Diagnostic Accuracy of a Combined Analysis of Cerebrospinal Fluid t-PrP, t-tau, p-tau, and Aβ42 in the Differential Diagnosis of Creutzfeldt-Jakob Disease from Alzheimer’s Disease with Emphasis on Atypical Disease Variants
Abstract
According to recent studies, the determination of cerebrospinal fluid (CSF) total tau (t-tau)/phosphorylated tau (p-tau) ratio and total prion protein (t-PrP) levels significantly improves the accuracy of the diagnosis of Alzheimer’s disease (AD) in atypical cases with clinical or laboratory features mimicking Creutzfeldt-Jakob disease (CJD). However, this has neither been validated nor tested in series including atypical CJD variants. Furthermore, the added diagnostic value of amyloid-β (Aβ)42 remains unclear. To address these issues, we measured t-PrP, 14-3-3, t-tau, p-tau, and Aβ42 CSF levels in 45 typical and 44 atypical/rapidly progressive AD patients, 54 typical and 54 atypical CJD patients, and 33 controls. CJD patients showed significantly lower CSF t-PrP levels than controls and AD patients. Furthermore, atypical CJD was associated with lower t-PrP levels in comparison to typical CJD. T-tau, 14-3-3, or t-PrP alone yielded, respectively, 80.6, 63.0, and 73.0% sensitivity and 75.3, 92.1, and 75% specificity in distinguishing AD from CJD. On receiver operating characteristic (ROC) curve analyses of biomarker combinations, the (t-tau×Aβ42)/(p-tau×t-PrP) ratio achieved the best accuracy, with 98.1% sensitivity and 97.7% specificity overall, and 96.2% sensitivity and 95.5% specificity for the “atypical” disease groups. Our results show that the combined analysis of CSF t-PrP, t-tau, p-tau, and Aβ42 is clinically useful in the differential diagnosis between CJD and AD. Furthermore, the finding of reduced CSF t-PrP levels in CJD patients suggest that, likewise Aβ42 in AD, CSF t-PrP levels reflect the extent of PrPc conversion into abnormal PrP (PrPSc) and the burden of PrPSc deposition in CJD.
INTRODUCTION
Creutzfeldt-Jakob disease (CJD) and Alzheimer’s disease (AD) are neurodegenerative disorders with overlapping clinical and laboratory features. CJD includes six major clinical-pathological subtypes that are largely determined by the genotype at the methionine (M)/valine (V) polymorphic codon 129 of the PRNP gene and the type (1 or 2) of pathological prion protein accumulating in the brain (namely MM1, MM2, MV1, MV2, VV1, and VV2) [1]. While the most common MM/MV1 subtype usually manifest with a subacute clinical course, atypical sporadic CJD variants such as MV 2K, MM 2C, and VV1 show a significantly slower clinical course, which may mimic AD and other neurodegenerative dementias [2]. On the other hand, although AD typically presents with a slowly progressive and well defined profile of cognitive decline, there also are well recognized variants of the disease with a rapid clinical progression and/or focal neurological signs manifesting relatively early in the disease course [3–6]. Cerebrospinal fluid (CSF) biomarker analysis is increasingly used in the diagnostic work-up of patients with neurodegenerative dementia. In particular, the search for elevated, above threshold, levels of protein 14-3-3 and/or t-tau is recommended by diagnostic criteria for CJD [7, 8], whereas a reduced concentration of amyloid-β peptide 1–42 (Aβ42) in combination with increased levels of both total (t-tau) and/or phosphorylated tau (p-tau) represents a well-established neurochemical profile in support of the clinical diagnosis of AD [9, 10]. However, the lack of full sensitivity and specificity of these biomarkers still represents a significant limitation. In particular, approximately 10% of AD patients have t-tau levels compatible with CJD and/or show a positive 14-3-3 assay [5, 6, 11–19], whereas a similar proportion of CJD cases, especially those of the MV2K, MM2C, and MM2T subtypes, is associated with a negative 14-3-3 test and/or with low t-tau levels [2, 19–21]. Finally, reduced Aβ42 CSF levels have also been found in CJD [22–27]. To improve the differential diagnosis between AD and CJD based on CSF biomarkers, some studies analyzed the performance of the combined analyses of multiple protein markers including t-tau and p-tau, but also total prion protein (t-PrP) [28–30]. Most significantly, the addition of t-PrP dosage and the calculation of the t-tau/(p-tau×t-PrP) ratio, also designated as “CJ factor”, has allowed, to date, the most accurate diagnosis in AD with clinical overlap with CJD [29]. However, these results have not been widely validated and there have been some divergent results between studies in terms oft-PrP CSF levels. Furthermore, the accuracy of the proposed CSF biomarker combination in the atypical variants of CJD has not been investigated as yet. In this study, we evaluated the diagnostic performance of CSF t-PrP levels either alone or in combination with other protein biomarkers, including Aβ42 in typical and atypical variants of both CJD and AD.
MATERIALS AND METHODS
Patient characterization
We analyzed 230 CSF samples submitted to the Neuropathology Laboratory at the Institute of Neurological Sciences of Bologna, including 33 controls, 89 AD patients, and 108 CJD patients. Clinical history and follow-up data were acquired for each patient. Brain magnetic resonance imaging (MRI) studies, inclusive of fluid attenuated inversion recovery (FLAIR) and diffusion weighted imaging (DWI) sequences, and electroencephalographic (EEG) recording were also available as part of routine clinical investigations.
The diagnosis of AD was made according to the 2011 National Institute on Aging and the Alzheimer’s Association workgroup guidelines [31]. In particular, after a clinical follow-up of at least 24 months, all 89 AD patients fulfilled criteria for probable AD dementia with high evidence of the AD pathophysiological process. Moreover, evidence for AD pathology of high severity was obtained by neuropathological examination in 6 cases [32].
