Living Alone with Alzheimer’s Disease: Data from SveDem, the Swedish Dementia Registry
Abstract
Background: Many people with Alzheimer’s disease (AD) live alone in their own homes. There is a lack of knowledge about whether these individuals receive the same quality of diagnostics and treatment for AD as patients who are cohabiting.
Objectives: To investigate the diagnostic work-up and treatment of community-dwelling AD patients who live alone.
Methods: We performed a cross-sectional cohort study based on data from the Swedish Dementia Registry (SveDem). We studied patients diagnosed with AD between 2007 and 2015 (n = 26,163). Information about drugs and comorbidities was acquired from the Swedish Prescribed Drug Register and the Swedish Patient Register.
Results: 11,878 (46%) patients lived alone, primarily older women. After adjusting for confounders, living alone was inversely associated with receiving computed tomography (OR 0.90; 95% CI 0.82–0.99), magnetic resonance imaging (OR 0.91; 95% CI 0.83–0.99), and lumbar puncture (OR 0.86; 95% CI 0.80–0.92). Living alone was also negatively associated with the use of cholinesterase inhibitors (OR 0.81; 95% CI 0.76; 0.87), memantine (OR 0.77; 95% CI 0.72; 0.83), and cardiovascular medication (OR 0.92; 0.86; 0.99). On the other hand, living alone was positively associated with the use of antidepressants (OR 1.15; 95% CI 1.08; 1.22), antipsychotics (OR 1.41; 95% CI 1.25; 1.58), and hypnotics and sedatives (OR 1.09; 95% CI 1.02; 1.17).
Conclusions: Solitary living AD patients do not receive the same extent of care as those who are cohabiting.
INTRODUCTION
An increasing lifespan together with changes in family structure due to the economic growth, reduced intergenerational living, and greater mobility has led to a higher number of older people who live alone in their homes [1]. In Sweden, the proportion of people aged 60 years and older in one-person households has increased from 23% in 1960 to 32% in 2012 [2]. Half of women older than 65 years live by themselves, which is about twice as high as the proportion of solitary living men [3]. While living with another person has been suggested to have a positive influence on physical and psychological health, living alone may lead to loneliness, depression, and a lower adherence to medical care [3–7].
The demographic changes also result in an increasing prevalence of age-related diseases, such as Alzheimer’s disease (AD), a syndrome characterized by cognitive decline that leads to patients’ dependency on caregivers [8]. There is no cure for AD, but treatment with cholinesterase inhibitors (ChEIs) and memantine helps to maintain patients’ cognition, behavioral, and functional status [8]. To estimate patients’ prognosis and plan health care and social resources, the correct diagnosis of AD is necessary and is usually performed as assessment of cognitive functions and by exclusion of other diseases [8]. Using biomarkers, such as hippocampal atrophy on magnetic resonance imaging (MRI) and analysis of cerebrospinal fluid by performing lumbar puncture (LP) increases the accuracy of the AD diagnosis [9].
It is estimated that 20–50% of AD patients live alone in their homes [2, 10–13]. Solitary living AD patients may be at higher risk for behavioral symptoms, having problems with safety, poor quality of nutrition, and limited access to health care [10, 12, 14]. There is a lack of data about whether people who live alone receive the same quality of diagnostics and treatment for AD. Capitalizing on a nationwide Swedish register of dementia patients, we aimed to study the association of living alone with the use of extended diagnostic work-up and prescription of drugs in AD patients.
METHODS
We performed a cross-sectional cohort study of AD patients registered in the quality register Swedish Dementia Registry (SveDem). Information about drugs and comorbidities was acquired from the Swedish Prescribed Drug Register and the Swedish Patient Register. Patients and their relatives were informed of the entry into SveDem and had a possibility to decline participation and to have their data removed at any time. Data were de-identified before analysis. This study was approved by the regional ethical review board in Stockholm, Sweden.
Registers
SveDem was established in May 2007 with the aim to register all patients at the time of the dementia diagnosis and monitor their care, as previously described in detail [15]. Briefly, patients are registered by physicians in a specialist or primary care unit with one of 8 dementia disorders: AD, mixed dementia with AD–vascular dementia (it will be further referred as mixed dementia), vascular dementia, dementia with Lewy bodies, frontotemporal dementia, Parkinson’s disease dementia, unspecified dementia, and other dementia types. At the time of dementia diagnosis, information about their age, gender, living condition, and performance of diagnostics tests is registered. Global cognitive status is assessed by Mini-Mental State Examination (MMSE) and its score is recorded.
