Assessing the Progression of Alzheimer’s Disease in Real-World Settings in Three European Countries
Abstract
Background:
There exists considerable variation in disease progression rates among patients with Alzheimer’s disease (AD).
Objective:
The primary objective of this observational study is to assess the progression of AD by characterizing cognitive, functional, and behavioral changes during the follow-up period between 6 and 24 months.
Methods:
A longitudinal prospective study with community-dwelling patients with an established clinical diagnosis of AD of mild to moderate severity was conducted in Germany, Spain and the UK. A sample of 616 patients from 69 sites was included.
Results:
Patients had a mean of 1.9 years (SD = 1.9) since AD diagnosis at study inclusion. Cognitive symptoms were reported to have first occurred a mean of 1.1 years (SD = 1.7) prior to AD diagnosis and 1.4 (SD = 1.8) years prior to AD treatment. Patients initially diagnosed with mild and moderate AD spent a median (95%CI) of 3.7 (2.8; 4.4) and 11.1 (6.1, ‘not reached’) years until progression to moderate and severe AD, respectively, according to the Mini-Mental State Examination (MMSE) scores. A mixed model developed for cognitive, functional, and neuropsychiatric scores, obtained from study patients at baseline and during follow-up period, showed progressive deterioration of AD patients over time.
Conclusion:
The study showed a deterioration of cognitive, functional, and neuropsychiatric functions during the follow-up period. Cognitive deterioration was slightly faster in patients with moderate AD compared to mild AD. The duration of moderate AD can be overestimated due to the use of retrospective data, lack of availability of MMSE scores in clinical charts and exclusion of patients at time of institutionalization.
INTRODUCTION
The worldwide prevalence of dementia is rapidly increasing as higher life expectancy rates continue to expand the proportion of the elderly (≥65 years) in the general population. The prevalence of Alzheimer’s disease (AD), the most common type of dementia, contributes to 50–75%of all dementia cases worldwide [1]. Currently, around 40–50 million people globally are living with dementia, with the numbers forecasted to double every two decades, and eventually expected to exceed 100 million by 2050 [2, 3]. Results from two recent meta-analyses suggest overall prevalence of dementia in Europe is 5–7%with variation observed across countries [4, 5]. National studies performed in the United Kingdom (UK) and Spain provide similar estimates in term of prevalence of dementia, around 6–8%in the UK [6] and 5%in Spain [7]. As of 2012, Germany had 1.4 million dementia patients and was one of the top 10 countries with the highest number of dementia cases worldwide [8]. Further evidence forecasts a 40%increase in dementia prevalence in Europe by 2030 [9]. AD results in an increasing burden to society that rises in direct proportion to the number of elderly persons in the population. Earlier identification, prevalence of comorbidities, and treatments may affect AD trajectory and outcomes, including resource utilization and healthcare costs [10, 11].
A mix of genetic, vascular, and lifestyle factors (e.g., age, smoking, low educational attainment, head injuries, diabetes, obesity, depression, and apolip-oprotein (APOE) ɛ4 are associated with increased risk of AD: and, statin use, light to moderate alcohol intake, physical and cognitive activities, social engagement, Mediterranean diet, and APOE ɛ2 are associated with decreased risk) modulate the risk of developing AD [9, 12–15]. A progressive decline in cognitive, behavioral, and functional skills is a key characteristic of AD, which helps in establishing a clinical diagnosis. Generally, functional impairment follows cognitive decline; however, there is considerable variability in progression rates among AD patients [16]. There are several studies performed to assess risk factors associated to rapid AD progression with variability of results. A recent meta-analysis to assess the risk factors associated to rapid cognitive decline concluded that APOE ɛ4, early onset, early appearance of extrapyramidal signs, high education level, and neuropsychiatric conditions might increase the risk of rapid cognitive decline while older age, diabetes, and multidrug therapy decreased the speed of cognitive decline in AD [17]. Earlier identification of risk factors of AD, some of which are modifiable, may alter the course of disease, which may expand personal autonomy and reduce socioeconomic and caregiver burden of AD.
Prospective observational studies are needed to characterize disease progression and transition points in terms of major care modifications, e.g., introduction of professional care and institutionalization. This may reflect on associated costs of care in this patient population according to the current medical practice.
The primary objective of this observational study is to assess the progression of AD over time, assessed by changes in patients’ cognitive and functional impairment, and behavioral symptoms over a follow-up period between 6 and 24 months.
MATERIALS AND METHODS
Study design and participants
A longitudinal prospective cohort study with primary data collection of community-dwelling patients (aged≥50 years) with an established clinical diagnosis of AD of mild to moderate severity was conducted in Germany, Spain, and the UK. A total of 616 pat-ients were enrolled at 69 different study sites along with their primary caregivers (defined as spending at least 7 hours a week caring for the patient) thr-ough neurologists, psychiatrists, and other specialists regularly managing AD patients. Diagnosis and clinical management of AD was made according to physicians’ routine clinical practice. Classification of AD severity was made according to the National Institute for Health and Clinical Excellence (NICE) clinical guideline’s classification [18] based on the Mini-Mental State Examination (MMSE) score (categorized as mild AD: 21–26 points; moderate AD: 10–20 points) [19, 20]. The Standardized MMSE incorporated explicit guidelines and instructions for administration and scoring of each item which improved reliability by reducing test-retest variance and inter-observer variance [21, 22].
Incident and prevalent mild to moderate AD patients were included in the study between October 2016 and December 2017. Study inclusion required that a reliable informant/caregiver agreed to attend study visits and answer questions pertaining to the patient. Additionally, patients had to be fluent and literate in the main language of the country of residence. Patients with a diagnosis of mild cognitive impairment, non-AD dementia, or patients without primary caregivers were excluded from the study. Patients were followed to assess cognitive and functional impairment and neuropsychiatric behavioral progression for a period of between 6 and 24 months after the enrolment of the first patient in the country. Follow-up period in each country was finalized once the first patient included in that country had reached the 24 months follow-up period. Institutional review boards (IRBs) approved the research protocol and written informed consent was collected from each patient/caregiver dyad.
At the baseline visit, socio-demographic information (for patient and caregiver), patient’s medical history, comorbidities and treatment history, family history of dementia, mild and or moderate AD diagnosis date, current medication and non-medication treatment interventions for AD were collected from patients’ clinical charts. All retrospective data prior to baseline (patient’s medical history, AD diagnosis date, family history, and prior medications used) were abstracted from patients’ charts. The same measures, with the exception of retrospective data, were collected at each 6-month visit (with a visit window of ±3 months). The assessment of patients’ cognitive and functional impairment and neuropsychological behavioral symptoms was performed at baseline and every 6 months using a unique battery of clinical outcome assessment (COA) instruments, that included the MMSE and the AD Assessment Scale-Cognitive subscale (ADAS-Cog) to measure cognitive impairment, the Neuropsychiatric Inventory-12 item scale (NPI-12) to address the presence and extent of neuropsychiatric behavioral symptoms [23], and the AD Cooperative Study-Activities of Daily Living Inventory 23-item (ADCS-ADL23) to measure functional impairment [24]. Although MMSE and ADAS-Cog have some limitations when being used in patients with mild AD due to ceiling effect, they are considered the gold standard measures for cognitive impairment assessment.