CJD patients were classified according to the updated WHO diagnostic criteria [7]. The largest group of “definite” CJD consisted of 83 autopsy-confirmed sporadic CJD and 8 genetic CJD, while the “probable” CJD group included 17 patients fulfilling the clinical criteria for possible CJD and showing a positive EEG and/or a positive DWI-MRI [7]. All CJD cases underwent PRNP genetic analysis, as described previously [33]. Moreover, PrPSc typing and CJD histotype classification was performed in all autopsied cases according to established methodologies and consensus criteria [34–35]. CSF samples, in both AD and CJD groups, were selected to include a significant number of patients with an atypical clinical presentation and/or anatypical CSF biomarker profile. In particular, we defined AD as atypical/rapidly progressive (a/rpAD) when at least one of the following features was present: (1) rapid cognitive decline or presence of additional motor signs at time of CSF analyses [29], (2) CSF t-tau >1200 pg/ml, or (3) a positive CSF 14-3-3 assay, whereas for CJD the classification of atypical required at least one among: (1) clinical course >2 years, (2) progressive cognitive decline without focal neurological signs (up to the time of CSF analyses), (3) CSF t-tau <1200 pg/ml, (4) borderline or negative CSF 14-3-3 assay. Finally, the control group included 33 age- and sex-matched subjects lacking any clinical or neuroradiological evidence of central nervous system disease.
In our population, there were 44 AD and 54 CJD patients manifesting an atypical clinical presentation and/or showing an atypical CSF biomarkers profile. Among the remaining patients, 45 were classified as typical AD and 54 as typical CJD.
Informed consent was obtained from all subjects included in the study according to the Declaration of Helsinki.
CSF biochemical analysis
CSF samples were obtained by lumbar puncture at L3/L4 or L4/L5 levels following a standard procedure. Samples were centrifuged at 1000×g for 10 min, divided into aliquots, and stored in polypropylene tubes at –80°C until analysis.
14-3-3 protein was detected by a western blot immunoassay using CSF controls with a weak or a strong 14-3-3 signal, respectively, as internal quality controls. The immunoreactivity signals were classified as negative when the 14-3-3 optical densitometric (OD) band was lower than the weakly positive control; borderline (or weakly positive), when the 14-3-3 OD was up to two times higher than the control, and positive when it was at least two times higher than the control [36].
We measured CSF t-tau, p-tau, and Aβ42, levels using commercially available enzyme-linked immunosorbent assay (ELISA) kits (INNOTEST htau-Ag, INNOTEST phosphorylated-Tau181, and INNOTEST Aβ1–42; Innogenetics/Fujirebio Europe) according to the manufacturer’s instructions. T-PrP CSF levels were determined using commercial Beta Prion Human Enzyme-Linked Immunoassay Test kits (AJ Roboscreen, Leipzig, Germany) according to the manufacturer’s instructions.
Statistical analyses
Statistical analysis was performed using SPSS software (version 21- IBM Analytics). Several combinations of biomarkers were analyzed. Depending on the data distribution, the Kruskal-Wallis test and the Mann-Whitney U test or the one-way ANOVA (followed by Tukey’s post hoc test) were used to test differences between AD patients, CJD patients, and controls regarding demographic data and biomarkers measurements. Data were expressed as mean±standard deviation (SD) or median and interquartile range (IQR). A Bonferroni correction was applied to multiple comparisons. Receiver operating characteristic analyses (ROC) were performed to establish the diagnostic accuracy, sensitivity, and specificity and the optimal cut-off value of each biomarker or combination of biomarkers. Differences were considered statistically significant at p < 0.05.
RESULTS
Demographic characteristics
In the control, AD, and CJD groups, mean ages and standard deviations were 61.6±10.9, 66.4±9.1, 66.4±9.0 years, respectively. Regarding sex, 42.4% controls, 59.6% AD patients, and 50.9% CJD patients were female. The molecular classification of CJD cases and their disease duration are summarized in Table 1. Atypical CJD cases presented a longer duration of the disease than typical CJD cases (p < 0.001). Table 2 shows the clinical and laboratory features of cases classified as a/rpAD.
CSF t-PrP levels (Fig. 1)
CSF t-PrP levels were significantly lower in CJD patients compared to AD patients (p < 0.001) and controls (p < 0.001), but did not differ significantly between AD and controls (p = 0.500). A value of t-PrP lower than 261 ng/ml distinguished CJD patients from AD patients with a 73.0% sensitivity and a 75.0% specificity. Samples of typical CJD cases showed higher CSF t-PrP levels than those of the atypical group (p = 0.005).
CSF biomarker values in AD and CJD
CSF biomarker data in the AD and CJD groups are summarized in Table 3.
There were statistically significant differences between CJD (all) and AD (all) patients regarding t-tau (p < 0.001), 14-3-3 (p < 0.001), Aβ42 (p < 0.001), t-tau/p-tau ratio (p < 0.001), t-tau/t-PrP (p < 0.001), Aβ42/p-tau (p < 0.001), Aβ42/(p-tau×t-PrP) (p < 0.001), Aβ42×t-tau/p-tau (p < 0.001), CJ factor (p < 0.001), and (Aβ42×t-tau)/(p-tau×t-PrP) (p < 0.001).
Results from ROC analysis for biomarker combinations are illustrated in Table 4 and Fig. 2. The (t-tau×Aβ42)/(p-tau×t-PrP) ratio achieved the best accuracy (area under the curve (AUC) = 0.995) in the discrimination of CJD from AD with 98.1% sensitivity and 97.7% specificity, using a cut-off value of 24.0. The accuracy, cut-off values, sensitivity and specificity of the other biomarker combinations are illustrated in Table 4.
Subgroup analysis
ROC analyses were also performed for the following comparisons: atypical CJD versus atypical/rapidly progressive AD; typical CJD versus atypical/rapidly progressive AD; atypical CJD versus typical AD.
Atypical CJD versus atypical/rapidly progressive AD
Table 5 illustrates the biomarker accuracy, cut-off values, sensitivity, and specificity for the comparison between atypical CJD and a/rpAD groups. There were no significant differences in CSF t-tau levels and 14-3-3 assay results between a/rp AD and atypical CJD (p = 0.057 and p = 0.323, respectively). In contrast, values of Aβ42, t-tau/p-tau ratio, t-tau/t-PrP, Aβ42/p-tau, Aβ42/(p-tau×t-PrP), Aβ42×t-tau/p-tau, CJ factor, (Aβ42×t-tau)/(p-tau×t-PrP) significantly differed between the two groups (p < 0.001). While the 1200 pg/ml cut-off and 14-3-3 test yielded only 61.1% and 25.9% sensitivity and 50.0% and 84.1% specificity, respectively, in the differential diagnosis, the (t-tau×Aβ42)/(p-tau×t-PrP) ratio best distinguished (AUC = 0.986) atypical CJD from a/rp AD with 96.2% sensitivity and 95.5% specificity with an optimal cut-off value of 24. A relatively good sensitivity and specificity, with values above 90%, was also obtained for three out four biomarkers (t-tau and p-tau plus t-PrP or Aβ42).