The Swedish Prescribed Drug Register contains information on all dispensed prescriptions since July 2005 at Swedish pharmacies to the entire Swedish population [16]. Drugs are coded according to the Anatomical Therapeutic Chemical (ATC) Classification system. The Swedish Patient Register covers in-patient and out-patient care in Sweden [17]. One main diagnosis and up to 21 additional diagnoses are registered with an International Classification of Diseases (ICD) code.
Definitions
At the time of dementia diagnosis, patients’ living condition was recorded as “living alone / living with another adult / don’t know” in SveDem. Performance of diagnostic tests was registered at the time of dementia diagnosis as yes / no / don’t know. Basic dementia-work up includes MMSE, clock test, blood chemistry test, and computerized tomography (CT), as defined by the Swedish National Board of Health and Welfare [15]. Extended dementia work-up comprises MRI, LP, neuropsychological testing, positron emission tomography, electroencephalography, and assessment by physio-, occupational-, or speech therapists. Extended diagnostic tests should be performed when a basic dementia work-up is not enough to reach a diagnosis. In the present study, we selected to study the use of LP, MRI, and neuropsychological testing as a part of the extended dementia work-up.
Drugs
We used data on drugs extracted from the Swedish Prescribed Drug Register at the time of dementia diagnosis and one year after. The following ATC codes were studied: ChEIs (N06DA), memantine (N06DX01), antidepressants (N06A), anxiolytics (N05B), antipsychotics (N05A), hypnotics and sedatives (N05C), and cardiovascular drugs (C01, C02, C03, C07, C08, C09, C10). We counted the drug if the prescription appeared at least once during the specified period.
Comorbidities
We used two measures of comorbidities: total number of drugs and Charlson Comorbidity Index [18, 19]. Total number of drugs was extracted from the Swedish Prescribed Drug Register at the time of dementia diagnosis, as used in previous studies [20–23], and captures all drugs, including dementia medication. Charlson Comorbidity Index was defined as a weighted sum of 17 diseases (acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer, mild liver disease, diabetes mellitus without chronic complications, diabetes mellitus with complications, hemiplegia or paraplegia, renal disease, cancer, moderate to severe liver disease, metastatic cancer, and AIDS).
