that have been targeted for stimulation and emphasize the potential of cholinergic forebrain stimulation. We conclude the article with the current challenges and unanswered questions that will have to be addressed for making DBS a standard-of-care strategy for the amelioration of memory and cognitive decline in neurodegenerative disorders.
MECHANISMS OF DBS ACTION
In conventional DBS for movement disorders, high frequency electrical stimulation (100–130 Hz, approximating the peak firing rate that neurons only reach for brief periods of times) is applied to the lead continuously in a predefined cyclic pattern, typically targeting the globus pallidus or subthalamic nucleus [24, 25]. The biophysical mechanisms of DBS are the subject of some debate, but stimulation parameters used for Parkinson’s disease appear overall to suppress the effect of the implanted area by overstimulating efferent targets [26]. Experimental and modelling data propose that axonic and somatic activity of electrically stimulated neurons are decoupled [3, 27]. Cathodic stimuli depolarize the cell membrane in a region proximal to the electrode with a surround hyperpolarization [28], whereas anodic stimulation induces a paradoxical effect whereby the somatic activity is inhibited and the synaptic output is enhanced [27, 29]. Locally near the stimulation lead, a depolarization block or repetitive activation of local inhibitory neurons, which can follow high stimulation rates, leads to a lack of somatic activation, whereas efferent axons maintain activation. The ameliorative effect on movement disorders requires stimulating at a higher frequency that the normal rates in the implanted nuclei. Typically, that means stimulating at rates over 100 Hz to produce a desired effect. The overall result is a reduced impact of the implanted region on its target areas.
Both monopolar and bipolar configurations can be used to stimulate nervous tissue [30, 31]. Monopolar electrical stimulation is achieved using a single stimulating electrode inserted into the neural tissue, and provides a counter electrode, or ground, located at a distance from the stimulating electrode [32]. In bipolar stimulation, two stimulating electrodes are present in a proximity to each other in the target tissue, and one electrode acts as a source, and the other as a sink [33]. The bipolar configuration results in less current spread than the monopolar stimulation (Fig. 1A), thereby making it preferable for small, selective target structures, particularly when spread of current outside the target structure is counterproductive, as is the case of basal ganglia nuclei with opposing functional properties in close proximity to each other [34, 35]. On the other hand, the threshold required for bipolar activation is higher, making it less power efficient, which can be an issue in clinical applications that wish to maximize battery life of the implantable pulse generator and frequent recharging is cumbersome. However, both monopolar and bipolar stimulation are relatively spatially selective for the activated leads [36].
Fig. 1
A) Schematic illustration of monopolar (left) and bipolar stimulation (right). Arrows represent current flow from the stimulating electrode. B) Schematic illustration of monophasic and biphasic stimulation. Current amplitude is represented as a function of time.
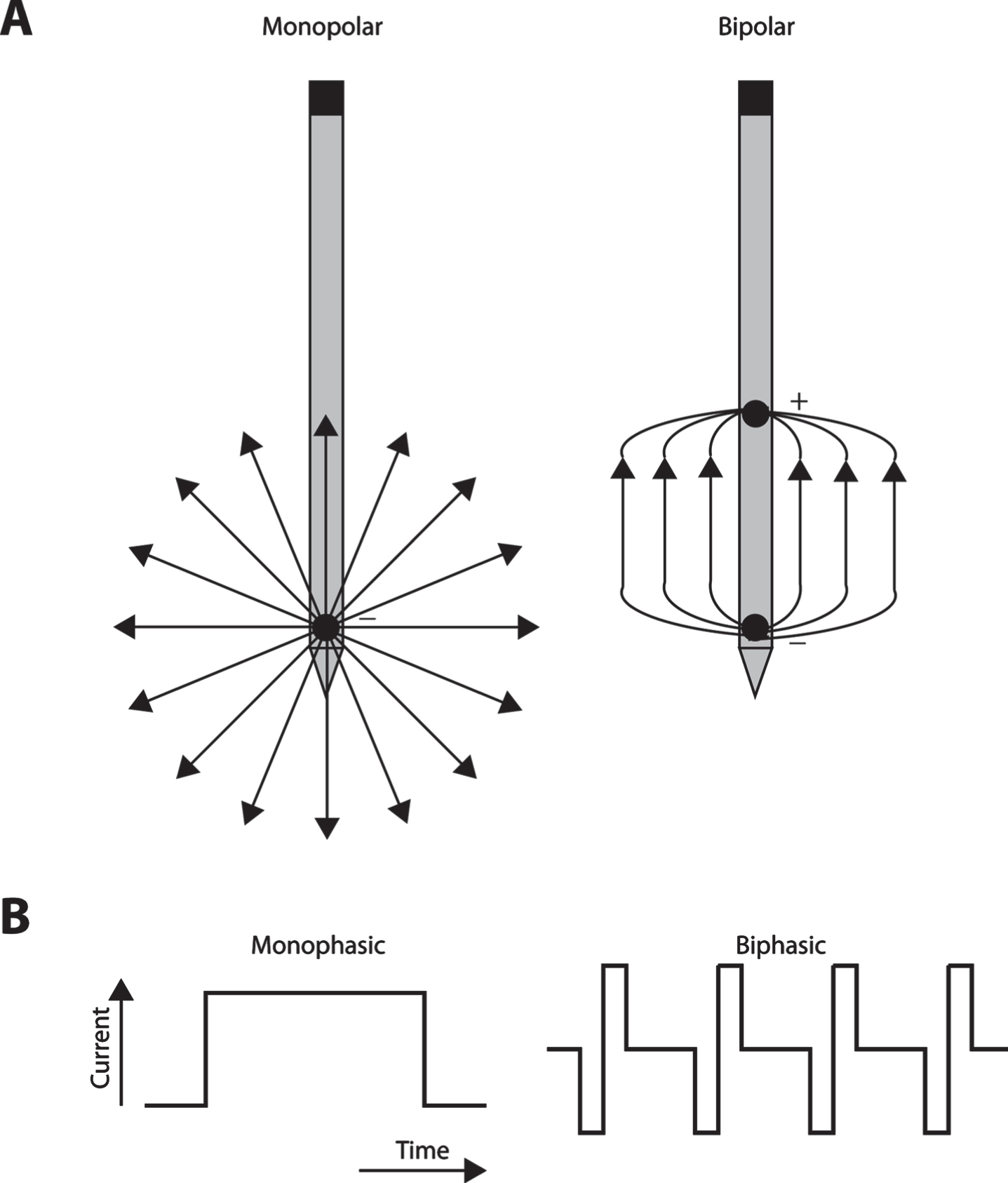
In most DBS applications, rectangular monophasic or biphasic voltage pulses are used to evoke the desired physiological effects (Fig. 1B). During a monophasic stimulation, either anodic or cathodic pulses are delivered to the neural tissue [37]. Most clinical stimulation devices can deliver monophasic pulses that induce current flow in only one direction. These devices use passive charge recovery to maintain the voltammetric necessity of zero net charge per pulse because otherwise sustained unidirectional current flow will either plate (deposit metal from the electrode) or gas (release molecular oxygen and hydrogen as a result of electrolysis) at the lead in the brain, both of which are undesirable [38]. In contrast, biphasic stimulation induces pulses of both anodic and cathodic phases, typically symmetrical rectangular pulses (Fig. 1B), in which the initial stimulation pulse is cathodic followed by anodic pulse leading to reversal of electrochemical processes during the stimulation phase with zero net charge in each pulse-pair. The duty cycle of stimulation is defined as the ratio of time that stimulation is applied or not. The duty cycle is a function of the duration of the pulse and frequency of stimulation and can vary for either monophasic or biphasic stimulation. Continuous modes of stimulation will have higher duty cycles compared to intermittent stimulation, when pulses are applied over a defined period of time followed by a gap in stimulation. In a conventional DBS paradigm, charge balanced biphasic stimulation is used consisting of cathodal stimulation phase followed by anodic reversal phase [39, 40]. In addition, nonrectangular pulses like Gaussian, triangular, and exponential decay waveforms have been experimentally tested, providing an alternative for maximizing axonal activation, while minimizing the energy required [41–43]. However, use of these optimized waveforms does not guarantee a significant difference in the clinical outcomes [41].
