Long-Term Exposure to PM2.5 and Cognitive Decline: A Longitudinal Population-Based Study
Abstract
Background:
A growing but contrasting evidence relates air pollution to cognitive decline. The role of cerebrovascular diseases in amplifying this risk is unclear.
Objectives:
1) Investigate the association between long-term exposure to air pollution and cognitive decline; 2) Test whether cerebrovascular diseases amplify this association.
Methods:
We examined 2,253 participants of the Swedish National study on Aging and Care in Kungsholmen (SNAC-K). One major air pollutant (particulate matter ≤2.5μm, PM2.5) was assessed yearly from 1990, using dispersion models for outdoor levels at residential addresses. The speed of cognitive decline (Mini-Mental State Examination, MMSE) was estimated as the rate of MMSE decline (linear mixed models) and further dichotomized into the upper (25%fastest cognitive decline), versus the three lower quartiles. The cognitive scores were used to calculate the odds of fast cognitive decline per levels of PM2.5 using regression models and considering linear and restricted cubic splines of 10 years exposure before the baseline. The potential modifier effect of cerebrovascular diseases was tested by adding an interaction term in the model.
Results:
We observed an inverted U-shape relationship between PM2.5 and cognitive decline. The multi-adjusted piecewise regression model showed an increased OR of fast cognitive decline of 81%(95%CI = 1.2–3.2) per interquartile range difference up to mean PM2.5 level (8.6μg/m3) for individuals older than 80. Above such level we observed no further risk increase (OR = 0.89;95%CI = 0.74–1.06). The presence of cerebrovascular diseases further increased such risk by 6%.
Conclusion:
Low to mean PM2.5 levels were associated with higher risk of accelerated cognitive decline. Cerebrovascular diseases further amplified such risk.
INTRODUCTION
Dementia and cognitive disorders represent major concerns for our aging societies [1]. According to the World Health Organization (WHO), 130 million people will be living with dementia by 2050 worldwide, posing major challenges at individual and societal levels [2]. In spite of decades of research in the field of cognitive dysfunctions, no curative treatments have been identified to date [3]. As a consequence, research has increasingly accumulated in the identification of modifiable risk and protective factors.
Growing evidence identifies the exposure to co-mmon environmental pollutants as risk factors for adverse health-related events, including cardio and cerebrovascular morbidity and mortality [4]. The Global Burden of Diseases (GBD 2015) included air pollution as a leading cause of global disease burden attributing 5.5 million premature deaths every year due to household and outdoor air pollution [5].
If the detrimental impact of air pollution on the re-spiratory, cardio, and cerebrovascular systems has been consistently pointed out, evidence on the negat-ive impact of air pollution on brain health has only recently started to accumulate [6–8]. Our group has shown a 50%increased hazard of dementia per interquartile range difference in average pollutant levels at residential address [9]. Similar results have been reported in a number of studies carried out acr-oss Europe, United States, and Canada [6, 10, 11].
If such effects have been observed for dementia, it is plausible to hypothesize similar findings for cog-nitive impairment. However, a recent systematic re-view concludes that the evidence on the association between air pollution exposure and cognitive impairment/decline is equivocal and that the studies may have led to inconsistent results, due to various mea-surements of cognitive function and insufficient fol-low-up time [12]. Focusing on cognitive impairment/decline can provide useful clinical insights since the prodromal phase of dementia is frequently featured by subtle cognitive changes and an accelerated cognitive decline is considered a risk condition for dementia [13]. In addition, considering the cognitive trajectory instead of a single time point impairment allows to capture the intra individual changes that characterize aging.
High air pollution levels have also well-established negative cerebrovascular repercussions [14]. Among others, the fine particulate matter (PM) with an aerodynamic diameter of < 2.5μm penetrates deeper in the respiratory system and may possibly enter into the circulation, thus exploiting its hazardous effect in the human body. In line with the close connection between stroke and cognitive dysfunctions [15, 16], it is also plausible to hypothesize that those diseases may play a role in further worsening the cognitive trajectories of people exposed to air pollution.
To test this hypothesis, we used data from a well-characterized population-based cohort study with spatially detailed data on long-term exposure to air pollution and longitudinal clinical assessments, including repeated measures of cognitive function.
METHODS
We gathered the data for this study from the Swe-dish National study on Aging and Care in Kungsholmen (SNAC-K), a population-based longitudinal study. The baseline assessment took place between 2001 and 2004 and eligible participants were reside-nts of the Kungsholmen district in central Stockholm aged 60 + . Overall, 3,363 (response rate: 73.3%) participants were examined at baseline and, since then, they have been followed-up regularly: every six years for the young-old cohorts (60–78 years) and every three years for older cohorts (78 +).
For the current study we excluded individuals who at baseline had dementia (n = 240), individuals with schizophrenia or developmental disorders (n = 17), individuals with a Mini-Mental State Examination (MMSE) lower than 24 (n = 103) and those with only the baseline assessment (n = 750). Thus, we ended up with a final sample of 2,253 participants.