Typical CJD versus atypical/rapidly progressive AD and atypical CJD versus typical AD
The biomarkers accuracy, cut-off values, sensitivity, and specificity for these comparisons are reported in Supplementary Tables 1 and 2. While the t-tau×Aβ42/p-tau ratio yielded the higher accuracy with 100% sensitivity and 97.7% specificity in the first comparison, the (t-tau×Aβ42)/(p-tau×t-PrP) ratio best distinguished typical AD from atypical CJD with 98.1% sensitivity and 100% specificity.
DISCUSSION
In this study we investigated for the first time the accuracy of CSF protein t-PrP, t-tau, 14-3-3, p-tau, and Aβ42 assays in the differential diagnosis between CJD and AD in a patient series including a high proportion of clinically atypical cases of both diseases. Furthermore, at variance with most previous studies which have mainly focused on t-tau, p-tau, and, to a lesser extent t-PrP, we also considered the added diagnostic value of Aβ42.
At variance with classic biomarkers such as t-tau, 14-3-3, p-tau, and Aβ42, there is still limited knowledge about t-PrP in the CSF in both controls and patients with neurodegenerative diseases. To date, few studies have measured CSF PrP levels in CJD, AD, and controls. Our finding of a statistically significant decrease in CSF t-PrP levels in CJD patients is consistent with those of other authors [29, 37–39]. Thus, as CSF Aβ42 levels inversely correlate with amyloid burden in AD, t-PrP levels likely reflect the extent of PrPc conversion into abnormal PrP (PrPSc) and the burden of PrPSc deposition in CJD. The latter may also explain our finding of an even higher reduction of t-PrP levels in atypical CJD cases. Indeed, atypical CJD variants such as MV 2K and MM 2C are usually associated with a relatively high amount of PrPSc accumulation involving major areas of the brain such as the cerebral cortex, basal ganglia, and thalamus.
Our results on t-PrP CSF levels in AD are at variance with both Dorey et al. [29] and Meyne et al. [40], who found either increased or decreased t-PrP levels in AD compared to controls. While the latter discrepancy has been attributed to the lack of specificity for the human prion protein of the assay used by Meyne et al. [40], the former may reflect differences in the selected patient populations. Whatever the case, further studies are needed to clarify the divergence, especially in view of the proposed putative role of PrPC in AD pathogenesis [29].
Regarding the diagnostic value of t-PrP in the discrimination of CJD from AD, we reported an AUC of 0.825, a sensitivity of 73%, and a specificity of 75% using a cut-off value of 261 ng/ml, which is slightly worse than that reported by Dorey et al. (AUC = 0.886, 82.1% sensitivity and 82.4% specificity) using the same kit and a very similar cut-off value (263 ng/ml). The small discrepancy likely reflects their findings of increased t-PrP levels compared to normal subjects in a subgroup of ADpatients.
In this study, we also addressed the possible diagnostic role of CSF Aβ42 and its derived ratios in the clinical distinction between CJD and AD. Van Everbroeck et al. [22] originally reported that Aβ42 levels are decreased in CJD, a finding that has subsequently been confirmed by others [22–27]. More recently, however, some studies showed that Aβ42 levels and Aβ42/p-tau ratio are significantly higher in CJD than in AD patients [21, 29, 41], thus indicating that the determination of Aβ42 levels might contribute to the differential diagnosis between the two diseases. In our CJD population, Aβ42 CSF levels were highly heterogeneous, with several cases of both typical and atypical CJD groups showing a lower than cut-off value. Whether this result simply reflects the burden of associated AD pathology, which has to be taken in account considering the mean age of death of CJD patients, or also depends on a pathogenic interaction between the two proteins, as it has recently been suggested [42, 43], remains to be seen.
Taken together, our results underline the added value of both Aβ42 and t-PrP CSF assays in the differential diagnosis between CJD and AD. Indeed, the addition of t-PrP and Aβ42 values to those of t-tau and p-tau in a novel ratio (t-tau×Aβ42)/(p-tau×t-PrP) distinguishes CJD from AD patients with greater accuracy (0.995 accuracy, 98.1% sensitivity, and 97.7% specificity) than all previously-described ratios such as CJ factor, t-tau/p-tau, and Aβ42/p-tau [21, 29, 30, 41]. Most importantly, the novel ratio also demonstrates superior diagnostic accuracy in the most difficult clinical scenario, which is the clinical distinction between atypical CJD and a/rpAD patients. Indeed, while CSF t-tau and 14-3-3 do not contribute at all to the differential diagnosis of such atypical variants (e.g., 61.1% and 25.9% sensitivity; 50% and 84.1% specificity), in the same situation the novel ratio yields 96.2% sensitivity and 95.5% specificity. We therefore strongly recommend extending the analyses to all four biomarkers in the presence of atypical clinical features and ambiguous results of standard CSF assays. Alternatively, particularly in a laboratory setting where the t-PrP assay is not available, Aβ42×t-tau/p-tau and t-tau/p-tau ratios remain the best biomarkers for accuracy, sensitivity, and specificity in distinguishing CJD from AD.
We are aware that one potential limitation of our study is the discrepancy in the number of neuropathologically-verified cases between CJD and AD. Nevertheless, all AD affected subjects included in this study fulfilled the diagnostic criteria of probable AD with high evidence of the pathophysiological process of AD. Furthermore, in all a/rpAD cases, the final diagnosis was formulated at follow-up after at least 2 years of further clinical observation.
In summary, while individually none of the major CSF proteins that reflect the specific molecular pathology of AD (p-tau and Aβ42) and CJD (PrP) or the associated neuronal damage (t-tau, 14-3-3) distinguish the two disorders with sufficient accuracy, various combinations of these markers significantly increase the diagnostic power. Among, them the (t-tau×Aβ42)/(p-tau×t-PrP) ratio best distinguishes CJD from AD patients and is especially recommended in the diagnostic work-up of patients presenting with atypical clinical features that are compatible with both diseases.