DBS is not cell-type specific, as electrical stimulation induces extracellular depolarization of all excitable structures in the target spatial region irrespective of biochemical composition and morphology, including fibers of passage, and hence lacks cell-type specificity, but it is spatially selective around the electrode lead. The activating function, or ability to elicit an all-or-none action potential response, drops off sharply at approximately 2 mm from the electrode lead for a typical current of 3 mA. The square root of the current scales with the radius of the activating function [36, 44, 45]. The spatial specificity of these fields is quite difficult to match with non-invasive stimulation [46]. To a first approximation, each DBS pulse elicits an all-or-none response in each excitable electrical element (soma, axon, dendrite) within range of the activating function and capable of following the stimulation pulse rate. DBS can be thought of as repetitively exciting the small spatially confined efferent elements to achieve effects [47].
Closed loop DBS, often referred to as feedback driven, provides an alternative for intracranial neurostimulation. Leads from one of the microelectrodes are connected to a programmer and are used as a sensor in detecting the real time electrophysiological fluctuations and changes in the single unit [48, 49], multi-unit [50], local field [51–53], or global field potentials [54] that occur during the onset of abnormal pattern of neural activity owing to pathological changes associated with the disease state. Stimulation parameters are adjusted dynamically in response to the ongoing changes in the neural activity by inbuilt algorithms and electrical stimulation is applied in a dose dependent manner in response to ongoing pathological neural activity reducing the adverse effects of intense stimulation. In practice, closed loop stimulation is currently approved for treating epilepsy, and there is an active debate about the import of the feedback driven.
Other future directions in DBS are, at present, aspirational. Artificial neural networks trained on input and output electrophysiological data can adapt to neural fluctuations based on learning and offer an ideal platform for developing stimulation predictors based on mathematical representation of stimulation evoked electrophysiological changes [55–57]. Artificial neural networks can learn nonlinear relationships [58] between sets of input and output data enabling the network to adapt to the inverse relationship between stimulation parameters and target neural response in the context of brain dynamic environment [59]. This can be achieved by a closed loop controller in determining parameters of stimulation to optimal therapeutic neural response, in real time [60]. As a proof of concept, a closed loop DBS controller relied on feedback extracted by fitting stimulation induced dopamine response to stimulation parameters in anesthetized rodents [61]. Machine learning techniques can be used to predict the stimulation parameters [62] as a transfer function, enabling the closed loop DBS controller to determine stimulation parameters to induce optimal therapeutic stimulation, which can be optimized for subject-specific therapeutic stimulation [63]. In a fuzzy-logic control system, time-varying spectral properties of the field potential are used as input by the digital signal processor [63]. Closed loop optogenetics DBS pre-clinical framework provides an opportunity for adding cell-type specific neuromodulation and circuit characterization [64]. Such future DBS application will provide a means to use activation of small specific spatial ensembles in the brain that cannot be activated with similar specificity otherwise, and to use this activation to counteract patterns of activity that are of neuropathological or psychiatric consequence.
DEMENTIA, ALZHEIMER’S DISEASE, AND THE CHOLINERGIC SYSTEM
Dementia is a worldwide health problem presenting with acquired loss of cognition in multiple cognitive and behavioral domains with progressive impairment of memory, executive functions including reasoning, judgement, and impulse control [65–67]. Progressive irreversible forms of dementia are typically of mixed etiology, including repeated ischemic insults as a result of stroke and vascular disorders, and an admixture of neurodegenerative pathologies including Lewy body disease, frontotemporal dementia, Huntington’s disease, and AD [68]. AD is a form of dementia presenting with cognitive deterioration and psychopathological symptoms and personality changes with molecular hallmarks of its presentation involving neurofibrillary tangles and amyloid plaques [69–72]. These pathological factors increase in parallel with the slow progression of the disease’s severity. Cholinesterase inhibitor drugs (donepezil, galantamine, rivastigmine) are the most commonly used frontline medications, which reduce the rate of breakdown of acetylcholine and prolong its action, with cognitive loss improvement peaking 3–6 months after therapy begins [73].
The cholinergic system is phylogenetically one of the oldest neuromodulator systems found in both vertebrates and invertebrates [74]. In the neocortex, the cholinergic system is implicated in cellular and synaptic functions owing to network dynamics during behavioral transition like sensory and cognitive behavior, wakefulness, sleep, detection and distraction to attention, memory, and recall [75–77]. Cholinergic neurons undergo moderate degenerative changes during aging, resulting in cholinergic hypofunction related to deterioration of memory with aging [78, 79].
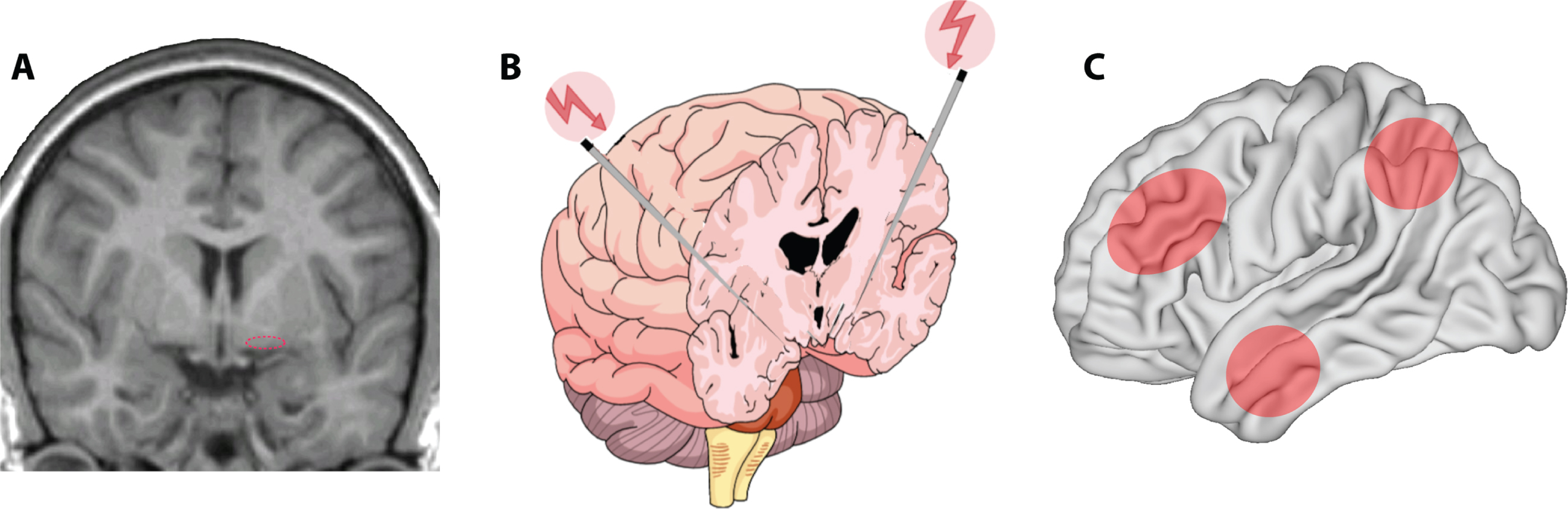
One of the most prevalent age-related neurodegenerative diseases is the cholinergic degeneration associated with AD, with a complex polygenic aberrant interaction of several molecular cascade mechanisms along with cardiovascular and lifestyle risk factors, characterized by progressive deterioration of memory and cognitive functions [80]. Therapeutic interventions have been tailored to modify, delay, and impede the progression of clinical manifestation of the disease state. The cholinergic hypothesis has translated the concept of the disease from its descriptive pathology to a mechanism of synaptic dysfunction, through detection of depleted presynaptic cholinergic markers in the cerebral cortex [81–84] and cell loss in the nucleus basalis complex [85]. Cholinergic and GABAergic axons originating from distinct subdivisions of the nucleus basalis (Fig. 2A) innervate exclusively all parts of the cerebral cortex, hippocampus, entorhinal cortex, amygdala, and the limbic structures [86, 87]. This innervation is most intense within the limbic system, moderate in association cortices, and least in the primary sensory areas. Depletion of cholinergic inputs in AD exhibits relatively uniform patterns of distribution. The hippocampus and the entorhinal cortex are the most severely affected areas and are correlated with memory loss; the temporal lobe is moderately affected, and sensory motor areas are the least affected [88]. Depletion of cholinergic inputs in laboratory animals impairs a broad array of functions like working memory, context-place memory, and sensory cortex plasticity [89–93]. Deterioration of dementia in AD also shows positive correlation with the degree of cholinergic depletion, reduction in choline acetyltransferase activity, and acetylcholine synthesis [94–99]. Therapeutic validation of cholinomimetics [100] and cholinesterase inhibitors [101, 102] has also been provided in enhancing memory functions. Postmortem examination has shown cholinergic denervation is associated with formation of neurofibrillary tangles in the nucleus basalis of Meynert [103, 104].