Statistical analysis
Statistical analyses were performed using SAS 9.2 (SAS Institute, NC, USA) software. Missing data has been described, but imputation methods have not been applied to maintain the use of real-world data. Descriptive statistics are presented by overall patient population and by AD severity at baseline (mild versus moderate). Comparisons by AD severity were made using Pearson χ2 test for categorical variables and Wilcoxon signed-rank sum test for continuous variables, using α= 0.05 as the significance level. Due to the variability in the follow-up period amongst study patients and successive follow-up visits with fewer and fewer patients, follow-up data, including changes in the cognitive, functional, and neuropsychiatric scores from baseline, concentrate on 12- and 18-month data.
Disease progression was assessed according to the changes obtained in MMSE scores during the follow-up period. Date of initial AD diagnosis and date of moderate AD diagnosis (if applicable) were collected and used to define time to progression. As initial or moderate AD diagnosis could have happened prior to the baseline visit, the time to progression could be longer than the current study follow-up period. Based on MMSE, disease progression in mild patients was defined when they reached the first MMSE score corresponding to moderate disease (10–20), and in moderate patients it was defined when they reached the first MMSE score corresponding to severe (< 10). Time to disease progression was assessed using Kaplan-Meier curves.
Finally, longitudinal data in each outcome (MMSE, ADAS-Cog, ADCS-ADL23, and NPI-12) was analyzed using mixed models for repeated measures (MMRM) to ascertain the significance of difference in the average levels of the study variable across time by AD status. Mixed models made use of all available longitudinal data and accommodated unequal numbers of observations per subject and unequal intervals between follow-up assessments. Adjustment for putative confounders such as age, gender, and others were considered. Model diagnostics such as Akaike Information Criteria (AIC) were used to assess the model fit.
RESULTS
Baseline characteristics
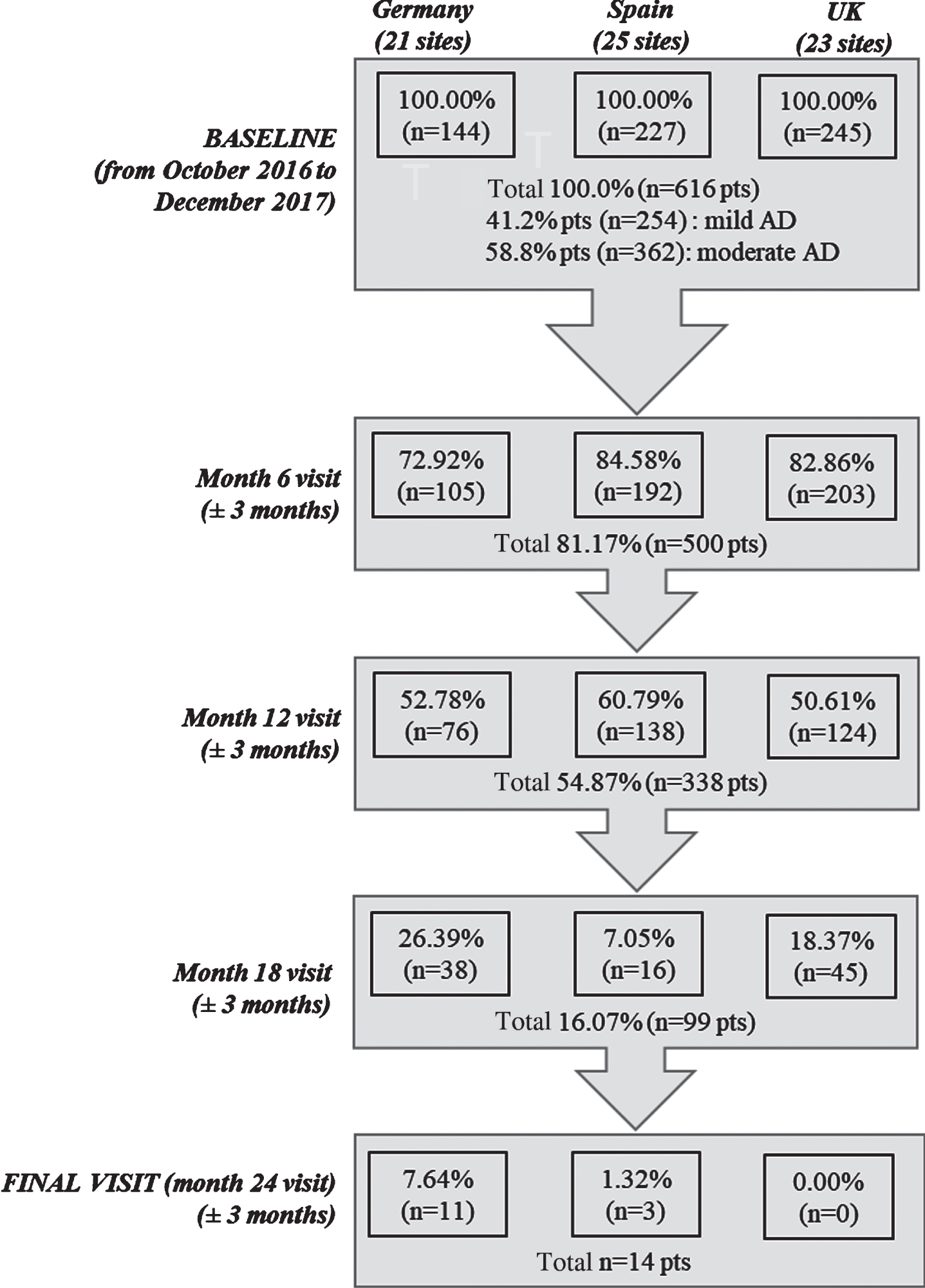
Overall, 616 mild to moderate AD patients and their caregivers (dyads) were included in the study, with 144 dyads enrolled from Germany (21 sites), 227 from Spain (25 sites), and 245 from the UK (23 sites). At baseline, 41.2%of patients were reported to have mild AD and 58.8%had moderate disease. Study follow-up in each country was stopped when the first patient in the corresponding country reached the 24 months of follow-up, as per study protocol. By the time that study was terminated, five-hundred patients (81.2%) participated in the month 6 visit, 338 (54.9%) at month 12, and 99 (16.1) at month 18. A total of 131 patients (21.2%) discontinued the study, excluding those patients who did not achieve the 24 months follow-up due to study termination. Commonly reported reasons for discontinuation inc-luded withdrawal of patient/legal representative or physician consent (25%), loss to follow-up (18%), permanent institutionalization (17%), death (16%), or other reasons (24%) (Fig. 1).