ACKNOWLEDGMENTS
The authors thank Italian neurologists for providing clinical information and Barbara Polischi, MSc for her valuable technical assistance.
This work was financially supported by the Italian Ministry of Health, grant RF2011-02351092, and by the Gino Galletti Foundation.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0740r2).
Appendices
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-160740.
REFERENCES
[1] | Parchi P , Giese A , Capellari S , Brown P , Schulz-Schaeffer W , Windl O , Zerr I , Budka H , Kopp N , Piccardo P , Poser S , Rojiani A , Streichemberger N , Julien J , Vital C , Ghetti B , Gambetti P , Kretzschmar H ((1999) ) Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46: , 224–233. |
[2] | Parchi P , Saverioni D ((2012) ) Molecular pathology, classification, and diagnosis of sporadic human prion disease variants. Folia Neuropathol 50: , 20–45. |
[3] | Carcaillon L , Peres K , Pere JJ , Helmer C , Orgogozo JM , Dartigues JF ((2007) ) Fast cognitive decline at the time of dementia diagnosis: A major prognostic factor for survival in the community. Dement Geriatr Cogn Disord 23: , 439–445. |
[4] | Reinwald S , Westner IM , Niedermaier N ((2004) ) Rapidly progressive Alzheimer’s disease mimicking Creutzfeldt-Jakob disease. J Neurol 251: , 1020–1022. |
[5] | Schmidt C , Haïk S , Satoh K , Rábano A , Martinez-Martin P , Roeber S , Brandel JP , Calero-Lara M , de Pedro-Cuesta J , Laplanche JL , Hauw JJ , Kretzschmar H , Zerr I ((2012) ) Rapidly progressive Alzheimer’s disease: A multicenter update. J Alzheimers Dis 30: , 751–756. |
[6] | Schmidt C , Wolff M , Weitz M , Bartlau T , Korth C , Zerr I ((2011) ) Rapidly progressive Alzheimer disease. Arch Neurol 68: , 1124–1130. |
[7] | Zerr I , Kallenberg K , Summers DM , Romero C , Taratuto A , Heinemann U , Breithaupt M , Varges D , Meissner B , Ladogana A , Schuur M , Haik S , Collins SJ , Jansen GH , Stokin GB , Pimentel J , Hewer E , Collie D , Smith P , Roberts H , Brandel JP , van Duijn C , Pocchiari M , Begue C , Cras P , Will RG , Sanchez-Juan P ((2009) ) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132: (Pt 10), 2659–2668. |
[8] | Parchi P , Capellari S ((2013) ) Prion disease: Diagnostic value of cerebrospinal fluid markers. Nat Rev Neurol 9: , 10–1. |
[9] | Henriksen K , O’Bryant SE , Hampel H , Trojanowski JQ , Montine TJ , Jeromin A , Blennow K , Lönneborg A , Wyss-Coray T , Soares H , Bazenet C , Sjögren M , Hu W , Lovestone S , Karsdal MA , Weiner MW , Blood-Based Biomarker Interest Group ((2014) ) The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement 10: , 115–131. |
[10] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[11] | Huang N , Marie SK , Livramento JA , Chammas R , Nitrini R ((2003) ) 14-3-3 protein in the CSF of patients with rapidly progressive dementia. Neurology 61: , 354–357. |
[12] | Jayaratnam S , Khoo AK , Basic D ((2008) ) Rapidly progressive Alzheimer’s disease and elevated 14-3-3 proteins in cerebrospinal fluid. Age Ageing 37: , 467–469. |
[13] | Kester MI , van der Vlies AE , Blankenstein MA , Pijnenburg YA , van Elk EJ , Scheltens P , van der Flier WM ((2009) ) CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology 73: , 1353–1358. |
[14] | Koric L , Felician O , Guedj E , Hubert AM , Mancini J , Boucraut J , Ceccaldi M ((2010) ) Could clinical profile influence CSF biomarkers in early-onset Alzheimer disease? Alzheimer Dis Assoc Disord 24: , 278–283. |
[15] | Mahmoudi R , Manckoundia P , Morrone I , Lang PO , Dramé M , Novella JL ((2010) ) Atypical case of Alzheimer’s disease mimicking Creutzfeldt-Jakob disease: Interest of cerebrospinal fluid biomarkers in the differential diagnosis. J Am Geriatr Soc 58: , 1821–1823. |
[16] | Snider BJ , Fagan AM , Roe C , Shah AR , Grant EA , Xiong C , Morris JC , Holtzman DM ((2009) ) Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol 66: , 638–645. |
[17] | Stoeck K , Sanchez-Juan P , Gawinecka J , Green A , Ladogana A , Pocchiari M , Sanchez-Valle R , Mitrova E , Sklaviadis T , Kulczycki J , Slivarichova D , Saiz A , Calero M , Knight R , Aguzzi A , Laplanche JL , Peoc’h K , Schelzke G , Karch A , van Duijn CM , Zerr I ((2012) ) Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: A longitudinal multicentre study over 10 years. Brain 135: (Pt 10), 3051–3061. |
[18] | Wallin AK , Blennow K , Zetterberg H , Londos E , Minthon L , Hansson O ((2010) ) CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology 74: , 1531–1537. |
[19] | Castellani RJ , Colucci M , Xie Z , Zou W , Li C , Parchi P , Capellari S , Pastore M , Rahbar MH , Chen SG , Gambetti P ((2004) ) Sensitivity of 14-3-3 protein test varies in subtypes of sporadic Creutzfeldt-Jakob disease. Neurology 63: , 436–442. |
[20] | Sanchez-Juan P , Green A , Ladogana A , Cuadrado-Corrales N , Sáanchez-Valle R , Mitrováa E , Stoeck K , Sklaviadis T , Kulczycki J , Hess K , Bodemer M , Slivarichová D , Saiz A , Calero M , Ingrosso L , Knight R , Janssens AC , van Duijn CM , Zerr I ((2006) ) CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology 67: , 637–643. |
[21] | Zanusso G , Fiorini M , Ferrari S , Gajofatto A , Cagnin A , Galassi A , Richelli S , Monaco S ((2011) ) Cerebrospinal fluid markers in sporadic Creutzfeldt-Jakob disease. Int J Mol Sci 12: , 6281–6292. |
[22] | Van Everbroeck B , Green AJ , Pals P , Martin JJ , Cras P ((1999) ) Decreased levels of amyloid-beta 1-42 in cerebrospinal fluid of Creutzfeldt-Jakob disease patients. J Alzheimers Dis 1: , 419–424. |
[23] | Otto M , Esselmann H , Schulz-Shaeffer W , Neumann M , Schröter A , Ratzka P , Cepek L , Zerr I , Steinacker P , Windl O , Kornhuber J , Kretzschmar HA , Poser S , Wiltfang J ((2000) ) Decreased beta-amyloid1-42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology 54: , 1099–1102. |