Fig. 2
A) Location of the nucleus basalis of Meynert (dotted ellipse) in the human brain. B) Targeted DBS for memory and cognitive enhancement. Schematic representation of bilateral deep brain stimulation of the nucleus basalis of Meynert. C) Target areas likely to undergo enhancement of activity after nucleus basalis stimulation include the prefrontal, parietal, and temporal association cortexes (shaded ellipses).
AD is likely caused by the interaction of amyloid-β (Aβ) oligomers with other biological factors. Familial AD is caused by mutations that lead to increased Aβ creation and/or neurofibrillary tangle deposition [105–110], while senile AD is related to impaired Aβ clearance [111]. Soluble Aβ oligomerizes [112, 113], and soluble oligomers bind multiple biological targets with high efficacy including noradrenergic, nicotinic cholinergic, and glutamatergic targets [114]. Human biomarker studies have related the decreased cerebrospinal fluid Aβ42 or decreased ratio Aβ42 to Aβ40 to dementia progression [113, 115–117], ostensibly because the soluble Aβ42 oligomers cluster and adhere to biological targets. The high affinity binding of soluble Aβ42 to nicotinic receptor targets [118] provides a plausible link between the cholinergic forebrain systems and the beta amyloid neuropathology. Tau pathology typically coexists with amyloid pathology and is exacerbated in apolipoprotein E type 4 allele carriers, who also experience greater amyloid pathologies [119]. It is thus hypothesized that Aβ drives tau pathology in most patients [120].
TARGETED NEURAL STRUCTURES FOR MEMORY AND COGNITIVE ENHANCEMENT
Structures that have been identified as targets of DBS for memory and cognitive enhancement include structures that make up the Papez circuit, brain areas projecting to the front lobe, and the cholinergic forebrain. We will examine these separately. The fornix, an important structure in the Papez circuit plays a critical role in episodic memory and is implicated in the cognitive and memory decline in AD. It was hypothesized therefore that fornix stimulation could improve memory function. The first use of DBS offered as a potential therapeutic regime was reported in a 50-year-old male patient with chronic resistant obesity implanted bilaterally with DBS electrodes in the hypothalamus. Stimulation of the hypothalamus resulted in an evoked recall of memory [121] through the ventral contacts of the stimulating electrode (3–5 V, 130 Hz, 0.6 ms pulse width) owing to the proximity of hypothalamus to the fornix. It was hypothesized that stimulation-induced corollary discharge to the fornix from the hypothalamus was responsible for such memory recall. It is well known that electrical stimulation of a wide range of brain regions, mainly in the temporal lobe, evoke memory experiences [122]. Nonetheless, the fornix results provided an insight for an elective procedure in 6 patients to investigate the efficacy of DBS of the fornix in mild form of AD [123]. After one year of stimulation, cognitive performance, evaluated with the Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-cog) showed mild deterioration by 4.7 points relative to baseline. Positron emission tomography (PET) scans revealed increased brain metabolism in a frontal-temporal-parietal-striatal-thalamic network and a frontal-temporal-parietal-occipital-hippocampal network [124]. No serious adverse events were reported in this group of patients. This paved a path for the advancement of DBS in targeting neural structures related to memory and cognitive disorders [125].
Entorhinal cortex has been proposed as an alternative to fornix for therapeutic target for memory and cognitive disorders associated with AD. In an initial study, six medically refractory epilepsy patients were studied had DBS in the entorhinal cortex. Four of six patients had additional electrodes implanted in the hippocampus. It was proposed that anterior entorhinal cortex stimulation during spatial navigation task increased efficiency in spatial memory when compared to hippocampal or no stimulation [125]. Interest in DBS targeting the entorhinal cortex has been supported by research in mouse models showing reduction in total tau content and phosphorylation in the cortex and hippocampus of AD mice [126, 127]. However strong counter-evidence has also been provided by follow up studies, showing that electrical stimulation of the entorhinal cortex and hippocampus (at 50 Hz) led to memory suppression rather than enhancement [128].
Frontal lobe networks have also been identified as targets that could improve cognitive function, as prefrontal cortical activity plays a central role in cognitive function [129], and regulating its inputs presents an attractive DBS target. Structures connected with frontal networks and co-active in cognitive processing, including the ventral internal capsule and ventral striatum, were targeted in a phase I study of three AD patients [130] as well as in patients previously implanted for the treatment of major depressive disorder (MDD) and OCD [131]. All three AD patients tested presented less cognitive decline than a matched comparison group from the Alzheimer’s Disease Neuroimaging Initiative using score trajectory slopes, based on the Clinical Dementia Rating–Sum of Boxes clinical measure. Minimal changes or increased metabolism, in frontal cortical regions after chronic DBS was seen with Fluoro-D-Glucose PET [130]. Results in MDD/OCD patients also showed improvement in cognitive control, evaluated with the Multi-Source Interference Task; subjects were on average faster with DBS stimulation on, without a speed-accuracy trade-off [131]. Experimental studies in animal models and pilot studies in humans have proposed an array of thalamic targets that could aid cognitive function through stimulation. These include the midline, central, and intralaminar nuclei [132–134]. The clinical utility of these targets in AD and age-related dementia remains to be seen.
CHOLINERGIC DBS STIMULATION
Cholinergic neurons in the nucleus basalis of Meynert undergo degeneration through the later stages of AD [135]. DBS of this area early in the progression of the disease therefore provides a promising target. The advantage of such a target, as opposed to that targeting the fornix, is that nucleus basalis stimulation is expected to enhance activity in multiple cortical areas, most importantly in the prefrontal, parietal, and temporal association cortexes (Fig. 2C), with cognitive enhancement involving all aspects of cortical processing. The first human study targeting the cholinergic forebrain was a four-week sham controlled double blind clinical trial, in which six patients with mild to moderate AD were implanted bilaterally in the nucleus basalis of Meynert (Fig. 2B). DBS was applied with a continuous stimulation protocol at a low frequency (20 pulse per second) followed by a 11-month follow up [136]. Measures of cognitive functioning generally indicated stable, or slow deterioration of cognitive function over a 12-month period (non-significant mean increase of 3 points in ADAS-cog, mean decrease of 0.5 points in MMSE). Analysis of cross-over results revealed slightly improved scores at the end of the-two stimulation period compared with the score at the end of the sham period. Glucose utilization in the entire cerebrum evaluated with PET also increased in all 4 patients imaged, by an average of 2.5%. Eight patients in total have now been stimulated with two-year outcomes published [136–138]. Long-term cognitive outcomes are not clearly better or worse than the null hypothesis, but stimulation was well tolerated and safe in the context of DBS.
Although these areas are well identified as being involved in higher cognitive functions and AD specifically, how DBS might tap into improving their function is not well understood. As discussed above, DBS for movement disorders typically impairs the output of the targeted region, but no current models propose hyperactive brain regions in AD. Thus, adopting the same basic approach to suppress the output from the region around the stimulated lead, should be expected to fail, no matter where electrodes are placed. A non-traditional approach using stimulation seeking instead to amplify function could, however, be used to target neuromodulatory agents. Brief phasic stimulation of forebrain acetylcholine release enhancing neural activity that occurs in reasonable temporal conjunction with the stimulation provides a working model. Such stimulation will alter the ensuing neural activity, alter the balance of excitation/inhibition, and cause neural plasticity observable at the synaptic and network levels. Additionally, nucleus basalis stimulation is likely to recruit effects of GABAergic neurons with ascending projections that are concurrently active with cholinergic neurons [139, 140], action which could not be substituted by cholinesterase inhibitors or other cholinergic drugs.