Fig. 1
Study flowchart. n, number of patients; pts, patients.
Patients recruited in the three countries had an overall mean age of 77.5 years (SD = 7.0) (Table 1). More than half of the patients were female, and the majority were not employed/retired (86.0%) and lived with a spouse/partner (75.3%). About one third of patients had a reported history of smoking and < 3%reported current smoking. Prevalence of concomitant diseases (occurring in > 10%of the population) included: hypertension (43.0%), diabetes mellitus (15.3%), hypercholesterolemia (14.6%), and depression (14.5%). One third of patients (33.9%) reported a family history of AD (Tables 1 and 2). Across the three countries, patients did not differ in age, had a similar employment status (74–88%were not employed/retired) and living situation (70–80%lived with their spouse/partner), the gender ratio was similar in Spain and Germany; however in the UK, more males were recruited (56.3%versus 43.7%).
Table 1
Baseline characteristics of patients by AD severity (mild and moderate)
| Characteristic | Parameter | Moderate [10–20] (N = 362) | Mild [21–26] (N = 254) | Overall population (N = 616) |
| Gender, n (%) | Female | 210 (58.01%) | 119 (46.85%) | 329 (53.41%) |
| Age, mean (SD) | Age in years | 77.43 (7.26) | 77.47 (6.71) | 77.45 (7.03) |
| Education level, n (%) | No formal education | 21 (5.80%) | 3 (1.18%) | 24 (3.90%) |
| Primary (1–6 years of education) | 101 (27.90%) | 41 (16.14%) | 142 (23.05%) | |
| Secondary/technical (7–13 years of education) | 163 (45.03%) | 146 (57.48%) | 309 (50.16%) | |
| University/higher education (greater than 13 years of education) | 65 (17.96%) | 58 (22.83%) | 123 (19.97%) | |
| Other | 7 (1.93%) | 6 (2.36%) | 13 (2.11%) | |
| Declined to answer | 2 (0.55%) | 0 (0.00%) | 2 (0.32%) | |
| Main working status (Former), n (%) | Full-time | 33 (9.12%) | 29 (11.42%) | 62 (10.06%) |
| Part-time | 7 (0.93%) | 8 (3.15%) | 15 (2.44%) | |
| Not employed/retired | 316 (87.29%) | 214 (84.25%) | 530 (86.04%) | |
| Declined to answer | 3 (0.83%) | 3 (1.18%) | 6 (0.97%) | |
| Other persons living with the patient (including caregiver if relevant), n (%)* | None, patient lives alone | 44 (12.15%) | 41 (16.14%) | 85 (13.80%) |
| Spouse or partner | 270 (74.59%) | 194 (76.38%) | 464 (75.32%) | |
| Other adults | 53 (14.64%) | 26 (10.24%) | 79 (12.82%) | |
| Children < 18 years of age | 7 (1.93%) | 3 (1.18%) | 10 (1.62%) | |
| Smoking status, n (%) | Current smoker | 7 (1.93%) | 10 (3.94%) | 17 (2.76%) |
| Ex-smoker | 109 (30.11%) | 79 (31.10%) | 188 (30.52%) | |
| Non-smoker | 236 (65.19%) | 155 (61.02%) | 391 (63.47%) | |
| Common chronic concomitant diseases, n (%)** | Hypercholesterolemia | 52 (14.36%) | 38 (14.96%) | 90 (14.61%) |
| Diabetes mellitus | 50 (13.81%) | 44 (17.32%) | 94 (15.26%) | |
| Hypertension | 149 (41.16%) | 116 (45.67%) | 265 (43.02%) | |
| Depression | 58 (16.02%) | 31 (12.20%) | 89 (14.45%) | |
| Outcome measures, mean score (SD) | MMSE | 16.65 (2.83) | 23.33 (1.68) | 19.40 (4.09) |
| ADAS-Cog | 33.82 (8.37) | 24.65 (6.47) | 30.04 (8.87) | |
| ADCS-ADL23 | 46.31 (15.80) | 54.41 (15.06) | 49.65 (15.99) | |
| NPI-12 | 13.19 (14.07) | 11.20 (12.32) | 12.37 (13.41) |
*Multi-response. **Chronic concomitant diseases reported in > 10%of the overall study population have been presented. SD, standard deviation; N, number; MMSE, Mini-Mental State Examination; ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive subscale; ADCS-ADL23, Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory-23 item; NPI-12, Neuropsychiatric Inventory-12 item.
Table 2
Initiation of cognitive symptoms, diagnosis of AD and treatment initiation by AD severity (mild and moderate)
| Characteristic | Parameter | Moderate [10–20] (N = 362) | Mild [21–26] (N = 254) | Overall population (N = 616) |
| Years between initiation of cognitive symptoms and AD diagnosis | Mean (SD) | 1.13 (1.60) | 1.16 (1.77) | 1.14 (1.67) |
| Range (min-max) | (0.00, 10.45) | (0.00, 11.34) | (0.00, 11.34) | |
| Years between initiation of cognitive symptoms and AD treatment | Mean (SD) | 1.45 (1.87) | 1.30 (1.71) | 1.39 (1.80) |
| Range (min-max) | (0.00, 13.53) | (0.00, 11.34) | (0.00, 13.53) | |
| Years between AD diagnosis and study inclusion | Mean (SD) | 2.10 (2.06) | 1.52 (1.71) | 1.86 (1.94) |
| Range (min-max) | (0.00, 11.10) | (0.00, 10.78) | (0.00, 11.10) | |
| Familial history of AD Criteria used to establish AD diagnosis | Reported | 126 (34.81%) | 83 (32.68%) | 209 (33.93%) |
| NINCDS-ADRDA | 207 (57.18%) | 133 (52.36%) | 340 (55.19%) | |
| DSM-IV | 99 (27.35%) | 94 (37.01%) | 193 (31.33%) | |
| AAN | 11 (3.04%) | 8 (3.15%) | 19 (3.08%) | |
| AHRQ | 5 (1.38%) | 6 (2.36%) | 11 (1.79%) | |
| DemTect | 33 (9.12%) | 28 (11.02%) | 61 (9.90%) | |
| Use of biomarkers or imaging tests since diagnosis | No biomarker or imaging test performed since diagnosis | 180 (49.72%) | 145 (57.09%) | 325 (52.76%) |
| At least one biomarker or imaging test performed since diagnosis | 155 (42.82%) | 99 (38.98%) | 254 (41.23%) | |
| At least one biomarker test performed since diagnosis*1 | 20 (12.9%) | 9 (9.09%) | 29 (11.42%) | |
| CSF Aβ42† | 7 (4.52%) | 2 (2.02%) | 9 (3.54%) | |
| CSF total tau† | 5 (3.23%) | 2 (2.02%) | 7 (2.76%) | |
| CSF phosphorylated tau† | 4 (2.58%) | 3 (3.03%) | 7 (2.76%) | |
| Other biomarkers† | 13 (8.39%) | 6 (6.06%) | 19 (7.48%) | |
| At least one imaging test performed since diagnosis**1 | 146 (94.19%) | 93 (93.94%) | 239 (94.09%) | |
| sMRI or fMRI† | 67 (43.23%) | 30 (30.91%) | 94 (38.19%) | |
| PIB Aβ PET† | 3 (1.94%) | 1 (1.01%) | 4 (1.57%) | |
| FDG PET† | 13 (8.39%) | 11 (11.11%) | 24 (9.45%) | |
| CT† | 79 (50.97%) | 59 (59.6%) | 138 (54.33%) | |
| Unknown | 27 (7.46%) | 10 (3.94%) | 37 (6.01%) |
*Biomarker tests include: Cerebrospinal fluid (CSF) Aβ42, CSF total tau, CSF phosphorylated tau and Other biomarkers. **Imaging test include: structural or functional magnetic resonance imaging (sMRI or fMRI), Pittsburgh compound B (PIB) Aβ positron emission tomography (PET) Fluorodeoxyglucose (18F) PET (FDG PET) and computed tomography (CT). †Multichoice option; 1Percentages calculated over the number of patients with at least one biomarker or imaging test performed.
