A Systematic Review of Longitudinal Studies Which Measure Alzheimer’s Disease Biomarkers
Abstract
Alzheimer’s disease (AD) is a progressive and fatal neurodegenerative disease, with no effective treatment or cure. A gold standard therapy would be treatment to slow or halt disease progression; however, knowledge of causation in the early stages of AD is very limited. In order to determine effective endpoints for possible therapies, a number of quantitative surrogate markers of disease progression have been suggested, including biochemical and imaging biomarkers. The dynamics of these various surrogate markers over time, particularly in relation to disease development, are, however, not well characterized. We reviewed the literature for studies that measured cerebrospinal fluid or plasma amyloid-β and tau, or took magnetic resonance image or fluorodeoxyglucose/Pittsburgh compound B-positron electron tomography scans, in longitudinal cohort studies. We summarized the properties of the major cohort studies in various countries, commonly used diagnosis methods and study designs. We have concluded that additional studies with repeat measures over time in a representative population cohort are needed to address the gap in knowledge of AD progression. Based on our analysis, we suggest directions in which research could move in order to advance our understanding of this complex disease, including repeat biomarker measurements, standardization and increased sample sizes.
INTRODUCTION
Alzheimer’s disease (AD) is characterized by progressive cognitive decline leading to dementia. It has been estimated that over 35.6 million people have dementia worldwide [1]. Continued high prevalence of AD [1] makes it a major public health issue, due to the high financial and emotional cost. AD is thought to be caused by neuronal death and brain atrophy [2]; this is often present alongside the accumulation of both tau tangles and amyloid–β (Aβ) plaques in the brain. Much debate has occurred about the order of events leading to neuronal death [3]; nevertheless, it is observed that both neurofibrillary tau tangles and Aβ plaques are present in the brains of AD patients postmortem [4]. Therefore, they have been widely employed as diagnostic markers of the disease and, concomitantly, possible quantitative measures of progression. Biomarkers, such as Aβ and tau monomers and oligomers, can be measured in the blood and cerebrospinal fluid (CSF).
Traditionally, measures of cognition have been used as clinical trial endpoints to assess treatments for AD [5, 6]. Current measures of cognitive decline have shown various degrees of sensitivity and specificity [7–9]. It has, however, been suggested that there is a neuropathological threshold beyond which any treatment will fail to affect cognition given the profound amount of brain atrophy developed [10]. Therefore, much research effort has been invested in preventative treatment, i.e. to stop neurodegeneration before it becomes too severe. Given that such a treatment will have to be administered prior to any signs of cognitive dysfunction in order for it to be effective, alternative clinical endpoints need to be established. The European Medicine Agency (EMA) and US Food and Drug Administration (FDA) have stated that the rate of disease progression could be linked to a biomarker indicative of underlying pathology [6, 11].
Biomarkers were added to the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) (NINCDS-ADRDA) diagnostic criteria in 2011 [12]. Therefore, a variety of quantitative, AD-specific measures have been characterized that it is hoped can aid in diagnosing the disease. In addition to their use as endpoints, it has also been suggested that clinical trial populations can be enriched by including those individuals exhibiting a biomarker profile indicative of future conversion. Defining this preclinical stage itself is a topic of debate in the field [13]. Despite their use in diagnostics and potential use in clinical trial enrichment, knowledge about the dynamics of these biomarkers over time is limited. An understanding of exactly when these become meaningful prognostic markers is imperative in order for them to be clinically useful.
Many biological markers of AD pathology have been characterized to date. AD patients have significantly lower Aβ42 and higher t-tau and p-tau in the CSF compared to controls [14]. Total Aβ burden can also be assessed in the brain via Pittsburgh compound B (PiB)-positron electron tomography (PET) scanning [15]. In addition, marked brain atrophy in AD and all-cause dementia cases can be observed with magnetic resonance imaging (MRI) scanning [16, 17]. Decreased glucose metabolism as assessed by fluorodeoxyglucose (FDG)-PET is also a hallmark of the disease [18]. Various studies have described the differences in these biomarkers between cognitively normal and AD patients. A previous systematic review and meta-analysis by Olsson et al. [14] assessed the utility of CSF and blood based markers in distinguishing between those with AD and controls, in addition to mild cognitive impairment (MCI) due to AD and stable MCI. An earlier systematic review by McGhee et al. [19] used an expanded set of criteria to identify biomarkers of interest, including any biomarker that could be used to describe the progression of AD. The work presented in this paper focuses on the longitudinal use of classical Aβ and tau markers, as well as MRI and PET, in cohort studies. In order to make the selected studies comparable to other investigations, those which have taken cognitive functional measures have also been highlighted.
METHODS
Search terms
In order to ensure that studies relevant to our analysis were identified, we conducted a review of the literature. We searched the US National Library of Medicine National Institutes of Health in 2015 for articles published in English between January 1995 and August 2015. The search terms are listed below:
A) Alzheimer disease[MeSH Terms] AND amyloid[MeSH Terms] AND (cohort study[MeSH Terms] OR cross-sectional study[MeSH Terms] OR longitudinal study[MeSH Terms]) (Search yielded 585 results).
B) Alzheimer disease[MeSH Terms] AND tau[All Fields] AND (cohort study[MeSH Terms] OR cross-sectional study[MeSH Terms] OR longitudinal study[MeSH Terms]) (Search yielded 443 results).
C) Alzheimer disease[MeSH Terms] AND (cohort study[MeSH Terms]) OR cross sectional study[MeSH Terms]) OR longitudinal study[MeSH Terms]) AND (positron emission tomography[MeSH Terms]) OR mri scan[MeSH Terms]) (Search yielded 854 results).
MeSH indexing is a system which places publications under categories of relevance; therefore, publications selected will be based on how they have been indexed. Cognitive testing was not used as a search term for this review, although methods used in the identified studies are discussed.
Inclusion criteria
We included studies which measured brain atrophy with MRI, amyloid levels with PiB-PET, tau levels as assessed by tau PET, CSF tau (phosphorylated tau or total tau), CSF Aβ (1–40, 1–42, or other variants), blood/plasma tau (phosphorylated tau, total tau, or antibodies to tau), blood Aβ (1–40, 1–42, or other variants or antibodies to amyloid-β), or autopsy data with tau or Aβ brain staining. Within this paper, we define “biomarker” as any of these measures, i.e., as a measure of biological state independent from clinical or cognitive measures. We included studies which measured 50 or more people at a minimum of 2 distinct points in time (longitudinal study).
Exclusion criteria
We excluded reviews and intervention studies (unless the placebo group fits the inclusion criteria). We excluded papers that were not written in English.
Analysis
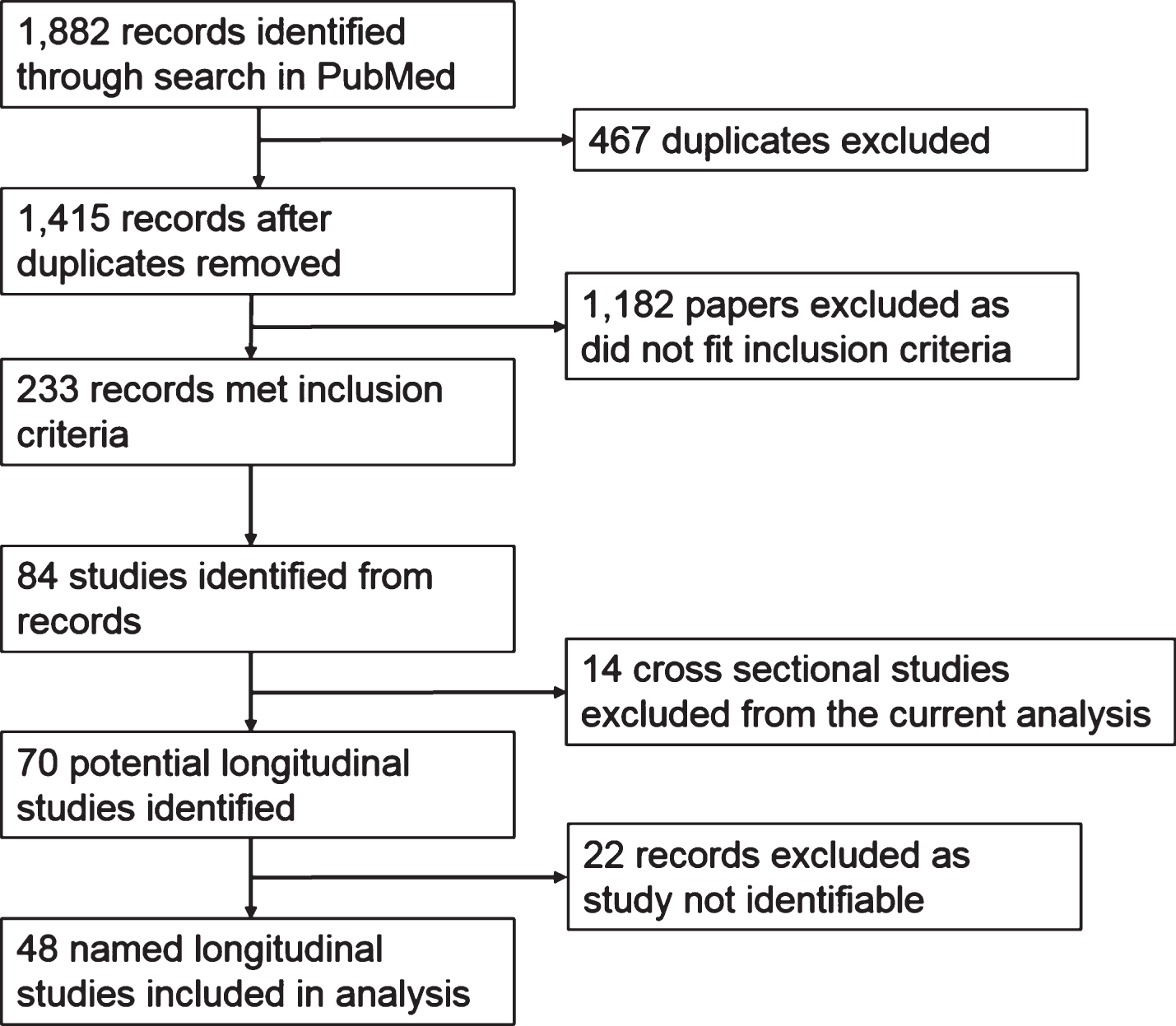
Studies were identified through reading the methods section of the paper. Analysis featured in this paper was performed on longitudinal studies (cross-sectional studies were omitted from analysis). In certain instances, the study populations were not precisely identified in the paper, in which case the papers were excluded from the analysis (see Fig. 1). Further information about the study, for example, location, sample size, cognitive testing method, diagnosis method, was obtained from reading the methods section of the paper. When more than one paper was published per study, a selection of papers was used for data extraction, including the first published and most recently published papers. For very large studies with more than 10 papers, relevant websites were consulted for information. In this analysis, we defined “number of participants” as the number of participants who had completed at least two biomarker assessments. We categorized the studies into groups, based on the way participants were selected or recruited. These can be summarized as: i) those which randomly recruited from memory clinic admissions; ii) those who selected healthy participants but with an unrepresentative bias toward family history; iii) those which followed patients who had existing white matter changes; iv) those who recruited from an existing population cohort; and v) those who recruited groups of participants based on diagnostic status. We collated the age and age distribution from the studies, noting the mean age and standard deviation within the overall study population, as well as for each diagnostic group (cognitively normal, mild cognitively impaired, and AD individuals). If these values were not presented for the overall population, but rather for the distinct diagnostic groups, the mean age reflects the weighted average from the individuals within the diagnostic groups present in the study.
Fig.1
Study Selection.
RESULTS
We identified 1,415 records after searching PubMed; of these, 233 met our inclusion criteria (Fig. 1). Given that any one individual study may have been considered in more than one publication, we attempted to identify those studies. In this case, we identified 70 longitudinal studies from the 233 publications. A total of 22 articles were omitted from analysis as the study name was not clearly identifiable. We therefore conducted the present analysis on the remaining 48 identified longitudinal studies as listed in Table 1.