We counted the disease if the respective ICD 10 code appeared at least once as the main or contributory diagnosis in the Swedish Patient Register between 2000 and the date of dementia diagnosis obtained from SveDem. We used ICD 10 codes as suggested by Quan [24], except for dementia, which was assigned to each patient automatically. The weight of each disease was defined according to Charlson [19].
Study sample
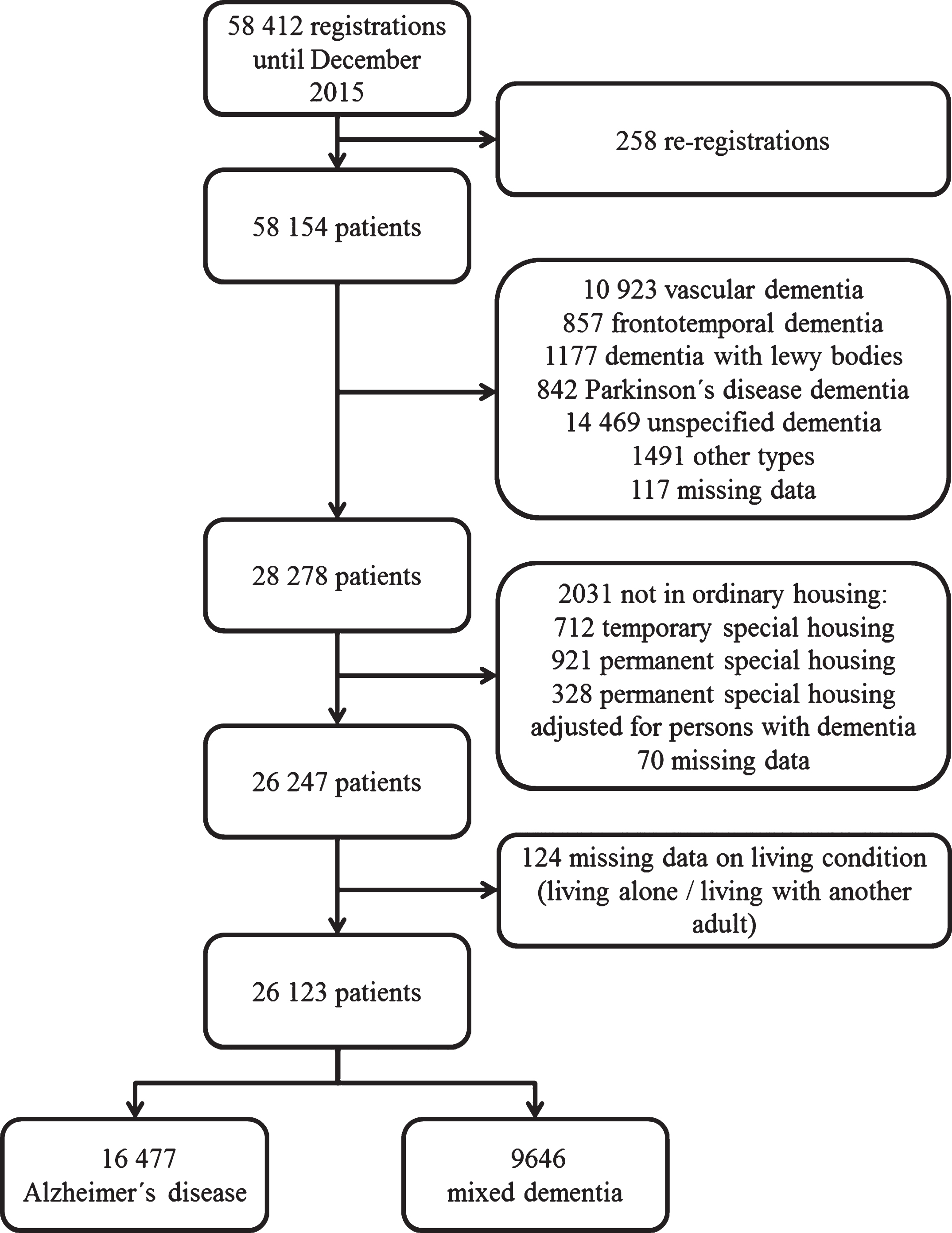
In order to have a representative sample of AD patients, we included those diagnosed with AD as well as mixed dementia. We studied patients registered to SveDem from May 2007 until December 2015 (n = 58, 412). We excluded duplicate cases (n = 258), patients with a diagnosis other than AD or mixed dementia (n = 29, 876), individuals who did not live in ordinary housing or had missing data on this variable (n = 2,013) and patients with missing data on living condition (n = 124). The final study sample consisted of 26,123 patients (Fig. 1).
Fig.1
Selection of the study population.
Statistical analysis
Descriptive data are presented as frequency (n, %), means and standard deviation (SD) or median and interquartile range (IQR). To compare characteristics of patients who lived alone versus those who lived with another adult, we used chi-square test for categorical variables, the independent-sample t test for continuous variables with normal distribution and Mann-Whitney test for continuous variables with skewed distribution.
We applied binary logistic regression to estimate odds ratios (ORs) with 95% confidence intervals (CIs) for associations of the use of diagnostic tests and drugs (as independent variables) with solitary living (as dependent variable). We adjusted for age, gender, diagnosis of mixed dementia, MMSE, and comorbidities. We ran two models with different measures of comorbidities: Model 1 with total number of drugs and Model 2 with Charlson Comorbidity index. We studied separate associations of 7 diagnostic tests (MMSE, clock test, blood chemistry test, CT, MRI, LP, and neuropsychological testing) and 7 different drug classes (ChEIs, memantine, antidepressants, anxiolytics, antipsychotics, hypnotics and sedatives, and cardiovascular drugs) and present results on 14 associations with solitary living, in each model. We used IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp. Armonk, NY) foranalysis.
RESULTS
From 26,123 patients (mean age 80 years, 62% women), 11,878 (46%) lived alone at the time of the diagnosis (Table 1). Solitary living patients were older (81 versus 77 years, p < 0.001), more frequently women (78% versus 49%, p < 0.001), had a slightly lower MMSE score (21.0 versus 21.5, p < 0.001), and were more often diagnosed with mixed dementia (41% versus 34%, p < 0.001). They received more drugs (5 versus 4, p < 0.001), but did not differ in Charlson Comorbidity Index.