All participants or a proxy (in the case of cognitively impaired persons) provided written informed consent. The Regional Ethical Review Board in Sto-ckholm, Sweden, approved the protocols of the SNAC-K study.
The results of this study are reported following the STROBE Recommendations.
Data collection
Data were collected at our research center or at home for those who were unable to come to the rese-arch center. Trained staff performed face-to-face interviews and clinical and laboratory examinations.
Data on age, sex, and education were obtained from the participants through a personal interview. Level of education was categorized into two groups: ele-mentary school and high school/university. Socioeconomic position was derived from the longest held occupation and categorized into two groups: blue collar and white collar workers. Data on smoking were categorized as current, former, or never smoker. Early retirement was defined as retirement before age 65. Body mass index (BMI) was obtained by dividing the participants’ weight by their squared height (kg/m2). Level of physical activity is based on a questionnaire administered to the participants, which assesses both the frequency and the intensity of these activities. Physical inactivity is defined as being physically act-ive for less than once a week in light and/or intensive activity. Depression was coded in keeping with the International Classification of Diseases, 10th edition (ICD-10). The dementia diagnosis was made in accordance with the DSM-IV following a three-step procedure. Briefly, an examining physician made a preliminary diagnosis, followed by a second, rev-iewing physician. In case of disagreement, a neurologist external to the data collection made the final diagnosis.
Assessment of global cognitive function and operationalization of fast cognitive decline
At each study examination, global cognitive function was assessed by the examining physician using the MMSE [17]. To estimate the rate of cognitive decline for each individual, we implemented a linear mixed model, where the MMSE was the dependent variable and the intercept and time of follow-up provided the fixed and random effect. Estimated slope va-lues were examined by quartiles. Based on the slopes quartiles participants were grouped into two mut-ually exclusive groups: 1) Slow/non-decliners, participants who belonged to the lower three quartiles of decline (reference group); 2) Fast decliners, participants who belonged to the upper quartile of decline.
Assessment of cerebrovascular disease burden
Comprehensive interviews and examinations from physicians, laboratory tests, use of medications, and registers from the Swedish National Patient Register were used to define diseases [18], in accordance with the International Classification of Diseases, 10th edition (ICD-10). For the present study we considered cerebrovascular diseases identified during the period 2001–2013 with the following ICD-10 codes: G45 (transient ischemic attack), G46 (vascular syndromes), I60–64 (hemorrhagic and ischemic stroke), I67 and I69 (cerebral vascular diseases).
Air pollution assessment
We estimated PM2.5 levels at the residential add-resses of participants with dispersion modeling based on local emission inventories, detailed elsewhere [19]. If the participants have moved, the exposure of air pollution was calculated based on the new residential address. Annual average PM2.5 levels were calculated using emission inventories, for the years 1990, 1995, 2000, 2005, and 2011. A Gaussian dispersion model was applied to the emission databases together with meteorology in climatology. To allow high resolution in vicinity of roads, a quadtree receptor grid was used. Annual average levels of PM2.5 for all the years 1990–2001/2004 period were obtained from linear interpolation for the years between each model simulation. Comparing the model calculated levels with yearly measurements at three curbside (traffic) monitoring sites and one urban background site in Stockholm City for the period 1990 –2011, r2-value of 0.86 for PM2.5 was obtained.
Statistical analyses
Multivariate logistic regression models were used to estimate odd ratios (ORs) and 95%confidence intervals (CIs) for fast cognitive decliners in relation to 10-year average PM2.5 exposures before baseline assessment. Since age is strongly associated with cognitive decline, we conducted stratified analyses by age (≤80 versus > 80 years). Potential confounders were defined a priori and chosen based on literature review, and included: age, sex, education, smoking, early retirement, physical activity, and socio-economic status. Further adjustments included: BMI and depression. The associations were analyzed first assuming a linear relationship. In a second stage, to assess departure from a linear trend, we modelled the continuous exposure using restricted cubic splines with 3 knots at fixed percentiles (10th, 50th, 90th) of its distribution. We then used piecewise regression models to allow generating two different slopes for specific values of the pollutant: one below the mean level of PM2.5 (8.6μg/m3) and one above this threshold. Finally, we tested the potential modifying effects of cerebrovascular diseases occurring any time during the follow-up period by adding a multiplicative interaction term in the model.
Sensitivity analyses
We repeated the logistic regression models considering a 5-year air pollution exposure window.
All statistical analyses were performed with Stata, version 16 (StataCorp, TX, USA).
RESULTS
At baseline, we excluded 750 individuals because of missing data in the MMSE. Those excluded were older, less educated, and more likely to be retired (p < 0.05 for all). The sample did not differ in terms of PM2.5 exposure level (p > 0.05). See Supplementary Table 1.
At study entry, mean (±SD) age of the population was 72.1 (±9.9), 63.5%were female, and 13.0%had an elementary level of education or below. Fast decliners (upper quartile, N = 564) were more likely to be older, female, and less educated (Table 1).