Table 1
List of longitudinal studies identified from systematic review of literature
| Study | Biomarkers | Number WITH 2 | Follow-up | No of measures | Location | Reference (identified |
| measured | + measures | length | from review) | |||
| ADAPET (Alzheimer’s Disease and Positron Emission Tomography) | MRI | 50 | 5 y | 4 | South Korea | [146, 147] |
| AddNeuroMed | MRI | 378 | 1 y | 3 | Europe | [104, 148] |
| ADNI 1 (Alzheimer’s Disease Neuroimaging Initiative) | CSF, blood, MRI, FDG, PiB-PET | 800 | 6 y | 7–9 | USA | [28–110] |
| AIBL (The Australian Imaging, Biomarker &Lifestyle Flagship Study of Ageing) | MRI, PiB-PET, blood | 1100 | 4.5 y | 3 | Australia | [110, 149–159] |
| Biobank facilities of the Institute Born-Bunge | CSF | 61 | minimum 30 days | 2 | Belgium | [139] |
| BIOCARD | CSF, MRI, Plasma | 349 | 17 y max | 2.4 MRI pp | USA | [121, 122] |
| BLSA (Baltimore Longitudinal Study of Aging) | MRI | 152 | 5.9 y | up to 10 | USA | [103, 105, 116–118] |
| Bordeaux-3City | MRI | 240 | 4 y | 2 | France | [160] |
| Brain Aging Project at the University of Kansas Alzheimer and Memory Program | MRI | 109 | 2 y | 2 | USA | [161] |
| Cardiovascular Health Study (CHS) Cognition Study | MRI (plasma in subset) | 2,101 | 7 y | 2-3 | USA | [162–168] |
| Cognitive Disorders Clinic at the National Hospital for Neurology and Neurosurgery, London, England | MRI | 73 | 1 y | 2 | UK | [169] |
| Davis Alzheimer’s Disease Center longitudinal cohort | MRI | 150 | 4 y | 2 | USA | [170] |
| DIAN (Dominantly Inherited Alzheimer Network) | CSF, PiB, FDG, PET, MRI | 122 | 1.1–2.1 y | 2+ | USA, Australia, Europe, Asia, and South America | [111] |
| Framingham Cohort study | MRI | 408 | 1-2 y | 2 | USA | [171–176] |
| German Dementia Competence Network | MRI | 66 | 21 months (Average 627 days) | 2 | Germany | [177] |
| Göteborg MCI study | CSF, MRI, SPECT, EEG, Plasma | 226 | 10 y | 3 | Sweden | [178–180] |
| IDADO (Improving the early Diagnosis of Alzheimer’s Disease and Other dementias) | MRI | 71 | 2 y | 2 | The Netherlands | [181] |
| Intervention study –RAGE Aβ | CSF, MRI, plasma | 133 | 18 months | 3 MRI | USA | [182] |
| Intervention study –Gal-Int-11 | MRI | 174 | 2 y | 2 | The Netherlands | [183] |
| Intervention study - Alzheimer’s Disease Cooperative Study MCI Donepezil/Vitamin E trial | MRI | 111 | 3 y | 2 | North America | [184] |
| Intervention study - Donepezil | MRI | 93 | 1 y | 2 | Japan | [185] |
| Intervention study - Donepezil | MRI | 88 | 1.5–2 y | 2 | USA | [186] |
| Intervention study - ELND005 | MRI | 82 | 6.5 y (78 months) | 5 | North America | [187] |
| Intervention study - Memantine | MRI | 114 | 1 y | 4 | France, Germany, Switzerland, and the UK | [188] |
| Intervention study - VITACOG | MRI | 76 | 2 y | 2 | UK | [189] |
| Intervention study - Elan | MRI | 52 | 10.9 months | 2 | 5 countries | [190] |
| Johns Hopkins Alzheimer’s Disease Research Center | MRI | 75 | 3, 6, 12 months | 4 | USA | [191–193] |
| Knight Alzheimer Disease Research Center | PiB-PET | 146 | 2.6 y mean | 2 | USA | [106, 194, 195] |
| Kuopio, Finland | plasma | 263 | 3 y | 2 | Finland | [107, 196, 197] |
| LADIS (Leukoaraiosis And DISability in the elderly) prospective study | MRI | 639 | 3 y | 2 | multinational European | [198–200] |
| Malmo | CSF | 119 | 1-2 y | 2 | Sweden | [201] |
| Malmo University Hospital Memory clinic | CSF | 52 | 6 months | 2 | Sweden | [202, 203] |
| Mayo Clinic Registry | MRI (plasma) | 160 | median 3.7 y | 2 | USA | [204–206, 108] |
| Mayo Clinic Study of Aging (MCSA) | MRI, FDG PET, amyloid PET | 219 | median 1.3 y | 2+ | USA | [109, 112–115] |
| Melbourne Healthy Aging Study and Austin Health Memory Disorders Clinic | PiB-PET | 185 | 3 y max | 2-3 | Australia | [207] |
| Memory Clinic at Huddinge University Hospital | MRI, SPECT, blood | 81 | mean 16 months | 2 | Sweden | [208] |
| Memory Clinic of Higashi Matsudo Municipal Hospital | CSF, MRI | 228 | 3 y | 2+ | Japan | [209] |
| MIRIAD (Minimum Interval Resonance Imaging in AD) | MRI | 69 | 1 y | 7 | UK | [119, 120] |
| Rush Alzheimer’s Disease Center (RADC) Clinic, the Religious Order Study &the Rush Memory and Aging Project | MRI | 58 | 5 y | 2 | USA | [210] |
| OASIS (Open Access Series of Imaging Studies) | MRI | 150 | 2 y (average 719 day) | 2+ | USA | [211–215] |
| OBAS (Oregon Brain Aging Study) | MRI | 105 | 6.4 y average | 5.8 | USA | [216–219] |
| Pitea River Valley Sweden | CSF | 192 | 1 y | 2 | Sweden | [220, 221] |
| Taipei Veterans General Hospital | MRI | 78 | 1 y | 3 | Taiwan | [222] |
| UCSF Memory and Aging Center (MAC) | MRI | 68 | 1 y | 2 | USA | [223] |
| Uppsala Longitudinal Study of Adult Men | Plasma | 630 | 7 y | 2 | Sweden | [124] |
| Vu University Medical Centre | CSF, MRI | 154 | 2 y | 2 | The Netherlands | [224–226] |
| Washington Heights-Inwood Columbia Aging Project | MRI | 303 | 4.5 y | 2 | USA | [227, 228] |
| Wisconsin Registry for Alzheimer’s Prevention | MRI | 108 | 4 y | 2 | USA | [125] |
Study location
Out of the 48 longitudinal studies identified, the majority took place in North America or Europe (Fig. 2). Nineteen studies took place in the USA, many being multi-site studies as, for example, the ADNI study (see insert, Fig. 2b). Only 4 studies were conducted outside of North America/Europe, and these took place in Japan, Taiwan, and South Korea. Seven studies took place in more than one country, and these were not always specified. Figure 3 shows the countries which were identified in the studies.
Fig.2
Study locations. Proportion of studies in each country. Countries in multinational studies included: France, Germany, Switzerland, and the United Kingdom. One article did not specify in which countries the study took place. One article specified multinational European study sites, 2 articles specified North American, and 1 study featured USA, Australia, Europe, and Argentina.
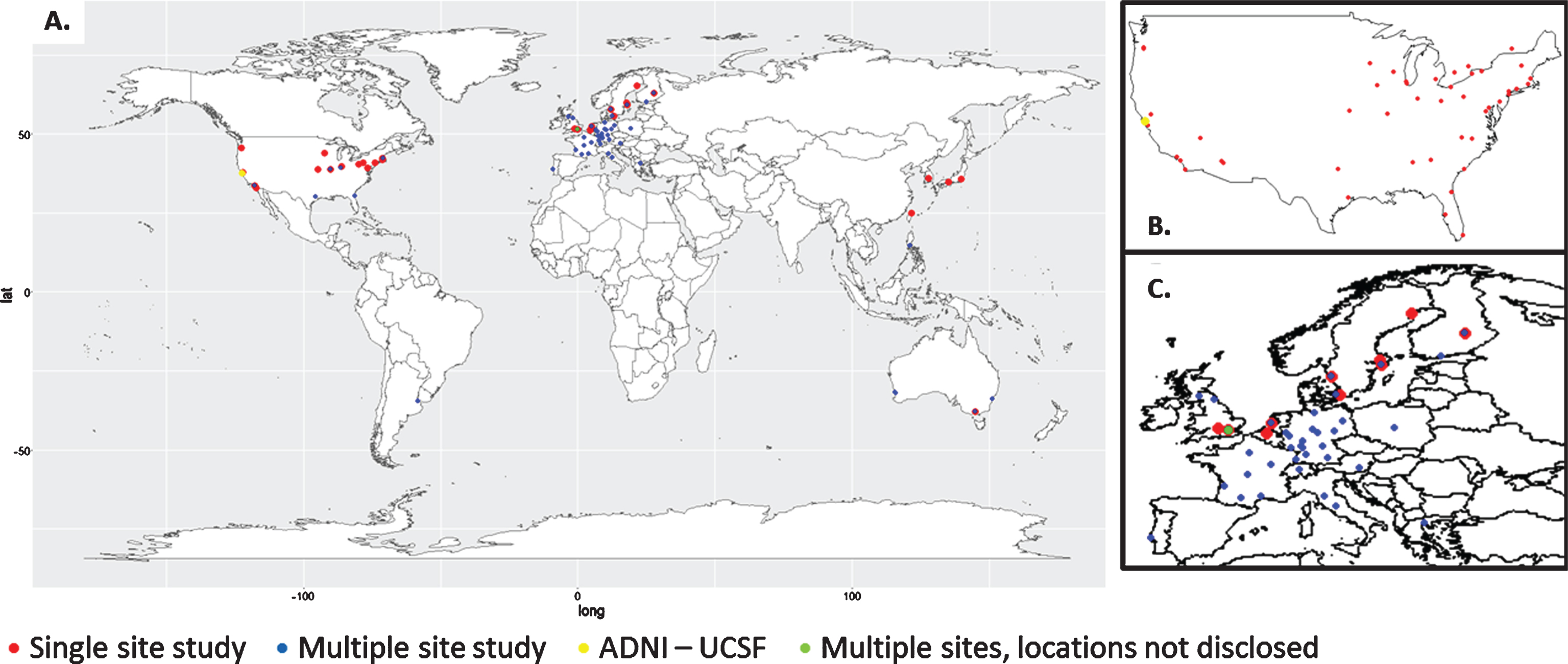
Fig.3
Study locations. A) Global distribution of studies identified in our systematic review. B) ADNI locations within the United States of America and Canada. C) Enlarged map of European studies.
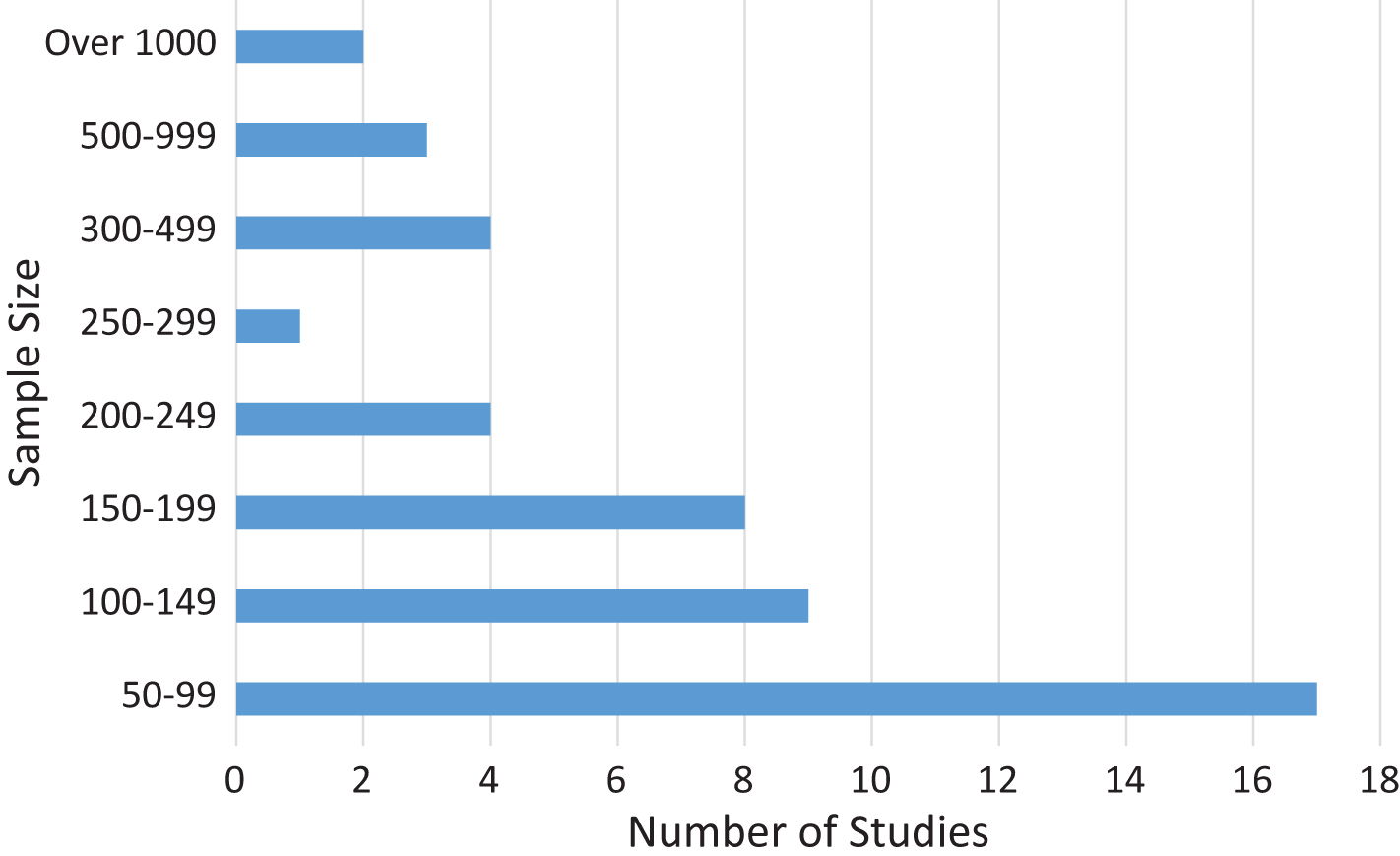
Fig.4
Sample size. The number of studies with sample size in the displayed range.
Sample size
Another important feature of the identified studies was the size of the sample that had undergone repeat assessments. While there were a number of large studies, over a third (35.4%) had less than 100 participants (studies with under 50 participants were excluded, see methods). Five studies had over 500 participants and of these only 2 had more than 1,000. Results are summarized in Fig. 4. This trend for low numbers in AD studies is most likely due to the cost involved in a number of the procedures being employed, such as MRI scanning [20].
Recruitment and selection
Given the small sample sizes within the identified studies, the recruitment and selection criteria employed were assessed. We categorized the studies into groups, based on the way recruited participants were selected, as summarized in the methods. The results are outlined in Table 2. The majority (68.45%) of the studies identified (33/48) had selectively recruited patients based on their diagnosis status. Seven studies recruited from an existing population cohort, and although this was seemingly representative of the original cohort, often the sample sizes were small –5 out of 7 having a sample of under 300 individuals. The remaining methods of study categorization are summarized in Table 2. They refer to different features of the population or address specific scientific questions. In general, there were few studies following a representative population cohort. This is likely due to the costs, length of time, and logistics involved in such studies.
Table 2
Recruitment and selection. The number of studies which used each recruitment method
| Type of recruitment | Number of |
| studies | |
| Consecutive memory clinic patients | 5 |
| Healthy but with bias toward family history of AD | 2 |
| Individuals with white matter changes | 1 |
| Subset of population cohort | 7 |
| Included based on disease status | 33 |
| Total | 48 |
Diagnosis methods used
Given that a large proportion of studies recruited participants based on the diagnosis of the participant, we considered which diagnosis criteria were commonly used. There was a good degree of consensus on this, with 37/48 studies using either the original or revised NINCDS-ADRDA diagnostic criteria for AD or probable AD [12]. Other criteria utilized included use of the Clinical Dementia Rating, for example using cut-points in the rating scale; this was done in 8 out of the 48 studies. The Diagnostic and Statistical Manual of Mental Disorders IV and III were referenced in 14/48 studies. A number of studies used different diagnosis methods, such as use of cut-points in cognitive scales and undefined “consensus diagnosis procedure”.
The diagnosis of MCI was described in 12 studies, and the Peterson criteria [21] were cited in 5 instances. In other cases, tailored criteria were described. Various methods for describing a cognitively healthy cohort were used, often featuring cognitive scores in the healthy or normal range. Overall, there was good concordance in the diagnosis techniques employed in the identified study populations.
Neuropsychological testing
Neuropsychological and cognitive testing can have numerous limitations in the context of clinical trial endpoints, including insensitivity at early stages [22]. Many tests are designed specifically to diagnose or assess dementia and are not specific to AD. Additionally, the wide variety of tests available are not always comparable, for example, they measure differing aspects of cognition or function. From the 48 studies identified, over 90 different tests were referenced. These tests were done in various combinations in the different studies. There appeared to be no pattern in the selection of the tests, other than the grouping into domains such as memory, executive function or attention.
The Mini-Mental State Examination (MMSE) was the most commonly used test out of all the identified studies (64.6%). This is not surprising, given its ease and speed of administration. It was designed to differentiate dementia from other psychiatric illnesses. It has demonstrated specificity in diagnosing dementia, but is not sensitive enough to diagnose AD [23]. The Alzheimer’s Disease Assessment Scale (ADAS) is a battery of tests which are used to assess cognitive and non-cognitive dysfunctions in people with AD [5]. The cognitive arm (ADAS-Cog) is made up of 11 tasks, which assess memory, language, praxis, attention among other cognitive abilities. Despite its common use in clinical trials of treatments for symptomatic patients, only 9 out of the 48 studies referred to using the ADAS-cog assessment. Other articles referred to a “comprehensive battery” or did not describe the tests in detail in the methods sections of the publications. Surprisingly, 3 out of the 48 studies do not reference use of cognitive tests. We were unable to discern if this was because they were not performed or if they were omitted from the publication.