Table 1
Characteristics of the patients (n = 26,123)
| Living alone | Living with another adult | p value | Missing (%) | |
| (n = 11,878) | (n = 14,245) | |||
| Basic characteristics | ||||
| Age, mean±SD | 81.2±7.2 | 77.0±7.6 | <0.001 | 0 |
| Females, n (%) | 9,294 (78.2) | 6,917 (48.6) | <0.001 | 0 |
| MMSE, mean±SD | 21.0±4.7 | 21.5±5.0 | <0.001 | 3.3 |
| Mixed dementia, n (%) | 4,810 (40.5) | 4,836 (33.9) | <0.001 | 0 |
| Basic diagnostic work-up, n (%) | ||||
| MMSE | 11,550 (97.2) | 13,736 (96.4) | <0.001 | 3.3 |
| Clock test | 10,550 (88.8) | 12,841 (90.1) | <0.001 | 1.2 |
| Blood test | 11,377 (95.8) | 13,666 (95.9) | 0.101 | 1.1 |
| CT | 10,372 (87.3) | 12,578 (88.3) | 0.006 | 1.1 |
| Extended diagnostic work-up, n (%) | ||||
| MRI | 1,334 (11.2) | 2,434 (17.1) | <0.001 | 2.0 |
| LP | 3,507 (29.5) | 6,084 (42.7) | <0.001 | 1.5 |
| Neuropsychological testing | 2,360 (19.9) | 3,617 (25.4) | <0.001 | 2.1 |
| Drugs, n (%) | ||||
| Cholinesterase inhibitors | 6,966 (58.6) | 9,825 (69.0) | <0.001 | |
| Memantine | 2,298 (19.3) | 3,466 (24.3) | <0.001 | |
| Cardiovascular drugs | 7,993 (67.3) | 9,307 (65.3) | 0.001 | |
| Antidepressant drugs | 4,147 (34.9) | 4,435 (31.1) | <0.001 | |
| Anxiolytic drugs | 2,466 (20.8) | 2,647 (18.6) | <0.001 | |
| Antipsychotic drugs | 896 (7.5) | 798 (5.6) | <0.001 | |
| Hypnotics and sedatives | 3,521 (29.6) | 3,375 (23.7) | <0.001 | |
| Comorbidities | ||||
| Charlson Comorbidity Index, median (IQR) | 2 (2) | 2 (2) | 0.06 | |
| Total number of drugs, median (IQR) | 5 (4) | 4 (4) | <0.001 |
SD, standard deviation; CT, computerized tomography; MRI, magnetic resonance imaging; LP, lumbar puncture; IQR, interquartile range.
Patients who lived alone received more frequently the MMSE test (97% versus 96%, p < 0.001), but less commonly the clock test (89% versus 90%, p < 0.001), CT (87% versus 88%, p = 0.01), LP (30% versus 43%, p < 0.001), MRI (11% versus 17%, p < 0.001), and neuropsychological testing (20% versus 25%, p < 0.001). They were treated to a lower extent with ChEIs (59% versus 69%; p < 0.001) and memantine (19% versus 24%; p < 0.01). On the other hand, they received more cardiovascular drugs (67% versus 65%; p = 0.001), antidepressants (35% versus 31%; p < 0.001), antipsychotics (8% versus 6%; p < 0.001), anxiolytics (21% versus 19%; p < 0.001), and hypnotics and sedatives (30% versus 24%; p < 0.001).
In multivariate analysis when controlled for age, gender, MMSE, diagnosis of mixed dementia, and total number of drugs (Table 2, Model 1), living alone was inversely associated with receiving CT (OR 0.90, 95% CI 0.82–0.99), MRI (OR 0.91, 95% CI 0.83–0.99), and LP (OR 0.86, 95% CI 0.80–0.92). Patients who lived alone had lower odds of being treated with ChEIs (OR 0.81; 95% CI 0.76; 0.87), memantine (OR 0.77; 95% CI 0.72; 0.83), and cardiovascular drugs (OR 0.92; 0.86; 0.99). On the other hand, living alone was associated with the use of antidepressants (OR 1.15; 95% CI 1.08; 1.22), antipsychotics (OR 1.41; 95% CI 1.25; 1.58), and hypnotics and sedatives (OR 1.09; 95% CI 1.02; 1.17). Models adjusting for Charlson Comorbidity Index gave similar results (Table 2, Model 2).