Table 1
Baseline characteristics of the study sample, overall and by cognitive decline
| No decliners (N = 1689) | Fast cognitive decliners (N = 564) | Total (N = 2253) | p | |
| Age (y, mean±SD) | 69.1±8.5 | 81.1±8.3 | 72.1±9.9 | <0.001 |
| Female, n (%) | 1055 (62.5) | 375 (66.5) | 1430 (63.5) | 0.086 |
| Education, n (%) | ||||
| Elementary (≤8 y) | 156 (9.3) | 136 (24.2) | 292 (13.0) | <0.001 |
| High school or above (>8 y) | 1533 (90.8) | 426 (75.6) | 1959 (87.0) | |
| Smoking, n (%) | ||||
| Never smoker | 756 (44.8) | 303 (53.8) | 1059 (47.0) | 0.001 |
| Former smoker | 695 (41.2) | 186 (33.0) | 881 (39.1) | |
| Current smoker | 230 (13.7) | 69 (12.3) | 299 (13.3) | |
| Physical activity, n (%) | ||||
| No or moderate | 1519 (67.4) | 1135 (67.2) | 384 (68.1) | <0.001 |
| High | 552 (24.5) | 476 (28.2) | 76 (13.5) | |
| Early retirement, n (%) | 1183 (70.1) | 544 (96.5) | 1727 (76.7) | <0.001 |
| SES status, n (%) | ||||
| Blue collar workers | 259 (15.4) | 180 (32.0) | 439 (19.5) | <0.001 |
| White collar workers | 1428 (84.6) | 381 (67.6) | 1809 (80.3) | |
| Stroke, n (%) | 240 (14.2) | 179 (31.7) | 419 (18.6) | <0.001 |
| MMSE (mean±SD) | 29.3±0.9 | 27.9±1.7 | 28.9±1.3 | <0.001 |
| PM2.5 (μg/m3, mean±SD) | 8.7±0.7 | 8.7±0.6 | 8.7±0.7 | 0.571 |
| IQR: 0.6μg/m3 |
MMSE, Mini-Mental State Examination; SD, standard deviation; PM2.5, Particulates matter with a diameter of 2.5μm or less; μg/m3, micrograms per cubic meter; IQR, interquartile range. Missing values: 14 in smoking, 17 in retirement, 5 in SES, 182 in physical activity, 9 in PM2.5.
We did not observe a linear association between the levels of pollutant and OR of fast cognitive decline after adjusting for possible confounders (p = 0.43). In the restricted cubic spline analysis, we observed a significant departure from linearity (p = 0.013 for the fully adjusted model). We detected an increased risk of fast cognitive decline associated with exposure to PM2.5, from low to mean levels (OR = 1.51, 95%CI: 1.10–2.07), especially in the old-est-old people (age > 80 years). The piece-wise regression model adjusted for potential confounders showed an increased OR of fast cognitive decline by as much as 81%per interquartile range difference up to a level of PM2.5 = 8.6μg/m3 (OR = 1.81, 95%CI:1.02–3.22). Above the level of 8.6μg/m3, we observed no further increase in such risk (OR = 0.89, 95%CI:0.74–1.06) (Fig. 1). Among those exposed to higher air pollution levels (above the average level), the survivors were (in comparison with those who died) more likely to be highly educated (more than 8 years schooling) (58.8%versus 35.5%; p < 0.001); more likely to be white collar (58.5%versus 45.3%; p = 0.001); less likely to be heavy drinker (48.3%versus 63.8%; p < 0.001) and more likely to perform physical activity (75.7%versus 53.6%; p < 0.001).
Fig. 1
Odds Ratios (ORs) and 95%confidence interval (CI) of fast cognitive decline by levels of PM2.5 during the 10 years before baseline by age groups. Estimates are odds ratios derived from logistic regression according to PM2.5 levels. PM2.5 is modelled by using: 1) restricted cubic splines with three knots (blue line), 2) piecewise linear spline (solid line) with one knot set at the median level pf PM2.5(8.6μg/m3) with 95%CI (dash lines). Models are adjusted for age, sex, education, smoking, socio-economic status, early retirement, and physical activity. The time exposure period is between 0–10 years before the baseline assessment. The reference group is considered the median exposure level in the entire population. The histogram represents the distribution of the exposure levels in the entire population.