[24] | Kapaki E , Kilidireas K , Paraskevas GP , Michalopoulou M , Patsouris E ((2001) ) Highly increased CSF tau protein and decreased beta-amyloid (1-42) in sporadic CJD: A discrimination from Alzheimer’s disease? J Neurol Neurosurg Psychiatry 71: , 401–403. |
[25] | Wiltfang J , Esselmann H , Smirnov A , Bibl M , Cepek L , Steinacker P , Mollenhauer B , Buerger K , Hampel H , Paul S , Neumann M , Maler M , Zerr I , Kornhuber J , Kretzschmar HA , Poser S , Otto M ((2003) ) Beta-amyloid peptides in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Ann Neurol 54: , 263–267. |
[26] | Mollenhauer B , Esselmann H , Roeber S , Schulz-Schaeffer WJ , Trenkwalder C , Bibl M , Steinacker P , Kretzschmar HA , Wiltfang J , Otto M ((2011) ) Different CSF β-amyloid processing in Alzheimer’s and Creutzfeldt-Jakob disease. J Neural Transm (Vienna) 118: , 691–697. |
[27] | Varges D , Jung K , Gawinecka J , Heinemann U , Schmitz M , von Ahsen N , Krasnianski A , Armstrong VW , Zerr I ((2011) ) Amyloid-β 1-42 levels are modified by apolipoprotein E ɛ4 in Creutzfeldt-Jakob disease in a similar manner as in Alzheimer’s disease. J Alzheimers Dis 23: , 717–726. |
[28] | Blennow K , Johansson A , Zetterberg H ((2005) ) Diagnostic value of 14-3-3beta immunoblot and T-tau/P-tau ratio in clinically suspected Creutzfeldt-Jakob disease. Int J Mol Med 16: , 1147–1149. |
[29] | Dorey A , Tholance Y , Vighetto A , Perret-Liaudet A , Lachman I , Krolak-Salmon P , Wagner U , Struyfs H , De Deyn PP , El-Moualij B , Zorzi W , Meyronet D , Streichenberger N , Engelborghs S , Kovacs GG , Quadrio I ((2015) ) Association of cerebrospinal fluid prion protein levels and the distinction between Alzheimer disease and Creutzfeldt-Jakob disease. JAMA Neurol 72: , 267–275. |
[30] | Skillbäck T , Rosén C , Asztely F , Mattsson N , Blennow K , Zetterberg H ((2014) ) Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: Results from the Swedish Mortality Registry. JAMA Neurol 71: , 476–483. |
[31] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[32] | Montine TJ , Phelps CH , Beach TG , Bigio EH , Cairns NJ , Dickson DW , Duyckaerts C , Frosch MP , Masliah E , Mirra SS , Nelson PT , Schneider JA , Thal DR , Trojanowski JQ , Vinters HV , Hyman BT , National Institute on Aging, Alzheimer’s Association ((2012) ) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 123: , 1–11. |
[33] | Jansen C , Parchi P , Capellari S , Ibrahim-Verbaas CA , Schuur M , Strammiello R , Corrado P , Bishop MT , van Gool WA , Verbeek MM , Baas F , van Saane W , Spliet WG , Jansen GH , van Duijn CM , Rozemuller AJ ((2012) ) Human prion diseases in the Netherlands (1998-2009): Clinical, genetic and molecular aspects. PLoS One 7: , e36333. |
[34] | Parchi P , de Boni L , Saverioni D , Cohen ML , Ferrer I , Gambetti P , Gelpi E , Giaccone G , Hauw JJ , Höftberger R , Ironside JW , Jansen C , Kovacs GG , Rozemuller A , Seilhean D , Tagliavini F , Giese A , Kretzschmar HA ((2012) ) Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: An inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol 124: , 517–529. |
[35] | Parchi P , Notari S , Weber P , Schimmel H , Budka H , Ferrer I , Haik S , Hauw JJ , Head MW , Ironside JW , Limido L , Rodriguez A , Ströbel T , Tagliavini F , Kretzschmar HA ((2009) ) Inter-laboratory assessment of PrPSc typing in Creutzfeldt-Jakob disease: A western blot study within the NeuroPrion Consortium. Brain Pathol 19: , 384–391. |
[36] | Baiardi S , Capellari S , Ladogana A , Strumia S , Santangelo M , Pocchiari M , Parchi P ((2015) ) Revisiting the Heidenhain variant of Creutzfeldt-Jakob disease: Evidence for prion type variability influencing clinical course and laboratory findings. J Alzheimers Dis 50: , 465–476. |
[37] | Llorens F , Ansoleaga B , Garcia-Esparcia P , Zafar S , Grau-Rivera O , López-González I , Blanco R , Carmona M , Yagüe J , Nos C , Del Río JA , Gelpí E , Zerr I , Ferrer I ((2013) ) PrP mRNA and protein expression in brain and PrP(c) in CSF in Creutzfeldt-Jakob disease MM1 and VV2. Prion 7: , 383–393. |
[38] | Schmitz M , Lüllmann K , Zafar S , Ebert E , Wohlhage M , Oikonomou P , Schlomm M , Mitrova E , Beekes M , Zerr I ((2014) ) Association of prion protein genotype and scrapie prion protein type with cellular prion protein charge isoform profiles in cerebrospinal fluid of humans with sporadic or familial prion diseases. Neurobiol Aging 35: , 1177–1188. |
[39] | Torres M , Cartier L , Matamala JM , Hernández N , Woehlbier U , Hetz C ((2012) ) Altered prion protein expression pattern in CSF as a biomarker for Creutzfeldt-Jakob disease. PLoS One 7: , e36159. |
[40] | Meyne F , Gloeckner SF , Ciesielczyk B , Heinemann U , Krasnianski A , Meissner B , Zerr I ((2009) ) Total prion protein levels in the cerebrospinal fluid are reduced in patients with various neurological disorders. J Alzheimers Dis 17: , 863–873. |
[41] | Struyfs H , Niemantsverdriet E , Goossens J , Fransen E , Martin JJ , De Deyn PP , Engelborghs S ((2015) ) Cerebrospinal fluid P-Tau181P: Biomarker for improved differential dementia diagnosis. Front Neurol 6: , 138. |
[42] | Ghoshal N , Cali I , Perrin RJ , Josephson SA , Sun N , Gambetti P , Morris JC ((2009) ) Codistribution of amyloid beta plaques and spongiform degeneration in familial Creutzfeldt-Jakob disease with the E200K-129M haplotype. Arch Neurol 66: , 1240–1246. |
[43] | Laurén J , Gimbel DA , Nygaard HB , Gilbert JW , Strittmatter SM ((2009) ) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457: , 1128–1132. |
Figures and Tables
Fig.1
Boxplots illustrate levels of the CSF total prion protein (t-PrP) in control, Alzheimer’s disease (AD), and Creutzfeldt-Jakob disease (CJD) populations. Central horizontal lines indicate median values. Boxes illustrate the ranges between lower and upper quartiles. Error bars represent the full ranges of data, excluding outliers, which are displayed separately as single dots.