Table 2
Associations of drugs and diagnostic tests with solitary living
| OR (95% CI) | ||
| Model 1 | Model 2 | |
| Basic diagnostic work-up | ||
| MMSE | 1.01 (1.00; 1.01)* | 1.00 (1.00; 1.01) |
| Clock test | 0.92 (0.82; 1.02) | 0.91 (0.82; 1.01) |
| Blood test | 0.92 (0.77; 1.10) | 0.93 (0.79; 1.09) |
| CT | 0.90 (0.82; 0.99)* | 0.89 (0.82; 0.97)* |
| Extended diagnostic work-up | ||
| MRI | 0.91 (0.83; 0.99)* | 0.90 (0.83; 0.98)* |
| LP | 0.86 (0.80; 0.92)** | 0.86 (0.81; 0.91)** |
| Neuropsychological testing | 0.97 (0.90; 1.04) | 0.97 (0.91; 1.04) |
| Drugs | ||
| Cholinesterase inhibitors | 0.81 (0.76; 0.87)** | 0.80 (0.76; 0.85)** |
| Memantine | 0.77 (0.72; 0.83)** | 0.75 (0.70; 0.80)** |
| Cardiovascular drugs | 0.92 (0.86; 0.99)* | 0.89 (0.84; 0.94)** |
| Antidepressant drugs | 1.15 (1.08; 1.22)** | 1.11 (1.05; 1.18)** |
| Anxiolytic drugs | 0.95 (0.89; 1.03) | 0.96 (0.89; 1.02) |
| Antipsychotic drugs | 1.41 (1.25; 1.58)** | 1.39 (1.24; 1.56)** |
| Hypnotics and sedatives | 1.09 (1.02; 1.17)* | 1.08 (1.01; 1.15)* |
CT, computerized tomography; MRI, magnetic resonance imaging; LP, lumbar puncture. Each variable in this table was entered separately into the model. Model 1 is adjusted for age, gender, MMSE, diagnosis of mixed dementia and total number of drugs. Model 2 is adjusted for age, gender, MMSE, diagnosis of mixed dementia and Charlson Comorbidity Index.
DISCUSSION
We found that 46% of AD patients lived alone at the time of dementia diagnosis, in particular older women. Living alone was associated with a lower utilization of imaging and biomarker tests and less frequent prescription of dementia drugs as well as cardiovascular medication. On the other hand, solitary living was related to the use of psychotropic drugs. This study suggests that people who live alone receive less optimal diagnostic work-up and treatment for AD and indicates inequality in distribution of resources in dementia care due to livingconditions.
The proportion of solitary living AD patients in our study is higher than reported in studies from the United States, France, Belgium, and in a previous study in Sweden (20–35%) [2, 10–13], but somewhat lower than in recent studies from Germany and Denmark (51–58%), which, however, included patients with all dementia disorders [25, 26]. The number of solitary living AD patients in our study may be underestimated due to two reasons. First, we excluded patients with unspecified dementia who may have had AD, but were not diagnosed with it, likely due to their high age, comorbidities, or even solitary living. Second, SveDem is estimated to cover 35% of incident dementia cases in Sweden [23]. It is possible that patients who live alone are less likely to be diagnosed and registered in SveDem and thus are not included in this study.
Our study suggests that living alone is a barrier to the treatment of AD patients with ChEIs and memantine. The inverse association of solitary living with the use of dementia medication persisted even after adjustment for the diagnosis of mixed dementia, which is itself related to a less common use of dementia drugs [20]. Possibly, the presence of a partner or a close caregiver influences therapeutic decisions, likely because they may insist on therapy, can secure adherence to drugs, and manage their side effects. Previous studies indicate that living alone is associated with non-adherence to drugs in patients with cognitive impairment [27]. However, ChEIs and memantine are at present the only symptomatic treatment against cognitive disturbances [8]. We propose that they should be prescribed irrespective of patients’ living condition. Patients who live alone would likely benefit from more support in the management of their medications, for example from a district nurse that can ensure adherence to drugs and monitor side effects.
In agreement with our previous report [20], solitary living patients had lower odds of being treated with cardiovascular medication. Further, we showed that living alone was associated with the use of antidepressants, antipsychotics, and hypnotics and sedatives. Previous studies indicate that being alone may lead to depression, manifestation of behavioral symptoms, and sleeping problems [2, 7, 28–31]. Another possibility is that physicians are more prone to prescribe psychotropic drugs to solitary living patients than to those who live with another adult. However, benefit from these drugs in AD patients is questionable. For example, some hypnotic drugs worsen cognition, there is little evidence that antidepressants are effective for treating depression in AD and antipsychotic drugs present with serious side effects including stroke [32–34]. We propose that improvement of living arrangements of AD patients could decrease the necessity for the prescription of psychotropic drugs.