Patients had a mean of 1.9 years (SD = 1.9) since AD diagnosis at baseline (study inclusion), with moderate AD patients having slightly longer duration than mild AD patients (a mean of 2.1 versus 1.5 years, respectively) (Table 2). Cognitive symptoms were reported to have first occurred a mean of 1.1 years (SD = 1.7) prior to AD diagnosis and 1.4 (SD = 1.8) prior to AD treatment. Physicians predominately used NINCDS-ADRDA (55.2%) and DSM-IV (31.3%) criteria to diagnose patients with AD. The use of at least one biomarker or imaging test since AD diagnosis was reported in 41.2%of patients, with the computed tomography (54.33%) and structural or functional magnetic resonance (38.19%) being the most commonly used tests among those patients that had at least one test performed since AD diagnosis (Table 2).
Cognitive, functional, and neuropsychiatric impairment progression by AD severity
At baseline, the mean (SD) MMSE score was 16.7 (2.8) among moderate AD patients and 23.3 (1.7) among patients with mild AD (p < 0.01), obtaining similar mean MMSE scores in all participant countries with mean scores ranging from 3.80 to 4.20 points (Fig. 2). Baseline scores also showed statistically significant differences (p < 0.01) between patients with mild and moderate AD in ADAS-Cog (24.7 (4.5) and 33.8 (8.4), respectively) and ADCS-ADL23 (54.4 (15.1) and 46.3 (15.8)) scores. Mean ADAS-Cog score was also similar across the countries, with mean ranging from 28.0 to 32.6 points. Figure 2 shows the evolution of unadjusted scores across the follow-up visits, showing a progressive deterioration in all scores. Baseline scores of MMSE, ADAS-Cog, ADCS-ADL23, and NPI-12 were compared missing according to the availability of follow-up data for each of these measures at 6, 12, and 18 months without showing statistically significant difference. Baseline cognitive function, cognitive impairment, functional deterioration, and neuropsychiatric deterioration at baseline was similar regardless of availability of follow-up data.
Fig. 2
Changes in MMSE, ADAS-Cog, ADCS-DL23, and NPI-12 scores over time from baseline stratifying by AD severity. Statistical comparisons performed to compare the change for the different tools between the different follow-up points. ***p < 0.001; **p < 0.01; *p < 0.05.
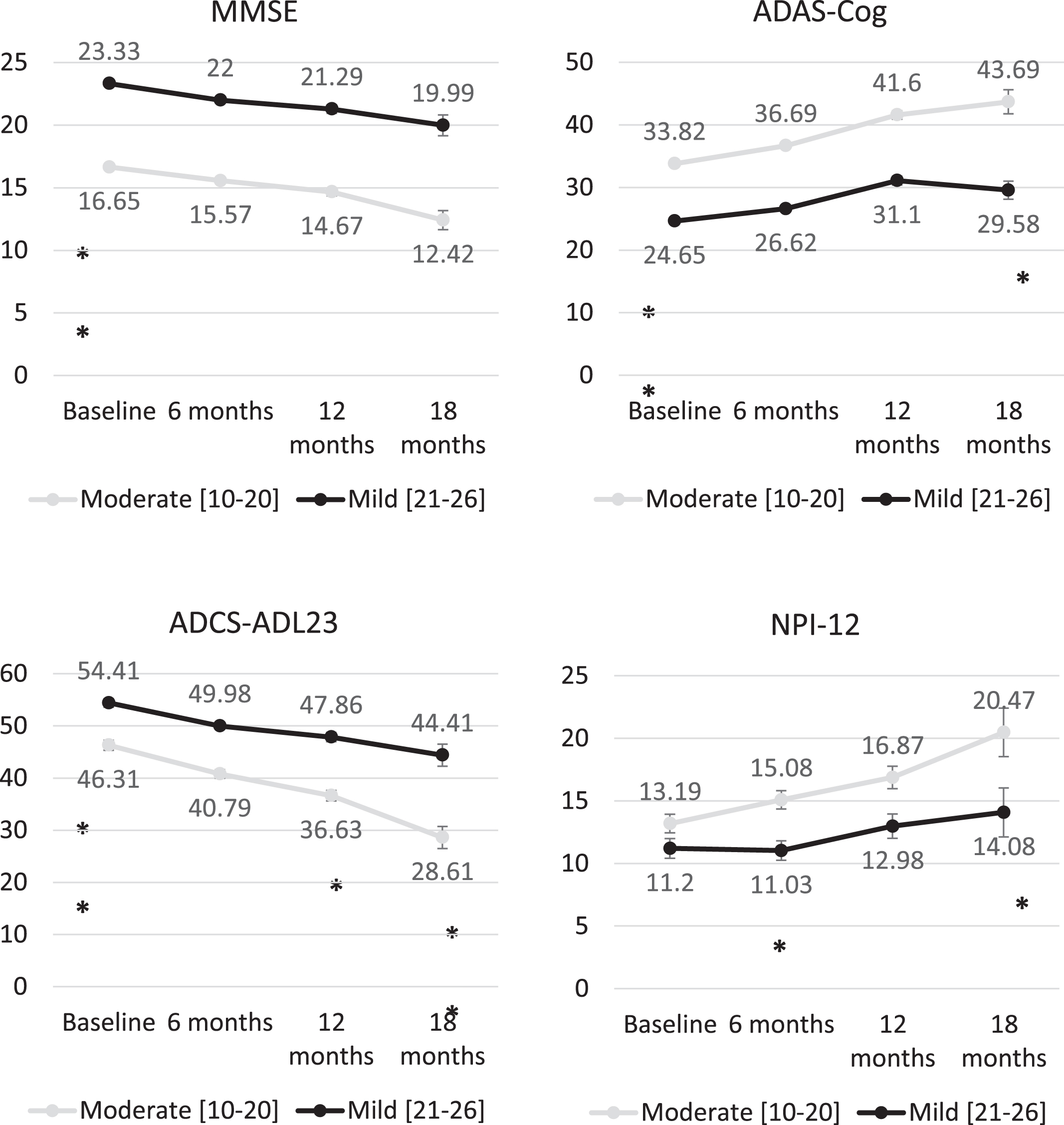
Table 3 shows the multivariable mixed model developed separately for cognitive, functional, and neuropsychiatric scores obtained from study patients at baseline and during whole study follow-up period. Patients from Germany had higher MMSE scores (p < 0.019), indicating better cognitive status, relative to patients from the UK. On the other hand, patients with unknown occupation (p < 0.01), those with physician-assessed moderate AD severity (p < 0.01), and patients with longer disease duration (time since diagnosis) had lower MMSE scores, corresponding to worse cognitive status. The model also shows a reduction of MMSE across the follow-up visits after adjusting for other covariates; with reductions of 1.1, 2.0, and 3.4 points at 6, 12, and 18 months (p < 0.01) respectively. The ADAS-Cog model shows a rapid change in scores across the follow-up period, increasing by 2.6, 7.9, and 8.0 points at 6, 12, and 18 months, respectively. Lower ADAS-Cog scores (indicating better cognitive status) were estimated for Germany (relative to the UK), for female patients, and for patients at higher age groups, as well as for patients with mild disease (versus moderate) according to physician assessment and patients with longer AD evolution. Changes over time in ADC-ADL23 and NPI-12 were similar to those obtained for MMSE, showing a gradual deterioration over the successive 6-month time periods assessed in the study.
