Could Altered Evoked Pain Responsiveness Be a Phenotypic Biomarker for Alzheimer’s Disease Risk? A Cross-Sectional Analysis of Cognitively Healthy Individuals
Abstract
Background:
This study evaluated whether the apolipoprotein ɛ4 (APOE4) allele, a genetic marker associated with increased risk of developing late-onset Alzheimer’s disease (AD), was associated with differences in evoked pain responsiveness in cognitively healthy subjects.
Objective:
The aim was to determine whether individuals at increased risk of late-onset AD based on APOE allele genotype differ phenotypically in their response to experimentally-induced painful stimuli compared to those who do not have at least one copy of the ɛ4 allele.
Methods:
Forty-nine cognitively healthy subjects aged 30–89 years old with the APOE4 allele (n = 12) and without (n = 37) were assessed for group differences in pain thresholds and affective (unpleasantness) responses to experimentally-induced thermal pain stimuli.
Results:
Statistically significant main effects of APOE4 status were observed for both the temperature at which three different pain intensity percepts were reached (p = 0.040) and the level of unpleasantness associated with each (p = 0.014). APOE4 positive participants displayed lower overall pain sensitivity than those who were APOE4 negative and also greater overall levels of pain unpleasantness regardless of intensity level.
Conclusion:
Cognitively healthy APOE4 carriers at increased risk of late-onset AD demonstrated reduced thermal pain sensitivity but greater unpleasantness to thermal pain stimuli relative to individuals at lower risk of late-onset AD. These results suggest that altered evoked pain perception could potentially be used as a phenotypic biomarker of late-onset AD risk prior to disease onset. Additional studies of this issue may be warranted.
INTRODUCTION
Earlier diagnosis of Alzheimer’s disease (AD) has been shown to reduce cost and improve patient outcomes despite the limited availability of current treatment options [1]. One method used to initiate earlier diagnosis is identifying AD prior to the onset of classical signs or symptoms, and biomarkers hold potential of serving this function. Currently recognized biomarkers of AD are invasive and not practical in the primary care setting where the majority of patients with memory concerns first seek care [1, 2]. An initial report of increased neurofibrillary tangles in olfactory bulb in people with AD spurred further studies directly investigating sensory processing in AD [3]. Since that early report, studies have demonstrated that changes in olfaction, taste, hearing, vision, and proprioception have the potential to serve as biomarkers of AD [4].
Current approaches used to identify individuals with AD include lumbar puncture with analysis of cerebrospinal fluid, magnetic resonance imaging, and positron emission tomography scans [1]. These approaches are invasive, costly, and not widely available or feasible in the primary care setting [5]. In contrast, measures of sensory changes are less invasive, more feasible, and readily available in primary care practices where most individuals present for initial diagnosis [2, 4]. Sensory processing for all senses (e.g., olfaction, vision, proprioception) involves multisystem physiological mechanisms in which stimuli activate sensory receptors, thereby initiating action potentials leading to central nervous system (CNS) processing and ultimately a perceptual response [6]. AD-related changes in the CNS have the potential to alter all such sensory processing [4]. Processing of pain stimuli in the CNS occurs in the lateral (sensory) and medial (emotional) brain networks with the integration of the rostral (behavioral) network in healthy individuals and at varying degrees of neurocognitive impairment, people may present with increased or decreased pain-related behaviors coupled with increased or decreased reports of pain intensity and pain unpleasantness [7]. Several studies have demonstrated that the perception of controlled experimentally-induced pain stimuli differs between cognitively healthy people and people with AD [7–11], which suggests that pain processing is altered in AD. However, changes in pain processing have not been directly linked to genetic markers for AD. Thus to date, the phenotypic association of the ɛ4 allele has not been considered in pain outcomes.