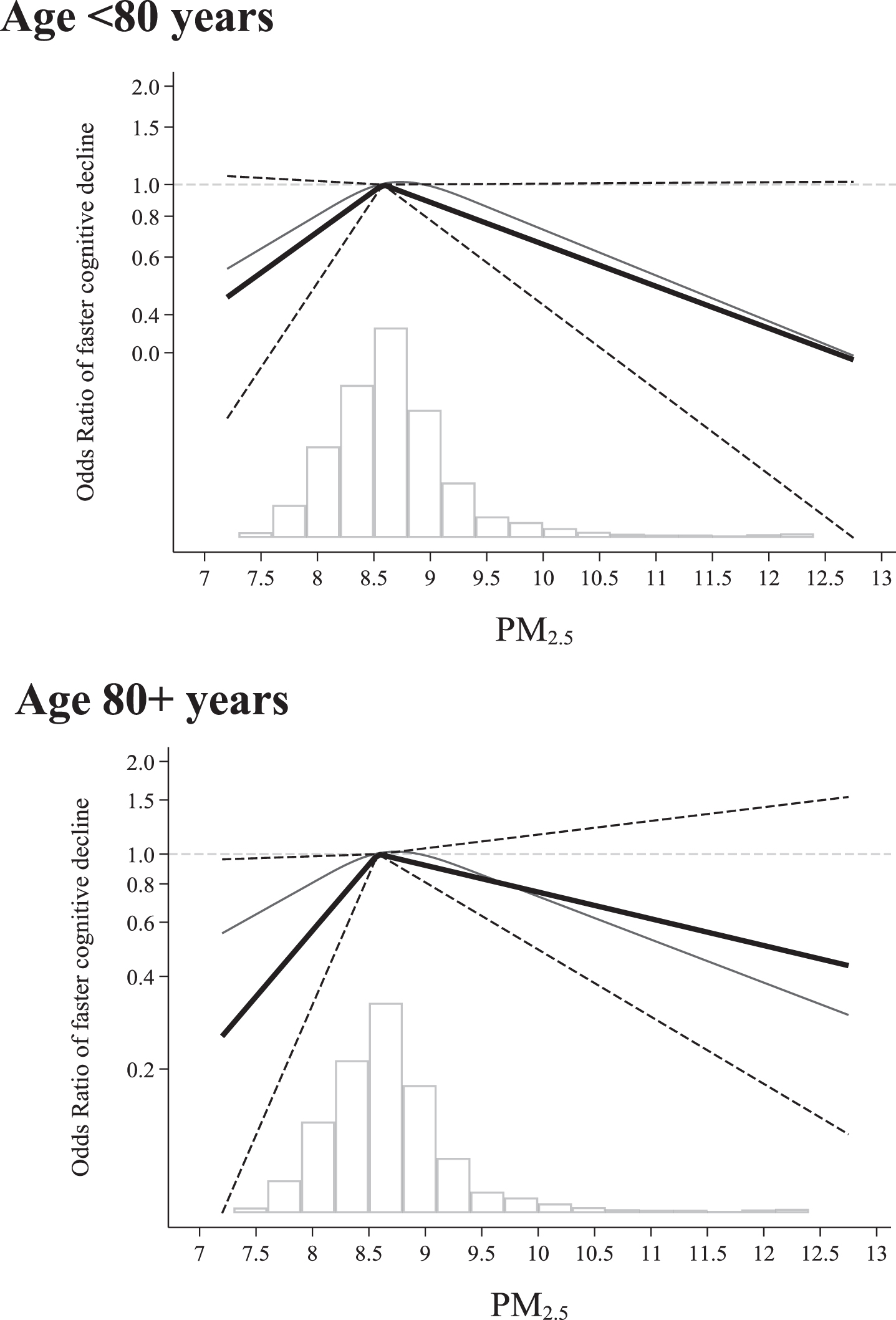
Table 2 shows the effect modification of cerebrova-scular diseases in the association between PM2.5 and cognitive decline. We detected a significant multi-plicative interaction between PM2.5 and cerebrova-scular diseases for PM2.5 levels ≤8.6μg/m3 (p < 0.001), but not for levels above. The presence of cerebrovascular diseases further increased the risk of fast cognitive decline particularly in the oldest-old (OR = 1.97; 95%CI:1.07–3.61). Further adjustment for depression and BMI did not modify the results (data not shown).
Table 2
Odds Ratios (ORs) and 95%confidence interval (CI) of fast cognitive decline by levels of PM2.5 during the 10 years before baseline by cerebrovascular diseases, overall and by age groups
| OR (95%CI) of fast cognitive decline | ||
| PM2.5≤8.6μg/m3 | PM2.5 > 8.6μg/m3 | |
| Overall | ||
| No cerebrovascular diseases | 1.46 (1.06–2.01) | 0.87 (0.76–1.01) |
| Yes cerebrovascular diseases | 1.57 (1.13–2.16) | 0.83 (0.65–1.06) |
| p for interaction <80 y | <0.001 | 0.395 |
| No cerebrovascular diseases | 1.31 (0.90–1.91) | 0.79 (0.62–1.00) |
| Yes cerebrovascular diseases | 1.41 (0.97–2.05) | 0.89 (0.66–1.19) |
| p for interaction 80 + y | <0.001 | 0.829 |
| No cerebrovascular diseases | 1.85 (1.06–3.37) | 0.93 (0.75–1.14) |
| Yes cerebrovascular diseases | 1.97 (1.07–3.61) | 0.74 (0.50–1.10) |
| p for interaction | <0.001 | 0.215 |
Estimates are odds ratios derived from logistic regression according to PM2.5 levels. Models are adjusted for age, sex, education, smoking, socio-economic status, early retirement, and physical activity. The time exposure period is between 0–10 years before the baseline assessment. The reference group is considered the median exposure level in the entire population.