Biomarkers measured
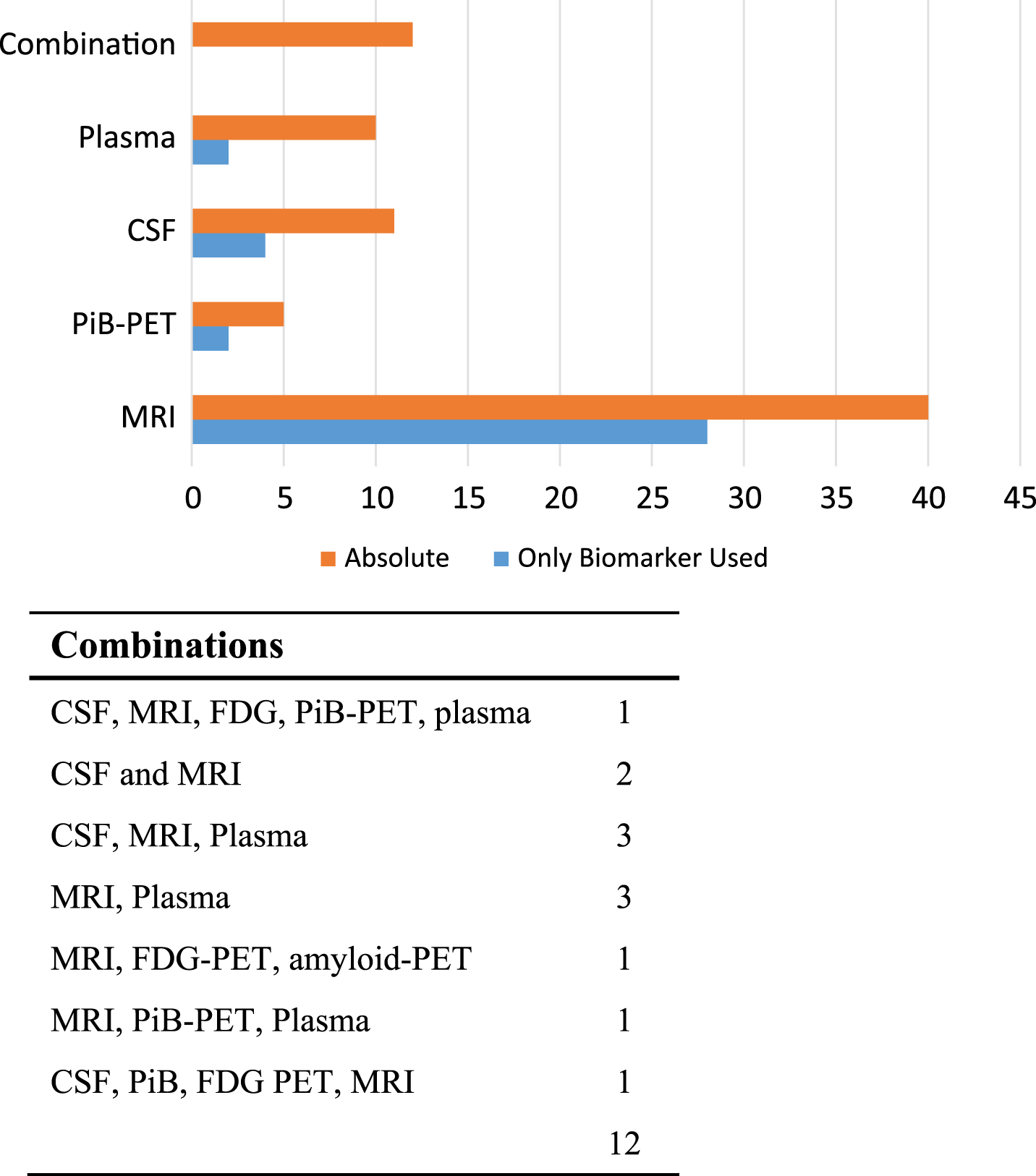
Of primary importance in this review were the different biomarkers which were measured in the studies. CSF and blood biomarker studies have been comprehensively reviewed recently by Olsson et al. [14]; however, these did not discriminate those which had performed repeat measures. It is somewhat surprising, that in total, only 48 named studies had performed repeat biomarker measurements. Monitoring the levels of these markers is highly desirable within clinical trial design, improving diagnosis and developing prognostic tools among other things. Even within the 48 studies, a majority of them (28/48) (Fig. 5) had only used MRI scans of whole brain or hippocampal atrophy, which is not a measure specific to AD. For instance, reduced hippocampal volume has been found in multiple conditions, including Parkinson’s disease, Huntington’s disease, and following traumatic brain injury [24, 25]. This may also be a result of the fact that many studies were interested in forms of dementia other than AD. Reduced glucose metabolism as assessed by FDG-PET has been shown to discriminate between regional differences in AD and normal subjects [26]. It has been shown to identify AD in 88% of cases, with a sensitivity of 94% and a specificity of 73% [27]. Despite this, only 3 of our selected studies (ADNI [28–110], DIAN [111] and the MCSA [109, 112–115]) made use of FDG-PET. CSF was assayed in only 9 studies. In other cases, reference to blood testing was made without always describing the full range of biomarkers that were analyzed. Our results are summarized in Fig. 5.
Fig.5
Biomarkers measured. Number of studies measuring each biomarker. The table presents the number of studies that measured a combination of biomarkers.
Number of time points measured and follow-up time
During our search, we observed that many studies had compared biomarker level at one point in time for diagnostic use only. However, these studies do not document the changes that occur over time. We assessed the number of repeat measures which had been taken from an individual (Fig. 6). Out of the 48 identified studies, 28 (58.33%) had performed measurements at just 2 separate times. There was often a lack of clarity in the literature, with a different number of repeats taken in different patients. This was due to various reasons, for example, death, drop out, and illness. Three studies (ADNI [28–110], BLSA [103, 105, 116–118] and MIRIAD [119, 120]) took more than 6 repeat measures. These were, however, limited to MRI measures of brain volume and atrophy, which are not specific measures of AD.
Fig.6
Length of follow-up and number of repeat measures. A) Number of studies with average follow-up in each interval. B) Number of studies with repeat measures.
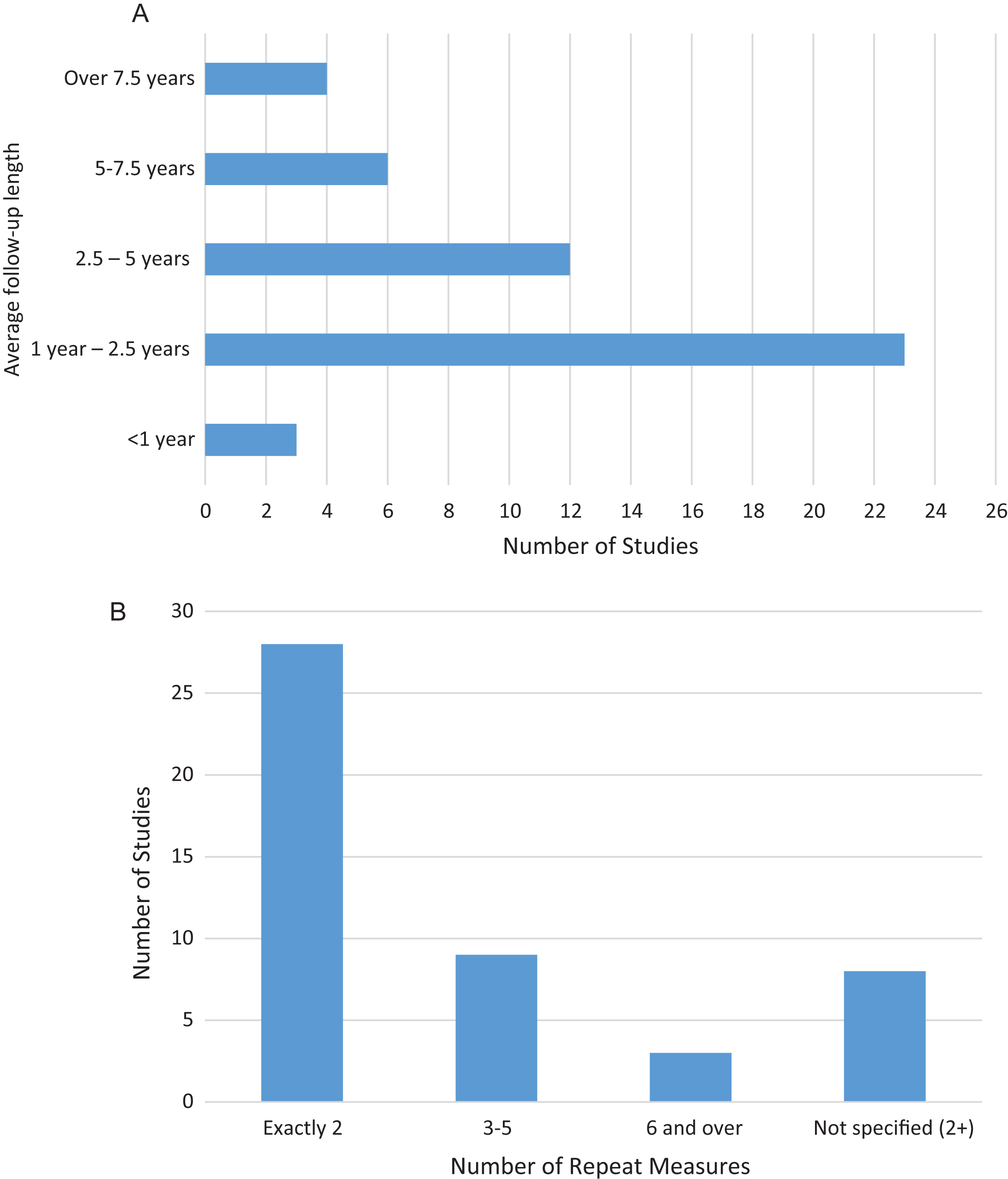
Another important feature of the follow-up measure is the amount of time between measurements. The average time between the initial and last measure taken in the studies is presented in Fig. 6. 26 out of the 48 studies took all measures in less than 2.5 years, representing a fairly short follow-up for a disease with such protracted development, often lasting decades. Only 10 studies return to patients at 5 years or more following the first measure. The longest described follow-up was 17 years in the BIOCARD study [121, 122]. We also found a lack of clarity surrounding repeat measures in the literature, with the precise timing of the protocols not being clearly described, with only average intervals for entire groups being presented. In most of the cases, the follow-up was not uniform between participants. It should be noted, however, that some studies were not complete at the time of writing, so the number of, and length of, follow-up measures may increase over time.
Average age of participants
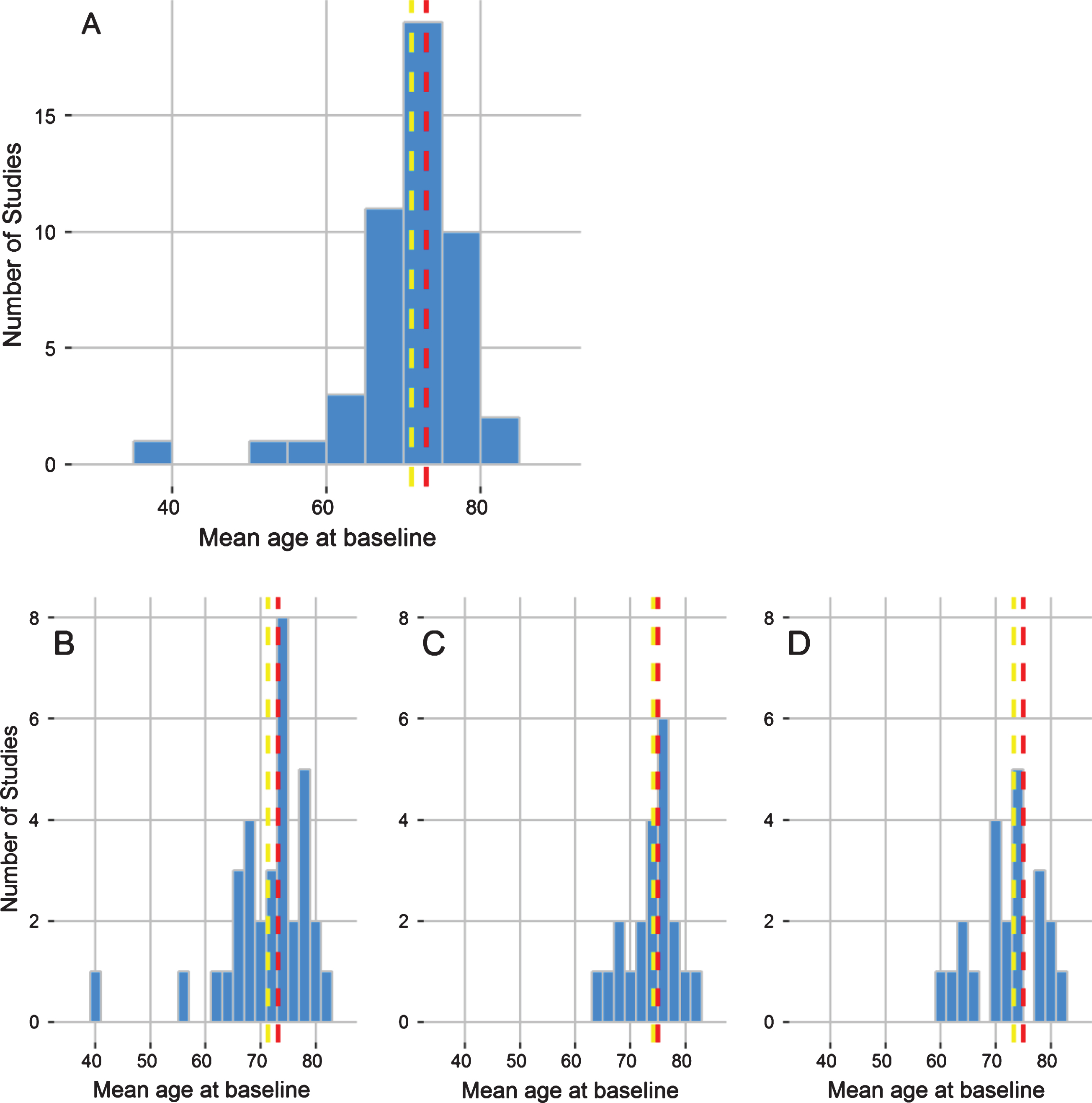
It has been postulated that the preclinical phase of AD can begin as early as 40 years old or younger [13, 123]; however, profiles of AD biomarkers in young individuals have not been characterized frequently. The average age of the participants in the studies we identified is 71 years, with a median age of 73 years (Fig. 7). One study identified included two birth-year cohorts performed in Uppsala, Sweden, where men were recruited for a 70-year-old cohort and 77-year-old cohort [124]. Although 19 out of the 48 studies (39.6%) recruited participants who were 60 years old or younger, it was very rare for any participants to be under 50 years old. One of the only studies to assess young individuals was the DIAN Study, which specifically recruits young healthy individuals, from families with a history of AD, to assess the progression of Early Onset AD (EOAD). Benzinger et al. presented findings for participants with an average age of 39.3 (SD 9.46) and 38.8 (SD 10.4). The other study which clearly utilized under 50s was the Wisconsin Registry for Alzheimer’s Prevention. Okonkwo et al. [125] also aim to study younger individuals from families with a history of AD.