The ɛ4 allele of apolipoprotein E (APOE) is a genetic marker associated with an increased incidence of developing late-onset AD, which typically affects those age 65 and older and is a risk factor identifiable long before the onset of AD symptoms [12–14]. The APOE gene encodes the production of a 299 amino acid ligand that is primarily synthesized in astrocytes of glial cells in the brain, the liver, and macrophages in peripheral tissue and is mostly responsible for transporting cholesterol [12–15]. APOE can take on three isoforms dictated by three polymorphic alleles—ɛ2, ɛ3, and ɛ4—resulting from a difference in only one or two amino acids [13, 14]. The most common allele, APOE ɛ3, is present in 50–90% of all populations; APOE ɛ4 occurs in about 5–35%; and APOE ɛ2, the least common allele, occurs in about 1–5% of the population [16–18]. Genetically, a person can be homozygous (i.e., ɛ4/ɛ4, ɛ3/ɛ3, and ɛ2/ɛ2) for one major subtype or heterozygous (ɛ4/ɛ3, ɛ3/ɛ2, and ɛ4/ɛ2) for two main subtypes, resulting in six different phenotypic populations [14]. The risk of developing AD differs between genetic profiles, as those with the greatest odds of developing AD include ɛ2/ɛ4, ɛ3/ɛ4, and ɛ4/ɛ4, while those with reduced odds include ɛ2/ɛ2 and ɛ2/ɛ3 [12, 14, 15]. A recent meta-analysis of the AD population estimated about 49% of individuals as being APOE ɛ4 carriers and about 10% as ɛ4/ɛ4 positive carriers, concluding that carrying at least one copy of the ɛ4 allele increases AD risk 3-fold when compared to healthy controls, with an almost 12-fold risk increase for those with two ɛ4 alleles [18].
The aim of the current study was to determine whether individuals at increased risk of late-onset AD based on APOE allele genotype differ phenotypically in their response to experimentally-induced painful stimuli compared to those who do not have at least one copy of the ɛ4 allele.
MATERIALS AND METHODS
Participants
Recruitment, enrollment, psychophysical testing, and cognitive assessments techniques are previously described [8]. English-speaking, verbally communicative participants aged 30–89 years were recruited between 2014 and 2017 from a Mid-south metropolitan area. The goal of the primary study was to examine sex differences in the psychophysical and neurophysical response to experimental thermal pain across the adult lifespan. Participants were excluded for the presence of chronic pain; cognitive impairment (Mini-Mental State Exam [MMSE] < 28) [19]; daily use of analgesic medication; or history of stroke, cancer, peripheral neuropathy, Raynaud’s disease, unstable medical conditions (e.g., severe restrictive or obstructive lung disease), insulin-dependent diabetes, current substance use disorders, or psychiatric diagnoses of bipolar disorder, major depressive disorder, schizophrenia; or presence of movement disorders including Parkinson’s disease and restless leg syndrome. Participants were considered free of chronic pain if not taking an analgesic medication within one-week of testing and not reporting a current acute or chronic pain condition requiring daily opioid analgesics.
All activities included in this research protocol meet criteria for minimal risk studies in individuals over age 18 as defined by U.S. Federal Regulations and as approved by the Vanderbilt University Institutional Review Board (IRB). Participants provided written informed consent approved by our IRB at the time of study enrollment. All participants were compensated US $100 for their time.
Screening and enrollment
Eligibility was assessed using a two-part screening process via telephone or an in-person visit. Participants underwent one hour of psychosocial assessment, including medication use, demographic information, Hollingshead Four-Factor Socioeconomic Status (SES) [20], and cognitive screening with the MMSE [19]. Participants were also assessed with the Brief Pain Inventory (BPI) [21], 5-item World Health Organization Well-Being Index [22], and the State-Trait Anxiety Inventory (STAI) [23]. Depression or anxiety as well as socioeconomic status may influence outcome measures and were included as potential confounders.