The analyses considering a 5-year window of air pollution exposure led to an attenuation of the association in the young-old cohort, but a slight stronger association in the older one (Supplementary Table 2).
DISCUSSION
We found residential PM2.5 to be associated with an increased risk of fast cognitive decline, but only at low to mean levels. The risk was particularly evident in the oldest-old individuals and it was amp-lified by the presence of cerebrovascular diseases. Notably, these findings are derived from a longitu-dinal population study in a central district of Stockholm, and the exposure levels were in general below the current European and US standards. Interestingly, we observed a flattening of the effect for PM2.5 levels above the mean PM2.5 value.
Emerging evidence has accumulated in the past years on the detrimental effect of air pollution on brain health, both in terms of cognitive decline and dementia [12, 20–22]. Several differences concerning the study design, in particular the assessment of air pollution exposure, and the cognitive tests used, prevent direct comparisons between our study and previous ones. However, according to a recent systematic review by Peters and colleagues on the rel-ationship between air pollution and cognitive decline/dementia, the evidence related with cognitive decline is equivocal [12]. Our results are in line with Weuve and colleagues who reported associations between PM2.5 and fast cognitive decline in a population-based study including 19,409 US women aged 70 to 81 years [23]. Conversely, in a clinical study on individuals who were part of the Alzheimer Disease Center in the US, no association was found between higher exposure to air pollution and cognitive impairment as assessed with the MMSE [24]. Similarly, in a study conducted in Northern Sweden by Oudin and colleagues, no association arose between long-term exposure to NOx and episodic memory change, investigated through a comprehensive neuropsychological battery comprising five different cognitive memory tests [25].
Interestingly, the evidence concerning dementia is more robust [12]. In spite of methodological differences between all these studies, in our view the findings as a whole point towards a deleterious effect of air pollution on the brain. In line with this, we were able to detect a steeper air pollution related cognitive decline in individuals exposed to low to mean air pollution levels in a residential area in central Stockholm, where the ambient air quality is comparatively good. This aspect raises important concerns for the expected consequences and brain repercussions in relation with environmental exposures in areas that present pollutants levels above the European and US standards. Notable, we observed an intriguing inverted U-shaped association between PM2.5 and cognitive decline. Namely, we found an increased risk from low to mean level of PM2.5 and then a flattening of such effect. Even if this seems counterintuitive, a similar shape when analyzing an exposure like air pollution has been already observed [26]. While saturation of metabolic pathways at high exposure levels seems very unlikely at these levels, a “healthy survivor” effect might be possible since a part of the study population already had superseded their average life expectancy at baseline. Interestingly, we observed that among those who were exposed to higher air pollution level, the survivors were more educated, more often white collars, less heavy drinkers and more often physically active, partially supporting this hypothesis. Finally, personal exposure may not be proportional to the estimated outdoor levels, because of differences in dwelling outdoors, and in other life habits. Future studies are needed to better investigate these issues and might also open new hypotheses on the brain repercussions on air pollution.
We here find evidence to support that the presence and the development of stroke may further increase the detrimental association between air pollution and cognitive decline. This latter observation is not surprising for a number of reasons. First, several large cohort studies have reported positive associations between long term exposure to ambient air pollution and cerebrovascular events [27, 28]. Stafoggia and colleagues demonstrated that higher PM2.5 levels were associated with an increased risk of cerebro-vascular events, including both ischemic and hemorrhagic stroke [29]. Similarly, in a recent study conducted in Sweden, Ljungman and colleagues found that long-term residential levels of locally emitted black carbon from traffic exhaust were associated with increased stroke incidence [30]. Animal studies have shown that exposure to particulate matter may alter the vascular endothelium accelerating carotid atherosclerosis and resulting in subsequent vasoconstriction [20]. It is also plausible to hypothesize that the pollutants may induce an increased systemic inflammatory response [31], triggering platelet activation and consequently resulting in thromboembolic events [32]. The exact biological mechanisms underlying these observations are not yet fully understood but it might be that a number of different bio-logical pathways (e.g., direct endothelium damage, systemic inflammation, platelet activation) are at play in facilitating the occurrence of stroke or transient ischemic attacks [8]. The cognitive consequences of cerebrovascular diseases are well-established: stroke doubles the risk of dementia [33] and can anticipate its onset by as much as 10 years. Consistent with this we observed an amplified cognitive decline risk in those people exposed to high level of air pollutants with concurrent cerebrovascular conditions. To dee-pen our understanding in the biological mechanisms behind these associations, it would be interesting to investigate the role of stroke subtypes to further exp-lore the nature of brain damage associated with air pollution. Interestingly, the results reported here are in line with recent studies, one of which from our group, that found a role of cardiovascular diseases in the development of dementia related to air pollution [9, 11]. In particular, we reported that up to 50%of dementia cases could be mediated by the development of stroke. Similarly, we now observe a possible biological interaction between air pollution and stroke in accelerating the decline in cognitive function.