Table 3
Mixed models for repeated measures to assess parameters related with MMSE, ADAS-Cog, ADC-ADL23, and NPI-12 total score
| Parameter (reference category) Intercept | Class | MMSE estimate (p) 20.962 (< 0.001) | ADAS-Cog estimate (p) 27.120 (< 0.001) | ADC-ADL23 estimate (p) 60.325 (< 0.001) | NPI-12 estimate (p) 10.703 (< 0.001) |
| Country (UK) | Germany | 1.756 (< 0.001) | –2.396 (0.008) | –4.963 (0.001) | |
| Spain | 0.017 (0.968) | 2.525 (0.002) | –3.954 (0.003) | ||
| Gender (male) | Female | – | –1.127 (0.103) | 4.808 (< 0.001) | –2.329 (0.028) |
| Age (< 73 years old) | Age 73–82 | – | –2.020 (0.017) | –2.517 (0.073) | |
| Age > 82 | – | –2.074 (0.040) | –6.795 (< 0.001) | ||
| Occupation (former) (primary or secondary sector) | Other/unknown | –1.323 (0.005) | – | – | |
| Tertiary sector | 0.200 (0.655) | – | – | ||
| Working status (retired) | Full-time | – | – | – | –2.662 (0.104) |
| Part-time | – | – | – | –7.377 (0.019) | |
| Not employed | – | – | – | –1.634 (0.283) | |
| Declined to answer | – | – | – | –0.252 (0.961) | |
| Height (cm) (> 172 cm) | 159–172 cm | – | – | 3.014 (0.031) | – |
| < 159 cm | – | – | 1.343 (0.482) | – | |
| Caregiver relationship(adult child) | Adult grandchild | 2.512 (0.242) | – | – | – |
| Close friend | 2.415 (0.209) | – | – | – | |
| Distant relative | 7.152 (0.021) | – | – | – | |
| Other | 1.428 (0.207) | – | – | – | |
| Sibling | 0.241 (0.832) | – | – | – | |
| Spouse/partner | 0.059 (0.889) | – | – | – | |
| Physician opinion about severity of AD (mild) | Moderate (including moderately severe) | –3.038 (< 0.001) | 7.193 (< 0.001) | –9.886 (< 0.001) | 2.140 (0.033) |
| AD diagnosis criteria DSM-IV | Yes | – | – | –2.651 (0.027) | – |
| Visit number (baseline) | Follow-up visit 1 | –1.132 (< 0.001) | 2.641 (< 0.001) | –4.719 (< 0.001) | 1.047 (0.051) |
| Follow-up visit 2 | –1.975 (< 0.001) | 7.895 (< 0.001) | –8.386 (< 0.001) | 2.546 (< 0.001) | |
| Follow-up visit 3 | –3.388 (< 0.001) | 7.992 (< 0.001) | –12.498 (< 0.001) | 3.564 (< 0.001) | |
| Time since clinical diagnosis of AD (< 0.65 years) | 0.65 to 2.61 years | –0.280 (0.503) | 0.985 (0.233) | –3.482 (0.011) | 2.760 (0.021) |
| > 2.61 years | –1.342 (0.006) | 3.563 (< 0.001) | –8.763 (< 0.001) | 4.189 (0.003) |
Values reported in the table correspond to correlation coefficient (p-value).