Thermal stimulation protocol (psychophysics)
The thermal stimulation protocol used in the study assessed two aspects of pain: the intensity of pain and the unpleasantness of pain. The protocol was a modification of the perceptual matching experimental mechanical pressure pain protocol used by Cole and colleagues [24] and used the Medoc Q-Sense™. This device evokes simulation of A-delta and C-fibers [25, 26]. The Medoc thermode (30×30 mm) was attached to the thenar eminence of the right hand of each participant, and participants were shown a 0–20 sensory pain intensity scale and asked to stop the heat stimulus (via clicking a computer mouse) when they felt “just noticeable pain,” “weak pain,” or “moderate pain” (with each percept tested in separate trials). Participants were then asked to rate the unpleasantness of the sensation at each pain intensity percept using a 0–20 unpleasantness scale with the following anchors: “0 = neutral,” “5 = slightly unpleasant,” “8 = unpleasant,” “11 = very unpleasant,” “16 = intolerable,” and “20 = extremely distressing”[27].
The baseline temperature was set as 30°C (a temperature not perceived as warm or cold [34]), and the thermode was programmed to deliver heat that increased at a rate of 4°C/s. We modeled our thermal stimulus delivery after Wager and colleagues’ paradigm in which each temperature stimulus began from baseline and ramped up and down at a moderate rate [19]. Subsequently, we recorded the temperature at which each participant reported the perceptions of just noticeable pain (indicator of pain threshold), weak pain, and moderate pain. Each participant completed three pseudorandomized trials consisting of two instances at each percept level. The average temperature (°C) across the three trials at each percept level was used in analyses (maximum temperature = 50°C). Immediately after indicating the first stimulus meeting criteria for each of the three percept levels, participants were asked to rate the unpleasantness associated with that stimulus level as described above. Ten seconds of rest was provided between each percept in each trial.
APOE4 status determination
Participants supplied 2 mL of saliva which was collected and stored in an Oragene™ saliva collection tube (https://www.dnagenotek.com/US/products/collection-human/oragene-discover/500-series/OGR-575.html) [28]. APOE genotype was determined using TaqMan™ assays (https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-assays.html#applications) [29]. For statis-tical analysis, participants were categorized as either being APOE4 positive (APOE ɛ3/ɛ4, ɛ4/ɛ4) or APOE4 negative (APOE ɛ2/ɛ2, ɛ2/ɛ3, ɛ3/ɛ3).
Statistical analysis
Frequency distributions were used to summarize nominal and ordinal data. Due to the skewness of many of the continuous distributions, median and inter-quartile range were used to summarize the continuously scaled measures. Mixed-effects (between-subject: APOE4 allele status; within-subject: three percept levels) general linear models tested the main and interaction effect of allele status (negative, positive) and percept level (just noticable pain, mild pain, moderate pain) on the temperature at which each level was reported and on the unpleasantness value of the pain at that level. Pairwise Mann-Whitney U tests were used to assess pairwise differences at each percept level. Distributions were square root transformed to meet the normal assumptions of the models used and to calculate Cohen’s d effect size statistics for the group differences at each threshold level. An alpha level of p < 0.05, set a priori, was used for statistical significance determinations.
RESULTS
Sample characteristics
A total of 92 participants were screened; 32 participants were excluded prior to enrollment for not meeting inclusion criteria; 60 participants were enrolled; of those, 1 participant was lost to follow-up, 7 participants were excluded for failure to provide sufficient salvia volume for genetic analysis, and 3 participants had incomplete data leaving a final sample of 49 (see Fig. 1). The majority of participants carried the ɛ3/ɛ3 alleles (n = 27, 55%), and the remaining allele distributions were ɛ3/ɛ4 (n = 10, 20%), ɛ2/ɛ3 (n = 8, 16%), ɛ2/ɛ2 (n = 2, 4%), and ɛ4/ɛ4 (n = 2, 4%). There were no participants with ɛ2/ɛ4 alleles. Slightly more than half the sample was male (n = 26, 53%) and a majority were white (n = 38, 81%). Sample characteristics are summarized in Table 1. Median age of the sample was 68.0 years (IQR: 48–80 and median MMSE score was 30.0 (IQR: 29–30). There were no statistically significant differences between APOE4 positive (n = 12) and APOE4 negative (n = 37) participants on any of the characteristics investigated including average pain and current pain scores on the BPI (p > 0.05, Table 1).
Fig. 1
CONSORT flow diagram.