Animal studies have established that phasic stimulation of the cholinergic basal forebrain enhances memory of sensory stimuli activated immediately prior, and that this is a natural role of the basal forebrain [141]. Repetitive application of a sensory stimulus followed by basal forebrain stimulation results in neuroplastic changes in the representation of that stimulus, and the immediate synaptic effects of that stimulation are now well described [142, 143]. These studies all utilized brief, phasic, pulse-train of electrical stimulation in the cholinergic basal forebrain. The role of more frequent phasic stimulation on executive function was not addressed in these studies.
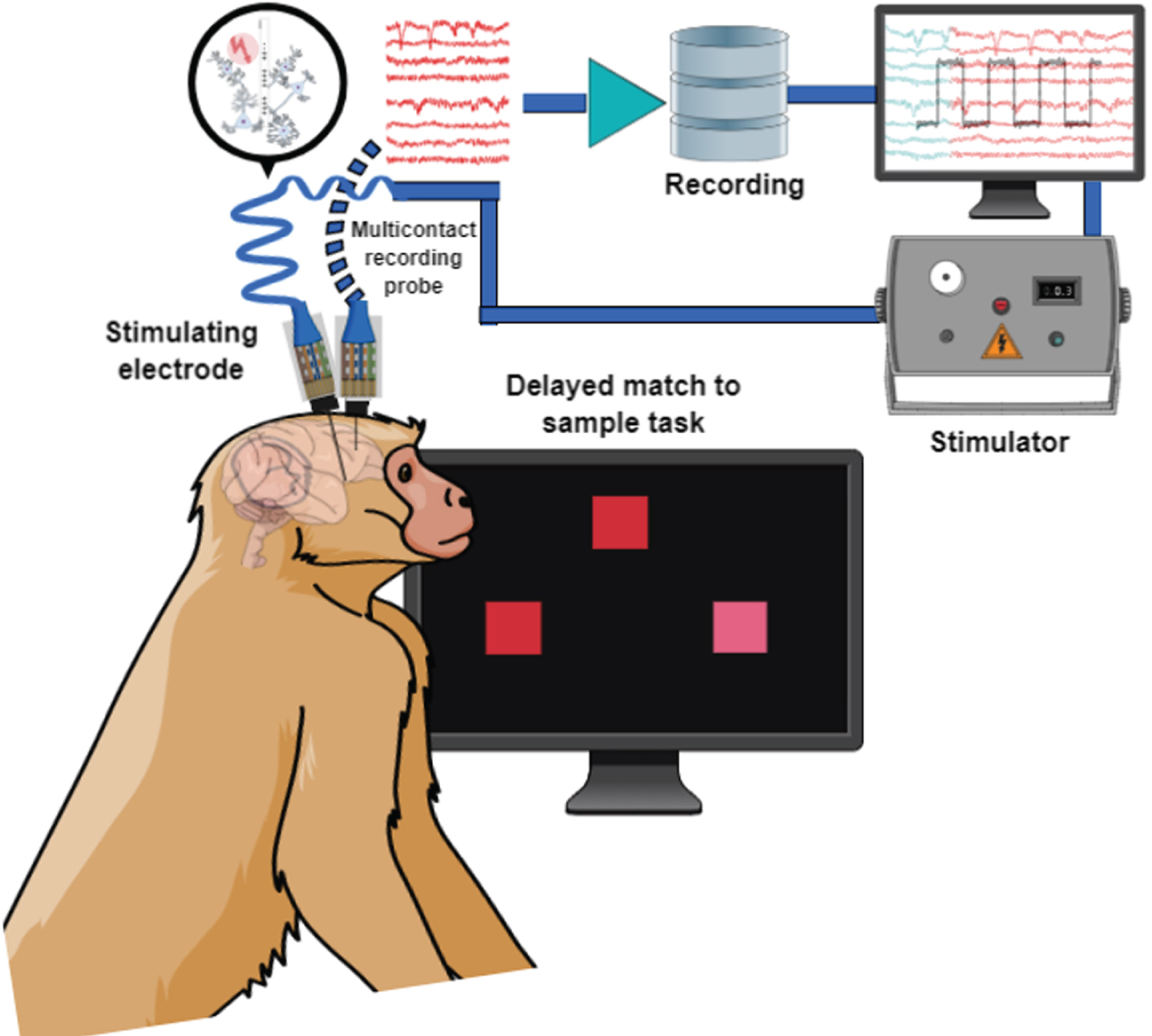
A series of recent studies in a non-human primate model, has targeted the nucleus basalis of Meynert [144–146]. The results show clear improvement in cognitive performance; however, the choice of stimulation parameters was critical, with intermittent stimulation delivering 1,200 stimulation pulses in 10–20 s of every minute being optimal (i.e., 80 Hz for 15 s, or 60 Hz for 20 s). On the other hand, continuous stimulation at comparable frequencies using any combination of frequency and intensity could not elicit short or long-term changes in cognitive function, while intermittent stimulation could. It is not implausible to think of the intermittent parameters as being comparable to a more frequent application of the phasic stimulation thoroughly explored in sensory cortex studies for its role in reinforcement and cortical plasticity. Simultaneous stimulation of the nucleus basalis and recording in downstream target areas such as the prefrontal cortex during execution of cognitive tasks (Fig. 3) have also began to reveal distinct changes in neural activity that improve working memory stability [147].
Fig. 3
Schematic illustration of simultaneous recording and stimulation in a nonhuman primate performing a behavioral task.
CHALLENGES AND FUTURE DIRECTIONS
A number of advances in technology offer promises for future treatment of memory and cognitive deficits. Noninvasive DBS using temporally interfering electric fields in which neurons are electrically stimulated at selective depths by interference between multiple electric fields provides such a future direction [46]. The application of optogenetics provides another avenue [64]. A major challenge in the translation of optogenetics tools in patients is its invasive nature in implanting an optical fiber and the chance of rejection complications. Additionally, opsins are foreign proteins that can trigger immune response leading to rejection, and the viral vector carrying optogenetics plasmid can trigger immune response. Neural rates inducible by optogenetic probes in principal neurons also remain low relative to those induced in most DBS studies. Until these technologies are mature for clinical use, optimizing DBS through more conventional methods, relying on electrode implantation, remains an urgent priority.
Current challenges of DBS that will have to be addressed include the implementation time of DBS in different stages of AD (early versus moderate AD). At this time, the earliest possible intervention appears most likely to confer the maximum benefits in arresting the progression of the condition; however, this will have to be empirically validated. The optimal frequency and duration of stimulation remains to be determined in human studies. For cholinergic stimulation, protocols that average 20 pulses/s either continuously or intermittently (e.g., 60 Hz for 20 s every minute) for a period of a few hours per day appear most effective. This is not a trivial problem, however, as the parameter space is large and the adjustment of parameters a laborious, trial-and-error method at present. This problem is compounded by the determination of whether electrode placement is optimal in a patient. If stimulation shows no clinical effect it is unclear if different parameters should be tried, or the electrode moved. Whether electrode placement is more advantageous unilaterally or bilaterally is also not clear, though the latter seems most promising. Although the small-sample patient studies reviewed emphasized that DBS was well tolerated, this is still an invasive procedure that carries inherent risks, including intracerebral hemorrhage, with an incidence estimated approximately at 1% –2%, seizures (1%), device infections (3% –8%), and other medical complications surrounding the surgery including deep venous thrombosis, pneumonia, and pulmonary embolism (< 2%) [148]. Adverse events may also include lead migration and fracture (2% –3%) and side effects from electrical stimulation [149]. Short and long-term safety outcomes of DBS for cognitive enhancement in human patients will have to be established.
Although these challenges may appear daunting at present, strides are being made by experimental and clinical studies. Wide adoption of the method has the potential to alter the standard of treatment for conditions such as AD, for which the best current treatment achieves modest impact, and progression of the disease is characterized by an inexorable decline of cognitive ability. If these conditions are met, the potential impact of DBS in cognitive function improvement may be no less than that in movement disorders.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health under award numbers R01 MH097695 and RF1 AG060754.
The authors have received stimulator devices from Boston Scientific through a Materials Transfer Agreement.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0425r2).