Strengths and limitations
The results of the present study are derived from a well-characterized, longitudinal population-based study with extensive repeated clinical evaluations, including in-depth assessments of co-occurring chr-onic diseases. In addition, the assessment of air pollution was spatially detailed and covered a period of more than 10 years before the baseline assessment. Some limitations need to be mentioned. First, although the MMSE represents a reliable and easy to administer cognitive test, it may not be sensitive enough to capture subtle cognitive changes. This has been demonstrated to be particularly true when studying cognitive aging in highly educated individuals [34]. A detailed neuropsychological battery would enable a deeper understanding of the process underlying the association between air pollution and cognitive function, and would allow further investigation into domain specific deficits, thus identifying specific brain areas more vulnerable to the damage of air pollution. It is also worth mentioning that a consensus does not exist on how to define and operationalize “fast cognitive decline” [35–37]. By using MMSE, a number of different approaches have been proposed. We adopted the linear mixed models to take full advantage of the longitudinal study design and the repeated measures of the MMSE and we accounted for the intercept when computing the slope. Regardless of the definition and operationalization of “fast decliners”, the identification of “at risk” groups of patients may help clinicians and families in planning a timely intervention of care. Second, the present findings come from an in-depth detailed population-based study but yet with relatively limited sample size when studying an exposure like air pollution. This has precluded us to perform in depth sensitivity analyses and better understand the role played by cerebrovascular diseases or other conditions. The latter limitation prevented us also to conduct a mediation analysis in order to disentangle whether stroke could mediate, rather than modify, the association of interest. Further studies with similar clinical characterization and larger sample size are needed to better address this relevant research question. Finally, SNAC-K includes older adults living in a central district of Stockholm who are of high socioeconomic status, fit, healthy and exposed to comparatively low air pollution levels, which might limit the generalizability of our results to other populations. However, given the above-mentioned characteristics, our results may represent an overall underestimation between air pollution and cognitive decline.
CONCLUSIONS
The focus in the field of cognitive disorders has recently increased on the contribution of environmental exposure and brain pathology, and we here demonstrated that increasing levels of air pollution (from low to mean) are associated with steeper cognitive decline. Future studies are warranted using a comprehensive neuropsychological assessment to confirm and further detail the association found in this study. Interestingly, we found evidence to support the contribution of cerebrovascular diseases in further accelerating the cognitive decline associated with air pollution.
Nine out of ten people around the world breathe polluted air [4]; this, coupled with the worldwide population aging, calls for effective strategies to reduce or delay the onset of dementia. The results from this and previous studies open new research avenues for dementia prevention.
ACKNOWLEDGMENTS
We thank the SNAC-K participants and the SNAC-K group for their collaboration in data collection and management.
This work was supported by the funders of the Swedish National study on Aging and Care; the Min-istry of Health and Social Affairs, Sweden; the par-ticipating County Councils and Municipalities; the Swedish Research Council; Karolinska Institutet (KID-funding), Stockholm, Sweden. Swedish Res-earch Council for Health, Working Life and Welfare. Chinese scholarship council (CSC).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0852r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200852.
REFERENCES
[1] | Grande G , Qiu C , Fratiglioni L ((2020) ) Prevention of dementia in an ageing world: Evidence and biological rationale. Ageing Res Rev 64: , 101045. |
[2] | World Health Organization (2015) Global action against dementia. |