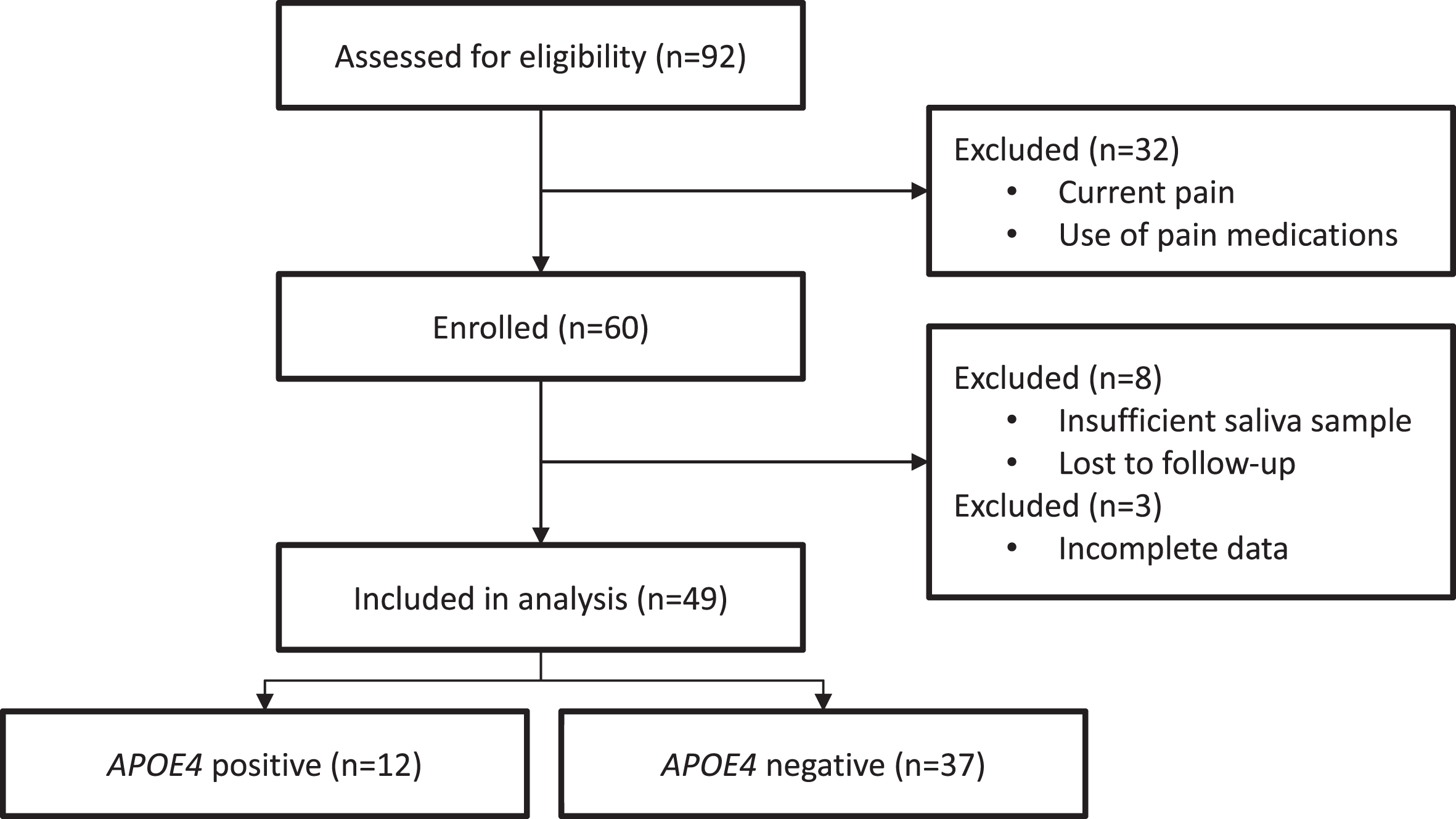
Table 1
Demographic and sample characteristics
| APOE4 | APOE4 | Total | p | |
| Negative (n = 37) | Positive (n = 12) | (n = 49) | ||
| n (%) | ||||
| Male | 20 (54.1) | 6 (50.0) | 26 (53.1) | 0.807 |
| White | 30 (85.7) | 8 (66/7) | 38 (80.9) | 0.148 |
| Median (IQR) | ||||
| Age | 68.0 (48–80) | 49.0 (33–80) | 65.0 (46–80) | 0.192 |
| aSES | 52.5 (49–58) | 58.0 (51–88) | 53.5 (49–60) | 0.131 |
| bMMSE | 30.0 (29-30) | 30 (29-30) | 30 (29-30) | 0.339 |
| cBPI-SF average pain | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0.783 |
| cBPI-SF pain right now | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0.817 |
| dWHO-5 | 19.0 (17–21) | 19.5 (16–21) | 19.0 (17–21) | 0.699 |
| eSTAI state Y form | 15.0 (15-16) | 15.0 (14-15) | 15.0 (15-15) | 0.544 |
| fSTAI trait | 47.0 (45–49) | 46.5 (44–49) | 47.0 (44–49) | 0.565 |
aHollingshead Four-Factor Measure of Socioeconomic Status (range = 8–66; 8 = lowest SES, 66 = highest SES); N = 40, Negative = 31, Positive = 9. bMMSE-Folstein Mini-Mental State Examination (range = 0–30; 0 = completely cognitively impaired, 30 = completely cognitively healthy). cBPI-SF-Brief Pain Inventory Short Form (range = 0–10; 0 = no pain, 10 = most pain); Current Pain N = 48, Negative = 37, Positive = 11. dWHO-5 Well-Being Index (range = 0–25, 25 = maximal well-being). eSTAI-Spielberger State Anxiety Inventory-STATE Y form (range 6–24; 6 = increased anxiety, 24 = least amount of anxiety). fSTAI-Spielberger Trait Anxiety Inventory (range = 20–80; 20 = increased anxiety, 80 = least amount of anxiety); N = 48, Negative = 36, Positive = 12.
APOE and pain