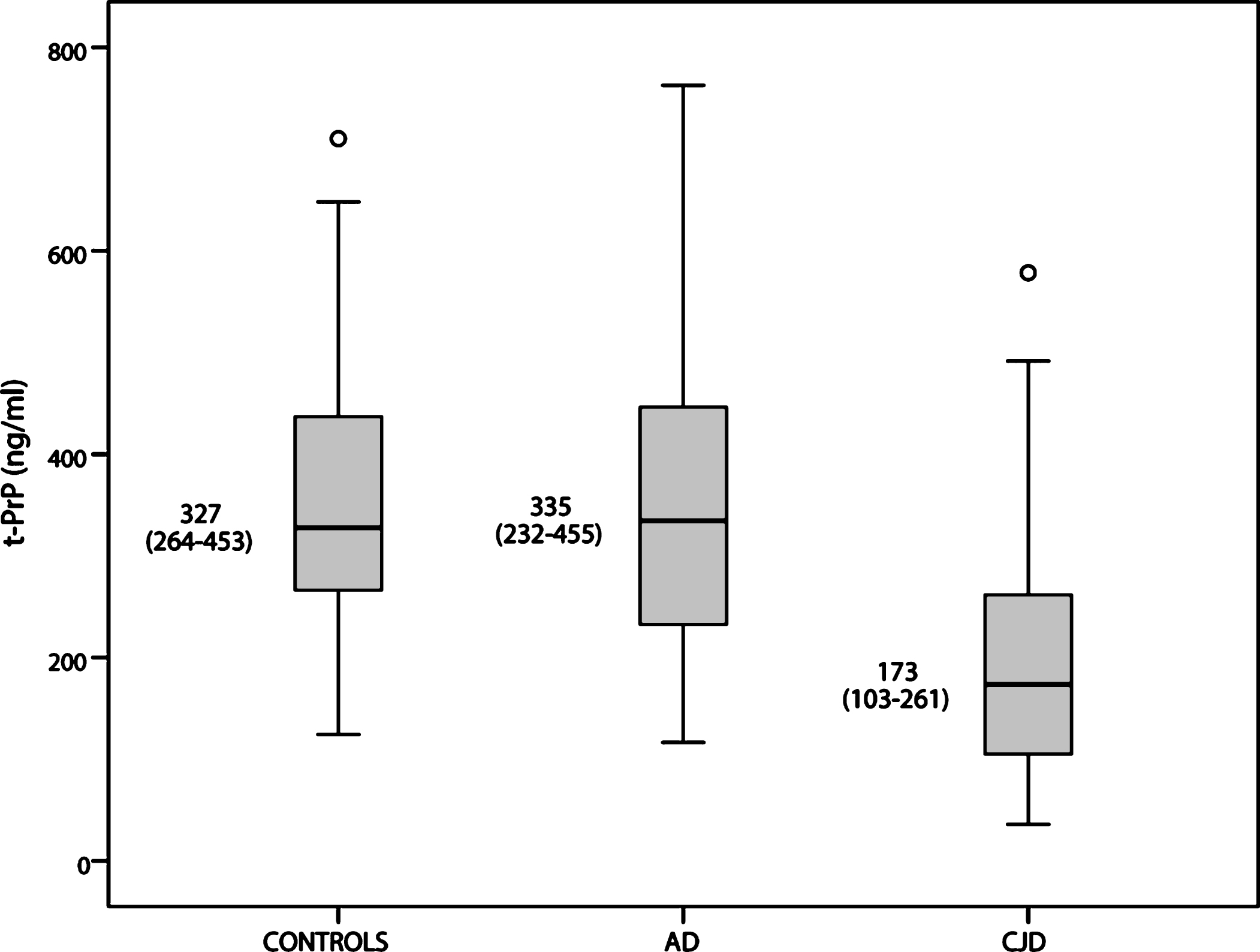
Fig.2
Receiver operating characteristics (ROC) curves illustrate sensitivity and specificity of various cerebrospinal fluid biomarker combinations. Area under the ROC curve is reported as area under the curve (AUC). The corresponding AUC values are also listed in Table 4.