Progression of AD (according to MMSE)
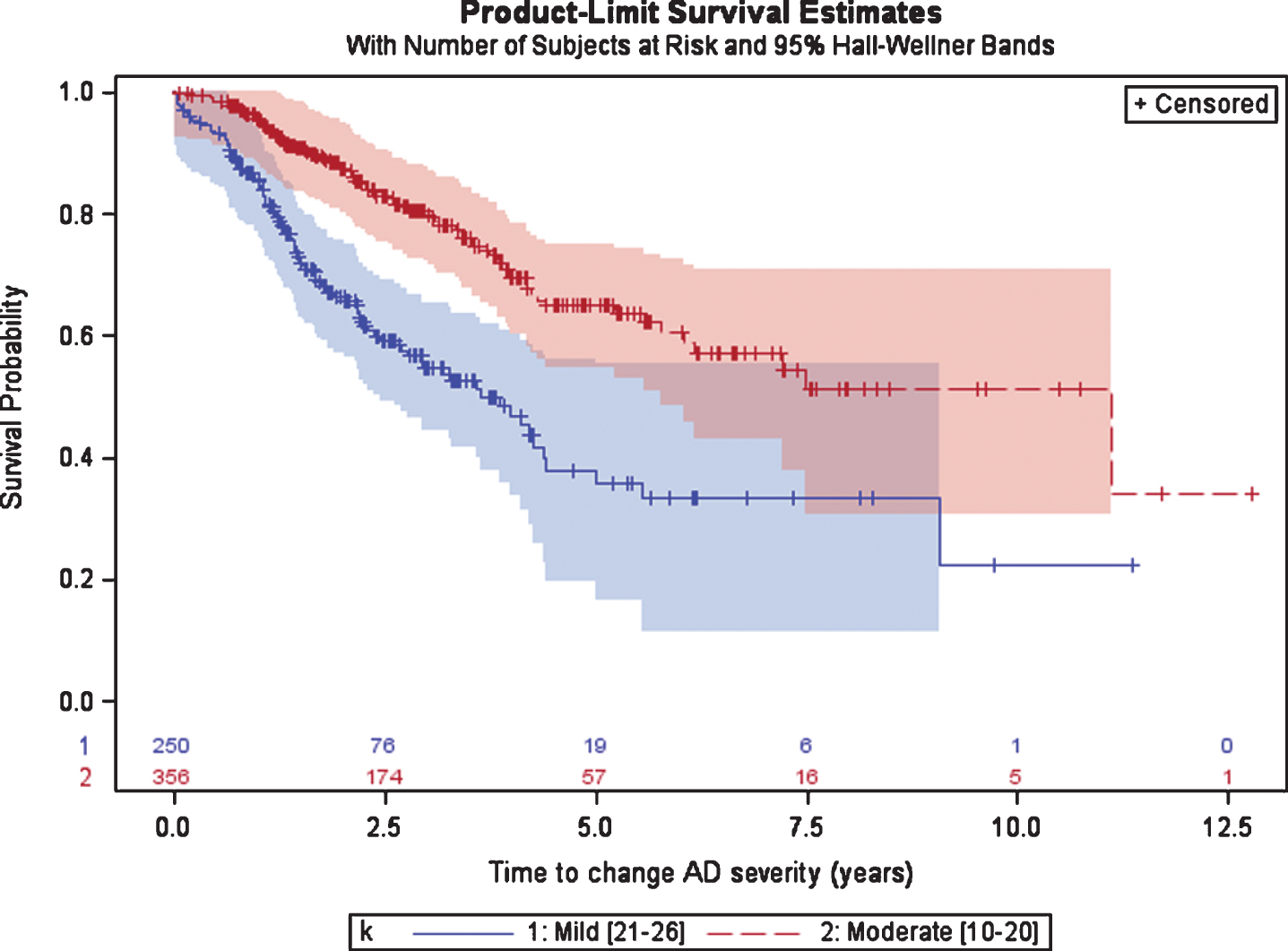
Patients initially diagnosed with mild AD spent a median (95%CI) of 3.7 (2.8; 4.4) years until progression to moderate AD as measured by the MMSE (Fig. 3). After diagnosis of moderate AD (either at the date of the first AD diagnosis or after a disease progression from mild to moderate) patients had a median of 11.1 years until progression to severe AD (MMSE < 10). The 95%CI for patients with moderate AD was not calculated given that more than 25%of patients did not progress to severe AD in the last visit.
Fig. 3
Time from diagnosis of mild or moderate AD to disease progression, defined as the first change in AD severity based on MMSE scale.
DISCUSSION
The current study provides an overview of the clinical course of mild to moderate AD including valuable data in terms of diagnosis and disease progression in patients treated according to routine clinical practices in three European countries.
Demographic characteristics of patients included in the current study, such as age, gender, and marital status/cohabitation, were observed to be similar to those collected in other longitudinal prospective studies conducted in the European population on mild-moderate AD [10, 26, 27]. Educational level observed in the study sample is also aligned with educational levels reported in participant countries for subjects 55 to 65 years old [28]. The same comparison for older populations is not possible due to lack of reference data in all participant countries. These results may suggest that the patients’ sample analyzed in the study would be representative of the general population affected by AD. The prevalence of vascular comorbidities (like hypertension, diabetes mellitus, and hypercholesterolemia), depression, and smoking history is also aligned with estimations presented in other studies [11, 27–29], These concomitant diseases represent established risk factors for AD incidence and may even be predictors of AD progression. Control and management of these risk factors offers a potential avenue for slowing down the progression of AD [30]. A recent systematic review and meta-analysis established high levels of low-density lipoprotein cholesterol as an AD risk factor [31]. In the present study, patients with hypercholesterolemia showed a lower deterioration during the one-year period, but differences did not reach the statistical significance in the regression models. The fact that the present study measured only total cholesterol instead of low-density lipoprotein cholesterol could explain these results not aligned with prior studies.
Prospective data collected in the current study de-monstrate the cognitive, functional, and neuropsychi-atric deterioration of patients with AD, even though the deterioration in these scales is also associated with other potential risk factors, such as living in the country, age, gender, AD severity, or time of AD evolution, among others. Cognitive impairment (ass-essed using MMSE and ADAS-Cog scores), functional impairment (assessed using the ADCS-ADL), and the neuropsychiatric impairment (assessed using the NPI-12) of patients with AD increased over the course of the study with a gradual deterioration over the successive 6-month time periods assessed in the study. MMSE scores decreased by 1.1, 2.0, and 3.4 points at 6, 12, and 18 months respectively, and ADAS-Cog scores increased by 2.6, 7.9, and 8.0 points at 6, 12, and 18 months; corresponding to a deterioration in both scales. The definition of change in MMSE considered clinically meaningful varies in literature, but it is generally accepted as an average annual decline of 1.4-4 points [32–34], with the rate of decline potentially depending on AD medication use. Comparable observational studies like the REseau sur la maladie d’Alzheimer FRançais (REAL.FR) cohort in France have also reported average declines of 2.4 points per year on the MMSE, 4.5 points on the ADAS-Cog, a worsening of ADL and NPI scores over time, and a variable rate of AD progression among subjects [34].
In the European ICTUS study, cognitive function declined non-linearly over time (MMSE: –1.5 points/first year, –2.5 points/second year; ADAS-Cog: +3.5 points/first year, + 4.8 points/second year), while the progression of behavioral disturbances (NPI scale) was linear [26]. In the initial CERAD population of AD patients, when acetylcholinesterase inhibitors were not available, an average annual decline of 3.4 points was obtained for the MMSE [33]. There was wide variability in individual rates of decline. Even with 4 years of follow-up, 15.8%of the patients had no clinically meaningful decline in MMSE score [33]. Schrag et al. also assessed changes in ADAS-Cog during a 6-month period and patients obtained mean changes of 3.1–3.8 points in ADAS-Cog domains [36]. The overall population in our study followed this trend of cognitive decline.
Despite the common use of acetylcholinesterase inhibitors and memantine, patients diagnosed with AD still have a clear unmet medical need, given that they are still showing an inevitable deterioration of cognitive, functional, and neuropsychiatric functions over time. Our study provides data about delays in AD diagnosis and treatments’ initiation, given that a specialist diagnosis occurred on average of 1.1 after symptoms occurrence and treatment was initiated a mean of 1.4 years after symptoms occurrence. Timely identification of AD will allow early treatment initiation, which may expand personal autonomy and reduce socioeconomic and caregiver burden [37]. The use of biomarkers for AD diagnosis is still uncommon in routine clinical practice and depends very much on specialist care. In our study, biomarkers and imaging tests’ employment was reported for 4.7%and 38.8%, respectively, of patients included in the study taking into account the period between diagnosis and baseline visit (a mean of 1.9 years after AD diagnosis). The identification of biomarkers and imaging tests for early diagnosis and the adherence to current neurology guidelines may thus be crucial for early intervention and identification of high-risk subjects [38]. Nevertheless, the possibility that the included patients have a biomarker or imaging tests performed prior to the AD diagnosis and not reported in the clinical chart should be considered as a potential bias to underestimate the use. In general, medical practice in Germany, only 34%of incident dementia cases had at least one contact with a neuropsychiatrist during the year of incidence. Only a minority (13.5%) of dementia patients was referred to radiology for imaging [39].