REFERENCES
[1] | Kogan M , McGuire M , Riley J ((2019) ) Deep brain stimulation for Parkinson disease. Neurosurg Clin N Am 30: , 137–146. |
[2] | Diestro JDB , Vesagas TS , Teleg RA , Aguilar JA , Anlacan JP , Jamora RDG ((2018) ) Deep brain stimulation for Parkinson disease in the Philippines: Outcomes of the Philippine Movement Disorder Surgery Center. World Neurosurg 115: , e650–e658. |
[3] | Gubellini P , Eusebio A , Oueslati A , Melon C , Kerkerian-Le Goff L , Salin P ((2006) ) Chronic high-frequency stimulation of the subthalamic nucleus and L-DOPA treatment in experimental parkinsonism: Effects on motor behaviour and striatal glutamate transmission. Eur J Neurosci 24: , 1802–1814. |
[4] | Elble RJ , Shih L , Cozzens JW ((2018) ) Surgical treatments for essential tremor. Expert Rev Neurother 18: , 303–321. |
[5] | Dallapiazza RF , Lee DJ , De Vloo P , Fomenko A , Hamani C , Hodaie M , Kalia SK , Fasano A , Lozano AM ((2019) ) Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry 90: , 474–482. |
[6] | Iorio-Morin C , Fomenko A , Kalia SK ((2020) ) Deep-brain stimulation for essential tremor and other tremor syndromes: A narrative review of current targets and clinical outcomes. Brain Sci 10: , 925. |
[7] | Duarte GS , Rodrigues FB , Prescott D , Ferreira J , Costa J ((2016) ) Deep brain stimulation for dystonia. Cochrane Database Syst Rev 2016: , CD012405. |
[8] | Eggink H , Toonen RF , van Zijl JC , van Egmond ME , Bartels AL , Brandsma R , Contarino MF , Peall KJ , van Dijk JMC , Oterdoom DLM , Beudel M , Tijssen MAJ ((2020) ) The effectiveness of deep brain stimulation in dystonia: A patient-centered approach. Tremor Other Hyperkinet Mov (N Y) 10: , 2. |
[9] | Rodrigues FB , Duarte GS , Prescott D , Ferreira J , Costa J ((2019) ) Deep brain stimulation for dystonia. Cochrane Database Syst Rev 1: , CD012405. |
[10] | Marceglia S , Rosa M , Servello D , Porta M , Barbieri S , Moro E , Priori A ((2018) ) Adaptive deep brain stimulation (aDBS) for tourette syndrome. Brain Sci 8: , 4. |
[11] | Casagrande SCB , Cury RG , Alho EJL , Fonoff ET ((2019) ) Deep brain stimulation in Tourette’s syndrome: Evidence to date. Neuropsychiatr Dis Treat 15: , 1061–1075. |
[12] | Kim W , Pouratian N ((2014) ) Deep brain stimulation for Tourette syndrome. Neurosurg Clin N Am 25: , 117–135. |
[13] | Blomstedt P , Sjöberg RL , Hansson M , Bodlund O , Hariz MI ((2013) ) Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg 80: , e245–53. |
[14] | Mian MK , Campos M , Sheth SA , Eskandar EN ((2010) ) Deep brain stimulation for obsessive-compulsive disorder: Past, present, and future. Neurosurg Focus 29: , E10. |
[15] | Arya S , Filkowski MM , Nanda P , Sheth SA ((2019) ) Deep brain stimulation for obsessive-compulsive disorder. Bull Menninger Clin 83: , 84–96. |
[16] | Pepper J , Hariz M , Zrinzo L ((2015) ) Deep brain stimulation versus anterior capsulotomy for obsessive-compulsive disorder: A review of the literature. J Neurosurg 122: , 1028–1037. |
[17] | Drobisz D , Damborská A ((2019) ) Deep brain stimulation targets for treating depression. Behav Brain Res 359: , 266–273. |
[18] | Dandekar MP , Fenoy AJ , Carvalho AF , Soares JC , Quevedo J ((2018) ) Deep brain stimulation for treatment-resistant depression: An integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry 23: , 1094–1112. |
[19] | Morishita T , Fayad SM , Higuchi Ma , Nestor KA , Foote KD ((2014) ) Deep brain stimulation for treatment-resistant depression: Systematic review of clinical outcomes. Neurotherapeutics 11: , 475–484. |
[20] | Xu DS , Ponce FA ((2016) ) Deep brain stimulation for Alzheimer’s disease. Curr Alzheimer Res 14: , 356–361. |
[21] | Laxton AW , Lozano AM ((2013) ) Deep brain stimulation for the treatment of alzheimer disease and dementias. World Neurosurg 80: , S28.e21–S28.e28. |
[22] | Mirzadeh Z , Bari A , Lozano AM ((2016) ) The rationale for deep brain stimulation in Alzheimer’s disease. J Neural Transm 123: , 775–783. |
[23] | Senova S , Chaillet A , Lozano AM ((2018) ) Fornical closed-loop stimulation for Alzheimer’s disease. Trends Neurosci 41: , 418–428. |
[24] | Mahlknecht P , Limousin P , Foltynie T ((2015) ) Deep brain stimulation for movement disorders: Update on recent discoveries and outlook on future developments. J Neurol 262: , 2583–2595. |
[25] | Wichmann T , DeLong MR ((2006) ) Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52: , 197–204. |
[26] | Vitek JL ((2002) ) Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord 17: , S69–S72. |
[27] | McIntyre CC , Grill WM , Sherman DL , Thakor VN ((2004) ) Cellular effects of deep brain stimulation: Model-based analysis of activation and inhibition. J Neurophysiol 91: , 1457–1469. |
[28] | Nowak LG , Bullier J ((1998) ) Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and initial segments. Exp Brain Res 118: , 489–500. |
[29] | McIntyre CC , Grill WM ((1999) ) Excitation of central nervous system neurons by nonuniform electric fields. Biophys J 76: , 878–888. |
[30] | Cheung T , Mamelak A , Tagliati M ((2013) ) Bipolar versus monopolar electrode configuration in thalamic DBS for essential tremor (P05.033). Neurology 80: (7 Suppl), P05. |
[31] | Basu I , Anderson W ((2014) ) Bipolar vs monopolar stimulation for cortical mapping: Which is better? Neurosurgery 75: , N16–17. |
[32] | Peckham PH , Ackermann DM ((2009) ) Implantable neural stimulators. In Neuromodulation, Krames ES, Peckham PH, Rezai AR, eds. Academic Press, San Diego, pp. 215-228. |
[33] | Deli G , Balas I , Nagy F , Balazs E , Janszky J , Komoly S , Kovacs N ((2011) ) Comparison of the efficacy of unipolar and bipolar electrode configuration during subthalamic deep brain stimulation. Parkinsonism Relat Disord 17: , 50–54. |
[34] | Rattay F ((1989) ) Analysis of models for extracellular fiber stimulation. IEEE Trans Biomed Eng 36: , 676–682. |
[35] | Basser PJ , Mattiello J , Lebihan D ((1994) ) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103: , 247–254. |
[36] | McIntyre CC , Mori S , Sherman DL , Thakor VN , Vitek JL ((2004) ) Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol 115: , 589–595. |
[37] | Cornelia C , Ulrich P , Johannes S , Sonja S ((1998) ) Electrophysiological considerations regarding electrical stimulation of motor cortex and brain stem in humans. Neurosurgery 42: , 527–532. |
[38] | Piallat B , Chabardès S , Devergnas A , Torres N , Allain M , Barrat E , Benabid AL ((2009) ) Monophasic but not biphasic pulses induce brain tissue damage during monopolar high-frequency deep brain stimulation. Neurosurgery 64: , 156–162. |
[39] | Popovych VO , Lysyansky B , Tass PA ((2017) ) Closed-loop deep brain stimulation by pulsatile delayed feedback with increased gap between pulse phases. Sci Rep 7: , 1033. |
[40] | Kent AR , Grill WM ((2012) ) Recording evoked potentials during deep brain stimulation: Development and validation of instrumentation to suppress the stimulus artefact. J Neural Eng 9: , 036004. |
[41] | Foutz TJ , McIntyre CC ((2010) ) Evaluation of novel stimulus waveforms for deep brain stimulation. J Neural Eng 7: , 066008. |
[42] | Sahin M , Tie Y ((2007) ) Non-rectangular waveforms for neural stimulation with practical electrodes. J Neural Eng 4: , 227–233. |