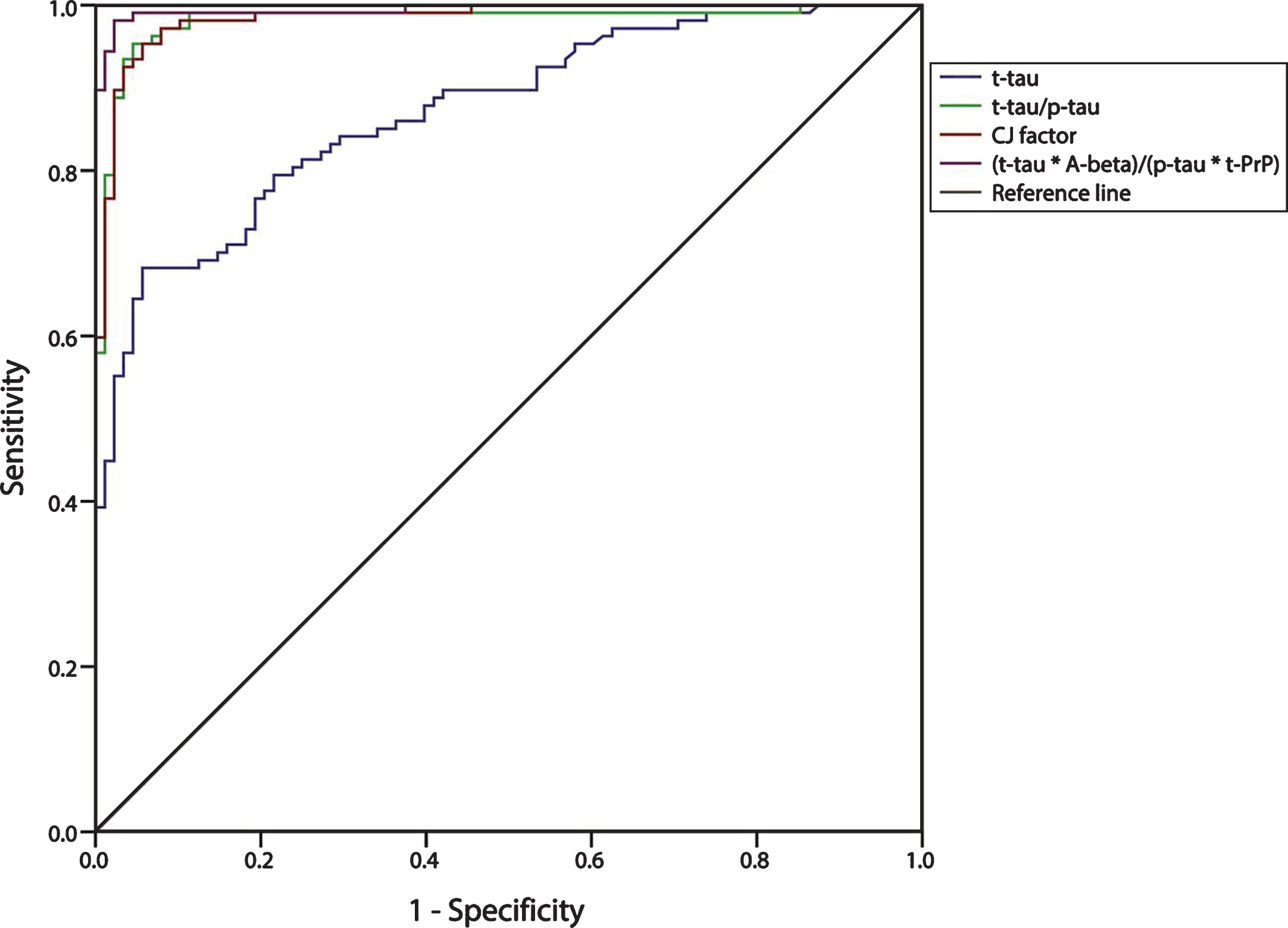
Table 1
Disease duration and histotype classification of CJD cases
| Typical CJD (N = 54) | Atypical CJD (N = 54) | Total CJD (N = 108) | |
| Disease duration (months) | N = 52 | N = 52 | N = 104 |
| Mean±SD | 5.0±4.0 | 18.6±14.8 | 11.8±12.7 |
| Min-Max | 1.0–20.0 | 1.0–60.0 | 1.0–60.0 |
| Median (IQR) | 4.0 (2.0–6.4) | 13.8 (6.5–24.8) | 6.3 (3.0–15.5) |
| Definite sporadic CJD | 51 | 32 | 83 |
| MM1 | 23 | 6 | 29 |
| MM1+2C | 8 | 5 | 13 |
| VV2 | 15 | 0 | 15 |
| MV 2K | 5 | 13 | 18 |
| MM 2C | 0 | 5 | 5 |
| MM 2T | 0 | 2 | 2 |
| VV1 | 0 | 1 | 1 |
| Definite genetic CJD | 1 | 7 | 8 |
| E200K-129MM(V) | 1 | 6 | 7 |
| R208H-129VV | 0 | 1 | 1 |
| Probable CJD | 2 | 15 | 17 |
| MM | 0 | 3 | 3 |
| MV | 2 | 12 | 14 |
Table 2
Clinical and biological features of atypical/rapidly progressive AD
| Time between first compliant | 26.3±25.6 (n = 44) |
| and LP (months) | |
| Clinical presentation | |
| Cognitive decline | 44/44 |
| Extrapyramidal signs | 9/44 |
| Pyramidal signs | 3/44 |
| Myoclonus | 5/44 |
| Cerebellar signs | 2/44 |
| Biomarkers Data | |
| t-tau >1200 pg/ml | 22/44 |
| Positive 14-3-3 | 7/44 |
| Genetic features | |
| APOE genotype§ | No E4 = 18 (58.1%) |
| One E4 = 8 (25.8%) | |
| E4/E4 = 5 (16%) | |
| FAD Mutations* | 2/13 |
| (APP, PSEN1, PSEN2) | (both in PSEN1) |
§patients (n = 31) with available DNA/informed consent for genetic analyses. *patients (n = 13) with early onset AD (<65 years) and/or a positive family history.