The psychophysical data for APOE4 positive and APOE4 negative participants are summarized in Table 2. Statistically significant main effects of increasing percept intensity were observed on both stimulus temperature and unpleasantness (p < 0.001). Thus, as expected, stimulus temperature and unpleasantness both increased as the targeted percept intensity increased from just noticeable to moderate pain. Our primary focus was on the main effects of APOE4 status. Statistically significant main effects of APOE4 status were noted for the temperature necessary to elicit the three targeted pain percepts (p = 0.040) and on the experience of unpleasantness of that pain (p = 0.014).
Table 2
Experience of pain between APOE4 negative and positive participants
| APOE4 Negative (n = 37) | APOE4 Positive (n = 12) | p* | Cohen’s d | |
| Temperature | Median °C (IQR) | Median °C (IQR) | ||
| Overall | 40.0 (35–45) | 42.0 (35–48) | 0.040 | 0.19 |
| Just noticeable pain | 34.0 (32–37) | 34.1 (32–36) | 0.806 | 0.16 |
| Weak pain | 41.0 (37–43) | 42.5 (39–46) | 0.139 | 0.54 |
| Moderate pain | 45.3 (43–48) | 47.9 (45–49) | 0.057 | 0.59 |
| Unpleasantness | Median (IQR) | Median (IQR) | ||
| Overall | 4.0 (0–7) | 5.5 (1–10) | 0.014 | 0.26 |
| Just noticeable pain | 0.0 (0-1) | 0.2 (0–2) | 0.395 | 0.23 |
| Weak pain | 4.0 (1–6) | 5.8 (4–8) | 0.060 | 0.52 |
| Moderate pain | 8.0 (6–10) | 10.0 (8–13) | 0.080 | 0.50 |
Mixed-effects analyses revealed statistically significant main effects of AOPE4 status for both temperature (p = 0.040) and unpleasantness (p = 0.014). * p-values are for Mann-Whitney tests at each pain threshold level. Values were square-root transformed to meet normal distribution assumptions of Cohen’s d.