[43] | Wongsarnpigoon A , Grill WM ((2010) ) Energy-efficient waveform shapes for neural stimulation revealed with a genetic algorithm. J Neural Eng 7: , 046009. |
[44] | Stoney SD , Thompson WD , Asanuma H ((1968) ) Excitation of pyramidal tract cells by intracortical microstimulation: Effective extent of stimulating current. J Neurophysiol 31: , 659–669. |
[45] | Murasugi CM , Salzman CD , Newsome WT ((1993) ) Microstimulation in visual area MT: Effects of varying pulse amplitude and frequency. J Neurosci 13: , 1719–1729. |
[46] | Grossman N , Bono D , Dedic N , Kodandaramaiah SB , Rudenko A , Suk HJ , Cassara AM , Neufeld E , Kuster N , Tsai LH , Pascual-Leone A , Boyden ES ((2017) ) Noninvasive deep brain stimulation via temporally interfering electric fields. Cell 169: , 1029–1041.e1016. |
[47] | McIntyre CC , Savasta M , Walter BL , Vitek JL ((2004) ) How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol 21: , 40–50. |
[48] | Lettieri C , Rinaldo S , Devigili G , Pauletto G , Verriello L , Budai R , Fadiga L , Oliynyk A , Mondani M , D’Auria S , Skrap M , Eleopra R ((2012) ) Deep brain stimulation: Subthalamic nucleus electrophysiological activity in awake and anesthetized patients. Clin Neurophysiol 123: , 2406–2413. |
[49] | Rosin B , Slovik M , Mitelman R , Rivlin-Etzion M , Haber SN , Israel Z , Vaadia E , Bergman H ((2011) ) Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72: , 370–384. |
[50] | Lemos Rodrigues MM , Skodda S , Parpaley Y , Hilker-Roggendorf R ((2016) ) EP 63. Spectral analysis and visualization of multi-unit activity in subthalamic nucleus in Parkinson’s as a tool for automated electrophysiological classification of basal ganglia structures during deep brain stimulation procedures. Clin Neurophysiol 127: , e201–e202. |
[51] | Little S , Pogosyan A , Neal S , Zavala B , Zrinzo L , Hariz M , Foltynie T , Limousin P , Ashkan K , FitzGerald J , Green AL , Aziz TZ , Brown P ((2013) ) Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74: , 449–457. |
[52] | Mamun KA , Mace M , Lutman ME , Stein J , Liu X , Aziz T , Vaidyanathan R , Wang S ((2015) ) Movement decoding using neural synchronization and inter-hemispheric connectivity from deep brain local field potentials. J Neural Eng 12: , 056011. |
[53] | Niketeghad S , Hebb AO , Nedrud J , Hanrahan SJ , Mahoor MH ((2014) ) Single trial behavioral task classification using subthalamic nucleus local field potential signals. In 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC 2014, pp. 3793-3796. |
[54] | Swann N , Poizner H , Houser M , Gould S , Greenhouse I , Cai W , Strunk J , George J , Aron AR ((2011) ) Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: A scalp EEG study in parkinson’s disease. J Neurosci 31: , 5721–5729. |
[55] | Chaturvedi A , Luján JL , McIntyre CC ((2013) ) Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J Neural Eng 10: , 056023. |
[56] | Basu I , Crocker B , Farnes K , Robertson MM , Paulk AC , Vallejo DI , Dougherty DD , Cash SS , Eskandar EN , Kramer MA , Widge AS ((2018) ) A neural mass model to predict electrical stimulation evoked responses in human and non-human primate brain. J Neural Eng 15: , 066012. |
[57] | Coyle D , Prasad G , McGinnity TM ((2009) ) Faster self-organizing fuzzy neural network training and a hyperparameter analysis for a brain-computer interface. IEEE Trans Syst Man Cybern B Cybern 39: , 1458–1471. |
[58] | Ghiassi M , Nangoy S ((2009) ) A dynamic artificial neural network model for forecasting nonlinear processes. Comput Ind Eng 57: , 287–297. |
[59] | Dura-Bernal S , Li K , Neymotin SA , Francis JT , Principe JC , Lytton WW ((2016) ) Restoring behavior via inverse neurocontroller in a lesioned cortical spiking model driving a virtual arm. Front Neurosci 10: , 28. |
[60] | Santaniello S , Fiengo G , Glielmo L , Grill WM ((2011) ) Closed-loop control of deep brain stimulation: A simulation study. IEEE Trans Neural Syst Rehabil Eng 19: , 15–24. |
[61] | Grahn PJ , Mallory GW , Khurram OU , Berry BM , Hachmann JT , Bieber AJ , Bennet KE , Min HK , Chang SY , Lee KH , Lujan JL ((2014) ) A neurochemical closed-loop controller for deep brain stimulation: Toward individualized smart neuromodulation therapies. Front Neurosci 8: , 169. |
[62] | Shah SA , Brown P , Gimeno H , Lin J-P , McClelland VM ((2020) ) Application of machine learning using decision trees for prognosis of deep brain stimulation of globus pallidus internus for children with dystonia. Front Neurol 11: , 825. |
[63] | Camara C , Warwick K , Bruña R , Aziz T , del Pozo F , Maestú F ((2015) ) A fuzzy inference system for closed-loop deep brain stimulation in Parkinson’s disease. J Med Syst 39: , 155. |
[64] | Montagni E , Resta F , Letizia A , Mascaro A , Pavone FS ((2019) ) Optogenetics in brain research: From a strategy to investigate physiological function to a therapeutic tool. Photonics 6: , 92. |
[65] | Butler R , Radhakrishnan R ((2012) ) Dementia. BMJ Clin Evid 2012: , 1001. |
[66] | Pompanin S , Jelcic N , Cecchin D , Cagnin A ((2014) ) Impulse control disorders in frontotemporal dementia: Spectrum of symptoms and response to treatment. Gen Hosp Psychiatry 36: , 760.e765–760.e767. |
[67] | Bidzan L , Bidzan M , Pąchalska M ((2012) ) Aggressive and impulsive behavior in Alzheimer’s disease and progression of dementia. Med Sci Monit 18: , CR182–189. |
[68] | Arvanitakis Z , Shah RC , Bennett DA ((2019) ) Diagnosis and management of dementia: Review. JAMA 322: , 1589–1599. |
[69] | Lacor PN , Buniel MC , Furlow PW , Clemente AS , Velasco PT , Wood M , Viola KL , Klein WL ((2007) ) Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27: , 796–807. |
[70] | Braak H , Alafuzoff I , Arzberger T , Kretzschmar H , Tredici K ((2006) ) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112: , 389–404. |
[71] | Braak H , Braak E ((1991) ) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: , 239–259. |
[72] | Ballatore C , Lee VMY , Trojanowski JQ ((2007) ) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8: , 663–672. |
[73] | Yoshida T , Ha-Kawa S , Yoshimura M , Nobuhara K , Kinoshita T , Sawada S ((2007) ) Effectiveness of treatment with donepezil hydrochloride and changes in regional cerebral blood flow in patients with Alzheimer’s disease. Ann Nucl Med 21: , 257–265. |
[74] | Semba K ((2004) ) Phylogenetic and ontogenetic aspects of the basal forebrain cholinergic neurons and their innervation of the cerebral cortex. Prog Brain Res 145: , 3–43. |
[75] | Hasselmo ME , Giocomo LM ((2006) ) Cholinergic modulation of cortical function. J Mol Neurosci 30, 133-135. |
[76] | Hasselmo ME , Sarter M ((2011) ) Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36: , 52–73. |
[77] | Dalley JW ((2004) ) Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex 14: , 922–932. |
[78] | Schliebs R , Arendt T ((2006) ) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm 113: , 1625–1644. |
[79] | Härtig W , Bauer A , Brauer K , Grosche J , Hortobágyi T , Penke B , Schliebs R , Harkany T ((2002) ) Functional recovery of cholinergic basal forebrain neurons under disease conditions: Old problems, new solutions? Rev Neurosci 13: , 95–165. |
[80] | de Bruijn RFAG , Ikram MA ((2014) ) Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med 12: , 130. |