Finally, the current study also provides data about disease progression, where patients with moderate AD stay longer at this stage compared with patients with mild AD. Differences in trends of time to disease progression according to AD severity has been shown among studies [16, 26], The multivariable models performed in the study did not identify significant risk factors associated with disease progression except for age or study country. The differences obtained among participant countries could be associated to selection bias of the participant sites and patients in each country.
The study was not designed for a 24-month follow-up, and main limitation in this study was related to discontinuation (21%in the overall sample) and study finalization after the 24 months of inclusion of the first patients in each country. However, the lack of follow-up visits due to study finalizations is not related to patient characteristics and it is not expected to bias the study results. Due to the high number of drops-out after 1 year of follow-up, there were a high number of censured data in disease progression, especially between patients with moderate AD. The high attrition rate is not unique to this study and is a common finding associated with longitudinal observational studies on AD. For instance, the multicenter prospective ICTUS [26] and REAL.FR [25] studies reported 31.5%and 40.5%drops-out after 2 years, respectively, and the reasons for discontinuations overlapped with the present study. Since AD is a progressive illness in mainly geriatric patients, factors attributed to aging and disease (e.g., death, institutionalization, loss of autonomy, etc.) are a frequently encountered causes of attrition in long-term studies [40]. Clinical trials can include, as interven-tion per protocol, further actions to reduce the attrition rate. But observational studies are limited to the routine clinical practice and higher attrition rate might be reached.
Despite the prospective design, the study also collected some data in a retrospective way based on clinical charts, most of them related to AD diagnosis (i.e., diagnostic tests used). Data collected using clinical charts is restricted to data available in clinical charts and it is impacted by limitations associated to retrospective data collection, e.g., limited use/documentation of standardized assessments. The collection of retrospective data relating to initial and moderate AD diagnosis allowed the use of survival analysis to describe time to progression and provide median values by severity. However, the criteria used to define disease progression in the retrospective data obtained from clinical charts might be different from the criteria used in the prospective part of the study. In addition, there are some sources of potential selection bias: the first is the fact that patients were included at any time during AD evolution can lead to a selection bias toward those with longer time in mild or moderate status, and the second is the fact that patient recruitment period took longer than initially expected. Finally, it is also important to mention that the inclusion of patients fluent in local language, in order to allow the response to the corresponding questionnaires, could limit the inclusion of subjects from certain communities or ethnicities, limiting the external validity of study results.
Currently, there is no cure for AD, and manage-ment of the disease is focused on symptomatic treatments and counseling to maintain functioning. In this context, larger real-world studies covering more geographical areas over a longer follow-up duration can help inform physicians on the pattern of AD progression and to understand factors that can help delay care dependency, reduce caregiver stress and burden and the socioeconomic burden on society.
This study allowed us to assess the natural history of AD due to the prospective design of the study with collection of a battery of cognitive, functional, and neuropsychiatric measures typically used as outcome parameters in clinical trials, and not usually collected in clinical practice. There was a typical deterioration of cognitive, functional, and neuropsychiatric functions during the follow-up period, and cognitive deterioration was slightly faster in patients with moderate AD compared to mild AD. The study showed a delay in the diagnosis and treatment initiation for AD patient, with negative consequences in an optimal early management of AD.
Limitations
The use of retrospective data, lack of availability of MMSE scores in clinical charts, and exclusion of patients at time of institutionalization should be considered in interpretation of the results. The duration of moderate AD can be overestimated due to limitations of the study.
ACKNOWLEDGMENTS
The authors thank the physicians involved in this project that contributed with data collection which made this study possible.
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1172r2).
REFERENCES
[1] | Atri A ((2019) ) The Alzheimer’s disease clinical spectrum: Diagnosis and management. Med Clin North Am 103: , 263–293. |
[2] | Prince M , Wimo A , Guerchet M , Ali G-C , Wu Y-T , Prina M ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Disease International, London. |
[3] | GBD2016 Dementia Collaborators ((2019) ) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: , 88–106. |
[4] | Niu H , Alvarez-Alvarez I , Guillen-Grima F , Aguinaga-Ontoso I ((2017) ) Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 32: , 523–532. |
[5] | Bacigalupo I , Mayer F , Lacorte E , Di Pucchio A , Marzolini F , Canevelli M , Di Fiandra T , Vanacore N ((2018) ) A systematic review and meta-analysis on the prevalence of dementia in europe: Estimates from the highest-quality studies adopting the DSM IV diagnostic criteria. J Alzheimers Dis 66: , 1471–1481. |
[6] | Matthews FE , Arthur A , Barnes LE , Bond J , Jagger C , Robinson L , Brayne C ((2013) ) A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. Lancet 382: , 1405–1412. |
[7] | Ponjoan A , Garre-Olmo J , Blanch J , Fages E , Alves-Cabratosa L , Marti-Lluch R , Comas-Cufi M , Parramon D , Garcia-Gil M , Ramos R ((2019) ) Epidemiology of dementia: Prevalence and incidence estimates using validated electronic health records from primary care. Clin Epidemiol 11: , 217–228. |
[8] | Doblhammer G , Fink A , Zylla S , Willekens F ((2015) ) Compression or expansion of dementia in Germany? An observational study of short-term trends in incidence and death rates of dementia between 2006/07 and 2009/10 based on German health insurance data. Alzheimers Res Ther 7: , 66–66. |
[9] | Prince M , Bryce R , Albanese E , Wimo A , Ribeiro W , Ferri CP ((2013) ) The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9: , 63–75.e62. |
[10] | Dodel R , Belger M , Reed C , Wimo A , Jones RW , Happich M , Argimon JM , Bruno G , Vellas B , Haroh JM ((2015) ) Determinants of societal costs in Alzheimer’s disease: GERAS study baseline results. Alzheimers Dement 11: (2015), 933–945. |