Old age has previously been shown to influence the number of performed diagnostic tests for reaching a dementia diagnosis [35]. In the present study, we found a lower utilization of CT as a part of the basic dementia work-up in patients who lived alone, which suggests a diagnostic investigation of a lower quality. The less frequent utilization of advanced tests, such as MRI and LP, further indicates a less intensive diagnostic work-up in solitary living patients. A partner or a close caregiver may influence diagnostic investigations as they can accompany patients to advanced examinations and insist on adequate tests. We suggest that solitary living persons should receive more support when undergoing investigations for the diagnosis of dementia.
This study is strengthened by a large sample size. Today, all memory clinics and 76% of primary care units in Sweden are affiliated to SveDem. Further, we used information on drugs and comorbidities derived from nationwide health registers that have a complete coverage in the country. A limitation of this study is a lack of information on how the living condition of patients varies with time. The living situation of solitary patients may change following the diagnosis of dementia, which can affect the pharmacological treatment of AD as well as diagnostic investigations, although in which direction is not clear. Future studies should investigate how the changes in living conditions following the diagnosis of AD influence its clinical management.
Another limitation is lacking information on socioeconomic and marital status, family members, geographic location, or type of care center. Moreover, SveDem covers approximately 35% cases of incident dementia [23] and it has not been studied how patients in SveDem differ from those who are not registered. Aspberg et al. suggested that patients included in a quality register are more likely to be men, younger, generally healthier, and of a higher socioeconomic status [36]. This may hold true for SveDem as well, which may limit the generalizability of our findings to a healthier and younger population that has more contact with health care, possibly leading to underestimation of the associations that we have found.
To conclude, almost half of AD patients in Sweden live alone at the time of dementia diagnosis. Our study suggests that the living arrangements influence the diagnostic work-up and therapy of AD patients and that solitary living patients are at a risk of receiving less optimal care. We emphasize that the living condition should not be a relevant factor when clinicians prescribe dementia drugs to AD patients. Further, we propose that interventions for supporting solitary living AD patients may decrease the use of antidepressants, antipsychotics, and hypnotics and sedatives in this group.
ACKNOWLEDGMENTS
The study was supported by the Swedish Research Council (grant 2012-2291), Demensfonden, Stiftelsen för Gamla Tjänarinnor, Swedish Association of Local Authorities and by the project “Sustainability for the National Institute of Mental Health” (grant LO1611), with a financial support from the Ministry of Education, Youth and Sports of the Czech Republic. The authors are grateful to SveDem for providing data for this study as well as many thanks to all the participants (patients, caregivers, and staff).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0102r1).
REFERENCES
[1] | Tomassini C , Glaser K , Wolf DA , Broese van Groenou MI , Grundy E ((2004) ) Living arrangements among older people: An overview of trends in Europe and the USA. Popul Trends 24–34. |
[2] | Wattmo C , Londos E , Minthon L ((2014) ) Solitary living in Alzheimer’s disease over 3 years: Association between cognitive and functional impairment and community-based services. Clin Interv Aging 9: , 1951–1962. |
[3] | Pimouguet C , Rizzuto D , Schon P , Shakersain B , Angleman S , Lagergren M , Fratiglioni L , Xu W ((2016) ) Impact of living alone on institutionalization and mortality: A population-based longitudinal study. Eur J Public Health 26: , 182–187. |