Table 3
Pain variables by APOE4 phenotypic subgroups
| Temperature Median °C (IQR) | |||
| Genotype | Just noticeable pain | Weak pain | Moderate pain |
| E2/E2 | 33.0 (32–34) | 42.0 (39–45) | 46.0 (44–48) |
| E2/E3 | 36.0 (34–38) | 41.4 (40–45) | 46.6 (45–48) |
| E3/E3 | 33.9 (33–35) | 41.0 (37–43) | 45.0 (43–47) |
| E3/E4 | 33.1 (32–35) | 42.5 (39–45) | 47.9 (46–48) |
| E4/E4 | 35.8 (35–36) | 44.2 (41–47) | 47.5 (45–50) |
| Unpleasantness Median (IQR) | |||
| Genotype | Just noticeable pain | Weak pain | Moderate pain |
| E2/E2 | 0.0 (0–0) | 4.0 (4–4) | 8.5 (8–9) |
| E2/E3 | 0.0 (0–2) | 4.5 (2–7) | 7.5 (6–10) |
| E3/E3 | 0.0 (0–1) | 3.6 (2–6) | 8.0 (6–10) |
| E3/E4 | 0. (0–1) | 5.8 (4–8) | 10.5 (8–13) |
| E4/E4 | 1.5 (1–2) | 5.7 (5–6) | 8.9 (9–9) |
The APOE4 positive participants were less pain sensitive overall, that is, they reported reaching the targeted pain percepts at a significantly higher temperature than did those who were APOE4 negative. However, when those pain percepts were reached, APOE4 positive participants reported that pain to be more unpleasant relative to APOE4 negative participants. As shown in Table 2, the interaction effects of APOE4 status and pain percept level in both models were not statistically significant (p > 0.05), yet as is apparent, the strongest effects of APOE4 status were observed at the weak and moderate pain levels (Cohen’s d = 0.50 to 0.59, Table 2). A follow-up analysis examining the effects of APOE4 on pain unpleasantness adjusting for differences in stimulus intensity at each percept level across genetic groups indicated that the pattern of results was little changed, with the APOE4 main effect becoming marginally significant (p < 0.09) but the overall effect size increasing slightly (from d = 0.26 to d = 0.33).
DISCUSSION
In this study, we found that cognitively healthy APOE4 positive individuals who are at increased genetic risk of late-onset AD exhibit significantly reduced sensitivity to evoked thermal pain relative to APOE4 negative individuals. However, when specific pain percepts are reached, APOE4 positive individuals report this pain to be more unpleasant than individuals without an APOE4 allele. To the best of our knowledge, this is the first report of an association of APOE4 allele status with an altered response to pain in a cognitively healthy sample of adults across the lifespan. Notably, the observed psychophysical results in this work mirror a recent study in which the authors used a very similar study design to test pain perception differences between cognitively healthy adults and people with diagnosed AD age 65 and older [10]. People with AD, as was the case for individuals with the APOE4 allele in the current work, required a higher stimulus temperature than cognitively healthy controls to report the perceptions of “warmth,” “mild pain,” and “moderate pain”. In context of this prior work, these present results suggest that thermal evoked pain testing could serve as a potential phenotypic biomarker of individuals at increased risk for AD. At minimum, further research on this issue appears to be warranted in a larger sample. Differences observed between APOE4 positive and APOE4 negative cognitively normal individuals in thermal percept detection levels and in pain unpleasantness at each percept may hint at possible mechanisms for how prodromal AD pathology may disrupt pain processing [8–11]. This possibility is consistent with other work suggesting that alterations in other sensory systems are potential phenotypic markers for subsequent AD risk [4].