[11] | Reed C , Happich M , Argimon JP , Haro JM , Wimo A , Bruno G , Dodel R , Jones RW , Vellas B Belger M ((2017) ) What drives country differences in cost of Alzheimer’s disease? An explanation from resource use in the GERAS Study. J Alzheimers Dis 57: , 797–812. |
[12] | Hersi M , Irvine B , Gupta P , Gomes J , Birkett N , Krewski D ((2017) ) Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 61: , 143–187. |
[13] | Scheltens P , Blennow K , Breteler MM , de Strooper B , Frisoni GB , Salloway S , Van der Flier WM ((2016) ) Alzheimer’s disease. Lancet 388: , 505–517. |
[14] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[15] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[16] | Doody RS , Pavlik V , Massman P , Rountree S , Darby E , Chan W ((2010) ) Predicting progression of Alzheimer’s disease. Alzheimers Res Ther 2: , 2. |
[17] | Song YN , Wang P , Xu W , Li JQ , Cao XP , Yu JT , Tan L ((2018) ) Risk factors of rapid cognitive decline in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. J Alzheimers Dis 66: , 497–515. |
[18] | National Institute for Health and Clinical Excellence (2011) Final appraisal determination - Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (review of NICE technology appraisal guidance 111) |
[19] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state“. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[20] | Perneczky R , Wagenpfeil S , Komossa K , Grimmer T , Diehl J , Kurz A ((2006) ) Mapping scores onto stages: Mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry 14: , 139–144. |
[21] | Molloy DW , Guyatt GH , Wilson DB , Duke R , Rees L , Singer J ((1991) ) Effect of tetrahydroaminoacridine on cognition, function and behaviour in Alzheimer’s disease. CMAJ 144: , 29–34. |
[22] | Molloy DW , Standish TI ((1997) ) A guide to the standardized Mini-Mental State Examination. Int Psychogeriatr 9: , 143–50. |
[23] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[24] | Marshall GA , Amariglio RE , Sperling RA , Rentz DM ((2012) ) Activities of daily living: Where do they fit in the diagnosis of Alzheimer’s disease? Neurodegener Dis Manag 2: , 483–491. |
[25] | Herdman M , Gudex C , Lloyd A , Janssen M , Kind P , Parkin D , Bonsel G , Badia X ((2011) ) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20: , 1727–1736. |
[26] | Vellas B , Hausner L , Frölich L , Cantet C , Gardette V , Reynish E , Gillette S , Agüera-Morales E , Auriacombe S , Boada M , Bullock R , Byrne J , Camus V , Cherubini A , Eriksdotter-Jönhagen M , Frisoni GB , Hasselbalch S , Jones RW , Martinez-Lage P , Rikkert MO , Tsolaki M , Ousset PJ , Pasquier F , Ribera-Casado JM , Rigaud AS , Robert P , Rodriguez G , Salmon E , Salva A , Scheltens P , Schneider A , Sinclair A , Spiru L , Touchon J , Zekry D , Winblad B , Andrieu S ((2012) ) Progression of Alzheimer disease in Europe: Data from the European ICTUS study. Curr Alzheimer Res 9: , 902–912. |
[27] | Wimo A , Reed C , Dodel R , Belger M , Jones RW , Happich M , Argimon JM , Bruno G , Novick D , Vellas B , Haro JM ((2013) ) The GERAS Study: A prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries - study design and baseline findings. J Alzheimers Dis 36: , 385–399. |
[28] | Organisation for Economic Co-operation and Development ((2020) ) Education at a Glance 2020: OECD Indicators. OECD Publishing, Paris. |
[29] | Menéndez E , Delgado E , Fernández-Vega F , Prieto MA , Bordiú E , Calle A , Carmena R , Castaño L , Catalá M , Franch J , Gaztambide S , Girbés J , Goday A , Gomis R , López-Alba A , Martínez-Larrad MT , Mora-Peces I , Ortega E , Rojo-Martínez G , Serrano-Ríos M , Urrutia I , Valdés S , Vázquez JA , Vendrell J , Soriguer F ((2016) ) Prevalence, diagnosis, treatment, and control of hypertension in Spain. Results of the [email protected] Study. Rev Esp Cardiol (Engl Ed) 69: , 572–578. |
[30] | Schmidt C , Wolff M , Weitz M , Bartlau T , Korth C , Zerr I ((2011) ) Rapidly progressive Alzheimer disease. Arch Neurol 68: , 1124–1130. |
[31] | Zhou Z , Liang Y , Zhang X , Xu J , Lin J , Zhang R , Kang K , Liu C , Zhao C , Zhao M ((2020) ) Low-density lipoprotein cholesterol and Alzheimer’s disease: A systematic review and meta-analysis. Front Aging Neurosci 12: , 5. |
[32] | Jones RW , Lebrec J , Kahle-Wrobleski K , Dell’Agnello G , Bruno G , Vellas B , Argimon JM , Dodel R , Haro JM , Wimo A , Reed C ((2017) ) Disease progression in mild dementia due to Alzheimer disease in an 18-month observational study (GERAS): The impact on costs and caregiver outcomes. Dement Geriatr Cogn Dis Extra 7: , 87–100. |
[33] | Clark CM , Sheppard L , Fillenbaum GG , Galasko D , Morris JC , Koss E , Mohs R , Heyman A ((1999) ) Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: A clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s disease. Arch Neurol 56: , 857–862. |
[34] | Howard R , Phillips P , Johnson T , O’Brien J , Sheehan B , Lindesay J , Bentham P , Burns A , Ballard C , Holmes C , McKeith I , Barber R , Dening T , Ritchie C , Jones R , Baldwin A , Passmore P , Findlay D , Hughes A , Macharouthu A , Banerjee S , Jones R , Knapp M , Brown RG , Jacoby R , Adams J , Griffin M , Gray R ((2011) ) Determining the minimum clinically important differences for outcomes in the DOMINO trial. Int J Geriatr Psychiatry 26: , 812–817. |
[35] | Gillette-Guyonnet S , Andrieu S , Nourhashemi F , Gardette V , Coley N , Cantet C , Gauthier S , Ousset PJ , Vellas B , REAL.FR study group ((2011) ) Long-term progression of Alzheimer’s disease in patients under antidementia drugs. Alzheimers Dement 7: , 579–592. |
[36] | Schrag A , Schott JM , Alzheimer’s Disease Neuroimaging Initia ((2012) ) What is the clinically relevant change on the ADAS-Cog? J Neurol Neurosurg Psychiatry 83: , 171–183. |
[37] | Dubois B , Padovani A , Scheltens P , Rossi A , Dell’Agnello G ((2016) ) Timely diagnosis for Alzheimer’s disease: A literature review on benefits and challenges. J Alzheimers Dis 49: , 617–631. |
[38] | Sorbi S , Hort J , Erkinjuntti T , Fladby T , Gainotti G , Gurvit H , Nacmias B , Pasquier F , Popescu BO , Rektorova I , Religa D , Rusina R , Rossor M , Schmidt R , Stefanova E , Warren JD , Scheltens P , EFNS Scientist Panel on Dementia and Cognitive Neurology ((2012) ) EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol 9: , 1159–1179. |
[39] | van den Bussche H , Wiese B , Koller D , Eisele M , Kaduszkiewicz H , Maier W , Glaeske G , Steinmann S , Wegscheider K , Schön G ((2011) ) Specialist involvement and referral patterns in ambulatory medical care for patients with dementia in Germany: Results of a claims data based case-control study. BMC Health Serv Res 11: , 148. |
[40] | Mohs RC , Schmeidler J , Aryan M ((2000) ) Longitudinal studies of cognitive, functional and behavioural change in patients with Alzheimer’s disease. Stat Med 19: , 1401–1409. |