[4] | Fratiglioni L , Wang HX , Ericsson K , Maytan M , Winblad B ((2000) ) Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet 355: , 1315–1319. |
[5] | Hakansson K , Rovio S , Helkala EL , Vilska AR , Winblad B , Soininen H , Nissinen A , Mohammed AH , Kivipelto M ((2009) ) Association between mid-life marital status and cognitive function in later life: Population based cohort study. BMJ 339: , b2462. |
[6] | Walker D , Beauchene RE ((1991) ) The relationship of loneliness, social isolation, and physical health to dietary adequacy of independently living elderly. J Am Diet Assoc 91: , 300–304. |
[7] | Stahl ST , Beach SR , Musa D , Schulz R ((2016) ) Living alone and depression: The modifying role of the perceived neighborhood environment. Aging Ment Health, 1–7. doi: 10.1080/13607863.2016.1191060 [Epub ahead of print]. |
[8] | Winblad B , Amouyel P , Andrieu S , Ballard C , Brayne C , Brodaty H , Cedazo-Minguez A , Dubois B , Edvardsson D , Feldman H , Fratiglioni L , Frisoni GB , Gauthier S , Georges J , Graff C , Iqbal K , Jessen F , Johansson G , Jonsson L , Kivipelto M , Knapp M , Mangialasche F , Melis R , Nordberg A , Rikkert MO , Qiu C , Sakmar TP , Scheltens P , Schneider LS , Sperling R , Tjernberg LO , Waldemar G , Wimo A , Zetterberg H ((2016) ) Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol 15: , 455–532. |
[9] | Jack CR , Knopman DS , Jagust WJ , Petersen RC , Weiner MW , Aisen PS , Shaw LM , Vemuri P , Wiste HJ , Weigand SD , Lesnick TG , Pankratz VS , Donohue MC , Trojanowski JQ ((2013) ) Update on hypothetical model of Alzheimer’s disease biomarkers. Lancet Neurol 12: , 207–216. |
[10] | Webber PA , Fox P , Burnette D ((1994) ) Living alone with Alzheimer’s disease: Effects on health and social service utilization patterns. Gerontologist 34: , 8–14. |
[11] | Soto M , Andrieu S , Gares V , Cesari M , Gillette-Guyonnet S , Cantet C , Vellas B , Nourhashemi F ((2015) ) Living alone with Alzheimer’s disease and the risk of adverse outcomes: Results from the Plan de Soin et d’Aide dans la maladie d’Alzheimer Study. J Am Geriatr Soc 63: , 651–658. |
[12] | Nourhashemi F , Amouyal-Barkate K , Gillette-Guyonnet S , Cantet C , Vellas B , Group RF ((2005) ) Living alone with Alzheimer’s disease: Cross-sectional and longitudinal analysis in the REAL.FR Study. J Nutr Health Aging 9: , 117–120. |
[13] | Mets T , De Deyn PP , Pals P , De Lepeleire J , Vandewoude M , Ventura M , Ivanoiu A , Albert A , Seghers AK , COGNOS group ((2013) ) COGNOS: Care for people with cognitive dysfunction: A national observational study. Alzheimer Dis Assoc Disord 27: , 123–132. |
[14] | Hansen ML , Waldorff FB , Waldemar G ((2011) ) Prognostic factors for weight loss over 1-year period in patients recently diagnosed with mild Alzheimer disease. Alzheimer Dis Assoc Disord 25: , 269–275. |
[15] | Religa D , Fereshtehnejad SM , Cermakova P , Edlund AK , Garcia-Ptacek S , Granqvist N , Hallback A , Kawe K , Farahmand B , Kilander L , Mattsson UB , Nagga K , Nordstrom P , Wijk H , Wimo A , Winblad B , Eriksdotter M ((2015) ) SveDem, the Swedish Dementia Registry - atool for improving the quality of diagnostics, treatment and careof dementia patients in clinical practice. PLoS One 10: , e0116538. |
[16] | Wettermark B , Hammar N , Fored CM , Leimanis A , Otterblad Olausson P , Bergman U , Persson I , Sundstrom A , Westerholm B , Rosen M ((2007) ) The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 16: , 726–735. |
[17] | Ludvigsson JF , Andersson E , Ekbom A , Feychting M , Kim JL , Reuterwall C , Heurgren M , Olausson PO ((2011) ) External review and validation of the Swedish national inpatient register. BMC Public Health 11: , 450. |
[18] | Schneeweiss S , Seeger JD , Maclure M , Wang PS , Avorn J , Glynn RJ ((2001) ) Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 154: , 854–864. |
[19] | Charlson ME , Pompei P , Ales KL , MacKenzie CR ((1987) ) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: , 373–383. |