This study does have some limitations. First, the use of a perceptual matching paradigm in which participants reported unpleasantness of pain at temperature intensities unique to each individual potentially leads to confounded assessment for this outcome. In future work, this potential confound can be overcome through using a series of fixed temperatures across participants. Another limitation is the small sample size, which reduced statistical power and likely adversely impacted ability to test the effect of APOE4 at each percept level individually. Further, analysis of differences between sex was not feasible given the unequal distribution between the APOE4 groups in this exploratory study. Despite these limitations, there are numerous strengths to the current pilot study. First, the mixed effect models did demonstrate statistically significant overall differences as a function of APOE4 status across the three percept levels. Moreover, the moderate effect sizes for between group differences noted at the mild pain and moderate pain percept levels suggest that APOE4-related differences in pain perception are likely to be clinically meaningful. We also note several aspects of this study that are novel. First, the NIH has initiated a focus on precision medicine, and this study is identifying brain mechanisms that may be useful for the prediction of AD. Next, our focus on a non-invasive biomarker (evoked pain responsiveness) as a predictor is unique. Finally, the feasibility of employing this predictor in primary care settings places it on the front lines of care. This study provides preliminary support for the possibility that there are linkages between altered pain processing and genetic markers for AD. Future studies should aim to replicate the current results in larger samples and with other types of experimental pain stimuli, such as a mechanical pressure pain or ischemic pain. As this study included a mostly white population future research should aim to include more ethnically and racially diverse groups who are at greater risk of AD and chronic pain.
As the prevalence of dementia is expected to triple over the next 30 years, more evidence is needed to understand the altered pain experience in people with AD [1, 30]. Carriers of the ɛ4 allele are at greater risk for amyloid deposition, impacting brain structures such as the hypothalamus and the prefrontal cortex, which may disrupt neural circuits mediating pain perception and behavioral expression that result in differences in psychophysical measurements of pain [24, 31, 32]. The results of this research demonstrate for the first time that alterations in evoked pain responsiveness may be a potential phenotypic marker for identifying those at risk for APOE4-related late-onset AD [4]. In the clinical context, the possibility that APOE4 allele status may alter the risk of pain-related suffering (either by directly increasing pain unpleasantness or delaying necessary medical care due to decreased pain sensitivity) irrespective of AD status may warrant further exploration. As previously mentioned, the use of precision medicine approaches continues to grow and the results from this study suggest the possibility that pain management plans may benefit from being individualized based on genotype.
ACKNOWLEDGMENTS
This work was supported by R21AG045735 from the NIH/NIA. The authors would like to acknowledge Curtis Roby, MA, for his editorial assistance.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1293r1).
REFERENCES
[1] | ((2020) ) 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 16: , 391–460. |
[2] | Wilkinson D , Stave C , Keohane D , Vincenzino O ((2004) ) The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: A multinational survey. J Int Med Res 32: , 149–159. |
[3] | Esiri MM , Wilcock GK ((1984) ) The olfactory bulbs in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 47: , 56–60. |
[4] | Romano 3rd RR , Carter MA , Monroe TB ((2020) ) Narrative review of sensory changes as a biomarker for Alzheimer’s disease. Biol Res Nurs, doi: 10.1177/1099800420947176 |
[5] | Iliffe S , Robinson L , Brayne C , Goodman C , Rait G , Manthorpe J , Ashley P , Iliffe S , Robinson L , Brayne C , Goodman C , Rait G , Manthorpe J , Ashley P ((2009) ) Primary care and dementia: 1. diagnosis, screening and disclosure. Int J Geriatr Psychiatry 24: , 895–901. |
[6] | Matlin MW , Foley HJ ((1992) ) Sensation and perception, 3rd ed, Allyn & Bacon, Needham Heights, MA, US. |