[3] | Winblad B , Amouyel P , Andrieu S , Ballard C , Brayne C , Brodaty H , Cedazo-Minguez A , Dubois B , Edvardsson D , Feldman H , Fratiglioni L , Frisoni GB , Gauthier S , Georges J , Graff C , Iqbal K , Jessen F , Johansson G , Jonsson L , Kivipelto M , Knapp M , Mangialasche F , Melis R , Nordberg A , Rikkert MO , Qiu C , Sakmar TP , Scheltens P , Schneider LS , Sperling R , Tjernberg LO , Waldemar G , Wimo A , Zetterberg H ((2016) ) Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol 15: , 455–532. |
[4] | Landrigan PJ , Fuller R , Acosta NJR , Adeyi O , Arnold R , Basu NN , Balde AB , Bertollini R , Bose-O’Reilly S , Boufford JI , Breysse PN , Chiles T , Mahidol C , Coll-Seck AM , Cropper ML , Fobil J , Fuster V , Greenstone M , Haines A , Hanrahan D , Hunter D , Khare M , Krupnick A , Lanphear B , Lohani B , Martin K , Mathiasen KV , McTeer MA , Murray CJL , Ndahimananjara JD , Perera F , Potocnik J , Preker AS , Ramesh J , Rockstrom J , Salinas C , Samson LD , Sandilya K , Sly PD , Smith KR , Steiner A , Stewart RB , Suk WA , van Schayck OCP , Yadama GN , Yumkella K , Zhong M ((2018) ) The Lancet Commission on pollution and health. Lancet 391: , 462–512. |
[5] | Cohen AJ , Brauer M , Burnett R , Anderson HR , Frostad J , Estep K , Balakrishnan K , Brunekreef B , Dandona L , Dandona R , Feigin V , Freedman G , Hubbell B , Jobling A , Kan H , Knibbs L , Liu Y , Martin R , Morawska L , Pope CA 3rd , Shin H , Straif K , Shaddick G , Thomas M , van Dingenen R , van Donkelaar A , Vos T , Murray CJL , Forouzanfar MH ((2017) ) Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 389: , 1907–1918. |
[6] | Chen H , Kwong JC , Copes R , Tu K , Villeneuve PJ , van Donkelaar A , Hystad P , Martin RV , Murray BJ , Jessiman B , Wilton AS , Kopp A , Burnett RT ((2017) ) Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 389: , 718–726. |
[7] | Cerza F , Renzi M , Gariazzo C , Davoli M , Michelozzi P , Forastiere F , Cesaroni G ((2019) ) Long-term exposure to air pollution and hospitalization for dementia in the Rome longitudinal study. Environ Health 18: , 72. |
[8] | Calderon-Garciduenas L , Azzarelli B , Acuna H , Garcia R , Gambling TM , Osnaya N , Monroy S , MR DELT, Carson JL , Villarreal-Calderon A , Rewcastle B ((2002) ) Air pollution and brain damage. Toxicol Pathol 30: , 373–389. |
[9] | Grande G , Ljungman PLS , Eneroth K , Bellander T , Rizzuto D ((2020) ) Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol 77: , 801–809. |
[10] | Oudin A , Andersson J , Sundstrom A , Nordin Adolfsson A , Oudin Astrom D , Adolfsson R , Forsberg B , Nordin M ((2019) ) Traffic-related air pollution as a risk factor for dementia: No clear modifying effects of APOEvarepsilon4 in the Betula Cohort. J Alzheimers Dis 71: , 733–740. |
[11] | Ilango SD , Chen H , Hystad P , van Donkelaar A , Kwong JC , Tu K , Martin RV , Benmarhnia T ((2020) ) The role of cardiovascular disease in the relationship between air pollution and incident dementia: A population-based cohort study. Int J Epidemiol 49: , 36–44. |
[12] | Peters R , Ee N , Peters J , Booth A , Mudway I , Anstey KJ ((2019) ) Air pollution and dementia: A systematic review. J Alzheimers Dis 70: , S145–S163. |
[13] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr. , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[14] | Lee KK , Miller MR , Shah ASV ((2018) ) Air pollution and stroke. J Stroke 20: , 2–11. |
[15] | Shang Y , Fratiglioni L , Marseglia A , Plym A , Welmer AK , Wang HX , Wang R , Xu W ((2020) ) Association of diabetes with stroke and post-stroke dementia: A population-based cohort study. Alzheimers Dement 16: , 1003–1012. |
[16] | Mijajlovic MD , Pavlovic A , Brainin M , Heiss WD , Quinn TJ , Ihle-Hansen HB , Hermann DM , Assayag EB , Richard E , Thiel A , Kliper E , Shin YI , Kim YH , Choi S , Jung S , Lee YB , Sinanovic O , Levine DA , Schlesinger I , Mead G , Milosevic V , Leys D , Hagberg G , Ursin MH , Teuschl Y , Prokopenko S , Mozheyko E , Bezdenezhnykh A , Matz K , Aleksic V , Muresanu D , Korczyn AD , Bornstein NM ((2017) ) Post-stroke dementia - a comprehensive review. BMC Med 15: , 11. |
[17] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[18] | Calderon-Larranaga A , Vetrano DL , Onder G , Gimeno-Feliu LA , Coscollar-Santaliestra C , Carfi A , Pisciotta MS , Angleman S , Melis RJ , Santoni G , Mangialasche F , Rizzuto D , Welmer AK , Bernabei R , Prados-Torres A , Marengoni A , Fratiglioni L ((2016) ) Assessing and measuring chronic multimorbidity in the older population: A proposal for its operationalization. J Gerontol A Biol Sci Med Sci 72: , 1417–1423. |
[19] | Segersson D , Eneroth K , Gidhagen L , Johansson C , Omstedt G , Nylen AE , Forsberg B ((2017) ) Health impact of PM10, PM2.5 and black carbon exposure due to different source sectors in Stockholm, Gothenburg and Umea, Sweden. Int J Environ Res Public Health 14: , 742. |