Fig.7
Age of participants. Histogram outlining the dispersion of mean age of each study population identified by the systematic review. The yellow line corresponds to the overall mean age, taken as the average of each study population, and the red line to the median age of study participants across the systematic review.
DISCUSSION
Our aim was to review the literature to identify cohort studies which incorporated longitudinal measurements of AD biomarkers and MRI imaging. The work presented builds upon the previous systematic reviews performed by Olsson et al. [14] and McGhee et al. [19], and focuses on well-established AD biomarkers (Aβ, tau, and PET markers) in cohorts comprised of at least 50 subjects. It has been 6 years since Jack et al. first proposed the hypothetical temporal model of AD progression [126]. Understanding the dynamics of these measures is of obvious importance for planning clinical trials of possible therapies in preclinical patients. This will also facilitate the development of mathematical models for the prediction of AD development and progression. However, finding data to support such models has proven difficult. We have observed that there is a lack of studies which measure AD biomarkers in a representative population cohort over long periods of time. This work adds to the recently published review by McGhee and colleagues [20], with a focus on characterizing the age and geographical location of patients with repeat measurements over time. In general, our findings agree with those of McGhee et al., in that there is not sufficient research to support the adoption of any pathological biomarker, a system for selecting biomarkers, such as that proposed by McGhee et al., should therefore be adopted in the future.
Although there appears to be a bias in the location of AD studies toward Europe and North America, these areas are those shown to be most affected by the increasing prevalence of dementia [127]. It is therefore not surprising that these are the most studied regions. However, while the incidence of AD seems to be stable or perhaps slightly declining in developed countries (possibly due to improved cardiovascular health) [128], the incidence of dementia in developing countries is predicted to increase [1]. More studies in these countries are therefore desirable. The small sample sizes of the cohorts followed was striking, with over a third following just 50–100 patients. This is most likely due to the large costs of biomarker measurement. In the cost analysis presented by Silverman et al., which is estimated from Medicare values, an FDG-PET scan was listed as $1,661, an MRI as up to $1,294.17, and neuropsychological testing as just $84.33 [20]. It is clear that cheaper alternatives to these imaging scans are desperately needed. Indeed, efforts are now focused on the development of blood based biomarkers [129]. If this is achieved, they will enable much larger populations to be screened for preclinical markers.
We found a paucity of representative population cohorts that measure biomarkers over time. This implies that there are limitations to what can be concluded from the identified studies on the temporal dynamics of biomarkers. Such information is highly desirable in any assessment of possible therapies. The most common method of recruitment for these studies is to select participants based on their diagnosis. This has led to a bias towards studying AD in patients who are either mildly cognitively impaired or those who have progressed to the disease state. There is therefore a lack of knowledge of the entire disease spectrum by age within the general population. Meaning that the process which causes AD may well be a part of aging rather than a pathogenic process. A number of the selected studies did not recruit an ethnically representative sample. For example, in the ADNI study, over 90% of participants had White Caucasian ancestry in comparison to the US population, where 63% have this ethnicity. In particular, Blacks, Asians, and Hispanics were underrepresented in this sample. Studies tended to exclude participants with health conditions other than AD. Given that AD is thought to be a disease of mixed pathology, and occurs concomitantly with other diseases of old age, it can be argued that studies of comorbid individuals are of real value. At present, they are scarce in the literature. It would be of further interest to compare the profile of early against late onset AD, with age as a central stratification in assessing the dynamics of change in biomarkers over time.
Braak et al. demonstrated that tau tangles and Aβ plaques can be identified in the brains of individuals from as young as age 20 and 40, respectively [123]. It is therefore of importance to connect this pathology with CSF, blood, and brain imaging markers. One important current challenge facing the development of preclinical preventative treatments for AD is assessing efficacy in a clinically healthy pre-onset population. This makes the dynamics of change in biomarker measures in younger populations of great importance. We found that the average age studied was 70–75 years old, with individuals under 50 rarely included in the studies identified. A relatively small number of existing studies have measured these biomarkers in young populations. Patenico et al. assessed a group of 21–63-year-olds for CSF biomarkers (Aβ, p-tau, and t-tau) and demonstrated that they were significantly different from older age groups [130]. Blomberg et al. assayed participants as young as 45 and found a positive correlation between CSF tau levels and age [131]. This was also demonstrated by Sjogren et al., who additionally set reference values for CSF tau in the age clusters 21–50, 51–70, and 71–93 years. They demonstrated different profiles in these age groups [132]. In order to build on these findings, larger populations of young cohorts should ideally be studied.
It was clear that the NINCDS-ARDRA criteria have been well adopted in published studies as these were widely used for diagnosis. This consensus in measurement techniques is important in drawing epidemiological comparisons between studies. Studies on variability in different clinician’s diagnosis (the recording of measurement error due to the person making the diagnosis) for the same patient at one point in time is of obvious importance. This has been done for the NINCDS-ARDA criteria; Lopez et al. found “fair” to “substantial” agreement between clinicians when they diagnosed 40 patients with blinded notes [133]. More recently, Khan et al. surveyed 2,618 patients and found high intra-class correlation for ADAS-Cog [134]. Repeated diagnosis by different clinicians at one point in time would also aid in our understanding of variance. Computerized tests would also help in reducing variance due to human subjectivity. A good understanding of variance in measurement (both biomarkers, cognitive tests and brain scans) is critical in evaluating useful clinical trial endpoints.
Progression of AD is frequently quantified by cognitive measures. As such they have been frequently used as an endpoint in published clinical trials [6]. However, we found that there is no consensus on which tests are most useful in monitoring disease progression, as a large variety of different tests were used in the identified studies (over 90). Tests were used in different combinations, making it difficult to compare results from the different studies. Furthermore, overall composite scores were calculated in different ways. The most commonly used test identified was the MMSE; however, this test has limited diagnostic accuracy [135] in addition to being an unreliable predictor of conversion to a disease state [136]. The ADAS-Cog also has a number of limitations including a non-linear relationship with disease progression and ceiling effects [137, 138]. Improvements in the sensitivity and specificity of these measures are therefore needed. At present, there is a lack of standardized cognitive measures in the field. Standardized cognitive measures, as well as a comprehensive understanding of the variance associated with them, is also critical to their use as clinical trial endpoints.
We found that MRI was performed far more frequently than any other biomarker measures. The main caveat associated with it, is that it is not a specific measure for AD, meaning that any abnormalities detected could signal other disease types [24, 25]. Amyloid-PET and FDG-PET scans are more specific to AD [26, 27], but were infrequently used in comparison to other measures. This is likely due to the more involved procedure as well as the need for expensive equipment. While CSF and blood were taken in a number of studies, different biomarkers were often measured with different assays, again leading to a lack of comparability between studies. Much work has been done on the diagnostic performance of CSF Aβ and tau. For example, Olsson et al. demonstrated that CSF T-tau, p-tau, Aβ42, NFL and plasma T-tau were strongly associated with AD [14]. However, future work should focus on the timing and order that these biomarkers become abnormal, to enable them to be more useful in a prognostic context. An understanding of the changes over time (i.e., dynamics by age and time) is also important for understanding measurement variance. CSF levels of Aβ1 - 42, T-tau, and P-tau were shown to be stable during a 30-day study [139], but longer time frames should also be considered. We found that it was most common to measure biomarkers at 2 points in time, with very few studies repeating measures more than twice. This can be attributed to the high costs of the measures used [20] or the invasiveness of CSF sample collection [140]. Earlier and more frequent measures would facilitate more robust conclusions to be drawn on the precise timing and etiology of AD pathology. In addition, they would help to define a pre-clinical AD profile to aid in the design of trials of possible therapies. However, it is recognized that because of the invasiveness of the procedure of sample collection patient compliance to repeated sampling may be understandably problematic.
As this work was not intended to be a full systematic review, some relevant studies may have been omitted from the analysis. More published articles could be screened, to identify studies excluded by our selection criteria. Several studies were omitted from our analysis as the study population was unclear in the published research. The study methods were also unclear in a number of papers, meaning that our findings may not be truly representative of the work completed. The search excluded all cause dementia studies, and this may have resulted in AD cases being missed from the analysis. It should also be noted that as MRI is not specifically an AD-related marker and that studies that have been identified in this review may have incorrectly classified non-AD dementia as AD, there may be further heterogeneity among the results. Tissue such as plasma and CSF may have been stored and not assayed in a number of studies. Future longitudinal analyses could, therefore, be performed on this, while the studies were not identified through reviewing the literature. Indeed, many longitudinal cohort studies freeze samples such as plasma for future analysis.
In general, there was a lack of clarity regarding reporting of the study methods in many articles, including the study location and populations sampled. Other aspects of the protocol such as recruitment criteria, cognitive tests used, and assessment schedules were sometimes not discussed in detail. A more thorough description of methods would help researchers draw comparisons between studies. Standardized reporting methods across cohort studies would help with this.
Despite the numerous limitations that presently exist within the field, there remains many new initiatives that aim to complete gaps in our current knowledge of the epidemiology of AD. The CHARIOT Register aims to enlist cognitively healthy participants from age 60 upwards, to be used as a basis for future research studies [141]. Dementias platform UK have bought together 31 UK based cohorts, in order to see if they can be useful to study dementia retrospectively [142]. The European Medical Information Framework-AD (EMIF-AD) also aims to connect Europe-wide cohorts for the benefit of AD research [143]. Other long term initiatives are taking place in Italy [144] and Japan [145] to follow-up healthy and diseased participants. Many of these new initiatives focus on collaboration between studies and data accessibility, so it is to be hoped that following a period of measurement standardization, the field will be in a good position to support clinical trials of possible therapies.
In summary, we have found that there are few studies that record longitudinal measures of AD biomarkers in well-defined and large cohorts of participants. This is of particular relevance to the development of preventative treatments, given the costs of trials that run over many years given the long period over which disease progression takes place. Therapies that induce small improvements in slowing progression would be better than no therapy at all – which is the current situation. To detect low efficacy in a relatively short time span of a few years in an as yet cognitively unimpaired population will require a much better understanding of the temporal dynamics of biomarker changes. Understanding of these temporal and age related changes will also help us to better understand the true etiology of this disease and therefore aid in the development of future treatments. At present, many new cohorts are being established, and more data pooling across studies is taking place in order to improve our knowledge. But it is clear from this review that urgent needs include better standardization in measurement, more precise determination of measurement error, better longitudinal follow-up of participants, and larger study population sizes.