[81] | Bowen DM , Smith CB , White P , Davison AN ((1976) ) Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99: , 459–496. |
[82] | Davies P , Maloney AJF ((1976) ) Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 308: , 1403. |
[83] | Coyle JT , Price DL , DeLong MR ((1983) ) Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 219: , 1184–1190. |
[84] | Terry VA Jr , Buccafusco JJ ((2003) ) The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 306: , 821–827. |
[85] | Mesulam MMM , Geula C ((1988) ) Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: Observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol 275: , 216–240. |
[86] | Mesulam MM , Geula C ((1994) ) Chemoarchitectonics of axonal and perikaryal acetylcholinesterase along information processing systems of the human cerebral cortex. Brain Res Bull 33: , 137–153. |
[87] | Mesulam MM , Hersh LB , Mash DC , Geula C ((1992) ) Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: A choline acetyltransferase study. J Comp Neurol 318: , 316–328. |
[88] | Geula C , Mesulam MM ((1996) ) Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cereb Cortex 6: , 165–177. |
[89] | Croxson PL , Kyriazis DA , Baxter MG ((2011) ) Cholinergic modulation of a specific memory function of prefrontal cortex. Nat Neurosci 14: , 1510–1512. |
[90] | Easton A , Fitchett AE , Eacott MJ , Baxter MG ((2011) ) Medial septal cholinergic neurons are necessary for context-place memory but not episodic-like memory. Hippocampus 21: , 1021–1027. |
[91] | Kilgard MP , Merzenich MM ((1998) ) Cortical map reorganization enabled by nucleus basalis activity. Science 279: , 1714–1718. |
[92] | Juliano SL , Ma W , Eslin D ((1991) ) Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Nat Acad Sci U S A 88: , 780–784. |
[93] | Webster HH , Hanisch U-KK , Dykes RW , Biesoldt D , Biesold D ((1991) ) Basal forebrain lesions with or without reserpine injection inhibit cortical reorganization in rat hindpaw primary somatosensory cortex following sciatic nerve section. Somatosen Motor Res 8: , 327–346. |
[94] | Perry EK , Blessed G , Tomlinson BE , Perry RH , Crow TJ , Cross AJ , Dockray GJ , Dimaline R , Arregui A ((1981) ) Neurochemical activities in human temporal lobe related to aging and Alzheimer-type changes. Neurobiol Aging 2: , 251–256. |
[95] | Francis PT , Palmer AM , Sims NR , Bowen DM , Davison AN , Esiri MM , Neary D , Snowden JS , Wilcock GK ((1985) ) Neurochemical studies of early-onset Alzheimer’s disease. N Engl J Med 313: , 7–11. |
[96] | Wilcock GK , Esiri MM , Bowen DM , Smith CCT ((1982) ) Alzheimer’s disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci 57: , 407–417. |
[97] | Sims NR , Bowen DM , Allen SJ , Smith CCT , Neary D , Thomas DJ , Davison AN ((1983) ) Presynaptic cholinergic dysfunction in patients with dementia. J Neurochem 40: , 503–509. |
[98] | Kaasinen V , Någren K , Järvenpää T , Roivainen A , Yu M , Oikonen V , Kurki T , Rinne JO ((2002) ) Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer’s disease. J Clin Psychopharmacol 22: , 615–620. |
[99] | Marcone A , Garibotto V , Moresco RM , Florea I , Panzacchi A , Carpinelli A , Virta JR , Tettamanti M , Borroni B , Padovani A , Bertoldo A , Herholz K , Rinne JO , Cappa SF , Perani D ((2012) ) [11]-MP4A PET cholinergic measurements in amnestic mild cognitive impairment, probable Alzheimer’s disease, and dementia with lewy bodies: A Bayesian method and voxel-based analysis. J Alzheimers Dis 31: , 387–399. |
[100] | Drachman DA , Leavitt J ((1974) ) Human memory and the cholinergic system: A relationship to aging? Arch Neurol 30: , 113–121. |
[101] | Summers WK , Majovski LV , Marsh GM , Tachiki K , Kling A ((1986) ) Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med 315: , 1241–1245. |
[102] | Brinkman SD , Gershon S ((1983) ) Measurement of cholinergic drug effects on memory in Alzheimer’s disease. Neurobiol Aging 4: , 139–145. |
[103] | Braak H , Del Tredici K ((2013) ) Reply: The early pathological process in sporadic Alzheimer’s disease. Acta Neuropathol 126: , 615–618. |
[104] | Mesulam MM ((2013) ) Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J Comp Neurol 521: , 4124–4144. |
[105] | Goate A , Chartier-Harlin MC , Mullan M , Brown J , Crawford F , Fidani L , Giuffra L , Haynes A , Irving N , James L , Mant R , Newton P , Rooke K , Roques P , Talbot C , Pericak-Vance M , Roses A , Williamson R , Rossor M , Owen M , Hardy J ((1991) ) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349: , 704–706. |
[106] | Mullan M , Crawford F , Axelman K , Houlden H , Lilius L , Winblad B , Lannfelt L ((1992) ) A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N–terminus of β–amyloid. Nat Genet 1: , 345–347. |
[107] | Felsenstein KM , Hunihan LW , Roberts SB ((1994) ) Altered cleavage and secretion of a recombinant β-APP bearing the Swedish familial Alzheimer’s disease mutation. Nat Genet 6: , 251–256. |
[108] | St. George-Hyslop PH , Tanzi RE , Polinsky RJ , Haines JL , Nee L , Watkins PC , Myers RH , Feldman RG , Pollen D , Drachman D , Growdon J , Bruni A , Foncin JF , Salmon D , Frommelt P , Amaducci L , Sorbi S , Piacentini S , Stewart GD , Hobbs WJ , Conneally PM , Gusella JF ((1987) ) The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science 235: , 885–890. |
[109] | Citron M , Oltersdorf T , Haass C , McConlogue L , Hung AY , Seubert P , Vigo-Pelfrey C , Lieberburg I , Selkoe DJ ((1992) ) Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature 360: , 672–674. |
[110] | Younkin SG ((1998) ) The role of Aβ42 in Alzheimer’s disease. J Physiol 92: , 289–292. |
[111] | Mawuenyega KG , Sigurdson W , Ovod V , Munsell L , Kasten T , Morris JC , Yarasheski KE , Bateman RJ ((2010) ) Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330: , 1774. |
[112] | Bernstein SL , Dupuis NF , Lazo ND , Wyttenbach T , Condron MM , Bitan G , Teplow DB , Shea JE , Ruotolo BT , Robinson VC , Bowers MT ((2009) ) Amyloid-β 2 protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem 1: , 326–331. |
[113] | Esparza TJ , Zhao H , Cirrito JR , Cairns NJ , Bateman RJ , Holtzman DM , Brody DL ((2013) ) Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol 73: , 104–119. |
[114] | Mroczko B , Groblewska M , Litman-Zawadzka A , Kornhuber J , Lewczuk P ((2018) ) Amyloid β oligomers (AβOs) in Alzheimer’s disease. J Neural Transm 125: , 177–191. |
[115] | Santos AN , Ewers M , Minthon L , Simm A , Silber RE , Blennow K , Prvulovic D , Hansson O , Hampel H ((2012) ) Amyloid-β oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer’s disease. J Alzheimers Dis 29: , 171–176. |
[116] | Reijs BLR , Ramakers IHGB , Köhler S , Teunissen CE , Koel-Simmelink M , Nathan PJ , Tsolaki M , Wahlund LO , Waldemar G , Hausner L , Vandenberghe R , Johannsen P , Blackwell A , Vanderstichele H , Verhey F , Visser PJ ((2017) ) Memory correlates of Alzheimer’s disease cerebrospinal fluid markers: A longitudinal cohort study. J Alzheimers Dis 60: , 1119–1128. |
[117] | Lewczuk P , Esselmann H , Otto M , Maler JM , Henkel AW , Henkel MK , Eikenberg O , Antz C , Krause WR , Reulbach U , Kornhuber J , Wiltfang J ((2004) ) Neurochemical diagnosis of Alzheimer’s dementia by CSF Aβ42, Aβ42/Aβ40 ratio and total tau. Neurobiol Aging 25: , 273–281. |