[20] | Hahad O , Lelieveld J , Birklein F , Lieb K , Daiber A , Munzel T ((2020) ) Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci 21: , 4306. |
[21] | Haghani A , Morgan TE , Forman HJ , Finch CE ((2020) ) Air pollution neurotoxicity in the adult brain: Emerging concets from experimental findings. J Alzheimers Dis 76: , 773–797. |
[22] | Attademo L , Bernardini F ((2020) ) Air pollution as risk factor for mental disorders: In search for a possible link with Alzheimer’s disease and schizophrenia. J Alzheimers Dis 6: , 825–830. |
[23] | Weuve J , Puett RC , Schwartz J , Yanosky JD , Laden F , Grodstein F ((2012) ) Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 172: , 219–227. |
[24] | Cleary EG , Cifuentes M , Grinstein G , Brugge D , Shea TB ((2018) ) Association of low-level ozone with cognitive decline in older adults. J Alzheimers Dis 61: , 67–78. |
[25] | Oudin A , Forsberg B , Lind N , Nordin S , Oudin Astrom D , Sundstrom A , Nordin M ((2017) ) Is long-term exposure to air pollution associated with episodic memory? A longitudinal study from Northern Sweden. Sci Rep 7: , 12789. |
[26] | Steenland K , Deddens JA ((2004) ) A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology 15: , 63–70. |
[27] | Shah AS , Lee KK , McAllister DA , Hunter A , Nair H , Whiteley W , Langrish JP , Newby DE , Mills NL ((2015) ) Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 350: , h1295. |
[28] | Brook RD , Rajagopalan S , Pope CA 3rd , Brook JR , Bhatnagar A , Diez-Roux AV , Holguin F , Hong Y , Luepker RV , Mittleman MA , Peters A , Siscovick D , Smith SC Jr. , Whitsel L , Kaufman JD , American Heart Association Council on Epidemiology and Prevention Council on the Kidney in Cardiovascular Disease and Council on Nutrition Physical Activity and Metabolism ((2010) ) Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121: , 2331–2378. |
[29] | Stafoggia M , Cesaroni G , Peters A , Andersen ZJ , Badaloni C , Beelen R , Caracciolo B , Cyrys J , de Faire U , de Hoogh K , Eriksen KT , Fratiglioni L , Galassi C , Gigante B , Havulinna AS , Hennig F , Hilding A , Hoek G , Hoffmann B , Houthuijs D , Korek M , Lanki T , Leander K , Magnusson PK , Meisinger C , Migliore E , Overvad K , Ostenson CG , Pedersen NL , Pekkanen J , Penell J , Pershagen G , Pundt N , Pyko A , Raaschou-Nielsen O , Ranzi A , Ricceri F , Sacerdote C , Swart WJ , Turunen AW , Vineis P , Weimar C , Weinmayr G , Wolf K , Brunekreef B , Forastiere F ((2014) ) Long-term exposure to ambient air pollution and incidence of cerebrovascular events: Results from 11 European cohorts within the ESCAPE project. Environ Health Perspect 122: , 919–925. |
[30] | Ljungman PLS , Andersson N , Stockfelt L , Andersson EM , Nilsson Sommar J , Eneroth K , Gidhagen L , Johansson C , Lager A , Leander K , Molnar P , Pedersen NL , Rizzuto D , Rosengren A , Segersson D , Wennberg P , Barregard L , Forsberg B , Sallsten G , Bellander T , Pershagen G ((2019) ) Long-term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect 127: , 107012. |
[31] | Block ML , Calderon-Garciduenas L ((2009) ) Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32: , 506–516. |
[32] | Li W , Dorans KS , Wilker EH , Rice MB , Ljungman PL , Schwartz JD , Coull BA , Koutrakis P , Gold DR , Keaney JF Jr. , Vasan RS , Benjamin EJ , Mittleman MA ((2017) ) Short-term exposure to ambient air pollution and biomarkers of systemic inflammation: The Framingham Heart Study. Arterioscler Thromb Vasc Biol 37: , 1793–1800. |
[33] | Kuzma E , Lourida I , Moore SF , Levine DA , Ukoumunne OC , Llewellyn DJ ((2018) ) Stroke and dementia risk: A systematic review and meta-analysis. Alzheimers Dement 14: , 1416–1426. |
[34] | Spering CC , Hobson V , Lucas JA , Menon CV , Hall JR , O’Bryant SE ((2012) ) Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer’s disease in ethnically diverse highly educated individuals: An analysis of the NACC database. J Gerontol A Biol Sci Med Sci 67: , 890–896. |
[35] | Marra C , Silveri MC , Gainotti G ((2000) ) Predictors of cognitive decline in the early stage of probable Alzheimer’s disease. Dement Geriatr Cogn Disord 11: , 212–218. |
[36] | Doody RS , Massman P , Dunn JK ((2001) ) A method for estimating progression rates in Alzheimer disease. Arch Neurol 58: , 449–454. |
[37] | Buccione I , Perri R , Carlesimo GA , Fadda L , Serra L , Scalmana S , Caltagirone C ((2007) ) Cognitive and behavioural predictors of progression rates in Alzheimer’s disease. Eur J Neurol 14: , 440–446. |