ACKNOWLEDGMENTS
This study was funded by the Janssen Prevention Center.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0261r2).
REFERENCES
[1] | Prince M , Bryce R , Albanese E , Wimo A , Ribeiro W , Ferri CP ((2013) ) The global prevalence of dementia:A systematic review andmetaanalysis. Alzheimers Dement 9: , 63–75.e62. |
[2] | Small DH , Mok SS , Bornstein JC ((2001) ) Alzheimer’s disease and Abeta toxicity: From top to bottom. Nat Rev Neurosci 2: , 595–598. |
[3] | Ittner LM , Gotz J ((2011) ) Amyloid-beta and tau–a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12: , 65–72. |
[4] | Braak H , Braak E ((1991) ) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: , 239–259. |
[5] | Rosen WG , Mohs RC , Davis KL ((1984) ) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141: , 1356–1364. |
[6] | Vellas B , Andrieu S , Sampaio C , Coley N , Wilcock G ((2008) ) Endpoints for trials in Alzheimer’s disease: A European task forceconsensus. Lancet Neurol 7: , 436–450. |
[7] | Cullen B , O’Neill B , Evans JJ , Coen RF , Lawlor BA ((2007) ) A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry 78: , 790–799. |
[8] | Lyness SA , Lee AY , Zarow C , Teng EL , Chui HC ((2014) ) 10-minute delayed recall from the modified mini-mental state test predicts Alzheimer’s disease pathology. J Alzheimers Dis 39: , 575–582. |
[9] | Ozer S , Young J , Champ C , Burke M ((2016) ) A systematic review of the diagnostic test accuracy of brief cognitive tests to detect amnestic mild cognitive impairment. Int J Geriatr Psychiatry 31: , 1139–1150. |
[10] | Sperling RA , Jack CR Jr , Aisen PS ((2011) ) Testing the right target and right drug at the right stage. Sci Transl Med 3: , 111cm133. |
[11] | FDA ((2013) ) Food and Drug Administration: Draft guidance forindustry. Alzheimer’s disease: Developing drugs for the treatmentof early stage disease, https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338287.pdf Accessed 6 May. |
[12] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’sdisease: Recommendations from the National Institute onAging-Alzheimer’s Association workgroups on diagnostic guidelinesfor Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[13] | Dubois B , Hampel H , Feldman HH , Scheltens P , Aisen P , Andrieu S , Bakardjian H , Benali H , Bertram L , Blennow K , Broich K , Cavedo E , Crutch S , Dartigues JF , Duyckaerts C , Epelbaum S , Frisoni GB , Gauthier S , Genthon R , Gouw AA , Habert MO , Holtzman DM , Kivipelto M , Lista S , Molinuevo JL , O’Bryant SE , Rabinovici GD , Rowe C , Salloway S , Schneider LS , Sperling R , Teichmann M , Carrillo MC , Cummings J , Jack CR Jr ((2016) ) Preclinical Alzheimer’s disease:Definition, natural history, and diagnostic criteria. Alzheimers Dement 12: , 292–323. |
[14] | Olsson B , Lautner R , Andreasson U , Ohrfelt A , Portelius E , Bjerke M , Holtta M , Rosen C , Olsson C , Strobel G , Wu E , Dakin K , Petzold M , Blennow K , Zetterberg H ((2016) ) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol 15: , 673–684. |
[15] | Klunk WE , Engler H , Nordberg A , Wang Y , Blomqvist G , Holt DP , Bergstrom M , Savitcheva I , Huang GF , Estrada S , Ausen B , Debnath ML , Barletta J , Price JC , Sandell J , Lopresti BJ , Wall A , Koivisto P , Antoni G , Mathis CA , Langstrom B ((2004) ) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55: , 306–319. |
[16] | Silbert LC , Quinn JF , Moore MM , Corbridge E , Ball MJ , Murdoch G , Sexton G , Kaye JA ((2003) ) Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology 61: , 487–492. |
[17] | Vemuri P , Jack CR Jr ((2010) ) Role of structural MRI in Alzheimer’s disease. Alzheimers Res Ther 2: , 23. |
[18] | de Leon MJ , Ferris SH , George AE , Christman DR , Fowler JS , Gentes C , Reisberg B , Gee B , Emmerich M , Yonekura Y , Brodie J , Kricheff II , Wolf AP ((1983) ) Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am J Neuroradiol 4: , 568–571. |
[19] | McGhee DJM , Ritchie CW , Thompson PA , Wright DE , Zajicek JP , Counsell CE ((2014) ) A systematic review of biomarkers for diseaseprogression in Alzheimer’s disease. PLoS One 9: , e88854. |
[20] | Silverman DH , Gambhir SS , Huang HW , Schwimmer J , Kim S , Small GW , Chodosh J , Czernin J , Phelps ME ((2002) ) Evaluating early dementia with and without assessment of regional cerebral metabolism by PET: A comparison of predicted costs and benefits. J Nucl Med 43: , 253–266. |
[21] | Petersen RC , Smith GE , Waring SC , Ivnik RJ , Tangalos EG , Kokmen E ((1999) ) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: , 303–308. |
[22] | Raghavan N , Samtani MN , Farnum M , Yang E , Novak G , Grundman M , Narayan V , DiBernardo A ((2013) ) The ADAS-Cog revisited: Novel composite scales based on ADAS-Cog to improve efficiency in MCI and early AD trials. Alzheimers Dement 9: , S21–S31. |
[23] | Creavin ST , Wisniewski S , Noel-Storr AH , Trevelyan CM , Hampton T , Rayment D , Thom VM , Nash KJ , Elhamoui H , Milligan R , Patel AS , Tsivos DV , Wing T , Phillips E , Kellman SM , Shackleton HL , Singleton GF , Neale BE , Watton ME , Cullum S ((2016) ) Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev 1: , CD011145. |
[24] | Geuze E , Vermetten E , Bremner JD ((2005) ) MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry 10: , 160–184. |
[25] | Fotuhi M , Do D , Jack C ((2012) ) Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8: , 189–202. |
[26] | Lopresti BJ , Klunk WE , Mathis CA , Hoge JA , Ziolko SK , Lu X , Meltzer CC , Schimmel K , Tsopelas ND , DeKosky ST , Price JC ((2005) ) Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: A comparative analysis. J Nucl Med 46: , 1959–1972. |
[27] | Silverman DH , Small GW , Chang CY , Lu CS , Kung De Aburto MA , Chen W , Czernin J , Rapoport SI , Pietrini P , Alexander GE , Schapiro MB , Jagust WJ , Hoffman JM , Welsh-Bohmer KA , Alavi A , Clark CM , Salmon E , de Leon MJ , Mielke R , Cummings JL , Kowell AP , Gambhir SS , Hoh CK , Phelps ME ((2001) ) Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA 286: , 2120–2127. |
[28] | Fleishman GM , Gutman BA , Fletcher PT , Thompson PM ((2015) ) Simultaneous longitudinal registration with group-wise similarity prior. Inf Process Med Imaging 24: , 746–757. |
[29] | Lebedev AV , Westman E , Van Westen GJ , Kramberger MG , Lundervold A , Aarsland D , Soininen H , Kloszewska I , Mecocci P , Tsolaki M , Vellas B , Lovestone S , Simmons A ((2014) ) Random Forest ensembles for detection and prediction of Alzheimer’s disease with a good between-cohort robustness. Neuroimage Clin 6: , 115–125. |
[30] | Altmann A , Tian L , Henderson VW , Greicius MD ((2014) ) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 75: , 563–573. |
[31] | Apostolova LG , Hwang KS , Avila D , Elashoff D , Kohannim O , Teng E , Sokolow S , Jack CR , Jagust WJ , Shaw L , Trojanowski JQ , Weiner MW , Thompson PM , Alzheimer’s Disease Neuroimaging Initiative ((2015) ) Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology 84: , 729–737. |
[32] | Beckett LA , Harvey DJ , Gamst A , Donohue M , Kornak J , Zhang H , Kuo JH ((2010) ) The Alzheimer’s Disease Neuroimaging Initiative: Annual change in biomarkers and clinical outcomes. Alzheimers Dement 6: , 257–264. |
[33] | Bernal-Rusiel JL , Greve DN , Reuter M , Fischl B , Sabuncu MR ((2013) ) Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage 66: , 249–260. |
[34] | Carmichael O , McLaren DG , Tommet D , Mungas D , Jones RN ((2013) ) Coevolution of brain structures in amnestic mild cognitive impairment. Neuroimage 66: , 449–456. |
[35] | Chang YL , Fennema-Notestine C , Holland D , McEvoy LK , Stricker NH , Salmon DP , Dale AM , Bondi MW ((2014) ) APOE interacts with age to modify rate of decline in cognitive and brain changes in Alzheimer’s disease. Alzheimers Dement 10: , 336–348. |
[36] | Chen K , Langbaum JB , Fleisher AS , Ayutyanont N , Reschke C , Lee W , Liu X , Bandy D , Alexander GE , Thompson PM , Foster NL , Harvey DJ , de Leon MJ , Koeppe RA , Jagust WJ , Weiner MW , Reiman EM ((2010) ) Twelve-month metabolic declines in probable Alzheimer’s disease and amnestic mild cognitive impairment assessed using an empirically pre-defined statistical region-of-interest: Findings from the Alzheimer’s Disease Neuroimaging Initiative. Neuroimage 51: , 654–664. |
[37] | Chen K , Roontiva A , Thiyyagura P , Lee W , Liu X , Ayutyanont N , Protas H , Luo JL , Bauer R , Reschke C , Bandy D , Koeppe RA , Fleisher AS , Caselli RJ , Landau S , Jagust WJ , Weiner MW , Reiman EM ((2015) ) Improved power for characterizing longitudinal amyloid-beta PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med 56: , 560–566. |
[38] | Chiang GC , Insel PS , Tosun D , Schuff N , Truran-Sacrey D , Raptentsetsang S , Jack CR Jr , Weiner MW ((2011) ) Identifying cognitively healthy elderly individuals with subsequent memory decline by using automated MR temporoparietal volumes. Radiology 259: , 844–851. |
[39] | Chincarini A , Bosco P , Gemme G , Esposito M , Rei L , Squarcia S , Bellotti R , Minthon L , Frisoni G , Scheltens P , Frolich L , Soininen H , Visser PJ , Nobili F ((2014) ) Automatic temporal lobe atrophy assessment in prodromal AD: Data from the DESCRIPA study. Alzheimers Dement 10: , 456–467. |
[40] | Clarkson MJ , Ourselin S , Nielsen C , Leung KK , Barnes J , Whitwell JL , Gunter JL , Hill DL , Weiner MW , Jack CR Jr , Fox NC ((2009) ) Comparison of phantom and registration scaling corrections using the ADNI cohort. Neuroimage 47: , 1506–1513. |
[41] | Da X , Toledo JB , Zee J , Wolk DA , Xie SX , Ou Y , Shacklett A , Parmpi P , Shaw L , Trojanowski JQ , Davatzikos C ((2014) ) Integration and relative value of biomarkers for prediction of MCI to AD progression: Spatial patterns of brain atrophy, cognitive scores, APOE genotype and CSF biomarkers. Neuroimage Clin 4: , 164–173. |
[42] | Davatzikos C , Bhatt P , Shaw LM , Batmanghelich KN , Trojanowski JQ ((2011) ) Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging 32: , 2322.e2319–2327. |
[43] | Dodge HH , Zhu J , Harvey D , Saito N , Silbert LC , Kaye JA , Koeppe RA , Albin RL ((2014) ) Biomarker progressions explain highervariability in stage-specific cognitive decline than baseline values in Alzheimerdisease. Alzheimers Dement 10: , 690–703. |
[44] | Fjell AM , Walhovd KB , Fennema-Notestine C , McEvoy LK , Hagler DJ , Holland D , Brewer JB , Dale AM ((2010) ) CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. J Neurosci 30: , 2088–2101. |
[45] | Fjell AM , Westlye LT , Grydeland H , Amlien I , Espeseth T , Reinvang I , Raz N , Dale AM , Walhovd KB ((2014) ) Accelerating cortical thinning: Unique to dementia or universal in aging? Cereb Cortex 24: , 919–934. |
[46] | Franko E , Joly O ((2013) ) Evaluating Alzheimer’s disease progression using rate of regional hippocampal atrophy. PLoS One 8: , e71354. |
[47] | Gomar JJ , Conejero-Goldberg C , Davies P , Goldberg TE ((2014) ) Extension and refinement of the predictive value of different classes of markers in ADNI: Four-year follow-up data. Alzheimers Dement 10: , 704–712. |
[48] | Guo H , Song X , Schmidt MH , Vandorpe R , Yang Z , LeBlanc E , Zhang J , Beyea S , Zhang Y , Rockwood K ((2014) ) Evaluation of whole brain health in aging and Alzheimer’s disease: A standard procedure for scoring an MRI-based brain atrophy and lesion index. J Alzheimers Dis 42: , 691–703. |
[49] | Guo LH , Alexopoulos P , Wagenpfeil S , Kurz A , Perneczky R ((2013) ) Brain size and the compensation of Alzheimer’s disease symptoms: A longitudinal cohort study. Alzheimers Dement 9: , 580–586. |
[50] | Haight TJ , Jagust WJ ((2012) ) Relative contributions of biomarkers in Alzheimer’s disease. Ann Epidemiol 22: , 868–875. |
[51] | Haight TJ , Landau SM , Carmichael O , Schwarz C , DeCarli C , Jagust WJ ((2013) ) Dissociable effects of Alzheimer disease and white matter hyperintensities on brain metabolism. JAMA Neurol 70: , 1039–1045. |
[52] | Holland D , Desikan RS , Dale AM , McEvoy LK ((2012) ) Rates of decline in Alzheimer disease decrease with age. PLoS One 7: , e42325. |
[53] | Hua X , Hibar DP , Lee S , Toga AW , Jack CR Jr , Weiner MW , Thompson PM ((2010) ) Sex and age differences in atrophic rates: An ADNI studywith n = 1368 MRI scans. Neurobiol Aging 31: , 1463–1480. |
[54] | Hua X , Lee S , Hibar DP , Yanovsky I , Leow AD , Toga AW , Jack CR Jr , Bernstein MA , Reiman EM , Harvey DJ , Kornak J , Schuff N , Alexander GE , Weiner MW , Thompson PM ((2010) ) Mapping Alzheimer’s disease progression in 1309 MRI scans: Power estimates for different inter-scan intervals. Neuroimage 51: , 63–75. |
[55] | Hua X , Lee S , Yanovsky I , Leow AD , Chou YY , Ho AJ , Gutman B , Toga AW , Jack CR Jr , Bernstein MA , Reiman EM , Harvey DJ , Kornak J , Schuff N , Alexander GE , Weiner MW , Thompson PM ((2009) ) Optimizing power to track brain degeneration in Alzheimer’s disease and mild cognitive impairment with tensor-based morphometry: An ADNI study of 515 subjects. Neuroimage 48: , 668–681. |
[56] | Jack CR Jr , Vemuri P , Wiste HJ , Weigand SD , Aisen PS , Trojanowski JQ , Shaw LM , Bernstein MA , Petersen RC , Weiner MW , Knopman DS ((2011) ) Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol 68: , 1526–1535. |
[57] | Jagust WJ , Bandy D , Chen K , Foster NL , Landau SM , Mathis CA , Price JC , Reiman EM , Skovronsky D , Koeppe RA ((2010) ) The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 6: , 221–229. |
[58] | Jedynak BM , Lang A , Liu B , Katz E , Zhang Y , Wyman BT , Raunig D , Jedynak CP , Caffo B , Prince JL ((2012) ) A computational neurodegenerative disease progression score: Method and results with the Alzheimer’s Disease Neuroimaging Initiative cohort. Neuroimage 63: , 1478–1486. |
[59] | Kantarci K , Gunter JL , Tosakulwong N , Weigand SD , Senjem MS , Petersen RC , Aisen PS , Jagust WJ , Weiner MW , Jack CR Jr ((2013) ) Focal hemosiderin deposits and beta-amyloid load in the ADNI cohort. Alzheimers Dement 9: , S116–S123. |