[118] | Wang H-Y , Lee DHS , Davis CB , Shank RP ((2002) ) Amyloid peptide Aβ1-42 binds selectively and with picomolar affinity to α7 nicotinic acetylcholine receptors. J Neurochem 75: , 1155–1161. |
[119] | van der Kant R , Goldstein LSB , Ossenkoppele R ((2020) ) Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci 21: , 21–35. |
[120] | Musiek ES , Holtzman DM ((2015) ) Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat Neurosci 18: , 800–806. |
[121] | Hamani C , McAndrews MP , Cohn M , Oh M , Zumsteg D , Shapiro CM , Wennberg RA , Lozano AM ((2008) ) Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 63: , 119–123. |
[122] | Curot J , Busigny T , Valton L , Denuelle M , Vignal JP , Maillard L , Chauvel P , Pariente J , Trebuchon A , Bartolomei F , Barbeau EJ ((2017) ) Memory scrutinized through electrical brain stimulation: A review of 80 years of experiential phenomena. Neurosci Biobehav Rev 78: , 161–177. |
[123] | Laxton AW , Tang-Wai DF , McAndrews MP , Zumsteg D , Wennberg R , Keren R , Wherrett J , Naglie G , Hamani C , Smith GS , Lozano AM ((2010) ) A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 68: , 521–534. |
[124] | Smith GS , Laxton AW , Tang-Wai DF , McAndrews MP , Diaconescu AO , Workman CI , Lozano AM ((2012) ) Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch Neurol 69: , 1141–1148. |
[125] | Suthana N , Haneef Z , Stern J , Mukamel R , Behnke E , Knowlton B , Fried I ((2012) ) Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med 366: , 502–510. |
[126] | Mann A , Gondard E , Tampellini D , Milsted JAT , Marillac D , Hamani C , Kalia SK , Lozano AM ((2018) ) Chronic deep brain stimulation in an Alzheimer’s disease mouse model enhances memory and reduces pathological hallmarks. Brain Stimul 11: , 435–444. |
[127] | Akwa Y , Gondard E , Mann A , Capetillo-Zarate E , Alberdi E , Matute C , Marty S , Vaccari T , Lozano AM , Baulieu EE , Tampellini D ((2018) ) Synaptic activity protects against AD and FTD-like pathology via autophagic-lysosomal degradation. Mol Psychiatry 23: , 1530–1540. |
[128] | Jacobs J , Miller J , Lee SA , Coffey T , Watrous AJ , Sperling MR , Sharan A , Worrell G , Berry B , Lega B , Jobst BC , Davis K , Gross RE , Sheth SA , Ezzyat Y , Das SR , Stein J , Gorniak R , Kahana MJ , Rizzuto DS ((2016) ) Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron 92: , 983–990. |
[129] | Riley MR , Constantinidis C ((2016) ) Role of prefrontal persistent activity in working memory. Front Syst Neurosci 9: , 181. |
[130] | Scharre DW , Weichart E , Nielson D , Zhang J , Agrawal P , Sederberg PB , Knopp MV , Rezai AR , Alzheimer’s Disease Neuroimaging Initiative ((2018) ) Deep brain stimulation of frontal lobe networks to treat Alzheimer’s disease. J Alzheimers Dis 62: , 621–633. |
[131] | Widge AS , Zorowitz S , Basu I , Paulk AC , Cash SS , Eskandar EN , Deckersbach T , Miller EK , Dougherty DD ((2019) ) Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nat Commun 10: , 1536. |
[132] | Xu J , Galardi MM , Pok B , Patel KK , Zhao CW , Andrews JP , Singla S , McCafferty CP , Feng L , Musonza ET , Kundishora AJ , Gummadavelli A , Gerrard JL , Laubach M , Schiff ND , Blumenfeld H ((2020) ) Thalamic stimulation improves postictal cortical arousal and behavior. J Neurosci 40: , 7343–7354. |
[133] | Redinbaugh MJ , Phillips JM , Kambi NA , Mohanta S , Andryk S , Dooley GL , Afrasiabi M , Raz A , Saalmann YB ((2020) ) Thalamus modulates consciousness via layer-specific control of cortex. Neuron 106: , 66–75 e12. |
[134] | Baker JL , Ryou JW , Wei XF , Butson CR , Schiff ND , Purpura KP ((2016) ) Robust modulation of arousal regulation, performance, and frontostriatal activity through central thalamic deep brain stimulation in healthy nonhuman primates. J Neurophysiol 116: , 2383–2404. |
[135] | Davis KL , Mohs RC , Marin D , Purohit DP , Perl DP , Lantz M , Austin G , Haroutunian V ((1999) ) Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA 281: , 1401–1406. |
[136] | Kuhn J , Hardenacke K , Lenartz D , Gruendler T , Ullsperger M , Bartsch C , Mai JK , Zilles K , Bauer A , Matusch A , Schulz RJ , Noreik M , Bührle CP , Maintz D , Woopen C , Häussermann P , Hellmich M , Klosterkötter J , Wiltfang J , Maarouf M , Freund HJ , Sturm V ((2015) ) Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol Psychiatry 20: , 353–360. |
[137] | Freund HJ , Kuhn J , Lenartz D , Mai JK , Schnell T , Klosterkoetter J , Sturm V ((2009) ) Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol 66: , 781–785. |
[138] | Hardenacke K , Hashemiyoon R , Visser-Vandewalle V , Zapf A , Freund HJ , Sturm V , Hellmich M , Kuhn J ((2016) ) Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia: Potential predictors of cognitive change and results of a long-term follow-up in eight patients. Brain Stimul 9: , 799–800. |
[139] | Walker LC , Price DL , Young WS 3rd ((1989) ) GABAergic neurons in the primate basal forebrain magnocellular complex. Brain Res 499: , 188–192. |
[140] | Kim T , Thankachan S , McKenna JT , McNally JM , Yang C , Choi JH , Chen L , Kocsis B , Deisseroth K , Strecker RE , Basheer R , Brown RE , McCarley RW ((2015) ) Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A 112: , 3535–3540. |
[141] | Guo W , Robert B , Polley DB ((2019) ) The cholinergic basal forebrain links auditory stimuli with delayed reinforcement to support learning. Neuron 103: , 1164–1177.e1166. |
[142] | Froemke RC , Merzenich MM , Schreiner CE ((2007) ) A synaptic memory trace for cortical receptive field plasticity. Nature 450: , 425–429. |
[143] | Froemke RC , Carcea I , Barker AJ , Yuan K , Seybold BA , Martins ARO , Zaika N , Bernstein H , Wachs M , Levis PA , Polley DB , Merzenich MM , Schreiner CE ((2013) ) Long-term modification of cortical synapses improves sensory perception. Nat Neurosci 16: , 79–88. |
[144] | Blake DT , Terry VA , Plagenhoef M , Constantinidis C , Liu R ((2017) ) Potential for intermittent stimulation of nucleus basalis of Meynert to impact treatment of Alzheimer’s disease. Commun Integr Biol 10: , e1389359. |
[145] | Liu R , Crawford J , Callahan PM , Terry VA , Constantinidis C , Blake DT ((2017) ) Intermittent stimulation of the nucleus basalis of Meynert improves working memory in adult monkeys. Curr Biol 27: , 2640–2646. |
[146] | Liu R , Crawford J , Callahan PM , Terry VA , Constantinidis C , Blake DT ((2018) ) Intermittent stimulation in the nucleus basalis of meynert improves sustained attention in rhesus monkeys. Neuropharmacology 137: , 202–210. |
[147] | Qi XL , Liu R , SinghB, BestueD, CompteA, Vazdarjanova AI , Blake DT , Constantinidis C ((2021) ) Nucleus basalis stimulation enhances working memory by stabilizing stimulus representations in primate prefrontal cortical activity. Cell Rep 36: , 109469. |
[148] | Hamani C , Richter E , Schwalb JM , Lozano AM ((2005) ) Bilateral subthalamic nucleus stimulation for Parkinson’s disease: A systematic review of the clinical literature. Neurosurgery 56: , 1313–1321; discussion 1321-1314. |
[149] | Lee DJ , Lozano CS , Dallapiazza RF , Lozano AM ((2019) ) Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg 131: , 333–342. |