Table 3
CSF biomarker data in the AD and CJD groups
| all AD | Typical AD | a/rpAD | all CJD | Typical CJD | Atypical CJD | |
| (N = 89) | (N = 45) | (N = 44) | (N = 108) | (N = 54) | (N = 54) | |
| t-PrP (ng/ml) | ||||||
| Median | 335 | 334 | 345 | 173 | 209 | 141 |
| (IQR) | (234–455) | (281–455) | (224–469) | (103–261) | (131–288) | (83–208) |
| t-tau (pg/ml) | ||||||
| Median | 822 | 697 | 1223 | 2489 | 7284 | 1390 |
| (IQR) | (582–1223) | (509–846) | (703–1668) | (1389–7344) | (3022–10004) | (914–2086) |
| p-tau (pg/ml) | ||||||
| Median | 104 | 90 | 122 | 49 | 55 | 46 |
| (IQR) | (77–140) | (74–115) | (81–151) | (37–68) | (41–72) | (34–65) |
| Aβ42 (pg/ml) | ||||||
| Median | 358 | 371 | 326 | 527 | 553 | 498 |
| (IQR) | (266–465) | (279–469) | (250–442) | (366–747) | (353–773) | (370–738) |
| 14-3-3 | ||||||
| N° positive | 7/89 | 0/45 | 7/44 | 68/108 | 54/54 | 14/54 |
| t-tau/p-tau | ||||||
| Median | 7.67 | 7.13 | 9.08 | 59.3 | 111 | 32.2 |
| (IQR) | (6.76–10.1) | (6.38–7.98) | (7.35–11.3) | (27.1–110) | (64.5–198) | (20.6–52.1) |
| t-tau/t-PrP | ||||||
| Median | 2.58 | 2.22 | 3.36 | 17.4 | 33.6 | 10.7 |
| (IQR) | (1.81–3.86) | (1.68–2.61) | (2.29–5.40) | (8.29–39.4) | (17.4–76.9) | (6.10–17.3) |
| Aβ42/p-tau | ||||||
| Median | 3.63 | 4.09 | 3.20 | 10.1 | 9.5 | 12.3 |
| (IQR) | (2.47–4.70) | (3.11–5.29) | (1.88–4.23) | (7.18–16.1) | (5.55–14.6) | (8.09–16.7) |
| Aβ42/ (p-tau×t-PrP) | ||||||
| Median | 0.010 | 0.011 | 0.009 | 0.062 | 0.053 | 0.071 |
| (IQR) | (0.007–0.018) | (0.008–0.019) | (0.005–0.016) | (0.035–0.107) | (0.026–0.090) | (0.044–0.144) |
| Aβ42×t-tau/p-tau | ||||||
| Median | 2870 | 2615 | 3089 | 27675 | 55348 | 15176 |
| (IQR) | (2143–3938) | (2010–3373) | (2344–4661) | (13490–59655) | (30720–121296) | (9099–27419) |
| t-tau/(p-tau×t-PrP) | ||||||
| Median | 0.024 | 0.022 | 0.032 | 0.370 | 0.637 | 0.223 |
| (IQR) | (0.018–0.037) | (0.018–0.031) | (0.019–0.045) | (0.161–0.778) | (0.238–1.345) | (0.131–0.460) |
| (t-tau×Aβ42)/(p-tau×t-PrP) | ||||||
| Median | 8.63 | 7.64 | 10.9 | 155 | 263 | 104 |
| (IQR) | (6.02–12.6) | (5.68–10.6) | (6.06–14.8) | (82.8–401) | (134–682) | (69.9–205) |
Table 4
Comparison of discriminatory power of CSF biomarkers to distinguish AD from CJD
| Biomarker | Area under | Cut-off value | Patients diagnosed | Sensitivity % | Specificity % | |
| the curve (AUC) | for CJD diagnosis | as having CJD | ||||
| CJD | AD | |||||
| t-PrP | 0.825±0.029 | <261 ng/ml | 81/108 | 24/89 | 73.0 | 75.0 |
| 14-3-3 | – | – | 68/108 | 7/89 | 63.0 | 92.1 |
| Aβ42/p-tau | 0.914±0.021 | >5.11 | 90/107 | 15/88 | 84.1 | 83.0 |
| Aβ42/(p-tau×t-PrP) | 0.956±0.014 | >0.022 | 94/107 | 10/88 | 87.9 | 88.6 |
| t-tau | 0.865±0.025 | >1200 pg/ml | 87/108 | 22/89 | 80.6 | 75.3 |
| t-tau/p-tau | 0.982±0.010 | >16.4 | 103/107 | 4/88 | 96.3 | 95.5 |
| t-tau/t-PrP | 0.945±0.016 | >5.50 | 98/108 | 8/89 | 90.7 | 91.0 |
| Aβ42×t-tau/p-tau | 0.984±0.008 | >6677 | 101/107 | 5/88 | 94.4 | 94.3 |
| t-tau/(p-tau×t-PrP) | 0.984±0.007 | >0.061 | 104/107 | 4/88 | 97.1 | 95.5 |
| (t-tau×Aβ42)/(p-tau×t-PrP) | 0.995±0.004 | >24.0 | 105/107 | 2/88 | 98.1 | 97.7 |
Table 5
Comparison of discriminatory power of CSF biomarkers in the distinction between atypical AD and atypical CJD
| Biomarker | Area under the | Cut-off value | Patients diagnosed | Sensitivity % | Specificity % | |
| curve (AUC) | for CJD diagnosis | as having CJD | ||||
| Atypical CJD | a/rp AD | |||||
| t-tau | 0.612±0.057 | >1200 pg/ml | 33/54 | 22/44 | 61.1 | 50.0 |
| 14-3-3 | – | – | 14/54 | 7/44 | 25.9 | 84.1 |
| Aβ42/p-tau | 0.945±0.025 | >4.70 | 48/53 | 4/44 | 90.6 | 90.9 |
| Aβ42/(p-tau×t-PrP) | 0.982±0.010 | >0.025 | 49/53 | 2/44 | 92.5 | 95.5 |
| t-tau/p-tau | 0.945±0.025 | >16.3 | 49/54 | 4/44 | 90.6 | 90.9 |
| t-tau/t-PrP | 0.852±0.040 | >5.27 | 45/54 | 8/44 | 83.3 | 81.8 |
| Aβ42×t-tau/p-tau | 0.958±0.020 | >6550 | 49/53 | 3/44 | 92.5 | 93.2 |
| t-tau/(p-tau×t-PrP) | 0.955±0.020 | >0.063 | 50/54 | 3/44 | 92.6 | 93.2 |
| (t-tau×Aβ42)/(p-tau×t-PrP) | 0.986±0.010 | >24.0 | 51/53 | 2/44 | 96.2 | 95.5 |