[60] | Landau SM , Fero A , Baker SL , Koeppe R , Mintun M , Chen K , Reiman EM , Jagust WJ ((2015) ) Measurement of longitudinal beta-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med 56: , 567–574. |
[61] | Landau SM , Lu M , Joshi AD , Pontecorvo M , Mintun MA , Trojanowski JQ , Shaw LM , Jagust WJ ((2013) ) Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 74: , 826–836. |
[62] | Lee GJ , Lu PH , Hua X , Lee S , Wu S , Nguyen K , Teng E , Leow AD , Jack CR Jr , Toga AW , Weiner MW , Bartzokis G , Thompson PM ((2012) ) Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biol Psychiatry 71: , 814–821. |
[63] | Leow AD , Yanovsky I , Parikshak N , Hua X , Lee S , Toga AW , Jack CR Jr , Bernstein MA , Britson PJ , Gunter JL , Ward CP , Borowski B , Shaw LM , Trojanowski JQ , Fleisher AS , Harvey D , Kornak J , Schuff N , Alexander GE , Weiner MW , Thompson PM ((2009) ) Alzheimer’s disease neuroimaging initiative: A one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage 45: , 645–655. |
[64] | Leung KK , Bartlett JW , Barnes J , Manning EN , Ourselin S , Fox NC ((2013) ) Cerebral atrophy in mild cognitive impairment and Alzheimer disease: Rates and acceleration. Neurology 80: , 648–654. |
[65] | Lillemark L , Sorensen L , Pai A , Dam EB , Nielsen M ((2014) ) Brain region’s relative proximity as marker for Alzheimer’s disease based on structural MRI. BMC Med Imaging 14: , 21. |
[66] | Lo RY , Hubbard AE , Shaw LM , Trojanowski JQ , Petersen RC , Aisen PS , Weiner MW , Jagust WJ ((2011) ) Longitudinal change of biomarkers in cognitive decline. Arch Neurol 68: , 1257–1266. |
[67] | Lo RY , Jagust WJ ((2012) ) Predicting missing biomarker data in a longitudinal study of Alzheimer disease. Neurology 78: , 1376–1382. |
[68] | Lo RY , Jagust WJ ((2013) ) Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord 27: , 343–350. |
[69] | Lo RY , Jagust WJ ((2012) ) Vascular burden and Alzheimer disease pathologic progression. Neurology 79: , 1349–1355. |
[70] | Marshall GA , Lorius N , Locascio JJ , Hyman BT , Rentz DM , Johnson KA , Sperling RA ((2014) ) Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer’s disease spectrum. J Alzheimers Dis 41: , 719–728. |
[71] | Mattila J , Koikkalainen J , Virkki A , Simonsen A , van Gils M , Waldemar G , Soininen H , Lotjonen J ((2011) ) A disease statefingerprint for evaluation of Alzheimer’s disease. JAlzheimers Dis 27: , 163–176. |
[72] | Mattsson N , Insel PS , Donohue M , Landau S , Jagust WJ , Shaw LM , Trojanowski JQ , Zetterberg H , Blennow K , Weiner MW ((2015) ) Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer’s disease. Brain 138: , 772–783. |
[73] | McEvoy LK , Holland D , Hagler DJ Jr , Fennema-Notestine C , Brewer JB , Dale AM ((2011) ) Mild cognitive impairment: Baseline and longitudinal structural MR imaging measures improve predictive prognosis. Radiology 259: , 834–843. |
[74] | Misra C , Fan Y , Davatzikos C ((2009) ) Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: Results from ADNI. Neuroimage 44: , 1415–1422. |
[75] | Morra JH , Tu Z , Apostolova LG , Green AE , Avedissian C , Madsen SK , Parikshak N , Toga AW , Jack CR Jr , Schuff N , Weiner MW , Thompson PM ((2009) ) Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. Neuroimage 45: , S3–15. |
[76] | Mouiha A , Duchesne S ((2012) ) Toward a dynamic biomarker model in Alzheimer’s disease. J Alzheimers Dis 30: , 91–100. |
[77] | Nazeri A , Ganjgahi H , Roostaei T , Nichols T , Zarei M ((2014) ) Imaging proteomics for diagnosis, monitoring and prediction of Alzheimer’s disease. Neuroimage 102 Pt 2: , 657–665. |
[78] | Nettiksimmons J , Harvey D , Brewer J , Carmichael O , DeCarli C , JackCR Jr , Petersen R , Shaw LM , Trojanowski JQ , Weiner MW , Beckett L ((2010) ) Subtypes based on cerebrospinal fluid and magneticresonance imaging markers in normal elderly predictcognitive decline. Neurobiol Aging 31: , 1419–1428. |
[79] | Rasmussen JM , Lakatos A , van Erp TG , Kruggel F , Keator DB , Fallon JT , Macciardi F , Potkin SG , Alzheimer’s Disease Neuroimaging Initiative ((2012) ) Empirical derivation of thereference region for computing diagnostic sensitive18fluorodeoxyglucose ratios in Alzheimer’s disease based onthe ADNI sample. Biochim Biophys Acta 1822: , 457–466. |
[80] | Risacher SL , Shen L , West JD , Kim S , McDonald BC , Beckett LA , Harvey DJ , Jack CR Jr , Weiner MW , Saykin AJ ((2010) ) Longitudinal MRI atrophy biomarkers: Relationship to conversion in the ADNI cohort. Neurobiol Aging 31: , 1401–1418. |
[81] | Roussotte FF , Gutman BA , Madsen SK , Colby JB , Narr KL , Thompson PM , Alzheimer’s Disease Neuroimaging Initiative ((2014) ) Apolipoprotein E epsilon 4 allele is associated with ventricular expansion rate and surface morphology in dementia and normal aging. Neurobiol Aging 35: , 1309–1317. |
[82] | Roy K , Pepin LC , Philiossaint M , Lorius N , Becker JA , Locascio JJ , Rentz DM , Sperling RA , Johnson KA , Marshall GA ((2014) ) Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer’s disease spectrum. J Alzheimers Dis 42: , 291–300. |
[83] | Runtti H , Mattila J , van Gils M , Koikkalainen J , Soininen H , Lotjonen J ((2014) ) Quantitative evaluation of disease progression in a longitudinal mild cognitive impairment cohort. J Alzheimers Dis 39: , 49–61. |
[84] | Shaffer JL , Petrella JR , Sheldon FC , Choudhury KR , Calhoun VD , Coleman RE , Doraiswamy PM ((2013) ) Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology 266: , 583–591. |
[85] | Skup M , Zhu H , Wang Y , Giovanello KS , Lin JA , Shen D , Shi F , Gao W , Lin W , Fan Y , Zhang H ((2011) ) Sex differences in grey matter atrophy patterns among AD and aMCI patients: Results from ADNI. Neuroimage 56: , 890–906. |
[86] | Tang X , Holland D , Dale AM , Younes L , Miller MI ((2014) ) Shape abnormalities of subcortical and ventricular structures in mild cognitive impairment and Alzheimer’s disease: Detecting, quantifying, and predicting. Hum Brain Mapp 35: , 3701–3725. |
[87] | Toledo JB , Bjerke M , Da X , Landau SM , Foster NL , Jagust W , Jack C Jr , Weiner M , Davatzikos C , Shaw LM , Trojanowski JQ ((2015) ) Nonlinear association between cerebrospinal fluid and florbetapir F-18 (-amyloid measures across the spectrum of Alzheimer disease. JAMA Neurol 72: , 571–581 |
[88] | Toledo JB , Da X , Weiner MW , Wolk DA , Xie SX , Arnold SE , Davatzikos C , Shaw LM , Trojanowski JQ ((2014) ) CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol 127: , 621–632. |
[89] | Toledo JB , Vanderstichele H , Figurski M , Aisen PS , Petersen RC , Weiner MW , Jack CR Jr , Jagust W , Decarli C , Toga AW , Toledo E , Xie SX , Lee VM , Trojanowski JQ , Shaw LM ((2011) ) Factors affecting Abeta plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol 122: , 401–413. |
[90] | Toledo JB , Xie SX , Trojanowski JQ , Shaw LM ((2013) ) Longitudinalchange in CSF Tau and Abeta biomarkers for up to 48 months inADNI. Acta Neuropathol 126: , 659–670. |
[91] | Tosun D , Schuff N , Shaw LM , Trojanowski JQ , Weiner MW ((2011) ) Relationship between CSF biomarkers of Alzheimer’s disease and rates of regional cortical thinning in ADNI data. J Alzheimers Dis 26: (Suppl 3), 77–90. |
[92] | Trzepacz PT , Yu P , Bhamidipati PK , Willis B , Forrester T , Tabas L , Schwarz AJ , Saykin AJ ((2013) ) Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement 9: , S95–S104.e101. |
[93] | Wee CY , Yap PT , Shen D ((2013) ) Prediction of Alzheimer’s disease and mild cognitive impairment using cortical morphological patterns. Hum Brain Mapp 34: , 3411–3425. |
[94] | Westman E , Aguilar C , Muehlboeck JS , Simmons A ((2013) ) Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer’s disease and mild cognitive impairment. Brain Topogr 26: , 9–23. |
[95] | Wolz R , Heckemann RA , Aljabar P , Hajnal JV , Hammers A , Lotjonen J , Rueckert D ((2010) ) Measurement of hippocampal atrophy using 4D graph-cut segmentation: Application to ADNI. Neuroimage 52: , 109–118. |
[96] | Zhang D , Liu J , Shen D ((2012) ) Temporally-constrained group sparse learning for longitudinal data analysis. Med Image Comput Comput Assist Interv 15: , 264–271. |
[97] | Zhang D , Shen D ((2012) ) Predicting future clinical changes of MCI patients using longitudinal and multimodal biomarkers. PLoS One 7: , e33182. |
[98] | Zhang N , Song X , Zhang Y , Chen W , D’Arcy RC , Darvesh S , Fisk JD , Rockwood K ((2011) ) An MRI brain atrophy and lesion index to assess the progression of structural changes in Alzheimer’s disease, mild cognitive impairment, and normal aging: A follow-up study. J Alzheimers Dis 26: (Suppl 3), 359–367. |
[99] | Zhou B , Nakatani E , Teramukai S , Nagai Y , Fukushima M ((2012) ) Risk classification in mild cognitive impairment patients for developing Alzheimer’s disease. J Alzheimers Dis 30: , 367–375. |
[100] | Spampinato MV , Rumboldt Z , Hosker RJ , Mintzer JE ((2011) ) Apolipoprotein E and gray matter volume loss in patients with mild cognitive impairment and Alzheimer disease. Radiology 258: , 843–852. |
[101] | Wang Y , Fan Y , Bhatt P , Davatzikos C ((2010) ) High-dimensional pattern regression using machine learning: From medical images to continuous clinical variables. Neuroimage 50: , 1519–1535. |
[102] | Fjell AM , Walhovd KB , Fennema-Notestine C , McEvoy LK , Hagler DJ , Holland D , Brewer JB , Dale AM ((2009) ) One-year brain atrophy evident in healthy aging. J Neurosci 29: , 15223–15231. |
[103] | Wang Y , Resnick SM , Davatzikos C ((2014) ) Analysis of spatio-temporal brain imaging patterns by Hidden Markov Models and serial MRI images. Hum Brain Mapp 35: , 4777–4794. |
[104] | Spulber G , Niskanen E , Macdonald S , Kivipelto M , Padilla DF , Julkunen V , Hallikainen M , Vanninen R , Wahlund LO , Soininen H ((2012) ) Evolution of global and local grey matter atrophy on serial MRI scans during the progression from MCI to AD. Curr Alzheimer Res 9: , 516–524. |
[105] | Davatzikos C , Xu F , An Y , Fan Y , Resnick SM ((2009) ) Longitudinal progression of Alzheimer’s-like patterns of atrophy in normal older adults: The SPARE-AD index. Brain 132: , 2026–2035. |
[106] | Harari O , Cruchaga C , Kauwe JS , Ainscough BJ , Bales K , Pickering EH , Bertelsen S , Fagan AM , Holtzman DM , Morris JC , Goate AM ((2014) ) Phosphorylated tau-A(42 ratio as a continuous trait for biomarker discovery for early-stage Alzheimer’s disease in multiplex immunoassay panels of cerebrospinal fluid. Biol Psychiatry 75: , 723–731. |
[107] | van Gils M , Koikkalainen J , Mattila J , Herukka S , Lotjonen J , Soininen H ((2010) ) Discovery and use of efficient biomarkers for objective disease state assessment in Alzheimer’s disease. Conf Proc IEEE Eng Med Biol Soc 2010: , 2886–2889. |
[108] | Jack CR Jr , Vemuri P , Wiste HJ , Weigand SD , Lesnick TG , Lowe V , Kantarci K , Bernstein MA , Senjem ML , Gunter JL , Boeve BF , Trojanowski JQ , Shaw LM , Aisen PS , Weiner MW , Petersen RC , Knopman DS ((2012) ) Shapes of the trajectories of 5 major biomarkers of Alzheimer disease. Arch Neurol 69: , 856–867. |
[109] | Whitwell JL , Wiste HJ , Weigand SD , Rocca WA , Knopman DS , Roberts RO , Boeve BF , Petersen RC , Jack CR Jr ((2012) ) Comparison of imaging biomarkers in the Alzheimer Disease Neuroimaging Initiative and the Mayo Clinic Study of Aging. Arch Neurol 69: , 614–622. |
[110] | Burnham SC , Faux NG , Wilson W , Laws SM , Ames D , Bedo J , Bush AI , Doecke JD , Ellis KA , Head R , Jones G , Kiiveri H , Martins RN , Rembach A , Rowe CC , Salvado O , Macaulay SL , Masters CL , Villemagne VL ((2014) ) A blood-based predictor for neocortical Abeta burden in Alzheimer’s disease: Results from the AIBL study. Mol Psychiatry 19: , 519–526. |
[111] | Benzinger TL , Blazey T , Jack CR Jr , Koeppe RA , Su Y , Xiong C , Raichle ME , Snyder AZ , Ances BM , Bateman RJ , Cairns NJ , Fagan AM , Goate A , Marcus DS , Aisen PS , Christensen JJ , Ercole L , Hornbeck RC , Farrar AM , Aldea P , Jasielec MS , Owen CJ , Xie X , Mayeux R , Brickman A , McDade E , Klunk W , Mathis CA , Ringman J , Thompson PM , Ghetti B , Saykin AJ , Sperling RA , Johnson KA , Salloway S , Correia S , Schofield PR , Masters CL , Rowe C , Villemagne VL , Martins R , Ourselin S , Rossor MN , Fox NC , Cash DM , Weiner MW , Holtzman DM , Buckles VD , Moulder K , Morris JC ((2013) ) Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A 110: , E4502–E4509. |
[112] | Jack CR Jr , Knopman DS , Weigand SD , Wiste HJ , Vemuri P , Lowe V , Kantarci K , Gunter JL , Senjem ML , Ivnik RJ , Roberts RO , Rocca WA , Boeve BF , Petersen RC ((2012) ) An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71: , 765–775. |
[113] | Jack CR Jr , Wiste HJ , Weigand SD , Knopman DS , Lowe V , Vemuri P , Mielke MM , Jones DT , Senjem ML , Gunter JL , Gregg BE , Pankratz VS , Petersen RC ((2013) ) Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81: , 1732–1740. |
[114] | Jones DT , Vemuri P , Murphy MC , Gunter JL , Senjem ML , Machulda MM , Przybelski SA , Gregg BE , Kantarci K , Knopman DS , Boeve BF , Petersen RC , Jack CR Jr ((2012) ) Non-stationarity in the “resting brain’s” modular architecture. PLoS One 7: , e39731. |
[115] | Knopman DS , Jack CR Jr , Wiste HJ , Weigand SD , Vemuri P , Lowe VJ , Kantarci K , Gunter JL , Senjem ML , Mielke MM , Roberts RO , Boeve BF , Petersen RC ((2013) ) Selective worsening of brain injury biomarker abnormalities in cognitively normal elderly persons with beta-amyloidosis. JAMA Neurol 70: , 1030–1038. |
[116] | Driscoll I , Beydoun MA , An Y , Davatzikos C , Ferrucci L , Zonderman AB , Resnick SM ((2012) ) Midlife obesity and trajectories of brain volume changes in older adults. Hum Brain Mapp 33: , 2204–2210. |
[117] | Driscoll I , Davatzikos C , An Y , Wu X , Shen D , Kraut M , Resnick SM ((2009) ) Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 72: , 1906–1913. |
[118] | Moffat SD , Szekely CA , Zonderman AB , Kabani NJ , Resnick SM ((2000) ) Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 55: , 134–136. |
[119] | Malone IB , Cash D , Ridgway GR , MacManus DG , Ourselin S , Fox NC , Schott JM ((2013) ) MIRIAD–Public release of a multiple time point Alzheimer’s MR imaging dataset. Neuroimage 70: , 33–36. |