[7] | Monroe TB , Gore JC , Chen LM , Mion LC , Cowan RL ((2012) ) Pain in people with Alzheimer disease: Potential applications for psychophysical and neurophysiological research. J Geriatr Psychiatry Neurol 25: , 240–255. |
[8] | Monroe TB , Beach PA , Bruehl SP , Dietrich MS , Rogers BP , Gore JC , Atalla SW , Cowan RL ((2017) ) The impact of Alzheimer’s disease on the resting state functional connectivity of brain regions modulating pain: A cross sectional study. J Alzheimers Dis 57, 57: , 71–83. |
[9] | Monroe TB , Fillingim RB , Bruehl SP , Rogers BP , Dietrich MS , Gore JC , Atalla SW , Cowan RL ((2018) ) Sex differences in brain regions modulating pain among older adults: A cross-sectional resting state functional connectivity study. Pain Med 19: , 1737–1747. |
[10] | Monroe TB , Gibson SJ , Bruehl SP , Gore JC , Dietrich MS , Newhouse P , Atalla S , Cowan RL ((2016) ) Contact heat sensitivity and reports of unpleasantness in communicative people with mild to moderate cognitive impairment in Alzheimer’s disease: A cross-sectional study. BMC Med 14: , 74. |
[11] | Romano RR , Anderson AR , Failla MD , Dietrich MS , Atalla S , Carter MA , Monroe TB ((2019) ) Sex differences in associations of cognitive function with perceptions of pain in older adults. J Alzheimers Dis 70: , 715–722. |
[12] | Najm R , Jones EA , Huang Y ((2019) ) Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer’s disease. Mol Neurodegener 14: , 24. |
[13] | Safieh M , Korczyn AD , Michaelson DM ((2019) ) ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med 17: , 64. |
[14] | Yamazaki Y , Zhao N , Caulfield TR , Liu CC , Bu G ((2019) ) Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat Rev Neurol 15: , 501–518. |
[15] | Maehlen MT , Provan SA , de Rooy DP , van der Helm-van Mil AH , Krabben A , Saxne T , Lindqvist E , Semb AG , Uhlig T , van der Heijde D , Mero IL , Olsen IC , Kvien TK , Lie BA ((2013) ) Associations between APOE genotypes and disease susceptibility, joint damage and lipid levels in patients with rheumatoid arthritis. PLoS One 8: , e60970. |
[16] | Mahley RW , Rall SC Jr. ((2000) ) Apolipoprotein E: Far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1: , 507–537. |
[17] | Verghese PB , Castellano JM , Holtzman DM ((2011) ) Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol 10: , 241–252. |
[18] | Ward A , Crean S , Mercaldi CJ , Collins JM , Boyd D , Cook MN , Arrighi HM ((2012) ) Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 38: , 1–17. |
[19] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[20] | Hollingshead AB ((1975) ) Four factor index of social status. Unpublished manuscript, Yale University, New Haven, CT. |
[21] | Cleeland CS , Ryan KM ((1994) ) Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 23: , 129–138. |
[22] | Hamilton M ((1960) ) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: , 56–62. |
[23] | Spielberger CD ((1971) ) Notes and comments trait-state anxiety and motor behavior. J Motor Behav 3: , 265–279. |
[24] | Cole LJ , Farrell MJ , Duff EP , Barber JB , Egan GF , Gibson SJ ((2006) ) Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain 129: , 2957–2965. |
[25] | Hunt SP , Koltzenburg M ((2005) ) The Neurobiology of Pain: (molecular and Cellular Neurobiology), Oxford University Press. |
[26] | Wager TD , Rilling JK , Smith EE , Sokolik A , Casey KL , Davidson RJ , Kosslyn SM , Rose RM , Cohen JD ((2004) ) Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303: , 1162–1167. |
[27] | Petzke F , Harris RE , Williams DA , Clauw DJ , Gracely RH ((2005) ) Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain 9: , 325–335. |
[28] | Oragene Discover, DNAgenotek, https://www.dnagenotek.com/US/products/collection-human/oragene-discover/500-series/OGR-575.html, |
[29] | TaqMan Assays for every qPCR application, ThermoFisher Scientific, https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-assays.html#applications |
[30] | Johannes CB , Le TK , Zhou X , Johnston JA , Dworkin RH ((2010) ) The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain 11: , 1230–1239. |
[31] | Scherder EJ , Sergeant JA , Swaab DF ((2003) ) Pain processing in dementia and its relation to neuropathology. Lancet Neurol 2: , 677–686. |
[32] | Stubbs B , Thompson T , Solmi M , Vancampfort D , Sergi G , Luchini C , Veronese N ((2016) ) Is pain sensitivity altered in people with Alzheimer’s disease? A systematic review and meta-analysis of experimental pain research. Exp Gerontol 82: , 30–38. |