[20] | Cermakova P , Fereshtehnejad SM , Johnell K , Winblad B , Eriksdotter M , Religa D ((2014) ) Cardiovascular medication burden in dementia disorders: A nationwide study of 19,743 dementia patients in the Swedish Dementia Registry. Alzheimers Res Ther 6: , 34. |
[21] | Cermakova P , Lund LH , Fereshtehnejad SM , Johnell K , Winblad B , Dahlstrom U , Eriksdotter M , Religa D ((2015) ) Heart failure and dementia: Survival in relation to types of heart failure and different dementia disorders. Eur J Heart Fail 17: , 612–619. |
[22] | Cermakova P , Johnell K , Fastbom J , Garcia-Ptacek S , Lund LH , Winblad B , Eriksdotter M , Religa D ((2015) ) Cardiovascular diseases in ∼30,000 patients in the Swedish Dementia Registry. J Alzheimers Dis 48: , 949–958. |
[23] | Cermakova P , Szummer K , Johnell K , Fastbom J , Winblad B , Eriksdotter M , Religa D ((2017) ) Management of acute myocardial infarction in patients with dementia: Data from SveDem, the Swedish Dementia Registry. J Am Med Dir Assoc 18: , 19–23. |
[24] | Quan H , Sundararajan V , Halfon P , Fong A , Burnand B , Luthi JC , Saunders LD , Beck CA , Feasby TE , Ghali WA ((2005) ) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: , 1130–1139. |
[25] | Fereshtehnejad SM , Johannsen P , Waldemar G , Eriksdotter M ((2015) ) Dementia diagnosis, treatment, and care in specialist clinics in two Scandinavian countries: A data comparison between the Swedish Dementia Registry (SveDem) and the Danish Dementia Registry. J Alzheimers Dis 48: , 229–239. |
[26] | Eichler T , Hoffmann W , Hertel J , Richter S , Wucherer D , Michalowsky B , Dreier A , Thyrian JR ((2016) ) Living alone with dementia: Prevalence, correlates and the utilization of health and nursing care services. J Alzheimers Dis 52: , 619–629. |
[27] | Maxwell CJ , Stock K , Seitz D , Herrmann N ((2014) ) Persistence and adherence with dementia pharmacotherapy: Relevance of patient, provider, and system factors. Can J Psychiatry 59: , 624–631. |
[28] | Calvo-Perxas L , Lopez-Pousa S , Vilalta-Franch J , Turro-Garriga O , Blankenburg M , Febrer L , Flaque M , Vallmajo N , Aguirregomozcorta M , Genis D , Casas I , Perkal H , Coromina J , Garre-Olmo J , Registryof Dementias of Girona Study Group ((2012) ) Central nervous system drug consumption depending on thetime between symptom onset and the diagnosis of Alzheimer’sdisease: An analysis by the Registry of Dementias of Girona. Dement Geriatr Cogn Disord 33: , 104–110. |
[29] | Henderson AS , Korten AE , Levings C , Jorm AF , Christensen H , Jacomb PA , Rodgers B ((1998) ) Psychotic symptoms in the elderly: A prospective study in a population sample. Int J Geriatr Psychiatry 13: , 484–492. |
[30] | Xiu-Ying H , Qian C , Xiao-Dong P , Xue-Mei Z , Chang-Quan H ((2012) ) Living arrangements and risk for late life depression: A meta-analysis of published literature. Int J Psychiatry Med 43: , 19–34. |
[31] | Hagg M , Houston B , Elmstahl S , Ekstrom H , Wann-Hansson C ((2014) ) Sleep quality, use of hypnotics and sleeping habits in different age-groups among older people. Scand J Caring Sci 28: , 842–851. |
[32] | Enache D , Winblad B , Aarsland D ((2011) ) Depression in dementia: Epidemiology, mechanisms, and treatment. Curr Opin Psychiatry 24: , 461–472. |
[33] | Urrestarazu E , Iriarte J ((2016) ) Clinical management of sleep disturbances in Alzheimer’s disease: Current and emerging strategies. Nat Sci Sleep 8: , 21–33. |
[34] | Douglas IJ , Smeeth L ((2008) ) Exposure to antipsychotics and risk of stroke: Self controlled case series study. BMJ 337: , a1227. |
[35] | Religa D , Spangberg K , Wimo A , Edlund AK , Winblad B , Eriksdotter-Jonhagen M ((2012) ) Dementia diagnosis differs in men and women and depends on age and dementia severity: Data from SveDem, the Swedish Dementia Quality Registry. Dement Geriatr Cogn Disord 33: , 90–95. |
[36] | Aspberg S , Stenestrand U , Koster M , Kahan T ((2013) ) Large differences between patients with acute myocardial infarction included in two Swedish health registers. Scand J Public Health 41: , 637–643. |