[120] | Smith SM , Rao A , De Stefano N , Jenkinson M , Schott JM , Matthews PM , Fox NC ((2007) ) Longitudinal and cross-sectional analysis of atrophy in Alzheimer’s disease: Cross-validation of BSI, SIENA and SIENAX. Neuroimage 36: , 1200–1206. |
[121] | Miller MI , Younes L , Ratnanather JT , Brown T , Trinh H , Lee DS , Tward D , Mahon PB , Mori S , Albert M ((2015) ) Amygdalar atrophy insymptomatic Alzheimer’s disease based on diffeomorphometry: TheBIOCARD cohort. Neurobiol Aging 36: (Suppl 1), S3–S10. |
[122] | Younes L , Albert M , Miller MI ((2014) ) Inferring changepoint times of medial temporal lobe morphometric change in preclinical Alzheimer’s disease. Neuroimage Clin 5: , 178–187. |
[123] | Braak H , Thal DR , Ghebremedhin E , Del Tredici K ((2011) ) Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol 70: , 960–969. |
[124] | Sundelof J , Giedraitis V , Irizarry MC , Sundstrom J , Ingelsson E , Ronnemaa E , Arnlov J , Gunnarsson MD , Hyman BT , Basun H , Ingelsson M , Lannfelt L , Kilander L ((2008) ) Plasma beta amyloid and the risk of Alzheimer disease and dementia in elderly men: A prospective, population-based cohort study. Arch Neurol 65: , 256–263. |
[125] | Okonkwo OC , Xu G , Dowling NM , Bendlin BB , Larue A , Hermann BP , Koscik R , Jonaitis E , Rowley HA , Carlsson CM , Asthana S , Sager MA , Johnson SC ((2012) ) Family history of Alzheimer disease predicts hippocampal atrophy in healthy middle-aged adults. Neurology 78: , 1769–1776. |
[126] | Jack CR Jr , Knopman DS , Jagust WJ , Shaw LM , Aisen PS , Weiner MW , Petersen RC , Trojanowski JQ ((2010) ) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: , 119–128. |
[127] | Ferri CP , Prince M , Brayne C , Brodaty H , Fratiglioni L , Ganguli M , Hall K , Hasegawa K , Hendrie H , Huang Y , Jorm A , Mathers C , Menezes PR , Rimmer E , Scazufca M ((2005) ) Global prevalence of dementia: A Delphi consensus study. Lancet 366: , 2112–2117. |
[128] | Wu YT , Fratiglioni L , Matthews FE , Lobo A , Breteler MM , Skoog I , Brayne C ((2016) ) Dementia in western Europe: Epidemiological evidence and implications for policy making. Lancet Neurol 15: , 116–124. |
[129] | Henriksen K , O’Bryant SE , Hampel H , Trojanowski JQ , Montine TJ , Jeromin A , Blennow K , Lonneborg A , Wyss-Coray T , Soares H , Bazenet C , Sjogren M , Hu W , Lovestone S , Karsdal MA , Weiner MW ((2014) ) The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement 10: , 115–131. |
[130] | Paternico D , Galluzzi S , Drago V , Bocchio-Chiavetto L , Zanardini R , Pedrini L , Baronio M , Amicucci G , Frisoni GB ((2012) ) Cerebrospinal fluid markers for Alzheimer’s disease in a cognitively healthy cohort of young and old adults. Alzheimers Dement 8: , 520–527. |
[131] | Blomberg M , Jensen M , Basun H , Lannfelt L , Wahlund LO ((2001) ) Cerebrospinal fluid tau levels increase with age in healthy individuals. Dement Geriatr Cogn Disord 12: , 127–132. |
[132] | Sjogren M , Vanderstichele H , Agren H , Zachrisson O , Edsbagge M , Wikkelso C , Skoog I , Wallin A , Wahlund LO , Marcusson J , Nagga K , Andreasen N , Davidsson P , Vanmechelen E , Blennow K ((2001) ) Tau and Abeta42 in cerebrospinal fluid from healthy adults 21-93 years of age: Establishment of reference values. Clin Chem 47: , 1776–1781. |
[133] | Lopez OL , Swihart AA , Becker JT , Reinmuth OM , Reynolds CF 3rd , Rezek DL , Daly FL 3rd ((1990) ) Reliability of NINCDS-ADRDA clinical criteria for the diagnosis of Alzheimer’s disease. Neurology 40: , 1517–1522. |
[134] | Khan A , Yavorsky C , DiClemente G , Opler M , Liechti S , Rothman B , Jovic S ((2013) ) Reliability of the Alzheimer’s disease assessment scale (ADAS-Cog) in longitudinal studies. Curr Alzheimer Res 10: , 952–963. |
[135] | Chapman KR , Bing-Canar H , Alosco ML , Steinberg EG , Martin B , Chaisson C , Kowall N , Tripodis Y , Stern RA ((2016) ) Mini Mental State Examination and Logical Memory scores for entry into Alzheimer’s disease trials. Alzheimers Res Ther 8: , 9. |
[136] | Arevalo-Rodriguez I , Smailagic N , Roque IFM , Ciapponi A , Sanchez-Perez E , Giannakou A , Pedraza OL , Bonfill Cosp X , Cullum S ((2015) ) Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 3: , CD010783. |
[137] | Doraiswamy PM , Kaiser L , Bieber F , Garman RL ((2001) ) The Alzheimer’s Disease Assessment Scale: Evaluation of psychometric properties and patterns of cognitive decline in multicenter clinical trials of mild to moderate Alzheimer’s disease. Alzheimer Dis Assoc Disord 15: , 174–183. |
[138] | Cano SJ , Posner HB , Moline ML , Hurt SW , Swartz J , Hsu T , Hobart JC ((2010) ) The ADAS-cog in Alzheimer’s disease clinical trials: Psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatry 81: , 1363–1368. |
[139] | Le Bastard N , Aerts L , Sleegers K , Martin JJ , Van Broeckhoven C , De Deyn PP , Engelborghs S ((2013) ) Longitudinal stability of cerebrospinal fluid biomarker levels: Fulfilled requirement for pharmacodynamic markers in Alzheimer’s disease. J Alzheimers Dis 33: , 807–822. |
[140] | Magin P , Juratowitch L , Dunbabin J , McElduff P , Goode S , Tapley A , Pond D ((2016) ) Attitudes to Alzheimer’s disease testing of Australian general practice patients: A cross-sectional questionnaire-based study. Int J Geriatr Psychiatry 31: , 361–366. |
[141] | Larsen ME , Curry L , Mastellos N , Robb C , Car J , Middleton LT ((2015) ) Development of the CHARIOT research register for the prevention of Alzheimer’s dementia and other late onset neurodegenerative diseases. PLoS One 10: , e0141806. |
[142] | DPUK, Dementias Platform UK - MRC, http://www.dementiasplatform.uk/, Accessed 24/05–2016. |
[143] | EMIF, The European Medical Information Framework-AD http://www.emif.eu/about/emif-ad, Accessed 24/05–2016. |
[144] | Cavedo E , Redolfi A , Angeloni F , Babiloni C , Lizio R , Chiapparini L , Bruzzone MG , Aquino D , Sabatini U , Alesiani M , Cherubini A , Salvatore E , Soricelli A , Vernieri F , Scrascia F , Sinforiani E , Chiarati P , Bastianello S , Montella P , Corbo D , Tedeschi G , Marino S , Baglieri A , De Salvo S , Carducci F , Quattrocchi CC , Cobelli M , Frisoni GB ((2014) ) The Italian Alzheimer’s Disease Neuroimaging Initiative (I-ADNI): Validation of structural MR imaging. J Alzheimers Dis 40: , 941–952. |
[145] | Iwatsubo T ((2010) ) Japanese Alzheimer’s Disease Neuroimaging Initiative: Present status and future. Alzheimers Dement 6: , 297–299. |
[146] | Kim YJ , Cho H , Kim YJ , Ki CS , Chung SJ , Ye BS , Kim HJ , Kim JH , Kim ST , Lee KH , Jeon S , Lee JM , Chin J , Kim JH , Na DL , Seong JK , Seo SW ((2015) ) Apolipoprotein e4 affects topographical changes in hippocampal and cortical atrophy in Alzheimer’s disease dementia: A five-year longitudinal study. J Alzheimers Dis 44: , 1075–1085. |
[147] | Cho H , Kim JH , Kim C , Ye BS , Kim HJ , Yoon CW , Noh Y , Kim GH , Kim YJ , Kim JH , Kim CH , Kang SJ , Chin J , Kim ST , Lee KH , Na DL , Seong JK , Seo SW ((2014) ) Shape changes of the basal ganglia and thalamus in Alzheimer’s disease: A three-year longitudinal study. J Alzheimers Dis 40: , 285–295. |
[148] | Simmons A , Westman E , Muehlboeck S , Mecocci P , Vellas B , Tsolaki M , Kloszewska I , Wahlund LO , Soininen H , Lovestone S , Evans A , Spenger C ((2011) ) The AddNeuroMed framework for multi-centre MRI assessment of Alzheimer’s disease: Experience from the first 24 months. Int J Geriatr Psychiatry 26: , 75–82. |
[149] | Pietrzak RH , Lim YY , Neumeister A , Ames D , Ellis KA , Harrington K , Lautenschlager NT , Restrepo C , Martins RN , Masters CL , Villemagne VL , Rowe CC , Maruff P ((2015) ) Amyloid-beta, anxiety, and cognitive decline in preclinical Alzheimer disease: A multicenter, prospective cohort study. JAMA Psychiatry 72: , 284–291. |
[150] | Ellis KA , Szoeke C , Bush AI , Darby D , Graham PL , Lautenschlager NT , Macaulay SL , Martins RN , Maruff P , Masters CL , McBride SJ , Pike KE , Rainey-Smith SR , Rembach A , Robertson J , Rowe CC , Savage G , Villemagne VL , Woodward M , Wilson W , Zhang P , Ames D ((2014) ) Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL). Int Psychogeriatr 26: , 543–554. |
[151] | Rembach A , Doecke JD , Roberts BR , Watt AD , Faux NG , Volitakis I , Pertile KK , Rumble RL , Trounson BO , Fowler CJ , Wilson W , Ellis KA , Martins RN , Rowe CC , Villemagne VL , Ames D , Masters CL , AIBL research group, Bush AI ((2013) ) Longitudinal analysis of serum copper and ceruloplasmin in Alzheimer’s disease. J Alzheimers Dis 34: , 171–182. |
[152] | Rembach A , Watt AD , Wilson WJ , Rainey-Smith S , Ellis KA , Rowe CC , Villemagne VL , Macaulay SL , Bush AI , Martins RN , Ames D , Masters CL , Doecke JD ((2014) ) An increased neutrophil-lymphocyte ratio in Alzheimer’s disease is a function of age and is weakly correlated with neocortical amyloid accumulation. J Neuroimmunol 273: , 65–71. |
[153] | Rembach A , Watt AD , Wilson WJ , Villemagne VL , Burnham SC , Ellis KA , Maruff P , Ames D , Rowe CC , Macaulay SL , Bush AI , Martins RN , Masters CL , Doecke JD ((2014) ) Plasma amyloid-beta levels are significantly associated with a transition toward Alzheimer’s disease as measured by cognitive decline and change in neocortical amyloid burden. J Alzheimers Dis 40: , 95–104. |
[154] | Dore V , Villemagne VL , Bourgeat P , Fripp J , Acosta O , Chetelat G , Zhou L , Martins R , Ellis KA , Masters CL , Ames D , Salvado O , Rowe CC ((2013) ) Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol 70: , 903–911. |
[155] | Villemagne VL , Burnham S , Bourgeat P , Brown B , Ellis KA , Salvado O , Szoeke C , Macaulay SL , Martins R , Maruff P , Ames D , Rowe CC , Masters CL ((2013) ) Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol 12: , 357–367. |
[156] | Rembach A , Faux NG , Watt AD , Pertile KK , Rumble RL , Trounson BO , Fowler CJ , Roberts BR , Perez KA , Li QX , Laws SM , Taddei K , Rainey-Smith S , Robertson JS , Vandijck M , Vanderstichele H , Barnham KJ , Ellis KA , Szoeke C , Macaulay L , Rowe CC , Villemagne VL , Ames D , Martins RN , Bush AI , Masters CL ((2014) ) Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer’s disease. Alzheimers Dement 10: , 53–61. |
[157] | Villain N , Chetelat G , Grassiot B , Bourgeat P , Jones G , Ellis KA , Ames D , Martins RN , Eustache F , Salvado O , Masters CL , Rowe CC , Villemagne VL ((2012) ) Regional dynamics of amyloid-beta deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: A voxelwise PiB-PET longitudinal study. Brain 135: , 2126–2139. |
[158] | Chetelat G , Villemagne VL , Pike KE , Baron JC , Bourgeat P , Jones G , Faux NG , Ellis KA , Salvado O , Szoeke C , Martins RN , Ames D , Masters CL , Rowe CC ((2010) ) Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain 133: , 3349–3358. |
[159] | Pietrzak RH , Lim YY , Ames D , Harrington K , Restrepo C , Martins RN , Rembach A , Laws SM , Masters CL , Villemagne VL , Rowe CC , Maruff P ((2015) ) Trajectories of memory decline in preclinical Alzheimer’s disease: Results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of ageing. Neurobiol Aging 36: , 1231–1238. |
[160] | Bernard C , Helmer C , Dilharreguy B , Amieva H , Auriacombe S , Dartigues JF , Allard M , Catheline G ((2014) ) Time course of brain volume changes in the preclinical phase of Alzheimer’s disease. Alzheimers Dement 10: , 143–151.e141. |
[161] | Burns JM , Honea RA , Vidoni ED , Hutfles LJ , Brooks WM , Swerdlow RH ((2012) ) Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim Biophys Acta 1822: , 333–339. |
[162] | Boyle CP , Raji CA , Erickson KI , Lopez OL , Becker JT , Gach HM , Longstreth WT Jr , Teverovskiy L , Kuller LH , Carmichael OT , Thompson PM ((2015) ) Physical activity, body mass index, and brainatrophy in Alzheimer’s disease. Neurobiol Aging 36: (Suppl 1), S194–S202. |
[163] | Fitzpatrick AL , Kuller LH , Lopez OL , Diehr P , O’Meara ES , Longstreth WT Jr , Luchsinger JA ((2009) ) Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Arch Neurol 66: , 336–342. |
[164] | Kuller LH , Lopez OL , Jagust WJ , Becker JT , DeKosky ST , Lyketsos C , Kawas C , Breitner JC , Fitzpatrick A , Dulberg C ((2005) ) Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 64: , 1548–1552. |
[165] | Lecci F , Junker B , Kuller LH , Lopez OL , Becker JT ((2015) ) Empirically derived trajectories to dementia over 15 years of follow-up identified by using mixed membership models. Am J Epidemiol 182: , 366–374. |
[166] | Lopez OL , Kuller LH , Mehta PD , Becker JT , Gach HM , Sweet RA , Chang YF , Tracy R , DeKosky ST ((2008) ) Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology 70: , 1664–1671. |
[167] | Newman AB , Fitzpatrick AL , Lopez O , Jackson S , Lyketsos C , Jagust W , Ives D , Dekosky ST , Kuller LH ((2005) ) Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 53: , 1101–1107. |
[168] | Podewils LJ , Guallar E , Beauchamp N , Lyketsos CG , Kuller LH , Scheltens P ((2007) ) Physical activity and white matter lesion progression: Assessment using MRI. Neurology 68: , 1223–1226. |
[169] | Ridha BH , Barnes J , van de Pol LA , Schott JM , Boyes RG , Siddique MM , Rossor MN , Scheltens P , Fox NC ((2007) ) Application of automated medial temporal lobe atrophy scale to Alzheimer disease. Arch Neurol 64: , 849–854. |
[170] | Maillard P , Carmichael O , Fletcher E , Reed B , Mungas D , DeCarli C ((2012) ) Coevolution of white matter hyperintensities and cognition in the elderly. Neurology 79: , 442–448. |
[171] | Debette S , Wolf PA , Beiser A , Au R , Himali JJ , Pikula A , Auerbach S , Decarli C , Seshadri S ((2009) ) Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology 73: , 2071–2078. |
[172] | Jefferson AL , Himali JJ , Beiser AS , Au R , Massaro JM , Seshadri S , Gona P , Salton CJ , DeCarli C , O’Donnell CJ , Benjamin EJ , Wolf PA , Manning WJ ((2010) ) Cardiac index is associated with brain aging: The Framingham Heart Study. Circulation 122: , 690–697. |
[173] | Jefferson AL , Massaro JM , Wolf PA , Seshadri S , Au R , Vasan RS , Larson MG , Meigs JB , Keaney JF Jr , Lipinska I , Kathiresan S , Benjamin EJ , DeCarli C ((2007) ) Inflammatory biomarkers are associated with total brain volume: The Framingham Heart Study. Neurology 68: , 1032–1038. |
[174] | Weinstein G , Beiser AS , Decarli C , Au R , Wolf PA , Seshadri S ((2013) ) Brain imaging and cognitive predictors of stroke and Alzheimer disease in the Framingham Heart Study. Stroke 44: , 2787–2794. |
[175] | Westwood AJ , Beiser A , Decarli C , Harris TB , Chen TC , He XM , Roubenoff R , Pikula A , Au R , Braverman LE , Wolf PA , Vasan RS , Seshadri S ((2014) ) Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology 82: , 1613–1619. |
[176] | Zade D , Beiser A , McGlinchey R , Au R , Seshadri S , Palumbo C , Wolf PA , DeCarli C , Milberg W ((2013) ) Apolipoprotein epsilon 4 allele modifies waist-to-hip ratio effects on cognition and brain structure. J Stroke Cerebrovasc Dis 22: , 119–125. |
[177] | Teipel SJ , Peters O , Heuser I , Jessen F , Maier W , Froelich L , Arlt S , Hull M , Gertz HJ , Kornhuber J , Wiltfang J , Thome J , Rienhoff O , Meindl T , Hampel H , Grothe M ((2011) ) Atrophy outcomes in multicentre clinical trials on Alzheimer’s disease: Effect of different processing and analysis approaches on sample sizes. World J Biol Psychiatry 12: (Suppl 1), 109–113. |
[178] | Bjerke M , Andreasson U , Rolstad S , Nordlund A , Lind K , Zetterberg H , Edman A , Blennow K , Wallin A ((2009) ) Subcortical vascular dementia biomarker pattern in mild cognitive impairment. Dement Geriatr Cogn Disord 28: , 348–356. |
[179] | Eckerstrom C , Olsson E , Klasson N , Berge J , Nordlund A , Bjerke M , Wallin A ((2015) ) Multimodal prediction of dementia with up to 10 years follow up: The Gothenburg MCI study. J Alzheimers Dis 44: , 205–214. |
[180] | Sagare AP , Deane R , Zetterberg H , Wallin A , Blennow K , Zlokovic BV ((2011) ) Impaired lipoprotein receptor-mediated peripheral binding of plasma amyloid-beta is an early biomarker for mild cognitive impairment preceding Alzheimer’s disease. J Alzheimers Dis 24: , 25–34. |
[181] | Schmand B , Rienstra A , Tamminga H , Richard E , van Gool WA , Caan MW , Majoie CB ((2014) ) Responsiveness of magnetic resonance imaging and neuropsychological assessment in memory clinic patients. J Alzheimers Dis 40: , 409–418. |
[182] | Galasko D , Bell J , Mancuso JY , Kupiec JW , Sabbagh MN , van Dyck C , Thomas RG , Aisen PS ((2014) ) Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology 82: , 1536–1542. |
[183] | van de Pol LA , van der Flier WM , Korf ES , Fox NC , Barkhof F , Scheltens P ((2007) ) Baseline predictors of rates of hippocampal atrophy in mild cognitive impairment. Neurology 69: , 1491–1497. |
[184] | Apostolova LG , Thompson PM , Green AE , Hwang KS , Zoumalan C , Jack CR Jr , Harvey DJ , Petersen RC , Thal LJ , Aisen PS , Toga AW , Cummings JL , Decarli CS ((2010) ) 3D comparison of low, intermediate, and advanced hippocampal atrophy in MCI. Hum Brain Mapp 31: , 786–797. |
[185] | Hashimoto M , Kazui H , Matsumoto K , Nakano Y , Yasuda M , Mori E ((2005) ) Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry 162: , 676–682. |
[186] | Wang L , Harms MP , Staggs JM , Xiong C , Morris JC , Csernansky JG , Galvin JE ((2010) ) Donepezil treatment and changes in hippocampal structure in very mild Alzheimer disease. Arch Neurol 67: , 99–106. |
[187] | Salloway S , Sperling R , Keren R , Porsteinsson AP , van Dyck CH , Tariot PN , Gilman S , Arnold D , Abushakra S , Hernandez C , Crans G , Liang E , Quinn G , Bairu M , Pastrak A , Cedarbaum JM ((2011) ) A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology 77: , 1253–1262. |
[188] | Wilkinson D , Fox NC , Barkhof F , Phul R , Lemming O , Scheltens P ((2012) ) Memantine and brain atrophy in Alzheimer’s disease: A 1-year randomized controlled trial. J Alzheimers Dis 29: , 459–469. |
[189] | Douaud G , Refsum H , de Jager CA , Jacoby R , Nichols TE , Smith SM , Smith AD ((2013) ) Preventing Alzheimer’s disease-related gray matteratrophy by B-vitamin treatment. Proc Natl Acad Sci U S A 110: , 9523–9528. |
[190] | Ridha BH , Anderson VM , Barnes J , Boyes RG , Price SL , Rossor MN , Whitwell JL , Jenkins L , Black RS , Grundman M , Fox NC ((2008) ) Volumetric MRI and cognitive measures in Alzheimer disease: Comparison of markers of progression. J Neurol 255: , 567–574. |
[191] | Soldan A , Pettigrew C , Li S , Wang MC , Moghekar A , Selnes OA , Albert M , O’Brien R ((2013) ) Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiol Aging 34: , 2827–2834. |
[192] | Nowrangi MA , Lyketsos CG , Leoutsakos JM , Oishi K , Albert M , Mori S , Mielke MM ((2013) ) Longitudinal, region-specific course of diffusion tensor imaging measures in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement 9: , 519–528. |
[193] | Fotenos AF , Snyder AZ , Girton LE , Morris JC , Buckner RL ((2005) ) Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 64: , 1032–1039. |
[194] | Vlassenko AG , Mintun MA , Xiong C , Sheline YI , Goate AM , Benzinger TL , Morris JC ((2011) ) Amyloid-beta plaque growth in cognitively normal adults: Longitudinal [11C]Pittsburgh compound B data. Ann Neurol 70: , 857–861. |
[195] | Brier MR , Thomas JB , Snyder AZ , Benzinger TL , Zhang D , Raichle ME , Holtzman DM , Morris JC , Ances BM ((2012) ) Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci 32: , 8890–8899. |
[196] | Seppala TT , Herukka SK , Hanninen T , Tervo S , Hallikainen M , Soininen H , Pirttila T ((2010) ) Plasma Abeta42 and Abeta40 as markers of cognitive change in follow-up: A prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry 81: , 1123–1127. |
[197] | Seppala TT , Koivisto AM , Hartikainen P , Helisalmi S , Soininen H , Herukka SK ((2011) ) Longitudinal changes of CSF biomarkers in Alzheimer’s disease. J Alzheimers Dis 25: , 583–594. |
[198] | Verdelho A , Madureira S , Ferro JM , Baezner H , Blahak C , Poggesi A , Hennerici M , Pantoni L , Fazekas F , Scheltens P , Waldemar G , Wallin A , Erkinjuntti T , Inzitari D ((2012) ) Physical activity prevents progression for cognitive impairment and vascular dementia: Results from the LADIS (Leukoaraiosis and Disability) study. Stroke 43: , 3331–3335. |
[199] | Verdelho A , Madureira S , Moleiro C , Ferro JM , O’Brien JT , Poggesi A , Pantoni L , Fazekas F , Scheltens P , Waldemar G , Wallin A , Erkinjuntti T , Inzitari D ((2013) ) Depressive symptoms predict cognitive decline and dementia in older people independently of cerebral white matter changes: The LADIS study. J Neurol Neurosurg Psychiatry 84: , 1250–1254. |
[200] | Ylikoski R , Jokinen H , Andersen P , Salonen O , Madureira S , Ferro J , Barkhof F , van der Flier W , Schmidt R , Fazekas F , Scheltens P , Waldemar G , Salvadori E , Pantoni L , Inzitari D , Erkinjuntti T ((2007) ) Comparison of the Alzheimer’s Disease Assessment ScaleCognitive Subscale and the Vascular Dementia Assessment Scale indifferentiating elderly individuals with different degrees ofwhite matter changes. The LADIS Study. Dement Geriatr CognDisord 24: , 73–81. |
[201] | Buchhave P , Blennow K , Zetterberg H , Stomrud E , Londos E , Andreasen N , Minthon L , Hansson O ((2009) ) Longitudinal study of CSF biomarkers in patients with Alzheimer’s disease. PLoS One 4: , e6294. |
[202] | Olsson B , Hertze J , Lautner R , Zetterberg H , Nagga K , Hoglund K , Basun H , Annas P , Lannfelt L , Andreasen N , Minthon L , Blennow K , Hansson O ((2013) ) Microglial markers are elevated in the prodromal phase of Alzheimer’s disease and vascular dementia. J Alzheimers Dis 33: , 45–53. |
[203] | Olsson B , Hertze J , Ohlsson M , Nagga K , Hoglund K , Basun H , Annas P , Lannfelt L , Andreasen N , Minthon L , Zetterberg H , Blennow K , Hansson O ((2013) ) Cerebrospinal fluid levels of heart fatty acid binding protein are elevated prodromally in Alzheimer’s disease and vascular dementia. J Alzheimers Dis 34: , 673–679. |
[204] | Jack CR Jr , Petersen RC , Xu Y , O’Brien PC , Smith GE , Ivnik RJ , Boeve BF , Tangalos EG , Kokmen E ((2000) ) Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55: , 484–489. |
[205] | Jack CR Jr , Shiung MM , Gunter JL , O’Brien PC , Weigand SD , Knopman DS , Boeve BF , Ivnik RJ , Smith GE , Cha RH , Tangalos EG , Petersen RC ((2004) ) Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62: , 591–600. |
[206] | Graff-Radford NR , Crook JE , Lucas J , Boeve BF , Knopman DS , Ivnik RJ , Smith GE , Younkin LH , Petersen RC , Younkin SG ((2007) ) Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol 64: , 354–362. |
[207] | Villemagne VL , Pike KE , Chetelat G , Ellis KA , Mulligan RS , Bourgeat P , Ackermann U , Jones G , Szoeke C , Salvado O , Martins R , O’Keefe G , Mathis CA , Klunk WE , Ames D , Masters CL , Rowe CC ((2011) ) Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 69: , 181–192. |
[208] | Wahlund LO , Julin P , Lannfelt L , Lindqvist J , Svensson L ((1999) ) Inheritance of the ApoE epsilon4 allele increases the rate of brain atrophy in dementia patients. Dement Geriatr Cogn Disord 10: , 262–268. |
[209] | Waragai M , Hata S , Suzuki T , Ishii R , Fujii C , Tokuda T , Arai H , Ohrui T , Higuchi S , Yoshida M , Igarashi K , Moriya M , Iwai N , Uemura K ((2014) ) Utility of SPM8 plus DARTEL (VSRAD) combined with magnetic resonance spectroscopy as adjunct techniques for screening and predicting dementia due to Alzheimer’s disease in clinical practice. J Alzheimers Dis 41: , 1207–1222. |
[210] | Stoub TR , Bulgakova M , Leurgans S , Bennett DA , Fleischman D , Turner DA , deToledo-Morrell L ((2005) ) MRI predictors of risk of incident Alzheimer disease: A longitudinal study. Neurology 64: , 1520–1524. |
[211] | Bachman AH , Lee SH , Sidtis JJ , Ardekani BA ((2014) ) Corpus callosum shape and size changes in early Alzheimer’s disease: A longitudinal MRI study using the OASIS brain database. J Alzheimers Dis 39: , 71–78. |
[212] | Li X , Coyle D , Maguire L , Watson DR , McGinnity TM ((2011) ) Gray matter concentration and effective connectivity changes in Alzheimer’s disease: A longitudinal structural MRI study. Neuroradiology 53: , 733–748. |
[213] | Bernal-Rusiel JL , Atienza M , Cantero JL ((2010) ) Determining the optimal level of smoothing in cortical thickness analysis: A hierarchical approach based on sequential statistical thresholding. Neuroimage 52: , 158–171. |
[214] | Zhu M , Wang X , Gao W , Shi C , Ge H , Shen H , Lin Z ((2014) ) Corpus callosum atrophy and cognitive decline in early Alzheimer’s disease: Longitudinal MRI study. Dement Geriatr Cogn Disord 37: , 214–222. |
[215] | Grydeland H , Westlye LT , Walhovd KB , Fjell AM ((2013) ) Improved prediction of Alzheimer’s disease with longitudinal white matter/gray matter contrast changes. Hum Brain Mapp 34: , 2775–2785. |
[216] | Carlson NE , Moore MM , Dame A , Howieson D , Silbert LC , Quinn JF , Kaye JA ((2008) ) Trajectories of brain loss in aging and the development of cognitive impairment. Neurology 70: , 828–833. |
[217] | Erten-Lyons D , Woltjer R , Kaye J , Mattek N , Dodge HH , Green S , Tran H , Howieson DB , Wild K , Silbert LC ((2013) ) Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology 81: , 977–983. |
[218] | Howieson DB , Camicioli R , Quinn J , Silbert LC , Care B , Moore MM , Dame A , Sexton G , Kaye JA ((2003) ) Natural history of cognitive decline in the old old. Neurology 60: , 1489–1494. |
[219] | Kaye JA , Moore MM , Dame A , Quinn J , Camicioli R , Howieson D , Corbridge E , Care B , Nesbit G , Sexton G ((2005) ) Asynchronousregional brain volume losses in presymptomatic to moderate AD. J Alzheimers Dis 8: , 51–56. |
[220] | Andreasen N , Minthon L , Clarberg A , Davidsson P , Gottfries J , Vanmechelen E , Vanderstichele H , Winblad B , Blennow K ((1999) ) Sensitivity, specificity, and stability of CSF-tau in AD in a community-based patient sample. Neurology 53: , 1488–1494. |
[221] | Andreasen N , Vanmechelen E , Van de Voorde A , Davidsson P , Hesse C , Tarvonen S , Raiha I , Sourander L , Winblad B , Blennow K ((1998) ) Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: A community based follow up study. J Neurol Neurosurg Psychiatry 64: , 298–305. |
[222] | Wang PN , Liu HC , Lirng JF , Lin KN , Wu ZA ((2009) ) Accelerated hippocampal atrophy rates in stable and progressive amnestic mild cognitive impairment. Psychiatry Res 171: , 221–231. |
[223] | Krueger CE , Dean DL , Rosen HJ , Halabi C , Weiner M , Miller BL , Kramer JH ((2010) ) Longitudinal rates of lobar atrophy in frontotemporal dementia, semantic dementia, and Alzheimer’s disease. Alzheimer Dis Assoc Disord 24: , 43–48. |
[224] | Bouwman FH , van der Flier WM , Schoonenboom NS , van Elk EJ , Kok A , Rijmen F , Blankenstein MA , Scheltens P ((2007) ) Longitudinal changes of CSF biomarkers in memory clinic patients. Neurology 69: , 1006–1011. |
[225] | Sluimer JD , Bouwman FH , Vrenken H , Blankenstein MA , Barkhof F , van der Flier WM , Scheltens P ((2010) ) Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: A longitudinal study. Neurobiol Aging 31: , 758–764. |
[226] | Jongbloed W , Kester MI , van der Flier WM , Veerhuis R , Scheltens P , Blankenstein MA , Teunissen CE ((2013) ) Discriminatory and predictive capabilities of enzyme-linked immunosorbent assay and multiplex platforms in a longitudinal Alzheimer’s disease study. Alzheimers Dement 9: , 276–283. |
[227] | Brickman AM , Schupf N , Manly JJ , Stern Y , Luchsinger JA , Provenzano FA , Narkhede A , Razlighi Q , Collins-Praino L , Artero S , Akbaraly TN , Ritchie K , Mayeux R , Portet F ((2014) ) APOE epsilon4 and risk for Alzheimer’s disease: Do regionally distributed white matter hyperintensities play a role? Alzheimers Dement 10: , 619–629. |
[228] | Brickman AM , Zahodne LB , Guzman VA , Narkhede A , Meier IB , Griffith EY , Provenzano FA , Schupf N , Manly JJ , Stern Y , Luchsinger JA , Mayeux R ((2015) ) Reconsidering harbingers of dementia: Progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol Aging 36: , 27–32. |



